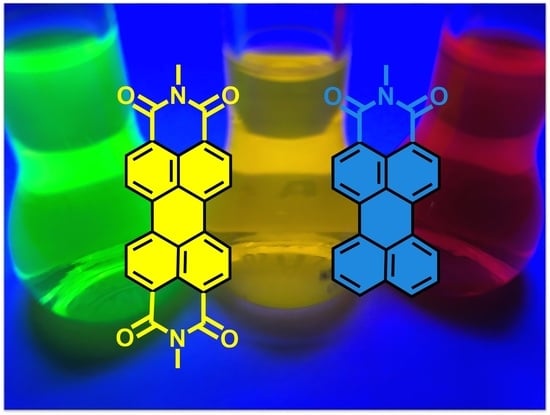
Recent Advances in Applications of Fluorescent Perylenediimide and Perylenemonoimide Dyes in Bioimaging, Photothermal and Photodynamic Therapy
Abstract
1. Introduction
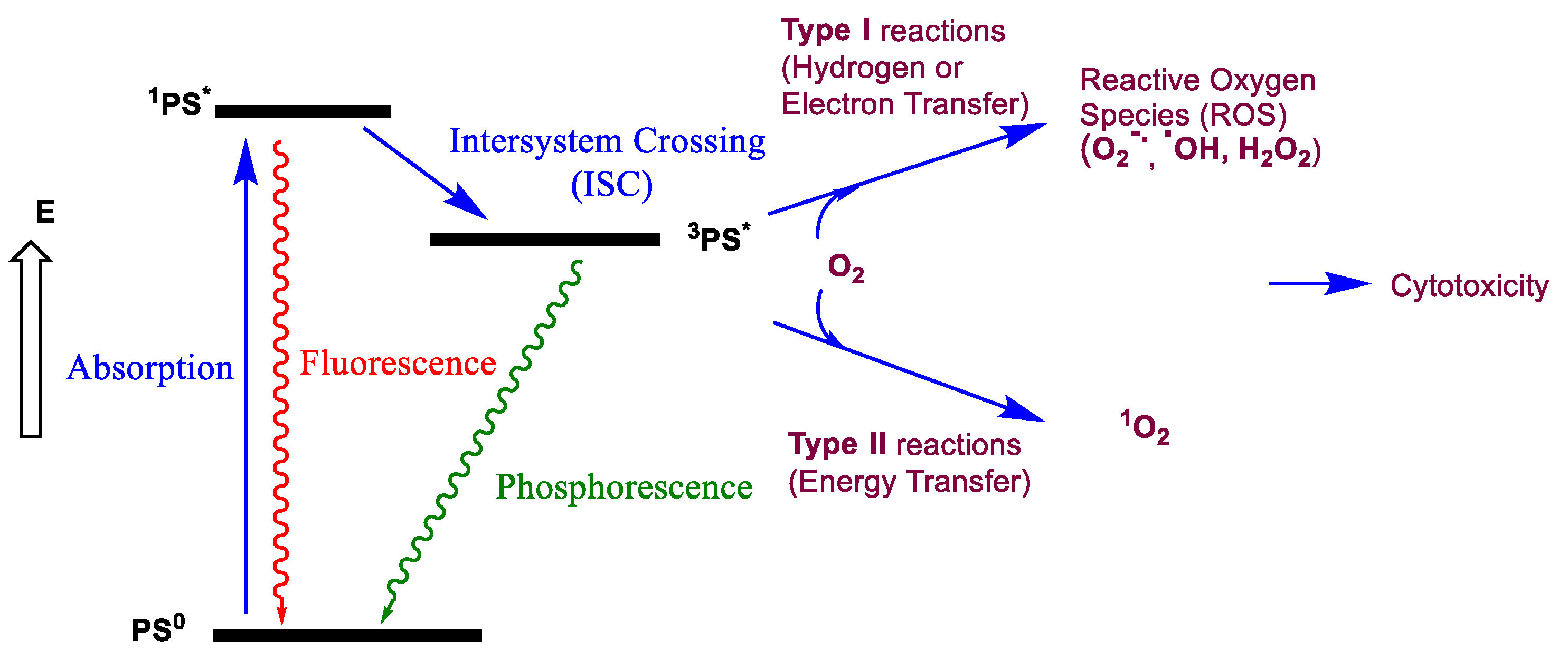
2. Presentation and Requirements for Photodynamic Therapy, Photothermal Therapy and Bioimaging Applications
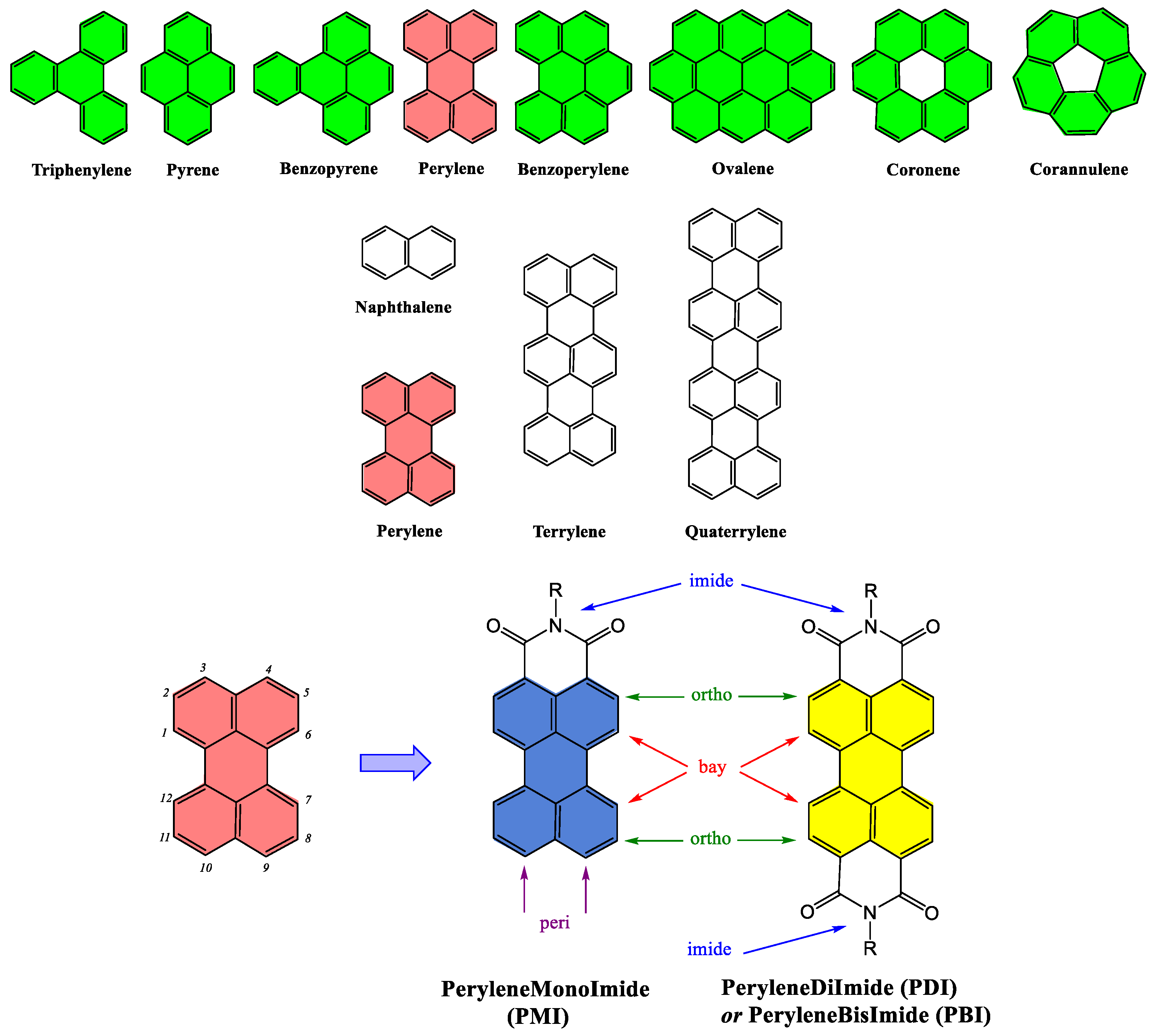
3. PDI and PMI Structures: Common Features and Specificities
3.1. Structural Elements for the Design of PDI and PMI Derivatives
3.2. Perylenediimide (PDI)-Based Building Blocks for Designing New Architectures
3.3. Perylenemonimide (PMI)-Based Building Blocks for Designing Architectures
4. Perylenediimide (PDI)-Based Systems for Bioimaging, PTT and PDT
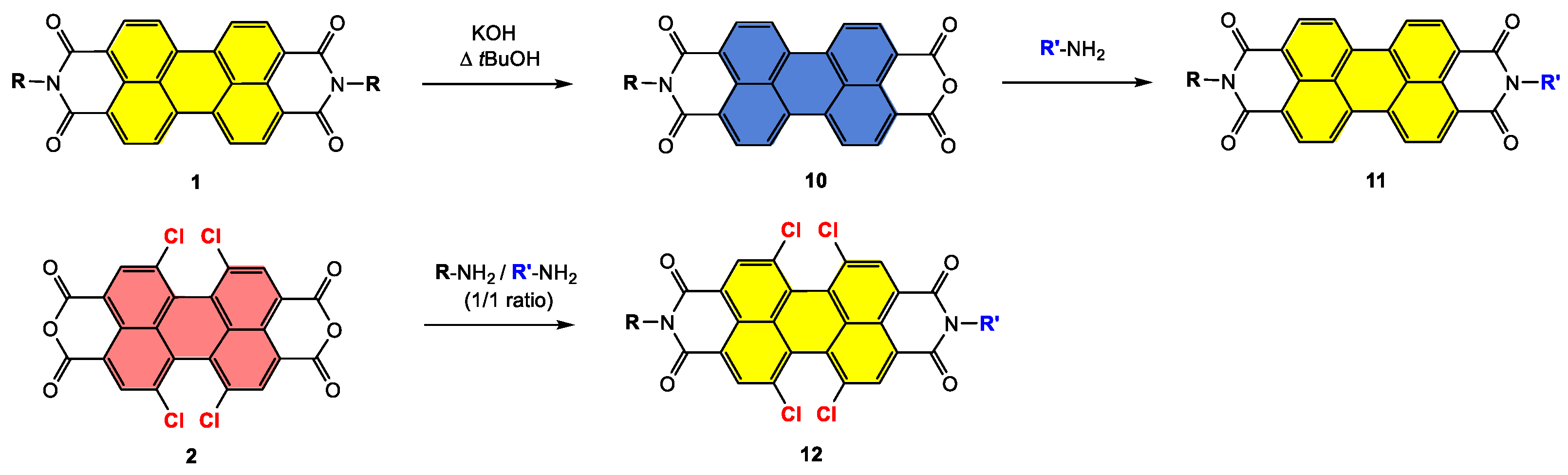
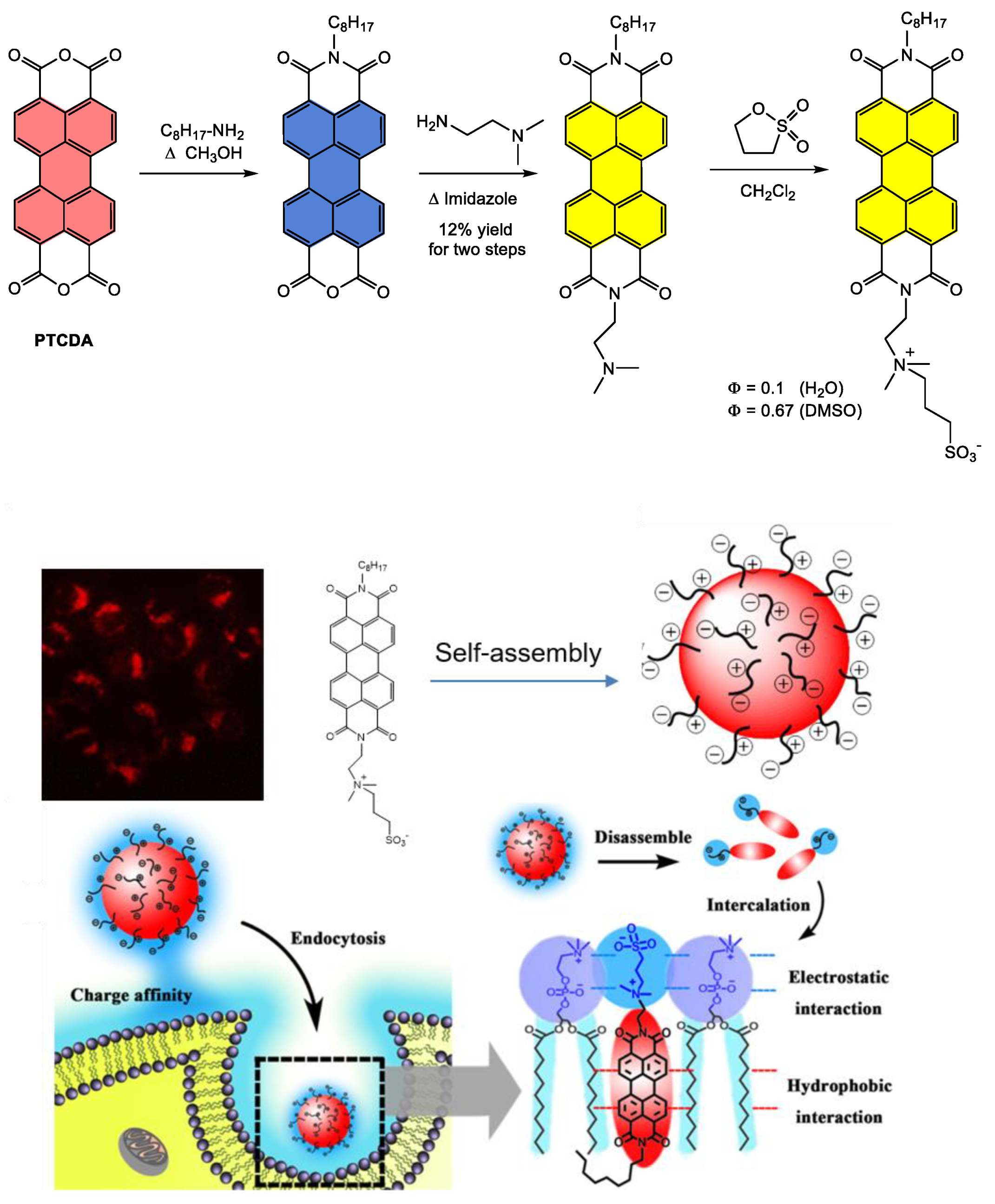
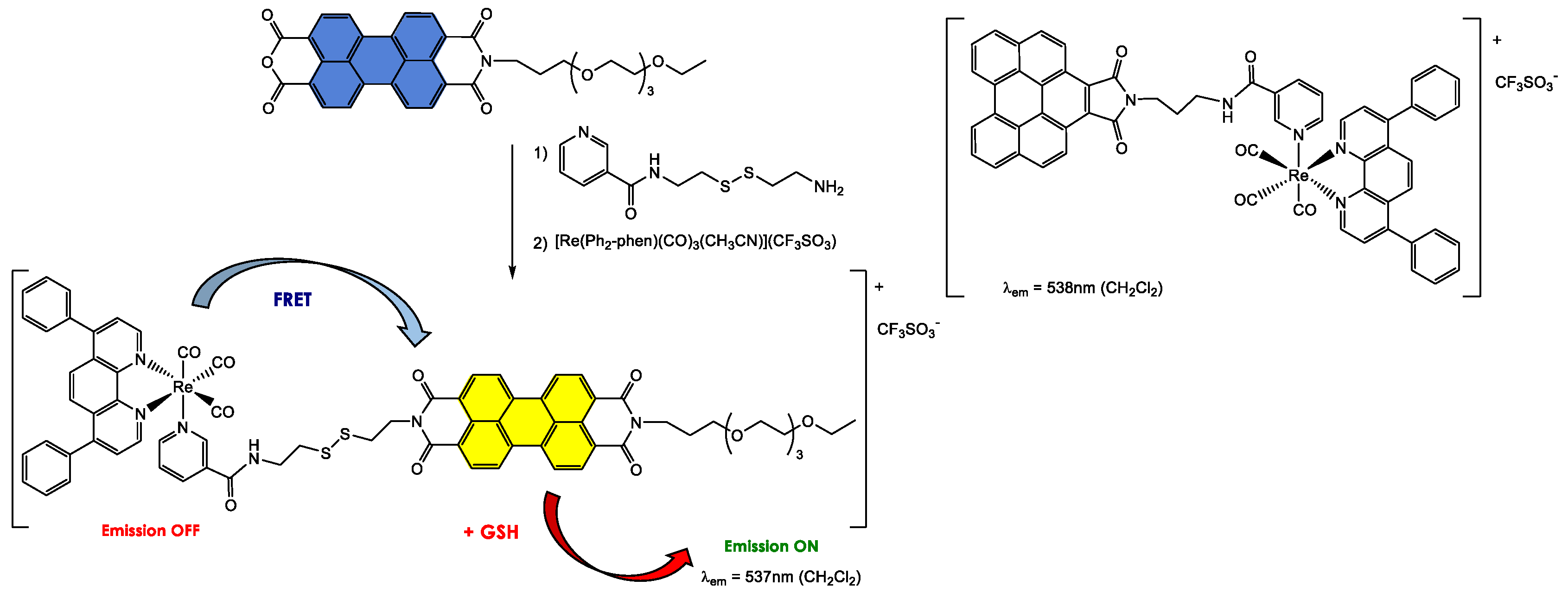
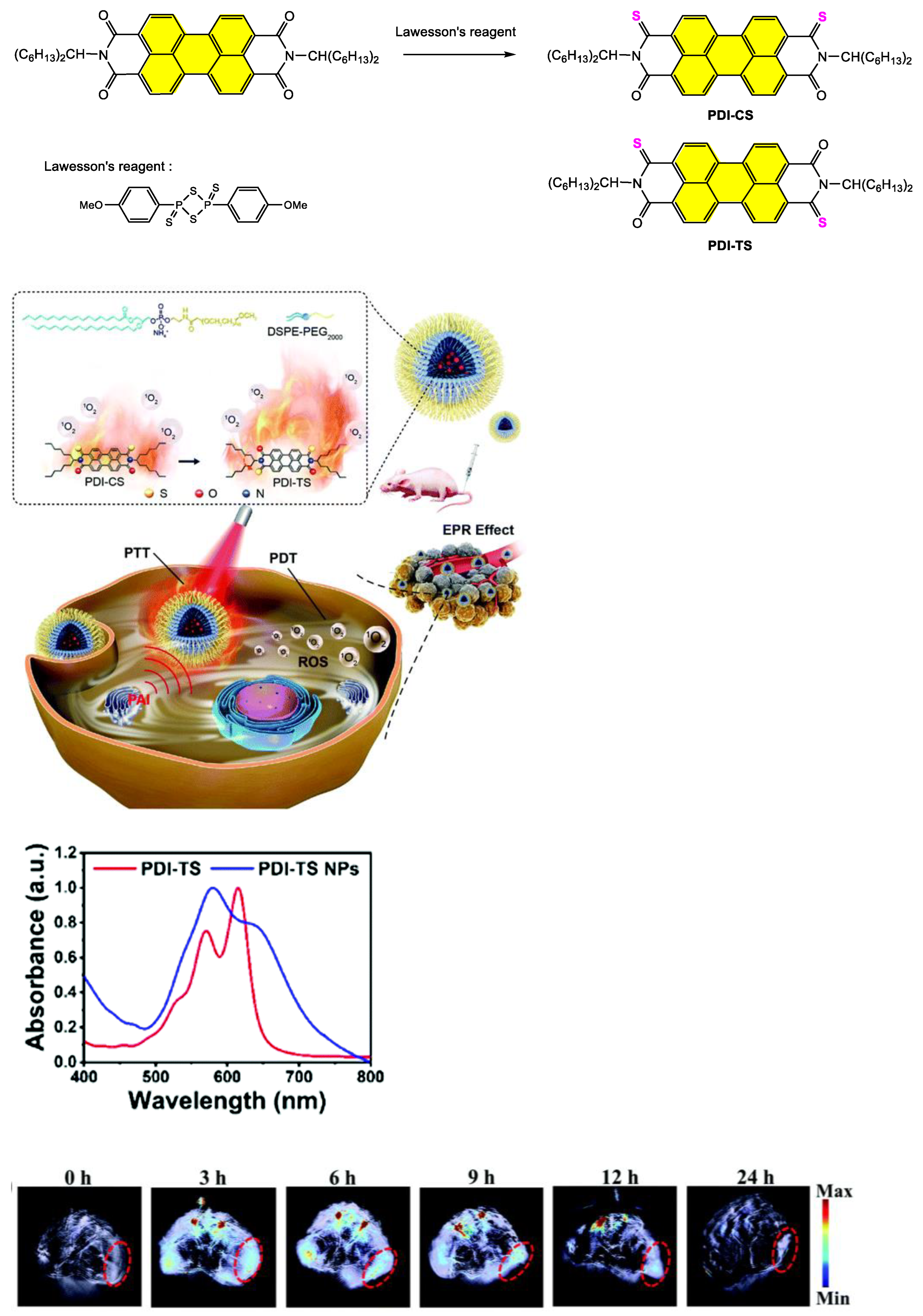
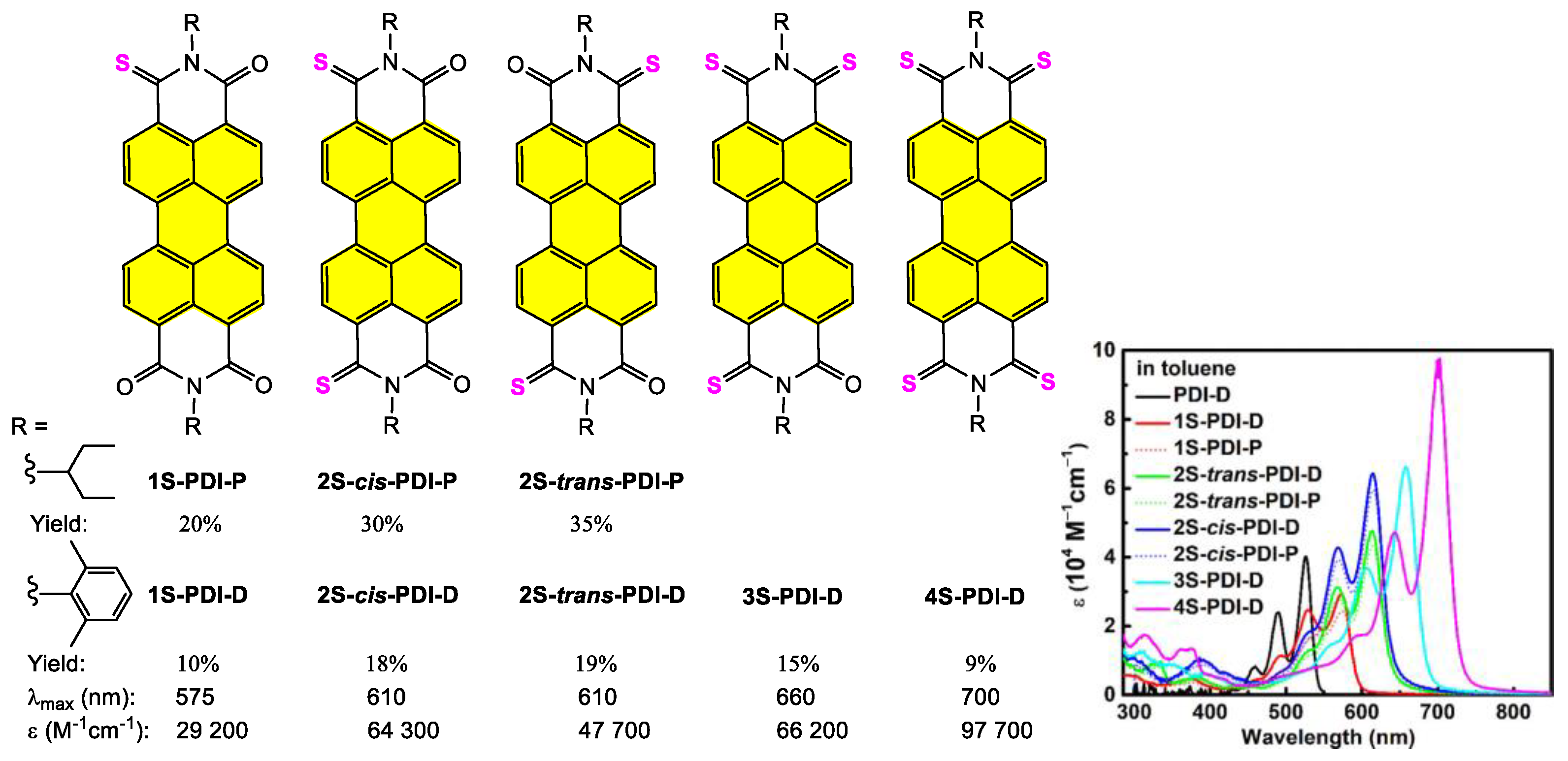
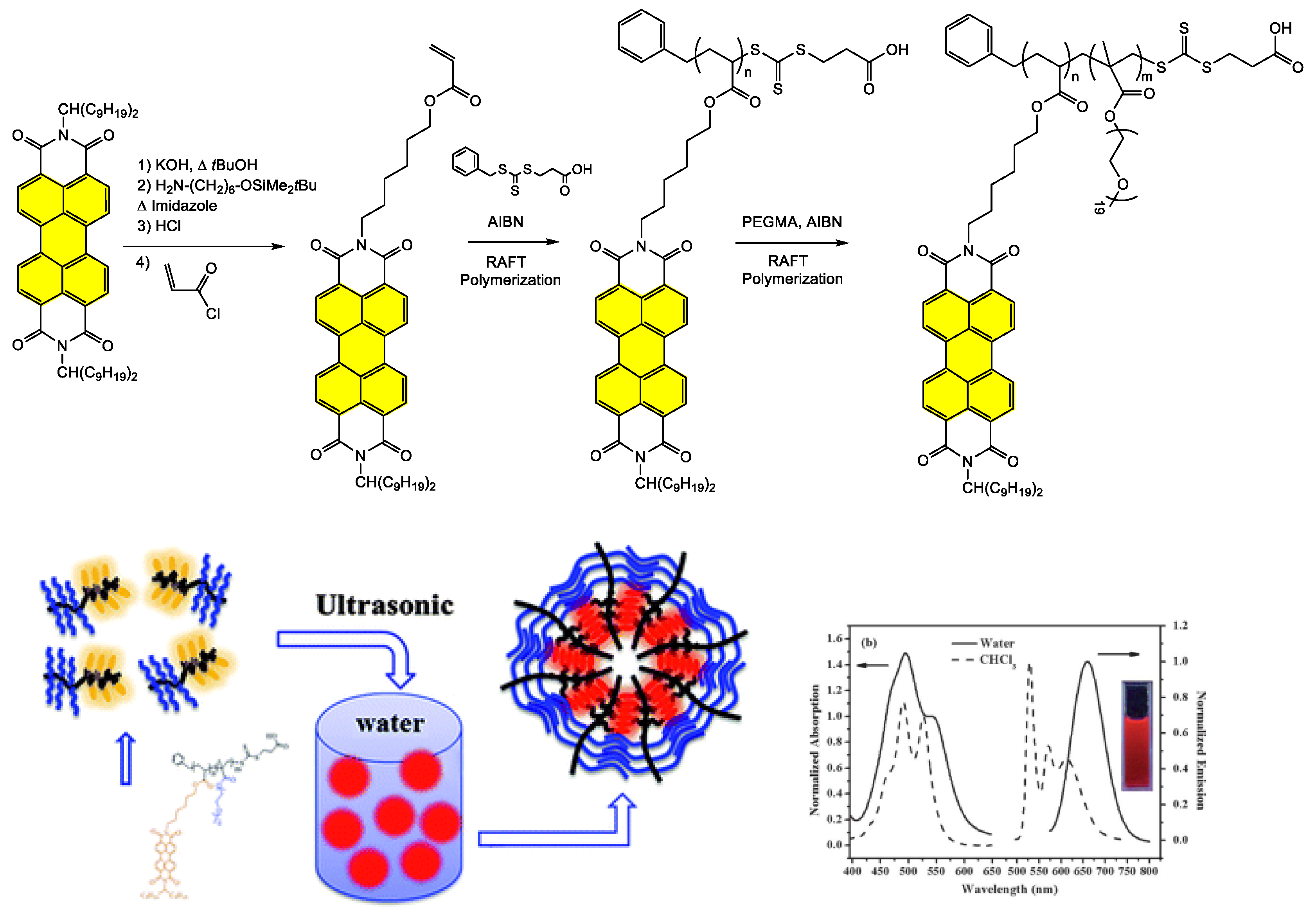
4.1. Synthesis and Applications of Unsubstituted Bay PDI Materials
4.1.1. Small-Molecule-Based Systems
4.1.2. Polymer-Based Systems
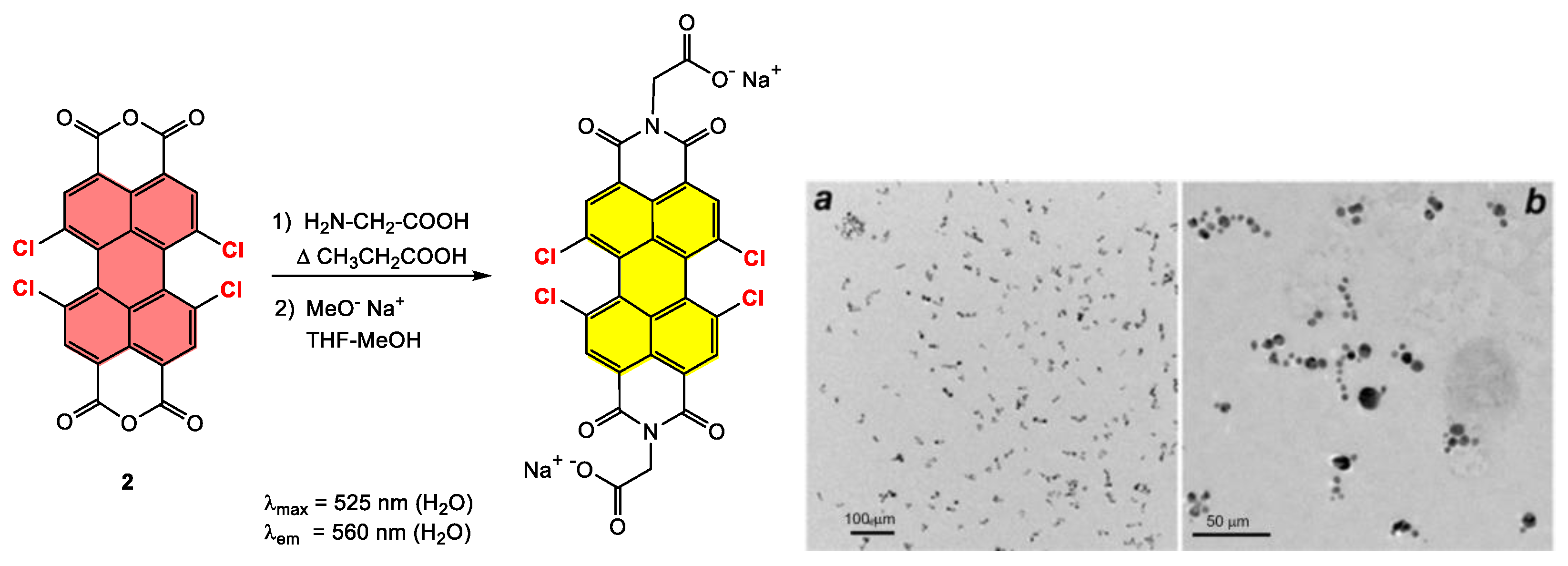
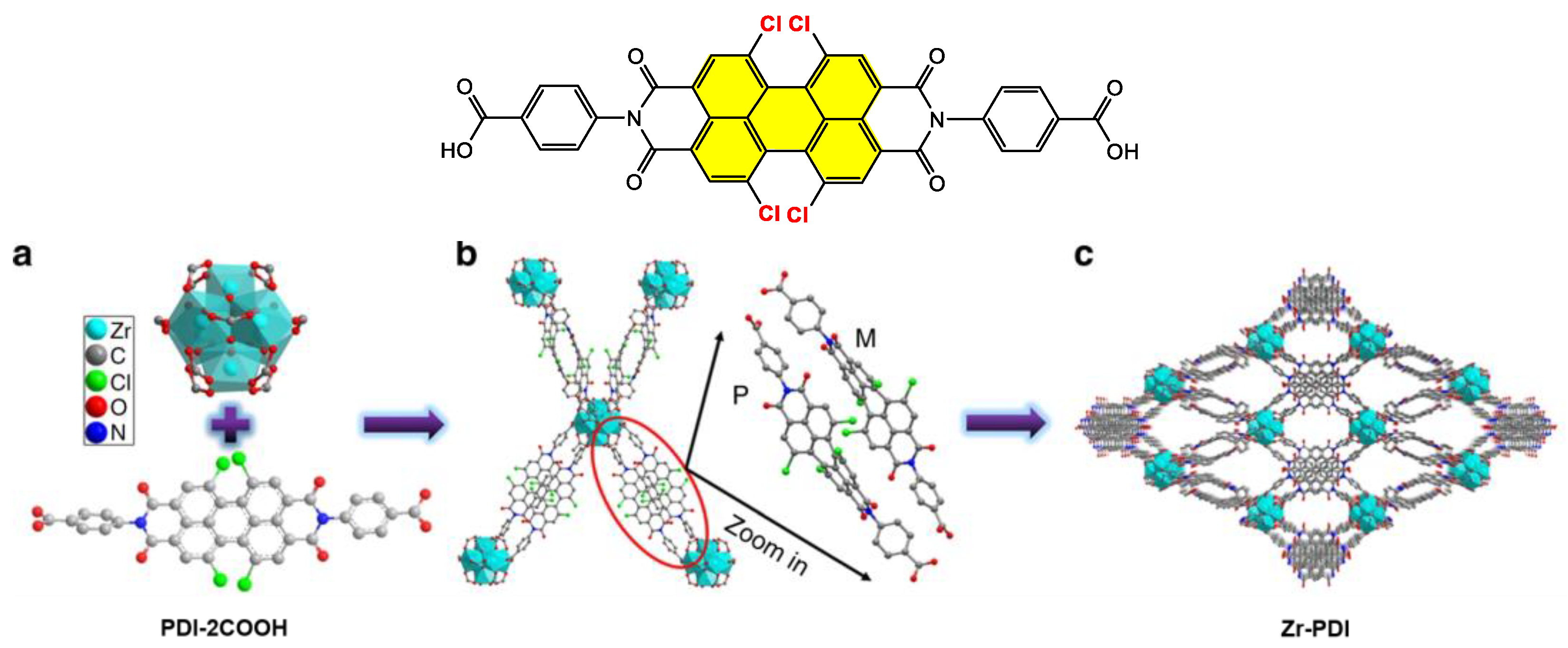
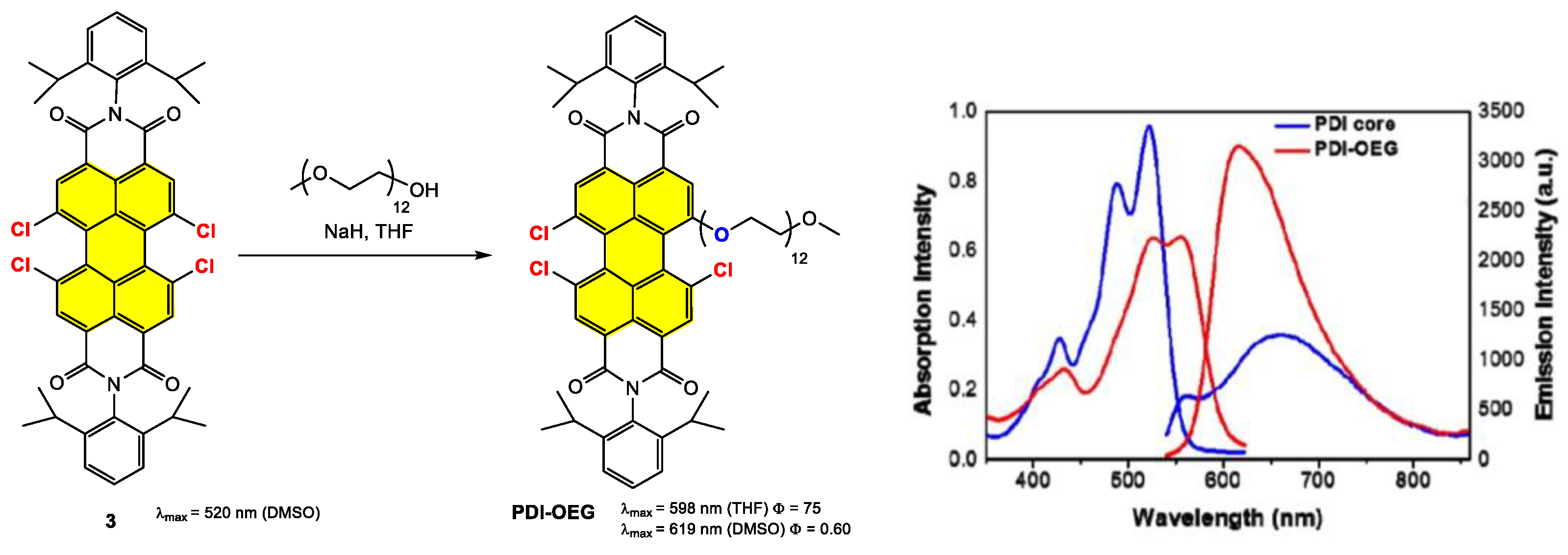
4.2. Synthesis and Applications of Tetrachloro Bay-Substituted PDI Materials
4.2.1. Small-Molecule-Based Systems
4.2.2. Polymer-Based Systems
Grafting the Chain Polymer in the Imide Position
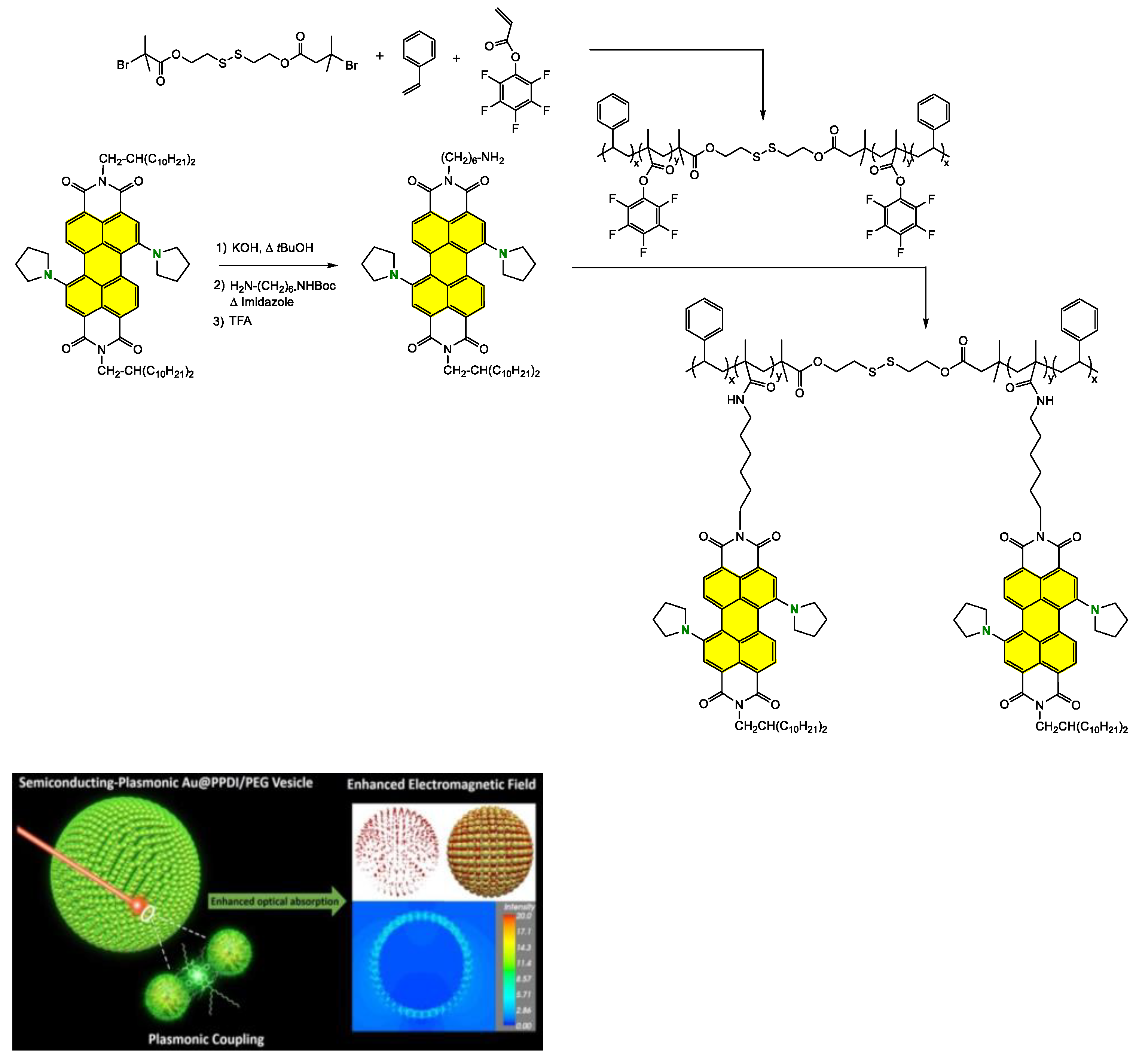
Grafting the Chain Polymer in the Bay Position
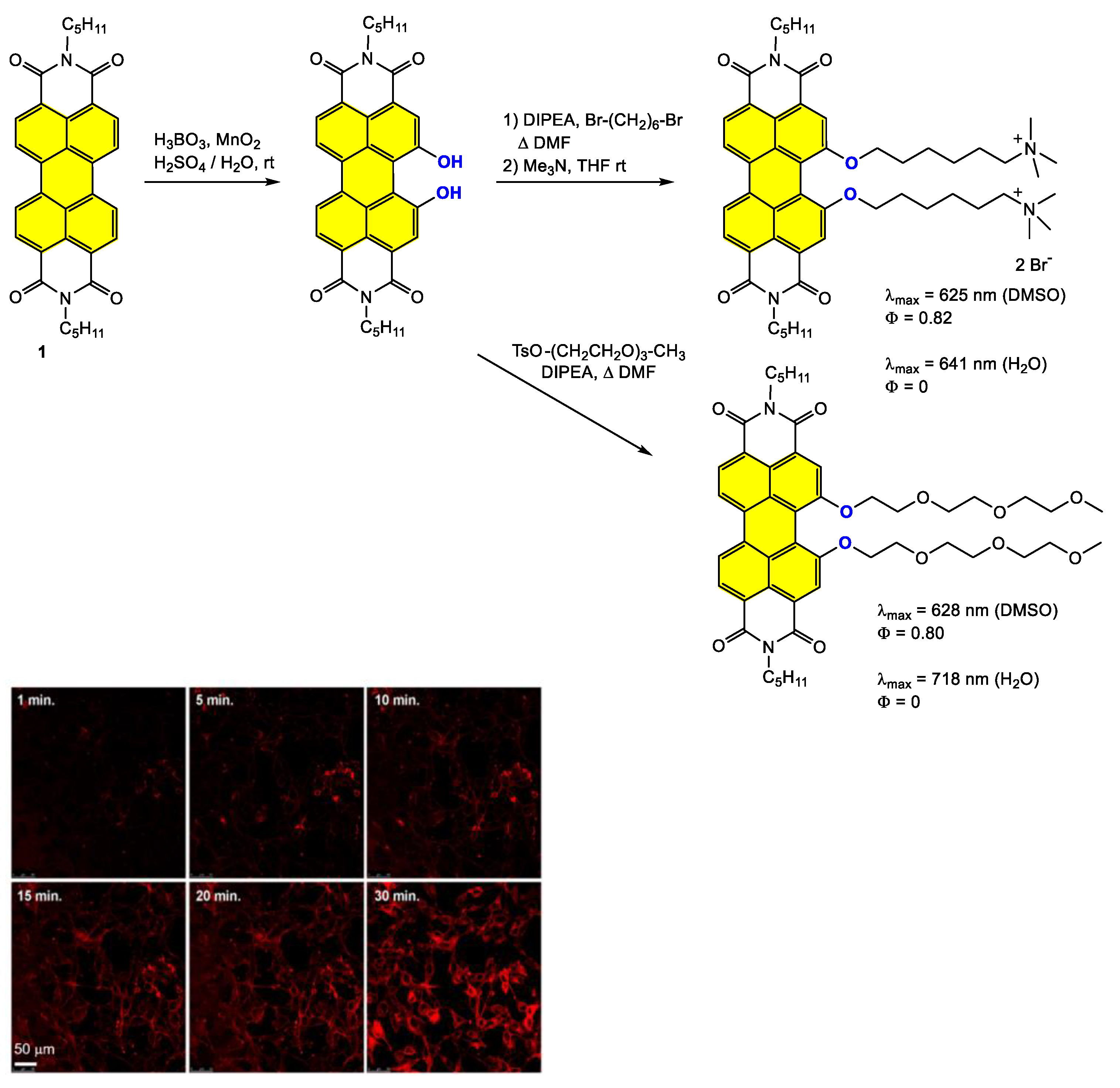
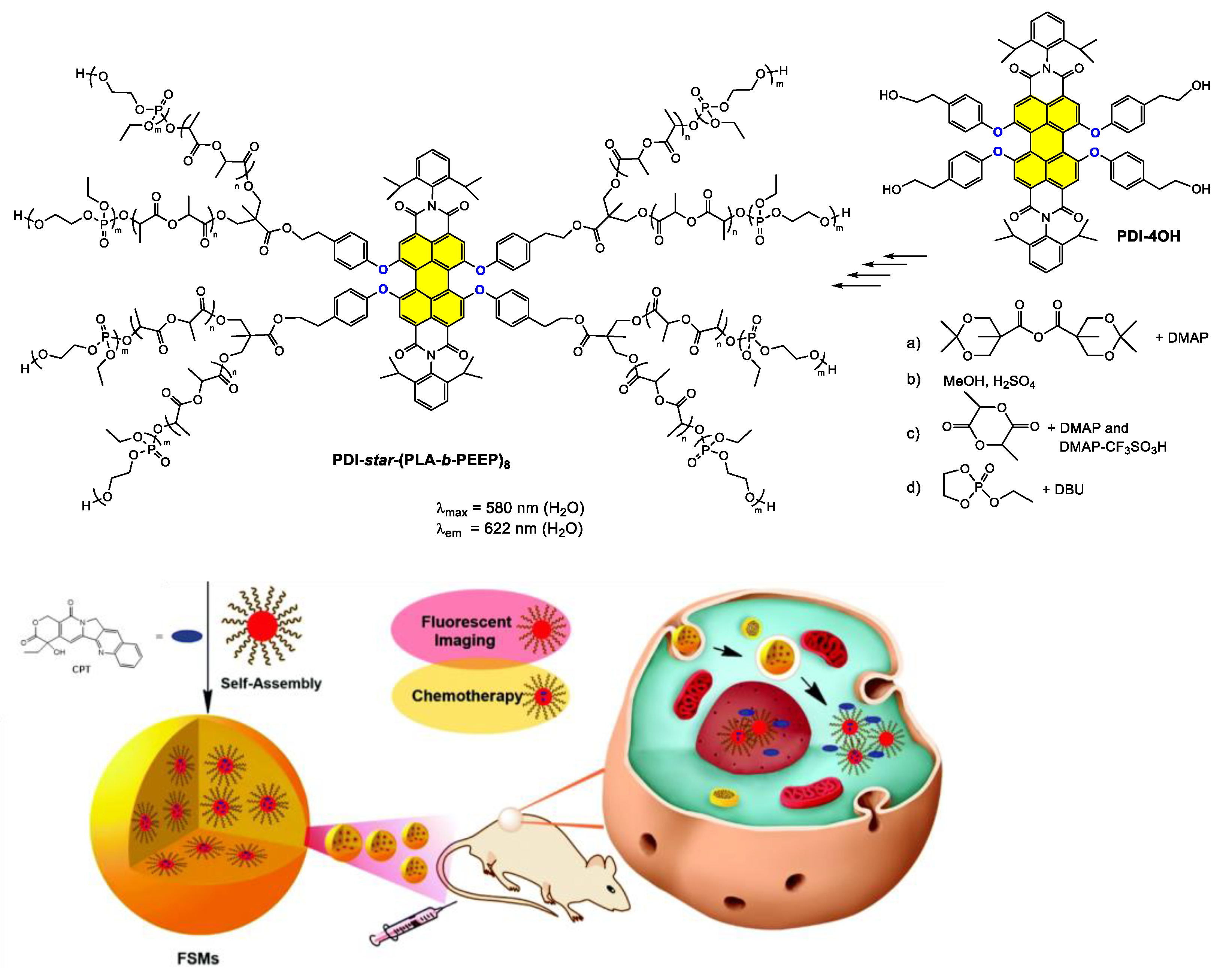
4.3. Synthesis and Applications of Dialkoxy and Tetraalkoxy Bay-Substituted PDI Materials
4.3.1. Small-Molecule-Based Systems
4.3.2. Polymer-Based Systems
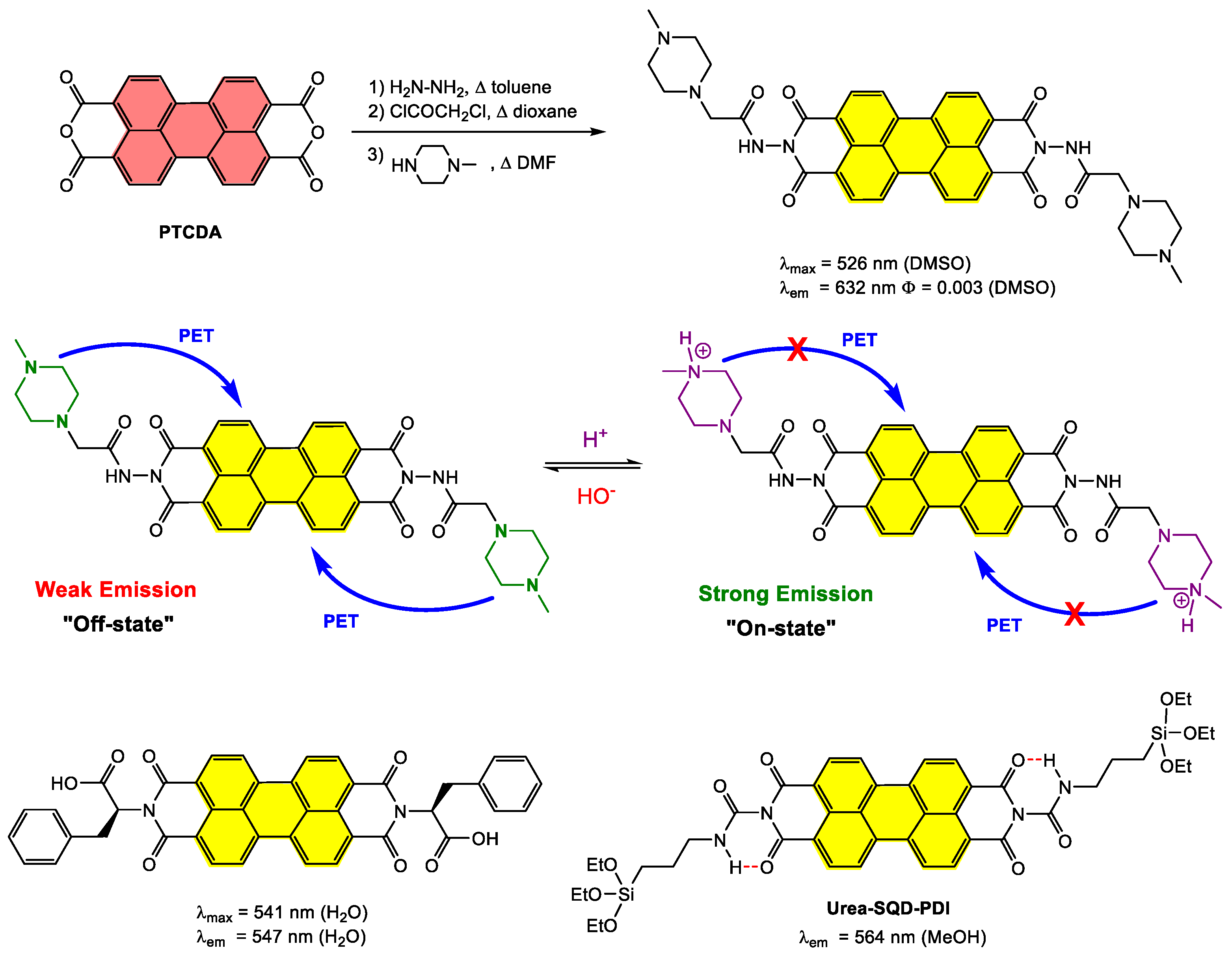
4.4. Synthesis and Applications of Amino-PDI Bay-Substituted Materials
4.4.1. Small-Molecule-Based Systems
4.4.2. Polymer-Based Systems
Grafting the Chain Polymer in the Imide Position
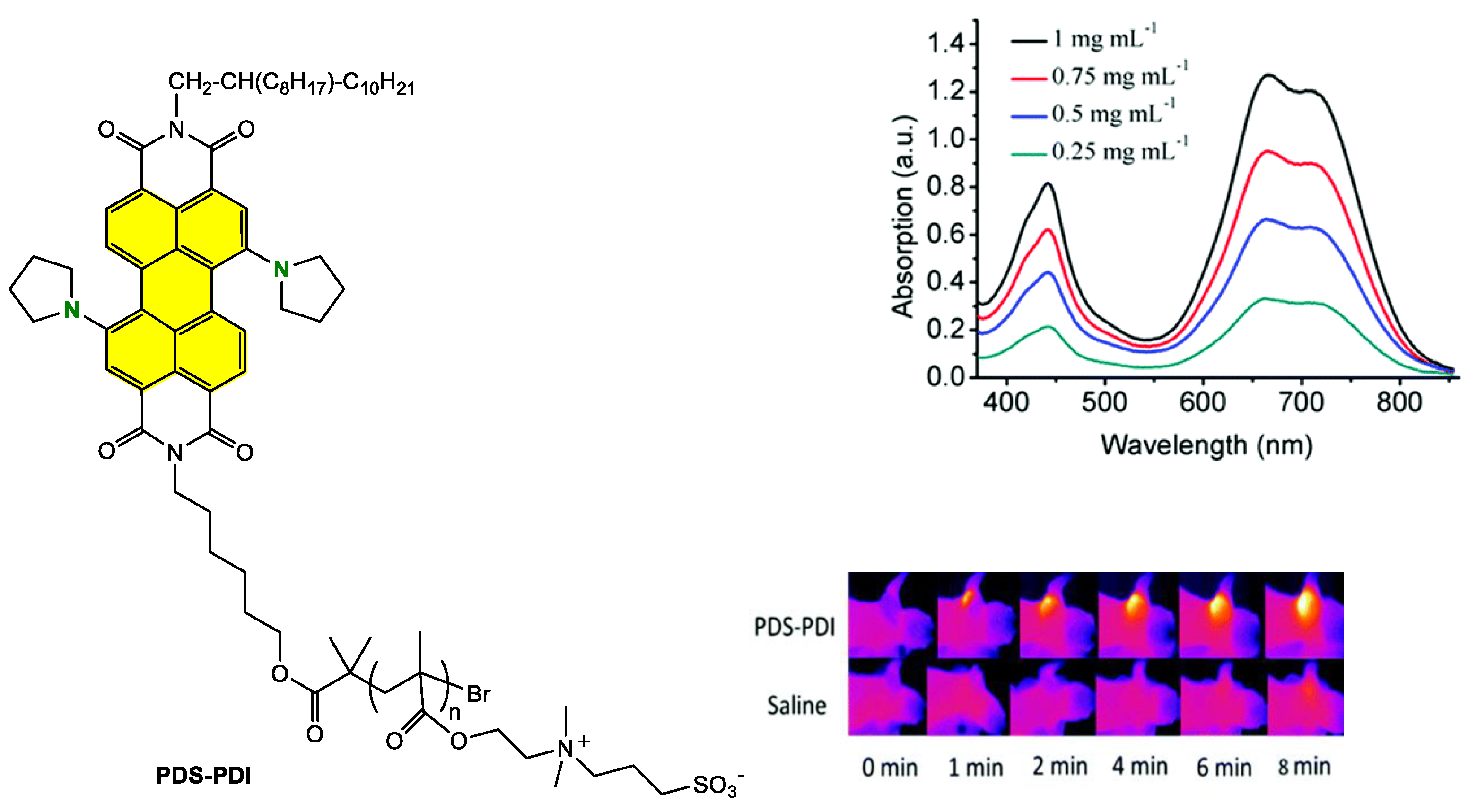
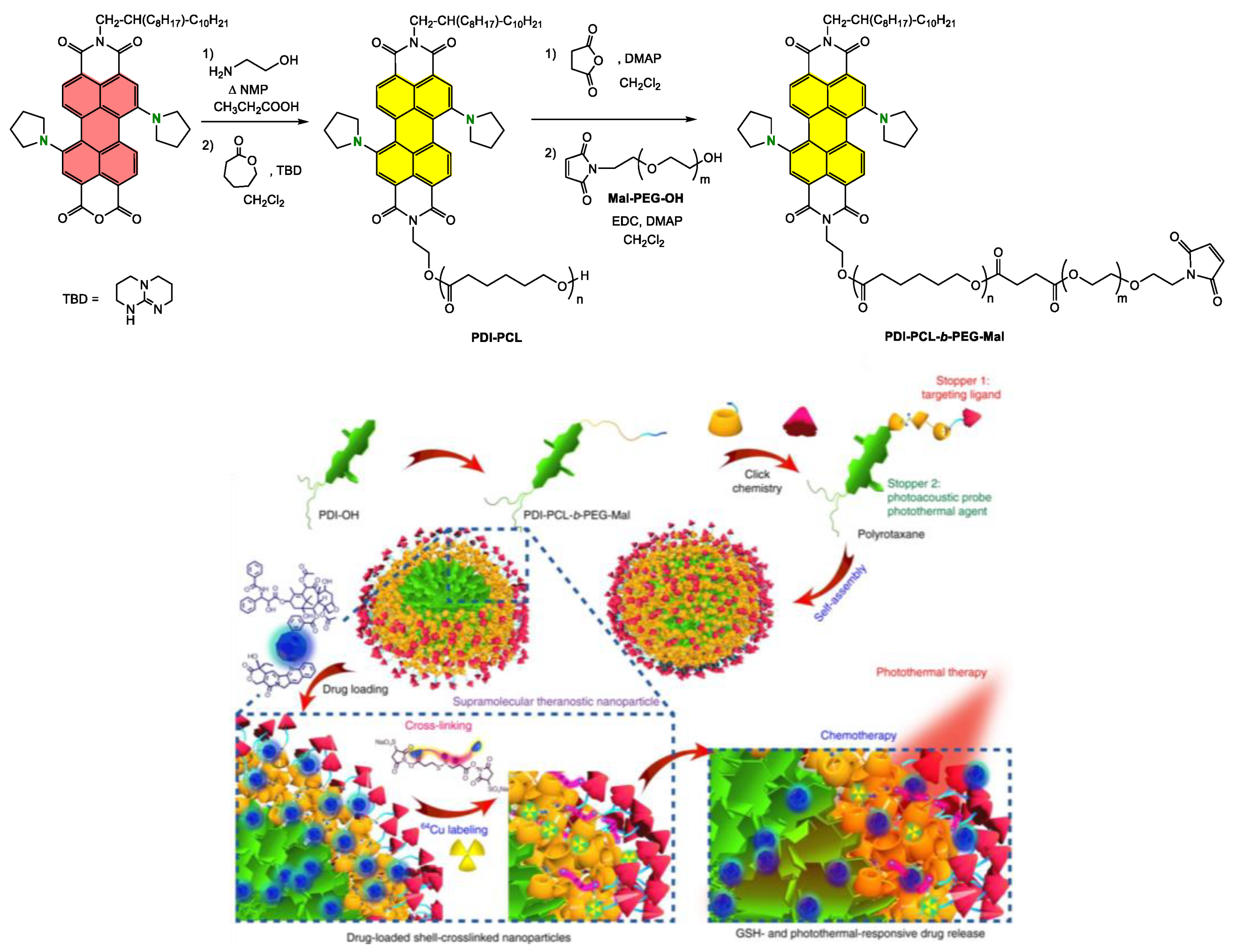
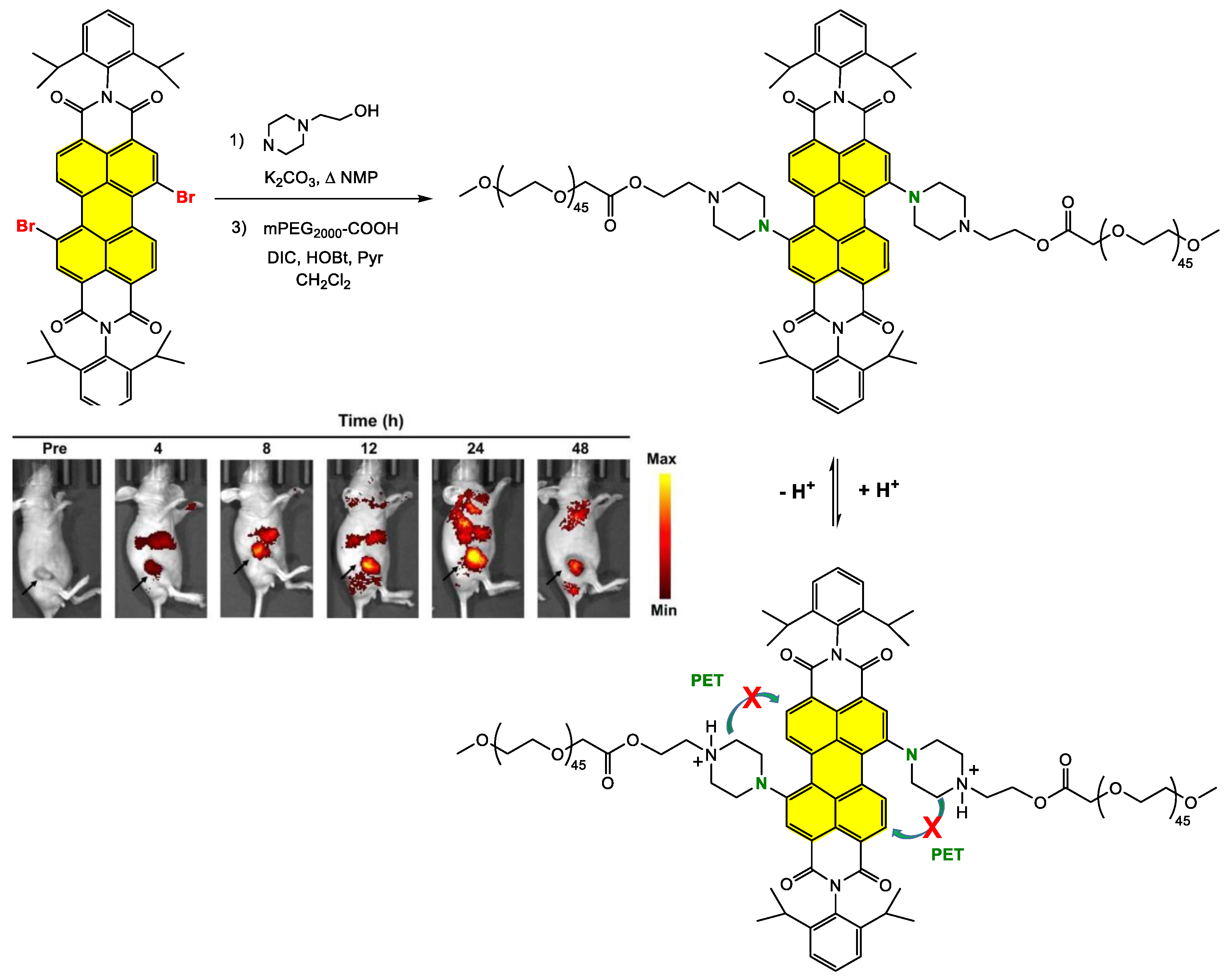
Grafting the Chain Polymer in the Bay Position
5. Perylenemonoimide (PMI)-Based Systems for Bioimaging and PDT
5.1. Synthesis and Applications of Unsubstituted Bay PMI Materials
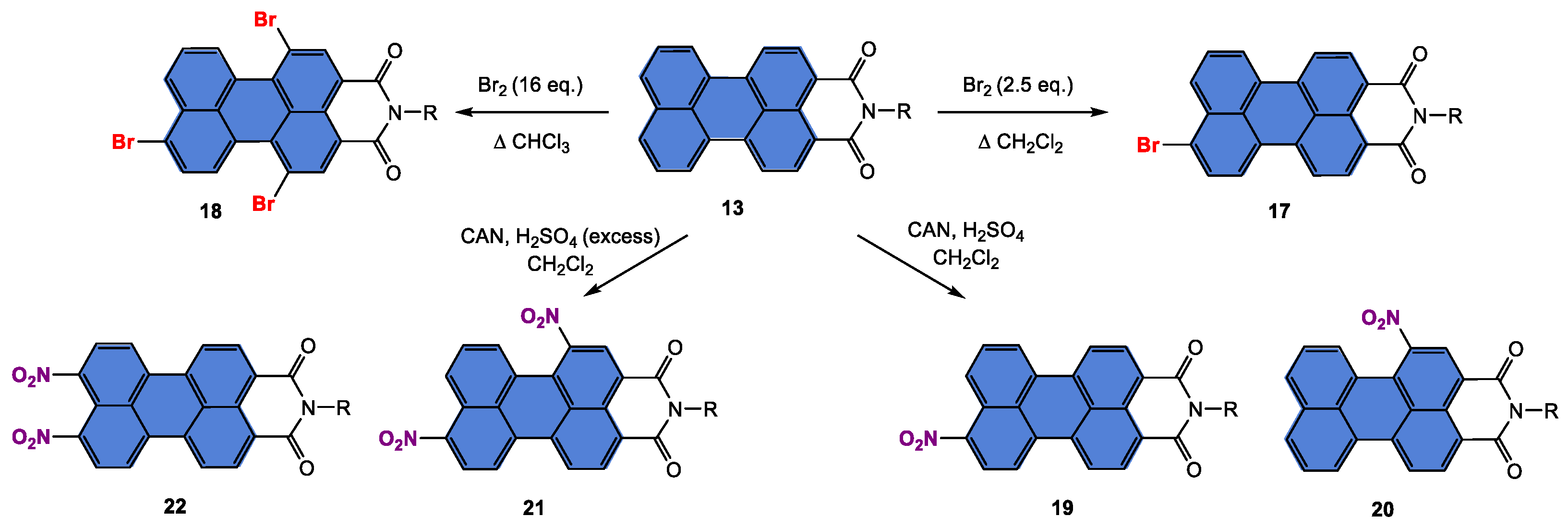
5.2. Synthesis and Applications of Substituted Bay and/or Peri PMI Materials
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Stępień, M.; Gońka, E.; Żyła, M.; Sprutta, N. Heterocyclic Nanographenes and Other Polycyclic Heteroaromatic Compounds: Synthetic Routes, Properties, and Applications. Chem. Rev. 2017, 117, 3479–3716. [Google Scholar] [CrossRef]
- Borissov, A.; Maurya, Y.K.; Moshniaha, L.; Wong, W.-S.; Żyła-Karwowska, M.; Stępień, M. Recent Advances in Heterocyclic Nanographenes and Other Polycyclic Heteroaromatic Compounds. Chem. Rev. 2022, 122, 565–788. [Google Scholar] [CrossRef] [PubMed]
- Buess, C.M.; Lawson, D.D. The Preparation, Reactions, and Properties of Triphenylenes. Chem. Rev. 1960, 60, 313–330. [Google Scholar] [CrossRef]
- Sonet, D.; Bibal, B. Triphenylene: A versatile molecular receptor. Tet. Lett. 2019, 60, 872–884. [Google Scholar] [CrossRef]
- Figueira-Duarte, T.M.; Müllen, K. Pyrene-Based Materials for Organic Electronics. Chem. Rev. 2011, 111, 7260–7314. [Google Scholar] [CrossRef]
- Zöphel, L.; Enkelmann, V.; Müllen, K. Tuning the HOMO–LUMO Gap of Pyrene Effectively via Donor–Acceptor Substitution: Positions 4,5 Versus 9,10. Org. Lett. 2013, 15, 804–807. [Google Scholar] [CrossRef]
- Chen, Q.; Thoms, S.; Stöttinger, S.; Schollmeyer, D.; Müllen, K.; Narita, A.; Basché, T. Dibenzo[hi,st]ovalene as Highly Luminescent Nanographene: Efficient Synthesis via Photochemical Cyclodehydroiodination, Optoelectronic Properties, and Single-Molecule Spectroscopy. J. Am. Chem. Soc. 2019, 141, 16439–16449. [Google Scholar] [CrossRef] [PubMed]
- Tran-Van, A.-F.; Wegner, H.A. Strategies in organic synthesis for condensed arenes, coronene, and graphene. Top. Curr. Chem. 2014, 349, 121–157. [Google Scholar] [CrossRef]
- Kumar, S.; Tao, Y.-T. Coronenes, Benzocoronenes and Beyond: Modern Aspects of Their Syntheses, Properties, and Applications. Chem. Asian J. 2021, 16, 621–647. [Google Scholar] [CrossRef] [PubMed]
- Nestoros, E.; Stuparu, M.C. Corannulene: A molecular bowl of carbon with multifaceted properties and diverse applications. Chem. Commun. 2018, 54, 6503–6519. [Google Scholar] [CrossRef] [PubMed]
- Muzammil, E.M.; Halilovic, D.; Stuparu, M.C. Synthesis of corannulene-based nanographenes. Commun. Chem. 2019, 2, 58. [Google Scholar] [CrossRef]
- Clar, E.; Kelly, W.; Laird, R.M. Die Synthesen des Terrylens und Quaterrylens und über das vermeintliche Quaterrylen von A. Zinke. Mon. Für Chem. 1956, 87, 391–398. [Google Scholar] [CrossRef]
- Biradar, M.R.; Bhosale, S.V.; Morajakar, P.P.; Bhosale, S.V. A review on energy storage devices based on rylene imide dyes: Synthesis, applications and challenges. Fuel 2022, 310, 122487. [Google Scholar] [CrossRef]
- Zhan, X.; Facchetti, A.; Barlow, S.; Marks, T.J.; Ratner, M.A.; Wasielewski, M.R.; Marder, S.R. Rylene and related diimides for organic electronics. Adv. Mater. 2011, 23, 268–284. [Google Scholar] [CrossRef]
- Liang, N.; Meng, D.; Wang, Z. Giant Rylene Imide-Based Electron Acceptors for Organic Photovoltaics. Acc. Chem. Res. 2021, 54, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Kardos, M. Über einige Aceanthrenchinon- und 1.9-Anthracen-Derivate. Ber. Dtsch. Chem. Ges. 1913, 46, 2085–2091. [Google Scholar] [CrossRef]
- Zollinger, H. Color Chemistry. Synthesis, Properties and Applications of Organic Dyes and Pigments, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Herbst, W.; Hunger, K.; Wilker, G.; Ohleier, H.; Winter, R. Industrial Organic Pigments: Production, Properties, Applications, 3rd ed.; Verlag, W.V., Ed.; Wiley-VCH Verlag: Weinheim, Germany, 2004. [Google Scholar] [CrossRef]
- Langhals, H. Cyclic Carboxylic Imide Structures as Structure Elements of High Stability. Novel Developments in Perylene Dye Chemistry. Heterocycles 1995, 40, 477–500. [Google Scholar] [CrossRef]
- Huang, C.; Barlow, S.; Marder, S.R. Perylene-3,4,9,10-tetracarboxylic acid diimides: Synthesis, physical properties, and use in organic electronics. J. Org. Chem. 2011, 76, 2386–2407. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Król, A.; Würthner, F. Progress in the synthesis of perylene bisimide dyes. Org. Chem. Front. 2019, 6, 1272–1318. [Google Scholar] [CrossRef]
- Rocard, L.; Goujon, A.; Hudhomme, P. Nitro-Perylenediimide: An Emerging Building Block for the Synthesis of Functional Organic Materials. Molecules 2020, 25, 1402. [Google Scholar] [CrossRef]
- Würthner, F. Perylene bisimide dyes as versatile building blocks for functional supramolecular architectures. Chem. Commun. 2004, 14, 1564–1579. [Google Scholar] [CrossRef]
- Würthner, F.; Saha-Möller, C.R.; Fimmel, B.; Ogi, S.; Leowanawat, P.; Schmidt, D. Perylene Bisimide Dye Assemblies as Archetype Functional Supramolecular Materials. Chem. Rev. 2016, 116, 962–1052. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Slattum, P.; Wang, C.; Zang, L. Self-Assembly of Perylene Imide Molecules into 1D Nanostructures: Methods, Morphologies, and Applications. Chem. Rev. 2015, 115, 11967–11998. [Google Scholar] [CrossRef]
- Li, J.; Li, P.; Fan, M.; Zheng, X.; Guan, J.; Yin, M. Chirality of Perylene Diimides: Design Strategies and Applications. Angew. Chem. Int. Ed. 2022, 61, e202202532. [Google Scholar] [CrossRef]
- Diacon, A.; Krupka, O.; Hudhomme, P. Fullerene-Perylenediimide (C60-PDI) Based Systems: An Overview and Synthesis of a Versatile Platform for Their Anchor Engineering. Molecules 2022, 27, 6522. [Google Scholar] [CrossRef]
- Sebastian, E.; Hariharan, M. Symmetry-Breaking Charge Separation in Molecular Constructs for Efficient Light Energy Conversion. ACS Energy Lett. 2022, 7, 696–711. [Google Scholar] [CrossRef]
- Kumar, S.; Shukla, J.; Kumar, Y.; Mukhopadhyay, P. Electron-poor arylenediimides. Org. Chem. Front. 2018, 5, 2254–2276. [Google Scholar] [CrossRef]
- Schaack, C.; Evans, A.M.; Ng, F.; Steigerwald, M.L.; Nuckolls, C. High-Performance Organic Electronic Materials by Contorting Perylene Diimides. J. Am. Chem. Soc. 2022, 144, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Król, A.; Shoyama, K.; Stolte, M.; Würthner, F. Naphthalene and perylene diimides–better alternatives to fullerenes for organic electronics? Chem. Commun. 2018, 54, 13763–13772. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Li, G.; Qi, T.; Huang, H. Aromatic imide/amide-based organic small-molecule emitters for organic light-emitting diodes. Mater. Chem. Front. 2020, 4, 1554–1568. [Google Scholar] [CrossRef]
- Quinn, J.T.E.; Zhu, J.; Li, X.; Wang, J.; Li, Y. Recent progress in the development of n-type organic semiconductors for organic field effect transistors. J. Mater. Chem. C 2017, 5, 8654–8681. [Google Scholar] [CrossRef]
- Li, C.; Wonneberger, H. Perylene Imides for Organic Photovoltaics: Yesterday, Today, and Tomorrow. Adv. Mater. 2012, 24, 613–636. [Google Scholar] [CrossRef] [PubMed]
- Kozma, E.; Catellani, M. Perylene diimides based materials for organic solar cells. Dye. Pigm. 2013, 98, 160–179. [Google Scholar] [CrossRef]
- Fernández-Lázaro, F.; Zink-Lorre, N.; Sastre-Santos, Á. Perylenediimides as non-fullerene acceptors in bulk-heterojunction solar cells (BHJSCs). J. Mater. Chem. A 2016, 4, 9336–9346. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, Y.; Zhang, Q.; Gao, X. Non-fullerene small molecule acceptors based on perylene diimides. J. Mater. Chem. A 2016, 4, 17604–17622. [Google Scholar] [CrossRef]
- Macedo, A.G.; Christopholi, L.P.; Gavim, A.E.X.; de Deus, J.F.; Teridi, M.A.M.; Yusoff, A.R.b.M.; da Silva, W.J. Perylene derivatives for solar cells and energy harvesting: A review of materials, challenges and advances. J. Mater. Sci. Mater. Electron. 2019, 30, 15803–15824. [Google Scholar] [CrossRef]
- Fujimoto, K.; Takahashi, M.; Izawa, S.; Hiramoto, M. Development of Perylene-Based Non-Fullerene Acceptors through Bay-Functionalization Strategy. Materials 2020, 13, 2148. [Google Scholar] [CrossRef]
- Zink-Lorre, N.; Font-Sanchis, E.; Sastre-Santos, Á.; Fernández-Lázaro, F. Perylenediimides as more than just non-fullerene acceptors: Versatile components in organic, hybrid and perovskite solar cells. Chem. Commun. 2020, 56, 3824–3838. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, Y.; Sun, C.; Xue, L.; Wang, H.; Zhang, Z.-G. Perylene-diimide derived organic photovoltaic materials. Sci. China Chem. 2022, 65, 462–485. [Google Scholar] [CrossRef]
- Sharma, V.; Koenig, J.D.B.; Welch, G.C. Perylene diimide based non-fullerene acceptors: Top performers and an emerging class featuring N-annulation. J. Mater. Chem. A 2021, 9, 6775–6789. [Google Scholar] [CrossRef]
- Shi, Q.; Wu, J.; Wu, X.; Peng, A.; Huang, H. Perylene Diimide-Based Conjugated Polymers for All-Polymer Solar Cells. Chem. Eur. J. 2020, 26, 12510–12522. [Google Scholar] [CrossRef] [PubMed]
- Soh, N.; Ueda, T. Perylene bisimide as a versatile fluorescent tool for environmental and biological analysis: A review. Talanta 2011, 85, 1233–1237. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, G.; Yang, B.; Ji, Q.; Xiang, W.; He, H.; Xu, Z.; Qi, C.; Li, S.; Yang, S.; et al. Review on application of perylene diimide (PDI)-based materials in environment: Pollutant detection and degradation. Sci. Total Environ. 2021, 780, 146483. [Google Scholar] [CrossRef] [PubMed]
- Görl, D.; Zhang, X.; Würthner, F. Molecular Assemblies of Perylene Bisimide Dyes in Water. Angew. Chem. Int. Ed. 2012, 51, 6328–6348. [Google Scholar] [CrossRef] [PubMed]
- Rostami-Tapeh-Esmail, E.; Golshan, M.; Salami-Kalajahi, M.; Roghani-Mamaqani, H. Perylene-3,4,9,10-tetracarboxylic diimide and its derivatives: Synthesis, properties and bioapplications. Dye. Pigm. 2020, 180, 108488. [Google Scholar] [CrossRef]
- Sun, M.; Müllen, K.; Yin, M. Water-soluble perylenediimides: Design concepts and biological applications. Chem. Soc. Rev. 2016, 45, 1513–1528. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xu, Z.; Yin, M. Perylenediimide-cored dendrimers and their bioimaging and gene delivery applications. Prog. Polym. Sci. 2015, 46, 25–54. [Google Scholar] [CrossRef]
- Chen, Y. Recent Advances in Excimer-Based Fluorescence Probes for Biological Applications. Molecules 2022, 27, 8628. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, X. Semiconducting Perylene Diimide Nanostructure: Multifunctional Phototheranostic Nanoplatform. Acc. Chem. Res. 2019, 52, 1245–1254. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, N.; Wang, Y.; Ling, G.; Zhang, P. Perylene diimide-based treatment and diagnosis of diseases. J. Mater. Chem. B 2021, 9, 8937–8950. [Google Scholar] [CrossRef]
- Feiler, L.; Langhals, H.; Polborn, K. Synthesis of perylene-3,4-dicarboximides—Novel highly photostable fluorescent dyes. Liebigs Ann. 1995, 1995, 1229–1244. [Google Scholar] [CrossRef]
- Roy, R.; Khan, A.; Chatterjee, O.; Bhunia, S.; Apurba, K. Perylene Monoimide as a Versatile Fluoroprobe: The Past, Present, and Future. Org. Mater. 2021, 3, 417–454. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Debele, T.A.; Peng, S.; Tsai, H.-C. Drug Carrier for Photodynamic Cancer Therapy. Int. J. Mol. Sci. 2015, 16, 22094–22136. [Google Scholar] [CrossRef] [PubMed]
- Foote, C.S. Definition of type I and type II photosensitized oxidation. Photochem. Photobiol. 1991, 54, 659. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef]
- Sun, H.; Guo, R.; Guo, Y.; Song, J.; Li, Z.; Song, F. Boosting Type-I and Type-II ROS Production of Water-Soluble Porphyrin for Efficient Hypoxic Tumor Therapy. Mol. Pharm. 2023, 20, 606–615. [Google Scholar] [CrossRef]
- Li, J.; Pu, K. Development of organic semiconducting materials for deep-tissue optical imaging, phototherapy and photoactivation. Chem. Soc. Rev. 2019, 48, 38–71. [Google Scholar] [CrossRef]
- Konan, Y.N.; Gurny, R.; Allémann, E. State of the art in the delivery of photosensitizers for photodynamic therapy. J. Photochem. Photobiol. B 2002, 66, 89–106. [Google Scholar] [CrossRef]
- dos Santos, A.F.; de Almeida, D.R.Q.; Terra, L.F.; Baptista, M.S.; Labriola, L. Photodynamic therapy in cancer treatment-An update review. J. Cancer Metastasis Treat. 2019, 5, 25. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, S.; Zhang, F. Near-infrared luminescence high-contrast in vivo biomedical imaging. Nat. Rev. Bioeng. 2023, 1, 60–78. [Google Scholar] [CrossRef]
- Smith, A.M.; Mancini, M.C.; Nie, S. Second window for in vivo imaging. Nat. Nanotech. 2009, 4, 710–711. [Google Scholar] [CrossRef]
- Schnermann, M.J. Organic dyes for deep bioimaging. Nature 2017, 551, 176–177. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.-N.; Yan, Y.; Zhao, J.; Yoon, J. Heavy-Atom-Free Photosensitizers: From Molecular Design to Applications in the Photodynamic Therapy of Cancer. Acc. Chem. Res. 2021, 54, 207–220. [Google Scholar] [CrossRef]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef] [PubMed]
- Giepmans, B.N.G.; Adams, S.R.; Ellisman, M.H.; Tsien, R.Y. The Fluorescent Toolbox for Assessing Protein Location and Function. Science 2006, 312, 217–224. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Zheng, K.; He, L.; Huang, W. Far-red to near infrared analyte-responsive fluorescent probes based on organic fluorophore platforms for fluorescence imaging. Chem. Soc. Rev. 2012, 42, 622–661. [Google Scholar] [CrossRef]
- Kowada, T.; Maeda, H.; Kikuchi, K. BODIPY-based probes for the fluorescence imaging of biomolecules in living cells. Chem. Soc. Rev. 2015, 44, 4953–4972. [Google Scholar] [CrossRef]
- Chen, M.; Yin, M. Design and development of fluorescent nanostructures for bioimaging. Prog. Polym. Sci. 2014, 39, 365–395. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ogawa, M.; Alford, R.; Choyke, P.L.; Urano, Y. New strategies for fluorescent probe design in medical diagnostic imaging. Chem. Rev. 2010, 110, 2620–2640. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, C.; Müllen, K. Beyond perylene diimides: Synthesis, assembly and function of higher rylene chromophores. J. Mater. Chem. C 2014, 2, 1938–1956. [Google Scholar] [CrossRef]
- Ji, C.; Cheng, W.; Yuan, Q.; Müllen, K.; Yin, M. From Dyestuff Chemistry to Cancer Theranostics: The Rise of Rylenecarboximides. Acc. Chem. Res. 2019, 52, 2266–2277. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, A.; Märkle, S.; Langhals, H. Lösliche Perylen-Fluoreszenzfarbstoffe mit hoher Photostabilität. Chem. Ber. 1982, 115, 2927–2934. [Google Scholar] [CrossRef]
- Langhals, H. Synthese von hochreinen Perylen-Fluoreszenzfarbstoffen in großen Mengen–gezielte Darstellung von Atrop-Isomeren. Chem. Ber. 1985, 118, 4641–4645. [Google Scholar] [CrossRef]
- Rajasingh, P.; Cohen, R.; Shirman, E.; Shimon, L.J.W.; Rybtchinski, B. Selective bromination of perylene diimides under mild conditions. J. Org. Chem. 2007, 72, 5973–5979. [Google Scholar] [CrossRef]
- Leroy-Lhez, S.; Baffreau, J.; Perrin, L.; Levillain, E.; Allain, M.; Blesa, M.-J.; Hudhomme, P. Tetrathiafulvalene in a Perylene-3,4:9,10-bis(dicarboximide)-Based Dyad: A New Reversible Fluorescence-Redox Dependent Molecular System. J. Org. Chem. 2005, 70, 6313–6320. [Google Scholar] [CrossRef]
- Perrin, L.; Hudhomme, P. Synthesis, Electrochemical and Optical Absorption Properties of New Perylene-3,4:9,10-bis(dicarboximide) and Perylene-3,4:9,10-bis(benzimidazole) Derivatives. Eur. J. Org. Chem. 2011, 2011, 5427–5440. [Google Scholar] [CrossRef]
- Würthner, F.; Stepanenko, V.; Chen, Z.; Saha-Möller, C.R.; Kocher, N.; Stalke, D. Preparation and characterization of regioisomerically pure 1,7-disubstituted perylene bisimide dyes. J. Org. Chem. 2004, 69, 7933–7939. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Chow, T.J. 1,7-Dinitroperylene bisimides: Facile synthesis and characterization as n-type organic semiconductors. Tet. Lett. 2010, 51, 5959–5963. [Google Scholar] [CrossRef]
- Meng, D.; Sun, D.; Zhong, C.; Liu, T.; Fan, B.; Huo, L.; Li, Y.; Jiang, W.; Choi, H.; Kim, T.; et al. High-Performance Solution-Processed Non-Fullerene Organic Solar Cells Based on Selenophene-Containing Perylene Bisimide Acceptor. J. Am. Chem. Soc. 2016, 138, 375–380. [Google Scholar] [CrossRef]
- El-Berjawi, R.; Hudhomme, P. Synthesis of a perylenediimide-fullerene C60 dyad: A simple use of a nitro leaving group for a Suzuki-Miyaura coupling reaction. Dye. Pigm. 2018, 159, 551–556. [Google Scholar] [CrossRef]
- Rocard, L.; Hatych, D.; Chartier, T.; Cauchy, T.; Hudhomme, P. Original Suzuki–Miyaura Coupling Using Nitro Derivatives for the Synthesis of Perylenediimide-Based Multimers. Eur. J. Org. Chem. 2019, 2019, 7635–7643. [Google Scholar] [CrossRef]
- Hruzd, M.; Rocard, L.; Goujon, A.; Allain, M.; Cauchy, T.; Hudhomme, P. Desymmetrization of Perylenediimide Bay Regions Using Selective Suzuki–Miyaura Reactions from Dinitro Substituted Derivatives. Chem. Eur. J. 2020, 26, 15881–15891. [Google Scholar] [CrossRef] [PubMed]
- Teraoka, T.; Hiroto, S.; Shinokubo, H. Iridium-Catalyzed Direct Tetraborylation of Perylene Bisimides. Org. Lett. 2011, 13, 2532–2535. [Google Scholar] [CrossRef] [PubMed]
- Battagliarin, G.; Li, C.; Enkelmann, V.; Müllen, K. 2,5,8,11-Tetraboronic Ester Perylenediimides: A Next Generation Building Block for Dye-Stuff Synthesis. Org. Lett. 2011, 13, 3012–3015. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; He, D.; Zhang, L.; Liu, Y.; Mo, X.; Lin, J.; Zhang, H.-J. Direct Synthesis of Large-Scale Ortho-Iodinated Perylene Diimides: Key Precursors for Functional Dyes. Org. Lett. 2017, 19, 5438–5441. [Google Scholar] [CrossRef]
- Kaiser, H.; Lindner, J.; Langhals, H. Synthese von nichtsymmetrisch substituierten Perylen-Fluoreszenzfarbstoffen. Chem. Ber. 1991, 124, 529–535. [Google Scholar] [CrossRef]
- Langhals, H.; Sprenger, S.; Brandherm, M.-T. Perylenamidine-imide dyes. Liebigs Ann. 1995, 1995, 481–486. [Google Scholar] [CrossRef]
- Wescott, L.D.; Mattern, D.L. Donor−σ−Acceptor Molecules Incorporating a Nonadecyl-Swallowtailed Perylenediimide Acceptor. J. Org. Chem. 2003, 68, 10058–10066. [Google Scholar] [CrossRef]
- Quante, H.; Müllen, K. Quaterrylenebis(dicarboximides). Angew. Chem. Int. Ed. Engl. 1995, 34, 1323–1325. [Google Scholar] [CrossRef]
- Altaş, A.; Gültekin, D.D.; Acar, M.; Cücü, E.; Karatay, A.; Elmalı, A.; Atalay, A.; Demircan, Ç.A.; Bozkaya, U.; Kazaz, C.; et al. Bay- and peri-functionalized donor-acceptor perylene monoimides via nitration and nucleophilic substitution/reduction pathway. Mater. Today Chem. 2022, 24, 100908. [Google Scholar] [CrossRef]
- Zagranyarski, Y.; Chen, L.; Zhao, Y.; Wonneberger, H.; Li, C.; Müllen, K. Facile transformation of perylene tetracarboxylic acid dianhydride into strong donor-acceptor chromophores. Org. Lett. 2012, 14, 5444–5447. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, N.I.; Said, A.I.; Toshkova, R.A.; Tzoneva, R.D.; Bojinov, V.B. A novel water-soluble perylenetetracarboxylic diimide as a fluorescent pH probe: Chemosensing, biocompatibility and cell imaging. Dye. Pigm. 2019, 160, 28–36. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, F.; Zhang, J.; Jiang, T.; Li, X.; Wu, J.; Ren, H. A water-soluble fluorescent pH probe based on perylene dyes and its application to cell imaging. Lumin. J. Biol. Chem. Lumin. 2016, 31, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Abdelhameed, M.; Aly, S.; Lant, J.T.; Zhang, X.; Charpentier, P. Energy/Electron Transfer Switch for Controlling Optical Properties of Silicon Quantum Dots. Sci. Rep. 2018, 8, 17068. [Google Scholar] [CrossRef]
- Ye, Y.; Zheng, Y.; Ji, C.; Shen, J.; Yin, M. Self-Assembly and Disassembly of Amphiphilic Zwitterionic Perylenediimide Vesicles for Cell Membrane Imaging. ACS Appl. Mater. Interfaces 2017, 9, 4534–4539. [Google Scholar] [CrossRef]
- Yip, A.M.-H.; Shum, J.; Liu, H.-W.; Zhou, H.; Jia, M.; Niu, N.; Li, Y.; Yu, C.; Lo, K.K.-W. Luminescent Rhenium(I)–Polypyridine Complexes Appended with a Perylene Diimide or Benzoperylene Monoimide Moiety: Photophysics, Intracellular Sensing, and Photocytotoxic Activity. Chem.—A Eur. J. 2019, 25, 8970–8974. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Chou, Y.-T.; Su, B.-K.; Wu, C.-C.; Wang, C.-H.; Chang, K.-H.; Ho, J.-A.A.; Chou, P.-T. Comprehensive Thione-Derived Perylene Diimides and Their Bio-Conjugation for Simultaneous Imaging, Tracking, and Targeted Photodynamic Therapy. J. Am. Chem. Soc. 2022, 144, 17249–17260. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, Y.; Jin, X.; Deng, Q.; Yin, Z.; Tong, S.; Qing, W.; Huang, Y. Regioisomer-manipulating thio-perylenediimide nanoagents for photothermal/photodynamic theranostics. J. Mater. Chem. B 2020, 8, 5535–5544. [Google Scholar] [CrossRef]
- Llewellyn, B.A.; Davies, E.S.; Pfeiffer, C.R.; Cooper, M.; Lewis, W.; Champness, N.R. Thionated perylene diimides with intense absorbance in the near-IR. Chem. Commun. 2016, 52, 2099–2102. [Google Scholar] [CrossRef]
- Wang, L.; Sun, C.; Li, S.; Jia, N.; Li, J.; Qu, F.; Goh, K.; Chen, Y. Perylene bisimide-incorporated water-soluble polyurethanes for living cell fluorescence labeling. Polymer 2016, 82, 172–180. [Google Scholar] [CrossRef]
- He, J.; Chen, H.; Guo, Y.; Wang, L.; Zhu, L.; Karahan, H.E.; Chen, Y. Polycondensation of a Perylene Bisimide Derivative and L-Malic Acid as Water-Soluble Conjugates for Fluorescent Labeling of Live Mammalian Cells. Polymers 2018, 10, 559. [Google Scholar] [CrossRef]
- Kulkarni, B.; Malhotra, M.; Jayakannan, M. Perylene-Tagged Polycaprolactone Block Copolymers and Their Enzyme-Biodegradable Fluorescent Nanoassemblies for Intracellular Bio-imaging in Cancer Cells. ACS Appl. Polym.Mater. 2019, 1, 3375–3388. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef]
- Zhang, E.; Liu, L.; Lv, F.; Wang, S. Design and Synthesis of Reactive Perylene Tetracarboxylic Diimide Derivatives for Rapid Cell Imaging. ACS Omega 2018, 3, 8691–8696. [Google Scholar] [CrossRef] [PubMed]
- Neelakandan, P.P.; Pan, Z.; Hariharan, M.; Zheng, Y.; Weissman, H.; Rybtchinski, B.; Lewis, F.D. Hydrophobic Self-Assembly of a Perylenediimide-Linked DNA Dumbbell into Supramolecular Polymers. J. Am. Chem. Soc. 2010, 132, 15808–15813. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yu, C. Fluorescence turn-on detection of a protein through the reduced aggregation of a perylene probe. Angew. Chem. Int. Ed. 2010, 49, 1485–1488. [Google Scholar] [CrossRef] [PubMed]
- Céspedes-Guirao, F.J.; Ropero, A.B.; Font-Sanchis, E.; Nadal, Á.; Fernández-Lázaro, F.; Sastre-Santos, Á. A water-soluble perylene dye functionalised with a 17β-estradiol: A new fluorescent tool for steroid hormones. Chem. Commun. 2011, 47, 8307–8309. [Google Scholar] [CrossRef]
- Wang, L.; Xu, L.; Neoh, K.G.; Kang, E.-T. Water-soluble highly fluorescent poly[poly(ethylene glycol) methyl ether methacrylate] for cell labeling. J. Mater. Chem. 2011, 21, 6502–6505. [Google Scholar] [CrossRef]
- Yang, Z.; Yuan, Y.; Jiang, R.; Fu, N.; Lu, X.; Tian, C.; Hu, W.; Fan, Q.; Huang, W. Homogeneous near-infrared emissive polymeric nanoparticles based on amphiphilic diblock copolymers with perylene diimide and PEG pendants: Self-assembly behavior and cellular imaging application. Polym. Chem. 2014, 5, 1372–1380. [Google Scholar] [CrossRef]
- Lemouchi, C.; Simonov, S.; Zorina, L.; Gautier, C.; Hudhomme, P.; Batail, P. Amino acid derivatives of perylenediimide and their N–H⋯O peptide bond dipoles-templated solid state assembly into stacks. Org. Biomol. Chem. 2011, 9, 8096–8101. [Google Scholar] [CrossRef] [PubMed]
- Yeshchenko, O.A.; Kutsevol, N.V.; Tomchuk, A.V.; Khort, P.S.; Kuziv, Y.I.; Hudhomme, P.; Krupka, O.M. Dextran-graft-PNIPAM / Au nanoparticles / perylenediimide hybrid system as thermosensitive optical switches and fluorescent labels for potential use in nanophotonics and biomedical applications. Opt. Mater. 2022, 131, 112753. [Google Scholar] [CrossRef]
- Lü, B.; Chen, Y.; Li, P.; Wang, B.; Müllen, K.; Yin, M. Stable radical anions generated from a porous perylenediimide metal-organic framework for boosting near-infrared photothermal conversion. Nat. Commun. 2019, 10, 767. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Chen, Q.; Ma, F.; Huang, Y.; Gao, Y.; Deng, Q.; Qiao, Z.-Y.; Xing, X.; Zhu, J.; et al. Regulating Twisted Skeleton to Construct Organ-Specific Perylene for Intensive Cancer Chemotherapy. Angew. Chem. Int. Ed. 2021, 60, 16215–16223. [Google Scholar] [CrossRef]
- Li, Q.; Hao, X.; Guo, J.; Ren, X.-K.; Xia, S.; Zhang, W.; Feng, Y. Multifunctional Gene Carriers Labeled by Perylene Diimide Derivative as Fluorescent Probe for Tracking Gene Delivery. Macromol. Rapid Commun. 2019, 40, e1800916. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-K.; Kim, R.; Prabhakaran, P.; Lee, K.-S. Highly biocompatible amphiphilic perylenediimide derivative for bioimaging. Opt. Mater. Express 2016, 6, 1420. [Google Scholar] [CrossRef]
- Schill, J.; van Dun, S.; Pouderoijen, M.J.; Janssen, H.M.; Milroy, L.-G.; Schenning, A.P.H.J.; Brunsveld, L. Synthesis and Self-Assembly of Bay-Substituted Perylene Diimide Gemini-Type Surfactants as Off-On Fluorescent Probes for Lipid Bilayers. Chem. Eur. J. 2018, 24, 7734–7741. [Google Scholar] [CrossRef]
- Menger, F.M.; Littau, C.A. Gemini surfactants: A new class of self-assembling molecules. J. Am. Chem. Soc. 1993, 115, 10083–10090. [Google Scholar] [CrossRef]
- Sun, M.; Yin, W.; Dong, X.; Yang, W.; Zhao, Y.; Yin, M. Fluorescent supramolecular micelles for imaging-guided cancer therapy. Nanoscale 2016, 8, 5302–5312. [Google Scholar] [CrossRef]
- Cheng, W.; Cheng, H.; Wan, S.; Zhang, X.; Yin, M. Dual-Stimulus-Responsive Fluorescent Supramolecular Prodrug for Antitumor Drug Delivery. Chem. Mater. 2017, 29, 4218–4226. [Google Scholar] [CrossRef]
- Yukruk, F.; Dogan, A.L.; Canpinar, H.; Guc, D.; Akkaya, E.U. Water-Soluble Green Perylenediimide (PDI) Dyes as Potential Sensitizers for Photodynamic Therapy. Org. Lett. 2005, 7, 2885–2887. [Google Scholar] [CrossRef]
- Fan, Q.; Cheng, K.; Yang, Z.; Zhang, R.; Yang, M.; Hu, X.; Ma, X.; Bu, L.; Lu, X.; Xiong, X.; et al. Perylene-diimide-based nanoparticles as highly efficient photoacoustic agents for deep brain tumor imaging in living mice. Adv. Mater. 2015, 27, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Kaur, S.; Kaur, S.; Bhargava, G.; Kumar, S.; Singh, P. Self-assembled nanofibers of perylene diimide for the detection of hypochlorite in water, bio-fluids and solid-state: Exogenous and endogenous bioimaging of hypochlorite in cells. J. Mater. Chem. B 2019, 8, 125–135. [Google Scholar] [CrossRef]
- Danilov, E.O.; Rachford, A.A.; Goeb, S.; Castellano, F.N. Evolution of the triplet excited state in Pt(II) perylenediimides. J. Phys. Chem. A 2009, 113, 5763–5768. [Google Scholar] [CrossRef]
- Prusakova, V.; McCusker, C.E.; Castellano, F.N. Ligand-Localized Triplet-State Photophysics in a Platinum(II) Terpyridyl Perylenediimideacetylide. Inorg. Chem. 2012, 51, 8589–8598. [Google Scholar] [CrossRef]
- Llewellyn, B.A.; Slater, A.G.; Goretzki, G.; Easun, T.L.; Sun, X.-Z.; Davies, E.S.; Argent, S.P.; Lewis, W.; Beeby, A.; George, M.W.; et al. Photophysics and electrochemistry of a platinum-acetylide disubstituted perylenediimide. Dalton Trans. 2013, 43, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.; Steffen, A.; Würthner, F. Near-IR phosphorescent ruthenium(II) and iridium(III) perylene bisimide metal complexes. Angew. Chem. Int. Ed. 2015, 54, 1570–1573. [Google Scholar] [CrossRef] [PubMed]
- Mari, C.; Huang, H.; Rubbiani, R.; Schulze, M.; Würthner, F.; Chao, H.; Gasser, G. Evaluation of Perylene Bisimide-Based RuII and IrIII Complexes as Photosensitizers for Photodynamic Therapy. Eur. J. Inorg. Chem. 2017, 2017, 1745–1752. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, R.; Wu, J.; Fan, Q.; Yung, B.C.; Niu, G.; Jacobson, O.; Wang, Z.; Liu, G.; Yu, G.; et al. Impact of Semiconducting Perylene Diimide Nanoparticle Size on Lymph Node Mapping and Cancer Imaging. ACS Nano 2017, 11, 4247–4255. [Google Scholar] [CrossRef]
- Cui, C.; Yang, Z.; Hu, X.; Wu, J.; Shou, K.; Ma, H.; Jian, C.; Zhao, Y.; Qi, B.; Hu, X.; et al. Organic Semiconducting Nanoparticles as Efficient Photoacoustic Agents for Lightening Early Thrombus and Monitoring Thrombolysis in Living Mice. ACS Nano 2017, 11, 3298–3310. [Google Scholar] [CrossRef]
- Tang, W.; Yang, Z.; Wang, S.; Wang, Z.; Song, J.; Yu, G.; Fan, W.; Dai, Y.; Wang, J.; Shan, L.; et al. Organic Semiconducting Photoacoustic Nanodroplets for Laser-Activatable Ultrasound Imaging and Combinational Cancer Therapy. ACS Nano 2018, 12, 2610–2622. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Yuan, P.; Wang, G.; Deng, W.; Tian, S.; Wang, C.; Lu, X.; Huang, W.; Fan, Q. High Density Glycopolymers Functionalized Perylene Diimide Nanoparticles for Tumor-Targeted Photoacoustic Imaging and Enhanced Photothermal Therapy. Biomacromolecules 2017, 18, 3375–3386. [Google Scholar] [CrossRef]
- Sun, P.; Wang, X.; Wang, G.; Deng, W.; Shen, Q.; Jiang, R.; Wang, W.; Fan, Q.; Huang, W. A perylene diimide zwitterionic polymer for photoacoustic imaging guided photothermal/photodynamic synergistic therapy with single near-infrared irradiation. J. Mater. Chem. B 2018, 6, 3395–3403. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Yang, Z.; Fu, X.; Yung, B.C.; Yang, J.; Mao, Z.; Shao, L.; Hua, B.; Liu, Y.; Zhang, F.; et al. Polyrotaxane-based supramolecular theranostics. Nat. Commun. 2018, 9, 766. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Fan, W.; Zou, J.; Tang, W.; Li, L.; He, L.; Shen, Z.; Wang, Z.; Jacobson, O.; Aronova, M.A.; et al. Precision Cancer Theranostic Platform by In Situ Polymerization in Perylene Diimide-Hybridized Hollow Mesoporous Organosilica Nanoparticles. J. Am. Chem. Soc. 2019, 141, 14687–14698. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Dai, Y.; Yin, C.; Fan, Q.; Zhang, W.; Song, J.; Yu, G.; Tang, W.; Fan, W.; Yung, B.C.; et al. Activatable Semiconducting Theranostics: Simultaneous Generation and Ratiometric Photoacoustic Imaging of Reactive Oxygen Species In Vivo. Adv. Mater. 2018, 30, 1707509. [Google Scholar] [CrossRef]
- Yang, Z.; Song, J.; Dai, Y.; Chen, J.; Wang, F.; Lin, L.; Liu, Y.; Zhang, F.; Yu, G.; Zhou, Z.; et al. Self-Assembly of Semiconducting-Plasmonic Gold Nanoparticles with Enhanced Optical Property for Photoacoustic Imaging and Photothermal Therapy. Theranostics 2017, 7, 2177–2185. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Wei, J.; Yin, M. Perylenediimide chromophore as an efficient photothermal agent for cancer therapy. Sci. Bull. 2018, 63, 101–107. [Google Scholar] [CrossRef]
- Li, J.; Liu, C.; Hu, Y.; Ji, C.; Li, S.; Yin, M. pH-responsive perylenediimide nanoparticles for cancer trimodality imaging and photothermal therapy. Theranostics 2020, 10, 166–178. [Google Scholar] [CrossRef]
- Aigner, D.; Borisov, S.M.; Petritsch, P.; Klimant, I. Novel near infra-red fluorescent pH sensors based on 1-aminoperylene bisimides covalently grafted onto poly(acryloylmorpholine). Chem. Commun. 2013, 49, 2139–2141. [Google Scholar] [CrossRef]
- Yang, Z.; Song, J.; Tang, W.; Fan, W.; Dai, Y.; Shen, Z.; Lin, L.; Cheng, S.; Liu, Y.; Niu, G.; et al. Stimuli-Responsive Nanotheranostics for Real-Time Monitoring Drug Release by Photoacoustic Imaging. Theranostics 2019, 9, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Makhloutah, A.; Hatych, D.; Chartier, T.; Rocard, L.; Goujon, A.; Felpin, F.-X.; Hudhomme, P. An investigation of palladium-catalyzed Stille-type cross-coupling of nitroarenes in perylenediimide series. Org. Biomol. Chem. 2022, 20, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Leroy-Lhez, S.; Perrin, L.; Baffreau, J.; Hudhomme, P. Perylenediimide derivatives in new donor–acceptor dyads. C. R. Chim. 2006, 9, 240–246. [Google Scholar] [CrossRef]
- Pal, K.; Sharma, V.; Sahoo, D.; Kapuria, N.; Koner, A.L. Large Stokes-shifted NIR-emission from nanospace-induced aggregation of perylenemonoimide-doped polymer nanoparticles: Imaging of folate receptor expression. Chem. Commun. 2018, 54, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.; Sharma, V.; Koner, A.L. Single-component white-light emission via intramolecular electronic conjugation-truncation with perylenemonoimide. Chem. Commun. 2017, 53, 7909–7912. [Google Scholar] [CrossRef]
- Li, C.; Schöneboom, J.; Liu, Z.; Pschirer, N.G.; Erk, P.; Herrmann, A.; Müllen, K. Rainbow perylene monoimides: Easy control of optical properties. Chemistry 2009, 15, 878–884. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, X.; Yuan, Z.; Zhang, H.; Luo, G.; Hu, Y.; Chen, Y. Construction of rylene near-infrared absorption dyes with azaperylene monoimide. Dye. Pigment. 2020, 173, 107930. [Google Scholar] [CrossRef]
- Mu, M.; Ke, X.; Cheng, W.; Li, J.; Ji, C.; Yin, M. Perylenemonoimide-Based Colorimetric Probe with High Contrast for Naked-Eye Detection of Fluoride Ions. Anal. Chem. 2022, 94, 11470–11475. [Google Scholar] [CrossRef]
- Busto, N.; García-Calvo, J.; Vicente Cuevas, J.; Herrera, A.; Mergny, J.-L.; Pons, S.; Torroba, T.; García, B. Influence of core extension and side chain nature in targeting G-quadruplex structures with perylene monoimide derivatives. Bioorg. Chem. 2021, 108, 104660. [Google Scholar] [CrossRef]
- Mengji, R.; Acharya, C.; Vangala, V.; Jana, A. A lysosome-specific near-infrared fluorescent probe for in vitro cancer cell detection and non-invasive in vivo imaging. Chem. Commun. 2019, 55, 14182–14185. [Google Scholar] [CrossRef]
- Kaloyanova, S.; Zagranyarski, Y.; Ritz, S.; Hanulová, M.; Koynov, K.; Vonderheit, A.; Müllen, K.; Peneva, K. Water-Soluble NIR-Absorbing Rylene Chromophores for Selective Staining of Cellular Organelles. J. Am. Chem. Soc. 2016, 138, 2881–2884. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Ni, D.; Cheng, W.; Ji, C.; Wang, Y.; Müllen, K.; Su, Z.; Liu, Y.; Chen, C.; Yin, M. Enzyme-Triggered Disassembly of Perylene Monoimide-based Nanoclusters for Activatable and Deep Photodynamic Therapy. Angew. Chem. Int. Ed. 2020, 59, 14014–14018. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yan, H.; Zhao, L.-X.; Zhang, G.-F.; Hu, Z.; Huang, Z.-L.; Zhu, M.-Q. A trident dithienylethene-perylenemonoimide dyad with super fluorescence switching speed and ratio. Nat. Commun. 2014, 5, 5709. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-X.; Xin, B.; Li, C.; Gong, W.-L.; Huang, Z.-L.; Tang, B.-Z.; Zhu, M.-Q. Photoswitchable polyfluorophores based on perylenemonoimide–dithienylethene conjugates as super-resolution MitoTrackers. J. Mater. Chem. C 2017, 5, 9339–9344. [Google Scholar] [CrossRef]
- Liu, J.-X.; Xin, B.; Li, C.; Xie, N.-H.; Gong, W.-L.; Huang, Z.-L.; Zhu, M.-Q. PEGylated Perylenemonoimide-Dithienylethene for Super-Resolution Imaging of Liposomes. ACS Appl. Mater. Interfaces 2017, 9, 10338–10343. [Google Scholar] [CrossRef] [PubMed]










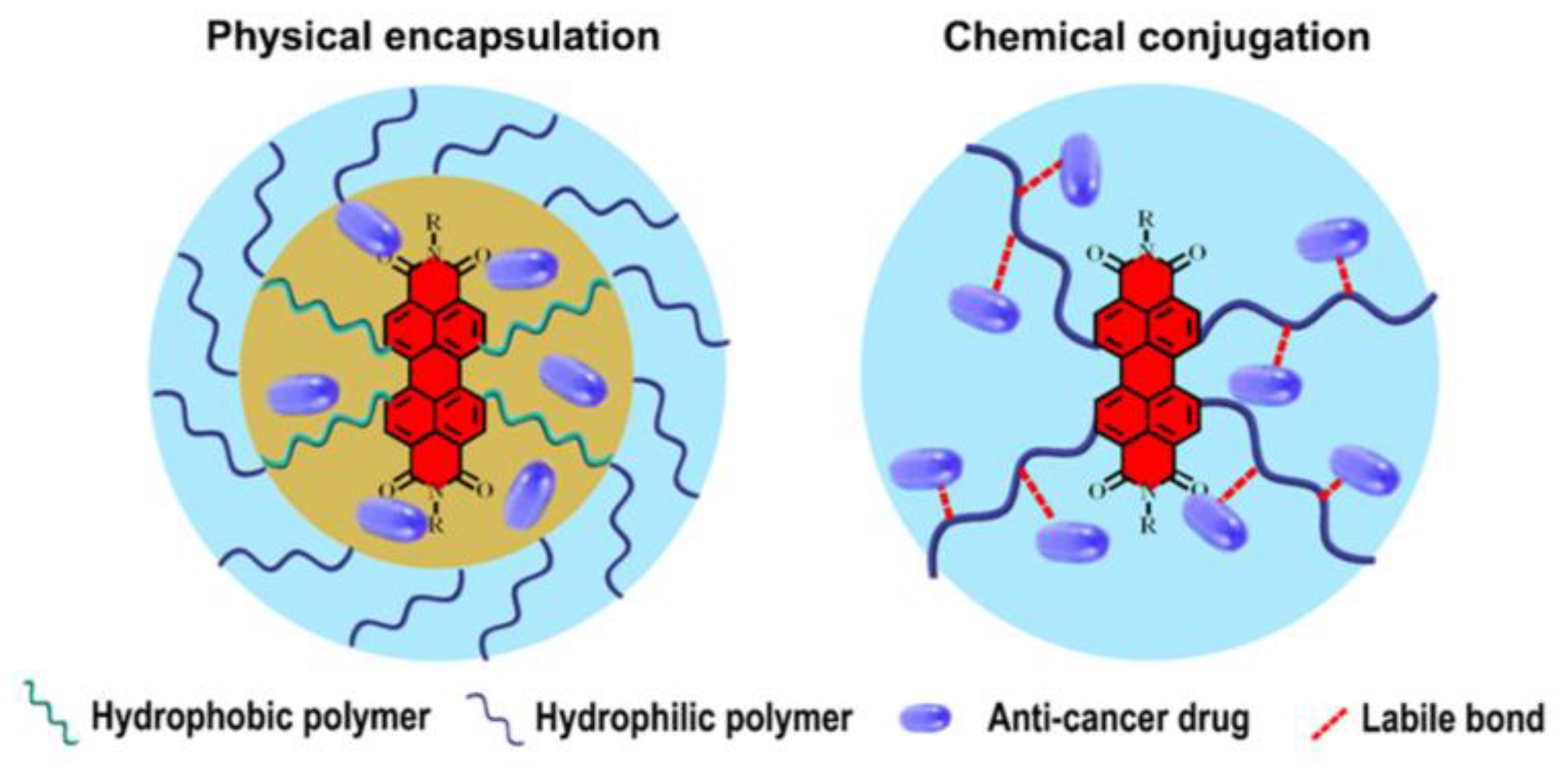
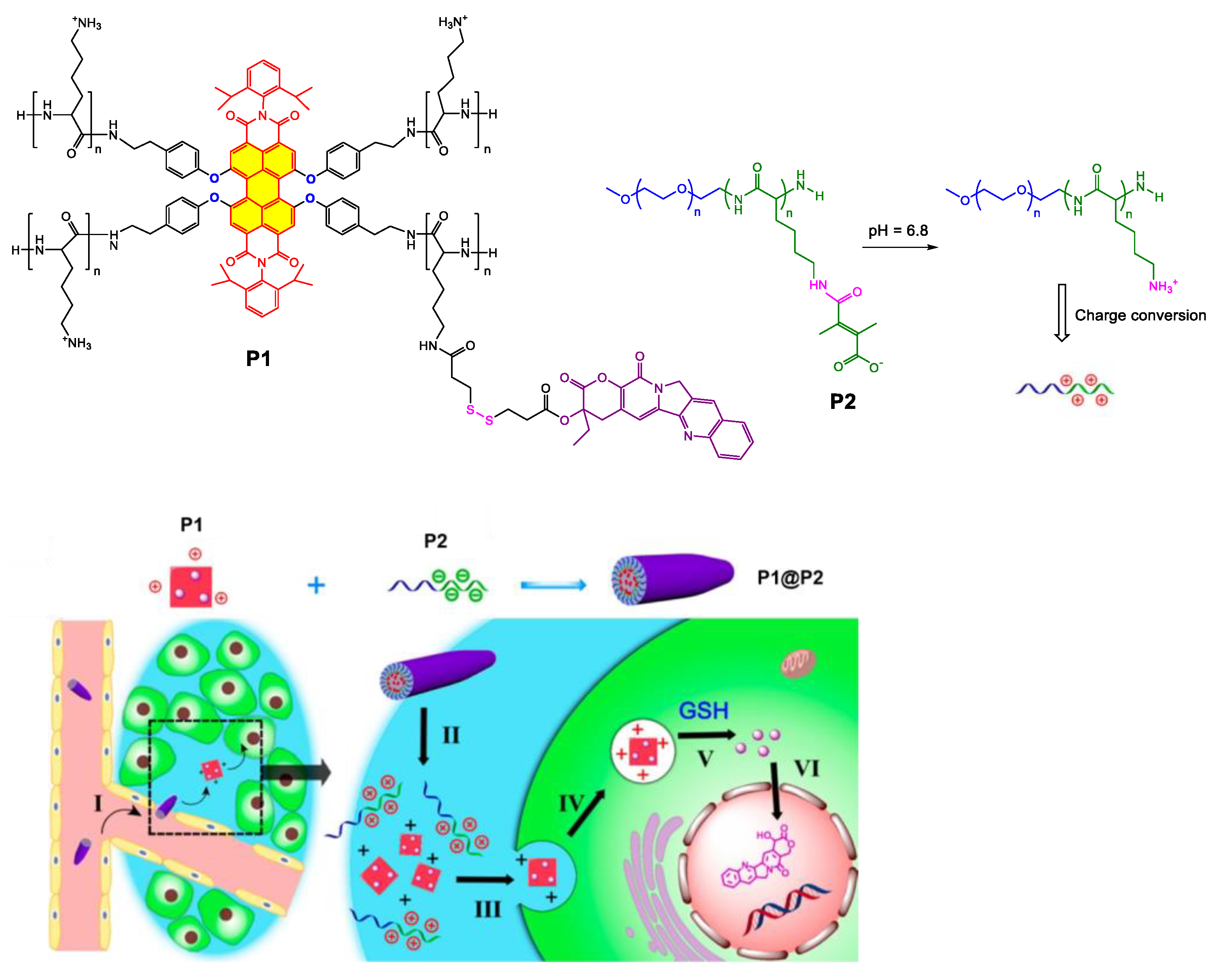
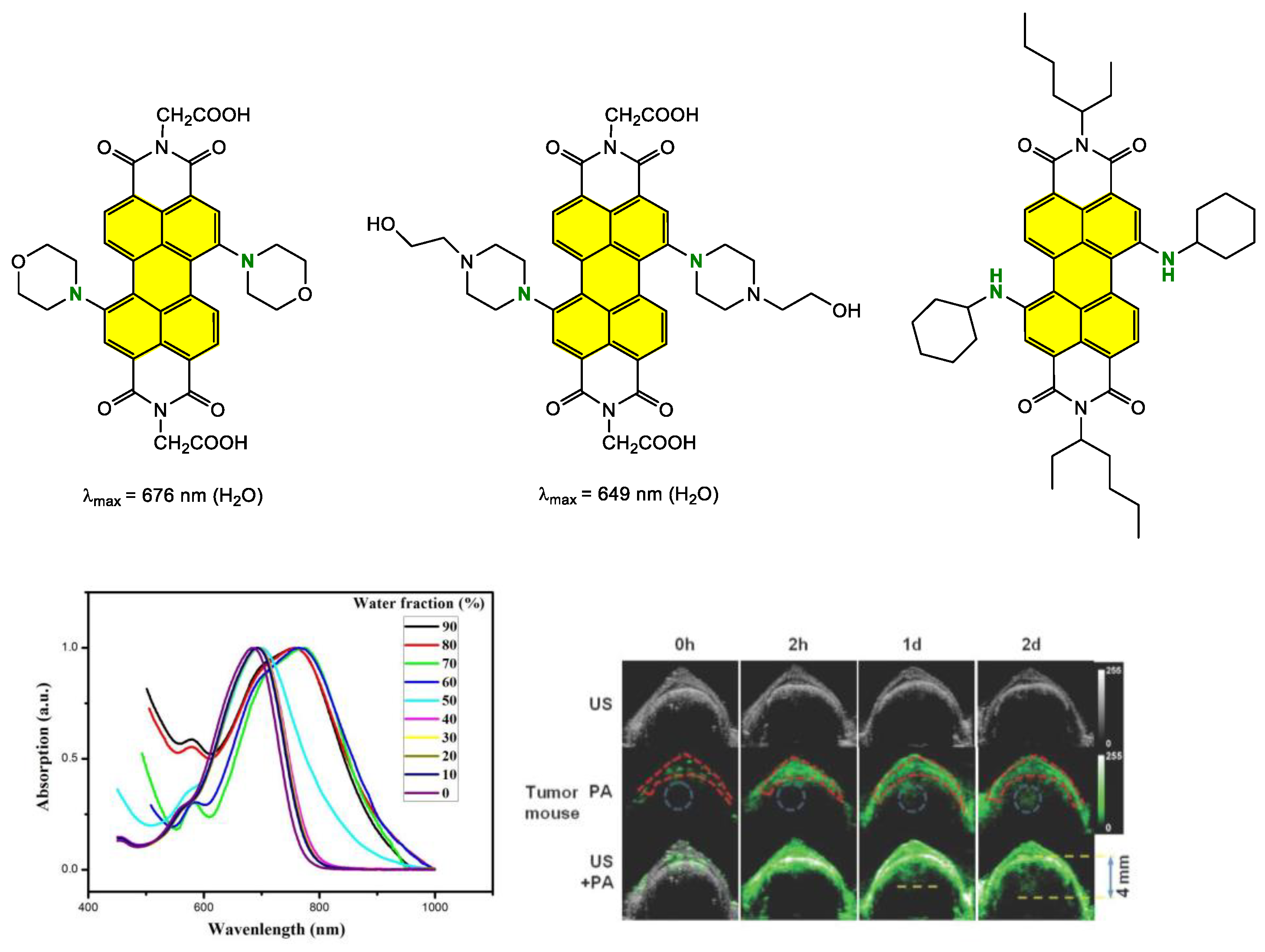
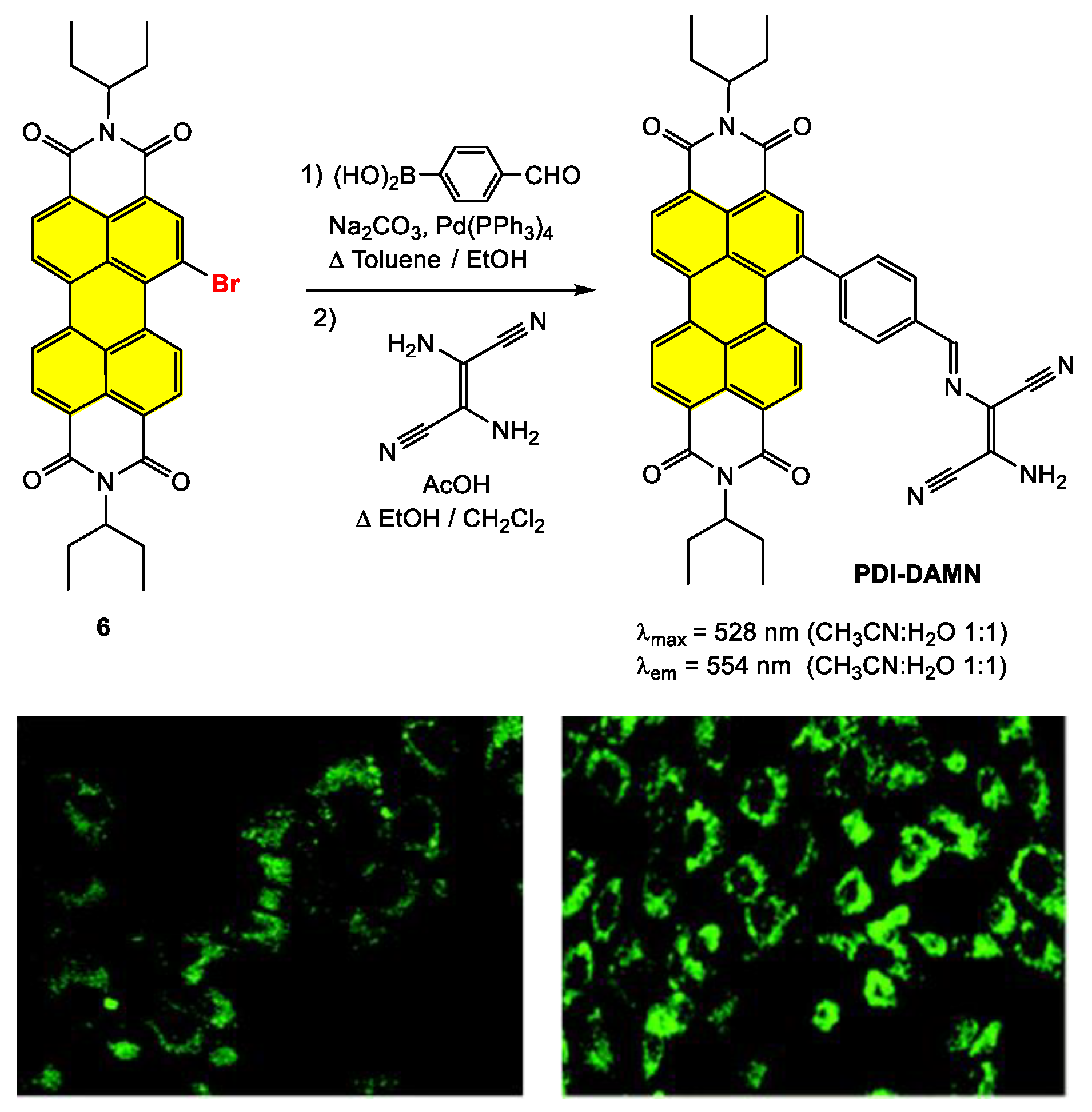
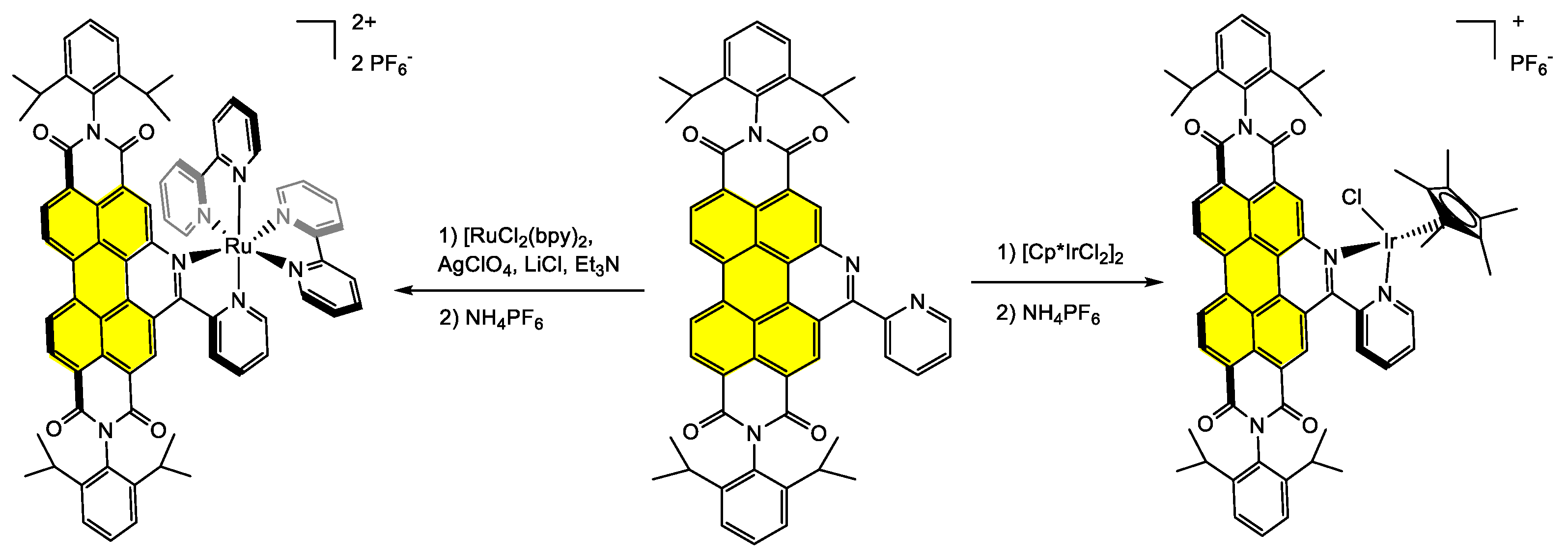

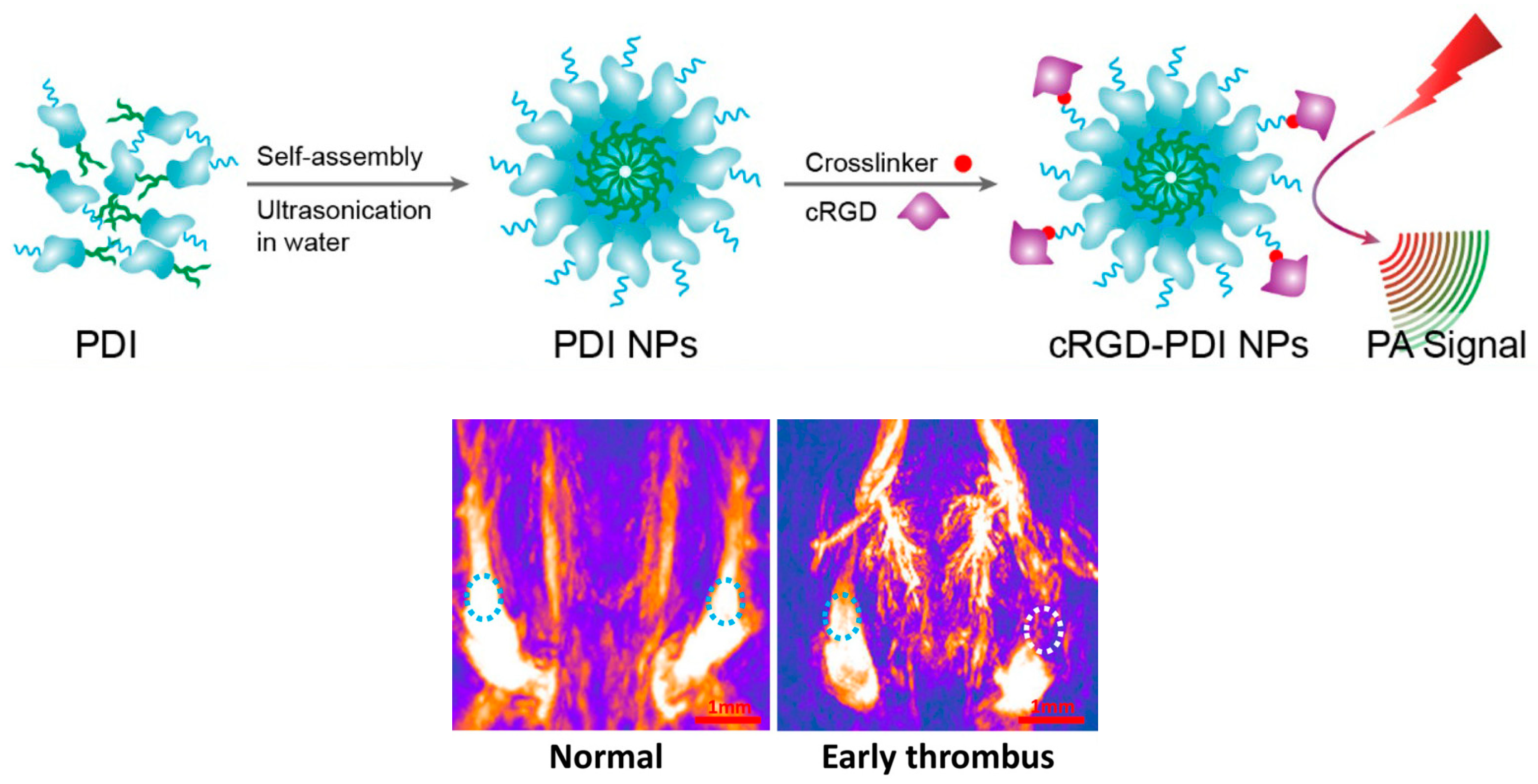
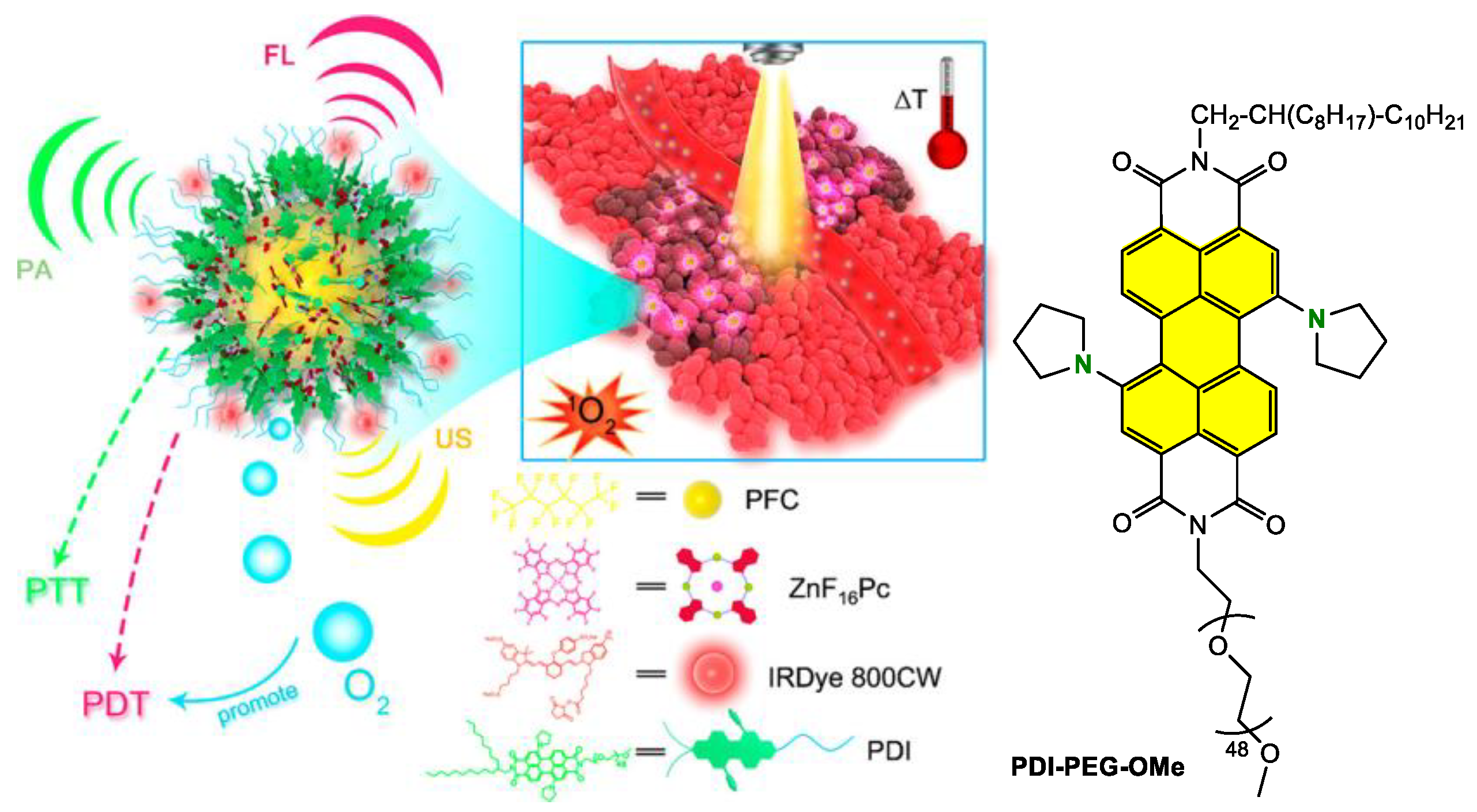
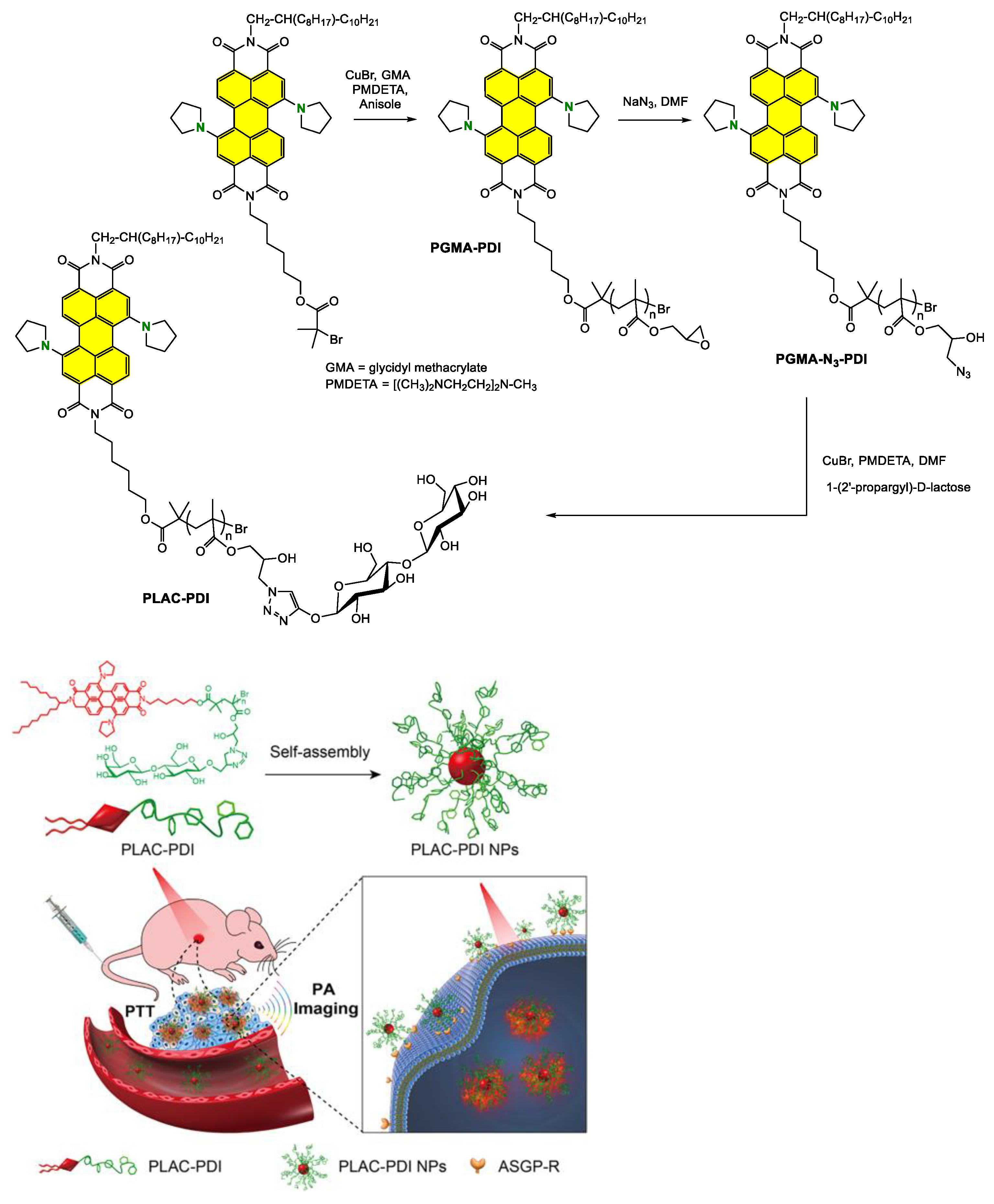



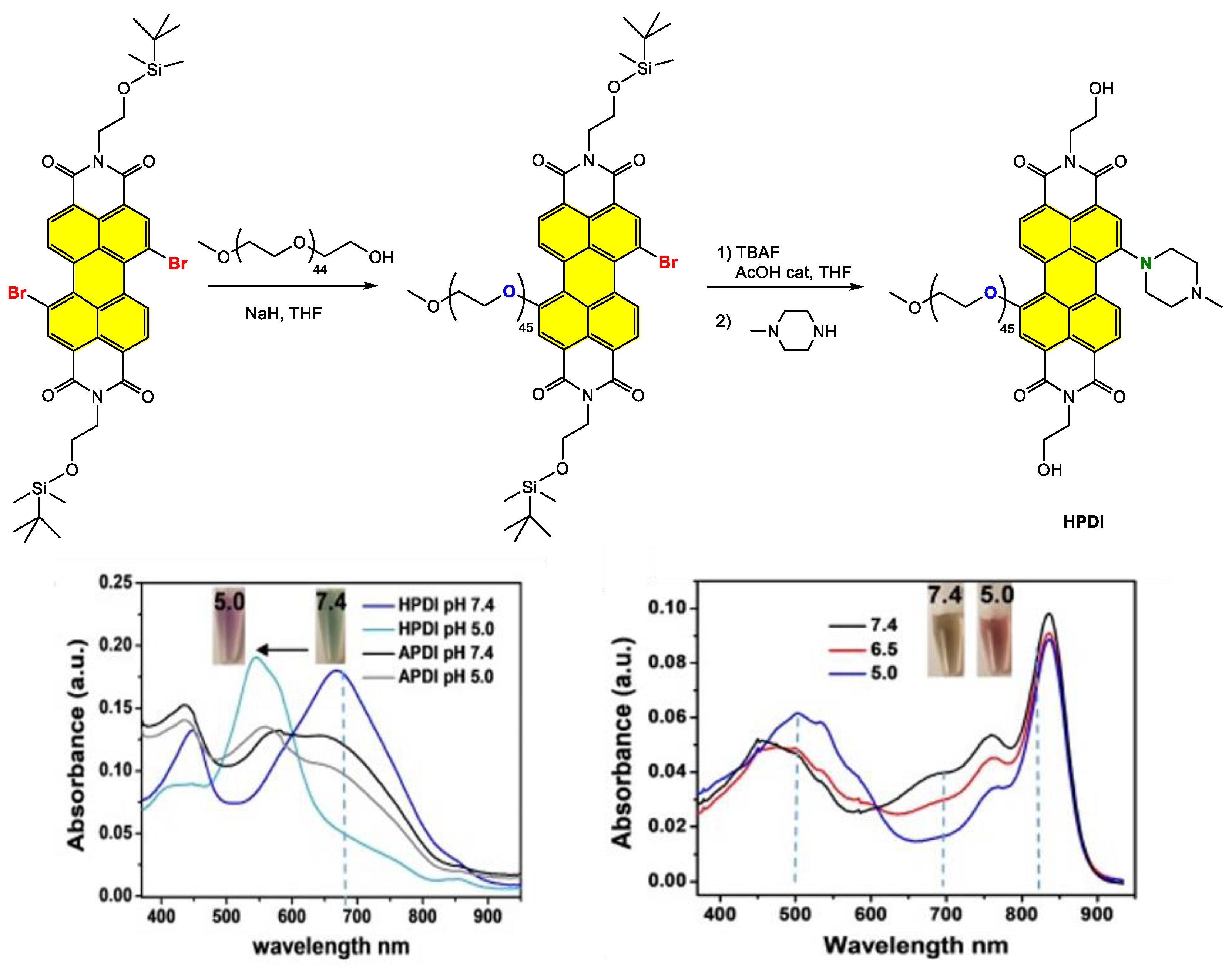
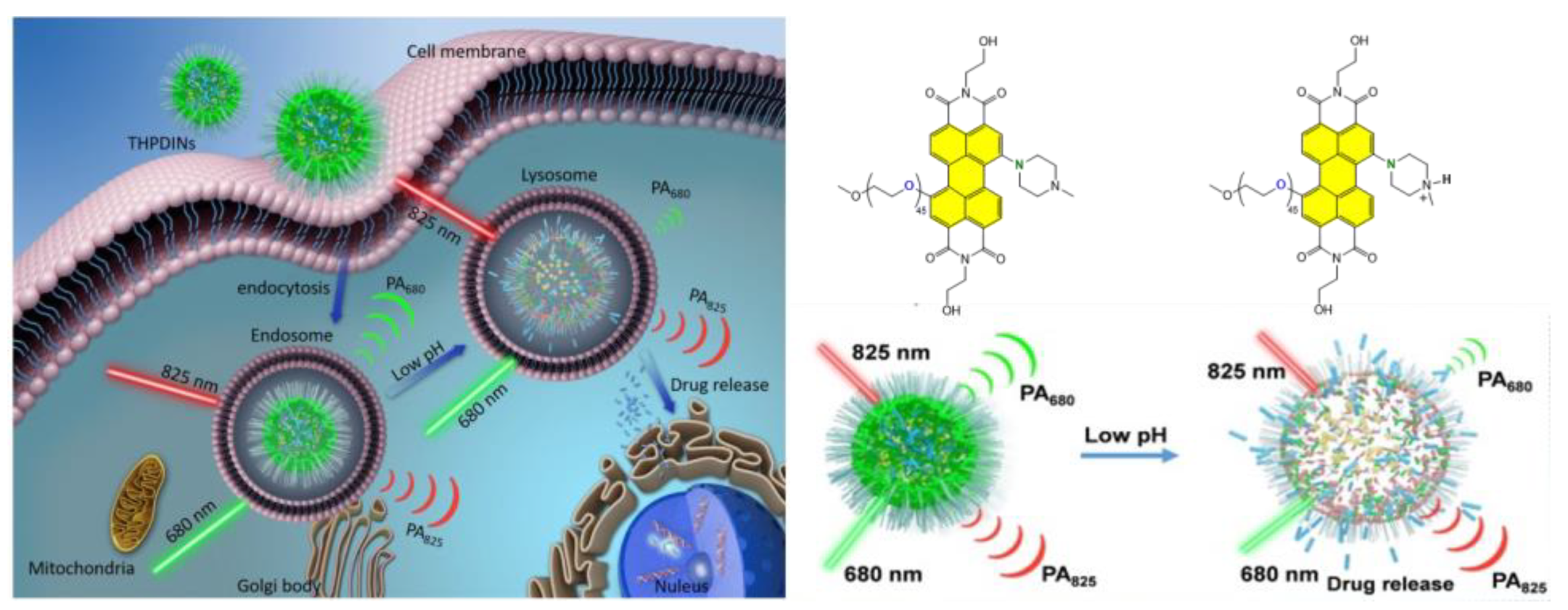
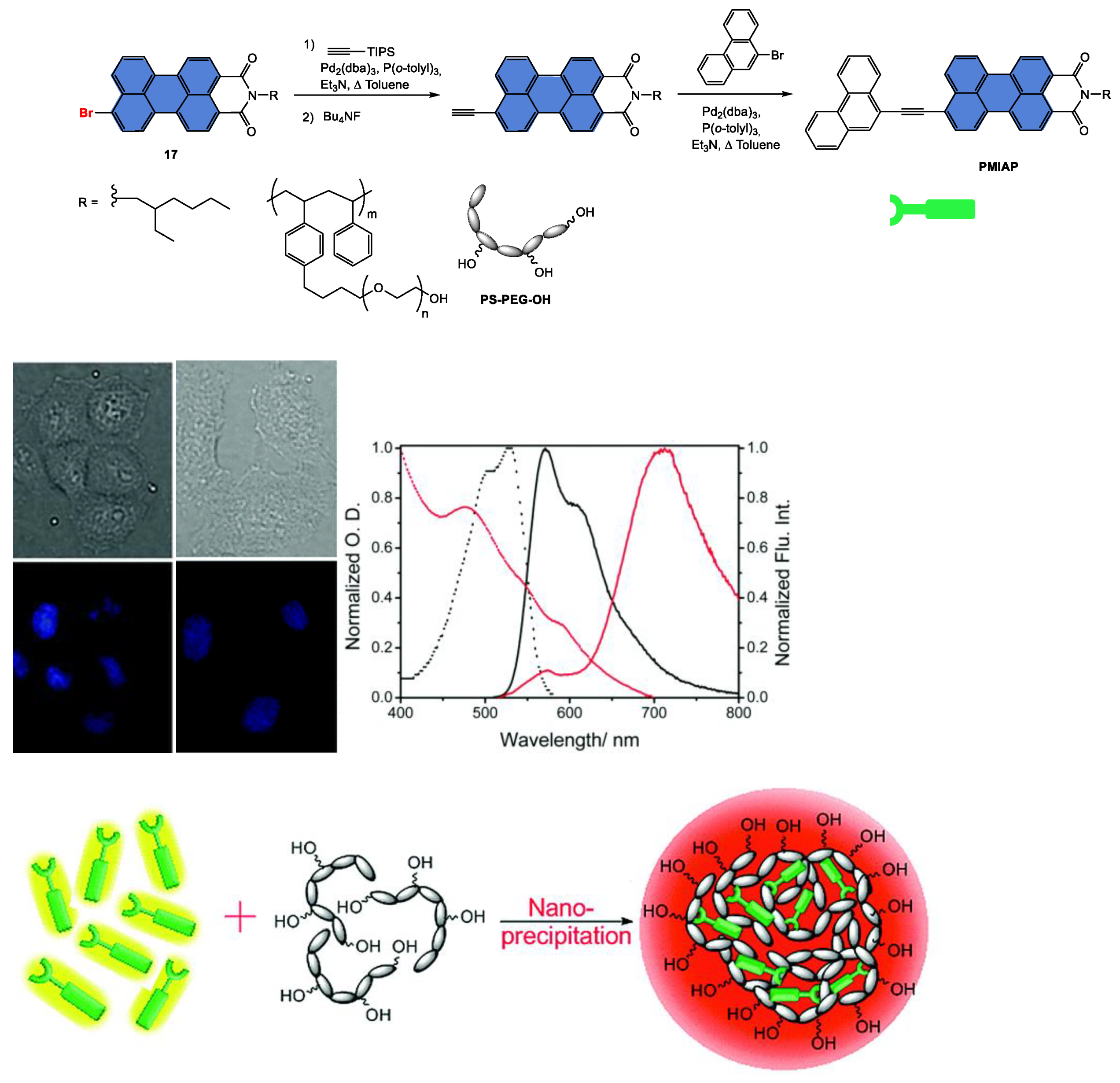
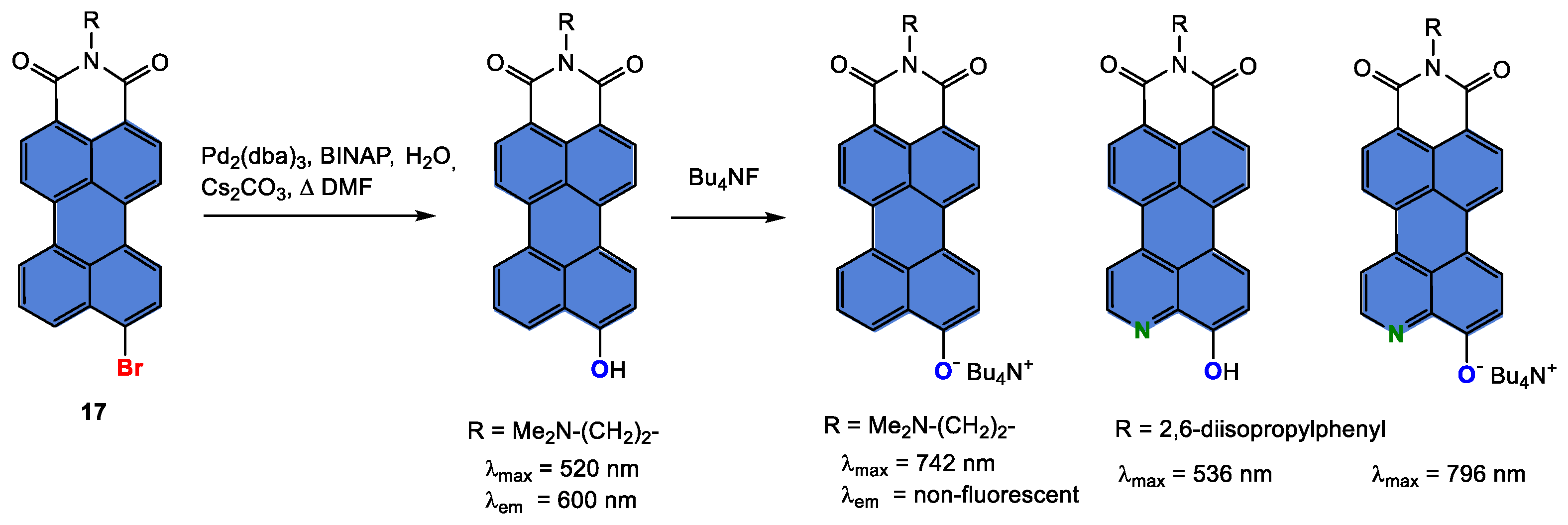
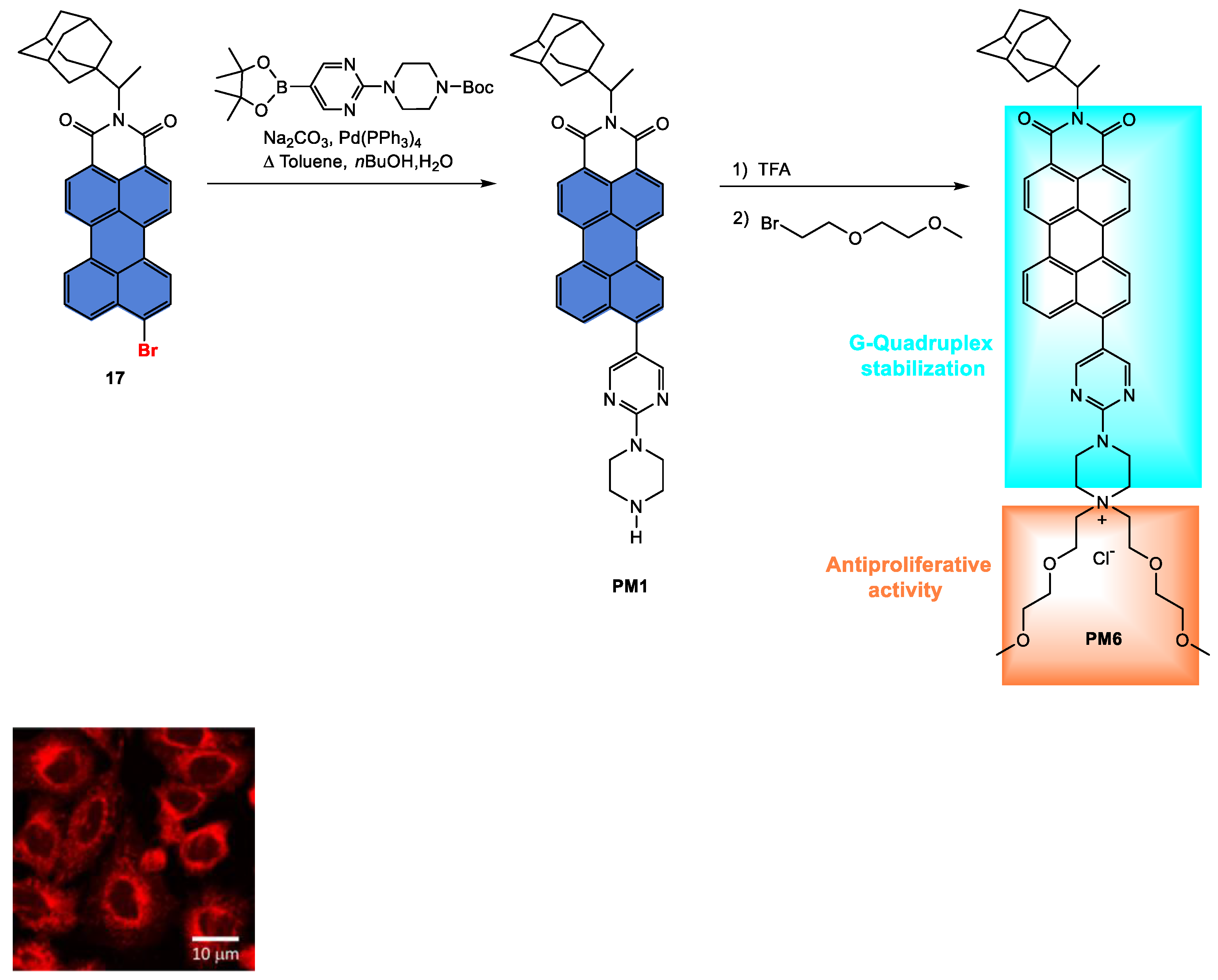
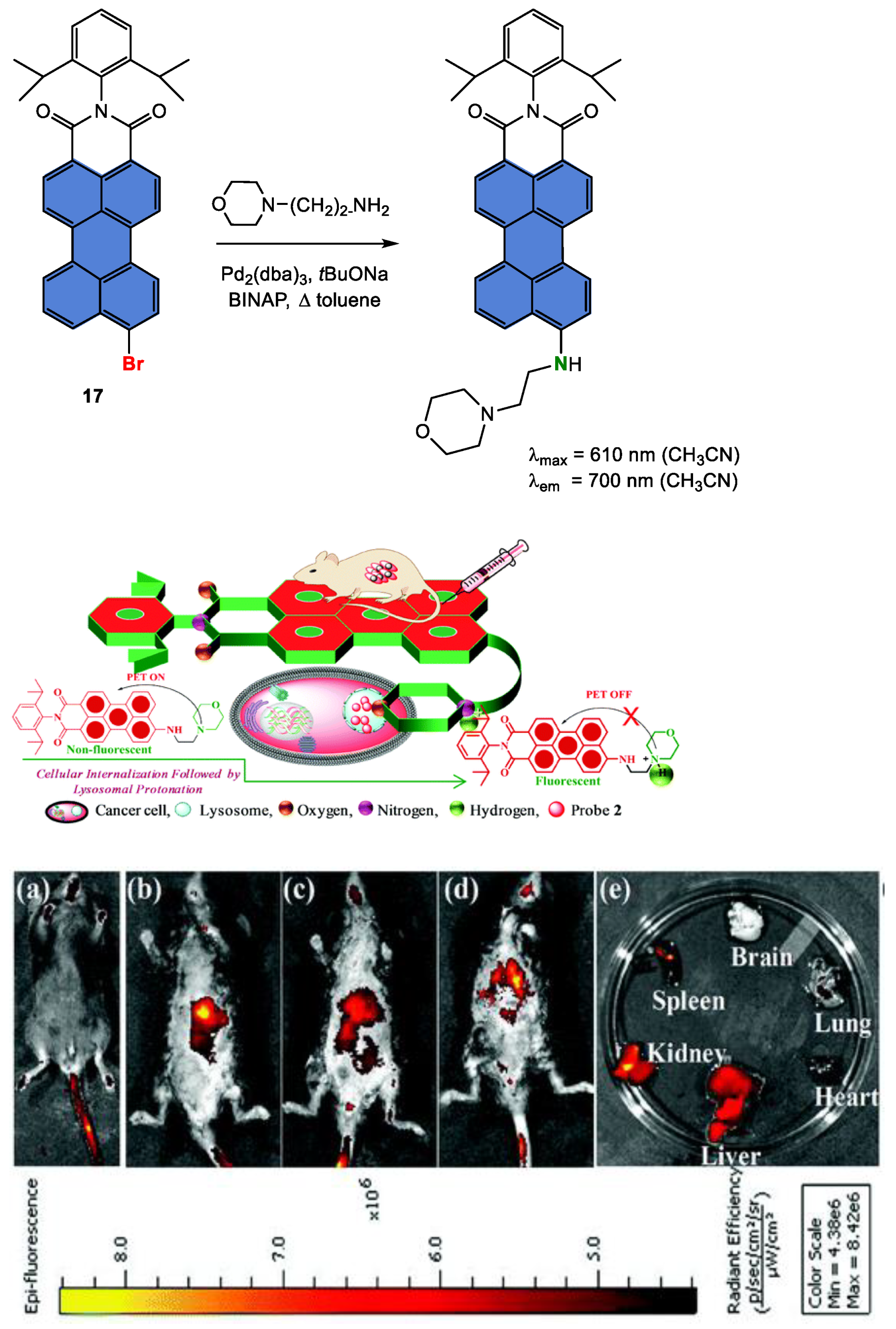

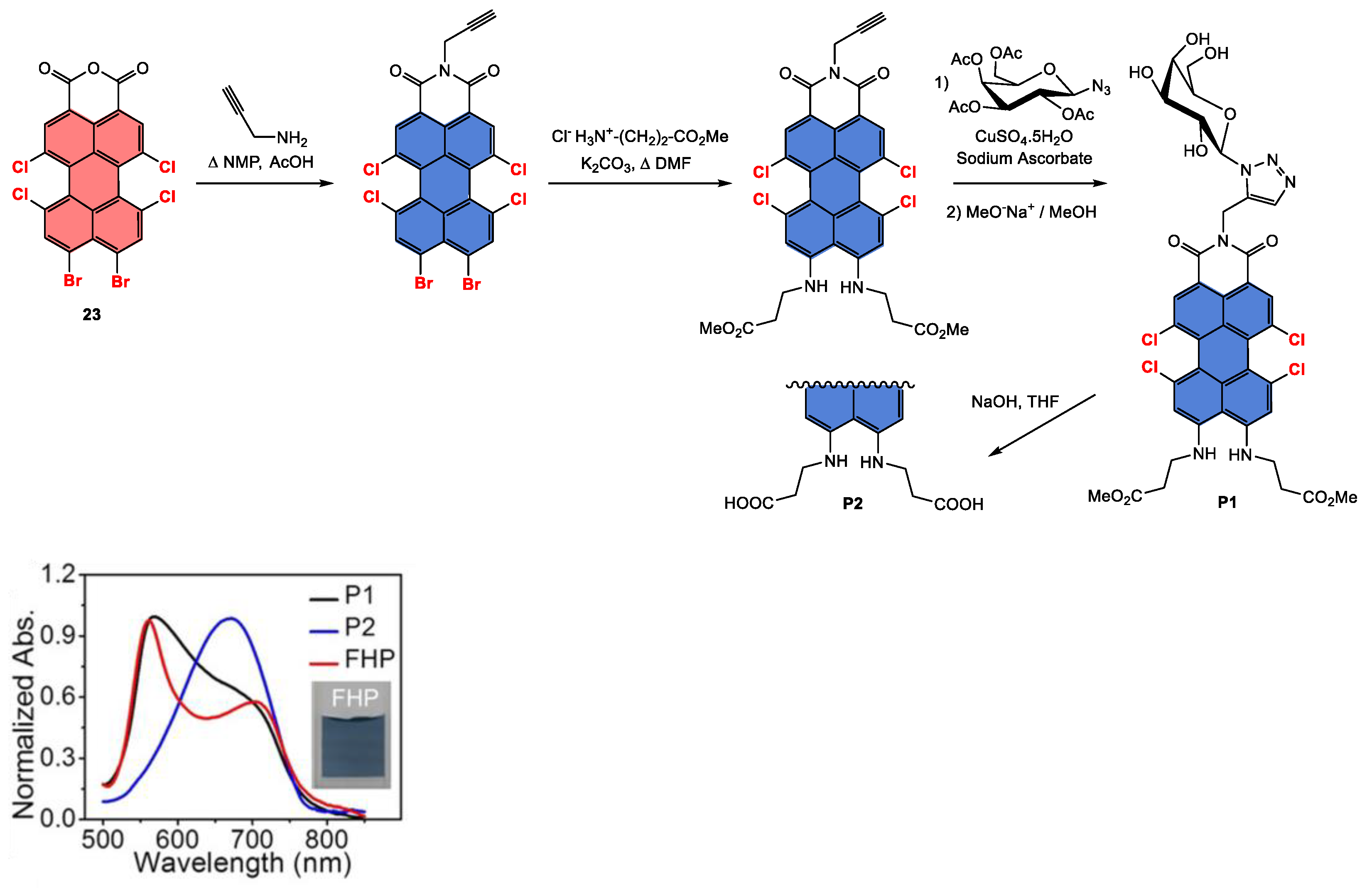
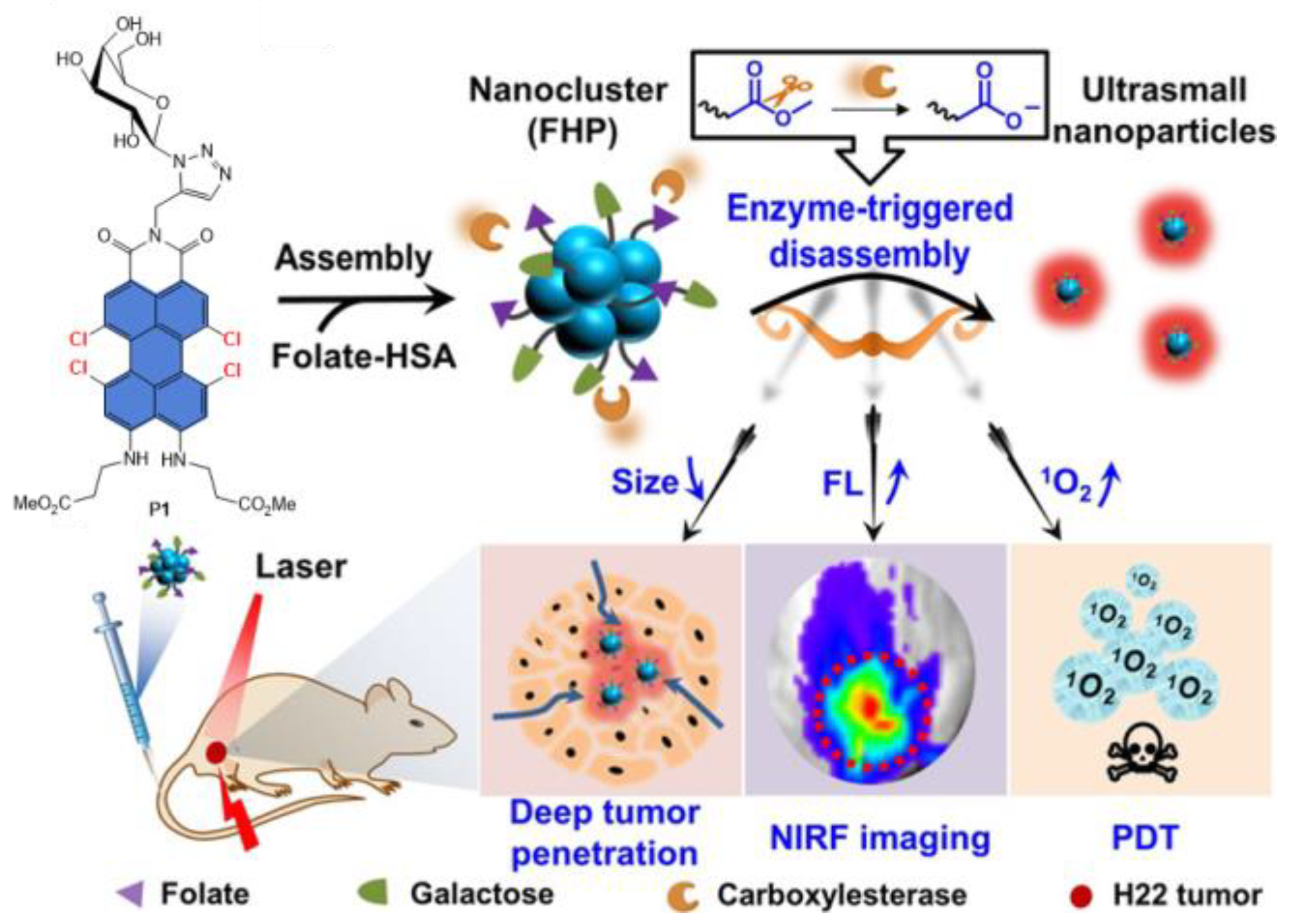
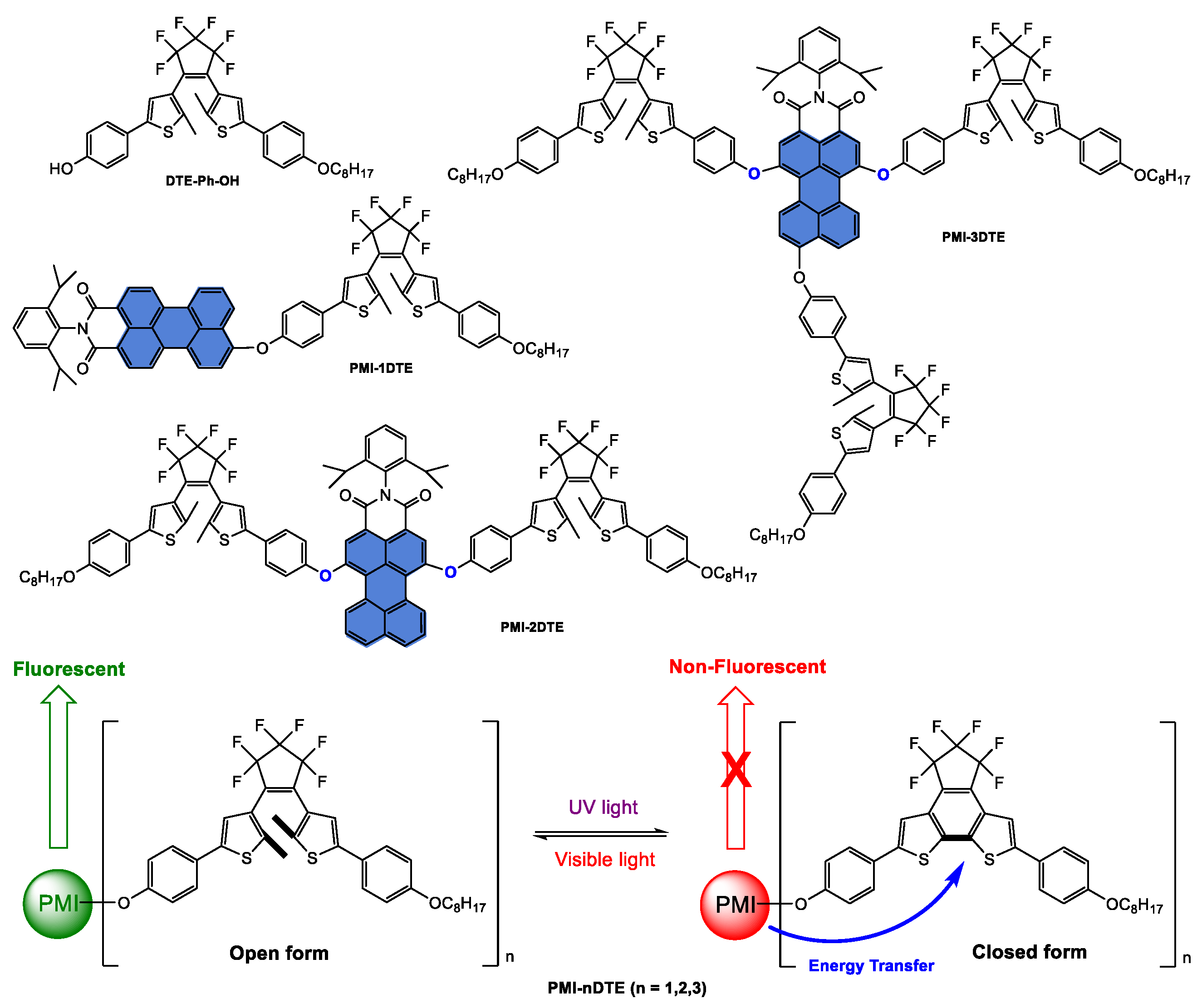
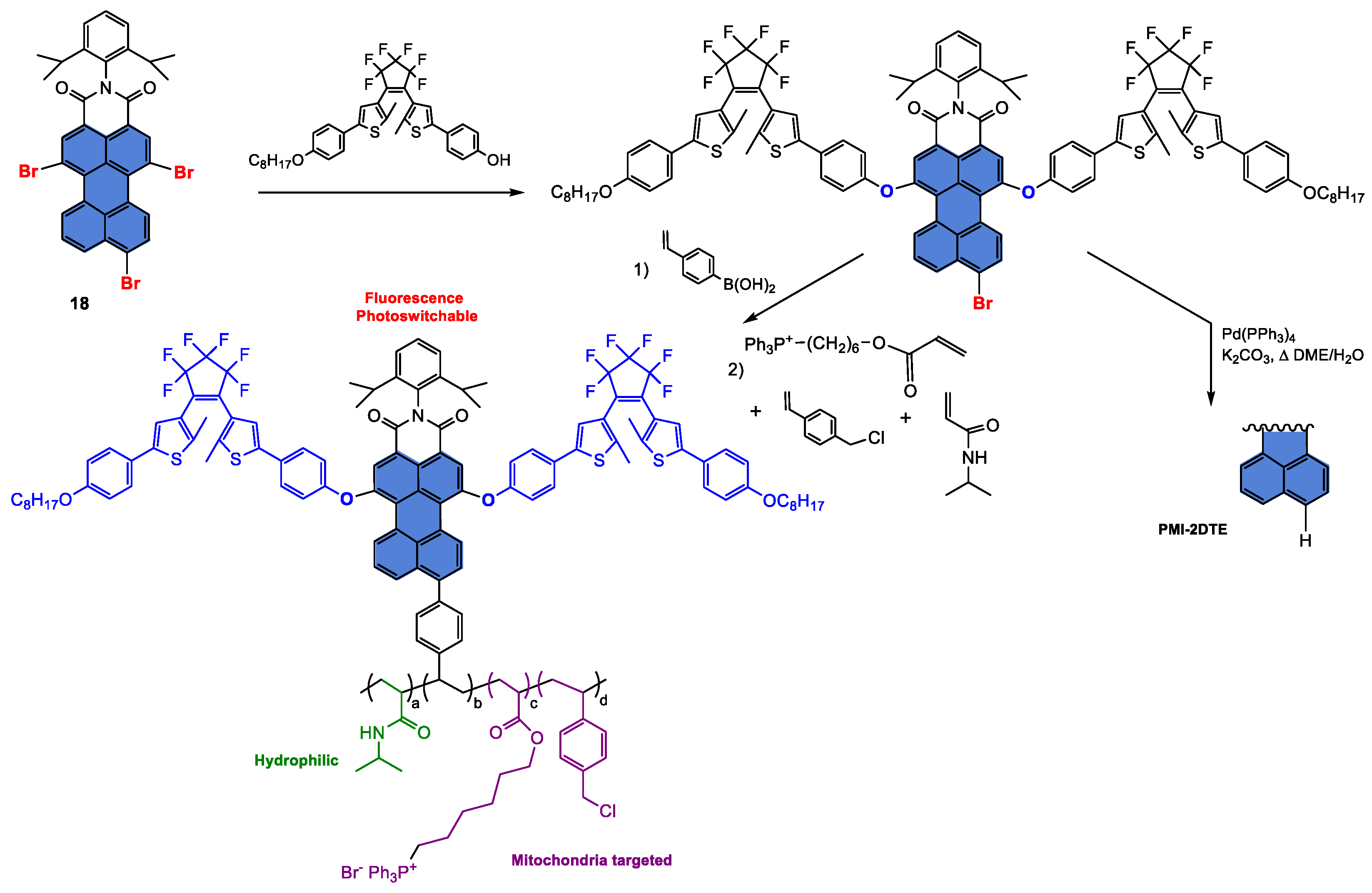
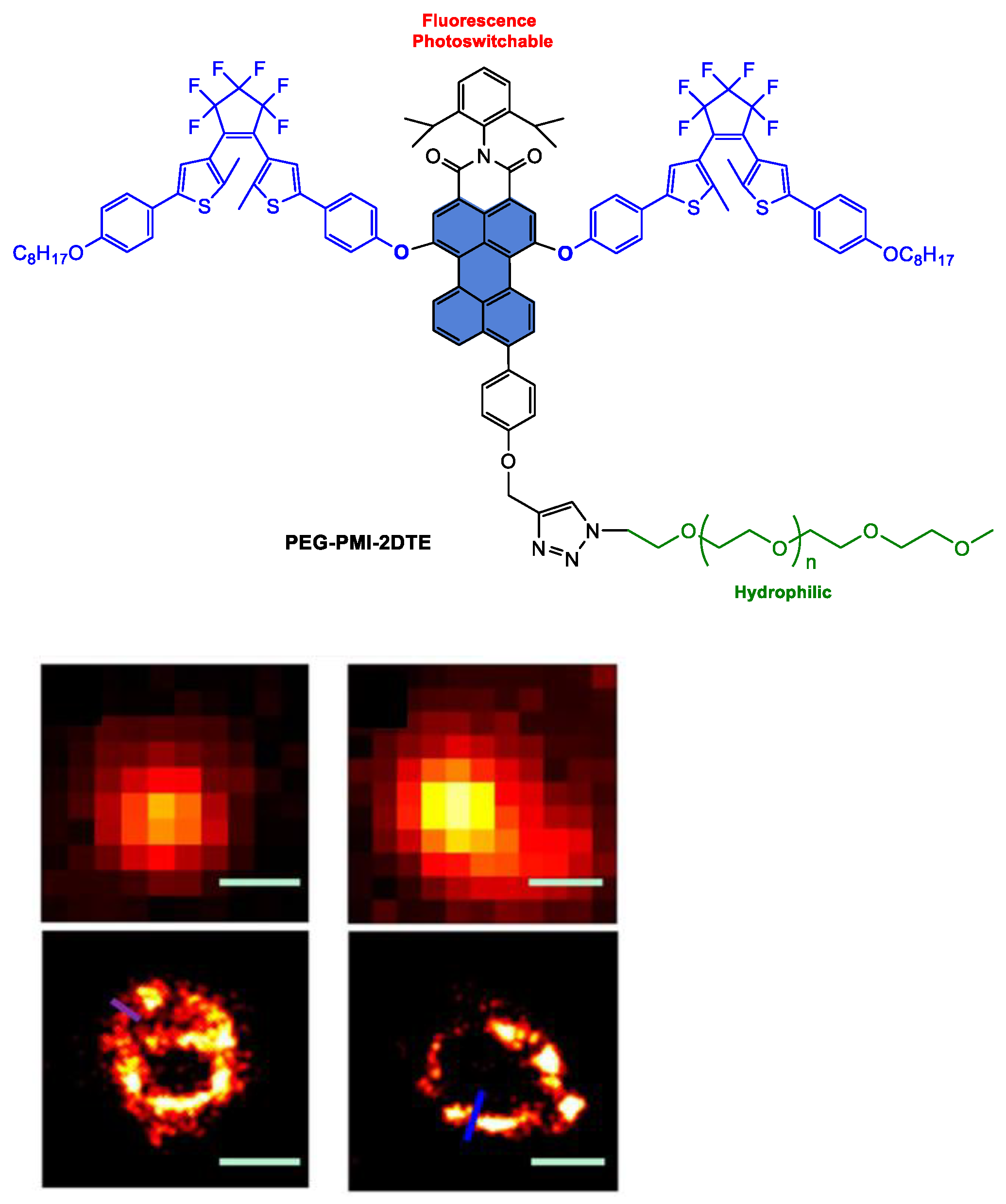
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krupka, O.; Hudhomme, P. Recent Advances in Applications of Fluorescent Perylenediimide and Perylenemonoimide Dyes in Bioimaging, Photothermal and Photodynamic Therapy. Int. J. Mol. Sci. 2023, 24, 6308. https://doi.org/10.3390/ijms24076308
Krupka O, Hudhomme P. Recent Advances in Applications of Fluorescent Perylenediimide and Perylenemonoimide Dyes in Bioimaging, Photothermal and Photodynamic Therapy. International Journal of Molecular Sciences. 2023; 24(7):6308. https://doi.org/10.3390/ijms24076308
Chicago/Turabian StyleKrupka, Oksana, and Piétrick Hudhomme. 2023. "Recent Advances in Applications of Fluorescent Perylenediimide and Perylenemonoimide Dyes in Bioimaging, Photothermal and Photodynamic Therapy" International Journal of Molecular Sciences 24, no. 7: 6308. https://doi.org/10.3390/ijms24076308
APA StyleKrupka, O., & Hudhomme, P. (2023). Recent Advances in Applications of Fluorescent Perylenediimide and Perylenemonoimide Dyes in Bioimaging, Photothermal and Photodynamic Therapy. International Journal of Molecular Sciences, 24(7), 6308. https://doi.org/10.3390/ijms24076308