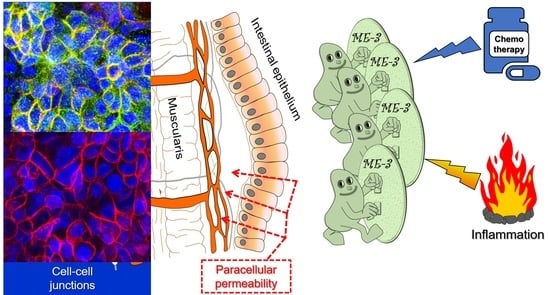
Protective Effect of Limosilactobacillus fermentum ME-3 against the Increase in Paracellular Permeability Induced by Chemotherapy or Inflammatory Conditions in Caco-2 Cell Models
Abstract
1. Introduction
2. Results
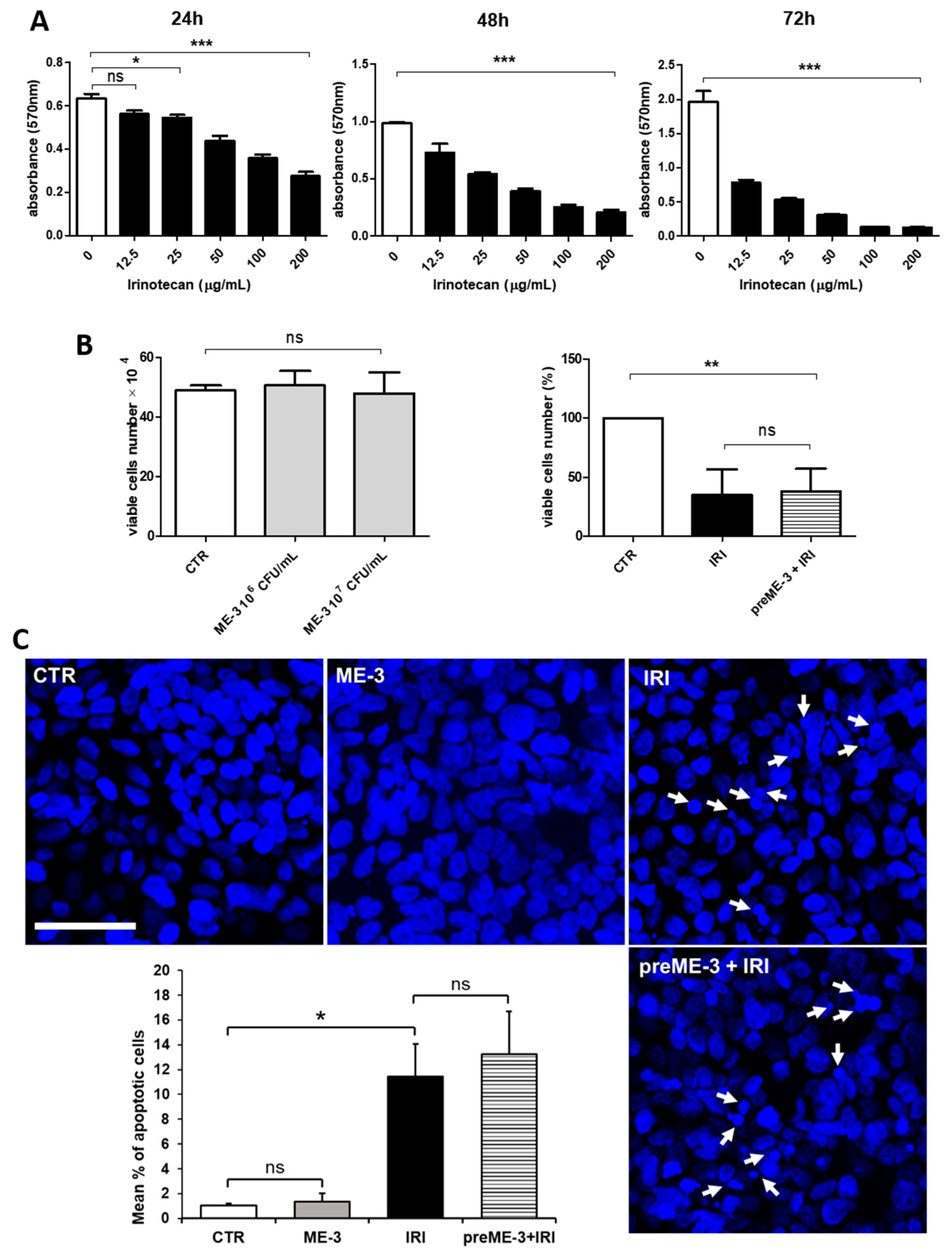
2.1. L. fermentum ME-3® Does Not Alter the Effects of Irinotecan on the Proliferation and Vitality of Caco-2 Cells
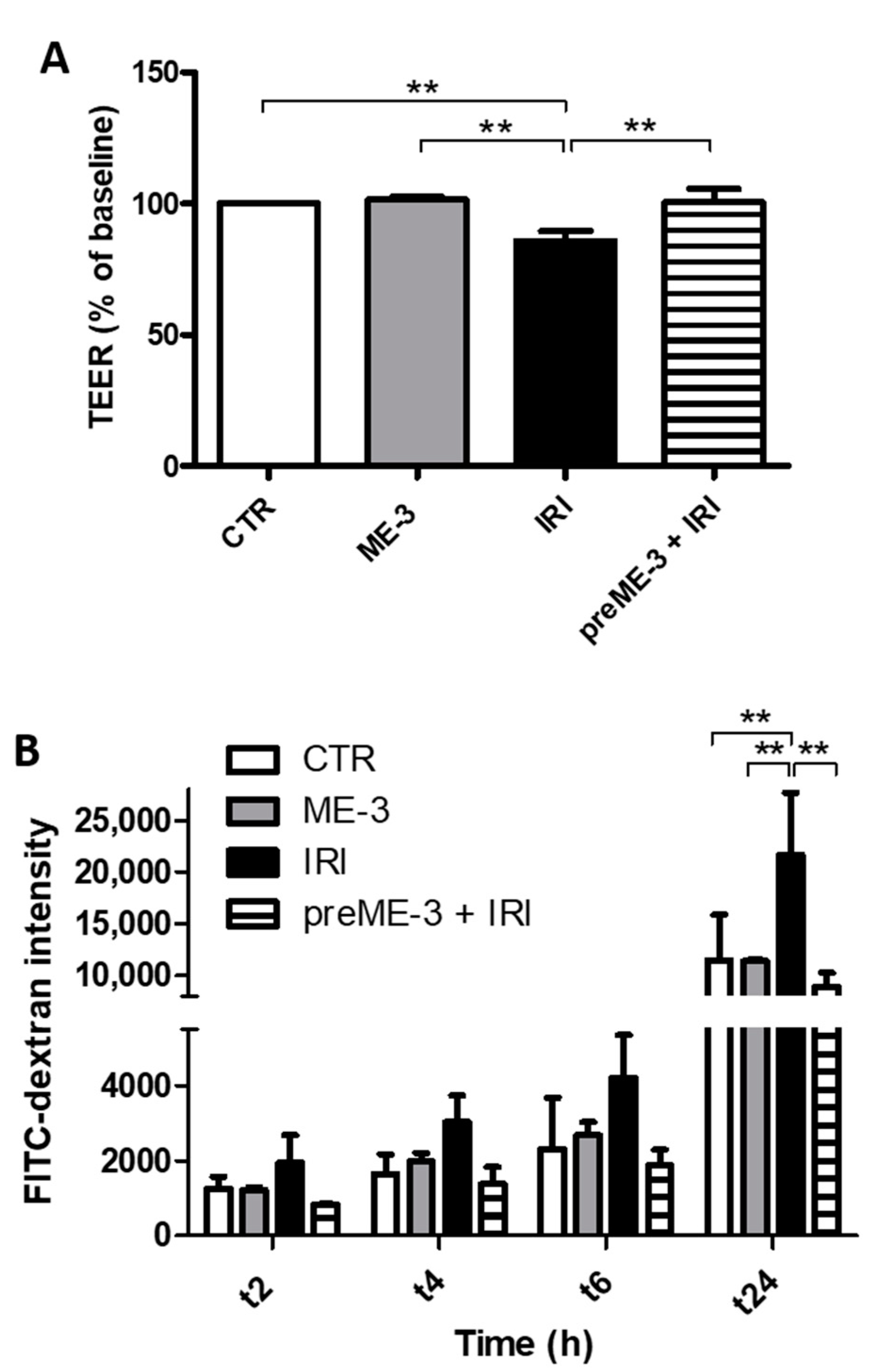
2.2. L. fermentum ME-3® Protects Caco-2 Monolayers from the Increase in Membrane Permeability Caused by Irinotecan Treatment
2.3. L. fermentum ME-3® Protects Caco-2 Cell Monolayers from the IRI-Induced Alteration of Tight Junction Integrity
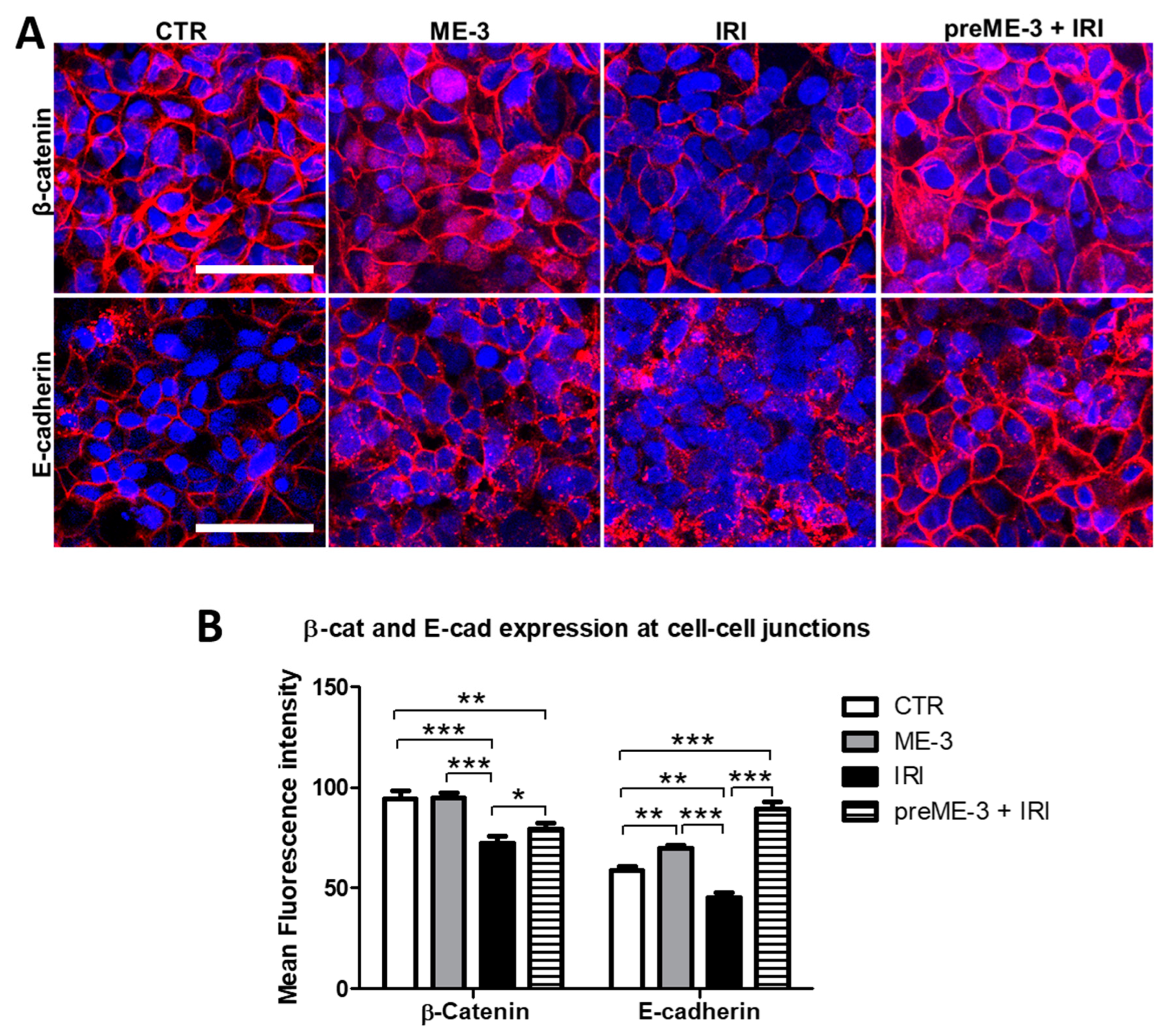
2.4. L. fermentum ME-3® Counteracts the IRI-Induced Alteration of Expression and Localization of Proteins Controlling the Adherens Junctions
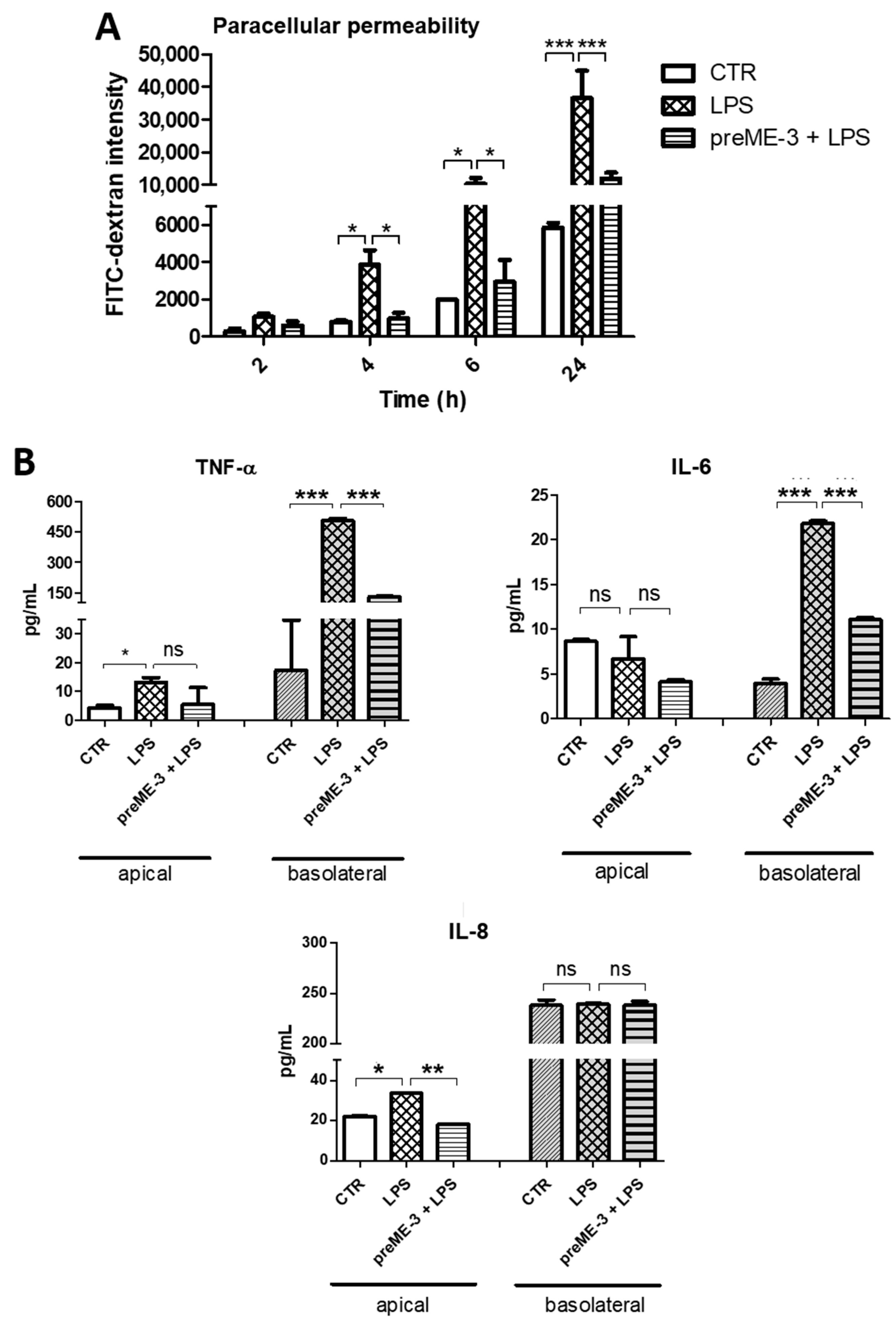
2.5. L. fermentum ME-3® Prevents the Increases in Membrane Permeability and Pro-Inflammatory Cytokine Release Caused by LPS-Stimulated Inflammation in Caco-2 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
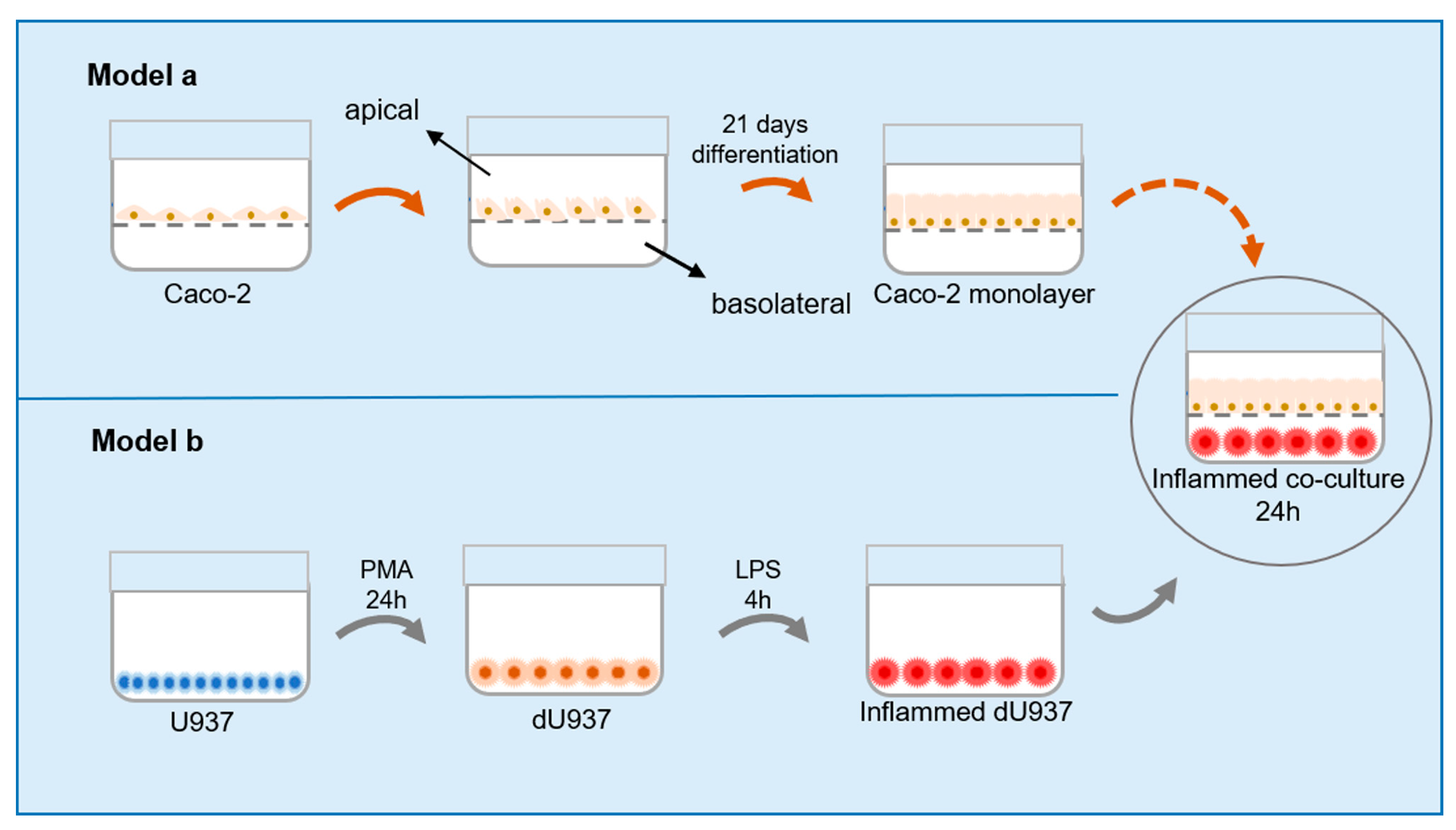
4.2. Cell Cultures and Cellular Models
4.3. Cytotoxicity Test
4.4. Permeability of Intestinal Barrier Evaluation
4.5. Transepithelial Electrical Resistance (TEER) Measurement
4.6. Paracellular Permeability Measurement by FITC-Dextran Flux Assay
4.7. Immunofluorescence Analyses and Confocal Laser Scanning Microscopy (CLSM)
4.8. Determination of Cytokines in the Intestinal Inflammation Model
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Soler, A.P.; Miller, R.D.; Laughlin, K.V.; Carp, N.Z.; Klurfeld, D.M.; Mullin, J.M. Increased tight junctional permeability is associated with the development of colon cancer. Carcinogenesis 1999, 20, 1425–1431. [Google Scholar] [CrossRef]
- Yu, L.C. Microbiota dysbiosis and barrier dysfunction in inflammatory bowel disease and colorectal cancers: Exploring a common ground hypothesis. J. Biomed. Sci. 2018, 25, 79. [Google Scholar] [CrossRef]
- Kouzu, K.; Tsujimoto, H.; Kishi, Y.; Ueno, H.; Shinomiya, N. Bacterial Translocation in Gastrointestinal Cancers and Cancer Treatment. Biomedicines 2022, 10, 380. [Google Scholar] [CrossRef]
- Genua, F.; Raghunathan, V.; Jenab, M.; Gallagher, W.M.; Hughes, D.J. The Role of Gut Barrier Dysfunction and Microbiome Dysbiosis in Colorectal Cancer Development. Front. Oncol. 2021, 11, 626349. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Kirby, J.; Reilly, C.M.; Luo, X.M. Leaky Gut As a Danger Signal for Autoimmune Diseases. Front. Immunol. 2017, 8, 598. [Google Scholar] [CrossRef] [PubMed]
- Scaldaferri, F.; Pizzoferrato, M.; Gerardi, V.; Lopetuso, L.; Gasbarrini, A. The gut barrier: New acquisitions and therapeutic approaches. J. Clin. Gastroenterol. 2012, 46, S12–S17. [Google Scholar] [CrossRef]
- Weber, C.R.; Turner, J.R. Inflammatory bowel disease: Is it really just another break in the wall? Gut 2007, 56, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Irrazabal, T.; Belcheva, A.; Girardin, S.E.; Martin, A.; Philpott, D.J. The multifaceted role of the intestinal microbiota in colon cancer. Mol. Cell 2014, 54, 309–320. [Google Scholar] [CrossRef]
- Bertocchi, A.; Carloni, S.; Ravenda, P.S.; Bertalot, G.; Spadoni, I.; Lo Cascio, A.; Gandini, S.; Lizier, M.; Braga, D.; Asnicar, F.; et al. Gut vascular barrier impairment leads to intestinal bacteria dissemination and colorectal cancer metastasis to liver. Cancer Cell 2021, 39, 708–724.e11. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.A. The role of tight junctions in cancer metastasis. Semin. Cell Dev. Biol. 2014, 36, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Yixia, Y.; Sripetchwandee, J.; Chattipakorn, N.; Chattipakorn, S.C. The alterations of microbiota and pathological conditions in the gut of patients with colorectal cancer undergoing chemotherapy. Anaerobe 2021, 68, 102361. [Google Scholar] [CrossRef] [PubMed]
- Nakao, T.; Kurita, N.; Komatsu, M.; Yoshikawa, K.; Iwata, T.; Utusnomiya, T.; Shimada, M. Irinotecan injures tight junction and causes bacterial translocation in rat. J. Surg. Res. 2012, 173, 341–347. [Google Scholar] [CrossRef]
- Hartsock, A.; Nelson, W.J. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim. Et Biophys. Acta 2008, 1778, 660–669. [Google Scholar] [CrossRef]
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef]
- Hollander, D.; Kaunitz, J.D. The "Leaky Gut": Tight Junctions but Loose Associations? Dig. Dis. Sci. 2020, 65, 1277–1287. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Mikelsaar, M.; Zilmer, M. Lactobacillus fermentum ME-3—An antimicrobial and antioxidative probiotic. Microb. Ecol. Health Dis. 2009, 21, 1–27. [Google Scholar]
- Kullisaar, T.; Songisepp, E.; Mikelsaar, M.; Zilmer, K.; Vihalemm, T.; Zilmer, M. Antioxidative probiotic fermented goats’ milk decreases oxidative stress-mediated atherogenicity in human subjects. Br. J. Nutr. 2003, 90, 449–456. [Google Scholar] [CrossRef]
- Songisepp, E.; Kals, J.; Kullisaar, T.; Mandar, R.; Hutt, P.; Zilmer, M.; Mikelsaar, M. Evaluation of the functional efficacy of an antioxidative probiotic in healthy volunteers. Nutr. J. 2005, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Kullisaar, T.; Shepetova, J.; Zilmer, K.; Songisepp, E.; Rehema, A.; Mikelsaar, M.; Zilmer, M. An antioxidant probiotic reduces postprandial lipemia and oxidative stress. Cent Eur. J. Biol. 2011, 6, 32–40. [Google Scholar] [CrossRef]
- Reyhanoglu, G.; Smith, T. Irinotecan; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Thomas, A.; Pommier, Y. Targeting Topoisomerase I in the Era of Precision Medicine. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 6581–6589. [Google Scholar] [CrossRef]
- Buckley, A.; Turner, J.R. Cell Biology of Tight Junction Barrier Regulation and Mucosal Disease. Cold Spring Harb. Perspect. Biol. 2018, 10, a029314. [Google Scholar] [CrossRef] [PubMed]
- Niessen, C.M. Tight junctions/adherens junctions: Basic structure and function. J. Investig. Dermatol. 2007, 127, 2525–2532. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, J.; John, A.; Lim, Y.C.; Kibria, K.M.K.; Mohiuddin, A.K.M.; Ming, L.C.; et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers 2022, 14, 1732. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T. The pathobiology of mucositis. Nature reviews. Cancer 2004, 4, 277–284. [Google Scholar] [PubMed]
- Boussios, S.; Pentheroudakis, G.; Katsanos, K.; Pavlidis, N. Systemic treatment-induced gastrointestinal toxicity: Incidence, clinical presentation and management. Ann. Gastroenterol. 2012, 25, 106–118. [Google Scholar]
- Shang, F.; Jiang, X.; Wang, H.; Chen, S.; Wang, X.; Liu, Y.; Guo, S.; Li, D.; Yu, W.; Zhao, Z.; et al. The inhibitory effects of probiotics on colon cancer cells: In vitro and in vivo studies. J. Gastrointest. Oncol. 2020, 11, 1224–1232. [Google Scholar] [CrossRef]
- Chrysostomou, D.; Roberts, L.A.; Marchesi, J.R.; Kinross, J.M. Gut Microbiota Modulation of Efficacy and Toxicity of Cancer Chemotherapy and Immunotherapy. Gastroenterology 2023, 164, 198–213. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, L.; Chen, S.; Guo, S.; Yue, T.; Hou, Q.; Feng, M.; Xu, H.; Liu, Y.; Wang, P.; et al. The administration of Escherichia coli Nissle 1917 ameliorates irinotecan-induced intestinal barrier dysfunction and gut microbial dysbiosis in mice. Life Sci. 2019, 231, 116529. [Google Scholar] [CrossRef] [PubMed]
- Sepp, E.; Smidt, I.; Stsepetova, J.; Roop, T.; Hutt, P.; Ratsep, M.; Mikelsaar, M. The effect of Lactobacillus fermentum ME-3 on the intestinal microbiota and urine polyamines content: A double-blind placebo-controlled pilot trial. J. Funct. Foods 2018, 48, 430–438. [Google Scholar] [CrossRef]
- Pelton, R. Lactobacillus fermentum ME-3: A Breakthrough in Glutathione Therapy. Integr. Med. 2022, 21, 54–58. [Google Scholar]
- Kullisaar, T.; Songisepp, E.; Aunapuu, M.; Kilk, K.; Arend, A.; Mikelsaar, M.; Rehema, A.; Zilmer, M. Complete glutathione system in probiotic Lactobacillus fermentum ME-3. Prikl. Biokhim. Mikrobiol. 2010, 46, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Kullisaar, T.; Zilmer, M.; Mikelsaar, M.; Vihalemm, T.; Annuk, H.; Kairane, C.; Kilk, A. Two antioxidative lactobacilli strains as promising probiotics. Int. J. Food Microbiol. 2002, 72, 215–224. [Google Scholar] [CrossRef]
- Medina-Diaz, I.M.; Ponce-Ruiz, N.; Rojas-Garcia, A.E.; Zambrano-Zargoza, J.F.; Bernal-Hernandez, Y.Y.; Gonzalez-Arias, C.A.; Barron-Vivanco, B.S.; Herrera-Moreno, J.F. The Relationship between Cancer and Paraoxonase 1. Antioxidants 2022, 11, 697. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, R. In Vitro Methods for Measuring the Permeability of Cell Monolayers. Methods Protoc. 2022, 5, 17. [Google Scholar] [CrossRef]
- Wardill, H.R.; Bowen, J.M.; Al-Dasooqi, N.; Sultani, M.; Bateman, E.; Stansborough, R.; Shirren, J.; Gibson, R.J. Irinotecan disrupts tight junction proteins within the gut: Implications for chemotherapy-induced gut toxicity. Cancer Biol. Ther. 2014, 15, 236–244. [Google Scholar] [CrossRef]
- Candelli, M.; Franza, L.; Pignataro, G.; Ojetti, V.; Covino, M.; Piccioni, A.; Gasbarrini, A.; Franceschi, F. Interaction between Lipopolysaccharide and Gut Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 6242. [Google Scholar] [CrossRef]
- de Waal, G.M.; de Villiers, W.J.S.; Forgan, T.; Roberts, T.; Pretorius, E. Colorectal cancer is associated with increased circulating lipopolysaccharide, inflammation and hypercoagulability. Sci. Rep. 2020, 10, 8777. [Google Scholar] [CrossRef]
- Chen, E.; Kalavar, A.; Bui-Thanh, N.-A.; Opekun, A.R.; White, D.; Rosen, D.; Graham, D.Y.; Rumbaut, R.E.; El-Serag, H.B.; Jiao, L. Serum Levels of Lipopolysaccharides and Risk of Advanced Colorectal Adenoma. Explor. Res. Hypothesis Med. 2020, 5, 47–52. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Ye, D.; Boivin, M.; Guo, S.; Hashimi, M.; Ereifej, L.; Ma, T.Y. Interleukin-6 modulation of intestinal epithelial tight junction permeability is mediated by JNK pathway activation of claudin-2 gene. PLoS ONE 2014, 9, e85345. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Huang, X.; Ma, Y.; Gao, M.; Wang, O.; Gao, T.; Shen, Y.; Liu, X. Interleukin-8 regulates endothelial permeability by down-regulation of tight junction but not dependent on integrins induced focal adhesions. Int. J. Biol. Sci. 2013, 9, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Suenaert, P.; Bulteel, V.; Lemmens, L.; Noman, M.; Geypens, B.; Van Assche, G.; Geboes, K.; Ceuppens, J.L.; Rutgeerts, P. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn’s disease. Am. J. Gastroenterol. 2002, 97, 2000–2004. [Google Scholar] [CrossRef]
- Aleman, R.S.; Moncada, M.; Aryana, K.J. Leaky Gut and the Ingredients That Help Treat It: A Review. Molecules 2023, 28, 619. [Google Scholar] [CrossRef]
- Boeing, T.; Speca, S.; de Souza, P.; Mena, A.M.; Bertin, B.; Desreumax, P.; Mota da Silva, L.; Faloni de Andrade, S.; Dubuqoy, L. The PPARgamma-dependent effect of flavonoid luteolin against damage induced by the chemotherapeutic irinotecan in human intestinal cells. Chem. -Biol. Interact. 2022, 351, 109712. [Google Scholar] [CrossRef]
- Ulgheri, F.; Spanu, P.; Deligia, F.; Loriga, G.; Fuggetta, M.P.; de Haan, I.; Chandgudge, A.; Groves, M.; Domling, A. Design, synthesis and biological evaluation of 1,5-disubstituted alpha-amino tetrazole derivatives as non-covalent inflammasome-caspase-1 complex inhibitors with potential application against immune and inflammatory disorders. Eur. J. Med. Chem. 2022, 229, 114002. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef]
- Putt, K.K.; Pei, R.; White, H.M.; Bolling, B.W. Yogurt inhibits intestinal barrier dysfunction in Caco-2 cells by increasing tight junctions. Food Funct. 2017, 8, 406–414. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Gregorio, A.; Serafino, A.; Krasnowska, E.K.; Superti, F.; Di Fazio, M.R.; Fuggetta, M.P.; Hammarberg Ferri, I.; Fiorentini, C. Protective Effect of Limosilactobacillus fermentum ME-3 against the Increase in Paracellular Permeability Induced by Chemotherapy or Inflammatory Conditions in Caco-2 Cell Models. Int. J. Mol. Sci. 2023, 24, 6225. https://doi.org/10.3390/ijms24076225
De Gregorio A, Serafino A, Krasnowska EK, Superti F, Di Fazio MR, Fuggetta MP, Hammarberg Ferri I, Fiorentini C. Protective Effect of Limosilactobacillus fermentum ME-3 against the Increase in Paracellular Permeability Induced by Chemotherapy or Inflammatory Conditions in Caco-2 Cell Models. International Journal of Molecular Sciences. 2023; 24(7):6225. https://doi.org/10.3390/ijms24076225
Chicago/Turabian StyleDe Gregorio, Alex, Annalucia Serafino, Ewa Krystyna Krasnowska, Fabiana Superti, Maria Rosa Di Fazio, Maria Pia Fuggetta, Ivano Hammarberg Ferri, and Carla Fiorentini. 2023. "Protective Effect of Limosilactobacillus fermentum ME-3 against the Increase in Paracellular Permeability Induced by Chemotherapy or Inflammatory Conditions in Caco-2 Cell Models" International Journal of Molecular Sciences 24, no. 7: 6225. https://doi.org/10.3390/ijms24076225
APA StyleDe Gregorio, A., Serafino, A., Krasnowska, E. K., Superti, F., Di Fazio, M. R., Fuggetta, M. P., Hammarberg Ferri, I., & Fiorentini, C. (2023). Protective Effect of Limosilactobacillus fermentum ME-3 against the Increase in Paracellular Permeability Induced by Chemotherapy or Inflammatory Conditions in Caco-2 Cell Models. International Journal of Molecular Sciences, 24(7), 6225. https://doi.org/10.3390/ijms24076225