CcNAC1 by Transcriptome Analysis Is Involved in Sudan Grass Secondary Cell Wall Formation as a Positive Regulator
Abstract
1. Introduction
2. Results
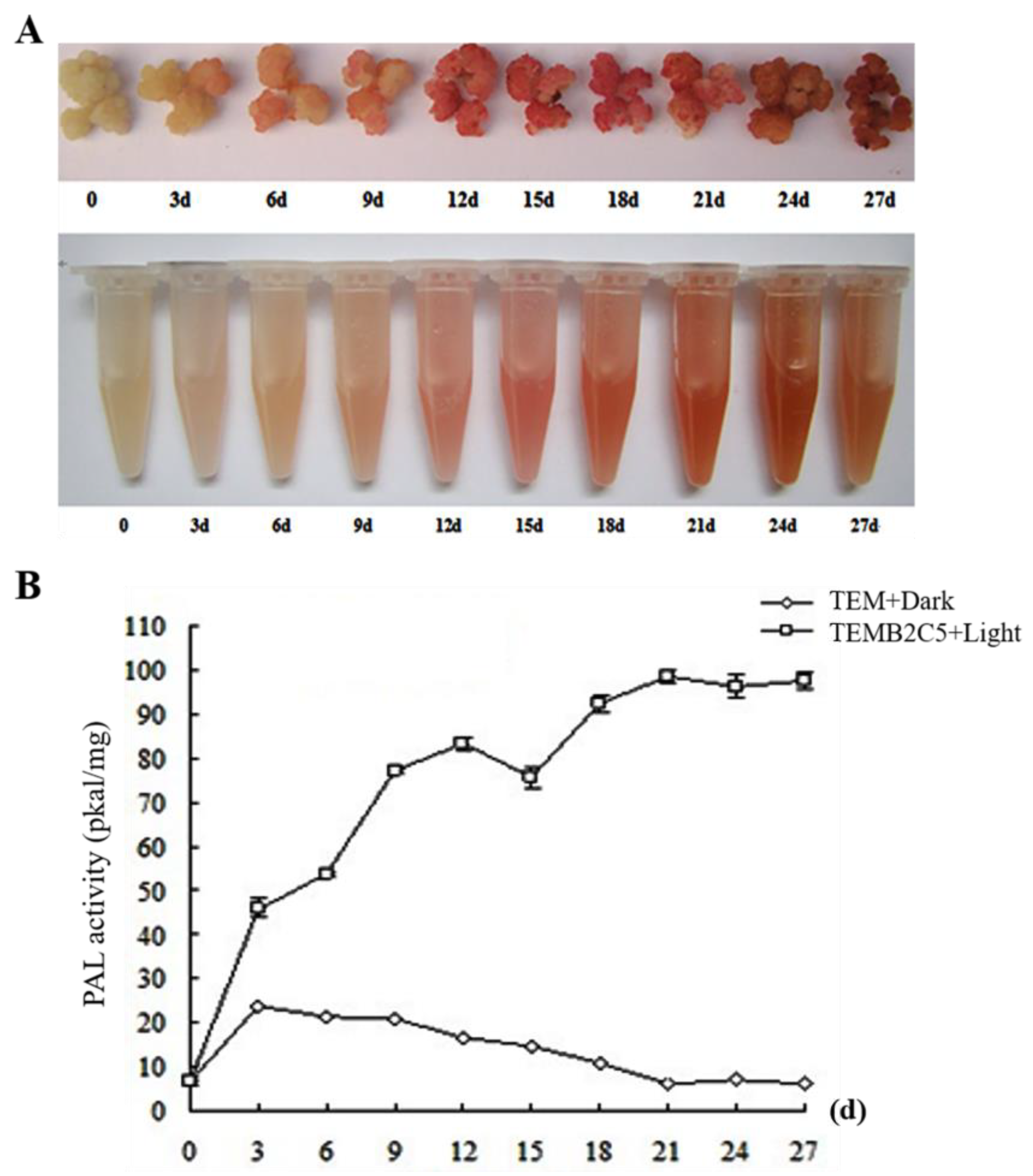
2.1. Induction of Secondary Cell Wall Establishment by Sudan Grass In Vitro
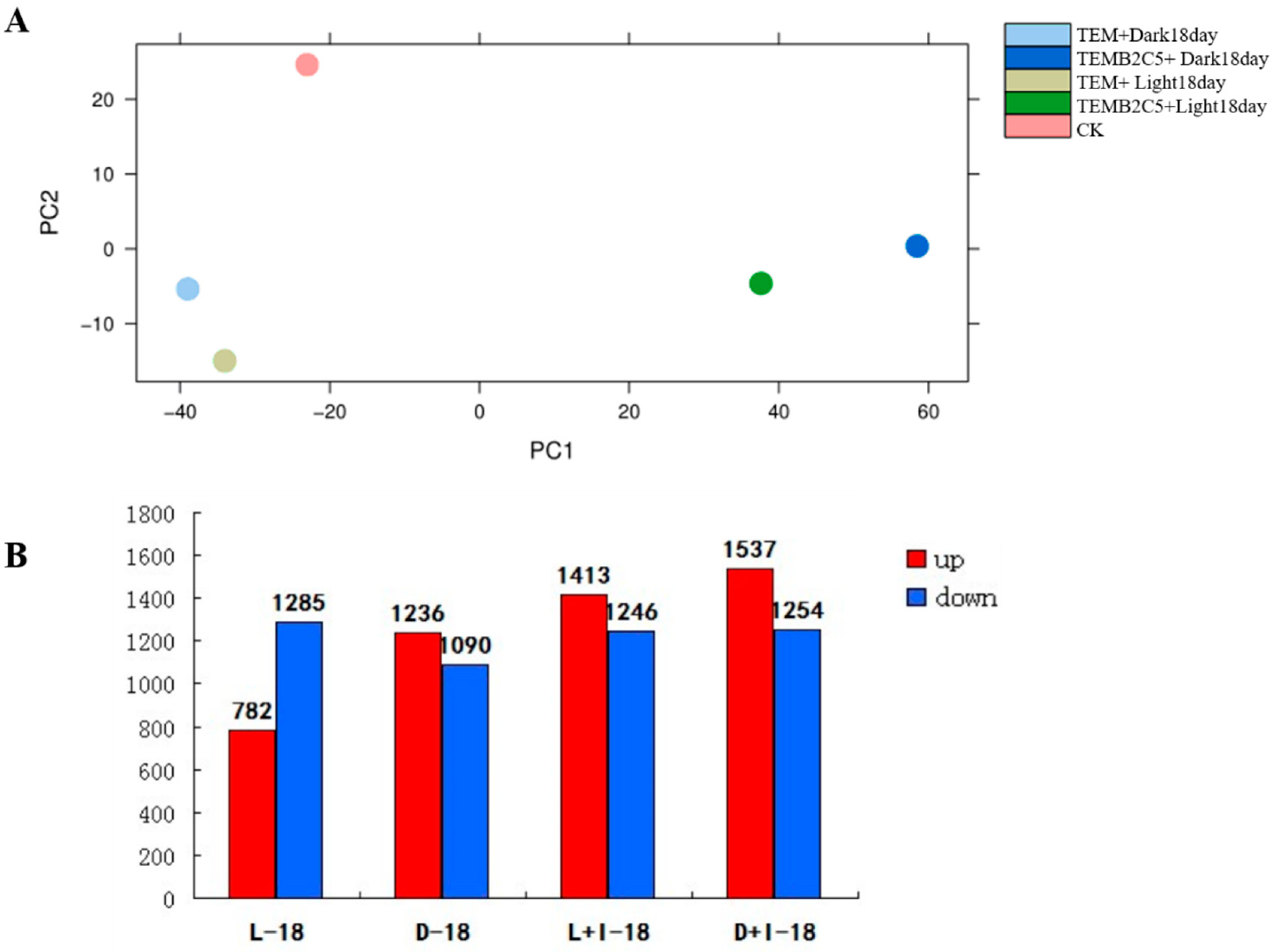
2.2. Transcriptome of Secondary Cell Wall Synthesis of Sudan Grass
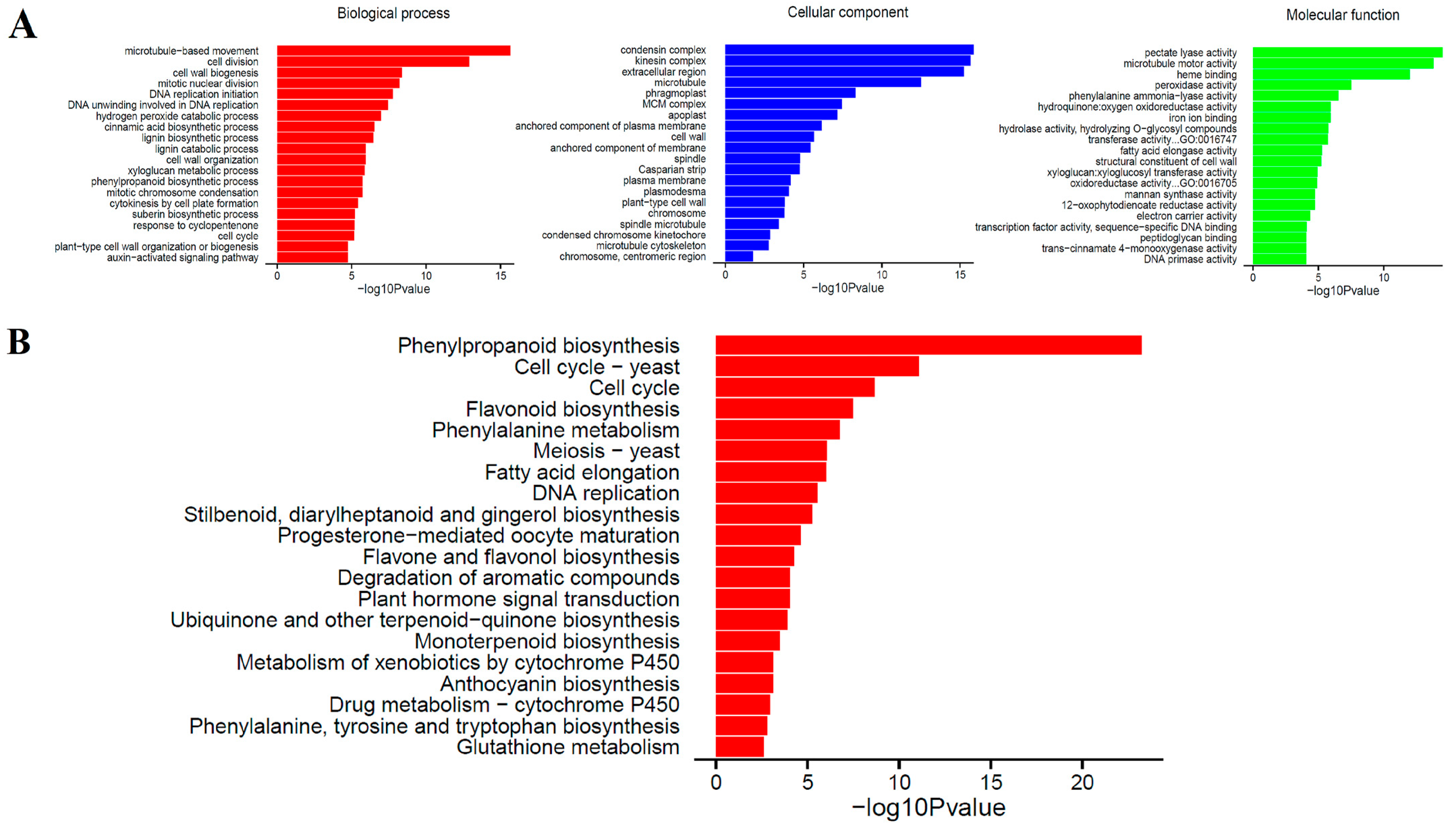
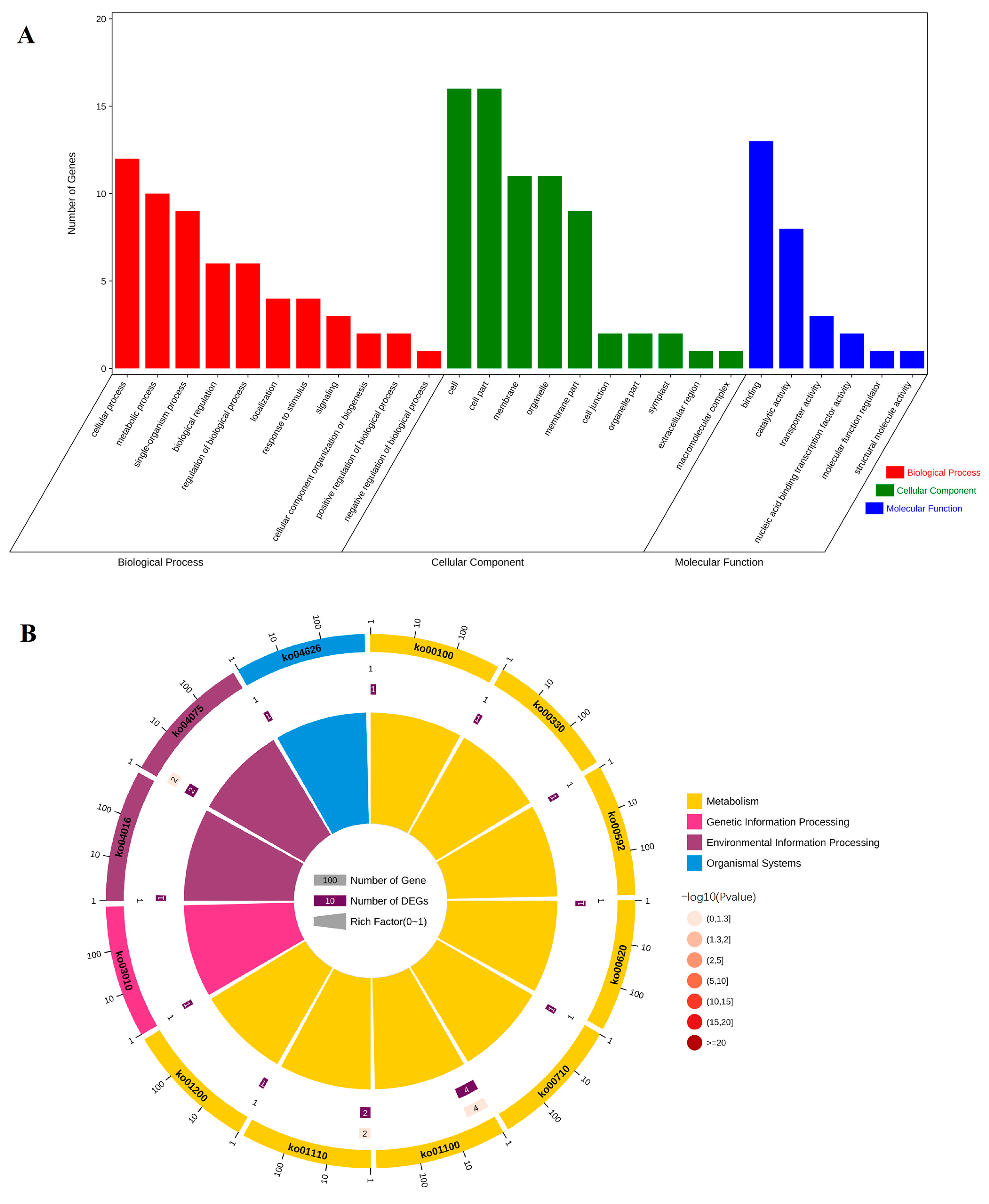
2.3. Functional Classification of Unigene of Secondary Cell Wall Synthesis of Sudan Grass
2.4. Analysis of CcNACs Family Genes Related to Secondary Cell Wall Synthesis
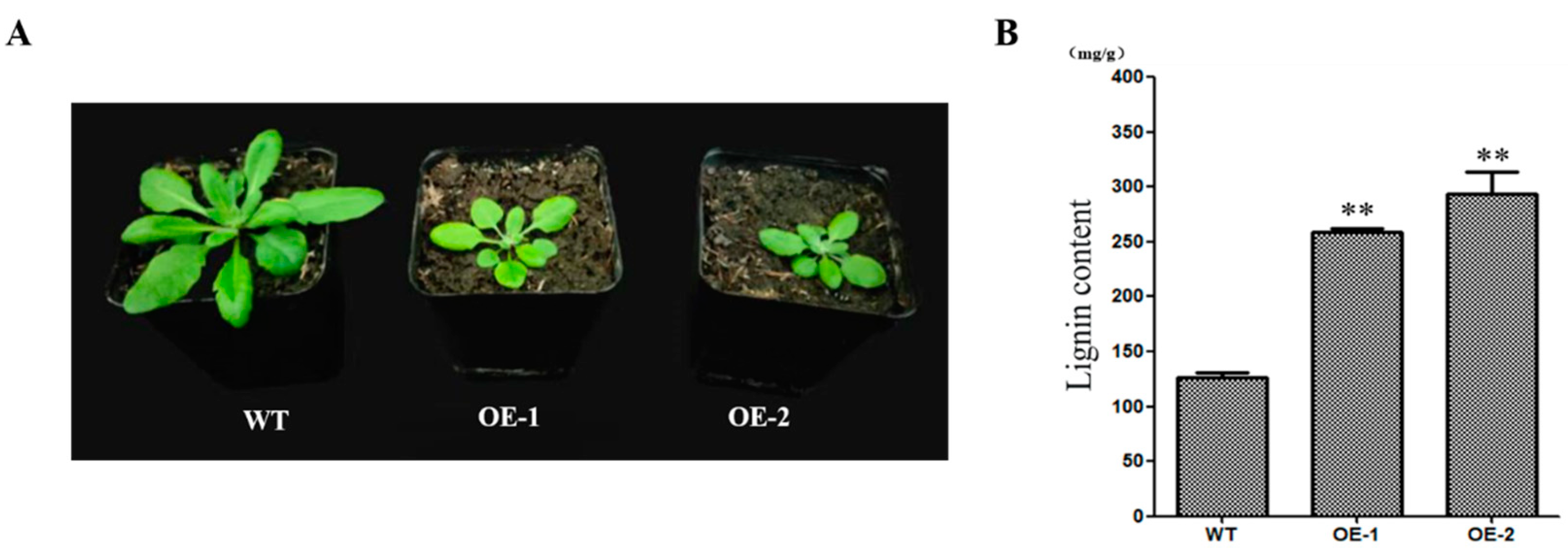
2.5. CcNAC1 Increases the Lignin Content of Plants
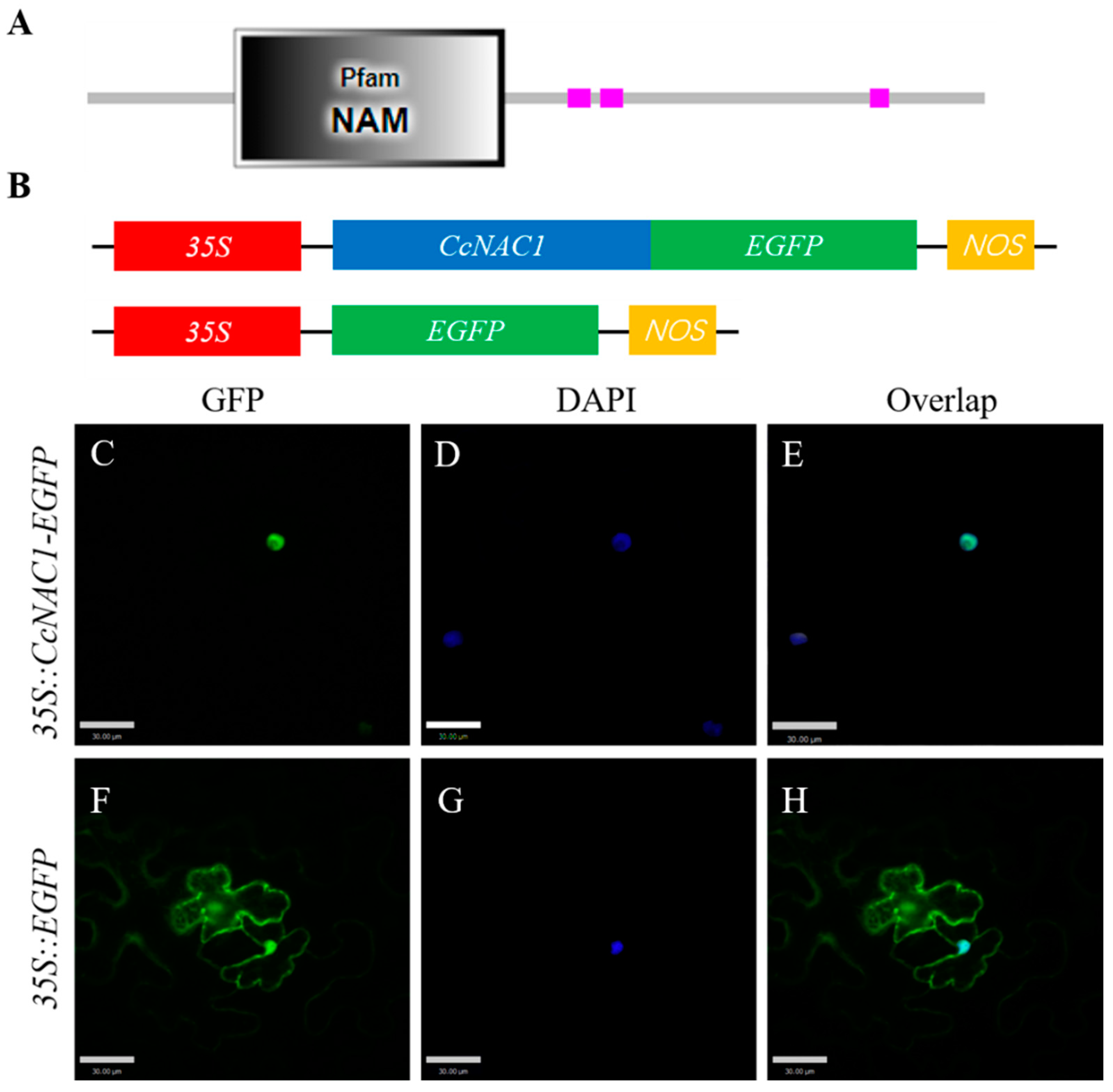
2.6. CcNAC1 Encodes a Nuclear Localization Protein
2.7. Candidate Interaction Proteins Analysis of CcNAC1
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Lignin Dyeing and Content Measurement Method
4.3. Phenylalanine Ammonia-Lyase (PAL) Activity Measurement Method
4.4. Transcriptome Sequencing and Analyses
4.5. Functional Classification of DEGs
4.6. Quantitative Real-Time PCR (qRT–PCR) Verification
4.7. Subcellular Localisation of CcNAC1 Protein
4.8. CcNAC1 Overexpression in Arabidopsis
4.9. Yeast Two-Hybrid Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kanani, J.; Lukefahr, S.D.; Stanko, R.L. Evaluation of tropical forage legumes (Medicago sativa, Dolichos lablab, Leucaena leucocephala and Desmanthus bicornutus) for growing goats. Small Rumin. Res. 2006, 65, 1–7. [Google Scholar] [CrossRef]
- Wolff, M.S.; Elliso, J.M.; Hao, Y.; Cockrum, R.R.; Austin, J.K.; Baraboo, M.; Burch, K.; Lee, J.H.; Maurer, T.; Patil, R.; et al. Diet shifts provoke complex and variable changes in the metabolic networks of the ruminal microbiome. Microbiome 2017, 5, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Duan, A.; Yang, X.; Feng, K.; Liu, J.; Xu, Z.; Xiong, A. Genome-wide analysis of NAC transcription factors and their response to abiotic stress in celery (Apium graveolens L.). Comput. Biol. Chem. 2020, 84, 107186. [Google Scholar] [CrossRef]
- Huang, C.; Li, L. Understanding of plant cell wall biosynthesis for utilization of lignocellulosic biomass resources. Chin. Sci. Bull. 2016, 61, 3623–3629. [Google Scholar] [CrossRef]
- Wang, H.; Dixon, R.A. On-off switches for secondary cell wall biosynthesis. Mol. Plant. 2012, 5, 297–303. [Google Scholar] [CrossRef]
- Zhong, R.; Ye, Z. Secondary cell walls: Biosynthesis, patterned deposition and transcriptional regulation. Plant Cell Physiol. 2015, 56, 195–214. [Google Scholar] [CrossRef]
- Nakano, Y.; Yamaguchi, M.; Endo, H.; Rejab, N.A.; Ohtani, M. NAC-MYB-based transcriptional regulation of secondary cell wall biosynthesis in land plants. Front. Plant Sci. 2015, 6, 288–306. [Google Scholar] [CrossRef]
- Kubo, M.; Udagawa, M.; Nishikubo, N.; Horiguchi, G.; Yamaguchi, M.; Ito, J.; Mimura, T.; Fukuda, H.; Demura, T. Transcription switches for protoxylem and metaxylem vessel formation. Gene Dev. 2005, 19, 1855–1861. [Google Scholar] [CrossRef]
- Wang, X.; Wang, D.; Xu, W.; Kong, L.; Ye, X.; Zhuang, Q.; Fan, D.; Luo, K. Histone methyltransferase ATX1 dynamically regulates fiber secondary cell wall biosynthesis in Arabidopsis inflorescence stem. Nucleic Acids Res. 2021, 49, 190–205. [Google Scholar] [CrossRef]
- Zhou, J.; Zhong, R.; Ye, Z. Arabidopsis NAC domain proteins, VND1 to VND5, are transcriptional regulators of secondary wall biosynthesis in vessels. PLoS ONE 2014, 9, e105726. [Google Scholar] [CrossRef]
- Ruonala, R.; Ko, D.; Helariutta, Y. Genetic networks in plant vascular development. Annu. Rev. Genet. 2017, 51, 335–359. [Google Scholar] [CrossRef]
- Ochando, I.; Gonzalez-Reig, S.; Ripoll, J.J.; Vera, A.; Martinez-Laborda, A. Alteration of the shoot radial pattern in Arabidopsis thaliana by a gain-of-function allele of the class Ⅲ HD-Zip gene INCURVATA4. Int. J. Dev. Biol. 2008, 52, 953–961. [Google Scholar] [CrossRef]
- Ohashi-Ito, K.; Iwamoto, K.; Fukuda, H. LOB DOMAINCONTAINING PROTEIN 15 positively regulates expression of VND7, a master regulator of tracheary elements. Plant Cell Physiol. 2018, 59, 989–996. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Ohtani, M.; Mitsuda, N.; Kubo, M.; Ohme-Takagi, M.; Fukuda, H.; Demura, T. VND-INTERACTING2, a NAC domain transcription factor, negatively regulates xylem vessel formation in Arabidopsis. Plant Cell 2010, 22, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Negi, S.; Tak, H.; Ganapathi, T.R. Xylem specific activation of 5′ upstream regulatory region of two NAC transcription factors (MusaVND6 and MusaVND7) in banana is regulated by SNBE-like sites. PLoS ONE 2018, 13, e0192852. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Demura, T.; Ye, Z. SND1, a NAC domain transcription factor, is a key regulator of secondary wall synthesis in fibers of Arabidopsis. Plant Cell 2006, 18, 3158–3170. [Google Scholar] [CrossRef] [PubMed]
- Mitsuda, N.; Iwase, A.; Yamamoto, H.; Yoshida, M.; Seki, M.; Shinozaki, K.; Ohme-Takagi, M. NAC transcription factors, NST1 and NST3, are key regulators of the formation of secondary walls in woody tissues of Arabidopsis. Plant Cell 2007, 19, 270–280. [Google Scholar] [CrossRef]
- Zhong, R.; Lee, C.; Ye, Z.H. Evolutionary conservation of the transcriptional network regulating secondary cell wall biosynthesis. Trends Plant Sci. 2021, 15, 625–632. [Google Scholar] [CrossRef]
- Kim, W.; Ko, J.; Kim, J.; Kim, J.; Bae, H.; Han, K. MYB46 directly regulates the gene expression of secondary wall-associated cellulose synthases in Arabidopsis. Plant J. 2013, 73, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Patzlaff, A.; McInnis, S.; Courtenay, A.; Surman, C.; Newman, L.J.; Smith, C.; Bevan, M.W.; Mansfield, S.; Whetten, R.W.; Sederoff, R.R.; et al. Characterisation of a pine MYB that regulates lignification. Plant J. 2003, 36, 743–754. [Google Scholar] [CrossRef]
- Goicoechea, M.; Lacombe, E.; Legay, S.; Mihaljevic, S.; Rech, P.; Jauneau, A.; Lapierre, C.; Pollet, B.; Verhaegen, D.; Chaubet-Gigot, N.; et al. EgMYB2, a new transcriptional activator from eucalyptus xylem, regulates secondary cell. Plant J. 2005, 43, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Geng, P.; Zhang, S.; Liu, J.; Zhao, C.; Wu, J.; Cao, Y.; Fu, C.; Han, X.; He, H.; Zhao, Q. MYB20, MYB42, MYB43, and MYB85 regulate phenylalanine and lignin biosynthesis during secondary cell wall formation. Plant Physiol. 2020, 182, 1272–1283. [Google Scholar] [CrossRef]
- McCarthy, R.L.; Zhong, R.; Ye, Z. MYB83 is a direct target of SND1 and Acts redundantly with MYB46 in the regulation of secondary cell wall biosynthesis in Arabidopsis. Plant Cell Physiol. 2009, 50, 1950–1964. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Lee, C.; Zhou, J.; McCarthy, L.R.; Ye, Z. A battery of transcription factors involved in the regulation of secondary cell wall biosynthesis in Arabidopsis. Plant Cell 2008, 20, 2763–2782. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Lee, C.; Zhong, R.; Ye, Z. MYB58 and MYB63 are transcriptional activators of the lignin biosynthetic pathway during secondary cell wall formation in Arabidopsis. Plant Cell 2009, 21, 248–266. [Google Scholar] [CrossRef]
- Xuan, H.; Huang, Y.; Zhou, L.; Deng, S.; Wang, C.; Xu, J.; Wang, H.; Zhao, J.; Guo, N.; Xing, H. Key Soybean Seedlings Drought-Responsive Genes and Pathways Revealed by Comparative Transcriptome Analyses of Two Cultivars. Int. J. Mol. Sci. 2022, 23, 2893. [Google Scholar] [CrossRef]
- Kim, M.H.; Tran, T.N.A.; Cho, J.S.; Park, E.J.; Lee, H.; Kim, D.G.; Hwang, S.; Ko, J.H. Wood transcriptome analysis of Pinus densiflora identifies genes critical for secondary cell wall formation and NAC transcription factors involved in tracheid formation. Tree Physiol. 2021, 4, 1289–1305. [Google Scholar] [CrossRef]
- Wong, M.M.; Cannon, C.H.; Wickneswari, R. Identification of lignin genes and regulatory sequences involved in secondary cell wall formation in Acacia auriculiformis and Acacia mangium via de novo transcriptome sequencing. BMC Genom. 2011, 12, 342–355. [Google Scholar] [CrossRef]
- Endo, H.; Yamaguchi, M.; Tamura, T.; Nakano, Y.; Nishikubo, N.; Yoneda, A.; Kato, K.; Kubo, M.; Kajita, S.; Katayama, Y. Multiple classes of transcription factors regulate the expression of VASCULAR-RELATED NAC-DOMAIN7, a master switch of xylem vessel differentiation. Plant Cell Physiol. 2015, 56, 242–254. [Google Scholar] [CrossRef]
- Doley, D. Effects of Shade on Xylem Development in Seedings of Eucalyptus Grandis Hill EX.Maiden. New Phytol. 2010, 82, 545–555. [Google Scholar] [CrossRef]
- Liu, J.; Meng, Y.; Lv, F.; Chen, J.; Ma, Y.; Wang, Y.; Chen, B.; Zhang, L.; Zhou, Z. Photosynthetic characteristics of the subtending leaf of cotton boll at different fruiting branch nodes and their relationships with lint yield and fiber quality. Front Plant Sci. 2015, 6, 747–757. [Google Scholar] [CrossRef]
- Steffens, B.; Rasmussen, A. The Physiology of Adventitious Roots. Plant Physiol. 2016, 170, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xie, Z.; Zhang, R.; Xu, P.; Liu, H.; Yang, H.; Doblin, M.S.; Bacic, A.; Li, L. Blue Light Regulates Secondary Cell Wall Thickening via MYC2/MYC4 Activation of the NST1-Directed Transcriptional Network in Arabidopsis. Plant Cell. 2018, 30, 2512–2528. [Google Scholar] [CrossRef] [PubMed]
- Fagard, M.; Desnos, T.; Desprez, T.; Goubet, F.; Refregier, G.; Mouille, G.; McCann, M.; Rayon, C.; Vernhettes, S.; Höfte, H. PROCUSTE1 encodes a cellulose synthase required for normal cell elongation specifically in roots and dark-grown hypocotyls of Arabidopsis. Plant Cell 2000, 12, 2409–2423. [Google Scholar] [CrossRef]
- Chen, S.; Sun, W.; Xiong, Y.; Jiang, Y.; Liu, X.; Liao, X.; Zhang, D.; Jiang, S.; Li, Y.; Liu, B.; et al. The Phoebe genome sheds light on the evolution of magnoliids. Hortic Res. 2020, 7, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Nagata, N.; Asami, T.; Yoshida, S. Brassinazole, an inhibitor of brassinosteroid biosynthesis, inhibits development of secondary xylem in cress plants (Lepidium sativum). Plant Cell Physiol. 2001, 42, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Fujioka, S.; Iwamoto, K.; Demura, T.; Takatsuto, S.; Yoshida, S.; Fukuda, H. Co-regulation of brassinosteroid biosynthesis-related genes during xylem cell differentiation. Plant Cell Physiol. 2007, 48, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, N.; Geng, Z.; Ji, M.; Wang, S.; Zhuang, Y.; Wang, D.; He, G.; Zhao, S.; Zhou, G.; et al. Integrated transcriptome and proteome analysis reveals brassinosteroid-mediated regulation of cambium initiation and patterning in woody stem. Hortic. Res. 2022, 9, uhab048. [Google Scholar] [CrossRef]
- Jia, K.; Wang, W.; Zhang, Q.; Jia, W. Cell Wall Integrity Signaling in Fruit Ripening. Int. J. Mol. Sci. 2023, 24, 4054. [Google Scholar] [CrossRef]
- Hossain, Z.; McGarvey, B.; Amyot, L.; Gruber, M.; Jung, J.; Hannoufa, A. DIMINUTO 1 affects the lignin profile and secondary cell wall formation in Arabidopsis. Planta 2012, 235, 485–498. [Google Scholar] [CrossRef]
- Du, J.; Gerttula, S.; Li, Z.; Zhao, S.; Liu, Y.; Liu, Y.; Lu, M.; Groover, A.T. Brassinosteroid regulation of wood formation in poplar. New Phytol. 2020, 225, 1516–1530. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, L.; Zola, J.; Aluru, M.; Ye, H.; Foudree, A.; Guo, H.; Anderson, S.; Aluru, S.; Liu, P.; et al. A brassinosteroid transcriptional network revealed by genome wide identification of BESI target genes in Arabidopsis thaliana. Plant J. 2011, 65, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Yang, C.; Wang, X. Brassinosteroids can regulate cellulose biosynthesis by controlling the expression of CESA genes in Arabidopsis. J. Exp. Bot. 2011, 62, 4495–4506. [Google Scholar] [CrossRef]
- Dong, N.; Lin, H. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef] [PubMed]
- Matus, T.; Loyola, R.; Vega, A.; Peña-Neira, A.; Bordeu, E.; Arce-Johnson, P.; Alcalde, A. Post-veraison sunlight exposure induces MYB-mediated transcriptional regulation of anthocyanin and flavonol synthesis in berry skins of Vitis vinifera. J. Exp. Bot. 2009, 60, 853–867. [Google Scholar] [CrossRef]
- Pandey, A.; Bhushan, A. Ultraviolet-B radiation: A potent regulator of flavonoids biosynthesis, accumulation and functions in plants. Curr. Sci. India 2020, 119, 176–185. [Google Scholar] [CrossRef]
- Wang, Y.; Chantreau, M.; Sibout, R.; Hawkins, S. Plant cell wall lignification and monolignol metabolism. Front Plant Sci. 2013, 4, 220–234. [Google Scholar] [CrossRef]
- Chen, Q.; Man, C.; Li, D.; Tan, H.; Xie, Y.; Huang, J. Arogenate Dehydratase Isoforms Differentially Regulate Anthocyanin Biosynthesis in Arabidopsis thaliana. Mol. Plant 2016, 9, 1609–1619. [Google Scholar] [CrossRef]
- Lee, M.H.; Jeon, H.S.; Kim, S.H.; Chung, J.H.; Roppolo, D.; Lee, H.J.; Cho, H.J.; Tobimatsu, Y.; Ralph, J.; Park, O.K. Lignin-based barrier restricts pathogens to the infection site and confers resistance in plants. EMBO J. 2019, 38, e101948. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.; Zhao, Z.; Ma, D.; Zhang, J.; Yang, Y.; Liu, Y.; Liu, H. COR27 and COR28 are Novel Regulators of the COP1–HY5 Regulatory Hub and Photomorphogenesis in Arabidopsis. Plant Cell 2020, 32, 3139–3154. [Google Scholar] [CrossRef]
- Sun, Y.; Han, Z.; Tang, J.; Hu, Z.; Chai, C.; Zhou, B.; Chai, J. Structure reveals that BAK1 as a co-receptor recognizes the BRI1-bound brassinolide. Cell Res. 2013, 23, 1326–1329. [Google Scholar] [CrossRef]
- Cheng, L.; Zhao, C.; Zhao, M.; Han, Y.; Li, S. Lignin Synthesis, Affected by Sucrose in Lotus (Nelumbo nucifera) Seedlings, Was Involved in Regulation of Root Formation in the Arabidopsis thanliana. Int. J. Mol. Sci. 2022, 23, 2250. [Google Scholar] [CrossRef]
- Lv, Z.; Zhou, D.; Shi, X.; Ren, J.; Zhang, H.; Zhong, C.; Kang, S.; Zhao, X.; Yu, H.; Wang, C. Comparative Multi-Omics Analysis Reveals Lignin Accumulation Affects Peanut Pod Size. Int. J. Mol. Sci. 2022, 23, 13533. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, C.; Liu, W.; Ma, Y.; Dong, L.; Tian, J.; Yu, Y.; Kong, Z. Augmin Antagonizes Katanin at Microtubule Crossovers to Control the Dynamic Organization of Plant Cortical Arrays. Curr. Biol. 2018, 28, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Liu, H.; Li, X.; Xiao, J.; Wang, S. Multiple phytohormones and phytoalexins are involved in disease resistance to Magnaporthe oryzae invaded from roots in rice. Physiol. Plant 2014, 152, 486–500. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xue, C.; Xue, D.; Zhao, J.; Gai, J.; Guo, N.; Xing, H. Overexpression of GmHsp90s, a heat shock protein 90 (Hsp90) gene family cloning from soybean, decrease damage of abiotic stresses in Arabidopsis thaliana. PLoS ONE 2013, 8, e69810. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Qian, C.; Lin, J.; Antwi-Boasiako, A.; Wu, J.; Liu, Z.; Mao, Z.; Zhong, X. CcNAC1 by Transcriptome Analysis Is Involved in Sudan Grass Secondary Cell Wall Formation as a Positive Regulator. Int. J. Mol. Sci. 2023, 24, 6149. https://doi.org/10.3390/ijms24076149
Huang Y, Qian C, Lin J, Antwi-Boasiako A, Wu J, Liu Z, Mao Z, Zhong X. CcNAC1 by Transcriptome Analysis Is Involved in Sudan Grass Secondary Cell Wall Formation as a Positive Regulator. International Journal of Molecular Sciences. 2023; 24(7):6149. https://doi.org/10.3390/ijms24076149
Chicago/Turabian StyleHuang, Yanzhong, Chen Qian, Jianyu Lin, Augustine Antwi-Boasiako, Juanzi Wu, Zhiwei Liu, Zhengfeng Mao, and Xiaoxian Zhong. 2023. "CcNAC1 by Transcriptome Analysis Is Involved in Sudan Grass Secondary Cell Wall Formation as a Positive Regulator" International Journal of Molecular Sciences 24, no. 7: 6149. https://doi.org/10.3390/ijms24076149
APA StyleHuang, Y., Qian, C., Lin, J., Antwi-Boasiako, A., Wu, J., Liu, Z., Mao, Z., & Zhong, X. (2023). CcNAC1 by Transcriptome Analysis Is Involved in Sudan Grass Secondary Cell Wall Formation as a Positive Regulator. International Journal of Molecular Sciences, 24(7), 6149. https://doi.org/10.3390/ijms24076149




