The Role of MicroRNA in the Pathogenesis of Diabetic Nephropathy
Abstract
1. Introduction
2. The Role of MicroRNA in the Pathogenesis of Diabetic Nephropathy
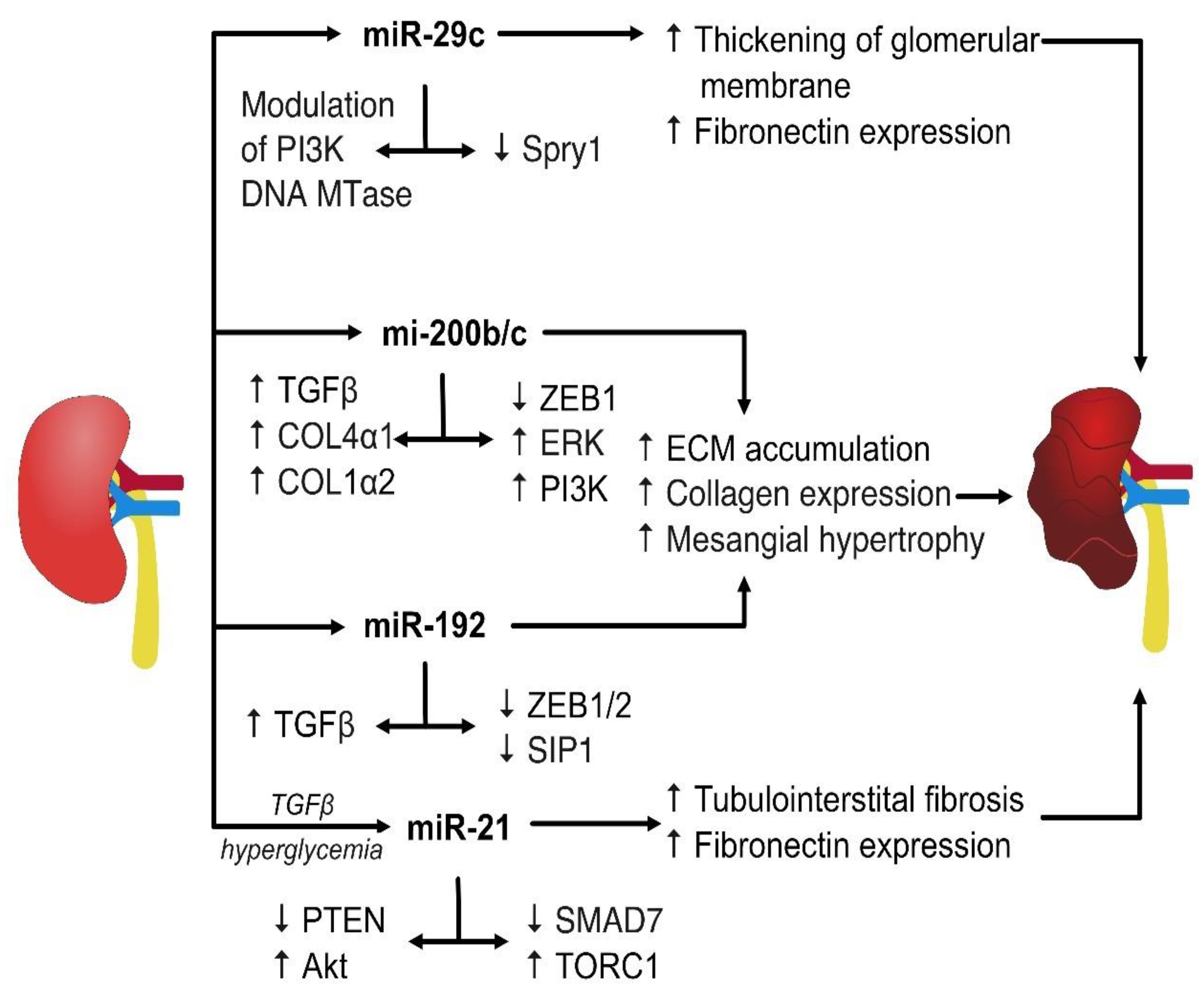
2.1. miRs Involved in Modulating TGF-β Signaling
2.2. miR Affecting Collagen Expression
2.3. miR Regulating the PTEN-Dependent Pathways
2.4. miR Affecting Other Pathways Involved in the Pathogenesis of the Disease
3. miRs That Elicit Protective Properties in Diabetic Nephropathy
4. miRs as Therapeutic Targets in Treatment and Prevention of DN
5. Select miR as Biomarkers of the Disease
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Report on Diabetes; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Huang, D.; Refaat, M.; Mohammedi, K.; Jayyousi, A.; Al Suwaidi, J.; Abi Khalil, C. Macrovascular Complications in Patients with Diabetes and Prediabetes. BioMed Res. Int. 2017, 2017, 7839101. [Google Scholar] [CrossRef] [PubMed]
- Valencia, W.M.; Florez, H. How to prevent the microvascular complications of type 2 diabetes be-yond glucose control. BMJ 2017, 356, i6505. [Google Scholar] [CrossRef]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef]
- Zhang, X.X.; Kong, J.; Yun, K. Prevalence of Diabetic Nephropathy among Patients with Type 2 Diabetes Mellitus in China: A Meta-Analysis of Observational Studies. J. Diabetes Res. 2020, 2020, 2315607. [Google Scholar] [CrossRef] [PubMed]
- Samsu, N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. BioMed Res. Int. 2021, 2021, 1497449. [Google Scholar] [CrossRef] [PubMed]
- Yarahmadi, A.; Shahrokhi, S.Z.; Mostafavi-Pour, Z.; Azarpira, N. MicroRNAs in diabetic nephropathy: From molecular mechanisms to new therapeutic targets of treatment. Biochem. Pharmacol. 2021, 189, 114301. [Google Scholar] [CrossRef]
- Kao, M.P.; Ang, D.S.; Pall, A.; Struthers, A.D. Oxidative stress in renal dysfunction: Mechanisms, clinical sequelae and therapeutic options. J. Hum. Hypertens. 2010, 24, 1–8. [Google Scholar] [CrossRef]
- Duni, A.; Liakopoulos, V.; Roumeliotis, S.; Peschos, D.; Dounousi, E. Oxidative Stress in the Pathogenesis and Evolution of Chronic Kidney Disease: Untangling Ariadne’s Thread. Int. J. Mol. Sci. 2019, 20, 3711. [Google Scholar] [CrossRef]
- Khazim, K.; Gorin, Y.; Cavaglieri, R.C.; Abboud, H.E.; Fanti, P. The antioxidant silybin prevents high glucose-induced oxidative stress and podocyte injury in vitro and in vivo. Am. J. Physiol. Renal. Physiol. 2013, 305, F691–F700. [Google Scholar] [CrossRef]
- Forbes, J.M.; Coughlan, M.T.; Cooper, M.E. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 2008, 57, 1446–1454. [Google Scholar] [CrossRef]
- Chawla, T.; Sharma, D.; Singh, A. Role of the renin angiotensin system in diabetic nephropathy. World J. Diabetes 2010, 1, 141–145. [Google Scholar] [CrossRef]
- Lim, A.K.; Tesch, G.H. Inflammation in diabetic nephropathy. Mediat. Inflamm. 2012, 2012, 146154. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Wang, L.; Zhang, Y.; Zhang, S.; Ning, L.; Zhao, J.; Cheng, G.; Liu, D.; Xiao, J.; Zhao, Z. Chemerin/ChemR23 axis promotes inflammation of glomerular endothelial cells in diabetic nephropathy. J. Cell. Mol. Med. 2019, 23, 3417–3428. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 2011, 7, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Navarro-González, J.F.; Mora-Fernández, C.; Muros de Fuentes, M.; García-Pérez, J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat. Rev. Nephrol. 2011, 7, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Morales, R.E.; Del Pino, M.D.; Valdivielso, J.M.; Ortiz, A.; Mora-Fernández, C.; Navarro-González, J.F. Inflammation in Diabetic Kidney Disease. Nephron 2019, 143, 12–16. [Google Scholar] [CrossRef]
- Kato, M.; Yuan, H.; Xu, Z.G.; Lanting, L.; Li, S.L.; Wang, M.; Hu, M.C.; Reddy, M.A.; Natarajan, R. Role of the Akt/FoxO3a pathway in TGF-beta1-mediated mesangial cell dysfunction: A novel mechanism related to diabetic kidney disease. J. Am. Soc. Nephrol. 2006, 17, 3325–3335. [Google Scholar] [CrossRef]
- Umezono, T.; Toyoda, M.; Kato, M.; Miyauchi, M.; Kimura, M.; Maruyama, M.; Honma, M.; Yagame, M.; Suzuki, D. Glomerular expression of CTGF, TGF-beta 1 and type IV collagen in diabetic nephropathy. J. Nephrol. 2006, 19, 751–757. [Google Scholar]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef]
- Kmiołek, T.; Rzeszotarska, E.; Wajda, A.; Walczuk, E.; Kuca-Warnawin, E.; Romanowska-Próchnicka, K.; Stypinska, B.; Majewski, D.; Jagodzinski, P.P.; Pawlik, A.; et al. The Interplay between Transcriptional Factors and MicroRNAs as an Important Factor for Th17/Treg Balance in RA Patients. Int. J. Mol. Sci. 2020, 21, 7169. [Google Scholar] [CrossRef]
- Bentwich, I.; Avniel, A.; Karov, Y.; Aharonov, R.; Gilad, S.; Barad, O.; Barzilai, A.; Einat, P.; Einav, U.; Meiri, E.; et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nat. Genet. 2005, 37, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Filipowicz, W.; Bhattacharyya, S.N.; Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat. Rev. Genet. 2008, 9, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Vishnoi, A.; Rani, S. MiRNA Biogenesis and Regulation of Diseases: An Overview. Methods Mol. Biol. 2017, 1509, 376. [Google Scholar] [CrossRef]
- Wu, L.; Fan, J.; Belasco, J.G. MicroRNAs direct rapid deadenylation of mRNA. Proc. Natl. Acad. Sci. USA 2006, 103, 4034–4039. [Google Scholar] [CrossRef] [PubMed]
- Huntzinger, E.; Izaurralde, E. Gene silencing by microRNAs: Contributions of translational repression and mRNA decay. Nat. Rev. Genet. 2011, 12, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Fabian, M.R.; Sonenberg, N. The mechanics of miRNA-mediated gene silencing: A look under the hood of miRISC. Nat. Struct. Mol. Biol. 2012, 19, 586–593. [Google Scholar] [CrossRef]
- Jonas, S.; Izaurralde, E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015, 16, 421–433. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, H.; Zhao, M.; Chang, C.; Lu, Q. Clinical significance of miRNAs in autoimmunity. J. Autoimmun. 2020, 109, 102438. [Google Scholar] [CrossRef]
- Dronavalli, S.; Duka, I.; Bakris, G.L. The pathogenesis of diabetic nephropathy. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 444–452. [Google Scholar] [CrossRef]
- Wada, J.; Makino, H. Inflammation and the pathogenesis of diabetic nephropathy. Clin. Sci. 2013, 124, 139–152. [Google Scholar] [CrossRef]
- Wu, H.; Kong, L.; Zhou, S.; Cui, W.; Xu, F.; Luo, M.; Li, X.; Tan, Y.; Miao, L. The Role of MicroRNAs in Diabetic Nephropathy. J. Diabetes Res. 2014, 2014, 920134. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Natarajan, R. MicroRNAs in diabetic nephropathy: Functions, biomarkers, and therapeutic targets. Ann. N. Y. Acad. Sci. 2015, 1353, 72–88. [Google Scholar] [CrossRef] [PubMed]
- Gholaminejad, A.; Abdul Tehrani, H.; Gholami Fesharaki, M. Identification of candidate microRNA biomarkers in diabetic nephropathy: A meta-analysis of profiling studies. J. Nephrol. 2018, 31, 813–831. [Google Scholar] [CrossRef] [PubMed]
- Neal, C.S.; Michael, M.Z.; Pimlott, L.K.; Yong, T.Y.; Li, J.Y.; Gleadle, J.M. Circulating microRNA expression is reduced in chronic kidney disease. Nephrol. Dial. Transplant. 2011, 26, 3794–3802. [Google Scholar] [CrossRef]
- Chen, N.X.; Kiattisunthorn, K.; O’Neill, K.D.; Chen, X.; Moorthi, R.N.; Gattone, V.H.; Allen, M.R.; Moe, S.M. Decreased microRNA is involved in the vascular remodeling abnormalities in chronic kidney disease (CKD). PLoS ONE 2013, 8, e64558. [Google Scholar] [CrossRef]
- Lu, Q.; Ma, Z.; Ding, Y.; Bedarida, T.; Chen, L.; Xie, Z.; Song, P.; Zou, M.H. Circulating miR-103a-3p contributes to angiotensin II-induced renal inflammation and fibrosis via a SNRK/NF-κB/p65 regulatory axis. Nat. Commun. 2019, 10, 2145. [Google Scholar] [CrossRef]
- Baricos, W.H.; CORTEZ, S.L.; Deboisblanc, M.; XIN, S. Transforming growth factor-β is a potent inhibitor of extracellular matrix degradation by cultured human mesangial cells. J. Am. Soc. Nephrol. 1999, 10, 790–795. [Google Scholar] [CrossRef]
- Meng, X.-M.; Tang, P.M.-K.; Li, J.; Lan, H.Y. TGF-β/Smad signaling in renal fibrosis. Front. Physiol. 2015, 6, 82. [Google Scholar] [CrossRef]
- Rocco, M.V.; Chen, Y.; Goldfarb, S.; Ziyadeh, F.N. Elevated glucose stimulates TGF-β gene expression and bioactivity in proximal tubule. Kidney Int. 1992, 41, 107–114. [Google Scholar] [CrossRef]
- López-Hernández, F.J.; López-Novoa, J.M. Role of TGF-β in chronic kidney disease: An integration of tubular, glomerular and vascular effects. Cell Tissue Res. 2012, 347, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Sudamrao Garud, M.; Anant Kulkarni, Y. Hyperglycemia to nephropathy via transforming growth factor beta. Curr. Diabetes Rev. 2014, 10, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Ziyadeh, F.N. Hyperglycemia and diabetic kidney disease: The case for transforming growth factor–β as a key mediator. Diabetes 1995, 44, 1139–1146. [Google Scholar] [CrossRef]
- Zhu, Y.; Casado, M.; Vaulont, S.; Sharma, K. Role of upstream stimulatory factors in regulation of renal transforming growth factor-β1. Diabetes 2005, 54, 1976–1984. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Arce, L.; Wang, M.; Putta, S.; Lanting, L.; Natarajan, R. A microRNA circuit mediates transforming growth factor-β1 autoregulation in renal glomerular mesangial cells. Kidney Int. 2011, 80, 358–368. [Google Scholar] [CrossRef]
- Kato, M.; Zhang, J.; Wang, M.; Lanting, L.; Yuan, H.; Rossi, J.J.; Natarajan, R. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-β-induced collagen expression via inhibition of E-box repressors. Proc. Natl. Acad. Sci. USA 2007, 104, 3432–3437. [Google Scholar] [CrossRef]
- Park, J.T.; Kato, M.; Yuan, H.; Castro, N.; Lanting, L.; Wang, M.; Natarajan, R. FOG2 protein down-regulation by transforming growth factor-β1-induced microRNA-200b/c leads to Akt kinase activation and glomerular mesangial hypertrophy related to diabetic nephropathy. J. Biol. Chem. 2013, 288, 22469–22480. [Google Scholar] [CrossRef]
- Alvarez, M.L.; Khosroheidari, M.; Eddy, E.; Kiefer, J. Role of microRNA 1207-5P and its host gene, the long non-coding RNA Pvt1, as mediators of extracellular matrix accumulation in the kidney: Implications for diabetic nephropathy. PLoS ONE 2013, 8, e77468. [Google Scholar] [CrossRef]
- Zhong, X.; Chung, A.C.; Chen, H.-Y.; Meng, X.-M.; Lan, H.Y. Smad3-mediated upregulation of miR-21 promotes renal fibrosis. J. Am. Soc. Nephrol. 2011, 22, 1668–1681. [Google Scholar] [CrossRef]
- Zhong, X.; Chung, A.C.K.; Chen, H.-Y.; Dong, Y.; Meng, X.; Li, R.; Yang, W.; Hou, F.; Lan, H. miR-21 is a key therapeutic target for renal injury in a mouse model of type 2 diabetes. Diabetologia 2013, 56, 663–674. [Google Scholar] [CrossRef]
- Mu, J.; Pang, Q.; Guo, Y.-H.; Chen, J.-G.; Zeng, W.; Huang, Y.-J.; Zhang, J.; Feng, B. Functional implications of microRNA-215 in TGF-β1-induced phenotypic transition of mesangial cells by targeting CTNNBIP1. PLoS ONE 2013, 8, e58622. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Koh, P.; Winbanks, C.; Coughlan, M.T.; McClelland, A.; Watson, A.; Jandeleit-Dahm, K.; Burns, W.C.; Thomas, M.C.; Cooper, M.E. miR-200a prevents renal fibrogenesis through repression of TGF-β2 expression. Diabetes 2011, 60, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Zhong, X.; Huang, X.R.; Meng, X.M.; You, Y.; Chung, A.C.; Lan, H.Y. MicroRNA-29b inhibits diabetic nephropathy in db/db mice. Mol. Ther. 2014, 22, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jim, B.; Ziyadeh, F.N. Diabetic nephropathy and transforming growth factor-β: Transforming our view of glomerulosclerosis and fibrosis build-up. In Seminars in Nephrology; Saunders: Philadelphia, PA, USA; pp. 532–543.
- Wang, J.; Gao, Y.; Ma, M.; Li, M.; Zou, D.; Yang, J.; Zhu, Z.; Zhao, X. Effect of miR-21 on renal fibrosis by regulating MMP-9 and TIMP1 in kk-ay diabetic nephropathy mice. Cell Biochem. Biophys. 2013, 67, 537–546. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Minto, A.W.; Wang, J.; Shi, Q.; Li, X.; Quigg, R.J. MicroRNA-377 is up-regulated and can lead to increased fibronectin production in diabetic nephropathy. FASEB J. 2008, 22, 4126–4135. [Google Scholar] [CrossRef]
- Long, J.; Wang, Y.; Wang, W.; Chang, B.H.; Danesh, F.R. MicroRNA-29c is a signature microRNA under high glucose conditions that targets Sprouty homolog 1, and its in vivo knockdown prevents progression of diabetic nephropathy. J. Biol. Chem. 2011, 286, 11837–11848. [Google Scholar] [CrossRef]
- Sabatel, C.; Cornet, A.M.; Tabruyn, S.P.; Malvaux, L.; Castermans, K.; Martial, J.A.; Struman, I. Sprouty1, a new target of the angiostatic agent 16K prolactin, negatively regulates angiogenesis. Mol. Cancer 2010, 9, 231. [Google Scholar] [CrossRef]
- Wang, B.; Komers, R.; Carew, R.; Winbanks, C.E.; Xu, B.; Herman-Edelstein, M.; Koh, P.; Thomas, M.; Jandeleit-Dahm, K.; Gregorevic, P. Suppression of microRNA-29 expression by TGF-β1 promotes collagen expression and renal fibrosis. J. Am. Soc. Nephrol. 2012, 23, 252–265. [Google Scholar] [CrossRef]
- Du, B.; Ma, L.-M.; Huang, M.-B.; Zhou, H.; Huang, H.-L.; Shao, P.; Chen, Y.-Q.; Qu, L.-H. High glucose down-regulates miR-29a to increase collagen IV production in HK-2 cells. FEBS Lett. 2010, 584, 811–816. [Google Scholar] [CrossRef]
- He, F.; Peng, F.; Xia, X.; Zhao, C.; Luo, Q.; Guan, W.; Li, Z.; Yu, X.; Huang, F. MiR-135a promotes renal fibrosis in diabetic nephropathy by regulating TRPC1. Diabetologia 2014, 57, 1726–1736. [Google Scholar] [CrossRef]
- Mehta, D.; Ahmmed, G.U.; Paria, B.C.; Holinstat, M.; Voyno-Yasenetskaya, T.; Tiruppathi, C.; Minshall, R.D.; Malik, A.B. RhoA interaction with inositol 1, 4, 5-trisphosphate receptor and transient receptor potential channel-1 regulates Ca2+ entry: Role in signaling increased endothelial permeability. J. Biol. Chem. 2003, 278, 33492–33500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Freedman, B.I.; Flekac, M.; Santos, E.; Hicks, P.J.; Bowden, D.W.; Efendic, S.; Brismar, K.; Gu, H.F. Evaluation of genetic association and expression reduction of TRPC1 in the development of diabetic nephropathy. Am. J. Nephrol. 2009, 29, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, B.D.; Hodakoski, C.; Barrows, D.; Mense, S.M.; Parsons, R.E. PTEN function: The long and the short of it. Trends Biochem. Sci. 2014, 39, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Lieberthal, W.; Levine, J.S. The role of the mammalian target of rapamycin (mTOR) in renal disease. J. Am. Soc. Nephrol. 2009, 20, 2493–2502. [Google Scholar] [CrossRef] [PubMed]
- Khokhar, M.; Roy, D.; Modi, A.; Agarwal, R.; Yadav, D.; Purohit, P.; Sharma, P. Perspectives on the role of PTEN in diabetic nephropathy: An update. Crit. Rev. Clin. Lab. Sci. 2020, 57, 470–483. [Google Scholar] [CrossRef]
- Kato, M.; Wang, L.; Putta, S.; Wang, M.; Yuan, H.; Sun, G.; Lanting, L.; Todorov, I.; Rossi, J.J.; Natarajan, R. Post-transcriptional Up-regulation of Tsc-22 by Ybx1, a Target of miR-216a, Mediates TGF-β-induced Collagen Expression in Kidney Cells*♦. J. Biol. Chem. 2010, 285, 34004–34015. [Google Scholar] [CrossRef]
- Kato, M.; Putta, S.; Wang, M.; Yuan, H.; Lanting, L.; Nair, I.; Gunn, A.; Nakagawa, Y.; Shimano, H.; Todorov, I. TGF-β activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat. Cell Biol. 2009, 11, 881–889. [Google Scholar] [CrossRef]
- Dey, N.; Das, F.; Mariappan, M.M.; Mandal, C.C.; Ghosh-Choudhury, N.; Kasinath, B.S.; Choudhury, G.G. MicroRNA-21 orchestrates high glucose-induced signals to TOR complex 1, resulting in renal cell pathology in diabetes. J. Biol. Chem. 2011, 286, 25586–25603. [Google Scholar] [CrossRef]
- McClelland, A.D.; Herman-Edelstein, M.; Komers, R.; Jha, J.C.; Winbanks, C.E.; Hagiwara, S.; Gregorevic, P.; Kantharidis, P.; Cooper, M.E. miR-21 promotes renal fibrosis in diabetic nephropathy by targeting PTEN and SMAD7. Clin. Sci. 2015, 129, 1237–1249. [Google Scholar] [CrossRef]
- Bera, A.; Das, F.; Ghosh-Choudhury, N.; Mariappan, M.M.; Kasinath, B.S.; Ghosh Choudhury, G. Reciprocal regulation of miR-214 and PTEN by high glucose regulates renal glomerular mesangial and proximal tubular epithelial cell hypertrophy and matrix expansion. Am. J. Physiol. Cell Physiol. 2017, 313, C430–C447. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, S.; Wu, D.; Liu, X.; Shi, M.; Wang, Y.; Zhang, F.; Ding, J.; Xiao, Y.; Guo, B. MicroRNA-22 promotes renal tubulointerstitial fibrosis by targeting PTEN and suppressing autophagy in diabetic nephropathy. J. Diabetes Res. 2018, 2018, 4728645. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Wang, S.S.; Han, Z.; Han, F.; Chang, Y.P.; Yang, Y.; Xue, M.; Sun, B.; Chen, L.M. Triptolide restores autophagy to alleviate diabetic renal fibrosis through the miR-141-3p/PTEN/Akt/mTOR pathway. Mol. Ther. Nucleic Acids 2017, 9, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, X.; Zhang, J.; Shi, J. MicroRNA-25 inhibits high glucose-induced apoptosis in renal tubular epithelial cells via PTEN/AKT pathway. Biomed. Pharmacother. 2017, 96, 471–479. [Google Scholar] [CrossRef]
- Fiorentino, L.; Cavalera, M.; Mavilio, M.; Conserva, F.; Menghini, R.; Gesualdo, L.; Federici, M. Regulation of TIMP3 in diabetic nephropathy: A role for microRNAs. Acta Diabetol. 2013, 50, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Lee, J.; Wang, Z.; Patel, V.B.; Fan, D.; Das, S.K.; Liu, G.C.; John, R.; Scholey, J.W.; Oudit, G.Y. Loss of TIMP3 selectively exacerbates diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2012, 303, F1341–F1352. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Q.; Wang, X.-X.; Yao, X.-M.; Zhang, D.-L.; Yang, X.-F.; Tian, S.-F.; Wang, N.-S. MicroRNA-195 promotes apoptosis in mouse podocytes via enhanced caspase activity driven by BCL2 insufficiency. Am. J. Nephrol. 2011, 34, 549–559. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Q.; Li, S. MicroRNA-218 promotes high glucose-induced apoptosis in podocytes by targeting heme oxygenase-1. Biochem. Biophys. Res. Commun. 2016, 471, 582–588. [Google Scholar] [CrossRef]
- Guo, J.; Li, J.; Zhao, J.; Yang, S.; Wang, L.; Cheng, G.; Liu, D.; Xiao, J.; Liu, Z.; Zhao, Z. MiRNA-29c regulates the expression of inflammatory cytokines in diabetic nephropathy by targeting tristetraprolin. Sci. Rep. 2017, 7, 2314. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, X.; Ding, S.; Chen, J.; Chen, T.; Chen, X.; Zha, H.; Yao, L.; He, X.; Peng, H. MicroRNA-451 regulates p38 MAPK signaling by targeting of Ywhaz and suppresses the mesangial hypertrophy in early diabetic nephropathy. FEBS Lett. 2012, 586, 20–26. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Y.; Wang, Z.; Wang, L.; Wei, X.; Zhang, B.; Wen, Z.; Fang, H.; Pang, Q.; Yi, F. Regulation of NADPH oxidase activity is associated with miRNA-25-mediated NOX4 expression in experimental diabetic nephropathy. Am. J. Nephrol. 2010, 32, 581–589. [Google Scholar] [CrossRef]
- Wang, L.P.; Geng, J.N.; Sun, B.; Sun, C.B.; Shi, Y.; Yu, X.Y. MiR-92b-3p is induced by advanced glycation end products and involved in the pathogenesis of diabetic nephropathy. Evid. Based Complement. Altern. Med. 2020, 2020, 6050874. [Google Scholar] [CrossRef]
- Li, D.; Lu, Z.; Jia, J.; Zheng, Z.; Lin, S. Changes in microRNAs associated with podocytic adhesion damage under mechanical stress. J. Renin Angiotensin Aldosterone Syst. 2013, 14, 97–102. [Google Scholar] [CrossRef]
- Lin, C.-L.; Lee, P.-H.; Hsu, Y.-C.; Lei, C.-C.; Ko, J.-Y.; Chuang, P.-C.; Huang, Y.-T.; Wang, S.-Y.; Wu, S.-L.; Chen, Y.-S. MicroRNA-29a promotion of nephrin acetylation ameliorates hyperglycemia-induced podocyte dysfunction. J. Am. Soc. Nephrol. 2014, 25, 1698–1709. [Google Scholar] [CrossRef]
- Long, J.; Wang, Y.; Wang, W.; Chang, B.H.; Danesh, F.R. Identification of microRNA-93 as a novel regulator of vascular endothelial growth factor in hyperglycemic conditions. J. Biol. Chem. 2010, 285, 23457–23465. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Q.; Guo, F.; Ma, X.; Ji, H.; Liu, F.; Zhao, Y.; Qin, G. MicroRNA-27a induces mesangial cell injury by targeting of PPARγ and its in vivo knockdown prevents progression of diabetic nephropathy. Sci. Rep. 2016, 6, 26072. [Google Scholar] [CrossRef]
- Maity, S.; Bera, A.; Ghosh-Choudhury, N.; Das, F.; Kasinath, B.S.; Choudhury, G.G. microRNA-181a downregulates deptor for TGFβ-induced glomerular mesangial cell hypertrophy and matrix protein expression. Exp. Cell Res. 2018, 364, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Zanchi, C.; Macconi, D.; Trionfini, P.; Tomasoni, S.; Rottoli, D.; Locatelli, M.; Rudnicki, M.; Vandesompele, J.; Mestdagh, P.; Remuzzi, G. MicroRNA-184 is a downstream effector of albuminuria driving renal fibrosis in rats with diabetic nephropathy. Diabetologia 2017, 60, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zeng, L.; Cao, C.; Lu, C.; Lian, W.; Han, J.; Zhang, X.; Zhang, J.; Tang, T.; Li, M. Long noncoding RNA MALAT1 regulates renal tubular epithelial pyroptosis by modulated miR-23c targeting of ELAVL1 in diabetic nephropathy. Exp. Cell Res. 2017, 350, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Duan, L.; Guo, T.; Gao, Y.; Tian, L.; Liu, J.; Wang, S.; Yang, J. Downregulation of miR-30c promotes renal fibrosis by target CTGF in diabetic nephropathy. J. Diabetes Its Complicat. 2016, 30, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Geng, J.; Zhou, Z.; Tian, J.; Li, X. MicroRNA-130b improves renal tubulointerstitial fibrosis via repression of Snail-induced epithelial-mesenchymal transition in diabetic nephropathy. Sci. Rep. 2016, 6, 20475. [Google Scholar] [CrossRef]
- Ying, S. MicroRNA Protocols, Methods in Molecular Biology, 3rd ed.; Humana Press: Totowa, HJ, USA, 2018. [Google Scholar]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef]
- Bhatt, K.; Lanting, L.L.; Jia, Y.; Yadav, S.; Reddy, M.A.; Magilnick, N.; Boldin, M.; Natarajan, R. Anti-Inflammatory Role of MicroRNA-146a in the Pathogenesis of Diabetic Nephropathy. J. Am. Soc. Nephrol. 2016, 27, 2277–2288. [Google Scholar] [CrossRef]
- Zhang, Y.; Le, X.; Zheng, S.; Zhang, K.; He, J.; Liu, M.; Tu, C.; Rao, W.; Du, H.; Ouyang, Y.; et al. MicroRNA-146a-5p-modified human umbilical cord mesenchymal stem cells enhance protection against diabetic nephropathy in rats through facilitating M2 macrophage polarization. Stem Cell Res. Ther. 2022, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.R.; Chen, B.P.; Chen, F.; Yang, S.X.; Zhu, C.Y.; Ma, Y.L.; Li, Y.; Shi, J. Exosomal microRNA-16-5p from human urine-derived stem cells ameliorates diabetic nephropathy through protection of podocyte. J. Cell. Mol. Med. 2021, 25, 10798–10813. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Kuang, G.; Wu, Y.; Ou, C. Emerging roles of exosomal miRNAs in diabetes mellitus. Clin. Transl. Med. 2021, 11, e468. [Google Scholar] [CrossRef]
- Wang, L.; Chopp, M.; Szalad, A.; Lu, X.; Zhang, Y.; Wang, X.; Cepparulo, P.; Lu, M.; Li, C.; Zhang, Z.G. Exosomes Derived From Schwann Cells Ameliorate Peripheral Neuropathy in Type 2 Diabetic Mice. Diabetes 2020, 69, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Chopp, M.; Wang, L.; Lu, X.; Szalad, A.; Zhang, Z.G. Exosomes derived from high-glucose-stimulated Schwann cells promote development of diabetic peripheral neuropathy. FASEB J. 2018, 31, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, R.M.; Akpovi, C.D.; Chen, L.; Vitale, M.L. Cholesterol metabolism and Cx43, Cx46, and Cx50 gap junction protein expression and localization in normal and diabetic and obese ob/ob and db/db mouse testes. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E21–E38. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Chopp, M.; Zhang, Z.G.; Liu, X.S. Emerging Roles of microRNAs as Biomarkers and Therapeutic Targets for Diabetic Neuropathy. Front. Neurol. 2020, 11, 558758. [Google Scholar] [CrossRef]
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering exosomes for targeted drug delivery. Theranostics 2021, 11, 3183–3195. [Google Scholar] [CrossRef]
- Fan, B.; Li, C.; Szalad, A.; Wang, L.; Pan, W.; Zhang, R.; Chopp, M.; Zhang, Z.G.; Liu, X.S. Mesenchymal stromal cell-derived exosomes ameliorate peripheral neuropathy in a mouse model of diabetes. Diabetologia 2020, 63, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.A.; Ludwig, R.G.; Garcia-Martin, R.; Brandão, B.B.; Kahn, C.R. Extracellular miRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab. 2019, 30, 656–673. [Google Scholar] [CrossRef] [PubMed]
- Lassandro, G.; Ciaccia, L.; Amoruso, A.; Palladino, V.; Palmieri, V.V.; Giordano, P. Focus on MicroRNAs as Biomarker in Pediatric Diseases. Curr. Pharm. Des. 2021, 27, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; How Huang, K.; Jen Lee, M.; Galas, D.J.; Wang, K. The MicroRNA Spectrum in 12 Body Fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef]
- Hunter, M.P.; Ismail, N.; Zhang, X.; Aguda, B.D.; Lee, E.J.; Yu, L.; Xiao, T.; Schafer, J.; Lee, M.-L.T.; Schmittgen, T.D.; et al. Detection of microRNA Expression in Human Peripheral Blood Microvesicles. PLoS ONE 2008, 3, e3694. [Google Scholar] [CrossRef]
- Chin, L.J.; Slack, F.J. A truth serum for cancer—MicroRNAs have major potential as cancer biomarkers. Cell Res. 2008, 18, 983–984. [Google Scholar] [CrossRef]
- Li, J.Y.; Yong, T.Y.; Michael, M.Z.; Gleadle, J.M. Review: The role of microRNAs in kidney disease. Nephrology 2010, 15, 599–608. [Google Scholar] [CrossRef]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032. [Google Scholar] [CrossRef]
- Franczyk, B.; Gluba-Brzózka, A.; Olszewski, R.; Parolczyk, M.; Rysz-Górzyńska, M.; Rysz, J. miRNA biomarkers in renal disease. Int. Urol. Nephrol. 2022, 54, 575–588. [Google Scholar] [CrossRef]
- Delić, D.; Eisele, C.; Schmid, R.; Baum, P.; Wiech, F.; Gerl, M.; Zimdahl, H.; Pullen, S.S.; Urquhart, R. Urinary Exosomal miRNA Signature in Type II Diabetic Nephropathy Patients. PLoS ONE 2016, 11, e0150154. [Google Scholar] [CrossRef]
- Argyropoulos, C.; Wang, K.; McClarty, S.; Huang, D.; Bernardo, J.; Ellis, D.; Orchard, T.; Galas, D.; Johnson, J. Urinary MicroRNA Profiling in the Nephropathy of Type 1 Diabetes. PLoS ONE 2013, 8, e54662. [Google Scholar] [CrossRef]
- Peng, H.; Zhong, M.; Zhao, W.; Wang, C.; Zhang, J.; Liu, X.; Li, Y.; Paudel, S.D.; Wang, Q.; Lou, T. Urinary miR-29 Correlates with Albuminuria and Carotid Intima-Media Thickness in Type 2 Diabetes Patients. PLoS ONE 2013, 8, e82607. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Matboli, M.; Aboushahba, R.; Bekhet, M.M.; Soliman, Y. Urinary exosomal microRNA panel unravels novel biomarkers for diagnosis of type 2 diabetic kidney disease. J. Diabetes Its Complicat. 2016, 30, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Colhoun, H.M.; Marcovecchio, M.L. Biomarkers of diabetic kidney disease. Diabetologia 2018, 61, 996–1011. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, A.; Guo, F.; Song, Y.; Jing, N.; Ding, X.; Pan, M.; Zhang, H.; Wang, J.; Wu, L.; et al. Urinary Exosomal MiRNA-4534 as a Novel Diagnostic Biomarker for Diabetic Kidney Disease. Front. Endocrinol. 2020, 11, 590. [Google Scholar] [CrossRef] [PubMed]
- Dewanjee, S.; Bhattacharjee, N. MicroRNA: A new generation therapeutic target in diabetic nephropathy. Biochem. Pharmacol. 2018, 155, 32–47. [Google Scholar] [CrossRef]
- Hua, F. New insights into diabetes mellitus and its complications: A narrative review. Ann. Transl. Med. 2020, 8, 1689. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Wang, J.; Zhang, L.; Sun, W.; Xu, X.; Zhang, K. Plasma miR-193a-3p can be a potential biomarker for the diagnosis of diabetic nephropathy. Ann. Clin. Biochem. 2020, 58, 141–148. [Google Scholar] [CrossRef]
| miRNA | Up/Downregulation | Target Protein or Pathway | Outcome | Model | Reference |
|---|---|---|---|---|---|
| miR-192 | Up | ZEB1/SIP1 | TGF- β -induced increased COL1α1 and COL1α2 expression | Mouse | [47] |
| miR-192 | Up | ZEB1/2 | Increased TGF-β signaling, increased fibrosis | Mouse | [46] |
| miR-216a | Up | PTEN, YBX1 | TGF-β-induced collagen expression | Mouse mesangial cells | [68] |
| miR-217 | Up | PTEN | Increases mesangial cell survival | Mouse mesangial cells | [69] |
| miR-200b/c | Up | ZEB1 | Mediates TGF-β1 autoregulation, increases COL1α2 and COL4α1 expression | Mouse | [46] |
| miR-200b/c | Up | FOG2, TGF-β | Akt kinase activation, glomerular mesangial hypertrophy | Mouse | [48] |
| miR-21 | Up | PTEN, PRAS40, TORC1 | Renal hypertrophy through Akt/TORC1 pathway | Human mesangial cells | [70] |
| miR-21 | Up | SMAD7 | activation of the TGF-β and NF-κB signaling pathways | Mouse | [51] |
| miR-21 | Up | SirT1 | TIMP3 downregulation | Mouse | [76] |
| miR-21 | Up | MMP9/TIMP1 | Increased collagen and FN expression | Mouse | [56] |
| miR-21 | Up | PTEN-SMAD7 | Regulation of renal tubular ECM via Smad3/Akt pathway | Mouse | [71] |
| miR-377 | Up | PAK1, SOD | Increased FN production | Mouse | [57] |
| miR-195 | Up | Bcl2 | Promotes apoptosis via enhanced caspase cascade | Mouse | [78] |
| miR-215 | Up | CTNNBIP1 | Promotes TGF-β driven mesangial cell phenotypic transition | Mesangial cells | [52] |
| miR-124 | Up | Integrin α3β1 | Downregulation of podocyte α3 and β1 that leads to adhesion damage under mechanical stress | Human podocytes | [84] |
| miR-29c | Up | Spry1 | Activation of Rho kinase, albuminuria, mesangial matrix accumulation | Mouse podocytes | [58] |
| miR-29c | Up | Tristetrapolin | Promotes inflammatory response via increase of TNF-α and IL-6 | Human plasma and urine | [80] |
| miR-1207-5p | Up | PMEPA1, G6PD, PDPK1 | ECM accumulation through TGF-β | Human podocytes | [49] |
| miR-135a | Up | TRPC1 | Increases FN and collagen synthesis | Human serum and kidney tissue, mouse | [62] |
| miR-200a | Down | TGF-β2 | Increased FN and collagen synthesis | Rat tubular epithelial cells | [53] |
| miR-141 | Down | TGF-β2 | Increased FN and collagen synthesis | Rat tubular epithelial cells | [53] |
| miR-29 | Down | Collagen I and collagen IV | Increased collagen production and fibrosis | Rat tubular epithelial cells, mesangial cells, podocytes | [60] |
| miR-29a | Down | COL4α1, COL4α2 | Excess collagen deposition | Human tubular cel line | [61] |
| miR-29a | Down | HDAC4 | Podocyte protein deacetylation and degradation as well as renal dysfunction | Mouse | [85] |
| miR-29b | Down | TGF-β/Smad3 | Upregulation of collagen matrix in mesangial cells | Mouse | [54] |
| miR-451 | Down | YWHAZ | Upregulation of p38 MAPK signaling, mesangial proliferation | Mouse mesangial cells | [81] |
| miR-25 | Down | NOX4 | Increased NOX4 expression | Rat | [82] |
| miR-93 | Down | VEGF-A | Increased fibrosis | Mouse, endothelial cells, podocytes | [86] |
| miR-22 | Up | PTEN | Suppression of autophagic flux and induction of Col IV and α-SMA expression | Rat renal cells | [73] |
| miR-27a | Up | PPARγ | Increased ECM accumulation, proteinuria | Rat | [87] |
| miR-92b-3p | Up | SMAD7 | Increased TGF-β signaling and renal fibrosis | Rat | [83] |
| miR-141-3p | Up | PTEN | Increased renal fibrosis through miR-141-3p/PTEN/Akt/mTOR pathway | Rat | [74] |
| miR-181a | Up | Deptor | Leads to TGFβ-induced mesangial cell hypertrophy and matrix protein FN expression | Rat mesangial cells | [88] |
| miR-184 | Up | LPP3 | Tubulointerstitial fibrosis and albuminuria | Rat | [89] |
| miR-214 | Up | PTEN | Cell hypertrophy and expression of the matrix protein FN | Mouse | [72] |
| miR-218 | Up | HO-1 | Acceleration of podocyte apoptosis through directly downregulating HO-1 and facilitating p38-MAPK activation | Mouse podocytes | [79] |
| miR-23c | Down | MALAT1 | Contributes to hyperglycemia-induced cell pyroptosis | Renal tubular epithelial cells | [90] |
| miR-25 | Down | PTEN/Akt | Oxidative stress and apoptosis in renal tubular epithelial cells | Renal biopsy tissue | [75] |
| miR-30c | Down | CTGF | Upregulation of CTFG results in renal fibrosis | Human kidney tubular epithelial cells | [91] |
| miR-130b | Down | Snail | Deregulated E-CADHERIN, VIMENTIN, COLLAGEN IV and α-smooth muscle actin (α-SMA), key mediators of thus affecting epithelial-to-mesenchymal transition | Rat | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szostak, J.; Gorący, A.; Durys, D.; Dec, P.; Modrzejewski, A.; Pawlik, A. The Role of MicroRNA in the Pathogenesis of Diabetic Nephropathy. Int. J. Mol. Sci. 2023, 24, 6214. https://doi.org/10.3390/ijms24076214
Szostak J, Gorący A, Durys D, Dec P, Modrzejewski A, Pawlik A. The Role of MicroRNA in the Pathogenesis of Diabetic Nephropathy. International Journal of Molecular Sciences. 2023; 24(7):6214. https://doi.org/10.3390/ijms24076214
Chicago/Turabian StyleSzostak, Joanna, Anna Gorący, Damian Durys, Paweł Dec, Andrzej Modrzejewski, and Andrzej Pawlik. 2023. "The Role of MicroRNA in the Pathogenesis of Diabetic Nephropathy" International Journal of Molecular Sciences 24, no. 7: 6214. https://doi.org/10.3390/ijms24076214
APA StyleSzostak, J., Gorący, A., Durys, D., Dec, P., Modrzejewski, A., & Pawlik, A. (2023). The Role of MicroRNA in the Pathogenesis of Diabetic Nephropathy. International Journal of Molecular Sciences, 24(7), 6214. https://doi.org/10.3390/ijms24076214






