A PCOS Paradox: Does Inositol Therapy Find a Rationale in All the Different Phenotypes?
Abstract
1. Introduction
2. Redefining PCOS: The Metabolic Alterations
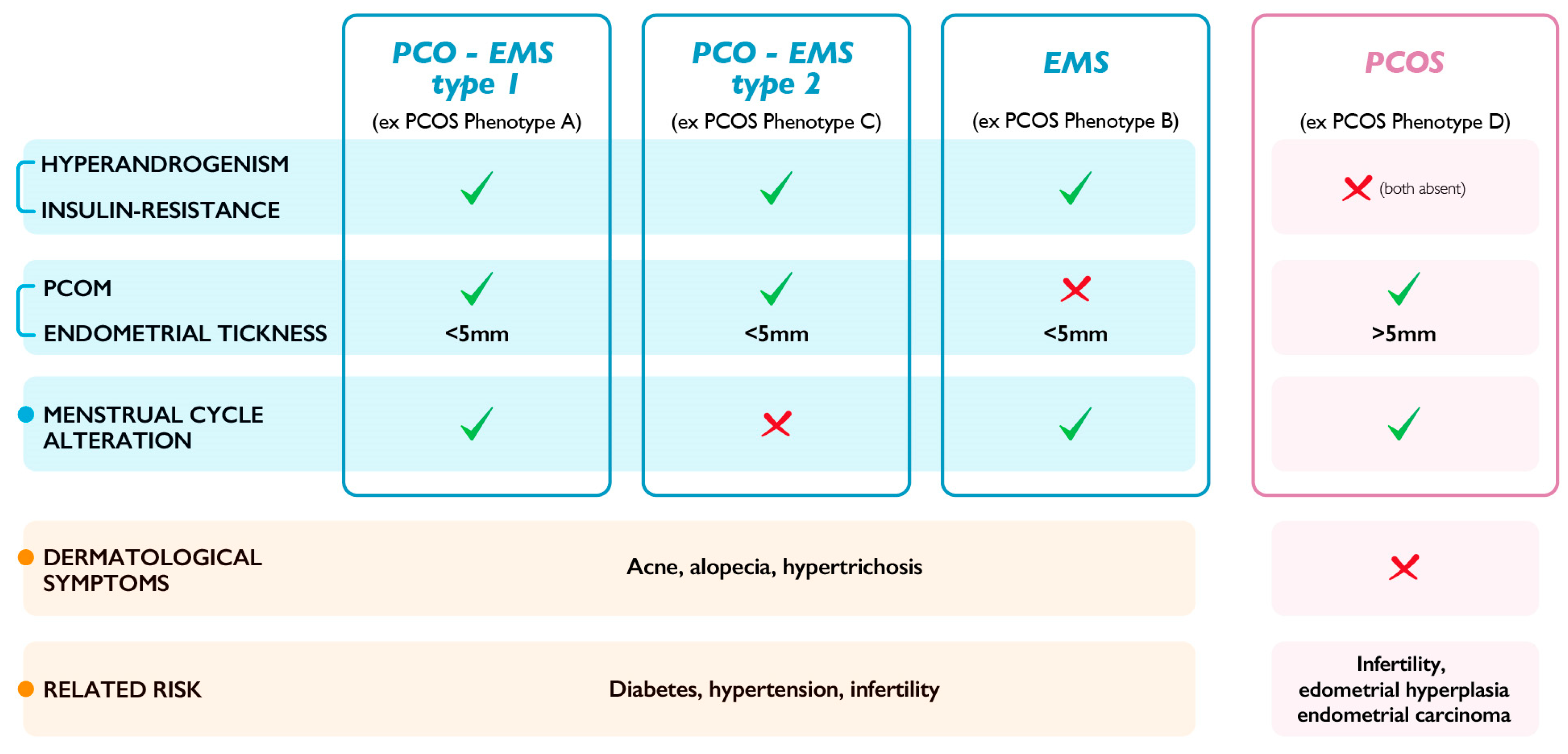
3. Alternative Classification for PCOS Phenotypes
4. Changing the Therapeutic Rationale
5. Final Considerations on Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joham, A.E.; Norman, R.J.; Stener-Victorin, E.; Legro, R.S.; Franks, S.; Moran, L.J.; Boyle, J.; Teede, H.J. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022, 10, 668–680. [Google Scholar] [CrossRef]
- Stein, I.F.; Leventhal, M.L. Amenorrhea associated with bilateral polycystic ovaries. Am. J. Obstet. Gynecol. 1935, 29, 181–191. [Google Scholar] [CrossRef]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Lizneva, D.; Suturina, L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 6–15. [Google Scholar] [CrossRef]
- Azziz, R. Polycystic Ovary Syndrome. Obstet. Gynecol. 2018, 132, 321–336. [Google Scholar] [CrossRef]
- Hoeger, K.M.; Dokras, A.; Piltonen, T. Update on PCOS: Consequences, Challenges, and Guiding Treatment. J. Clin. Endocrinol. Metab. 2021, 106, e1071–e1083. [Google Scholar] [CrossRef]
- Armanini, D.; Boscaro, M.; Bordin, L.; Sabbadin, C. Controversies in the Pathogenesis, Diagnosis and Treatment of PCOS: Focus on Insulin Resistance, Inflammation, and Hyperandrogenism. Int. J. Mol. Sci. 2022, 23, 4110. [Google Scholar] [CrossRef] [PubMed]
- Tosi, F.; Bonora, E.; Moghetti, P. Insulin resistance in a large cohort of women with polycystic ovary syndrome: A comparison between euglycaemic-hyperinsulinaemic clamp and surrogate indexes. Hum. Reprod. 2017, 32, 2515–2521. [Google Scholar] [CrossRef] [PubMed]
- Cassar, S.; Misso, M.L.; Hopkins, W.G.; Shaw, C.S.; Teede, H.J.; Stepto, N.K. Insulin resistance in polycystic ovary syndrome: A systematic review and meta-analysis of euglycaemic-hyperinsulinaemic clamp studies. Hum. Reprod. 2016, 31, 2619–2631. [Google Scholar] [CrossRef]
- Calvo, F.; Karras, B.T.; Phillips, R.; Kimball, A.M.; Wolf, F. Diagnoses, syndromes, and diseases: A knowledge representation problem. AMIA Annu. Symp. Proc. 2003, 2003, 802. [Google Scholar]
- Azziz, R.; Carmina, E.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Futterweit, W.; Janssen, O.E.; Legro, R.S.; Norman, R.J.; Taylor, A.E.; et al. Positions statement: Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: An Androgen Excess Society guideline. J. Clin. Endocrinol. Metab. 2006, 91, 4237–4245. [Google Scholar] [CrossRef]
- Zawadzki, J.K.; Dunaif, A. Diagnostic Criteria for Polycystic Ovary Syndrome: Towards a Rational Approach; Blackwell Scientific Publications: Boston, MA, USA, 1992. [Google Scholar]
- Tosi, F.; Villani, M.; Migazzi, M.; Faccin, G.; Garofalo, S.; Fiers, T.; Kaufman, J.M.; Bonora, E.; Moghetti, P. Insulin-Mediated Substrate Use in Women With Different Phenotypes of PCOS: The Role of Androgens. J. Clin. Endocrinol. Metab. 2021, 106, e3414–e3425. [Google Scholar] [CrossRef]
- Unluhizarci, K.; Karaca, Z.; Kelestimur, F. Role of insulin and insulin resistance in androgen excess disorders. World J. Diabetes 2021, 12, 616–629. [Google Scholar] [CrossRef]
- Moghetti, P.; Tosi, F. Insulin resistance and PCOS: Chicken or egg? J. Endocrinol. Investig. 2021, 44, 233–244. [Google Scholar] [CrossRef]
- Moghetti, P.; Tosi, F.; Bonin, C.; Di Sarra, D.; Fiers, T.; Kaufman, J.M.; Giagulli, V.A.; Signori, C.; Zambotti, F.; Dall’Alda, M.; et al. Divergences in insulin resistance between the different phenotypes of the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2013, 98, E628–E637. [Google Scholar] [CrossRef]
- Dai, S.; Zhang, H.; Yang, F.; Shang, W.; Zeng, S. Effects of IGF-1 on the Three-Dimensional Culture of Ovarian Preantral Follicles and Superovulation Rates in Mice. Biology 2022, 11, 833. [Google Scholar] [CrossRef]
- Di Guardo, F.; Ciotta, L.; Monteleone, M.; Palumbo, M. Male Equivalent Polycystic Ovarian Syndrome: Hormonal, Metabolic, and Clinical Aspects. Int. J. Fertil. Steril. 2020, 14, 79–83. [Google Scholar] [CrossRef]
- Cannarella, R.; Condorelli, R.A.; Mongioì, L.M.; La Vignera, S.; Calogero, A.E. Does a male polycystic ovarian syndrome equivalent exist? J. Endocrinol. Investig. 2018, 41, 49–57. [Google Scholar] [CrossRef]
- Pkhaladze, L.; Russo, M.; Unfer, V.; Nordio, M.; Basciani, S.; Khomasuridze, A. Treatment of lean PCOS teenagers: A follow-up comparison between Myo-Inositol and oral contraceptives. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7476–7485. [Google Scholar] [CrossRef]
- Jin, P.; Xie, Y. Treatment strategies for women with polycystic ovary syndrome. Gynecol. Endocrinol. 2018, 34, 272–277. [Google Scholar] [CrossRef]
- Amiri, M.; Nahidi, F.; Bidhendi-Yarandi, R.; Khalili, D.; Tohidi, M.; Ramezani Tehrani, F. A comparison of the effects of oral contraceptives on the clinical and biochemical manifestations of polycystic ovary syndrome: A crossover randomized controlled trial. Hum. Reprod. 2020, 35, 175–186. [Google Scholar] [CrossRef]
- Kumar, Y.; Kotwal, N.; Singh, Y.; Upreti, V.; Somani, S.; Hari Kumar, K.V.S. A randomized, controlled trial comparing the metformin, oral contraceptive pills and their combination in patients with polycystic ovarian syndrome. J. Fam. Med. Prim. Care 2018, 7, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, S.; Ganie, M.A.; Amin, S.; Shah, Z.A.; Bhat, I.A.; Yousuf, S.D.; Jeelani, H.; Kawa, I.A.; Fatima, Q.; Rashid, F. Oral contraceptive use increases risk of inflammatory and coagulatory disorders in women with Polycystic Ovarian Syndrome: An observational study. Sci. Rep. 2019, 9, 10182. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Zhou, H.; Hu, M.; Feng, H. Effect of Diet on Insulin Resistance in Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2020, 105, 3346–3360. [Google Scholar] [CrossRef]
- Barrea, L.; Arnone, A.; Annunziata, G.; Muscogiuri, G.; Laudisio, D.; Salzano, C.; Pugliese, G.; Colao, A.; Savastano, S. Adherence to the Mediterranean Diet, Dietary Patterns and Body Composition in Women with Polycystic Ovary Syndrome (PCOS). Nutrients 2019, 11, 2278. [Google Scholar] [CrossRef]
- Mirabelli, M.; Chiefari, E.; Arcidiacono, B.; Corigliano, D.M.; Brunetti, F.S.; Maggisano, V.; Russo, D.; Foti, D.P.; Brunetti, A. Mediterranean Diet Nutrients to Turn the Tide against Insulin Resistance and Related Diseases. Nutrients 2020, 12, 1066. [Google Scholar] [CrossRef]
- Kite, C.; Lahart, I.M.; Afzal, I.; Broom, D.R.; Randeva, H.; Kyrou, I.; Brown, J.E. Exercise, or exercise and diet for the management of polycystic ovary syndrome: A systematic review and meta-analysis. Syst. Rev. 2019, 8, 51. [Google Scholar] [CrossRef]
- Szczuko, M.; Kikut, J.; Szczuko, U.; Szydłowska, I.; Nawrocka-Rutkowska, J.; Ziętek, M.; Verbanac, D.; Saso, L. Nutrition Strategy and Life Style in Polycystic Ovary Syndrome-Narrative Review. Nutrients 2021, 13, 2452. [Google Scholar] [CrossRef] [PubMed]
- Witchel, S.F.; Oberfield, S.E.; Peña, A.S. Polycystic Ovary Syndrome: Pathophysiology, Presentation, and Treatment With Emphasis on Adolescent Girls. J. Endocr. Soc. 2019, 3, 1545–1573. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.; Ma, Y.; Xiao, J.; Luo, G.; Li, Y.; Wu, D. Multi-system reproductive metabolic disorder: Significance for the pathogenesis and therapy of polycystic ovary syndrome (PCOS). Life Sci. 2019, 228, 167–175. [Google Scholar] [CrossRef]
- Yen, H.; Chang, Y.T.; Yee, F.J.; Huang, Y.C. Metformin Therapy for Acne in Patients with Polycystic Ovary Syndrome: A Systematic Review and Meta-analysis. Am. J. Clin. Dermatol. 2021, 22, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Reiser, E.; Lanbach, J.; Böttcher, B.; Toth, B. Non-Hormonal Treatment Options for Regulation of Menstrual Cycle in Adolescents with PCOS. J. Clin. Med. 2022, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Hanem, L.G.E.; Stridsklev, S.; Júlíusson, P.B.; Salvesen, Ø.; Roelants, M.; Carlsen, S.M.; Ødegård, R.; Vanky, E. Metformin Use in PCOS Pregnancies Increases the Risk of Offspring Overweight at 4 Years of Age: Follow-Up of Two RCTs. J. Clin. Endocrinol. Metab. 2018, 103, 1612–1621. [Google Scholar] [CrossRef]
- Luque-Ramírez, M.; Ortiz-Flores, A.E.; Nattero-Chávez, L.; Escobar-Morreale, H.F. A safety evaluation of current medications for adult women with the polycystic ovarian syndrome not pursuing pregnancy. Expert Opin. Drug Saf. 2020, 19, 1559–1576. [Google Scholar] [CrossRef]
- Laganà, A.S.; Forte, G.; Bizzarri, M.; Kamenov, Z.A.; Bianco, B.; Kaya, C.; Gitas, G.; Alkatout, I.; Terzic, M.; Unfer, V. Inositols in the ovaries: Activities and potential therapeutic applications. Expert Opin. Drug Metab. Toxicol. 2022, 18, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Kamenov, Z.; Gateva, A. Inositols in PCOS. Molecules 2020, 25, 5566. [Google Scholar] [CrossRef]
- Hernandez Marin, I.; Picconi, O.; Laganà, A.S.; Costabile, L.; Unfer, V. A multicenter clinical study with myo-inositol and alpha-lactalbumin in Mexican and Italian PCOS patients. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3316–3324. [Google Scholar] [CrossRef]
- Laganà, A.S.; Garzon, S.; Casarin, J.; Franchi, M.; Ghezzi, F. Inositol in Polycystic Ovary Syndrome: Restoring Fertility through a Pathophysiology-Based Approach. Trends Endocrinol. Metab. 2018, 29, 768–780. [Google Scholar] [CrossRef]
- Monastra, G.; Unfer, V.; Harrath, A.H.; Bizzarri, M. Combining treatment with myo-inositol and D-chiro-inositol (40:1) is effective in restoring ovary function and metabolic balance in PCOS patients. Gynecol. Endocrinol. 2017, 33, 1–9. [Google Scholar] [CrossRef]
- Benelli, E.; Del Ghianda, S.; Di Cosmo, C.; Tonacchera, M. A Combined Therapy with Myo-Inositol and D-Chiro-Inositol Improves Endocrine Parameters and Insulin Resistance in PCOS Young Overweight Women. Int. J. Endocrinol. 2016, 2016, 3204083. [Google Scholar] [CrossRef]
- Nordio, M.; Basciani, S.; Camajani, E. The 40:1 myo-inositol/D-chiro-inositol plasma ratio is able to restore ovulation in PCOS patients: Comparison with other ratios. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5512–5521. [Google Scholar] [CrossRef] [PubMed]
- Monastra, G.; Vucenik, I.; Harrath, A.H.; Alwasel, S.H.; Kamenov, Z.A.; Laganà, A.S.; Monti, N.; Fedeli, V.; Bizzarri, M. PCOS and Inositols: Controversial Results and Necessary Clarifications. Basic Differences Between D-Chiro and Myo-Inositol. Front. Endocrinol. 2021, 12, 660381. [Google Scholar] [CrossRef] [PubMed]
- Nestler, J.E.; Jakubowicz, D.J.; Reamer, P.; Gunn, R.D.; Allan, G. Ovulatory and metabolic effects of D-chiro-inositol in the polycystic ovary syndrome. N. Engl. J. Med. 1999, 340, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Cheang, K.I.; Baillargeon, J.P.; Essah, P.A.; Ostlund, R.E., Jr.; Apridonize, T.; Islam, L.; Nestler, J.E. Insulin-stimulated release of D-chiro-inositol-containing inositolphosphoglycan mediator correlates with insulin sensitivity in women with polycystic ovary syndrome. Metabolism 2008, 57, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Nordio, M.; Bezerra Espinola, M.S.; Bilotta, G.; Capoccia, E.; Montanino Oliva, M. Long-Lasting Therapies with High Doses of D-chiro-inositol: The Downside. J. Clin. Med. 2023, 12, 390. [Google Scholar] [CrossRef] [PubMed]
- Palomba, S. Is fertility reduced in ovulatory women with polycystic ovary syndrome? An opinion paper. Hum Reprod 2021, 36, 2421–2428. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Forte, G.; Montanino Oliva, M.; Laganà, A.S.; Unfer, V. Melatonin and Myo-Inositol: Supporting Reproduction from the Oocyte to Birth. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Palomba, S.; Daolio, J.; La Sala, G.B. Oocyte Competence in Women with Polycystic Ovary Syndrome. Trends Endocrinol Metab 2017, 28, 186–198. [Google Scholar] [CrossRef]
- Palomba, S.; Piltonen, T.T.; Giudice, L.C. Endometrial function in women with polycystic ovary syndrome: A comprehensive review. Hum Reprod Update 2021, 27, 584–618. [Google Scholar] [CrossRef]
- Palomba, S.; de Wilde, M.A.; Falbo, A.; Koster, M.P.; La Sala, G.B.; Fauser, B.C. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update 2015, 21, 575–592. [Google Scholar] [CrossRef]
- Montanino Oliva, M.; Buonomo, G.; Calcagno, M.; Unfer, V. Effects of myo-inositol plus alpha-lactalbumin in myo-inositol-resistant PCOS women. J. Ovarian Res. 2018, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Unfer, V.; Dewailly, D.; Kamenov, Z.A.; Diamanti-Kandarakis, E.; Laganà, A.S.; Nestler, J.E.; Soulage, C.O. Inositols in Polycystic Ovary Syndrome: An Overview on the Advances. Trends Endocrinol. Metab. 2020, 31, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, G.; Kandaraki, E.A.; Garidou, A.; Koutsaki, M.; Papalou, O.; Diamanti-Kandarakis, E.; Peppa, M. Tailoring treatment for PCOS phenotypes. Expert Rev. Endocrinol. Metab. 2021, 16, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Conway, G.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Franks, S.; Gambineri, A.; Kelestimur, F.; Macut, D.; Micic, D.; Pasquali, R.; et al. The polycystic ovary syndrome: A position statement from the European Society of Endocrinology. Eur. J. Endocrinol. 2014, 171, P1–P29. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unfer, V.; Dinicola, S.; Russo, M. A PCOS Paradox: Does Inositol Therapy Find a Rationale in All the Different Phenotypes? Int. J. Mol. Sci. 2023, 24, 6213. https://doi.org/10.3390/ijms24076213
Unfer V, Dinicola S, Russo M. A PCOS Paradox: Does Inositol Therapy Find a Rationale in All the Different Phenotypes? International Journal of Molecular Sciences. 2023; 24(7):6213. https://doi.org/10.3390/ijms24076213
Chicago/Turabian StyleUnfer, Vittorio, Simona Dinicola, and Michele Russo. 2023. "A PCOS Paradox: Does Inositol Therapy Find a Rationale in All the Different Phenotypes?" International Journal of Molecular Sciences 24, no. 7: 6213. https://doi.org/10.3390/ijms24076213
APA StyleUnfer, V., Dinicola, S., & Russo, M. (2023). A PCOS Paradox: Does Inositol Therapy Find a Rationale in All the Different Phenotypes? International Journal of Molecular Sciences, 24(7), 6213. https://doi.org/10.3390/ijms24076213






