Synthesis and Characterization of Tetraphenylethene AIEgen-Based Push–Pull Chromophores for Photothermal Applications: Could the Cycloaddition–Retroelectrocyclization Click Reaction Make Any Molecule Photothermally Active?
Abstract
1. Introduction
2. Results and Discussion
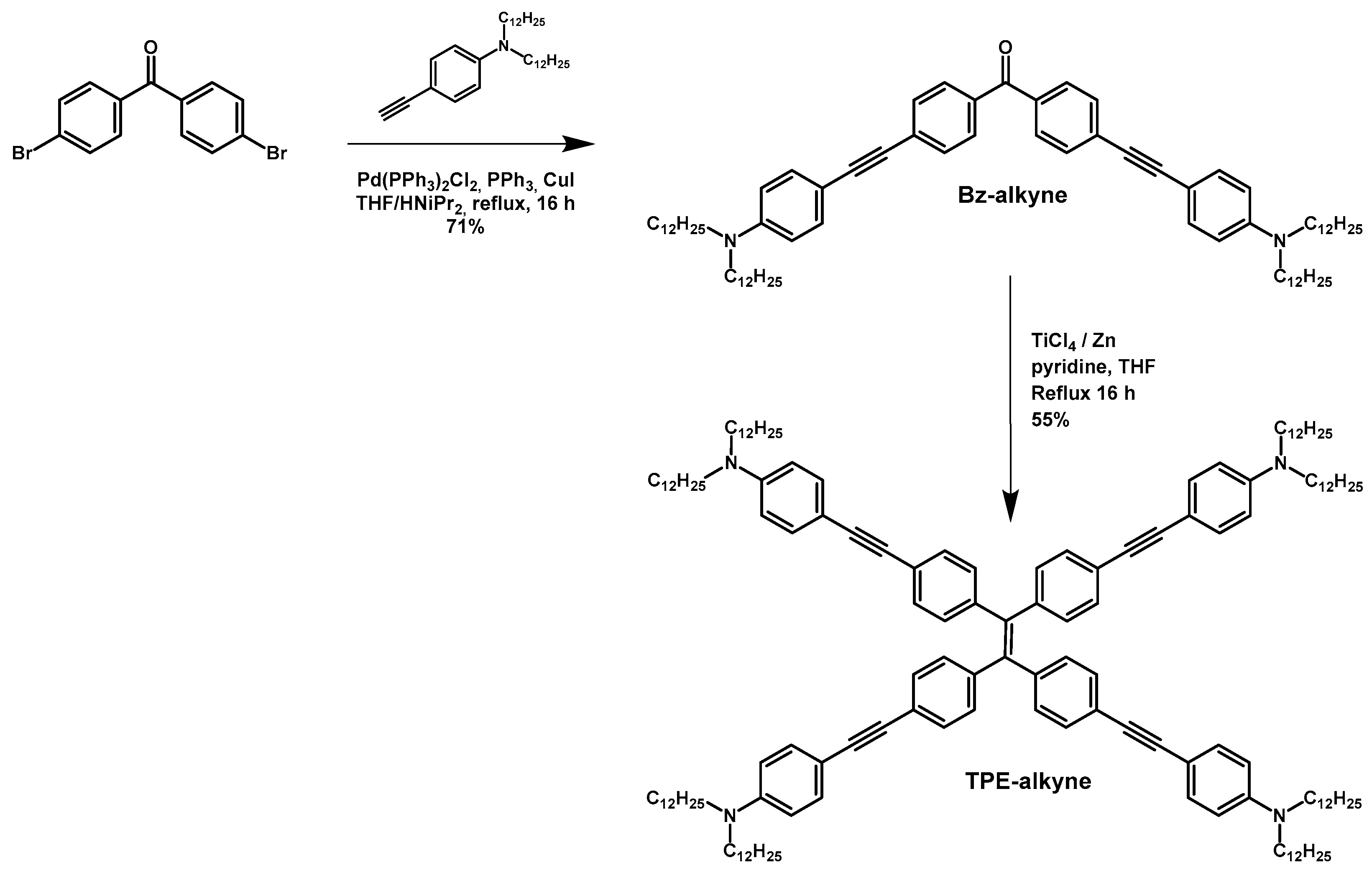
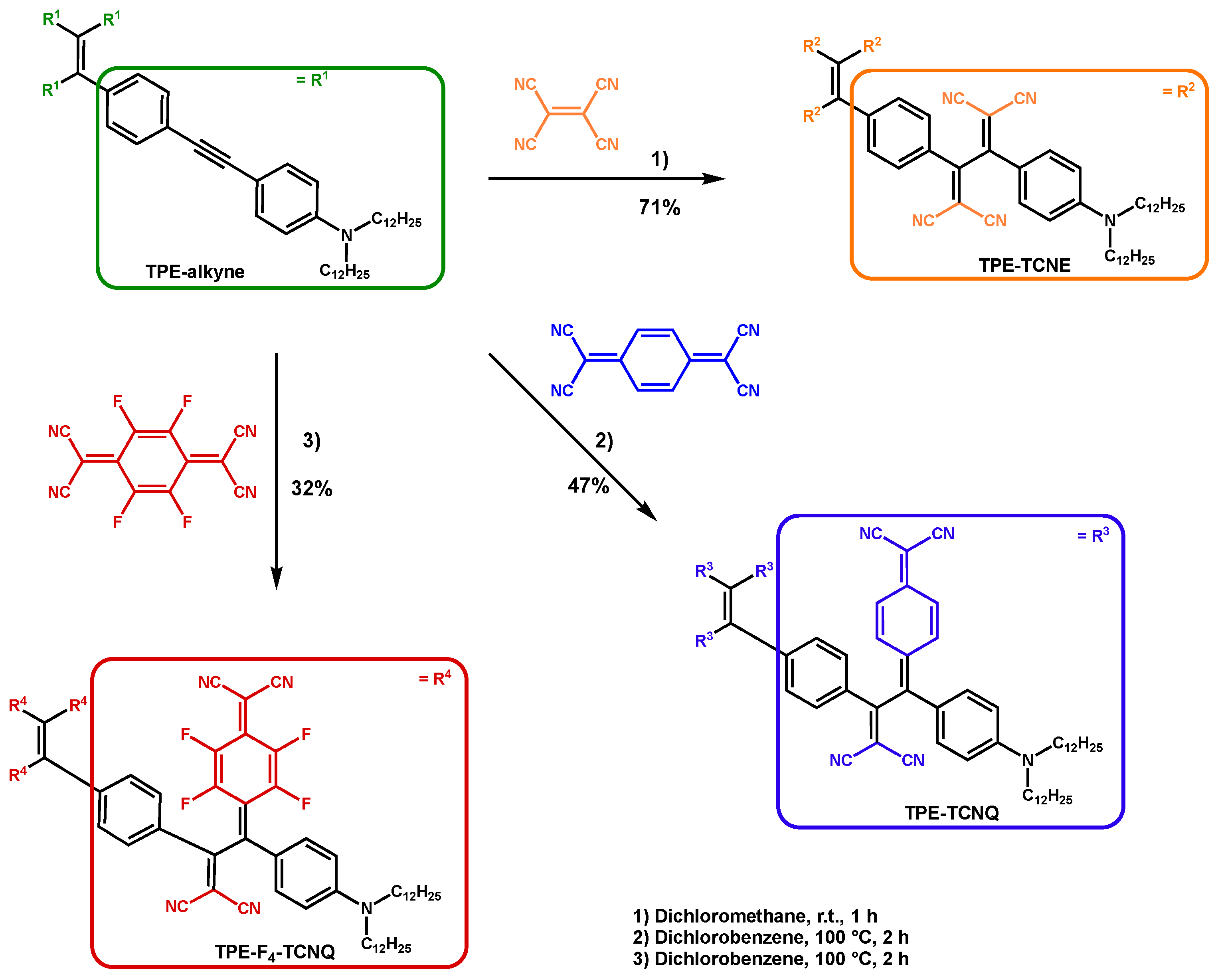
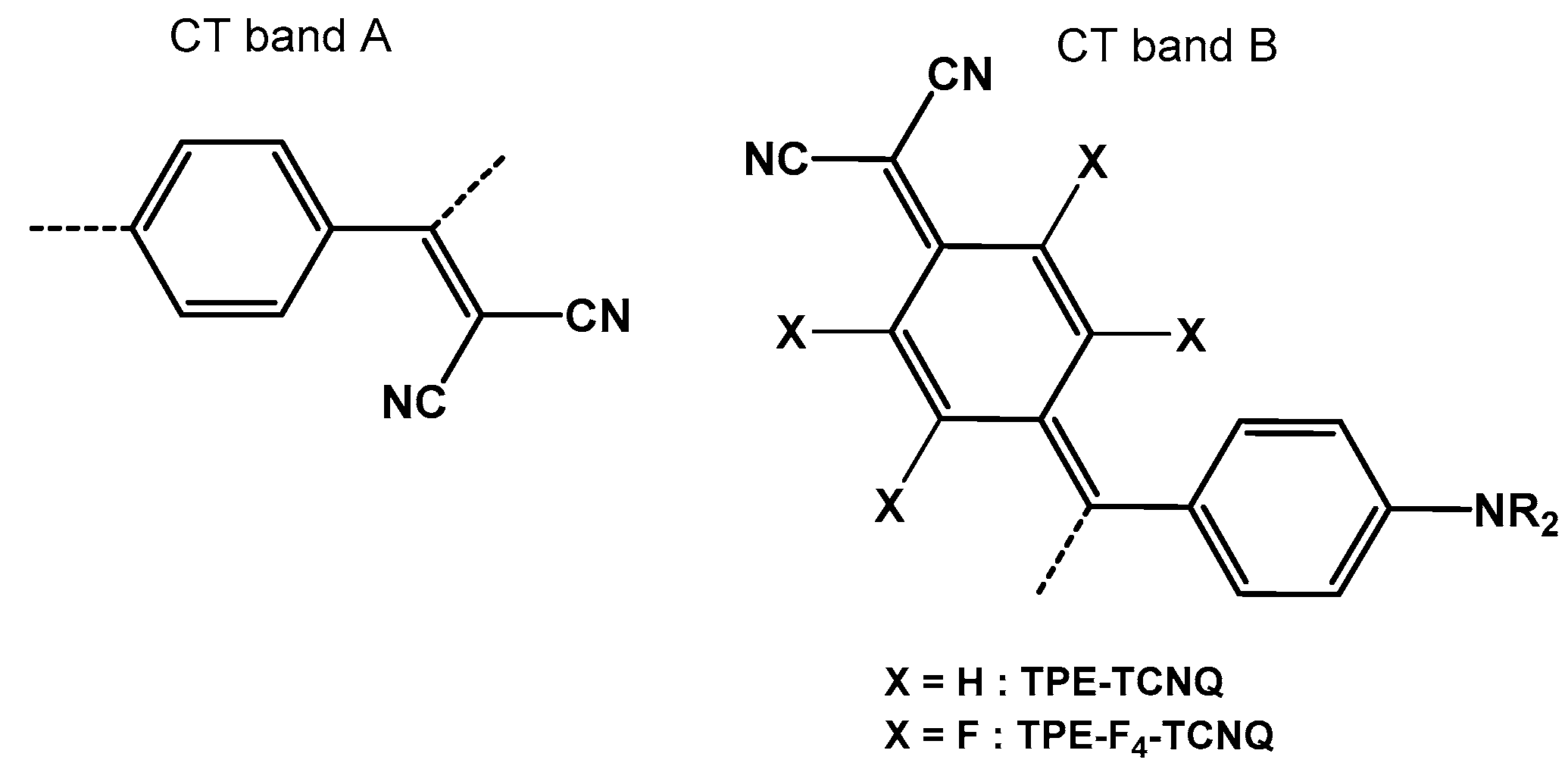
2.1. Synthesis
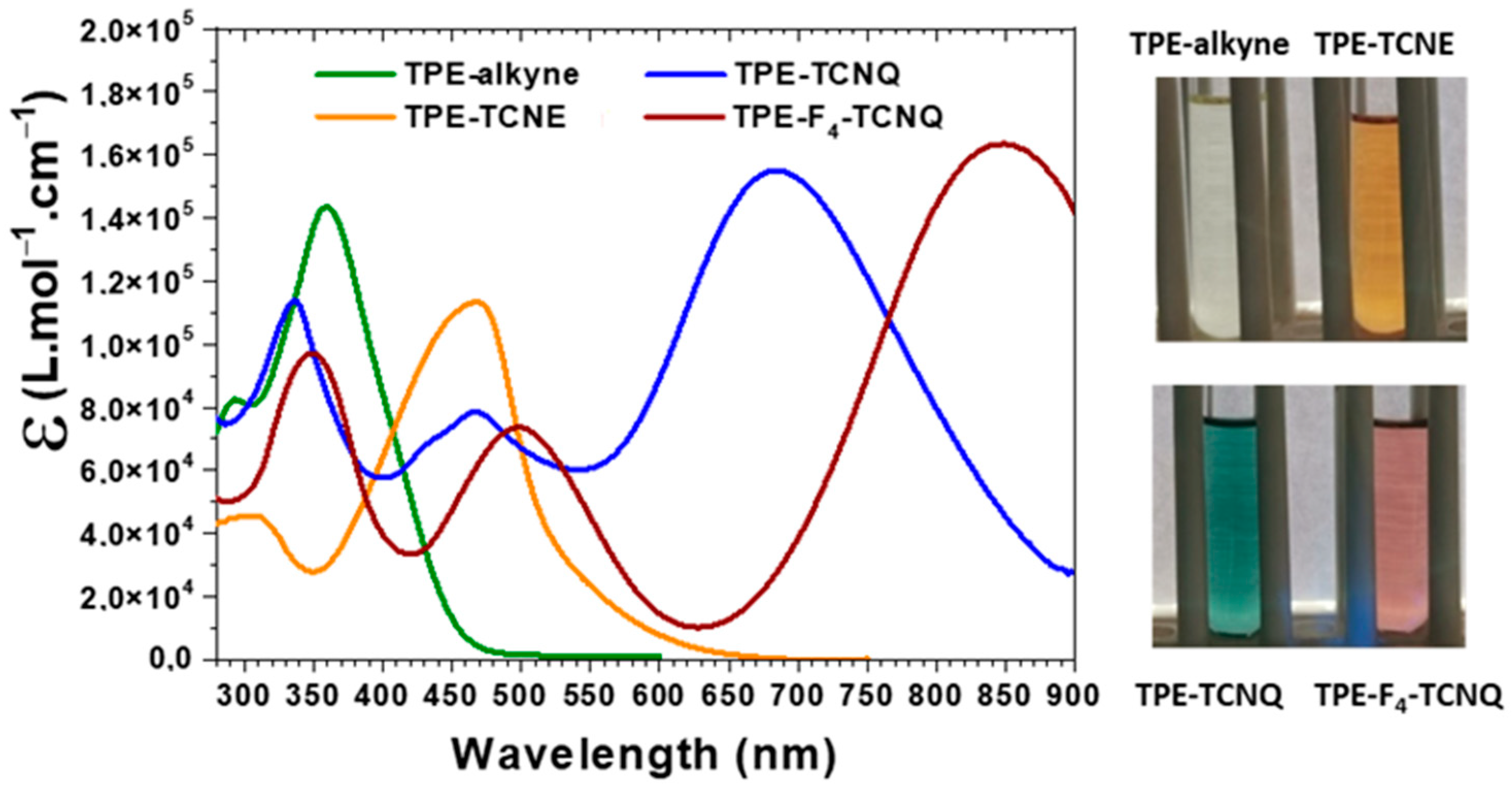
2.2. Photophysical Properties
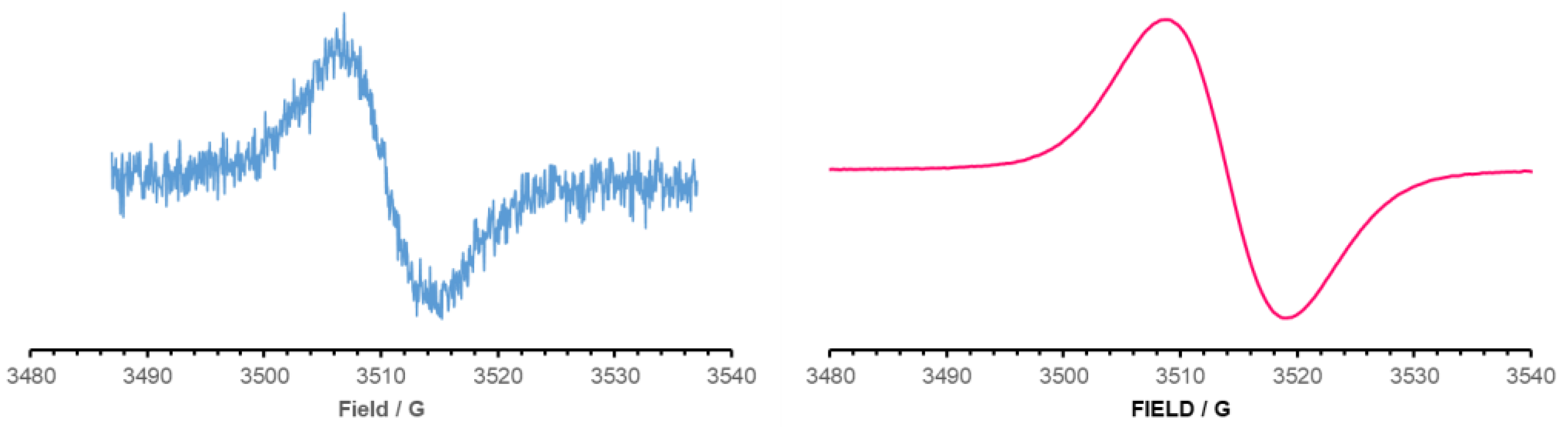
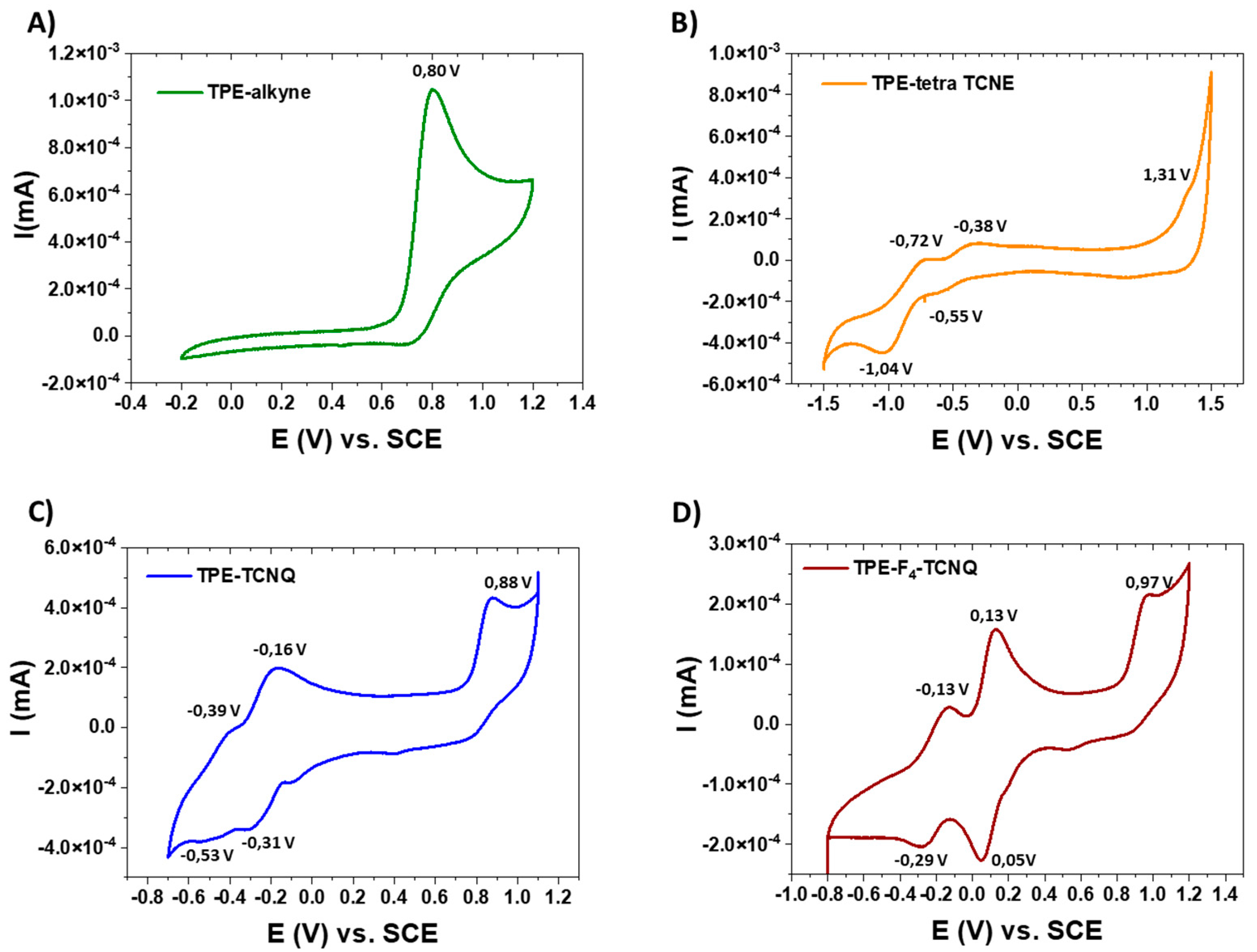
2.3. Electrochemistry
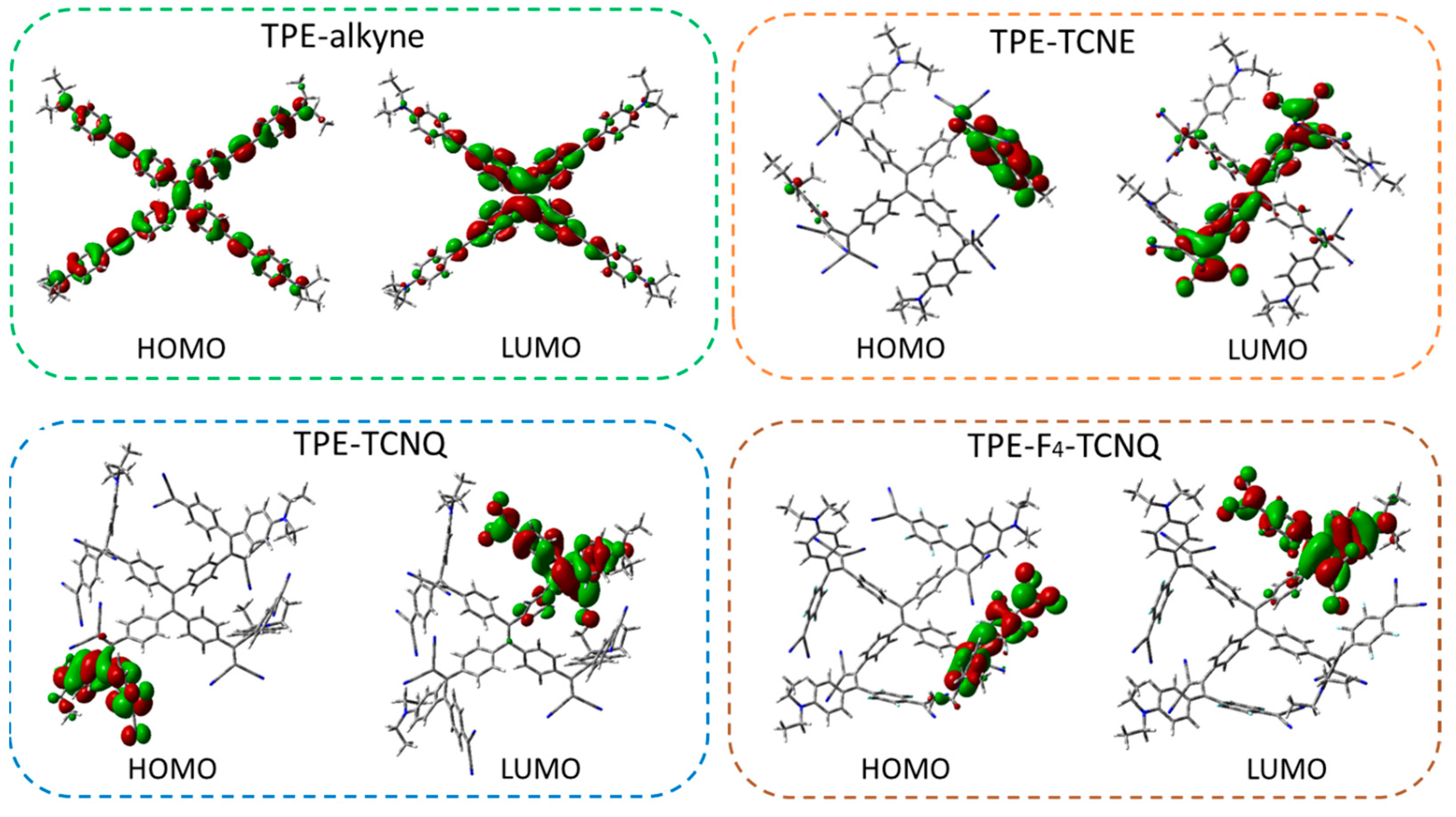
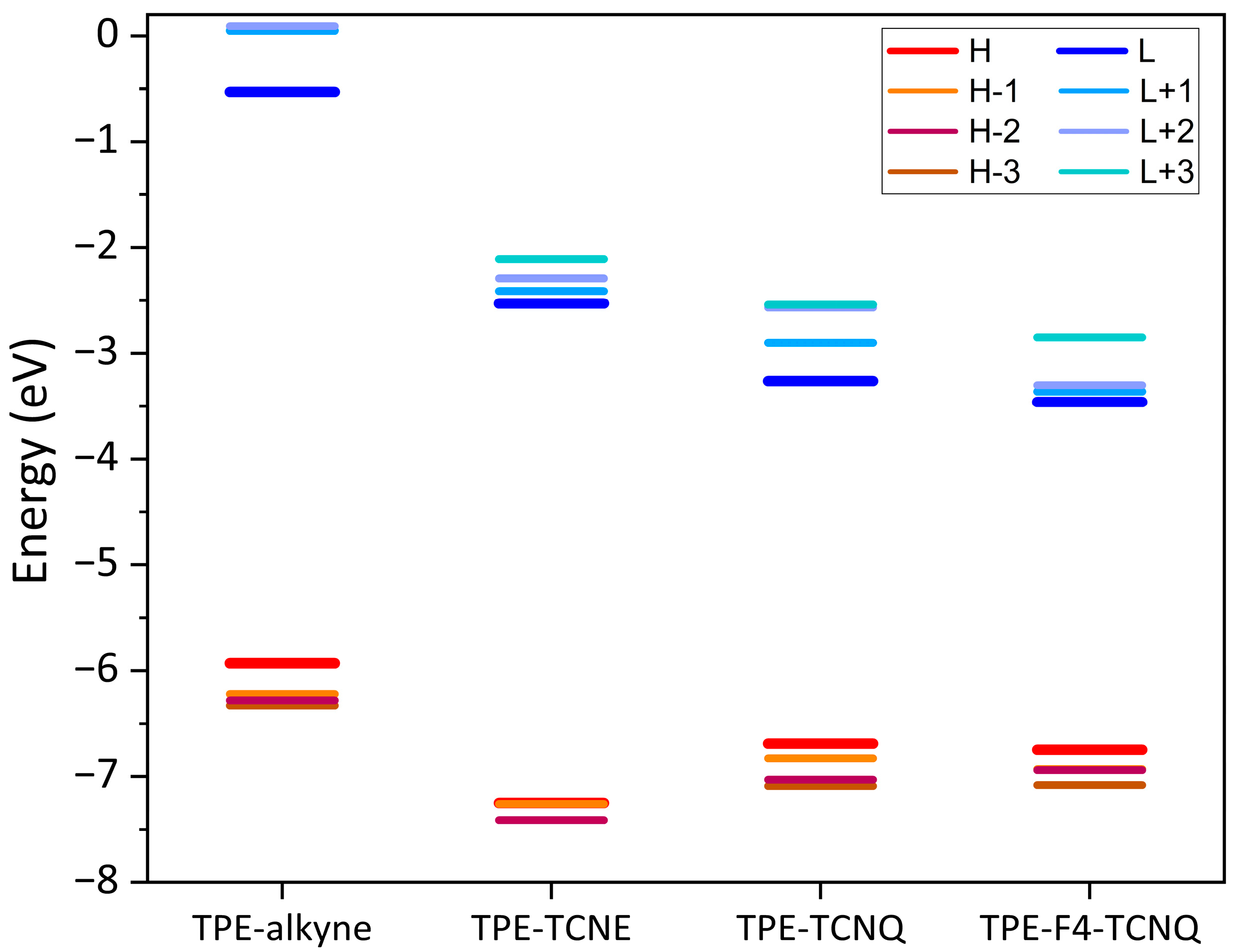
2.4. Electronic Structure Calculations
2.5. Excited-State Calculations
2.6. Photothermal Properties and Thermal Stability
2.7. Concluding Remarks
3. Methods and Materials
3.1. Materials
3.2. Characterization Methods
3.3. Calculations Details
3.4. Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Braye, E.H.; Hübel, W.; Caplier, I. New Unsaturated Heterocyclic Systems. I. J. Am. Chem. Soc. 1961, 83, 4406–4413. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Zhang, Y.; Li, Y.; Li, Y.; Li, Y.; Feng, Z.; Fan, X.; Qian, J.; Lin, H. A historical review of aggregation-induced emission from 2001 to 2020: A bibliometric analysis. Aggregate 2022, 3, e152. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, H.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: New Vistas at the Aggregate Level. Angew. Chem. Int. Ed. Engl. 2020, 59, 9888–9907. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Du, L.; Ma, C.; Leung, N.L.C.; Niu, Y.; Qin, A.; Sun, J.; Peng, Q.; Sung, H.H.Y.; et al. Drawing a clear mechanistic picture for the aggregation-induced emission process. Mater. Chem. Front. 2019, 3, 1143–1150. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Turley, A.T.; Wang, L.; McGonigal, P.R.; Tu, Y.; Li, Y.; Wang, Z.; Kwok, R.T.K.; Lam, J.W.Y.; et al. Aggregate Science: From Structures to Properties. Adv. Mater. 2020, 32, 2001457. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Duan, Y.; Liu, B. Precise Molecular Design for High-Performance Luminogens with Aggregation-Induced Emission. Adv. Mater. 2020, 32, 1903530. [Google Scholar] [CrossRef]
- Wan, Q.; Li, Y.; Ding, K.; Xie, Y.; Fan, J.; Tong, J.; Zeng, Z.; Li, Y.; Zhao, C.; Wang, Z.; et al. Aggregation Effect on Multiperformance Improvement in Aryl-Armed Phenazine-Based Emitters. J. Am. Chem. Soc. 2023, 145, 1607–1616. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, G.; Zhao, G.; Wang, X.; Tian, W.; Sun, Y. Novel benzonitrile-based AIE host with high triplet energy for highly efficient solution-processed blue TADF OLEDs. Dyes Pigm. 2023, 210, 111037. [Google Scholar] [CrossRef]
- Hwang, J.; Nagaraju, P.; Cho, M.J.; Choi, D.H. Aggregation-induced emission luminogens for organic light-emitting diodes with a single-component emitting layer. Aggregate 2023, 4, e199. [Google Scholar] [CrossRef]
- Amro, K.; Thakur, A.K.; Rolland, M.; Van Der Lee, A.; Lemaur, V.; Lazzaroni, R.; Rault-Berthelot, J.; Poriel, C.; Hirsch, L.; Clément, S.; et al. Linking triptycene to silole: A fruitful association. Mater. Chem. Front. 2020, 4, 2006–2017. [Google Scholar] [CrossRef]
- Anitha, O.; Mathivanan, M.; Tharmalingam, B.; Thiruppathiraja, T.; Ghorai, S.; Natarajan, R.; Thiagarajan, V.; Lakshmipathi, S.; Murugesapandian, B. Multi-stimuli responsiveness of pyrimidine bishydrazone: AIE, tuneable luminescence, white light emission, mechanochromism, acidochromism and its anticounterfeiting applications. Dyes Pigm. 2023, 212, 111091. [Google Scholar] [CrossRef]
- Guo, X.; Song, T.; Chen, D.; Zhu, J.; Li, Z.; Xia, Q.; Wang, L.; Yang, W. Multi Stimuli-Responsive Aggregation-Induced Emission Active Polymer Platform Based on Tetraphenylethylene-Appended Maleic Anhydride Terpolymers. ACS Appl. Mater. Interfaces 2023, 15, 3543–3557. [Google Scholar] [CrossRef]
- Deng, D.-D.; Zou, Y.; Chen, Z.; Liu, S.; Yang, Y.; Pu, S. Finely regulated benzothiadiazole derivatives: Aggregation-induced emission (AIE), hypso- or bathochromic mechanofluorochromic behaviors, and multilevel information encryption applications. Dyes Pigm. 2023, 211, 111051. [Google Scholar] [CrossRef]
- Ahangar, A.A.; Ahmad, I.; Dar, A.A. AIE in the halogenated anils and their utilization as fluorescent probes for explosive nitro-aromatics. New J. Chem. 2023, 47, 4775–4783. [Google Scholar] [CrossRef]
- Amro, K.; Clément, S.; Déjardin, P.; Douglas, W.E.; Gerbier, P.; Janot, J.-M.; Thami, T. Supported thin flexible polymethylhydrosiloxane permeable films functionalised with silole groups: New approach for detection of nitroaromatics. J. Mater. Chem. 2010, 20, 7100–7103. [Google Scholar] [CrossRef]
- Zhang, Q.; Yin, B.; Hao, J.; Ma, L.; Huang, Y.; Shao, X.; Li, C.; Chu, Z.; Yi, C.; Wong, S.H.D.; et al. An AIEgen/graphene oxide nanocomposite (AIEgen@GO)-based two-stage “turn-on” nucleic acid biosensor for rapid detection of SARS-CoV-2 viral sequence. Aggregate 2023, 4, e195. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zhang, P.; Shen, Q.; Zhou, Y.; Wang, Z.; Xu, Y.; Meng, L.; Dang, D.; Ben, Z.T. AIE nanocrystals: Emerging nanolights with ultra-high brightness for biological application. Coord. Chem. Rev. 2023, 477, 214944. [Google Scholar] [CrossRef]
- Luo, W.; Tan, Y.; Gui, Y.; Yan, D.; Wang, D.; Tang, B.Z. Near-Infrared-Emissive AIE Bioconjugates: Recent Advances and Perspectives. Molecules 2022, 27, 3914. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, Y.; Xu, R.; Xu, Y.; Dang, D.; Shen, Q.; Meng, L.; Tang, B.Z. Seeing the unseen: AIE luminogens for super-resolution imaging. Coord. Chem. Rev. 2022, 451, 214279. [Google Scholar] [CrossRef]
- Yan, D.; Qin, Y.; Yan, S.; Sun, P.; Wang, Y.; Wang, D.; Tang, B.Z. Near-infrared emissive AIE nanoparticles for biomedical applications: From the perspective of different nanocarriers. Particuology 2023, 74, 103–118. [Google Scholar] [CrossRef]
- Chua, M.H.; Chin, K.L.O.; Loh, X.J.; Zhu, Q.; Xu, J. Aggregation-Induced Emission-Active Nanostructures: Beyond Biomedical Applications. ACS Nano 2023, 17, 1845–1878. [Google Scholar] [CrossRef] [PubMed]
- Ingle, J.; Basu, S. Mitochondria Targeted AIE Probes for Cancer Phototherapy. ACS Omega 2023, 8, 8925–8935. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, Z.; Ge, J.; Deng, Y.; Ding, F.; Hu, L.; Wang, H. A deep-red emission AIE fluorescent probes based on coumarin for imaging lipid droplets in living cells. J. Mol. Struct. 2023, 1277, 134847. [Google Scholar] [CrossRef]
- Ingle, J.; Sengupta, P.; Basu, S. Illuminating Sub-Cellular Organelles by Small Molecule AIEgens. ChemBioChem 2023, 24, e202200370. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, S.; Liu, Z.; Cao, W.; Li, G.; Gao, W.; Tang, B. Novel AIE Probe for In Situ Imaging of Protein Sulfonation to Assess Cigarette Smoke-Induced Inflammatory Damage. Anal. Chem. 2023, 95, 1967–1974. [Google Scholar] [CrossRef]
- Kotras, C.; Fossepre, M.; Roger, M.; Gervais, V.; Richeter, S.; Gerbier, P.; Ulrich, S.; Surin, M.; Clement, S. A cationic tetraphenylethene as a light-up supramolecular probe for DNA G-quadruplexes. Front. Chem. 2019, 7, 493. [Google Scholar] [CrossRef]
- Arribat, M.; Remond, E.; Richeter, S.; Gerbier, P.; Clement, S.; Cavelier, F. Silole amino acids with aggregation-induced emission features synthesized by hydrosilylation. Eur. J. Org. Chem. 2019, 2019, 2275–2281. [Google Scholar] [CrossRef]
- Pan, Z.; Wang, Y.; Chen, N.; Cao, G.; Zeng, Y.; Dong, J.; Liu, M.; Ye, Z.; Li, Y.; Huang, S.; et al. Aggregation-Induced emission photosensitizer with lysosomal response for photodynamic therapy against cancer. Bioorg. Chem. 2023, 132, 106349. [Google Scholar] [CrossRef]
- Wang, X.; Xue, K.; Wang, X.; Zhao, Y.; Deng, J.; Yang, L.; Liang, J.; Li, Y.; Qi, Z. An aggregation-induced emission photosensitizer with efficient singlet oxygen generation capacity for mitochondria targeted photodynamic therapy. Dyes Pigm. 2023, 213, 111181. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, L.; Chen, S.; Pu, Z.; Gu, M.; Shen, Y. Highly Efficient Photodynamic Therapy with Mitochondria-Targeting Aggregation-Induced Emission Photosensitizer for Retinoblastoma. Adv. Healthc. Mater. 2023, 12, 2202219. [Google Scholar] [CrossRef]
- Qu, R.; Zhen, X.; Jiang, X. Emerging designs of aggregation-induced emission agents for enhanced phototherapy applications. CCS Chem. 2022, 4, 401–419. [Google Scholar] [CrossRef]
- Lin, Y.; Yi, M.; Guan, X.; Chen, E.; Yang, L.; Li, S.; Li, Y.; Zhang, L. “Two birds with one stone” strategy for the lung cancer therapy with bioinspired AIE aggregates. J. Nanobiotechnol. 2023, 21, 49. [Google Scholar] [CrossRef]
- Jiang, W.; Cheng, C.; Qiu, X.; Chen, L.; Guo, X.; Luo, Y.; Wang, J.; Wang, J.; Xie, Z.; Li, P.; et al. Peptide Supramolecular Assembly-Instructed In Situ Self-Aggregation for Stratified Targeting Sonodynamic Therapy Enhancement of AIE Luminogens. Adv. Sci. 2023, 10, 2204989. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Chen, L.; Chen, Q.; Hu, R.; Xu, X.; Wang, Y.; Li, J.; Feng, S.; Dong, C.; Zhang, X.-L.; et al. Engineered Phage with Aggregation-Induced Emission Photosensitizer in Cocktail Therapy against Sepsis. Adv. Mater. 2023, 35, 2208578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, X.; Yuan, C.; Pang, X.; Shangguan, P.; Liu, Y.; Han, L.; Sun, J.; Lam, J.W.Y.; Liu, Y.; et al. Near-Infrared Aggregation-Induced Emission Luminogens for In Vivo Theranostics of Alzheimer’s Disease. Angew. Chem. Int. Ed. 2023, 62, e202211550. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Structural and process controls of AIEgens for NIR-II theranostics. Chem. Sci. 2020, 12, 3427–3436. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Koo, S.; Sun, Y.; Liu, Y.; Liu, X.; Pan, Y.; Zhang, Z.; Du, M.; Lu, S.; et al. Versatile Types of Inorganic/Organic NIR-IIa/IIb Fluorophores: From Strategic Design toward Molecular Imaging and Theranostics. Chem. Rev. 2022, 122, 209–268. [Google Scholar] [CrossRef]
- Xu, C.; Pu, K. Second near-infrared photothermal materials for combinational nanotheranostics. Chem. Soc. Rev. 2021, 50, 1111–1137. [Google Scholar] [CrossRef] [PubMed]
- Shao, A.; Xie, Y.; Zhu, S.; Guo, Z.; Zhu, S.; Guo, J.; Shi, P.; James, T.D.; Tian, H.; Zhu, W.H. Far-Red and Near-IR AIE-Active Fluorescent Organic Nanoprobes with Enhanced Tumor-Targeting Efficacy: Shape-Specific Effects. Angew. Chem. Int. Ed. Engl. 2015, 54, 7275–7280. [Google Scholar] [CrossRef]
- Gu, X.; Yao, J.; Zhang, G.; Zhang, C.; Yan, Y.; Zhao, Y.; Zhang, D. New electron-donor/acceptor-substituted tetraphenylethylenes: Aggregation-induced emission with tunable emission color and optical-waveguide behavior. Chem. Asian J. 2013, 8, 2362–2369. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Lee, M.M.S.; Zhang, Z.; Sung, H.H.Y.; Williams, I.D.; Kwok, R.T.K.; Lam, J.W.Y.; Wang, D.; Tang, B.Z. Facile synthesis of AIEgens with wide color tunability for cellular imaging and therapy. Chem. Sci. 2019, 10, 3494–3501. [Google Scholar] [CrossRef]
- Ajayaghosh, A. Donor–acceptor type low band gap polymers: Polysquaraines and related systems. Chem. Soc. Rev. 2003, 32, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Jia, T.; Kang, B.; Li, F.; Fahlman, M.; Wang, Y. Nitrile-substituted QA derivatives: New acceptor materials for solution-processable organic bulk heterojunction solar cells. Adv. Energy Mater. 2011, 1, 431–439. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Michinobu, T.; Diederich, F. The [2+2] Cycloaddition-Retroelectrocyclization (CA-RE) Click Reaction: Facile Access to Molecular and Polymeric Push-Pull Chromophores. Angew. Chem. Int. Ed. 2018, 57, 3552–3577. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Ito, S. Azulene-based donor-acceptor systems: Synthesis, optical, and electrochemical properties. Chem.-Eur. J. 2017, 23, 16696–16709. [Google Scholar] [CrossRef]
- Bruce, M.I.; Rodgers, J.R.; Snow, M.R.; Swincer, A.G. Cyclopentadienyl-ruthenium and -osmium chemistry. Cleavage of tetracyanoethylene under mild conditions: X-ray crystal structures of [Ru{η3-C(CN)2CPhCC(CN)2}(PPh3)(η-C5H5)] and [Ru{C[C(CN)2]CPhC(CN)2}-(CNBut)(PPh3)(η-C5H5)]. J. Chem. Soc. Chem Comm. 1981, 6, 271–272. [Google Scholar] [CrossRef]
- Cai, C.; Liakatas, I.; Wong, M.-S.; Bösch, M.; Bosshard, C.; Günter, P.; Concilio, S.; Tirelli, N.; Suter, U.W. Donor−Acceptor-Substituted Phenylethenyl Bithiophenes: Highly Efficient and Stable Nonlinear Optical Chromophores. Org. Lett. 1999, 1, 1847–1849. [Google Scholar] [CrossRef]
- Wu, X.; Wu, J.; Liu, Y.; Jen, A.K.Y. Highly Efficient, Thermally and Chemically Stable Second Order Nonlinear Optical Chromophores Containing a 2-Phenyl-tetracyanobutadienyl Acceptor. J. Am. Chem. Soc. 1999, 121, 472–473. [Google Scholar] [CrossRef]
- Michinobu, T.; Boudon, C.; Gisselbrecht, J.P.; Seiler, P.; Frank, B.; Moonen, N.N.; Gross, M.; Diederich, F. Donor-substituted 1,1,4,4-tetracyanobutadienes (TCBDS): New chromophores with efficient intramolecular charge-transfer interactions by atom-economic synthesis. Chem. Eur. J. 2006, 12, 1889–1905. [Google Scholar] [CrossRef]
- Michinobu, T.; May, J.C.; Lim, J.H.; Boudon, C.; Gisselbrecht, J.-P.; Seiler, P.; Gross, M.; Biaggio, I.; Diederich, F. A new class of organic donor–acceptor molecules with large third-order optical nonlinearities. Chem. Commun. 2005, 6, 737–739. [Google Scholar] [CrossRef] [PubMed]
- Kivala, M.; Boudon, C.; Gisselbrecht, J.P.; Enko, B.; Seiler, P.; Muller, I.B.; Langer, N.; Jarowski, P.D.; Gescheidt, G.; Diederich, F. Organic super-acceptors with efficient intramolecular charge-transfer interactions by [2+2] cycloadditions of TCNE, TCNQ, and F4-TCNQ to donor-substituted cyanoalkynes. Chem. Eur. J. 2009, 15, 4111–4123. [Google Scholar] [CrossRef] [PubMed]
- Kivala, M.; Boudon, C.; Gisselbrecht, J.-P.; Seiler, P.; Gross, M.; Diederich, F. A novel reaction of 7,7,8,8-tetracyanoquinodimethane (TCNQ): Charge-transfer chromophores by [2 + 2] cycloaddition with alkynes. Chem. Commun. 2007, 45, 4731–4733. [Google Scholar] [CrossRef]
- Reutenauer, P.; Kivala, M.; Jarowski, P.D.; Boudon, C.; Gisselbrecht, J.P.; Gross, M.; Diederich, F. New strong organic acceptors by cycloaddition of TCNE and TCNQ to donor-substituted cyanoalkynes. Chem. Commun. 2007, 46, 4898–4900. [Google Scholar] [CrossRef] [PubMed]
- Patil, Y.; Misra, R. Diketopyrrolopyrrole-Based and Tetracyano-Bridged Small Molecules for Bulk Heterojunction Organic Solar Cells. Chem.-Asian J. 2018, 13, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Rout, Y.; Chauhan, V.; Misra, R. Synthesis and Characterization of Isoindigo-Based Push-Pull Chromophores. J. Org. Chem. 2020, 85, 4611–4618. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S.; More, V.G.; Jangale, A.D.; Bhosale, S.V.; Bhosale, R.S.; Puyad, A.L.; Chen, J.-Y.; Li, J.-L.; Bhosale, S.V.; Gupta, A.; et al. A series of V-shaped small molecule non-fullerene electron acceptors for efficient bulk-heterojunction devices. Dyes Pigm. 2019, 171, 107677. [Google Scholar] [CrossRef]
- Rao, P.S.; Puyad, A.L.; Bhosale, S.V.; Bhosale, S.V. Triphenylamine-merocyanine-based D1-A1-π-A2/A3-D2 chromophore system: Synthesis, optoelectronic, and theoretical studies. Int. J. Mol. Sci. 2019, 20, 1621. [Google Scholar] [CrossRef]
- Rout, Y.; Misra, R.; Singhal, R.; Biswas, S.; Sharma, G.D. Phenothiazine-based small-molecule organic solar cells with power conversion efficiency over 7% and open circuit voltage of about 1.0 V using solvent vapor annealing. Phys. Chem. Chem. Phys. 2018, 20, 6321–6329. [Google Scholar] [CrossRef]
- Srinivasa Rao, P.; Gupta, A.; Bhosale, S.V.; Bilic, A.; Xiang, W.; Evans, R.A.; Bhosale, S.V. Donor-acceptor-acceptor-based non-fullerene acceptors comprising terminal chromen-2-one functionality for efficient bulk-heterojunction devices. Dyes Pigm. 2017, 146, 502–511. [Google Scholar] [CrossRef]
- Gautam, P.; Misra, R.; Sharma, G.D. Dicyanoquinodimethane-substituted benzothiadiazole for efficient small-molecule solar cells. Phys. Chem. Chem. Phys. 2016, 18, 7235–7241. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Washino, Y.; Murata, K.; Nozaki, N.; Matsumoto, H.; Michinobu, T. [2+2] Cycloaddition-retroelectrocyclization reactivity and thin film transistor performances of carbazole-based platinum polyyne polymers. Mater. Chem. Phys. 2022, 281, 125861. [Google Scholar] [CrossRef]
- Leliège, A.; Blanchard, P.; Rousseau, T.; Roncali, J. Triphenylamine/Tetracyanobutadiene-Based D-A-D π-Conjugated Systems as Molecular Donors for Organic Solar Cells. Org. Lett. 2011, 13, 3098–3101. [Google Scholar] [CrossRef] [PubMed]
- Raheem, A.A.; Murugan, P.; Shanmugam, R.; Praveen, C. Azulene Bridged π-Distorted Chromophores: The Influence of Structural Symmetry on Optoelectrochemical and Photovoltaic Parameters. ChemPlusChem 2021, 86, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Philippe, C.; Melan, J.; Barsella, A.; Vives, T.; Leroux, Y.R.; Robin-Le Guen, F.; Lemiegre, L.; Jacquemin, D.; Gauthier, S.; Trolez, Y. A comprehensive study of tetracyanobutadiene push-pull chromophores derived from γ-pyranylidene. Tetrahedron Chem. 2023, 5, 100036. [Google Scholar] [CrossRef]
- Pokladek, Z.; Ripoche, N.; Betou, M.; Trolez, Y.; Mongin, O.; Olesiak-Banska, J.; Matczyszyn, K.; Samoc, M.; Humphrey, M.G.; Blanchard-Desce, M.; et al. Linear Optical and Third-Order Nonlinear Optical Properties of Some Fluorenyl- and Triarylamine-Containing Tetracyanobutadiene Derivatives. Chem. Eur. J. 2016, 22, 10155–10167. [Google Scholar] [CrossRef]
- Ripoche, N.; Betou, M.; Philippe, C.; Trolez, Y.; Mongin, O.; Dudek, M.; Pokladek, Z.; Matczyszyn, K.; Samoc, M.; Sahnoune, H.; et al. Two-photon absorption properties of multipolar triarylamino/tosylamido 1,1,4,4-tetracyanobutadienes. Phys. Chem. Chem. Phys. 2021, 23, 22283–22297. [Google Scholar] [CrossRef]
- Mammadova, F.; Inyurt, F.C.; Barsella, A.; Dengiz, C. Cyano-rich donor-acceptor-donor-type NLOphores containing dialkylated triazene and aniline groups. Dyes Pigm. 2023, 209, 110894. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, D.; Gao, H.; Zhang, J.; Xing, Y.; Yang, Z.; Cao, H.; He, W. Third-order nonlinear optical properties of the “clicked” closed-ring spiropyrans. Dyes Pigm. 2019, 162, 451–458. [Google Scholar] [CrossRef]
- Miao, Z.; Han, H.; Wang, D.; Gao, H.; Gu, J.; Hu, H. Nonlinear optical and energy-level modulation of organic alkynes by click chemistry. Tetrahedron 2016, 72, 4039–4046. [Google Scholar] [CrossRef]
- Yang, L.; Li, L.; Gao, H.; Wang, D.; Yang, Z.; Cao, H.; He, W. Photoacoustic effect of azo derivatives modified by click reagents and parceled by liposomes. Dyes Pigm. 2020, 172, 107822. [Google Scholar] [CrossRef]
- Gao, H.; Zhao, Z.; Liu, W.; Wang, D.; He, W.; Cao, H.; Yang, Z. Novel application of NIR photoacoustic absorbing dyes in thermosensitive micelles. Dyes Pigm. 2019, 164, 319–326. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, D.; Gao, H.; Yang, Z.; Cao, H.; He, W. Photoacoustic effect of near-infrared absorbing fullerene derivatives with click moieties. Dyes Pigm. 2019, 164, 182–187. [Google Scholar] [CrossRef]
- Li, L.; Wang, D.; Wang, L.; Ramella, D.; Wang, H.; Gao, H.; Zhang, J.; Xing, Y.; Li, B.; Yang, Z.; et al. The photoacoustic effect of near-infrared absorbing porphyrin derivatives prepared via click chemistry. Dyes Pigm. 2018, 148, 501–507. [Google Scholar] [CrossRef]
- Xu, A.-P.; Han, H.-H.; Lu, J.; Yang, P.-P.; Gao, Y.-J.; An, H.-W.; Zhanng, D.; Li, L.-Z.; Zhang, J.-P.; Wang, D.; et al. Charge transfer NIR dyes for improved photoacoustic effect. Dyes Pigm. 2016, 125, 392–398. [Google Scholar] [CrossRef]
- Shi, H.; Gu, R.; Xu, W.; Huang, H.; Xue, L.; Wang, W.; Zhang, Y.; Si, W.; Dong, X. Near-Infrared Light-Harvesting Fullerene-Based Nanoparticles for Promoted Synergetic Tumor Phototheranostics. ACS Appl. Mater. Interfaces 2019, 11, 44970–44977. [Google Scholar] [CrossRef]
- Tang, B.; Qin, A.; Han, P.; Zhang, G.; Xu, H. Preparation of Organic Near-Infrared Photothermal Materials and Its Application. CN115,043,756, 13 September 2022. [Google Scholar]
- Bhusanur, D.I.; Nadimetla, D.N.; Harmalkar, S.S.; Bhosale, R.S.; Puyad, A.L.; Wagalgave, S.M.; Bhosale, S.V.; Bhosale, S.V. Synthesis, crystal structure and supramolecular self-assembly of tetraphenylethylene subunit appended isoindigo derivatives. J. Mol. Struct. 2022, 1255, 132452. [Google Scholar] [CrossRef]
- Philippe, C.; Coste, M.; Bretonniere, Y.; Lemiegre, L.; Ulrich, S.; Trolez, Y. Quadruple Functionalization of a Tetraphenylethylene Aromatic Scaffold with Ynamides or Tetracyanobutadienes: Synthesis and Optical Properties. Eur. J. Org. Chem. 2022, 2022, e202200049. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, C.; Wu, W.; Wang, F.; Du, L.; Zhang, X.; Xiong, Y.; He, X.; Cai, Y.; Kwok, R.T.K.; et al. Highly efficient photothermal nanoagent achieved by harvesting energy via excited-state intramolecular motion within nanoparticles. Nat. Commun. 2019, 10, 768. [Google Scholar] [CrossRef]
- Fesser, P.; Iacovita, C.; Wäckerlin, C.; Vijayaraghavan, S.; Ballav, N.; Howes, K.; Gisselbrecht, J.-P.; Crobu, M.; Boudon, C.; Stöhr, M.; et al. Visualizing the Product of a Formal Cycloaddition of 7,7,8,8-Tetracyano-p-quinodimethane (TCNQ) to an Acetylene-Appended Porphyrin by Scanning Tunneling Microscopy on Au(111). Chem. Eur. J. 2011, 17, 5246–5250. [Google Scholar] [CrossRef]
- Liu, X.; Wang, D.; Gao, H.; Yang, Z.; Xing, Y.; Cao, H.; He, W.; Wang, H.; Gu, J.; Hu, H. Nonlinear optical properties of symmetrical and asymmetrical porphyrin derivatives with click chemistry modification. Dyes Pigm. 2016, 134, 155–163. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, W.; Xing, Y.; Gao, H.; Wang, X.; Zhao, Y.; Yang, H. Energy-level modulation of organic alkynes by click chemistry. Tetrahedron 2013, 69, 895–901. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Li, Q.; Guo, Z.; He, Z.; Zhang, H.; Ma, C.; Gao, J.; Zhao, Y.; Wang, D. Preparation of Polyphenylene Ring Derivative Dyes with Wide Wave Absorption Properties and Their Performance Study. Molecules 2022, 27, 5551. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, X.; Zhao, X.; Li, Q.; Zhao, Y.; Guo, Z.; He, Z.; Zhang, H.; Gao, J.; Miao, Z. Preparation of symmetrical and asymmetrical multi-phenylene ring nonlinear optical materials with click chemical modifications and their properties. Tetrahedron 2022, 127, 132992. [Google Scholar] [CrossRef]
- Dar, A.H.; Gowri, V.; Gopal, A.; Muthukrishnan, A.; Bajaj, A.; Sartaliya, S.; Selim, A.; Ali, E.M.; Jayamurugan, G. Designing of Push-Pull Chromophores with Tunable Electronic and Luminescent Properties Using Urea as the Electron Donor. J. Org. Chem. 2019, 84, 8941–8947. [Google Scholar] [CrossRef] [PubMed]
- Simon Marques, P.; Castan, J.M.A.; Raul, B.A.L.; Londi, G.; Ramirez, I.; Pshenichnikov, M.S.; Beljonne, D.; Walzer, K.; Blais, M.; Allain, M.; et al. Triphenylamine/Tetracyanobutadiene-Based π-Conjugated Push-Pull Molecules End-Capped with Arene Platforms: Synthesis, Photophysics, and Photovoltaic Response. Chem.-Eur. J. 2020, 26, 16422–16433. [Google Scholar] [CrossRef]
- Philippe, C.; Bui, A.T.; Beau, M.; Bloux, H.; Riobe, F.; Mongin, O.; Roisnel, T.; Cordier, M.; Paul, F.; Lemiegre, L.; et al. Synthesis and Photophysical Properties of 1,1,4,4-Tetracyanobutadienes Derived from Ynamides Bearing Fluorophores. Chem.-Eur. J. 2022, 28, e202200025. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density-Functional Theory of Atoms and Molecules; Clarendon Press: Oxford, UK, 1989. [Google Scholar]
- Runge, E.; Gross, E.K.U. Density-functional theory for time-dependent systems. Phys. Rev. Lett. 1984, 52, 997–1000. [Google Scholar] [CrossRef]
- Stratmann, R.E.; Scuseria, G.E.; Frisch, M.J. An efficient implementation of time-dependent density-functional theory for the calculation of excitation energies of large molecules. J. Chem. Phys. 1998, 109, 8218–8224. [Google Scholar] [CrossRef]
- Trickey, S.B. Recent Advances in Density Functional Methods—Part I by Delano P. Chong. Int. J. Quantum Chem. 1999, 72, 155–156. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Density Functionals with Broad Applicability in Chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Adamo, C.; Jacquemin, D. The calculations of excited-state properties with Time-Dependent Density Functional Theory. Chem. Soc. Rev. 2013, 42, 845–856. [Google Scholar] [CrossRef]
- Martin, R.L. Natural transition orbitals. J. Chem. Phys. 2003, 118, 4775–4777. [Google Scholar] [CrossRef]
- Etienne, T.; Assfeld, X.; Monari, A. Toward a Quantitative Assessment of Electronic Transitions’ Charge-Transfer Character. J. Chem. Theory Comput. 2014, 10, 3896–3905. [Google Scholar] [CrossRef] [PubMed]
- Etienne, T.; Assfeld, X.; Monari, A. New Insight into the Topology of Excited States through Detachment/Attachment Density Matrices-Based Centroids of Charge. J. Chem. Theory Comput. 2014, 10, 3906–3914. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Zhang, G.; Xu, H.; Hu, R.; Qin, A.; Tang, B.Z. Organic near infrared photothermal materials with temperatures up to 450°C constructed by cycloaddition-retroelectrocyclization click reaction. ChemRxiv 2022, 1–32. [Google Scholar] [CrossRef]
- Banziger, S.D.; Clendening, R.A.; Oxley, B.M.; Ren, T. Spectroelectrochemical and Computational Analysis of a Series of Cycloaddition-Retroelectrocyclization-Derived Donor-Acceptor Chromophores. J. Phys. Chem. B 2020, 124, 11901–11909. [Google Scholar] [CrossRef]
- Gautam, P.; Maragani, R.; Misra, R. Tuning the HOMO-LUMO gap of donor-substituted benzothiazoles. Tetrahedron Lett. 2014, 55, 6827–6830. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, D.; Wang, X.; Liang, P.; Mi, Y.; Yang, H. Efficient modification of pyrene-derivative featuring third-order nonlinear optics via the click post-functionalization. Tetrahedron Lett. 2013, 54, 4859–4864. [Google Scholar] [CrossRef]
- Liang, P.; Du, Z.; Wang, D.; Yang, Z.; Sheng, H.; Liang, S.; Cao, H.; He, W.; Yang, H. Optoelectronic and Self-assembly Properties of Porphyrin Derivatives with Click Chemistry Modification. ChemPhysChem 2014, 15, 3523–3529. [Google Scholar] [CrossRef]
- Mi, Y.; Liang, P.; Jin, Z.; Wang, D.; Yang, Z. Synthesis and Third-Order Nonlinear Optical Properties of Triphenylene Derivatives Modified by Click Chemistry. ChemPhysChem 2013, 14, 4102–4108. [Google Scholar] [CrossRef] [PubMed]
- Misra, R.; Gautam, P. Tuning of the HOMO-LUMO gap of donor-substituted symmetrical and unsymmetrical benzothiadiazoles. Org. Biomol. Chem. 2014, 12, 5448–5457. [Google Scholar] [CrossRef] [PubMed]
- Rout, Y.; Gautam, P.; Misra, R. Unsymmetrical and Symmetrical Push-Pull Phenothiazines. J. Org. Chem. 2017, 82, 6840–6845. [Google Scholar] [CrossRef] [PubMed]
- Rout, Y.; Jang, Y.; Gobeze, H.B.; Misra, R.; D’Souza, F. Conversion of Large-Bandgap Triphenylamine-Benzothiadiazole to Low-Bandgap, Wide-Band Capturing Donor-Acceptor Systems by Tetracyanobutadiene and/or Dicyanoquinodimethane Insertion for Ultrafast Charge Separation. J. Phys. Chem. C 2019, 123, 23382–23389. [Google Scholar] [CrossRef]
- Sharma, R.; Maragani, R.; Misra, R. C3-Symmetric star shaped donor-acceptor truxenes: Synthesis and photophysical, electrochemical and computational studies. New J. Chem. 2018, 42, 882–890. [Google Scholar] [CrossRef]
- Sharma, R.; Thomas, M.B.; Misra, R.; D’Souza, F. Strong Ground- and Excited-State Charge Transfer in C3-Symmetric Truxene-Derived Phenothiazine-Tetracyanobutadine and Expanded Conjugates. Angew. Chem. Int. Ed. 2019, 58, 4350–4355. [Google Scholar] [CrossRef]
- Zhang, W.-S.; Wang, D.; Cao, H.; Yang, H. Energy level tunable pre-click functionalization of [60]fullerene for nonlinear optics. Tetrahedron 2014, 70, 573–577. [Google Scholar] [CrossRef]
- Zhang, Z.; Gou, G.; Wan, J.; Li, H.; Wang, M.; Li, L. Synthesis, Structure, and Significant Energy Gap Modulation of Symmetrical Silafluorene-Cored Tetracyanobutadiene and Tetracyanoquinodimethane Derivatives. J. Org. Chem. 2022, 87, 2470–2479. [Google Scholar] [CrossRef]
- Coulson, D.R.; Satek, L.C.; Grim, S.O. Tetrakis(Triphenylphosphine)Palladium(0). In Inorganic Syntheses; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1990; Volume 28, pp. 107–109. [Google Scholar]
- Meier, H.; Muehling, B.; Kolshorn, H. Red- and blue-shifts in oligo(1,4-phenyleneethynylene)s having terminal donor-acceptor substitutions. Eur. J. Org. Chem. 2004, 5, 1033–1042. [Google Scholar] [CrossRef]
- Makula, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV−Vis spectra. J. Phys. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- COMSOL Multiphysics, V. 6.0. COMSOL AB: Stockholm, Sweden. Available online: www.comsol.com (accessed on 1 January 2023).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 Rev. D.01; Gaussian, Inc.: Wallington, CT, USA, 2016. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef] [PubMed]





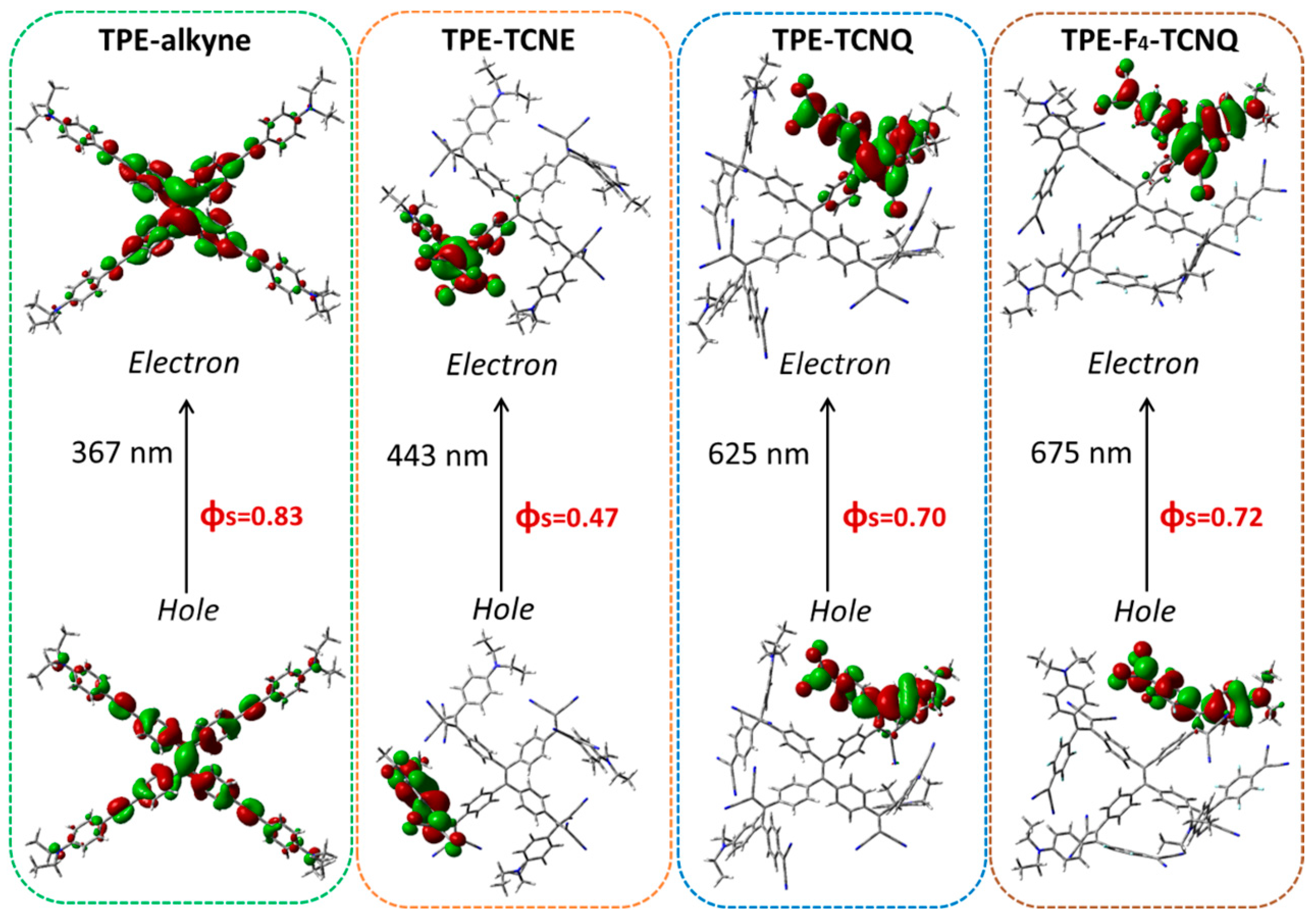
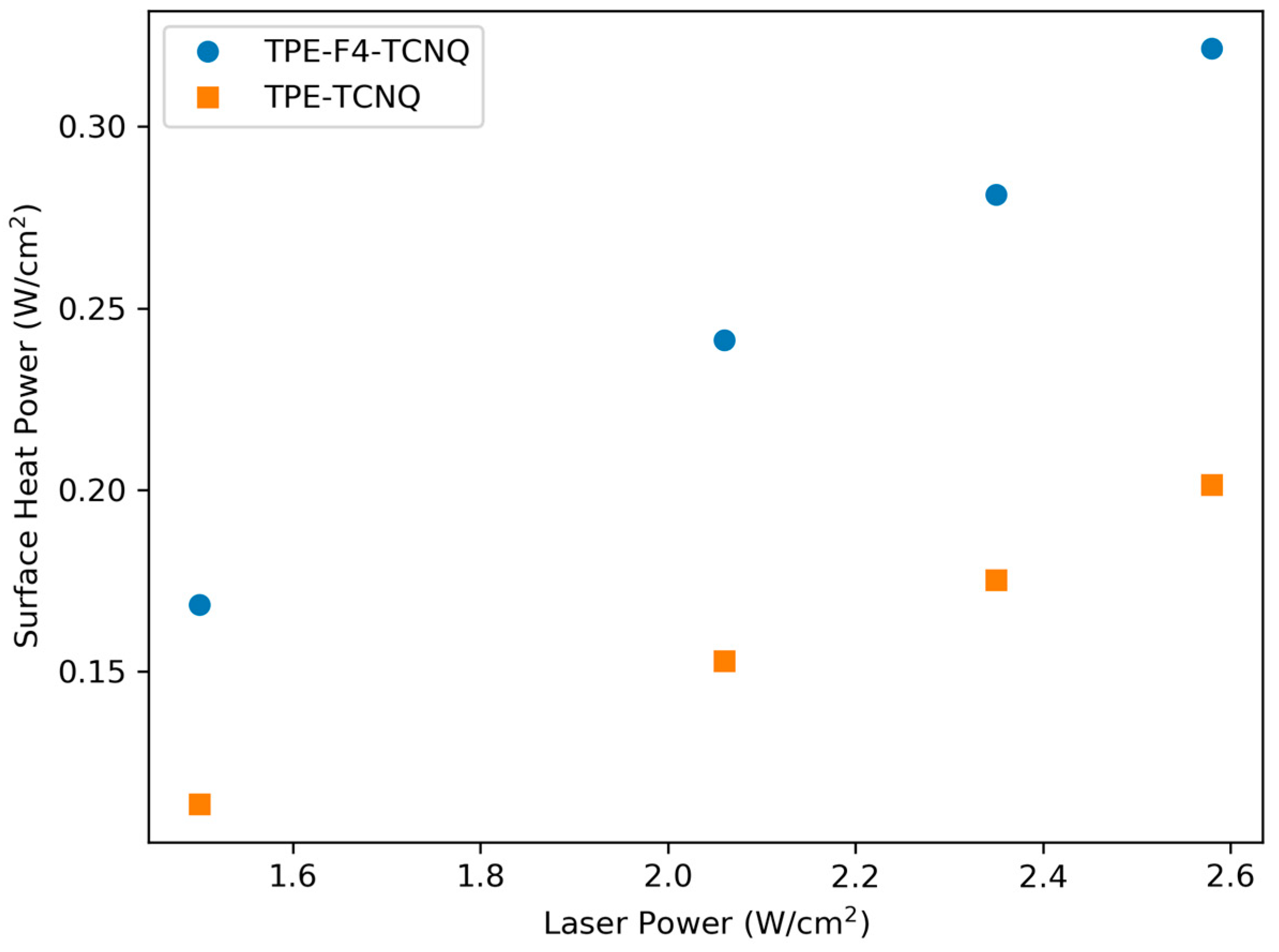
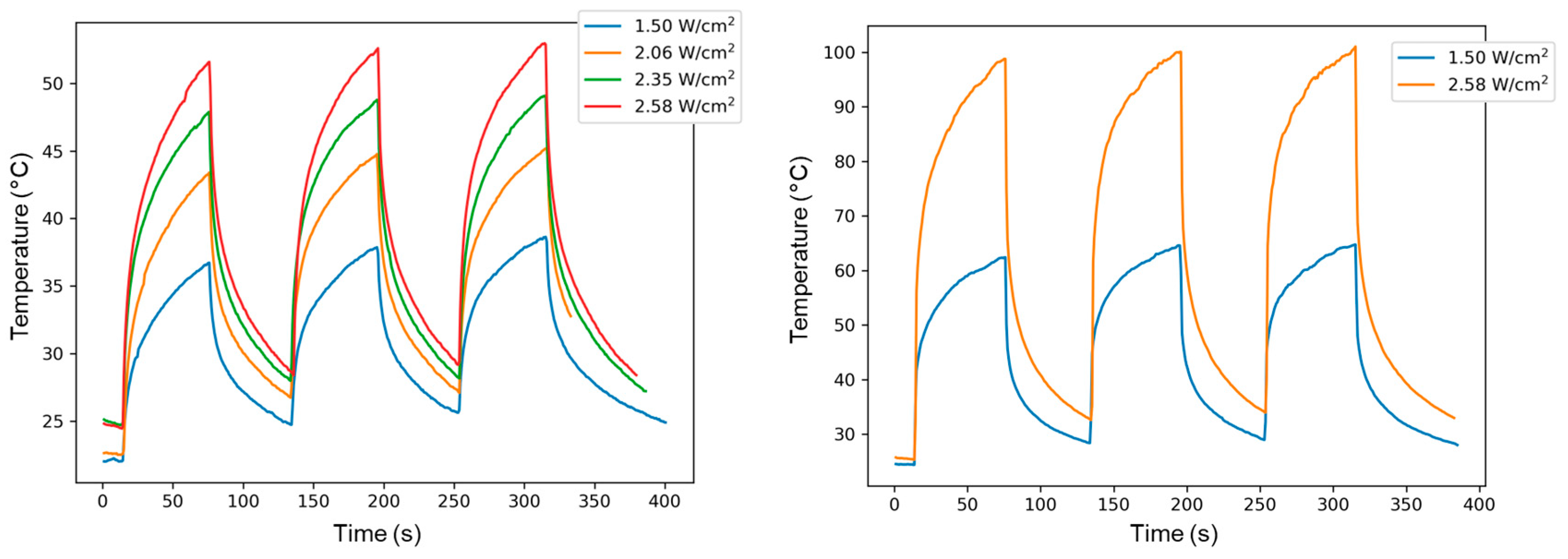
| TPE-Alkyne | TPE-TCNE | TPE-TCNQ | TPE-F4-TCNQ | |
|---|---|---|---|---|
| Absorbance in solution a (nm)/(ε (L.mol−1.cm−1)) | 294 (82,400) 359 (143,400) | 307/(45,600) 469 (113,400) | 337 (113,900) 468 (78,500) 688 (155,000) | 348 (97,500) 497 (73,600) 849 (163,400) |
| Absorbance as thin films (nm) | - | 476 | 471; 738 | 498; 864 |
| λem max (solution a/aggregated b) | 582/560/- | ≈700/786 | - | - |
| Φsol (%) a | 13% | - | - | - |
| Φaggr (%) b | 21% | ND | - | - |
| λonset (nm) c | 460 | 540 | 900 | - |
| Eg opt (eV) d | 2.70 | 2.30 | 1.38 | - |
| TPE-Alcyne | TPE-TCNE | TPE-TCNQ | TPE-F4-TCNQ | |
|---|---|---|---|---|
| E1/2 (V) a | - | −0.45 | −0.23 | +0.09 |
| −0.88 | −0.46 | −0.21 | ||
| Ep b | +0.80 | +1.31 | +0.88 | +0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roger, M.; Bretonnière, Y.; Trolez, Y.; Vacher, A.; Arbouch, I.; Cornil, J.; Félix, G.; De Winter, J.; Richeter, S.; Clément, S.; et al. Synthesis and Characterization of Tetraphenylethene AIEgen-Based Push–Pull Chromophores for Photothermal Applications: Could the Cycloaddition–Retroelectrocyclization Click Reaction Make Any Molecule Photothermally Active? Int. J. Mol. Sci. 2023, 24, 8715. https://doi.org/10.3390/ijms24108715
Roger M, Bretonnière Y, Trolez Y, Vacher A, Arbouch I, Cornil J, Félix G, De Winter J, Richeter S, Clément S, et al. Synthesis and Characterization of Tetraphenylethene AIEgen-Based Push–Pull Chromophores for Photothermal Applications: Could the Cycloaddition–Retroelectrocyclization Click Reaction Make Any Molecule Photothermally Active? International Journal of Molecular Sciences. 2023; 24(10):8715. https://doi.org/10.3390/ijms24108715
Chicago/Turabian StyleRoger, Maxime, Yann Bretonnière, Yann Trolez, Antoine Vacher, Imane Arbouch, Jérôme Cornil, Gautier Félix, Julien De Winter, Sébastien Richeter, Sébastien Clément, and et al. 2023. "Synthesis and Characterization of Tetraphenylethene AIEgen-Based Push–Pull Chromophores for Photothermal Applications: Could the Cycloaddition–Retroelectrocyclization Click Reaction Make Any Molecule Photothermally Active?" International Journal of Molecular Sciences 24, no. 10: 8715. https://doi.org/10.3390/ijms24108715
APA StyleRoger, M., Bretonnière, Y., Trolez, Y., Vacher, A., Arbouch, I., Cornil, J., Félix, G., De Winter, J., Richeter, S., Clément, S., & Gerbier, P. (2023). Synthesis and Characterization of Tetraphenylethene AIEgen-Based Push–Pull Chromophores for Photothermal Applications: Could the Cycloaddition–Retroelectrocyclization Click Reaction Make Any Molecule Photothermally Active? International Journal of Molecular Sciences, 24(10), 8715. https://doi.org/10.3390/ijms24108715








