Neurotrophins: Neuroimmune Interactions in Human Atopic Diseases
Abstract
1. Introduction
2. Brain-Derived Neurotrophic Factor
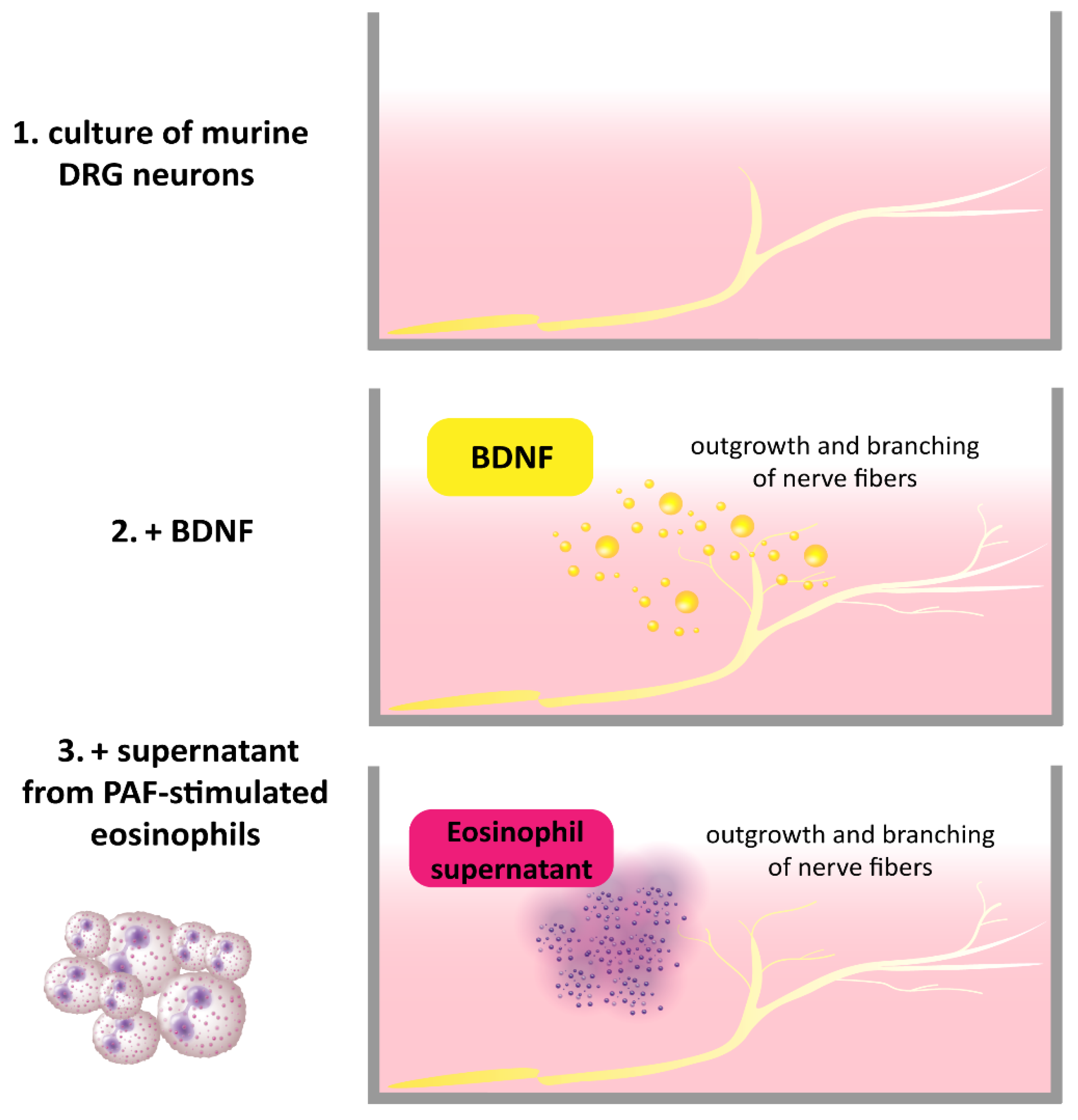
2.1. Atopic Dermatitis and BDNF
2.2. BDNF in Allergic Rhinitis
2.3. BDNF in Allergic Asthma
3. Nerve Growth Factor
3.1. Skin Inflammation and NGF
3.2. NGF in Allergic Rhinitis and Asthma
3.3. NGF and Allergic Rhinoconjunctivitis
4. NT-3 and NT-4/5
5. Neuronal Effectors
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nockher, W.A.; Renz, H. Neurotrophins in allergic diseases: From neuronal growth factors to intercellular signaling molecules. J. Allergy Clin. Immunol. 2006, 117, 583–589. [Google Scholar] [CrossRef]
- Lambiase, A.; Bracci-Laudiero, L.; Bonini, S.; Bonini, S.; Starace, G.; D’Elios, M.M.; de Carli, M.; Aloe, L. Human CD4+ T cell clones produce and release nerve growth factor and express high-affinity nerve growth factor receptors. J. Allergy Clin. Immunol. 1997, 100, 408–414. [Google Scholar] [CrossRef]
- Kerschensteiner, M.; Gallmeier, E.; Behrens, L.; Leal, V.V.; Misgeld, T.; Klinkert, W.E.; Kolbeck, R.; Hoppe, E.; Oropeza-Wekerle, R.L.; Bartke, I.; et al. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: A neuroprotective role of inflammation? J. Exp. Med. 1999, 189, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Amorim, D.; Iglesias-Martínez-Almeida, M.; Rivera-Baltanás, T.; Fernández-Palleiro, P.; Freiría-Martínez, L.; Rodríguez-Jamardo, C.; Comís-Tuche, M.; Del Vallejo-Curto, M.C.; Álvarez-Ariza, M.; López-García, M.; et al. The Role of the Second Extracellular Loop of Norepinephrine Transporter, Neurotrophin-3 and Tropomyosin Receptor Kinase C in T Cells: A Peripheral Biomarker in the Etiology of Schizophrenia. Int. J. Mol. Sci. 2021, 22, 8499. [Google Scholar] [CrossRef]
- Torcia, M.; Bracci-Laudiero, L.; Lucibello, M.; Nencioni, L.; Labardi, D.; Rubartelli, A.; Cozzolino, F.; Aloe, L.; Garaci, E. Nerve Growth Factor Is an Autocrine Survival Factor for Memory B Lymphocytes. Cell 1996, 85, 345–356. [Google Scholar] [CrossRef]
- Leon, A.; Buriani, A.; Dal Toso, R.; Fabris, M.; Romanello, S.; Aloe, L.; Levi-Montalcini, R. Mast cells synthesize, store, and release nerve growth factor. Proc. Natl. Acad. Sci. USA 1994, 91, 3739–3743. [Google Scholar] [CrossRef]
- Guseva, D.; Rüdrich, U.; Kotnik, N.; Gehring, M.; Patsinakidis, N.; Agelopoulos, K.; Ständer, S.; Homey, B.; Kapp, A.; Gibbs, B.F.; et al. Neuronal branching of sensory neurons is associated with BDNF-positive eosinophils in atopic dermatitis. Clin. Exp. Allergy 2020, 50, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.; Appel, E.; Baruch, R.; Herz, U.; Botchkarev, V.; Paus, R.; Brodie, C.; Renz, H. Role of nerve growth factor in a mouse model of allergic airway inflammation and asthma. Eur. J. Immunol. 1998, 28, 3240–3251. [Google Scholar] [CrossRef]
- Garaci, E.; Caroleo, M.C.; Aloe, L.; Aquaro, S.; Piacentini, M.; Costa, N.; Amendola, A.; Micera, A.; Caliò, R.; Perno, C.F.; et al. Nerve growth factor is an autocrine factor essential for the survival of macrophages infected with HIV. Proc. Natl. Acad. Sci. USA 1999, 96, 14013–14018. [Google Scholar] [CrossRef]
- Manti, S.; Brown, P.; Perez, M.K.; Piedimonte, G. The Role of Neurotrophins in Inflammation and Allergy. Vitam. Horm. 2017, 104, 313–341. [Google Scholar] [CrossRef]
- Samah, B.; Porcheray, F.; Gras, G. Neurotrophins modulate monocyte chemotaxis without affecting macrophage function. Clin. Exp. Immunol. 2008, 151, 476–486. [Google Scholar] [CrossRef]
- Peng, W.-M.; Maintz, L.; Allam, J.-P.; Raap, U.; Gütgemann, I.; Kirfel, J.; Wardelmann, E.; Perner, S.; Zhao, W.; Fimmers, R.; et al. Increased circulating levels of neurotrophins and elevated expression of their high-affinity receptors on skin and gut mast cells in mastocytosis. Blood 2013, 122, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Raap, U.; Goltz, C.; Deneka, N.; Bruder, M.; Renz, H.; Kapp, A.; Wedi, B. Brain-derived neurotrophic factor is increased in atopic dermatitis and modulates eosinophil functions compared with that seen in nonatopic subjects. J. Allergy Clin. Immunol. 2005, 115, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- Raap, U.; Fokkens, W.; Bruder, M.; Hoogsteden, H.; Kapp, A.; Braunstahl, G.-J. Modulation of neurotrophin and neurotrophin receptor expression in nasal mucosa after nasal allergen provocation in allergic rhinitis. Allergy 2008, 63, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Lommatzsch, M.; Schloetcke, K.; Klotz, J.; Schuhbaeck, K.; Zingler, D.; Zingler, C.; Schulte-Herbrüggen, O.; Gill, H.; Schuff-Werner, P.; Virchow, J.C. Brain-derived neurotrophic factor in platelets and airflow limitation in asthma. Am. J. Respir. Crit. Care Med. 2005, 171, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Joachim, R.A.; Noga, O.; Sagach, V.; Hanf, G.; Fliege, H.; Kocalevent, R.D.; Peters, E.M.; Klapp, B.F. Correlation between immune and neuronal parameters and stress perception in allergic asthmatics. Clin. Exp. Allergy 2008, 38, 283–290. [Google Scholar] [CrossRef]
- Virchow, J.C.; Julius, P.; Lommatzsch, M.; Luttmann, W.; Renz, H.; Braun, A. Neurotrophins are increased in bronchoalveolar lavage fluid after segmental allergen provocation. Am. J. Respir. Crit. Care Med. 1998, 158, 2002–2005. [Google Scholar] [CrossRef]
- Prakash, Y.S.; Thompson, M.A.; Pabelick, C.M. Brain-derived neurotrophic factor in TNF-alpha modulation of Ca2+ in human airway smooth muscle. Am. J. Respir. Cell Mol. Biol. 2009, 41, 603–611. [Google Scholar] [CrossRef]
- Skaper, S.D. Nerve growth factor: A neuroimmune crosstalk mediator for all seasons. Immunology 2017, 151, 1–15. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Dahinden, C.A. Effect of nerve growth factor on the release of inflammatory mediators by mature human basophils. Blood 1992, 79, 2662–2669. [Google Scholar] [CrossRef]
- Solomon, A.; Aloe, L.; Pe’er, J.; Frucht-Pery, J.; Bonini, S.; Levi-Schaffer, F. Nerve growth factor is preformed in and activates human peripheral blood eosinophils. J. Allergy Clin. Immunol. 1998, 102, 454–460. [Google Scholar] [CrossRef] [PubMed]
- La Sala, A.; Corinti, S.; Federici, M.; Saragovi, H.U.; Girolomoni, G. Ligand activation of nerve growth factor receptor TrkA protects monocytes from apoptosis. J. Leukoc. Biol. 2000, 68, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Hamada, A.; Watanabe, N.; Ohtomo, H.; Matsuda, H. Nerve growth factor enhances survival and cytotoxic activity of human eosinophils. Br. J. Haematol. 1996, 93, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Noga, O.; Englmann, C.; Hanf, G.; Grützkau, A.; Guhl, S.; Kunkel, G. Activation of the specific neurotrophin receptors TrkA, TrkB and TrkC influences the function of eosinophils. Clin. Exp. Allergy 2002, 32, 1348–1354. [Google Scholar] [CrossRef]
- Sin, A.Z.; Roche, E.M.; Togias, A.; Lichtenstein, L.M.; Schroeder, J.T. Nerve growth factor or IL-3 induces more IL-13 production from basophils of allergic subjects than from basophils of nonallergic subjects. J. Allergy Clin. Immunol. 2001, 108, 387–393. [Google Scholar] [CrossRef]
- Ikoma, A.; Steinhoff, M.; Ständer, S.; Yosipovitch, G.; Schmelz, M. The neurobiology of itch. Nat. Rev. Neurosci. 2006, 7, 535–547. [Google Scholar] [CrossRef]
- Peters, E.M.J.; Liezmann, C.; Spatz, K.; Daniltchenko, M.; Joachim, R.; Gimenez-Rivera, A.; Hendrix, S.; Botchkarev, V.A.; Brandner, J.M.; Klapp, B.F. Nerve growth factor partially recovers inflamed skin from stress-induced worsening in allergic inflammation. J. Investig. Dermatol. 2011, 131, 735–743. [Google Scholar] [CrossRef]
- Foster, E.L.; Simpson, E.L.; Fredrikson, L.J.; Lee, J.J.; Lee, N.A.; Fryer, A.D.; Jacoby, D.B. Eosinophils increase neuron branching in human and murine skin and in vitro. PLoS ONE 2011, 6, e22029. [Google Scholar] [CrossRef]
- Kinkelin, I.; Mötzing, S.; Koltenzenburg, M.; Bröcker, E.B. Increase in NGF content and nerve fiber sprouting in human allergic contact eczema. Cell Tissue Res. 2000, 302, 31–37. [Google Scholar] [CrossRef]
- Toyoda, M.; Nakamura, M.; Makino, T.; Morohashi, M. Localization and content of nerve growth factor in peripheral blood eosinophils of atopic dermatitis patients. Clin. Exp. Allergy 2003, 33, 950–955. [Google Scholar] [CrossRef]
- Raap, U.; Werfel, T.; Goltz, C.; Deneka, N.; Langer, K.; Bruder, M.; Kapp, A.; Schmid-Ott, G.; Wedi, B. Circulating levels of brain-derived neurotrophic factor correlate with disease severity in the intrinsic type of atopic dermatitis. Allergy 2006, 61, 1416–1418. [Google Scholar] [CrossRef]
- Bonini, S.; Lambiase, A.; Angelucci, F.; Magrini, L.; Manni, L.; Aloe, L. Circulating nerve growth factor levels are increased in humans with allergic diseases and asthma. Proc. Natl. Acad. Sci. USA 1996, 93, 10955–10960. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.; Lommatzsch, M.; Lewin, G.R.; Virchow, J.C.; Renz, H. Neurotrophins: A link between airway inflammation and airway smooth muscle contractility in asthma? Int. Arch. Allergy Immunol. 1999, 118, 163–165. [Google Scholar] [CrossRef]
- Shi, Y.; Jin, Y.; Guo, W.; Chen, L.; Liu, C.; Lv, X. Blockage of nerve growth factor modulates T cell responses and inhibits allergic inflammation in a mouse model of asthma. Inflamm. Res. 2012, 61, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Nassenstein, C.; Braun, A.; Erpenbeck, V.J.; Lommatzsch, M.; Schmidt, S.; Krug, N.; Luttmann, W.; Renz, H.; Virchow, J.C. The neurotrophins nerve growth factor, brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4 are survival and activation factors for eosinophils in patients with allergic bronchial asthma. J. Exp. Med. 2003, 198, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, M.; Segatto, M.; Bruscolini, A.; Abicca, I.; Cavaliere, C.; Lambiase, A. Changes of NGF pathway in allergic rhinoconjunctivitis: A conjunctival allergen challenge study. Allergy 2019, 74, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Raap, U.; Kapp, A. Neurotrophins in healthy and diseased skin. G. Ital. Dermatol. Venereol. 2010, 145, 205–211. [Google Scholar] [PubMed]
- Rost, B.; Hanf, G.; Ohnemus, U.; Otto-Knapp, R.; Groneberg, D.A.; Kunkel, G.; Noga, O. Monocytes of allergics and non-allergics produce, store and release the neurotrophins NGF, BDNF and NT-3. Regul. Pept. 2005, 124, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Raap, U.; Deneka, N.; Bruder, M.; Kapp, A.; Wedi, B. Differential up-regulation of neurotrophin receptors and functional activity of neurotrophins on peripheral blood eosinophils of patients with allergic rhinitis, atopic dermatitis and nonatopic subjects. Clin. Exp. Allergy 2008, 38, 1493–1498. [Google Scholar] [CrossRef]
- Quarcoo, D.; Fischer, T.C.; Peckenschneider, N.; Groneberg, D.A.; Welker, P. High abundances of neurotrophin 3 in atopic dermatitis mast cell. J. Occup. Med. Toxicol. 2009, 4, 8. [Google Scholar] [CrossRef]
- İsmi, O.; Özcan, C.; Karabacak, T.; Polat, G.; Vayisoğlu, Y.; Güçlütürk, T.; Görür, K. Local Effect of Neurotrophin-3 in Neuronal Inflammation of Allergic Rhinitis: Preliminary Report. Balkan Med. J. 2015, 32, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Szczepankiewicz, A.; Rachel, M.; Sobkowiak, P.; Kycler, Z.; Wojsyk-Banaszak, I.; Schöneich, N.; Skibinska, M.; Bręborowicz, A. Serum neurotrophin-3 and neurotrophin-4 levels are associated with asthma severity in children. Eur. Respir. J. 2012, 39, 1035–1037. [Google Scholar] [CrossRef]
- Grewe, M.; Vogelsang, K.; Ruzicka, T.; Stege, H.; Krutmann, J. Neurotrophin-4 production by human epidermal keratinocytes: Increased expression in atopic dermatitis. J. Investig. Dermatol. 2000, 114, 1108–1112. [Google Scholar] [CrossRef]
- Siiskonen, H.; Harvima, I. Mast Cells and Sensory Nerves Contribute to Neurogenic Inflammation and Pruritus in Chronic Skin Inflammation. Front. Cell. Neurosci. 2019, 13, 422. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Takamori, K. Itch and nerve fibers with special reference to atopic dermatitis: Therapeutic implications. J. Dermatol. 2014, 41, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.P.; Mühl, S.; Pogatzki-Zahn, E.M.; Agelopoulos, K.; Ständer, S. Intraepidermal Nerve Fiber Density: Diagnostic and Therapeutic Relevance in the Management of Chronic Pruritus: A Review. Dermatol. Ther. 2016, 6, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Takamori, K. Peripheral itch sensitization in atopic dermatitis. Allergol. Int. 2022, 71, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Kido, M.; Takeuchi, S.; Esaki, H.; Hayashida, S.; Furue, M. Scratching behavior does not necessarily correlate with epidermal nerve fiber sprouting or inflammatory cell infiltration. J. Dermatol. Sci. 2010, 58, 130–135. [Google Scholar] [CrossRef]
- Fölster-Holst, R.; Papakonstantinou, E.; Rüdrich, U.; Buchner, M.; Pite, H.; Gehring, M.; Kapp, A.; Weidinger, S.; Raap, U. Childhood atopic dermatitis-Brain-derived neurotrophic factor correlates with serum eosinophil cationic protein and disease severity. Allergy 2016, 71, 1062–1065. [Google Scholar] [CrossRef]
- Polakowski, N.; Terol, M.; Hoang, K.; Nash, I.; Laverdure, S.; Gazon, H.; Belrose, G.; Mesnard, J.-M.; Césaire, R.; Péloponèse, J.-M.; et al. HBZ stimulates brain-derived neurotrophic factor/TrkB autocrine/paracrine signaling to promote survival of human T-cell leukemia virus type 1-Infected T cells. J. Virol. 2014, 88, 13482–13494. [Google Scholar] [CrossRef]
- D’Onofrio, M.; de Grazia, U.; Morrone, S.; Cuomo, L.; Spinsanti, P.; Frati, L.; Gulino, A.; Ragona, G. Expression of neurotrophin receptors in normal and malignant B lymphocytes. Eur. Cytokine Netw. 2000, 11, 283–291. [Google Scholar]
- Jin, P.; Andiappan, A.K.; Quek, J.M.; Lee, B.; Au, B.; Sio, Y.Y.; Irwanto, A.; Schurmann, C.; Grabe, H.J.; Suri, B.K.; et al. A functional brain-derived neurotrophic factor (BDNF) gene variant increases the risk of moderate-to-severe allergic rhinitis. J. Allergy Clin. Immunol. 2015, 135, 1486–1493.e8. [Google Scholar] [CrossRef] [PubMed]
- Andiappan, A.K.; Parate, P.N.; Anantharaman, R.; Suri, B.K.; Wang, D.Y.; Chew, F.T. Genetic variation in BDNF is associated with allergic asthma and allergic rhinitis in an ethnic Chinese population in Singapore. Cytokine 2011, 56, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Wussow, A.; Cryer, A.; Schmeck, B.; Noga, O.; Zweng, M.; Peiser, C.; Dinh, Q.T.; Heppt, W.; Groneberg, D.A. Neuronal plasticity in persistent perennial allergic rhinitis. J. Occup. Environ. Med. 2005, 47, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Prakash, Y.S.; Martin, R.J. Brain-derived neurotrophic factor in the airways. Pharmacol. Ther. 2014, 143, 74–86. [Google Scholar] [CrossRef]
- Lommatzsch, M.; Niewerth, A.; Klotz, J.; Schulte-Herbrüggen, O.; Zingler, C.; Schuff-Werner, P.; Virchow, J.C. Platelet and plasma BDNF in lower respiratory tract infections of the adult. Respir. Med. 2007, 101, 1493–1499. [Google Scholar] [CrossRef]
- Drake, M.G.; Scott, G.D.; Blum, E.D.; Lebold, K.M.; Nie, Z.; Lee, J.J.; Fryer, A.D.; Costello, R.W.; Jacoby, D.B. Eosinophils increase airway sensory nerve density in mice and in human asthma. Sci. Transl. Med. 2018, 10, eaar8477. [Google Scholar] [CrossRef]
- Aloe, L. Rita Levi-Montalcini and the discovery of NGF, the first nerve cell growth factor. Arch. Ital. Biol. 2011, 149, 175–181. [Google Scholar] [CrossRef]
- Cohen, S.; Levi-Montalcini, R.; Hamburger, V. A NERVE GROWTH-STIMULATING FACTOR ISOLATED FROM SARCOM AS 37 AND 180. Proc. Natl. Acad. Sci. USA 1954, 40, 1014–1018. [Google Scholar] [CrossRef]
- Otten, U.; Ehrhard, P.; Peck, R. Nerve growth factor induces growth and differentiation of human B lymphocytes. Proc. Natl. Acad. Sci. USA 1989, 86, 10059–10063. [Google Scholar] [CrossRef]
- Meng, J.; Li, Y.; Fischer, M.J.M.; Steinhoff, M.; Chen, W.; Wang, J. Th2 Modulation of Transient Receptor Potential Channels: An Unmet Therapeutic Intervention for Atopic Dermatitis. Front. Immunol. 2021, 12, 696784. [Google Scholar] [CrossRef]
- Oetjen, L.K.; Mack, M.R.; Feng, J.; Whelan, T.M.; Niu, H.; Guo, C.J.; Chen, S.; Trier, A.M.; Xu, A.Z.; Tripathi, S.V.; et al. Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell 2017, 171, 217–228.e13. [Google Scholar] [CrossRef]
- Ständer, S.; Yosipovitch, G. Substance P and neurokinin 1 receptor are new targets for the treatment of chronic pruritus. Br. J. Dermatol. 2019, 181, 932–938. [Google Scholar] [CrossRef]
- Kobayashi, H.; Gleich, G.J.; Butterfield, J.H.; Kita, H. Human eosinophils produce neurotrophins and secrete nerve growth factor on immunologic stimuli. Blood 2002, 99, 2214–2220. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.M.J.; Michenko, A.; Kupfer, J.; Kummer, W.; Wiegand, S.; Niemeier, V.; Potekaev, N.; Lvov, A.; Gieler, U. Mental stress in atopic dermatitis--neuronal plasticity and the cholinergic system are affected in atopic dermatitis and in response to acute experimental mental stress in a randomized controlled pilot study. PLoS ONE 2014, 9, e113552. [Google Scholar] [CrossRef] [PubMed]
- Lewin, G.R.; Nykjaer, A. Pro-neurotrophins, sortilin, and nociception. Eur. J. Neurosci. 2014, 39, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.O.; Perestrelo, T.; Almeida, R.D. PROneurotrophins and CONSequences. Mol. Neurobiol. 2018, 55, 2934–2951. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, S.; Daniltchenko, M.; Tobin, D.J.; Hagen, E.; Hunt, S.P.; Klapp, B.F.; Arck, P.C.; Peters, E.M.J. Further exploring the brain-skin connection: Stress worsens dermatitis via substance P-dependent neurogenic inflammation in mice. J. Investig. Dermatol. 2008, 128, 434–446. [Google Scholar] [CrossRef]
- Yamaguchi, J.; Aihara, M.; Kobayashi, Y.; Kambara, T.; Ikezawa, Z. Quantitative analysis of nerve growth factor (NGF) in the atopic dermatitis and psoriasis horny layer and effect of treatment on NGF in atopic dermatitis. J. Dermatol. Sci. 2009, 53, 48–54. [Google Scholar] [CrossRef]
- Dou, Y.-C.; Hagströmer, L.; Emtestam, L.; Johansson, O. Increased nerve growth factor and its receptors in atopic dermatitis: An immunohistochemical study. Arch. Dermatol. Res. 2006, 298, 31–37. [Google Scholar] [CrossRef]
- Raap, U.; Braunstahl, G.-J. The role of neurotrophins in the pathophysiology of allergic rhinitis. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 8–13. [Google Scholar] [CrossRef]
- O’Connor, T.M.; O’Connell, J.; O’Brien, D.I.; Goode, T.; Bredin, C.P.; Shanahan, F. The role of substance P in inflammatory disease. J. Cell. Physiol. 2004, 201, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, A.; Bonini, S.; Micera, A.; Magrini, L.; Bracci-Laudiero, L.; Aloe, L. Increased plasma levels of nerve growth factor in vernal keratoconjunctivitis and relationship to conjunctival mast cells. Investig. Ophthalmol. Vis. Sci. 1995, 36, 2127–2132. [Google Scholar]
- Lin, C.; Li, W.; Fan, X. S1P promotes corneal trigeminal neuron differentiation and corneal nerve repair via upregulating nerve growth factor expression in a mouse model. Open Life Sci. 2022, 17, 1324–1332. [Google Scholar] [CrossRef]
- Lewin, G.R.; Barde, Y.A. Physiology of the neurotrophins. Annu. Rev. Neurosci. 1996, 19, 289–317. [Google Scholar] [CrossRef] [PubMed]
- Sekimoto, M.; Tsuji, T.; Matsuzaki, J.; Chamoto, K.; Koda, T.; Nemoto, K.; Degawa, M.; Nishimura, S.; Nishimura, T. Functional expression of the TrkC gene, encoding a high affinity receptor for NT-3, in antigen-specific T helper type 2 (Th2) cells. Immunol. Lett. 2003, 88, 221–226. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef]
- Reed-Geaghan, E.G.; Wright, M.C.; See, L.A.; Adelman, P.C.; Lee, K.H.; Koerber, H.R.; Maricich, S.M. Merkel Cell-Driven BDNF Signaling Specifies SAI Neuron Molecular and Electrophysiological Phenotypes. J. Neurosci. 2016, 36, 4362–4376. [Google Scholar] [CrossRef]
- Bennett, D.L. Neurotrophic factors: Important regulators of nociceptive function. Neuroscientist 2001, 7, 13–17. [Google Scholar] [CrossRef]
- Rukwied, R.; Mayer, A.; Kluschina, O.; Obreja, O.; Schley, M.; Schmelz, M. NGF induces non-inflammatory lo-calized and lasting mechanical and thermal hypersensitivity in human skin. Pain 2010, 148, 407–413. [Google Scholar] [CrossRef]
- Rukwied, R.; Weinkauf, B.; Main, M.; Obreja, O.; Schmelz, M. Inflammation meets sensitization--an explanation for spontaneous nociceptor activity? Pain 2013, 154, 2707–2714. [Google Scholar] [CrossRef] [PubMed]
- Rukwied, R.R.; Main, M.; Weinkauf, B.; Schmelz, M. NGF sensitizes nociceptors for cowhage—But not hista-mine-induced itch in human skin. J. Investig. Dermatol. 2013, 133, 268–270. [Google Scholar] [CrossRef]
- Solinski, H.J.; Rukwied, R.; Schmelz, M. Microinjection of pruritogens in NGF-sensitized human skin. Sci. Rep. 2021, 11, 21490. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Smith, M.T. Neurotrophins and Neuropathic Pain: Role in Pathobiology. Molecules 2015, 20, 10657–10688. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, M.; Ahmad, F.; Pandey, A.; Datsi, A.; AlHammadi, A.; Al-Khawaga, S.; Al-Malki, A.; Meng, J.; Alam, M.; Buddenkotte, J. Neuroimmune communication regulating pruritus in atopic dermatitis. J. Allergy Clin. Immunol. 2022, 149, 1875–1898. [Google Scholar] [CrossRef]
- Tavares-Ferreira, D.; Shiers, S.; Ray, P.R.; Wangzhou, A.; Jeevakumar, V.; Sankaranarayanan, I.; Cervantes, A.M.; Reese, J.C.; Chamessian, A.; Copits, B.A.; et al. Spatial transcriptomics of dorsal root ganglia identifies molecular signatures of human nociceptors. Sci. Transl. Med. 2022, 14, eabj8186. [Google Scholar] [CrossRef]
- Solinski, H.J.; Dranchak, P.; Oliphant, E.; Gu, X.; Earnest, T.W.; Braisted, J.; Inglese, J.; Hoon, M.A. Inhibition of natriuretic peptide receptor 1 reduces itch in mice. Sci. Transl. Med. 2019, 11, eaav5464. [Google Scholar] [CrossRef]
- Keefe, K.M.; Sheikh, I.S.; Smith, G.M. Targeting Neurotrophins to Specific Populations of Neurons: NGF, BDNF, and NT-3 and Their Relevance for Treatment of Spinal Cord Injury. Int. J. Mol. Sci. 2017, 18, 548. [Google Scholar] [CrossRef]
- Oetjen, L.K.; Kim, B.S. Interactions of the immune and sensory nervous systems in atopy. FEBS J. 2018, 285, 3138–3151. [Google Scholar] [CrossRef]
- Blake, K.J.; Jiang, X.R.; Chiu, I.M. Neuronal Regulation of Immunity in the Skin and Lungs. Trends Neurosci. 2019, 42, 537–551. [Google Scholar] [CrossRef]
- Zhou, X.; Nai, Q.; Chen, M.; Dittus, J.D.; Howard, M.J.; Margiotta, J.F. Brain-derived neurotrophic factor and trkB signaling in parasympathetic neurons: Relevance to regulating alpha7-containing nicotinic receptors and synaptic function. J. Neurosci. 2004, 24, 4340–4350. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Clark, T.E.; Undem, B.J. Neural control of the lower airways: Role in cough and airway inflammatory disease. Handb. Clin. Neurol. 2022, 188, 373–391. [Google Scholar] [CrossRef] [PubMed]
- Undem, B.J.; Taylor-Clark, T. Mechanisms underlying the neuronal-based symptoms of allergy. J. Allergy Clin. Immunol. 2014, 133, 1521–1534. [Google Scholar] [CrossRef] [PubMed]
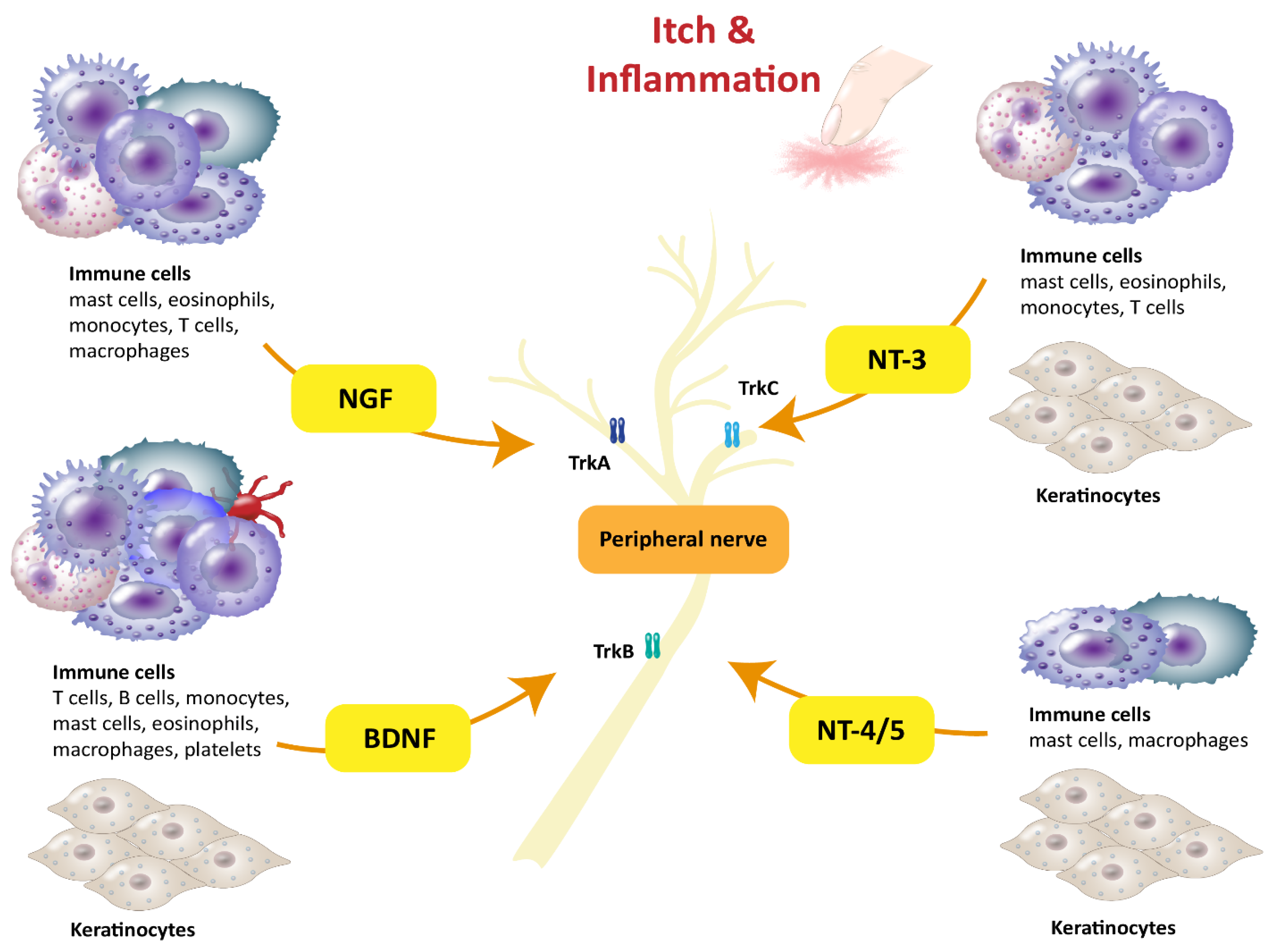
| Neurotrophins | Expressing Immune Cells in Humans | Functions and Upregulation in Allergic Diseases | Refs. |
|---|---|---|---|
| BDNF | Eosinophils, T cells, B cells, macrophages, monocytes, mast cells, platelets | Atopic dermatitis
| [3,7,11,12,13,14,15,16,17,18] |
| NGF | Eosinophils, T cells, macrophages, monocytes, mast cells | Skin inflammation
| [2,14,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36] |
| NT-3 | Eosinophils, T cells, monocytes, mast cells |
| [4,17,37,38,39,40,41,42] |
| NT-4/5 | Macrophages, mast cells |
| [11,35,37,39,42,43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weihrauch, T.; Limberg, M.M.; Gray, N.; Schmelz, M.; Raap, U. Neurotrophins: Neuroimmune Interactions in Human Atopic Diseases. Int. J. Mol. Sci. 2023, 24, 6105. https://doi.org/10.3390/ijms24076105
Weihrauch T, Limberg MM, Gray N, Schmelz M, Raap U. Neurotrophins: Neuroimmune Interactions in Human Atopic Diseases. International Journal of Molecular Sciences. 2023; 24(7):6105. https://doi.org/10.3390/ijms24076105
Chicago/Turabian StyleWeihrauch, Tobias, Maren M. Limberg, Natalie Gray, Martin Schmelz, and Ulrike Raap. 2023. "Neurotrophins: Neuroimmune Interactions in Human Atopic Diseases" International Journal of Molecular Sciences 24, no. 7: 6105. https://doi.org/10.3390/ijms24076105
APA StyleWeihrauch, T., Limberg, M. M., Gray, N., Schmelz, M., & Raap, U. (2023). Neurotrophins: Neuroimmune Interactions in Human Atopic Diseases. International Journal of Molecular Sciences, 24(7), 6105. https://doi.org/10.3390/ijms24076105






