Priming Potato Plants with Melatonin Protects Stolon Formation under Delayed Salt Stress by Maintaining the Photochemical Function of Photosystem II, Ionic Homeostasis and Activating the Antioxidant System
Abstract
1. Introduction
2. Results
2.1. The Growth Parameters of Potato Plants
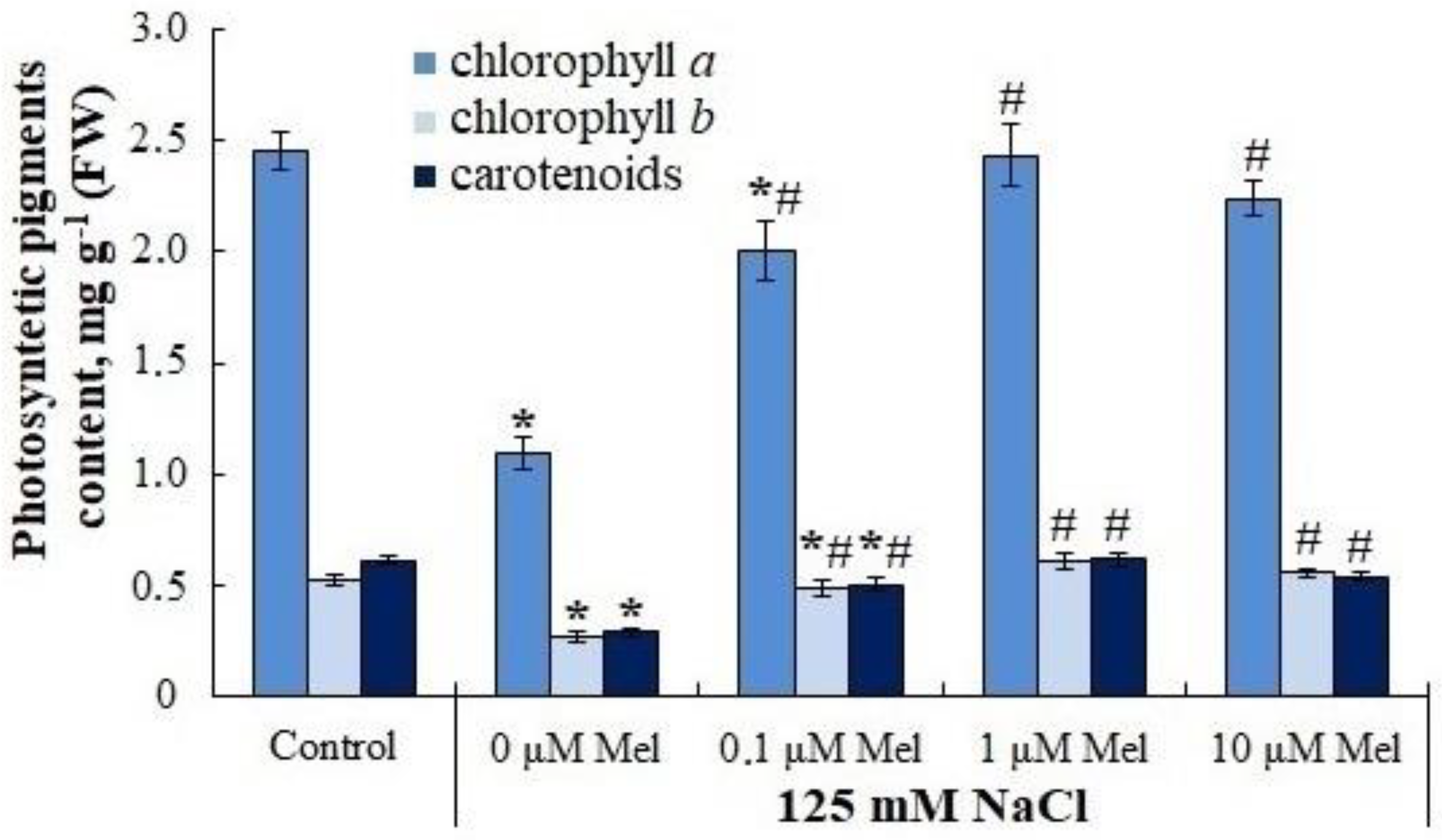
2.2. The Content of Photosynthetic Pigments in Potato Leaves
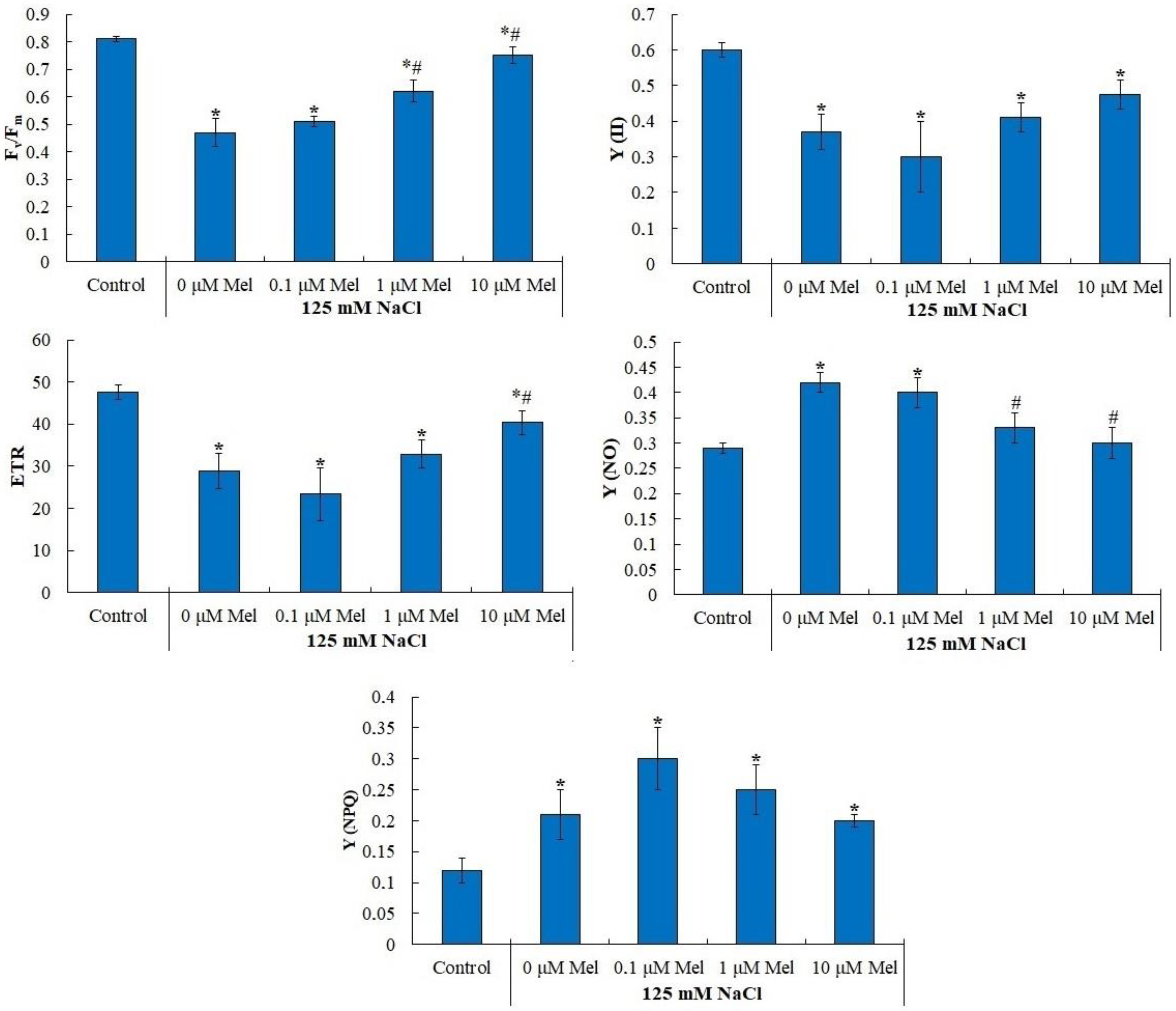
2.3. Primary Photosynthetic Processes in Potato Leaves
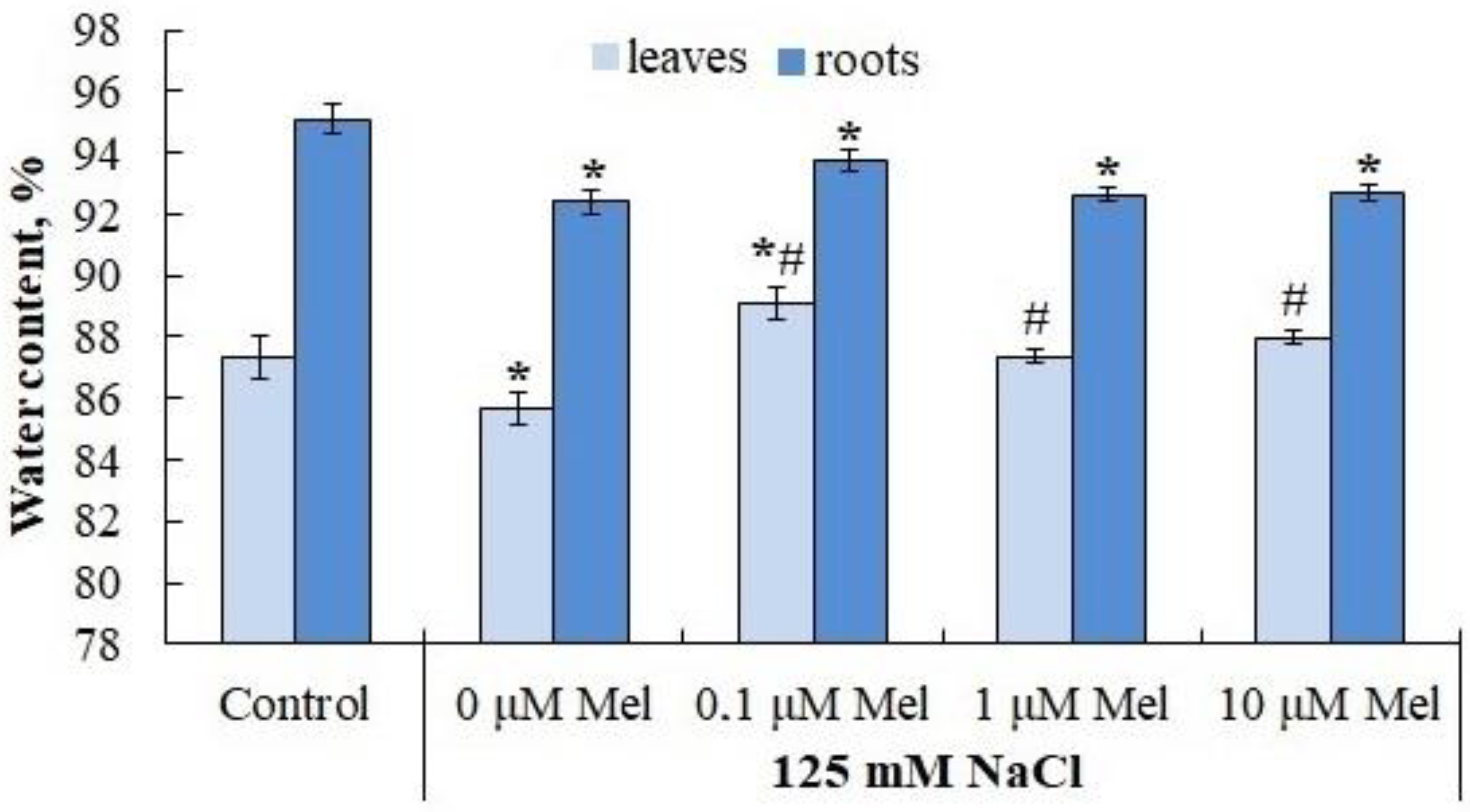
2.4. Water Content and Leaf Cell Sap Osmotic Potential
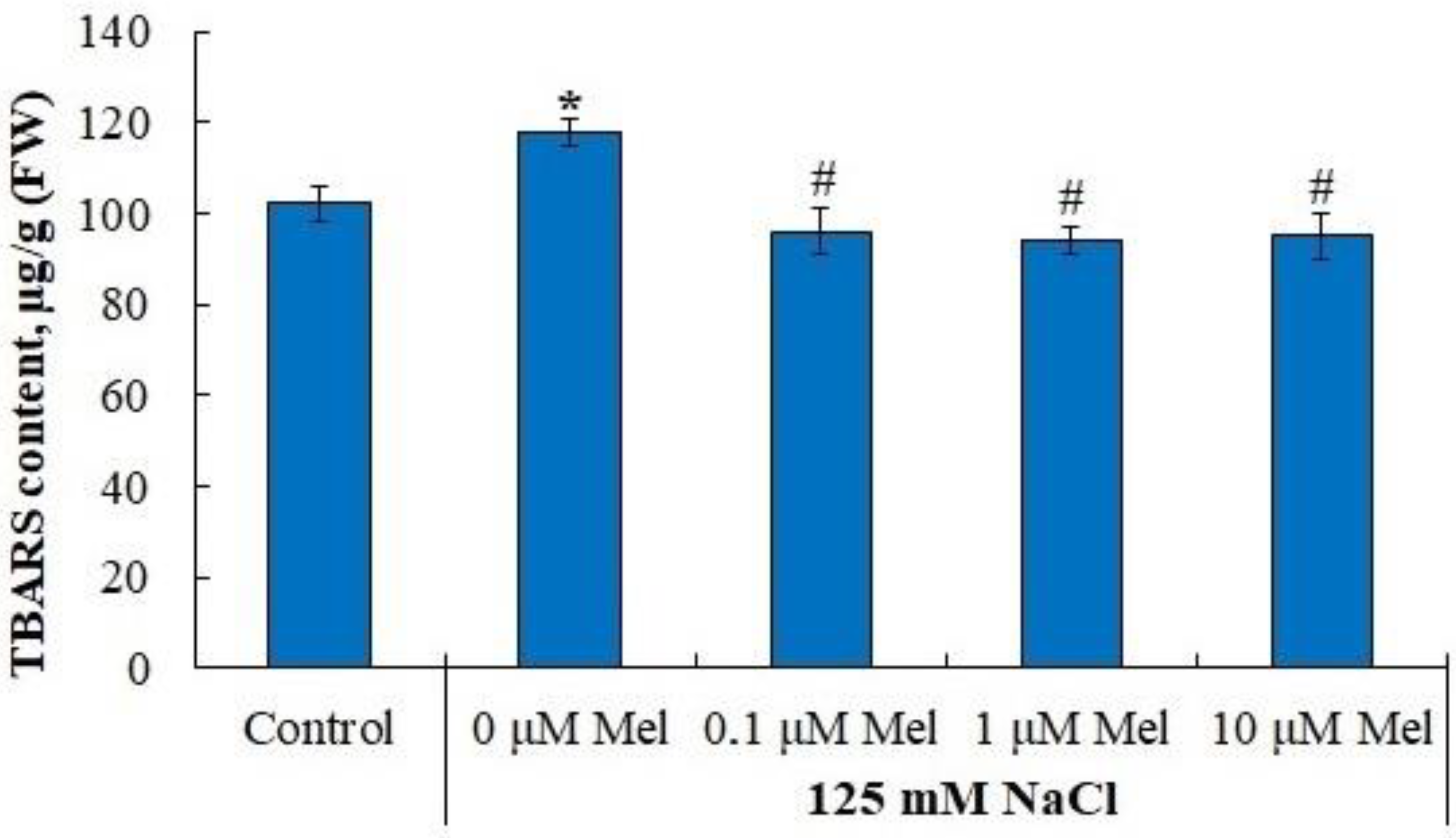
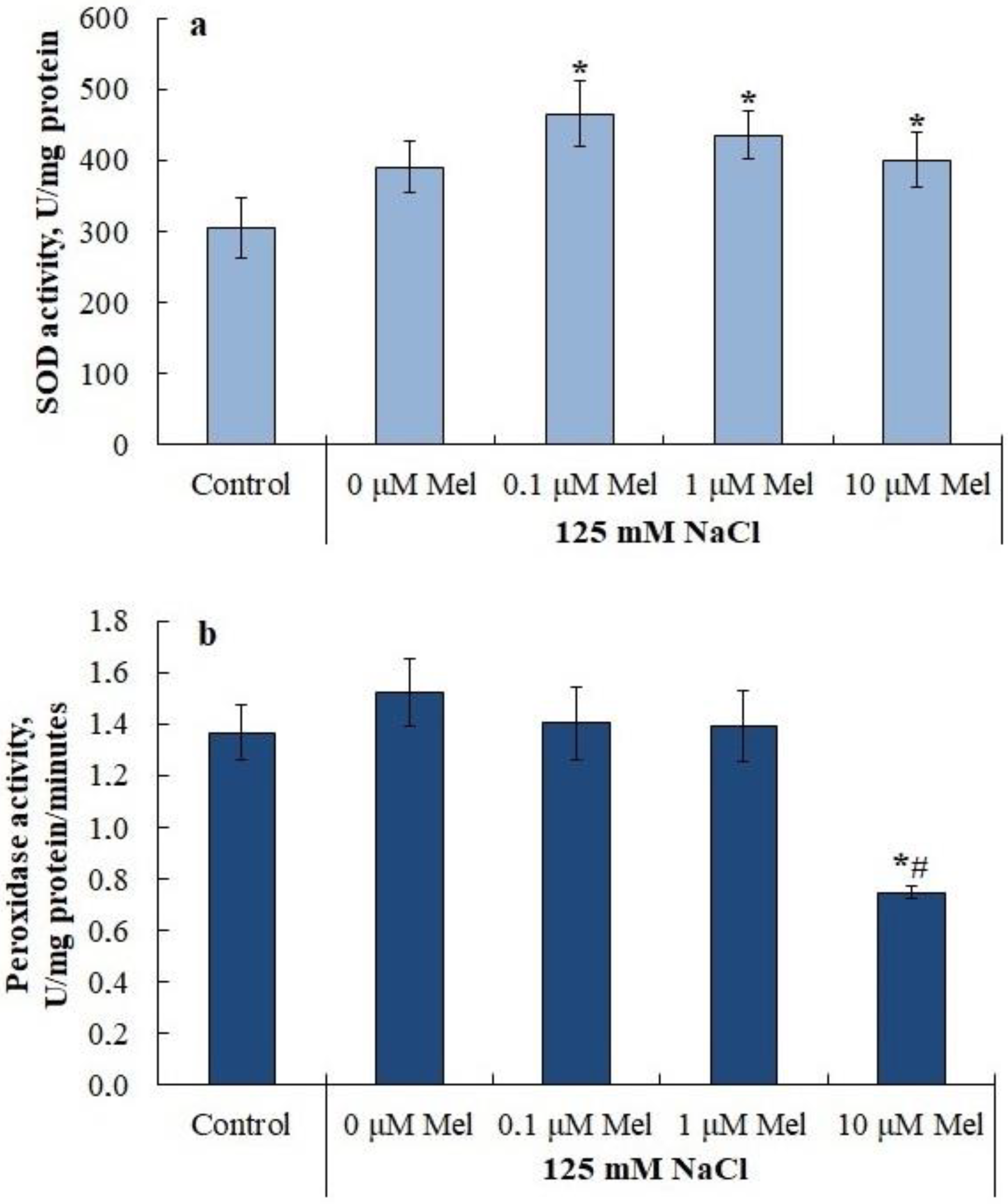
2.5. Lipid Peroxidation u Antioxidant Enzyme Activity in Potato Leaves
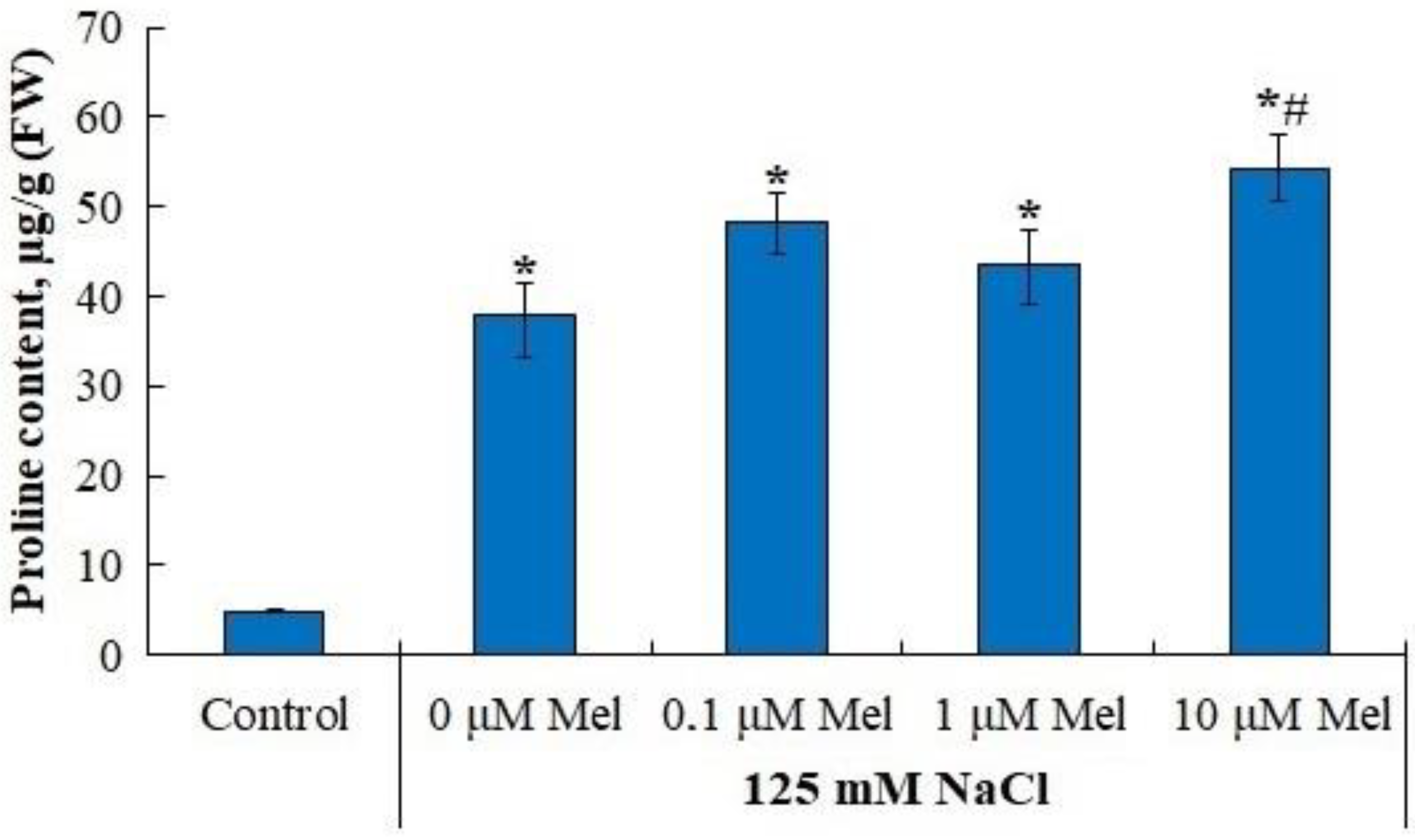
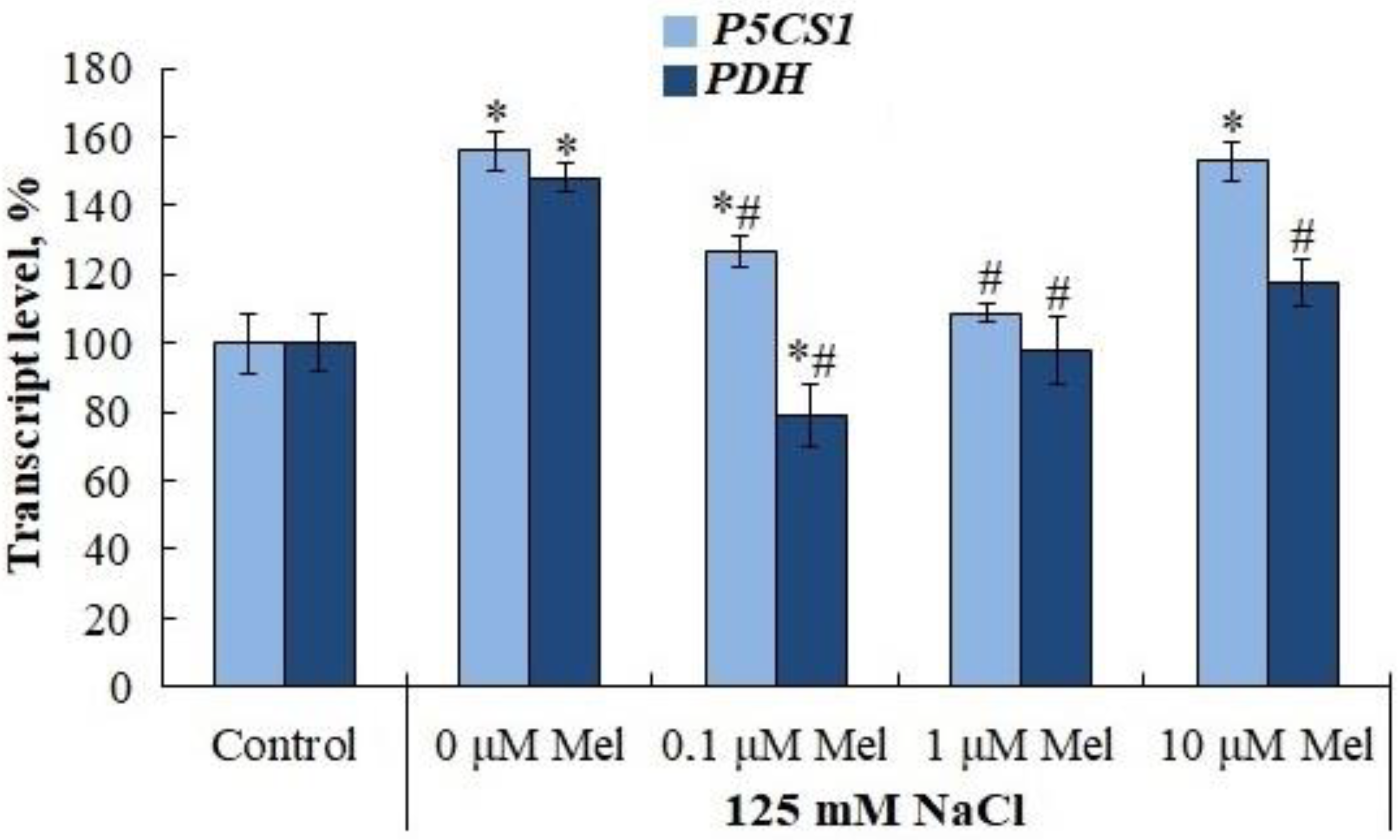
2.6. Proline and Flavonoid Content in Potato Leaves
2.7. Effect of Salt Stress and Melatonin on the Content of Sodium and Potassium Ions in Roots, Stems and Leaves of Potato Plants
2.8. Effect of Melatonin on Na+/H+ Antiporter Gene Transcript Levels of the Tonoplast (NHX1to NHX3) and Plasmalemma (SOS1) in Solanum Tuberosum Plants under Saline Conditions
3. Discussion
3.1. Potato Stolon Formation and Plant Water Status
3.2. Contents of Sodium, Chlorine and Potassium Ions in Leaves, Stem and Roots of Potato Plants
3.3. Basic Photosynthetic Pigments and Photochemical Activity of Photosystem II
3.4. Components of Antioxidant System and Proline Accumulation
4. Materials and Methods
4.1. Plant Growth and Experimental Design
4.2. Determination of Growth Parameters
4.3. Determining the Fresh and Dry Weight, and Water Content
4.4. Determining the Osmotic Potential in Potato Plants
4.5. Determination of Photosynthetic Pigments
4.6. Determination of Chlorophyll Fluorescence
4.7. Evaluating of the Lipid Peroxidation Level
4.8. Determination of Proline Content
4.9. Determination of the Total Content of Flavonoids
- D—the optical density of the test solution;
- DS—the optical density of the rutin solution;
- m—the mass of raw materials, g;
- mS—the mass of rutin, g;
- —the dilution factor of the test solution (1250);
- —dilution factor of rutin solution (2500);
- W—weight loss during drying of raw materials, %.
4.10. Determination of the Activity of Antioxidant Enzymes
4.11. The Determination of Total Protein Content
4.12. Analysis of Elemental Composition of Potato Plants
4.13. RNA Isolation and cDNA Synthesis
4.14. The Selection of Target Genes for qRT-PCR Analysis and Primer Design
4.15. Quantitative RT-PCR Analysis of Target Gene Expression
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of salt-induced land degradation and restoration. Nat. Resour. Forum. 2014, 38, 282–295. [Google Scholar] [CrossRef]
- Sharma, D.K.; Singh, A. Salinity research in india-achievements, challenges and future prospects. Water Energy Int. 2015, 58, 35–45. [Google Scholar]
- Machado, R.M.A.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth. management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Lutaladio, N.; Castaldi, L. Potato: The Hidden Treasure. J. Food Compost. Anal. 2009, 22, 491–493. [Google Scholar] [CrossRef]
- Hao, S.; Wang, Y.; Yan, Y.; Liu, Y.; Wang, J.; Chen, S. A Review on plant responses to salt stress and their mechanisms of salt resistance. Horticulturae 2021, 7, 13. [Google Scholar] [CrossRef]
- Athar, H.-u.-R.; Zulfiqar, F.; Moosa, A.; Ashraf, M.; Zafar, Z.U.; Zhang, L.; Ahmed, N.; Kalaji, H.M.; Nafees, M.; Hossain, M.A.; et al. Salt stress proteins in plants. Front. Plant Sci. 2022, 13, 999058. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, X.; Lv, W.; Yang, Y. Molecular mechanisms of plant responses to salt stress. Front. Plant Sci. 2022, 13, 934877. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Breusegem, F.V. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Munns, R.; Gilliham, M. Salinity tolerance of crops—What is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, L.; Li, H.; Zhang, S.; Fu, X.; Zhai, X.; Yang, N.; Shen, J.; Li, R.; Li, D. Exogenous melatonin promotes the salt tolerance by removing active oxygen and maintaining ion balance in wheat (Triticum aestivum L.). Front. Plant Sci. 2022, 12, 787062. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. Functions of melatonin in plants. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Kholodova, V.P.; Vasil’ev, S.V.; Efimova, M.V.; Voronin, P.Y.; Rakhmankulova, Z.F.; Danilova, E.Y.; Kuznetsov, V.V. Exogenous melatonin protects canola plants from toxicity of excessive copper. Russ. J. Plant Physiol. 2018, 65, 882–889. [Google Scholar] [CrossRef]
- Murch, S.J.; Erland, L.A.E. A Systematic review of melatonin in plants: An example of evolution of literature. Front. Plant Sci. 2021, 12, 683047. [Google Scholar] [CrossRef] [PubMed]
- Rehaman, A.; Mishra, A.K.; Ferdose, A.; Per, T.S.; Hanief, M.; Jan, A.T.; Asgher, M. Melatonin in plant defense against abiotic stress. Forests 2021, 12, 1404. [Google Scholar] [CrossRef]
- Zeng, W.; Mostafa, S.; Lu, Z.; Jin, B. Melatonin-mediated abiotic stress tolerance in plants. Front. Plant Sci. 2022, 13, 847175. [Google Scholar] [CrossRef]
- Khanna, K.; Bhardwaj, R.; Alam, P.; Reiter, R.J.; Ahmad, P. Phytomelatonin: A master regulator for plant oxidative stress management. Plant Physiol. Biochem. 2023, 196, 260–269. [Google Scholar] [CrossRef]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef]
- Zhan, H.; Nie, X.; Zhang, T.; Li, S.; Wang, X.; Du, X.; Tong, W.; Song, W. Melatonin: A small molecule but important for salt stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 709. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, H.; Chen, D.; Hao, G. Genetic and evolutionary dissection of melatonin response signaling facilitates the regulation of plant growth and stress responses. J. Pineal Res. 2023, 11, e12850. [Google Scholar] [CrossRef]
- Yang, X.; Chen, J.; Ma, Y.; Huang, M.; Qiu, T.; Bian, H.; Han, N.; Wang, J. Function, mechanism, and application of plant melatonin: An update with a focus on the cereal crop, barley (Hordeum vulgare L.). Antioxidants 2022, 11, 634. [Google Scholar] [CrossRef]
- Mannino, G.; Pernici, C.; Serio, G.; Gentile, C.; Bertea, C.M. Melatonin and phytomelatonin: Chemistry, biosynthesis, metabolism, distribution and bioactivity in plants and animals. Int. J. Mol. Sci. 2021, 22, 9996. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernandez-Ruiz, J. Melatonin: A new plant hormone and/or a plant master regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, C.; Xu, L.; Niu, H.; Liu, Q.; Huang, Y.; Lv, G.; Yang, H.; Li, M. Melatonin and indole-3-acetic acid synergistically regulate plant growth and stress resistance. Cells J. 2022, 11, 3250. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, K.; Chaudhary, R.; Sarwar, A.; Ahmad, B.; Gul, A.; Hano, C.; Abbasi, B.H.; Anjum, S. Melatonin as master regulator in plant growth, development and stress alleviator for sustainable agricultural production: Current status and future perspectives. Sustainability 2021, 13, 294. [Google Scholar] [CrossRef]
- Wei, J.; Li, D.X.; Zhang, J.R.; Shan, C.; Rengel, Z.; Song, Z.B.; Chen, Q. Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J. Pineal Res. 2018, 65, e12500. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Elkelish, A.; Dafea, M.; Khan, M.; Arnao, M.B.; Abdelhamid, M.T.; El-Ezz, A.A.; Almoneafy, A.l.; Mahmoud, A.; Awad, M.; et al. Role of melatonin in plant tolerance to soil stressors: Salinity, ph and heavy metals. Molecules 2020, 25, 5359. [Google Scholar] [CrossRef]
- Jiang, D.; Lu, B.; Liu, L.; Duan, W.; Meng, Y.; Li, Y.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H.; et al. Exogenous melatonin improves the salt tolerance of cotton by removing active oxygen and protecting photosynthetic organs. BMC Plant Biol. 2021, 21, 331. [Google Scholar] [CrossRef]
- Wang, J.; Lv, P.; Yan, D.; Zhang, Z.; Xu, X.; Wang, T.; Wang, Y.; Peng, Z.; Yu, C.; Gao, Y.; et al. Exogenous melatonin improves seed germination of wheat (Triticum aestivum L.) under salt stress. Int. J. Mol. Sci. 2022, 23, 8436. [Google Scholar] [CrossRef]
- Khan, T.A.; Saleem, M.; Fariduddin, O. Recent advances and mechanistic insights on melatonin-mediated salt stress signaling in plants. Plant Physiol. Biochem. 2022, 188, 97–107. [Google Scholar] [CrossRef]
- Liu, T.; Xing, G.; Chen, Z.; Zhai, X.; Wei, X.; Wang, C.; Li, T.; Zheng, S. Effect of exogenous melatonin on salt stress in cucumber: Alleviating effect and molecular basis. Biotechnol. Biotechnol. Equip. 2022, 36, 818–827. [Google Scholar] [CrossRef]
- Wei, L.; Zhao, H.; Wang, B.; Wu, X.; Lan, R.; Huang, X.; Chen, B.; Jiang, C.; Wang, J.; Liu, Y.; et al. Exogenous melatonin improves the growth of rice seedlings by regulating redox balance and ion homeostasis under salt stress. J. Plant Growth Regul. 2020, 41, 2108–2121. [Google Scholar] [CrossRef]
- Huang, X.; Tanveer, M.; Min, Y.; Shabala, S. Melatonin as a regulator of plant ionic homeostasis: Implications for abiotic stress tolerance. J. Exp. Bot. 2022, 73, 5886–5902. [Google Scholar] [CrossRef]
- Aranega-Bou, P.; de la O Leyva, M.; Finiti, I.; García-Agustín, P.; González-Bosch, C. Priming of plant resistance by natural compounds. Hexanoic acid as a model. Front. Plant Sci. 2014, 5, 488. [Google Scholar] [CrossRef] [PubMed]
- Charng, Y.-Y.; Mitra, S.; Yu, S.-J. Maintenance of abiotic stress memory in plants: Lessons learned from heat acclimation. Plant Cell. 2023, 35, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Bulgakov, V.P.; Wu, H.-C.; Jinn, T.-L. Coordination of ABA and chaperone signaling in plant stress responses. Trends Plant Sci. 2019, 24, 636–651. [Google Scholar] [CrossRef] [PubMed]
- Rajora, N.; Vats, S.; Raturi, G.; Thakral, V.; Kaur, S.; Rachappanavar, V.; Kumar, M.; Kesarwani, A.K.; Sonah, H.; Sharma, T.R.; et al. Seed priming with melatonin: A promising approach to combat abiotic stress in plants. Plant Stress. 2022, 4, 100071. [Google Scholar] [CrossRef]
- Yang, Z.; Zhi, P.; Chang, C. Priming seeds for the future: Plant immune memory and application in crop protection. Front. Plant Sci. 2022, 13, 961840. [Google Scholar] [CrossRef]
- Bahcesular, B.; Yildirim, E.D.; Karaçocuk, M.; Kulak, M.; Karaman, S. Seed priming with melatonin effects on growth, essential oil compounds and antioxidant activity of basil (Ocimum basilicum L.) under salinity stress. Ind. Crops Prod. 2020, 146, 112165. [Google Scholar] [CrossRef]
- Han, Q.-H.; Huang, B.; Ding, C.-B.; Zhang, Z.-W.; Chen, Y.-E.; Hu, C.; Zhou, L.-J.; Huang, Y.; Liao, J.-Q.; Yuan, S.; et al. Effects of melatonin on anti-oxidative systems and photosystem II in cold-stressed rice seedlings. Front. Plant Sci. 2017, 8, 785. [Google Scholar] [CrossRef]
- Kolomeichuk, L.V.; Efimova, M.V.; Zlobin, I.E.; Kreslavski, V.D.; Murgan, O.K.; Kovtun, I.S.; Khripach, V.A.; Kuznetsov, V.V.; Allakhverdiev, S.I. Epibrassinolide alleviates the toxic effects of NaCl on photosynthetic processes in potato plants. Photosynth. Res. 2020, 146, 151–163. [Google Scholar] [CrossRef]
- Daia, L.; Lia, J.; Harmensd, H.; Zhenge, X.; Zhang, C. Melatonin enhances drought resistance by regulating leaf stomatal behaviour, root growth and catalase activity in two contrasting rapeseed (Brassica napus L.) genotypes. Plant Physiol. Biochem. 2020, 149, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Varghese, N.; Alyammahi, O.; Nasreddine, S.; Alhassani, A.; Gururani, M.A. Melatonin positively influences the photosynthetic machinery and antioxidant system of Avena sativa during salinity stress. Plants 2019, 8, 610. [Google Scholar] [CrossRef] [PubMed]
- Sheikhalipour, M.; Mohammadi, S.A.; Esmaielpour, B.; Zareei, E.; Muhittin Kulak, M.; Ali, S.; Nouraein, M.; Bahrami, M.K.; Gohari, G.; Fotopoulos, V. Exogenous melatonin increases salt tolerance in bitter melon by regulating ionic balance, antioxidant system and secondary metabolism-related genes. BMC Plant Biol. 2022, 22, 380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Shi, Z.; Zhang, X.; Zheng, S.; Wang, J.; Mo, J. Alleviating effects of exogenous melatonin on salt stress in cucumber. Sci. Hortic. 2020, 262, 109070. [Google Scholar] [CrossRef]
- Yin, Z.; Lu, J.; Meng, S.; Liu, Y.; Mostafa, I.; Qi, M.; Li, T. Exogenous melatonin improves salt tolerance in tomato by regulating photosynthetic electron flux and the ascorbate–glutathione cycle. J. Plant Interact. 2019, 14, 453–463. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, A.; Li, X.; Kou, M.; Wang, W.; Chen, X.; Xu, T.; Zhu, M.; Ma, D.; Li, Z.; et al. Melatonin-stimulated triacylglycerol breakdown and energy turnover under salinity stress contributes to the maintenance of plasma membrane H+–ATPase activity and K+/Na+ homeostasis in sweet potato. Front. Plant Sci. 2018, 9, 256. [Google Scholar] [CrossRef]
- Björkman, O.; Demmig-Admas, B. Photon Yield of O2 Evolution and chlorophyll fluorescence characteristics at 77 k among vascular plants of diverse origins. Plantation 1987, 170, 489–504. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, P.K. Soil salinity and food security in India. Front. Sustain. Food Syst. 2020, 4, 533781. [Google Scholar] [CrossRef]
- Chourasia, K.N.; Lal, M.K.; Tiwari, R.K.; Dev, D.; Kardile, H.B.; Patil, V.U.; Kumar, A.; Vanishree, G.; Kumar, D.; Bhardwaj, V.; et al. Salinity stress in potato: Understanding physiological, biochemical and molecular responses. Life 2021, 11, 545. [Google Scholar] [CrossRef]
- Dobránszki, J.; Magyar-Tábori, K.; Hudák, I. In vitro Tuberization in Hormone-free systems on Solidified Medium and Dormancy of Potato Microtubers. Fruit Veg. Cereal Sci. Biotechnol. 2008, 2, 82–94. [Google Scholar]
- Gao, W.; Feng, Z.; Bai, Q.; He, J.; Wang, Y. Melatonin-mediated regulation of growth and antioxidant capacity in salt-tolerant naked oat under salt stress. Int. J. Mol. Sci. 2019, 20, 1176. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Saleem, M.; Fariduddin, Q. Melatonin influences stomatal behavior, root morphology, cell viability, photosynthetic responses, fruit yield, and fruit quality of tomato plants exposed to salt stress. J. Plant Growth Regul. 2022, 1–25. [Google Scholar] [CrossRef]
- Yang, X.L.; Xu, X.; Li, D.; Gao, X.; Li, T.L.; Wang, R. Effect of melatonin priming on photosynthetic capacity of tomato leaves under low-temperature stress. Photosynthetica 2018, 56, 884–892. [Google Scholar] [CrossRef]
- Szafranska, K.; Reiter, R.J.; Posmyk, M.M. Melatonin improves the photosynthetic apparatus in pea leaves stressed by paraquat via chlorophyll breakdown regulation and its accelerated de novo synthesis. Front. Plant Sci. 2017, 8, 878. [Google Scholar] [CrossRef]
- Heuer, B.; Nadler, A. Growth and development of potatoes under salinity and water deficit. Aust. J. Agric. Res. 1995, 46, 1477–1486. [Google Scholar] [CrossRef]
- Ishikawa, T.; Shabala, S. Control of xylem Na+ loading and transport to the shoot in rice and barley as a determinant of differential salinity stress tolerance. Physiol. Plant. 2019, 165, 619–631. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Chaoxing, H. Effect of Arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Sci. Hortic. 2011, 127, 228–233. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Salinity stress enhances color parameters, bioactive leaf pigments, vitamins, polyphenols, flavonoids and antioxidant activity in selected Amaranthus leafy vegetables. J. Sci. Food Agric. 2019, 99, 2275–2284. [Google Scholar] [CrossRef]
- Charfeddine, M.; Charfeddine, S.; Ghazala, I.; Bouaziz, D.; Bouzid, R.G. Investigation of the response to salinity of transgenic potato plants overexpressing the transcription factor StERF94. J. Biosci. 2019, 44, 141. [Google Scholar] [CrossRef]
- Sidhu, G.P.S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Alterations in photosynthetic pigments, protein, and carbohydrate metabolism in a wild plant Coronopus didymus L. (Brassicaceae) under lead stress. Acta Physiol. Plant. 2017, 39, 176. [Google Scholar] [CrossRef]
- Sherin, G.; Aswathi, K.P.; Puthur, R.J.T. Photosynthetic functions in plants subjected to stresses are positively influenced by priming. Plant Stress. 2022, 4, 100079. [Google Scholar] [CrossRef]
- Pardo-Hernández, M.; López-Delacalle, M.; Rivero, R.M. ROS and NO regulation by melatonin under abiotic stress in plants. Antioxidants 2020, 9, 1078. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin against environmental plant stressors: A review. Curr. Protein Pept. Sci. 2021, 22, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Allakhverdiev, S.I.; Murata, N. Protein synthesis is the primary target of reactive oxygen species in the photoinhibition of photosystem II. Physiologia Plantarum. 2011, 142, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernandez-Ruiz, J.; Cano, A. Role of melatonin and nitrogen metabolism in plants: Implications under nitrogen-excess or nitrogen-low. Int. J. Mol. Sci. 2022, 23, 15217. [Google Scholar] [CrossRef]
- Efimova, M.V.; Kolomeichuk, L.V.; Boyko, E.V.; Malofii, M.K.; Vidershpan, A.N.; Plyusnin, I.N.; Golovatskaya, I.F.; Murgan, O.K.; Kuznetsov, V.V. Physiological mechanisms of Solanum tuberosum L. plants’ tolerance to chloride salinity. Russ. J. Plant Physiol. 2018, 65, 394–403. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Plant Cell Membranes; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Meth. Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Gage, T.B.; Wendei, S.H. Quantitative determination of certain flavonol 3-glycosides. Anal. Chem. 1950, 22, 708–711. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Efimova, M.V.; Khripach, V.A.; Boyko, E.V.; Malofii, M.K.; Kolomeichuk, L.V.; Murgan, O.K.; Vidershpun, A.N.; Mukhamatdinova, E.A.; Kuznetsov, V.V. The priming of potato plants induced by brassinosteroids reduces oxidative stress and increases salt tolerance. Dokl. Biol. Sci. 2018, 478, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Esen, A. A simple method for quantitative, semiquantitative, and qualitative assay of protein. Anal. Biochem. 1978, 89, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Nicot, N.; Hausman, J.F.; Hoffmann, L.; Evers, D. Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J. Exp. Bot. 2005, 56, 2907–2914. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Stem Length, cm | Root Length, cm | Total Leaf Area, cm2 | Number of Stolons, Unit | Total Weight of the Plant, g | |
|---|---|---|---|---|---|---|
| NaCl, mM | Melatonin, µM | |||||
| 0 | 0 | 10.00 ± 0.46 | 16.60 ± 0.73 | 15.55 ± 1.59 | 2.33 ± 0.37 | 2.47 ± 0.18 |
| 125 | 0 | 8.00 ± 0.26 * | 13.47 ± 0.13 * | 5.34 ± 0.34 * | 0.39 ± 0.13 * | 1.49 ± 0.10 * |
| 125 | 0.1 | 9.17 ± 0.31 | 12.43 ± 0.72 * | 4.22 ± 0.43 * | 1.43 ± 0.37 *,# | 1.50 ± 0.14 * |
| 125 | 1.0 | 7.89 ± 0.20 * | 14.30 ± 0.96 * | 4.70 ± 0.45 * | 1.50 ± 0.34 *,# | 1.49 ± 0.10 * |
| 125 | 10.0 | 8.63 ± 0.50 | 13.00 ± 0.65 * | 4.83 ± 0.28 * | 1.50 ± 0.33 *,# | 1.59 ± 0.08 * |
| Treatment | Ion Content in Different Parts of the Plant | ||||||
|---|---|---|---|---|---|---|---|
| NaCl, mM | Melatonin, µM | Leaves | % | Stems | % | Roots | % |
| Na+, mg/g (DW) | |||||||
| 0 | 0 | 1.10 ± 0.10 | 100 | 2.55 ± 0.21 | 100 | 1.51 ± 0.06 | 100 |
| 125 | 0 | 81.44 ± 0.25 * | 7404 | 81.94 ± 3.58 * | 3213 | 39.51 ± 1.48 * | 2617 |
| 125 | 0.1 | 52.01 ± 0.58 *,# | 4728 | 45.35 ± 2,81 *,# | 1778 | 44.64 ± 4.91 * | 2956 |
| 125 | 1.0 | 25.53 ± 3.36 *,# | 2321 | 56.52 ± 3.53 *,# | 2216 | 28.87 ± 1.12 *,# | 1912 |
| 125 | 10.0 | 35.54 ± 1.54 *,# | 3231 | 58.73 ± 2.12 *,# | 2303 | 27.68 ± 2.88 *,# | 1833 |
| Cl−, mg/g (DW) | |||||||
| 0 | 0 | 8.60 ± 1.01 | 100 | 7.91 ± 0.46 | 100 | 10.26 ± 0.85 | 100 |
| 125 | 0 | 59.50 ± 6.89 * | 692 | 108.49 ± 7.64 * | 1372 | 55.69 ± 3.29 * | 543 |
| 125 | 0.1 | 59.67 ± 5.17 * | 694 | 70.94 ± 2.35 *,# | 897 | 75.79 ± 6.14 *,# | 739 |
| 125 | 1.0 | 44.80 ± 2.80 *,# | 521 | 69.70 ± 4.72 *,# | 881 | 36.72 ± 3.51 *,# | 358 |
| 125 | 10.0 | 61.47 ± 6.19 * | 715 | 82.40 ± 2.75 *,# | 1042 | 40.83 ± 3.62 *,# | 398 |
| K+, mg/g (DW) | |||||||
| 0 | 0 | 50.33 ± 1.33 | 100 | 55.08 ± 2.06 | 100 | 52.50 ± 2.90 | 100 |
| 125 | 0 | 29.16 ± 0.34 * | 58 | 39.30 ± 1.99 * | 71 | 38.90 ± 3.81 * | 74 |
| 125 | 0.1 | 29.65 ± 0.97 * | 59 | 50.48 ± 3.50 # | 92 | 53.60 ± 5.40 # | 102 |
| 125 | 1.0 | 34.39 ± 3.33 *,# | 68 | 45.50 ± 2.35 * | 83 | 54.60 ± 2.60 # | 104 |
| 125 | 10.0 | 46.31 ± 2.03 *,# | 92 | 57.78 ± 2.63 # | 105 | 32.50 ± 2.31 * | 62 |
| Treatment | SK+ Na+ (K+/Na+ in the Leaves)/ (K+/Na+ in the Roots) | SK+ Na+ (K+/Na+ in the Stems)/ (K+/Na+ in the Roots) | |
|---|---|---|---|
| NaCl, mM | Melatonin, µM | ||
| 0 | 0 | 8.98 ± 0.65 | 4.27 ± 0.28 |
| 125 | 0 | 0.35 ± 0.028 * | 0.49 ± 0.03 * |
| 125 | 0.1 | 0.45 ± 0.031 * | 0.87 ± 0.05 *,# |
| 125 | 1.0 | 0.71 ± 0.055 *,# | 1.04 ± 0.09 *,# |
| 125 | 10.0 | 1.11 ± 0.09 *,# | 0.84 ± 0.06 *,# |
| Treatment | NHX1, Rel. Units | % | NHX2, Rel. Units | % | NHX3, Rel. Units | % | SOS1, Rel. Units | % | |
|---|---|---|---|---|---|---|---|---|---|
| NaCl, mM | Melatonin, µM | ||||||||
| 0 | 0 | 0.0049 ± 0.0004 | 100 | 0.0006 ± 0.0001 | 100 | 0.0008 ± 0.0001 | 100 | 0.00016 ± 0.000017 | 100 |
| 125 | 0 | 0.0054 ± 0.0003 | 110 | 0.0014 ± 0.0001 * | 233 | 0.0026 ± 0.0001 * | 325 | 0.00027 ± 0.000024 * | 164 |
| 125 | 0.1 | 0.0050 ± 0.0002 | 101 | 0.0015 ± 0.0003 * | 250 | 0.0026 ± 0.0003 * | 325 | 0.00021 ± 0.000013 * | 127 |
| 125 | 1.0 | 0.0036 ± 0.0002 *,# | 74 | 0.0010 ± 0.0001 * | 155 | 0.0022 ± 0.0003 * | 275 | 0.00026 ± 0.000015 * | 158 |
| 125 | 10.0 | 0.0037 ± 0.0004 *,# | 76 | 0.0012 ± 0.0002 * | 195 | 0.0026 ± 0.0002 * | 325 | 0.00034 ± 0.000032 * | 211 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efimova, M.V.; Danilova, E.D.; Zlobin, I.E.; Kolomeichuk, L.V.; Murgan, O.K.; Boyko, E.V.; Kuznetsov, V.V. Priming Potato Plants with Melatonin Protects Stolon Formation under Delayed Salt Stress by Maintaining the Photochemical Function of Photosystem II, Ionic Homeostasis and Activating the Antioxidant System. Int. J. Mol. Sci. 2023, 24, 6134. https://doi.org/10.3390/ijms24076134
Efimova MV, Danilova ED, Zlobin IE, Kolomeichuk LV, Murgan OK, Boyko EV, Kuznetsov VV. Priming Potato Plants with Melatonin Protects Stolon Formation under Delayed Salt Stress by Maintaining the Photochemical Function of Photosystem II, Ionic Homeostasis and Activating the Antioxidant System. International Journal of Molecular Sciences. 2023; 24(7):6134. https://doi.org/10.3390/ijms24076134
Chicago/Turabian StyleEfimova, Marina V., Elena D. Danilova, Ilya E. Zlobin, Lilia V. Kolomeichuk, Olga K. Murgan, Ekaterina V. Boyko, and Vladimir V. Kuznetsov. 2023. "Priming Potato Plants with Melatonin Protects Stolon Formation under Delayed Salt Stress by Maintaining the Photochemical Function of Photosystem II, Ionic Homeostasis and Activating the Antioxidant System" International Journal of Molecular Sciences 24, no. 7: 6134. https://doi.org/10.3390/ijms24076134
APA StyleEfimova, M. V., Danilova, E. D., Zlobin, I. E., Kolomeichuk, L. V., Murgan, O. K., Boyko, E. V., & Kuznetsov, V. V. (2023). Priming Potato Plants with Melatonin Protects Stolon Formation under Delayed Salt Stress by Maintaining the Photochemical Function of Photosystem II, Ionic Homeostasis and Activating the Antioxidant System. International Journal of Molecular Sciences, 24(7), 6134. https://doi.org/10.3390/ijms24076134






