Insights into Canine Infertility: Apoptosis in Chronic Asymptomatic Orchitis
Abstract
1. Introduction
2. Results
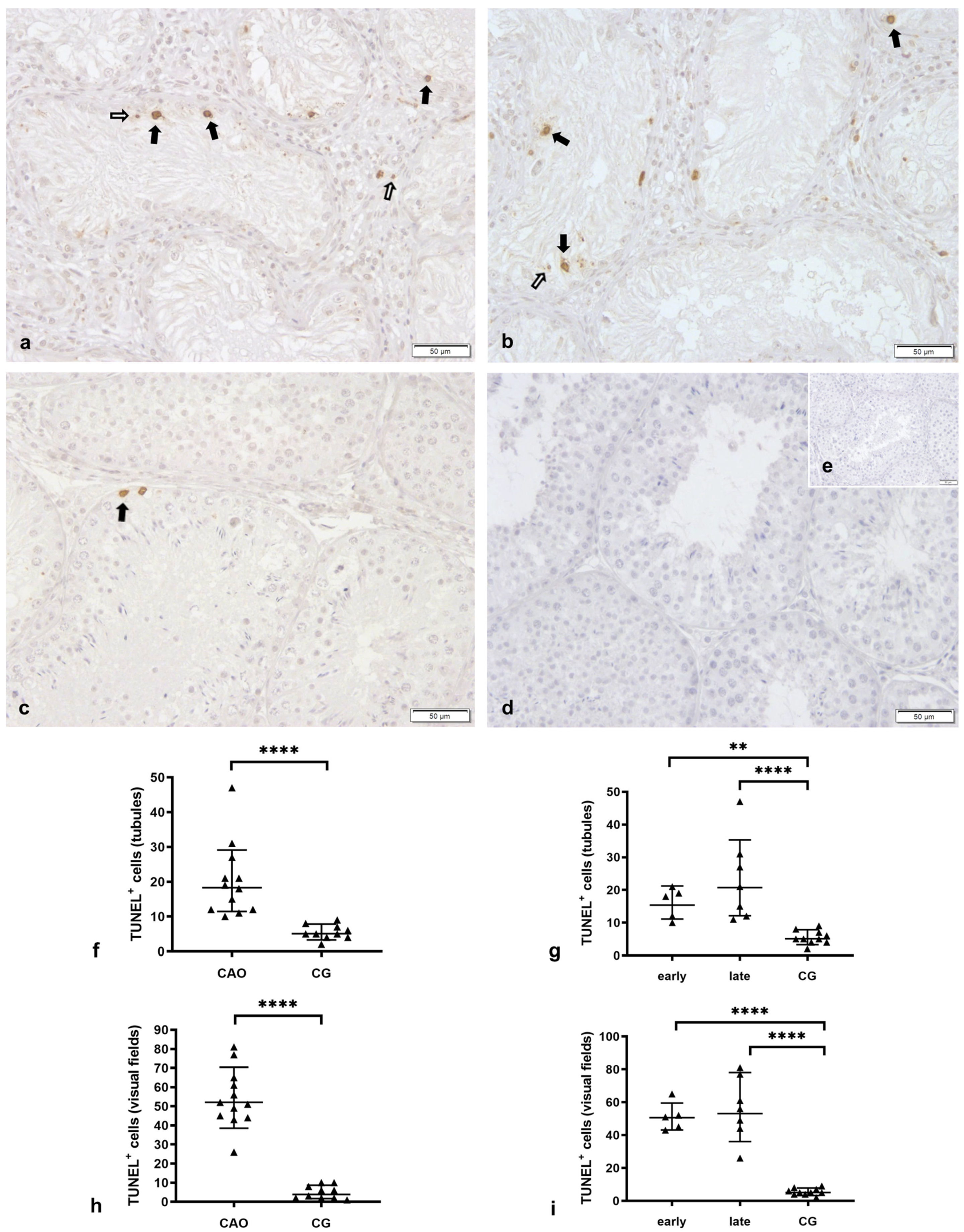
2.1. Number of Apoptotic Cells by TUNEL Method
Apoptosis Correlates with the Degree of Spermatogenesis Disturbance
2.2. Intrinsic Pathway of Apoptosis
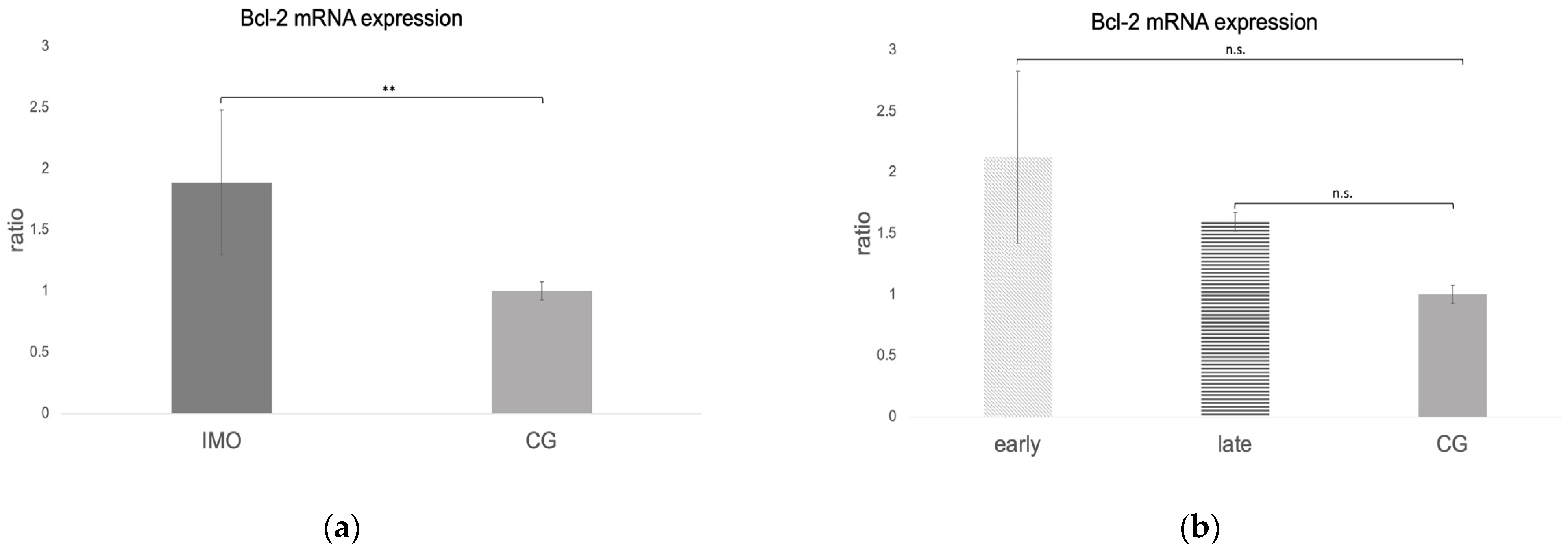
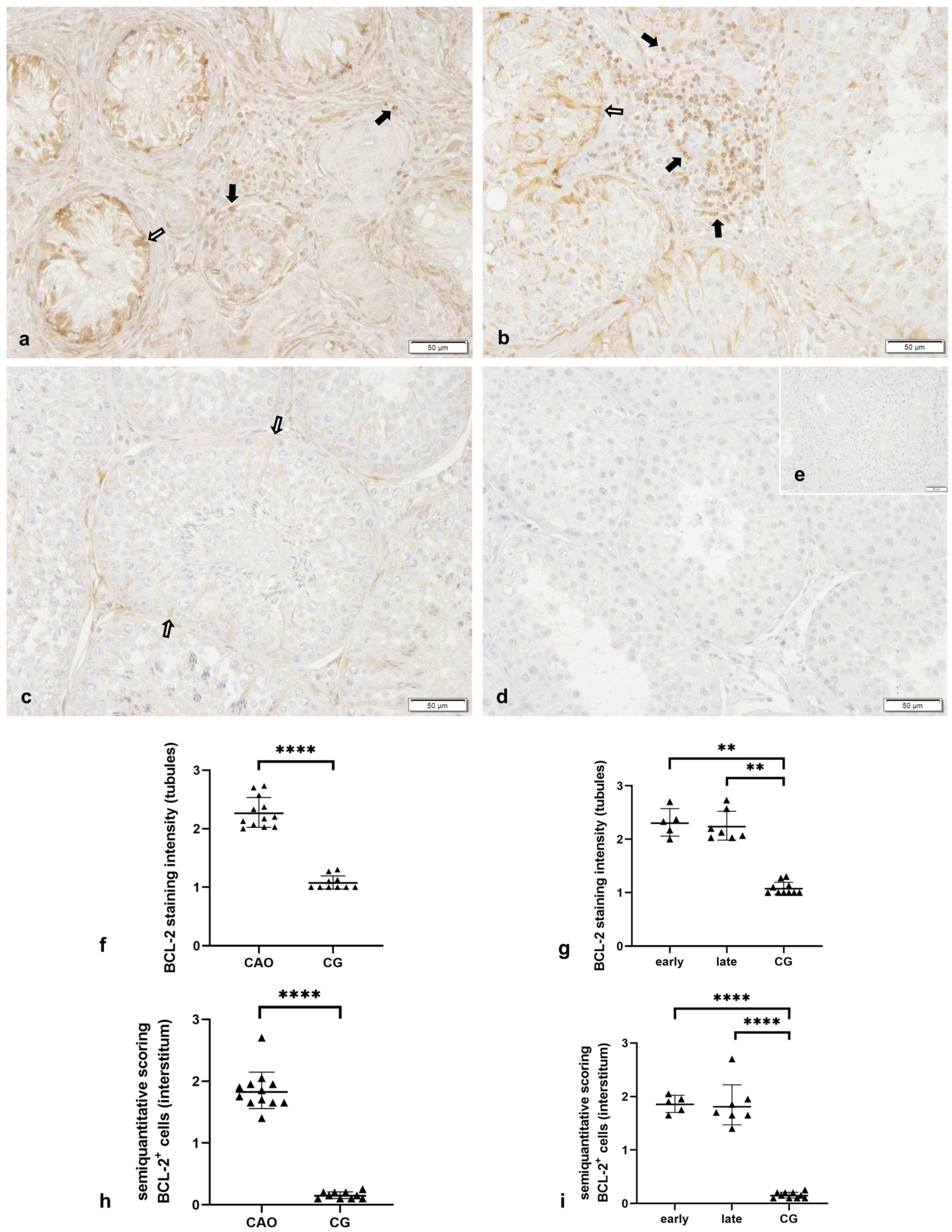
2.2.1. Bcl-2 Is Upregulated in CAO
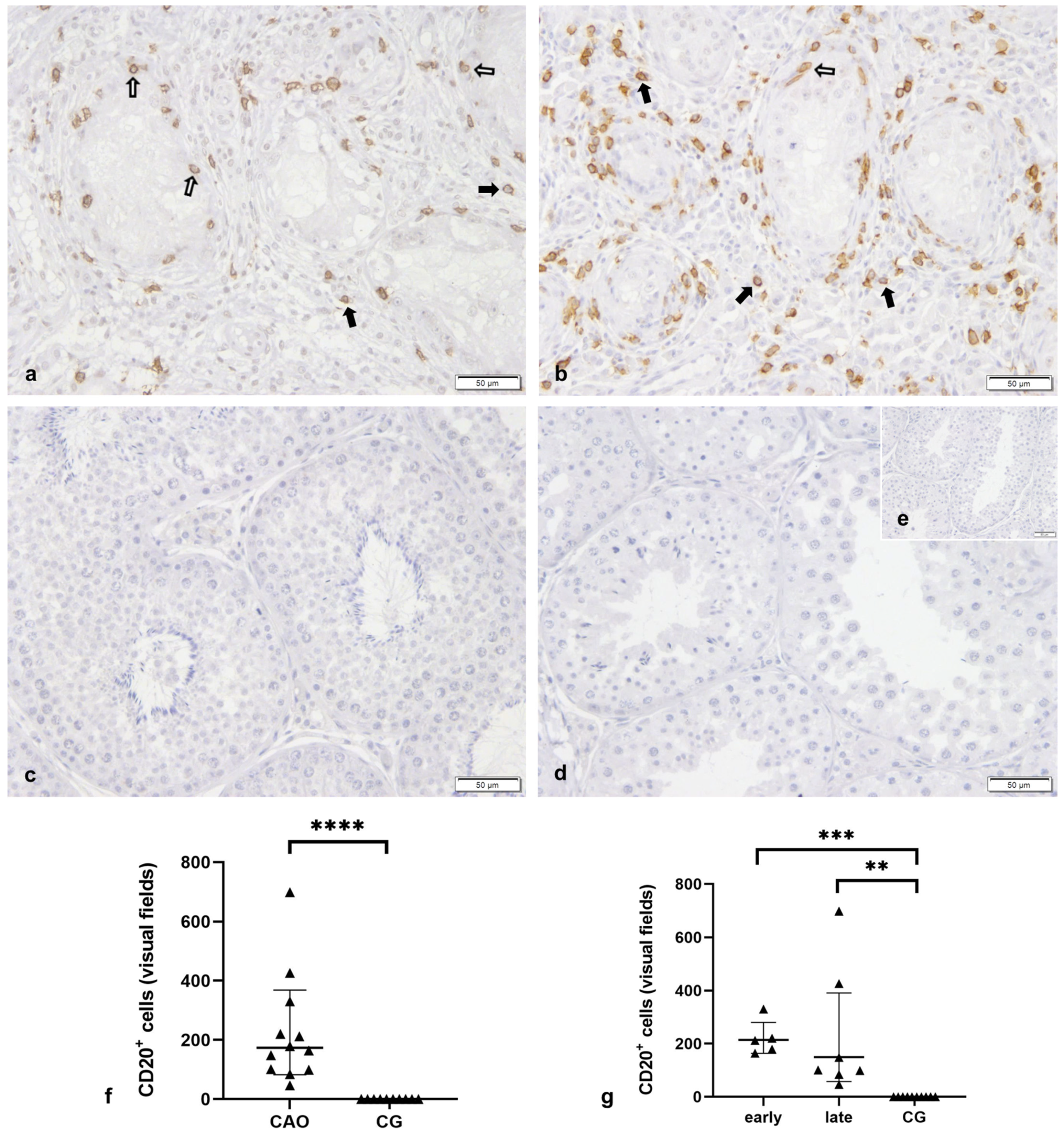
2.2.2. Bcl-2 Upregulation in CAO Is Associated with CD20-Expressing B Lymphocytes
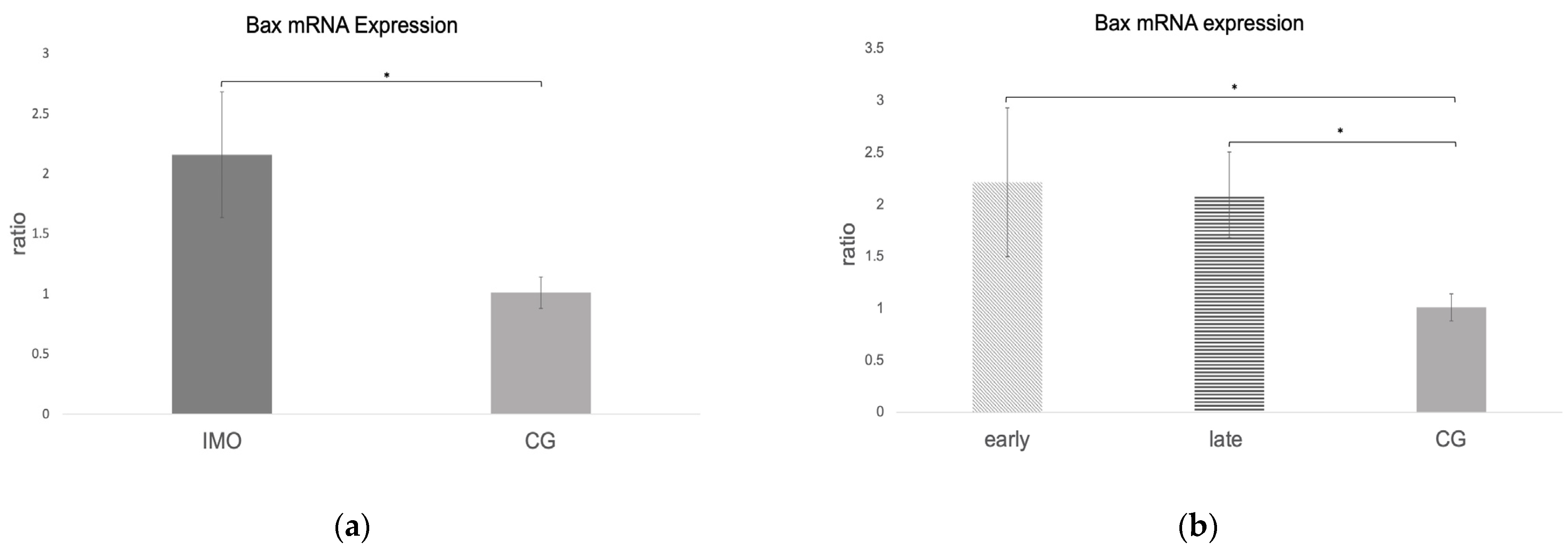
2.2.3. Bax and Caspase 9 mRNA Expression Is Increased in CAO
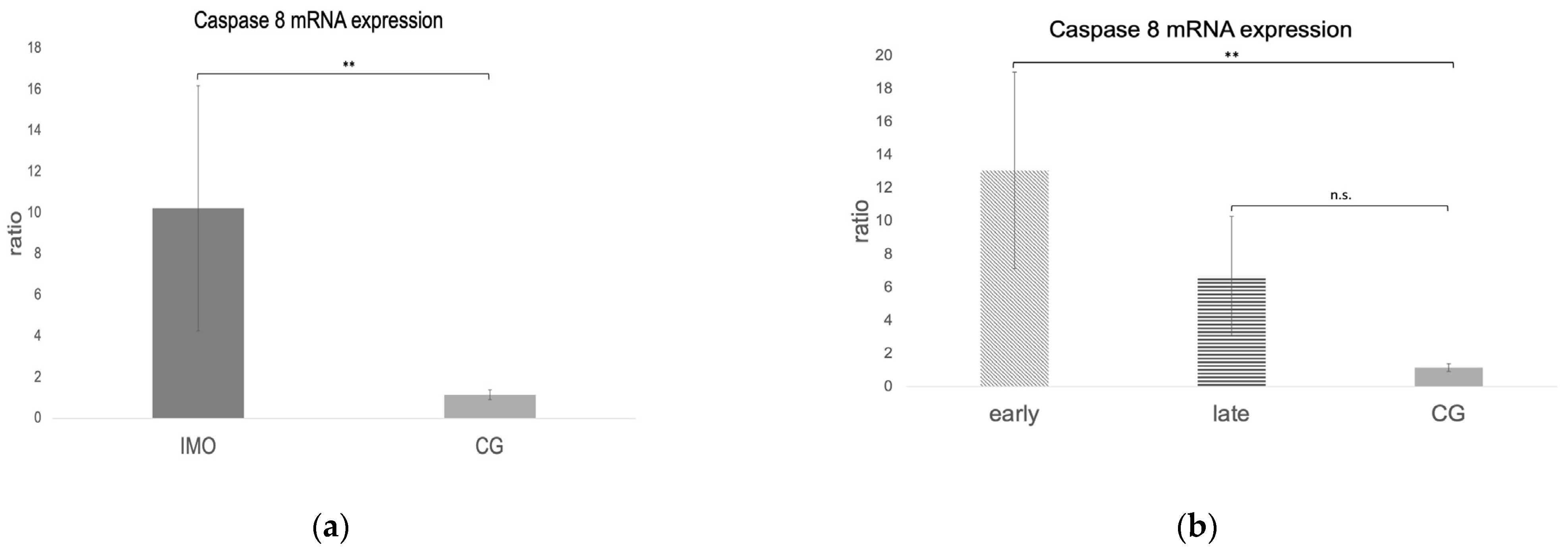
2.3. Extrinsic Pathway
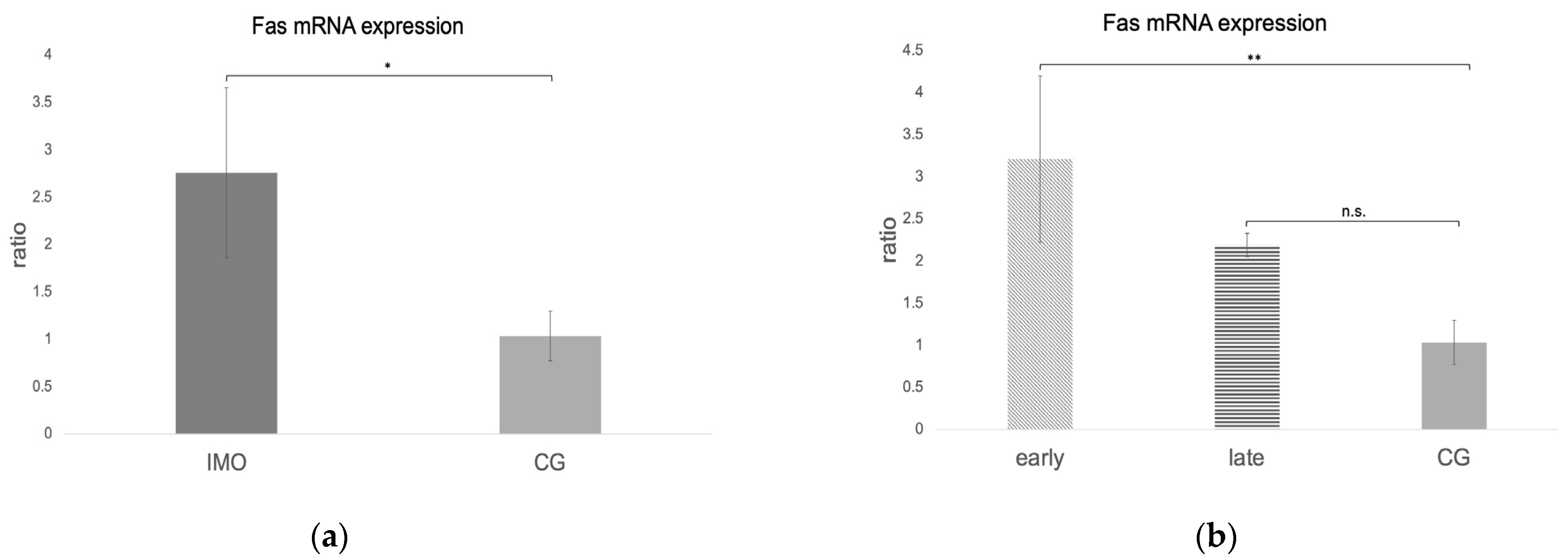
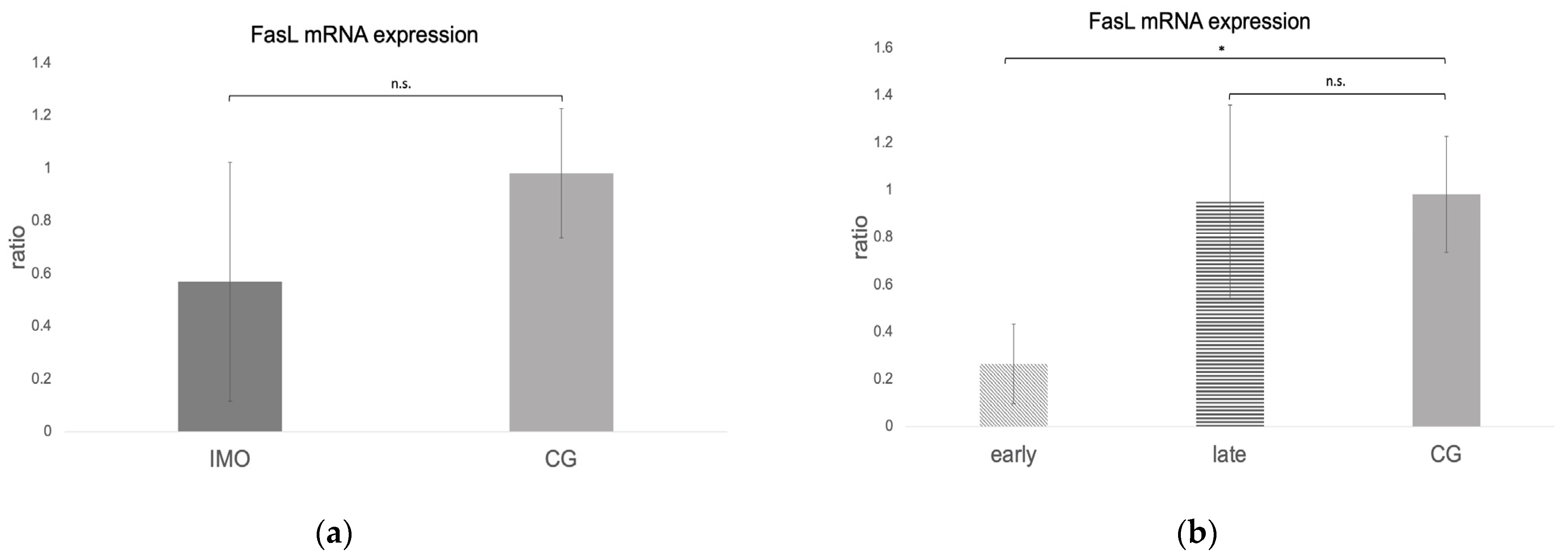
Fas, FasLigand, and Caspase 8 mRNA Expression
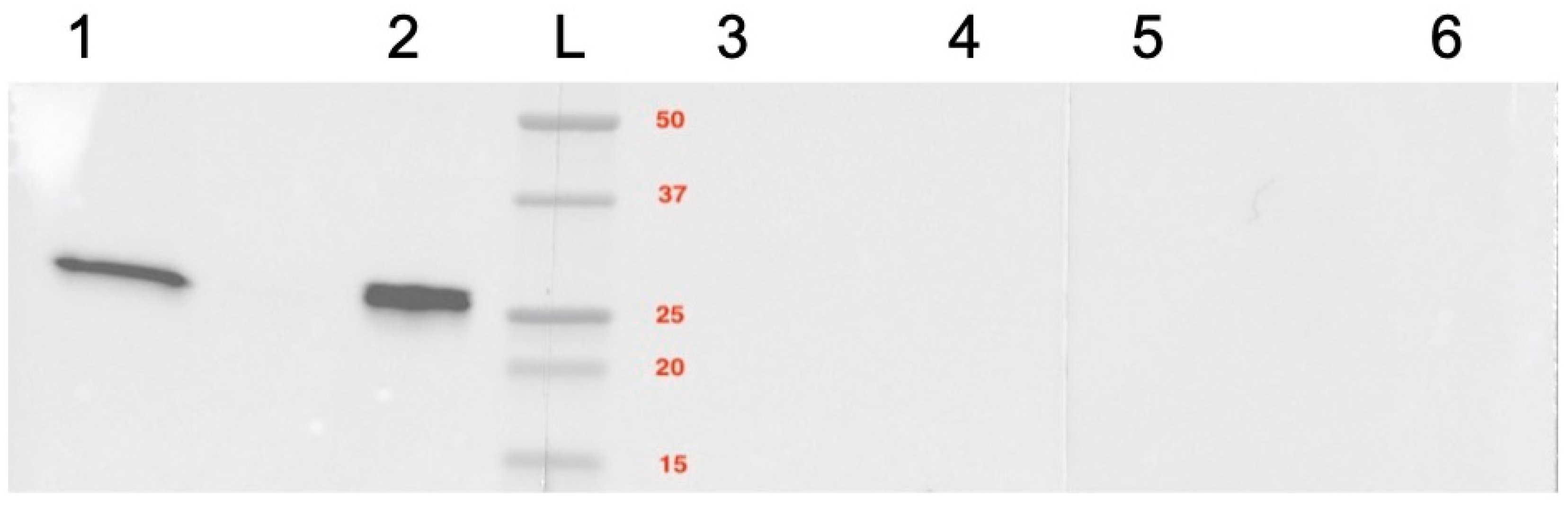
2.4. Caspase 3 mRNA and Protein Expression
3. Discussion
4. Materials and Methods
4.1. Study Design, Animals, and Grouping
4.2. Study Design, Sample Collection, and Processing
4.3. Quantitative Real-Time PCR (RT-qPCR)
4.4. Protein Extraction and Western Blot Analyses
4.5. TUNEL Method, Procedure, and Evaluation
4.6. Immunohistochemistry and Evaluation of Bcl-2, CD20, and Caspase 3 Staining
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romagnoli, S. Two Common Causes of Infertility in the Male Dog. Proc. World Small Anim. Vet. Assoc. 2006, 687–690. [Google Scholar]
- Behrens Mathiesen, C.; Körber, H.; Goericke-Pesch, S. Inflammatory Changes in Dogs with Azoospermia—A Normal Finding? Reprod. Dom. Anim. 2017, 52, 8–9. [Google Scholar]
- Goericke-Pesch, S.; Wehrend, A. Determination of the Alkaline Phosphatase in Canine Seminalplasma to Differentiate Azoospermia and Incomplete Ejaculation (German). Tierärztl. Prax. 2008, 36, A24. [Google Scholar]
- Romagnoli, S.; Bonaccini, P.; Stelletta, C.; Garolla, A.; Menegazzo, M.; Foresta, C.; Mollo, A.; Milani, C.; Gelli, D. Clinical Use of Testicular Fine Needle Aspiration Cytology in Oligozoospermic and Azoospermic Dogs. Reprod. Domest. Anim. 2009, 44 (Suppl. 2), 329–333. [Google Scholar] [CrossRef] [PubMed]
- Goericke-Pesch, S.; Reifarth, L.; Behrens Mathiesen, C.; Schuler, G.; Umbach, A.K.; Korber, H. Chronic Immune-Mediated Orchitis Is the Major Cause of Acquired Non-Obstructive Azoospermia in Dogs. Front. Vet. Sci. 2022, 9, 865967. [Google Scholar] [CrossRef] [PubMed]
- Fritz, T.E.; Lombard, S.A.; Tyler, S.A.; Norris, W.P. Pathology and Familial Incidence of Orchitis and Its Relation to Thyroiditis in a Closed Beagle Colony. Exp. Mol. Pathol. 1976, 24, 142–158. [Google Scholar] [CrossRef]
- Pröbstl, C.; Umbach, A.; Beineke, A.; Korber, H.; Goericke-Pesch, S. Immune Cell Characterization in Spontaneous Autoimmune Orchitis in Dogs. Theriogenology 2022, 187, 219–226. [Google Scholar] [CrossRef]
- Allen, W.E.; Longstaffe, J.A. Spermatogenic Arrest Associated with Focal Degenerative Orchitis in Related Dogs. J. Small Anim. Pract. 1982, 23, 337–343. [Google Scholar] [CrossRef]
- Allen, W.E.; Patel, J.R. Autoimmune Orchitis in Two Related Dogs. J. Small Anim. Pract. 1982, 23, 713–718. [Google Scholar] [CrossRef]
- Casal, M.L. Canine Autoimmune Orchitis. Clin. Theriogenol. 2012, 4, 251–254. [Google Scholar]
- Davidson, A.P.; von Dehn, B.J.; Schlafer, D.H. Adult-Onset Lymphoplasmacytic Orchitis in a Labrador Retriever Stud Dog. Top. Companion Anim. Med. 2015, 30, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Matschurat, C.; Rode, K.; Hollenbach, J.; Wolf, K.; Urhausen, C.; Beineke, A.; Gunzel-Apel, A.R.; Brehm, R. Impaired Spermatogenesis, Tubular Wall Disruption, Altered Blood-Testis Barrier Composition and Intratubular Lymphocytes in an Infertile Beagle Dog—A putative Case of Autoimmune Orchitis. Histol. Histopathol. 2019, 34, 525–535. [Google Scholar] [CrossRef]
- Metcalfe, S.S.; Gunn, I.M.; Champness, K.A. Azoospermia in Two Labrador Retrievers. Aust. Vet. J. 1999, 77, 570–573. [Google Scholar] [CrossRef]
- Tung, K.S.; Ellis, L.E.; Childs, G.V.; Dufau, M. The Dark Mink: A Model of Male Infertility. Endocrinology 1984, 114, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, R.M.; Yoon, S.R.; Akpovi, C.D.; Silvas, E.; Vitale, M.L. Defects in the Regulatory Clearance Mechanisms Favor the Breakdown of Self-Tolerance during Spontaneous Autoimmune Orchitis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R743–R762. [Google Scholar] [CrossRef] [PubMed]
- Furbeth, C.; Hubner, G.; Thoenes, G.H. Spontaneous Immune Complex Orchitis in Brown Norway Rats. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1989, 57, 37–45. [Google Scholar] [CrossRef]
- Tung, K.S.; Teuscher, C. Mechanisms of Autoimmune Disease in the Testis and Ovary. Hum. Reprod. Update 1995, 1, 35–50. [Google Scholar] [CrossRef]
- Schuppe, H.C.; Pilatz, A.; Hossain, H.; Meinhardt, A.; Bergmann, M.; Haidl, G.; Weidner, W. Orchitis and Male Infertility. Urologe A 2010, 49, 629–635. [Google Scholar] [CrossRef]
- Pilatz, A.; Fijak, M.; Wagenlehner, F.; Schuppe, H.C. Orchitis. Urologe A 2019, 58, 697–710. [Google Scholar] [CrossRef]
- Silva, C.A.; Cocuzza, M.; Carvalho, J.F.; Bonfa, E. Diagnosis and Classification of Autoimmune Orchitis. Autoimmun. Rev. 2014, 13, 431–434. [Google Scholar] [CrossRef]
- Fijak, M.; Meinhardt, A. The Testis in Immune Privilege. Immunol. Rev. 2006, 213, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Fijak, M.; Pilatz, A.; Hedger, M.P.; Nicolas, N.; Bhushan, S.; Michel, V.; Tung, K.S.K.; Schuppe, H.C.; Meinhardt, A. Infectious, Inflammatory and ‘Autoimmune’ Male Factor Infertility: How Do Rodent Models Inform Clinical Practice? Hum. Reprod. Update 2018, 24, 416–441. [Google Scholar] [CrossRef]
- Jacobo, P.; Guazzone, V.A.; Theas, M.S.; Lustig, L. Testicular Autoimmunity. Autoimmun. Rev. 2011, 10, 201–204. [Google Scholar] [CrossRef]
- Lustig, L.; Guazzone, V.A.; Theas, M.S.; Pleuger, C.; Jacobo, P.; Perez, C.V.; Meinhardt, A.; Fijak, M. Pathomechanisms of Autoimmune Based Testicular Inflammation. Front. Immunol. 2020, 11, 583135. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Rodriguez, J.; Martinez-Garcia, C. Spontaneous Germ Cell Death in the Testis of the Adult Rat Takes the Form of Apoptosis: Re-Evaluation of Cell Types That Exhibit the Ability to Die during Spermatogenesis. Cell Prolif. 1996, 29, 13–31. [Google Scholar] [CrossRef]
- Billig, H.; Furuta, I.; Rivier, C.; Tapanainen, J.; Parvinen, M.; Hsueh, A.J. Apoptosis in Testis Germ Cells: Developmental Changes in Gonadotropin Dependence and Localization to Selective Tubule Stages. Endocrinology 1995, 136, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Huckins, C. The Morphology and Kinetics of Spermatogonial Degeneration in Normal Adult Rats: An Analysis Using a Simplified Classification of the Germinal Epithelium. Anat. Rec. 1978, 190, 905–926. [Google Scholar] [CrossRef]
- Oakberg, E.F. A Description of Spermiogenesis in the Mouse and Its Use in Analysis of the Cycle of the Seminiferous Epithelium and Germ Cell Renewal. Am. J. Anat. 1956, 99, 391–413. [Google Scholar] [CrossRef]
- Lee, J.; Richburg, J.H.; Younkin, S.C.; Boekelheide, K. The Fas System Is a Key Regulator of Germ Cell Apoptosis in the Testis. Endocrinology 1997, 138, 2081–2088. [Google Scholar] [CrossRef]
- Rodriguez, I.; Ody, C.; Araki, K.; Garcia, I.; Vassalli, P. An Early and Massive Wave of Germinal Cell Apoptosis Is Required for the Development of Functional Spermatogenesis. EMBO J. 1997, 16, 2262–2270. [Google Scholar] [CrossRef]
- Blanco-Rodriguez, J. A matter of death and life: The Significance of Germ Cell Death during Spermatogenesis. Int. J. Androl. 1998, 21, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Koji, T.; Hishikawa, Y. Germ Cell Apoptosis and Its Molecular Trigger in Mouse Testes. Arch. Histol. Cytol. 2003, 66, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Theas, M.S.; Rival, C.; Lustig, L. Germ Cell Apoptosis in Autoimmune Orchitis: Involvement of the Fas-FasL System. Am. J. Reprod. Immunol. 2003, 50, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Theas, M.S.; Rival, C.; Dietrich, S.J.; Guazzone, V.A.; Lustig, L. Death Receptor and Mitochondrial Pathways Are Involved in Germ Cell Apoptosis in an Experimental Model of Autoimmune Orchitis. Hum. Reprod. 2006, 21, 1734–1742. [Google Scholar] [CrossRef] [PubMed]
- Jacobo, P.V.; Fass, M.; Perez, C.V.; Jarazo-Dietrich, S.; Lustig, L.; Theas, M.S. Involvement of Soluble Fas Ligand in Germ Cell Apoptosis in Testis of Rats Undergoing Autoimmune Orchitis. Cytokine 2012, 60, 385–392. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Cohen, G.M. Caspases: The Executioners of Apoptosis. Biochem. J. 1997, 326 Pt 1, 1–16. [Google Scholar] [CrossRef]
- Ashe, P.C.; Berry, M.D. Apoptotic Signaling Cascades. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 199–214. [Google Scholar] [CrossRef]
- Zimmermann, K.C.; Bonzon, C.; Green, D.R. The Machinery of Programmed Cell Death. Pharmacol. Ther. 2001, 92, 57–70. [Google Scholar] [CrossRef]
- Ashkenazi, A.; Dixit, V.M. Death Receptors: Signaling and Modulation. Science 1998, 281, 1305–1308. [Google Scholar] [CrossRef]
- Itoh, N.; Yonehara, S.; Ishii, A.; Yonehara, M.; Mizushima, S.; Sameshima, M.; Hase, A.; Seto, Y.; Nagata, S. The Polypeptide Encoded by the Cdna for Human Cell Surface Antigen Fas Can Mediate Apoptosis. Cell 1991, 66, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.C.; Hahne, M.; Schroeter, M.; Frei, K.; Fontana, A.; Villunger, A.; Newton, K.; Tschopp, J.; Strasser, A. Activation of Fas by Fasl Induces Apoptosis by a Mechanism That Cannot Be Blocked by Bcl-2 or Bcl-x(L). Proc. Natl. Acad. Sci. USA 1999, 96, 14871–14876. [Google Scholar] [CrossRef] [PubMed]
- Krajewski, S.; Tanaka, S.; Takayama, S.; Schibler, M.J.; Fenton, W.; Reed, J.C. Investigation of the Subcellular Distribution of the Bcl-2 Oncoprotein: Residence in the Nuclear Envelope, Endoplasmic Reticulum, and Outer Mitochondrial Membranes. Cancer Res. 1993, 53, 4701–4714. [Google Scholar] [PubMed]
- Hockenbery, D.; Nunez, G.; Milliman, C.; Schreiber, R.D.; Korsmeyer, S.J. Bcl-2 is an Inner Mitochondrial Membrane Protein That Blocks Programmed Cell Death. Nature 1990, 348, 334–336. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Bhalla, K.; Kim, C.N.; Ibrado, A.M.; Cai, J.; Peng, T.I.; Jones, D.P.; Wang, X. Prevention of Apoptosis by Bcl-2: Release of Cytochrome C from Mitochondria Blocked. Science 1997, 275, 1129–1132. [Google Scholar] [CrossRef]
- Orrenius, S. Mitochondrial Regulation of Apoptotic Cell Death. Toxicol. Lett. 2004, 149, 19–23. [Google Scholar] [CrossRef]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome C and Datp-Dependent Formation of Apaf-1/Caspase-9 Complex Initiates an Apoptotic Protease Cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef]
- Kuerban, M.; Naito, M.; Hirai, S.; Terayama, H.; Qu, N.; Musha, M.; Ikeda, A.; Koji, T.; Itoh, M. Involvement of Fas/Fas-L and Bax/Bcl-2 Systems in Germ Cell Death Following Immunization with Syngeneic Testicular Germ Cells in Mice. J. Androl. 2012, 33, 824–831. [Google Scholar] [CrossRef]
- Tedder, T.F.; Klejman, G.; Schlossman, S.F.; Saito, H. Structure of the Gene Encoding the Human B Lymphocyte Differentiation Antigen Cd20 (B1). J. Immunol. 1989, 142, 2560–2568. [Google Scholar] [CrossRef]
- Stashenko, P.; Nadler, L.M.; Hardy, R.; Schlossman, S.F. Characterization of a Human B Lymphocyte-Specific Antigen. J. Immunol. 1980, 125, 1678–1685. [Google Scholar] [CrossRef]
- Pentikainen, V.; Erkkila, K.; Dunkel, L. Fas Regulates Germ Cell Apoptosis in the Human Testis In Vitro. Am. J. Physiol. 1999, 276, E310–E316. [Google Scholar] [CrossRef]
- Henning, H.; Masal, C.; Herr, A.; Wolf, K.; Urhausen, C.; Beineke, A.; Beyerbach, M.; Kramer, S.; Gunzel-Apel, A.R. Effect of Short-Term Scrotal Hyperthermia on Spermatological Parameters, Testicular Blood Flow and Gonadal Tissue in Dogs. Reprod. Domest. Anim. 2014, 49, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, E.; Hori, T.; Tsutsui, T. Azoospermia of Dogs with Apoptotic Germ Cells and Leydig Cells. J. Vet. Med. Sci. 2000, 62, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Croci, M.; Dettwiler, M.; Vaughan, L.; Guscetti, F. Immunohistochemical Expression of Bax and Bak in Canine Non-Neoplastic Tissues. Vet. J. 2013, 198, 131–140. [Google Scholar] [CrossRef]
- Saraste, A.; Pulkki, K. Morphologic and Biochemical Hallmarks of Apoptosis. Cardiovasc. Res. 2000, 45, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Gavrieli, Y.; Sherman, Y.; Ben-Sasson, S.A. Identification of Programmed Cell Death In Situ via Specific Labeling of Nuclear DNA Fragmentation. J. Cell Biol. 1992, 119, 493–501. [Google Scholar] [CrossRef]
- Majtnerova, P.; Rousar, T. An Overview of Apoptosis Assays Detecting DNA Fragmentation. Mol. Biol. Rep. 2018, 45, 1469–1478. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Chen, Z.; Huang, X. Long-Term Reproductive Consequences of No-Scalpel Vasectomy in Beagles. J. Huazhong Univ. Sci. Technol. Med. Sci. 2012, 32, 899–905. [Google Scholar] [CrossRef]
- Mirzayans, R.; Murray, D. Do Tunel and Other Apoptosis Assays Detect Cell Death in Preclinical Studies? Int. J. Mol. Sci. 2020, 21, 9090. [Google Scholar] [CrossRef] [PubMed]
- Theas, M.S.; Rival, C.; Jarazo-Dietrich, S.; Jacobo, P.; Guazzone, V.A.; Lustig, L. Tumour Necrosis Factor-Alpha Released by Testicular Macrophages Induces Apoptosis of Germ Cells in Autoimmune Orchitis. Hum. Reprod. 2008, 23, 1865–1872. [Google Scholar] [CrossRef]
- He, X.; Wu, J.; Yuan, L.; Lin, F.; Yi, J.; Li, J.; Yuan, H.; Shi, J.; Yuan, T.; Zhang, S.; et al. Lead Induces Apoptosis in Mouse Tm3 Leydig Cells through the Fas/Fasl Death Receptor Pathway. Environ. Toxicol. Pharmacol. 2017, 56, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ren, X.; Hu, X.; Zhou, L.; Zhang, C.; Zhang, M. Cadmium-Induced Apoptosis through Reactive Oxygen Species-Mediated Mitochondrial Oxidative Stress and the Jnk Signaling Pathway in Tm3 Cells, a Model of Mouse Leydig Cells. Toxicol. Appl. Pharmacol. 2019, 368, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Aoyama-Ishikawa, M.; Uemura, M.; Yamashita, H.; Koga, Y.; Terashima, M.; Usami, M.; Kotani, J.; Hirata, J. Interleukin-18 Levels and Mouse Leydig Cell Apoptosis during Lipopolysaccharide-Induced Acute Inflammatory Conditions. J. Reprod. Immunol. 2020, 141, 103167. [Google Scholar] [CrossRef]
- Beumer, T.L.; Roepers-Gajadien, H.L.; Gademan, I.S.; Lock, T.M.; Kal, H.B.; De Rooij, D.G. Apoptosis Regulation in the Testis: Involvement of Bcl-2 Family Members. Mol. Reprod. Dev. 2000, 56, 353–359. [Google Scholar] [CrossRef]
- Oltvai, Z.N.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 Heterodimerizes In Vivo with a Conserved Homolog, Bax, That Accelerates Programmed Cell Death. Cell 1993, 74, 609–619. [Google Scholar] [CrossRef]
- Korsmeyer, S.J.; Shutter, J.R.; Veis, D.J.; Merry, D.E.; Oltvai, Z.N. Bcl-2/Bax: A Rheostat That Regulates an Anti-Oxidant Pathway and Cell Death. Semin. Cancer Biol. 1993, 4, 327–332. [Google Scholar] [PubMed]
- Russell, L.D.; Chiarini-Garcia, H.; Korsmeyer, S.J.; Knudson, C.M. Bax-Dependent Spermatogonia Apoptosis Is Required for Testicular Development and Spermatogenesis. Biol. Reprod. 2002, 66, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Si, M.; Li, X.; Zou, H.; Gu, J.; Yuan, Y.; Liu, X.; Liu, Z.; Bian, J. Zearalenone Induces Apoptosis of Rat Sertoli Cells through Fas-Fas Ligand and Mitochondrial Pathway. Environ. Toxicol. 2019, 34, 424–433. [Google Scholar] [CrossRef]
- Lee, Y.S.; Yoon, H.J.; Oh, J.H.; Park, H.J.; Lee, E.H.; Song, C.W.; Yoon, S. 1,3-Dinitrobenzene Induces Apoptosis in Tm4 Mouse Sertoli Cells: Involvement of the C-Jun N-Terminal Kinase (Jnk) Mapk Pathway. Toxicol. Lett. 2009, 189, 145–151. [Google Scholar] [CrossRef]
- Aslani, F.; Sebastian, T.; Keidel, M.; Frohlich, S.; Elsasser, H.P.; Schuppe, H.C.; Klug, J.; Mahavadi, P.; Fijak, M.; Bergmann, M.; et al. Resistance to Apoptosis and Autophagy Leads to Enhanced Survival in Sertoli Cells. Mol. Hum. Reprod. 2017, 23, 370–380. [Google Scholar] [CrossRef]
- Reifarth, L.; Körber, H.; Goericke-Pesch, S. Analysis of Putative Stem Cell Markers in Dogs with Spontaneous Immune Mediated Orchitis. Reprod. Domest. Anim. 2022, 57, 12. [Google Scholar]
- Hockenbery, D.M.; Zutter, M.; Hickey, W.; Nahm, M.; Korsmeyer, S.J. Bcl2 Protein Is Topographically Restricted in Tissues Characterized by Apoptotic Cell Death. Proc. Natl. Acad. Sci. USA 1991, 88, 6961–6965. [Google Scholar] [CrossRef]
- Nunez, G.; Hockenbery, D.; McDonnell, T.J.; Sorensen, C.M.; Korsmeyer, S.J. Bcl-2 Maintains B Cell Memory. Nature 1991, 353, 71–73. [Google Scholar] [CrossRef]
- McDonnell, T.J.; Deane, N.; Platt, F.M.; Nunez, G.; Jaeger, U.; McKearn, J.P.; Korsmeyer, S.J. Bcl-2-Immunoglobulin Transgenic Mice Demonstrate Extended B Cell Survival and Follicular Lymphoproliferation. Cell 1989, 57, 79–88. [Google Scholar] [CrossRef]
- McDonnell, T.J.; Nunez, G.; Platt, F.M.; Hockenberry, D.; London, L.; McKearn, J.P.; Korsmeyer, S.J. Deregulated Bcl-2-Immunoglobulin Transgene Expands a Resting but Responsive Immunoglobulin M and D-Expressing B-Cell Population. Mol. Cell Biol. 1990, 10, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Vaux, D.L.; Cory, S.; Adams, J.M. Bcl-2 Gene Promotes Haemopoietic Cell Survival and Cooperates with C-Myc to Immortalize Pre-B Cells. Nature 1988, 335, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Voutsadakis, I.A. Apoptosis and the Pathogenesis of Lymphoma. Acta Oncol. 2000, 39, 151–156. [Google Scholar] [CrossRef]
- Sen, R. Control of B Lymphocyte Apoptosis by the Transcription Factor Nf-Kappab. Immunity 2006, 25, 871–883. [Google Scholar] [CrossRef]
- Bruun Jensen, H.; Engelhard Holm, J.; Körber, H.; Goericke-Pesch, S. Expression of Connexin 43 and Androgen Receptor in Testes of Azoospermic Dogs. Reprod. Domest. Anim. 2018, 53, 5. [Google Scholar]
- Vazquez, M.I.; Catalan-Dibene, J.; Zlotnik, A. B Cells Responses and Cytokine Production Are Regulated by Their Immune Microenvironment. Cytokine 2015, 74, 318–326. [Google Scholar] [CrossRef]
- Perez, C.V.; Sobarzo, C.M.; Jacobo, P.V.; Pellizzari, E.H.; Cigorraga, S.B.; Denduchis, B.; Lustig, L. Loss of Occludin Expression and Impairment of Blood-Testis Barrier Permeability in Rats with Autoimmune Orchitis: Effect of Interleukin 6 on Sertoli Cell Tight Junctions. Biol. Reprod. 2012, 87, 122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yin, Y.; Wang, G.; Liu, Z.; Liu, L.; Sun, F. Interleukin-6 Disrupts Blood-Testis Barrier through Inhibiting Protein Degradation or Activating Phosphorylated Erk in Sertoli Cells. Sci. Rep. 2014, 4, 4260. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xia, W.; Mruk, D.D.; Wang, C.Q.; Yan, H.H.; Siu, M.K.; Lui, W.Y.; Lee, W.M.; Cheng, C.Y. Tumor Necrosis Factor {Alpha} Reversibly Disrupts the Blood-Testis Barrier and Impairs Sertoli-Germ Cell Adhesion in the Seminiferous Epithelium of Adult Rat Testes. J. Endocrinol. 2006, 190, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Pröbstl, C.; Körber, H.; Goericke-Pesch, S. Germ Cell Apoptosis Induced by Tumor Necrosis Factor (Tnf) Alpha in Spontaneous Autoimmune Orchitis in Dogs Is Mainly Triggered by Interaction with Tnf Receptor 2 on Spermatogonia. Reprod. Domest. Anim. 2022, 57, 18. [Google Scholar]
- Kim, S.K.; Yoon, Y.D.; Park, Y.S.; Seo, J.T.; Kim, J.H. Involvement of the Fas-Fas Ligand System and Active Caspase-3 in Abnormal Apoptosis in Human Testes with Maturation Arrest and Sertoli Cell-Only Syndrome. Fertil. Steril. 2007, 87, 547–553. [Google Scholar] [CrossRef]
- Jhun, H.; Park, H.J.; Lee, R.; Song, H.; Hur, T.Y.; Lee, S.; Park, J.K.; Lee, W.Y. Germ Cell-Specific Apoptosis by Extracellular Clusterin in Cryptorchid Dog Testes. Anim. Reprod. Sci. 2018, 193, 158–164. [Google Scholar] [CrossRef]
- Almeida, C.; Correia, S.; Rocha, E.; Alves, A.; Ferraz, L.; Silva, J.; Sousa, M.; Barros, A. Caspase Signalling Pathways in Human Spermatogenesis. J. Assist. Reprod. Genet. 2013, 30, 487–495. [Google Scholar] [CrossRef]
- Bellgrau, D.; Gold, D.; Selawry, H.; Moore, J.; Franzusoff, A.; Duke, R.C. A Role for Cd95 Ligand in Preventing Graft Rejection. Nature 1995, 377, 630–632. [Google Scholar] [CrossRef]
- Griffith, T.S.; Brunner, T.; Fletcher, S.M.; Green, D.R.; Ferguson, T.A. Fas Ligand-Induced Apoptosis as a Mechanism of Immune Privilege. Science 1995, 270, 1189–1192. [Google Scholar] [CrossRef]
- Head, J.R.; Neaves, W.B.; Billingham, R.E. Immune Privilege in the Testis. I. Basic Parameters of Allograft Survival. Transplantation 1983, 36, 423–431. [Google Scholar] [CrossRef]
- Jarazo Dietrich, S.; Fass, M.I.; Jacobo, P.V.; Sobarzo, C.M.; Lustig, L.; Theas, M.S. Inhibition of Nos-No System Prevents Autoimmune Orchitis Development in Rats: Relevance of No Released by Testicular Macrophages in Germ Cell Apoptosis and Testosterone Secretion. PLoS ONE 2015, 10, e0128709. [Google Scholar] [CrossRef]
- Yazdani, I.; Ghazi-Khansari, M.; Saeedi Saravi, S.S.; Nobakht, M.; Majdani, R.; Rezayat, S.M.; Mousavi, S.E.; Yari, A.; Dehpour, A.R. Nortriptyline Protects Testes against Germ Cell Apoptosis and Oxidative Stress Induced by Testicular Ischaemia/Reperfusion. Andrologia 2017, 49, e12605. [Google Scholar] [CrossRef] [PubMed]
- Lysiak, J.J.; Zheng, S.; Woodson, R.; Turner, T.T. Caspase-9-Dependent Pathway to Murine Germ Cell Apoptosis: Mediation by Oxidative Stress, Bax, and Caspase 2. Cell Tissue Res. 2007, 328, 411–419. [Google Scholar] [CrossRef]
- Riesenbeck, A.; Völger, D.; Hoffmann, B. Praxisnahe Beurteilung von Rüdensperma zur Bestimmung von VitalitäTsparametern. Tierärztliche Praxis 2001, 29, 116–120. [Google Scholar]
- Goericke-Pesch, S.; Spang, A.; Schulz, M.; Ozalp, G.; Bergmann, M.; Ludwig, C.; Hoffmann, B. Recrudescence of Spermatogenesis in the Dog Following Downregulation Using a Slow Release Gnrh Agonist Implant. Reprod. Domest. Anim. 2009, 44 (Suppl. 2), 302–308. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time Rt-Pcr. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Urhausen, C.; Beineke, A.; Piechotta, M.; Karre, I.; Beyerbach, M.; Gunzel-Apel, A.R. Apoptosis in the Uterotubal Junction and Oviductal Isthmus during the Estrous Cycle of the Bitch. Anat. Rec. 2011, 294, 342–348. [Google Scholar] [CrossRef]
- Rempel, L.M.; Korber, H.; Reichler, I.M.; Balogh, O.; Goericke-Pesch, S. Investigations on the Potential Role of Prostaglandin E2 in Canine Uterine Inertia. Theriogenology 2021, 175, 134–147. [Google Scholar] [CrossRef]
- Pillai-Kastoori, L.; Heaton, S.; Shiflett, S.D.; Roberts, A.C.; Solache, A.; Schutz-Geschwender, A.R. Antibody Validation for Western Blot: By the User, for the User. J. Biol. Chem. 2020, 295, 926–939. [Google Scholar] [CrossRef]
- Körber, H.; Goericke-Pesch, S. Expression of Ptgs2, Pgfs and Ptgfr during Downregulation and Restart of Spermatogenesis Following Gnrh Agonist Treatment in the Dog. Cell Tissue Res. 2019, 375, 531–541. [Google Scholar] [CrossRef]
- Sorenmo, K.U.; Goldschmidt, M.H.; Shofer, F.S.; Goldkamp, C.; Ferracone, J. Evaluation of Cyclooxygenase-1 and Cyclooxygenase-2 Expression and the Effect of Cyclooxygenase Inhibitors in Canine Prostatic Carcinoma. Vet. Comp. Oncol. 2004, 2, 13–23. [Google Scholar] [CrossRef] [PubMed]


| Primer | Accession Nr. | Forward Sequence (5′→3′) | Reverse Sequence (5′→3′) | Amplicon Length (bp) | Efficiency |
|---|---|---|---|---|---|
| Bcl-2 | NM_ 001002949.1 | ATGTGTGTGGAGAGCGTCAAC | GCCAGGAGAAGTCAAACAGAGG | 175 | 1.98 |
| Bax | NM_ 001003011.1 | TCAAGCGCATCGGAGATGAAC | TCGAAGGAAGTCCAGTGTCCAG | 248 | 1.98 |
| Fas | XM_ 022410445.1 | AGACCCGGAATACCAAGTGCAG | GGATGAGGACGCAAAACCACAG | 180 | 1.91 |
| FasL | NM_ 001287153.1 | CAGCGAAAGGCATGTAGCACC | CACCCCAGAGACAAGGGCAAT | 169 | 2.05 |
| Caspase 3 | NM_ 001003042.1 | TCCAGTCACTTTGTGCGATGC | CCAAACCAAACCAAACCAACCC | 218 | 2.02 |
| Caspase 8 | NM_ 001048029.1 | GCTTCAGATACCAGGCAGAGC | CTCCCGGCTCAAGAGAAACTTA | 115 | 2.19 |
| Caspase 9 | NM_ 001031633.1 | TGTCTAGTTTGCCCACTCCCAG | TGCGAAACAGCATTAGCGACC | 184 | 1.98 |
| GAPDH | NM_001003142 | GGCCAAGAGGGTCATCATCTC | GGGGCCGTCCACGGTCTTC | 229 | 1.93 |
| ß-actin | AF484115.1 | GCTGTGCTGTCCCTGTATG | GCGTACCCCTCATAGATGG | 98 | 1.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morawietz, J.; Körber, H.; Packeiser, E.-M.; Beineke, A.; Goericke-Pesch, S. Insights into Canine Infertility: Apoptosis in Chronic Asymptomatic Orchitis. Int. J. Mol. Sci. 2023, 24, 6083. https://doi.org/10.3390/ijms24076083
Morawietz J, Körber H, Packeiser E-M, Beineke A, Goericke-Pesch S. Insights into Canine Infertility: Apoptosis in Chronic Asymptomatic Orchitis. International Journal of Molecular Sciences. 2023; 24(7):6083. https://doi.org/10.3390/ijms24076083
Chicago/Turabian StyleMorawietz, Judith, Hanna Körber, Eva-Maria Packeiser, Andreas Beineke, and Sandra Goericke-Pesch. 2023. "Insights into Canine Infertility: Apoptosis in Chronic Asymptomatic Orchitis" International Journal of Molecular Sciences 24, no. 7: 6083. https://doi.org/10.3390/ijms24076083
APA StyleMorawietz, J., Körber, H., Packeiser, E.-M., Beineke, A., & Goericke-Pesch, S. (2023). Insights into Canine Infertility: Apoptosis in Chronic Asymptomatic Orchitis. International Journal of Molecular Sciences, 24(7), 6083. https://doi.org/10.3390/ijms24076083





