Abstract
Esophageal adenocarcinoma (EAC) is rapidly increasing in incidence and is associated with a poor prognosis. Barrett’s esophagus (BE) is a known precursor of esophageal adenocarcinoma. This review aims to explore Barrett’s esophagus, esophageal adenocarcinoma, and the progression from the former to the latter. An overview of the definition, diagnosis, epidemiology, and risk factors for both entities are presented, with special attention being given to the areas of debate in the literature. The progression from Barrett’s esophagus to esophageal adenocarcinoma is reviewed and the relevant molecular pathways are discussed. The definition of Barrett’s esophagus remains debated and without international consensus. This, alongside other factors, has made establishing the true prevalence of Barrett’s esophagus challenging. The degree of dysplasia can be a histological challenge, but is necessary to guide clinical management. The progression of BE to EAC is likely driven by inflammatory pathways, pepsin exposure, upregulation of growth factor pathways, and mitochondrial changes. Surveillance is maintained through serial endoscopic evaluation, with shorter intervals recommended for high-risk features.
1. Introduction
Barrett’s esophagus (BE) is defined by the American College of Gastroenterology (ACG) as intestinal metaplasia (IM) of the distal esophageal squamous epithelium and is a known precursor to esophageal adenocarcinoma (EAC) [1]. Although the true prevalence is difficult to ascertain, approximately two percent of adults are affected by BE, with 0.5–1% going on to develop EAC per year [2]. Gastroesophageal reflux disease (GERD) is thought to be a pathologic driver behind BE, yet 40% of patients diagnosed with BE report no symptoms of GERD at the time of diagnosis [3].
EAC is characterized by a strong male predominance, with a rapidly increasing incidence in the West, now surpassing the incidence of esophageal squamous cell carcinoma (ESCC) in several European, North American, and Oceanic countries [4]. Incidence increases with age, and certain genetic factors may play a role in the development of EAC from BE. The prognosis of EAC is strongly related to the stage at the time of diagnosis; however, due to most cases being diagnosed with the late-stage disease, the prognosis is poor, with a 20% overall survival rate at five years.
The purpose of this review is to summarize the current literature regarding EAC risk in patients with BE and to highlight the unanswered questions regarding these topics for future research.
2. Materials and Methods
A literature search of the Ovid Medline/PubMed database was conducted for papers published up to December 2022. The keywords used in the search included combinations of the American and English spellings of Barrett esophagus and esophagus neoplasm or adenocarcinoma. Studies were excluded if they were abstracts, not in English, or not peer reviewed. The references were examined for relevant articles and to ensure a comprehensive review of the literature. The articles were then interpreted and summarized into a cohesive review.
3. Definition and Diagnosis
BE is generally defined as columnar metaplasia of the distal esophagus; however, there is controversy concerning the diagnostic criteria for this disease process [5]. This is mainly in regard to whether IM is necessary for the diagnosis. IM is generally characterized by the presence of goblet cells; however, while the presence of goblet cells indicates intestinal metaplasia, not all intestinal metaplastic epithelia contain goblet cells [6]. The ACG, American Gastroenterological Association (AGA), and European Society of Gastrointestinal Endoscopy (ESGE) define BE as a change in the normal esophageal squamous mucosa, greater than 1 cm, that is visible endoscopically and demonstrates IM on biopsy, while the guidelines of the British Society of Gastroenterology (BSG), and Japan Esophageal Society (JES) do not require IM, and BE may be diagnosed with cardiac, intestinal, or oxyntic columnar mucosa on biopsy [3,7,8,9,10].
It is important to consider that differentiating goblet cells from pseudogoblet cells, which occur when columnar cells become distended with mucin and acquire a shape that mimics goblet cells, can be challenging. There is no histochemical or immunohistochemical stains that can reliably identify goblet cells or differentiate them from pseudogoblet cells when they are not apparent on standard H&E stain [11,12]. The difficulty in differentiating goblet and pseudogoblet cells was demonstrated with poor interobserver agreement in a study between seven GI pathologists [6].
In addition to the histologic challenges in the diagnosis, the risk of sampling bias is high. Studies have demonstrated a mosaic distribution of goblet cells within the areas of columnar metaplasia [13,14]. This results in a variation of goblet cell density, and therefore detection rate, depending on the location within the esophagus. The probability of detecting goblet cells has been demonstrated to increase proportionally with the number of biopsies obtained at the time of endoscopy [15]. In an observational study, a minimum of eight biopsies was deemed optimal to diagnose IM in patients with a columnar-lined esophagus on endoscopy. IM was diagnosed at a rate of 68% when eight biopsies were taken compared to 35% when only four biopsies were taken [16]. In addition to a sampling error, the presence and density of goblet cells can fluctuate with time and disease progression. In a study of 43 patients with less than 3 cm of columnar mucosa and no intestinal metaplasia on initial biopsy, Jones et al. demonstrated that 23% of the patients with suspected short-segment (<3 cm) BE had intestinal metaplasia on the repeat biopsy [17]. Kim et al. found that with the repeat biopsy six weeks after study entry and the initial biopsy, 21 (18%) of the 116 patients who met the criteria for BE did so only on one of the two exams [18].
The inclusion of IM in the diagnostic criteria of BE stems from the data supporting a significantly increased risk of dysplasia and EAC in patients with IM. It is argued that IM should be required for the diagnosis until columnar metaplasia without IM is proven to have a significant risk of progression to EAC. Chandrasoma et al. examined 214 patients with columnar metaplasia and demonstrated that IM was present in all 55 cases of dysplasia and/or EAC [19]. A large study of over 8500 patients in the North Ireland Cancer Registry demonstrated a hazard ratio (HR) of 3.54 [95% confidence interval (CI) 2.09–6.00] of developing BE-related adenocarcinoma in patients with IM compared to those without [20]. Advocates for requiring IM for the diagnosis of BE also point to the serious changes in quality of life that result from being diagnosed and labelled with a neoplastic process such as BE [21,22]. An analysis of the University of Chicago Medical Center database over 21 years concluded that the removal of goblet cells from the diagnostic criteria of BE would result in an ~150% increase in BE diagnoses; additionally, none of the patients without goblet cells (n = 118) developed EAC or dysplasia, with a mean 5.8 years of follow-up and 2.8 repeat endoscopies [23].
On the other hand, Kelty et al. found a similar risk of EAC in patients with columnar epithelia with and without IM (4.5% versus 3.6%, respectively) in a United Kingdom (UK)-based study, with a median follow-up period of 12 years [24]. Some have argued that sampling could explain the differing results between the Chandrasoma et al. and Kelty et al. studies, with the prior having a rigorous biopsy protocol compared to the latter [5,25]. Takubo et al. classified the mucosa surrounding early mucosal EAC obtained by endoscopic mucosal resection in 141 cases. Overall, 70% of the primary EAC were adjacent to cardiac/fundic-type mucosa as opposed to intestinal-type mucosa [26]. Similarly, Liu et al. found no significant differences in the DNA content abnormalities between samples with and without IM [27].
In summation, due to the concerns with the accurate identification of the presence of IM and the uncertainty regarding whether other mucosal types can progress to dysplasia and EAC, the BSG concluded that the presence of IM is not a defining prerequisite for BE, but that the presence of IM should be taken into account when considering clinical surveillance and follow-up [8]. On the other hand, the AGA and ACG concluded that the diagnosis of BE should include the presence of IM due to the lack of data supporting the risk of the malignant transformation of non-intestinal mucosa [3,7].
EAC is a gland-forming tumor most commonly with tubular, tubulopapillary, or papillary growth patterns. Well-differentiated tumors show >95% gland formation, moderately differentiated tumors show 50–95% gland formation, and poorly differentiated tumors show <50% gland formation [28]. The diagnosis of EAC is typically confirmed via an endoscopic biopsy. Endoscopic ultrasound (EUS) is currently the procedure of choice to determine the depth of the local invasion and clinical T-staging [29]. In a systematic review, EUS had a 91% true positive rate at differentiating T1/2 from T3/4 tumors, which is critical for clinical management [30]. EUS, computed tomography (CT), and fluorodeoxyglucose positron emission tomography (FDG-PET) are the principal modalities for evaluating regional lymph node metastases [29].
4. Epidemiology and Risk Factors
4.1. Epidemiology
The true prevalence of BE is difficult to estimate due to the sparsity of studies reporting upper esophageal endoscopic findings in the healthy adult population [25]. Multiple studies have attempted to estimate the prevalence of BE, which are summarized in Table 1. Cameron et al. conducted a prospective autopsy study over 18 months, in which 733 autopsies were examined, with seven cases of long segment BE, defined by the authors as an extension of the squamous columnar junction >3 cm above its normal location. The autopsies were predominantly male (61%), and no age distribution was reported. The authors noted that only three of the seven cases had intestinal metaplasia. These findings would suggest that BE was present in approximately 0.4% of the population. This was a significantly higher prevalence than they had estimated by using the number of clinically diagnosed cases from their local shared medical data system [31]. Two European studies in the mid-2000s attempted to estimate the prevalence of BE in the general population through the upper endoscopy of a random population. Ronkainen et al. (conducted in Sweden, n = 1000) and Zagari et al. (conducted in Italy, n = 1033) both required the presence of goblet cells for the diagnosis of BE, with a prevalence of 1.6% and 1.3%, respectively [32,33]. The study population of Zagari et al. was representative of the general Italian population in terms of age, sex, smoking, alcohol-use, and obesity, except for an under representation of participants younger than 35 years old [33]. The Ronkainen et al. study population was 51% female, with an average age of 53.5 years [32]. The risk factors for BE vary greatly between different populations, making these estimates unlikely to be accurate for any specific population [34,35].

Table 1.
Summary of discussed studies estimating Barrett’s Esophagus prevalence.
In 2020, esophageal cancer was ranked the seventh highest in incidence and sixth in mortality amongst all cancers worldwide, with over 600,000 new cases and 540,000 deaths [36]. Approximately 70% of esophageal cancer cases occur in men with a 2–3-fold increase in incidence and mortality compared to women [36]. Esophageal squamous cell carcinoma (ESCC) remains the most common worldwide, but there has been a dramatic epidemiologic shift since the 1990s, with the incidence of EAC surpassing that of ESCC in North America, Europe, and Australia [37]. This has been attributed to the decreased rates of heavy alcohol consumption and smoking, and the increasing rates of obesity [38,39]. A landmark paper by Pohl et al. in 2005 demonstrated that the incidence of EAC in the United States (US) increased by six-fold between 1975–2001, and that it was a true increase, as opposed to being due to overdiagnosis or reclassification [40]. EAC cases and deaths in the US are projected to continue increasing until 2030 [41]. Similarly, the incidence of EAC has been projected to rise dramatically during the 2005–2030 time period across high-income countries. By 2030, it is estimated that 1 in 100 men in the UK and the Netherlands may be diagnosed with EAC [42]. In the US, the incidence of EAC increased from 0.4 per 100,000 to 2.8 per 100,000 between 1973 and 2012; 92% of the cases diagnosed during that time were in persons aged >50 years [37].
4.2. Barrett’s Esophagus Risk Factors
Clinical, demographic, and lifestyle factors have been reported to affect the risk of BE. GERD has long been identified as the main risk factor for BE, yet 40% of patients do not reports any symptoms of GERD at the time of BE diagnosis [3,8]. On the other hand, an increasing severity and frequency of GERD symptoms has been shown to increase the risk of BE [43,44,45]. Similarly, an earlier age, <30, of GERD symptom onset increases the risk [46]. Studies in human studies, along with rat models, have demonstrated a role for reflux bile in the development of PPI-resistant BE [47]. Many studies have demonstrated an increased risk of BE with hiatal hernias [43,44]. A meta-analysis by Andrici et al. demonstrated the increased risk of BE with hiatal hernia, independent of GERD or BMI [48]. Aspirin has been shown to have an inverse association with developing BE. The use of >325 mg of Aspirin per day was associated with a 0.56 OR of developing BE in a case-control study by Omer et al. [49]. On the other hand, multiple studies have demonstrated Helicobacter pylori positive persons to have a decreased risk of developing BE [46,50,51,52]. The protective effect of H. pylori is hypothesized to be due to decreased gastric acid production and a decreased likelihood of damaging acidic reflux into the esophagus [51]. The current data support white race, male sex, and age > 50 as risk factors for the development of BE [3,43]. A recent meta-analysis of the BE risk factors of over 300,000 individuals demonstrated that the prevalence of BE for various populations was as follows: low-risk general population, 0.8%; obesity, 1.9%; GERD, 3%; age > 50, 6.1%; male sex, 6.8%; GERD in addition to the presence of any other risk factor, 12.2%; and family history of BE or EAC, 23.4% [35]. Smoking has been considered a modifiable risk factor for BE, while alcohol consumption has had mixed results, with some studies demonstrating an increased risk with heavy alcohol consumption (>50 g/day) [53]. Central adiposity has been associated with an increased OR (1.98) of developing BE [34]. The association persisted even after adjusting for BMI.
4.3. Esophageal Adenocarcinoma Risk Factors
BE is the precursor lesion to EAC, with a progression from erosive esophagitis to BE, low-grade dysplasia (LGD), high-grade dysplasia (HGD), and finally EAC [54]. Two landmark studies established GERD as a risk factor for EAC in the mid-to-late 1990s [55,56]. The pooled data from five case-control studies demonstrated a 6.24 OR of developing EAC in individuals who had heartburn for at least 20 years compared to those with no heartburn [57]. Studies examining the cancer-protective potential of proton pump inhibitors (PPI) have produced inconsistent results [58,59,60,61]. In 2013, a meta-analysis by Singh et al. pooled seven observational studies with a 71% reduced risk of EAC and/or high-grade dysplasia in patients with BE [59]. Hu et al. performed an updated meta-analysis in 2017 that included two additional studies; however, no dysplasia- or cancer-protective effects were identified in the analysis [60]. Due to these inconsistencies, Chen et al. conducted a metanalysis in 2021 with three additional studies. PPI use was associated with a 0.47 OR of BE progressing to high-grade dysplasia or EAC [61]. Anti-reflux treatment is not limited to medical treatment alone; a meta-analysis of 11 studies revealed evidence that anti-reflux surgery may prevent EAC better than anti-reflux medication, although this effect was only statistically significant when the studies published after the year 2000 (n = 4) were pooled. However, the analysis was based on a limited sample size with possible bias and confounding factors [62].
The association between EAC and other medication has also been examined; statins, aspirin, and non-steroidal anti-inflammatory drugs (NSAIDs) have been demonstrated to have inverse associations with the risk of developing EAC [4]. Mechanistically, the protective effect of aspirin and NSAIDs is thought to be mediated through the inhibition of the COX pathways. Immunohistochemical studies have demonstrated COX-2 expression in BE, with an elevation in expression from LGD, HGD, and eventually to EAC [63]. In the general population, a meta-analysis of nine observational studies (two cohort, seven case-control) by Corely et al. demonstrated a protective effect of any aspirin and NSAID use for the risk of developing EAC (OR = 0.67; 95% CI, 0.51–0.87) in the pooled analysis [64]. Stratified analysis was included in the study, aspirin use was protective (OR = 0.5; CI, 0.38–0.66) and NSAIDs had a borderline protective association (OR = 0.75; 95% CI, 0.54–1.0), but EAC and ESCC were both included in this analysis. Similarly, Abnet et al. performed a meta-analysis in which aspirin and NSAID use were inversely associated with EAC (OR 0.64 (0.52–0.79) and 0.65 (0.50–0.85), respectively) [65]. Analysis of the data from randomized controlled trials (RCTs), where the primary outcomes were the prevention of vascular events, provided further evidence for the decreased incidence and mortality from adenocarcinomas with the use of aspirin [66,67]. Furthermore, in a prospective Dutch study of 570 BE patients, NSAID use was associated with a reduced risk of neoplastic progression (HR = 0.47; 95% CI 0.24–0.93) [68]. Statin use was also associated with a reduced risk (HR = 0.46; 95% CI 0.21–0.99). The protective effect was increased with the combined use of both (HR = 0.22; 95% CI 0.06–0.85) [68]. Other case-control studies have also supported the association between statin use and a reduction in EAC risk [69].
Meta-analyses from the late 2000s and early 2010s demonstrated an inverse association between H. pylori and EAC [70,71,72]. A recent prospective German cohort (n = 9511) demonstrated similar findings, with a 0.35 hazard ratio of H. Pylori-infected individuals developing EAC during the mean 14 years of observation [73]. Tobacco smoking has been well-established as a strong risk factor for developing EAC. The analysis of the primary data from ten population-based case-control and two cohort studies by Cook et al. revealed that the risk of EAC doubled with ever smoking compared to never smoking (OR 1.96; 95% CI 1.64 to 2.34) [74]. Importantly, this risk does not appear to return to that of never-smokers, even after >20 years of smoking cessation [75]. Alcohol, on the other hand, has not been associated with an increased risk of EAC, even with heavy alcohol consumption (seven or more drinks per day of alcohol) [76]. The meta-analysis and pooled analysis of epidemiologic studies have demonstrated an increased BMI to be associated with an increased risk of EAC in a linear dose-response trend [39,77]. This association does not remain after an adjustment for abdominal obesity. However, abdominal adiposity is independently associated with an increased risk of EAC, even after an adjustment for BMI [34,78].
The association between diet and EAC has been particularly challenging to assess. Due to the relatively low prevalence of EAC, the majority of studies are case-control in design. Such studies are prone to recall bias. The analysis of specific dietary components is difficult due to a high correlation of macronutrients with one another, which suggests that the evaluation of dietary patterns may be more informative than that of individual macronutrients [79]. As there exists a large spectrum of foods and dietary patterns across different population, the results of such studies often have poor generalizability. In an effort to improve the aforementioned limitations, the Factors Influencing the Barrett’s Adenocarcinoma Relationship (FINBAR) study was a population-based study performed in Ireland that evaluated the association between a spectrum of food intake and nutrients with BE and EAC risk within the same population. Fruit consumption was associated with a decreased risk of BE and EAC, while the intake of dietary fat was associated with an increased risk of BE and EAC [80,81]. Three large cohort studies have evaluated diet as a risk for EAC. The NIH-AARP cohort found no association between the total fruit and vegetable intake, total fat intake, and subtypes with a risk of EAC [82,83]. Similarly, vegetable and/or fruit consumption was not associated with a risk of EAC in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort [84]. The prospective Netherlands Cohort Study was the only of the cohorts to demonstrate a protective effect of vegetables and citrus fruits against EAC, with the protection conferred more in smokers than nonsmokers [85]. Multiple metanalyses of the available observational studies, the majority of which are case-control, have demonstrated a positive association between meat consumption and EAC; however, this association was only present in the case-control studies and no association was demonstrated in the cohort studies [86,87]. In summation, the conclusions regarding the association between diet and EAC are restricted by the limited data from high quality prospective cohort studies.
The discussed risk factors for BE and EAC are summarized in Table 2.

Table 2.
Risk factors for development of Barrett’s Esophagus (BE) and Esophageal Adenocarcinoma (EAC).
5. Progression of BE to EAC
Esophageal epithelia accumulate mutations, chromosomal changes, and multiple clonal populations of cells with age [88]. However, the majority of these mutations are unlikely to drive cancer development due to having only neutral or mildly deleterious functional consequences. Cancer-driving mutations occur in stem or proliferating cells and lead to positive selection and clonal expansion [89]. In discussing the clonal evolution of cancers, the argument of gradualism versus punctuated equilibrium has emerged into consideration [90]. Gradualism describes the gradual evolution of malignant clones through stepwise genetic alterations, with multiple clonal expansions and phenotypic changes. In the case of BE, although there is stepwise histologic progression from nondysplastic BE to LGD to HGD to EAC, the timing between these steps varies greatly between patients [91]. Additionally, biopsy samples of BE have, over time, revealed a low number of stable mutations [92]; this suggests a punctuated equilibrium as an alternative evolutionary model. Punctuated equilibrium refers to pre-malignant clones gradually accumulating mutations over time, without significant clonal expansion or phenotypic change, until one obtains the necessary mutations to enable clonal expansion and sudden phenotypic change [93]. Gradualism and punctuated equilibria are not mutually exclusive and the concurrent occurrence of both is consistent with the heterogeneity of both BE and EAC [79]. The relevance of cancer evolution, clinically, lies in the potential for its use to stratify patients and conduct timely surveillance and early intervention for the highest-risk patients [79].
Pre-cancerous lesions, such as BE, are areas of histologic change associated with an increased risk of cancer. The increased risk of cancer can depend on the specific histologic changes. While BE is the only known precursor lesion to EAC, half of patients diagnosed with EAC in a Mayo Clinic cohort did not have any detectable BE, and the majority of patients with EAC did not have a prior BE diagnosis in a North Ireland Cohort [94,95]. As previously noted in the text, these findings bring into debate whether all cases of EAC arise from BE [26]. In BE, non-dysplastic lesions have been demonstrated in large population-based studies to have a very low risk of progression to EAC (less than 0.3% per year) [96]. This risk increases with the development of LGD and further with the development of HGD.
Histologically, LGD is characterized by nuclear enlargement, cell elongation, hyperchromasia, and stratification with retained nuclear polarity and architecture, while HGD exhibits a greater degree of cytologic atypia and architectural abnormalities [2]. In clinical practice, there remains a high degree of variability and only moderate agreement in the diagnosis and grading of dysplasia in BE [97,98,99]. This was demonstrated in an international study of 51 participating pathologists and a four pathologists reference panel examining 55 esophageal biopsy cases. There was excellent concordance for nondysplastic BE (79%) and HGD (71%), but intermediate concordance for low-grade dysplasia (42%), and indefinite for dysplasia (23%) [100]. Diagnosis can also be particularly challenging in differentiating between LGD and regenerative changes, seen with active inflammation, ulceration, or post-ulcer healing, and between HGD and EAC. Additionally, disagreements exist between pathologists, particularly between those in North America and Europe and those in Japan, in regard to the histologic criteria used to diagnosis and differentiate HGD and early adenocarcinoma [101].
A meta-analysis of 24 studies including over 2600 individuals with LGD reported an annual incidence rate of 0.54% for EAC and 1.73% for EAC or HGD [102]. However, there was significant heterogeneity between the studies. When dysplasia is confirmed by expert pathologists, significantly higher rates of progression have been noted. This is likely due to the overdiagnosis of LGD by general pathologists compared to experienced GI pathologists [103]. In a landmark study, Duits et al. evaluated 293 patients with LGD, in which 73% were downstaged to non-dysplastic BE or indefinite for dysplasia. Endoscopic follow-up was conducted in 264 patients (90%) with a median follow-up of 39 months. For patients with confirmed LGD, the risk of developing EAC or HGD was 9.1% per patient-year. On the other hand, those downstaged to nondysplastic BE or indefinite for dysplasia had a risk of progression of 0.6% and 0.9% per patient-year, respectively [104]. These results are in-line with other studies. In the control arm of the RCT by Phoa et al., which examined radiofrequency ablation (RFA) versus endoscopic surveillance in patients with LGD, 26.5% of the patients developed EAC or HGD over a 30 month follow-up period [105]. Similarly, in a separate RFA RCT, 14% of LGD patients in the control group developed HGD after one year, whilst none developed EAC during that time [106]. HGD is an actionable diagnosis with surveillance not being recommended [3]. The annual incidence of EAC in HGD is reported to be 6%, but studies examining the natural history of the diagnosis are limited [107]. In addition to examining the effect of the presence of dysplasia on the risk of developing EAC, others have examined the extent of the dysplastic. Srivastna et al. studied the long-term outcomes and prognostic value of dysplasia in 77 individuals with dysplastic BE. The individuals were followed for progression to EAC. The extent of LGD was associated with an increased risk of developing EAC, but the extent of HGD was not [108]. This has suggested that once any degree of HGD develops, the individual is at an elevated risk of developing EAC regardless of the extent of the HGD.
The underlying molecular changes that drive this transition remain largely unknown. It has been hypothesized that reflux events and/or inflammatory mechanisms mediate the reactivation of the signaling pathways central to the development of embryonic columnar esophageal epithelium [109]. The Hedgehog pathway has been implicated in this process. The Hedgehog pathway is active during the development of the columnar epithelium of the embryonic esophagus and is normally downregulated in the adult squamous esophageal epithelium [110]. However, the Hedgehog ligand sonic Hedgehog (SHH) is upregulated in BE epithelia [111]. The exposure of squamous esophageal epithelia to the acid and bile components of refluxate induces SHH expression. Clemons et al. have shown that the ectopic expression of SHH induces the columnar differentiation of squamous esophageal epithelium [109,112]. It is thought that SHH expression upregulates the expression of SOX9 via the BMP4-mediated pathways in a paracrine fashion between the epithelia and mesenchyme [109,111]. SOX9 expression is thought to drive columnar metaplasia in the epithelium.
Several other developmental pathways have been shown to play a role in the development of BE, including TGF-beta/BMP, Notch, and Wnt/beta-catenin [109]. The TGF-beta/BMP family of proteins are highly expressed during the embryonic development of multiple organs, including the gastrointestinal tract. Milano et al. have shown that the in vitro exposure of squamous epithelial cells to BMP4 induces the expression of columnar markers [113]. BMP4 is a downstream target of SHH, and the expression of SHH in mouse esophageal epithelium drives the stromal BMP4 expression, which subsequently increases the expression of columnar-specific cytokeratins, likely via SOX9 expression, as previously discussed [111]. The Notch and Wnt/beta-catenin pathways play essential roles in intestinal differentiation [114,115]. In the mouse esophagus, the activated Wnt signaling causes features of dysplasia, but interestingly, not metaplasia [115]. Beta-catenin is upregulated in human esophageal epithelium, which demonstrates HGD, but not in BE without features of HGD. This suggests that the Wnt/beta-catenin pathway does not contribute significantly to the BE pathogenesis, but may play a larger role in the dysplastic changes and malignant transformation [115].
The intrinsic and extrinsic inflammatory signaling pathways likely drive the progression of EAC from BE, including the NF-kappaB and IL-6/STAT3 pathways [109]. The inflammation driven by the chronic exposure to gastric refluxate likely induces oxidative stress and the activation of intrinsic inflammatory cascades [116]. Gibson et al. have demonstrated that the IL-6/STAT3 pathway is active in biopsies from patients with BE and EAC, and the increasing IL-6 expression is correlated with the degree of dysplasia in BE [117]. Figure 1 summarizes the discussed molecular pathways.
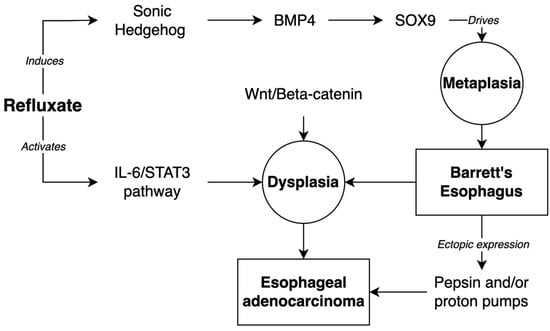
Figure 1.
Molecular pathways of development of Barrett’s Esophagus and progression to Esophageal Adenocarcinoma.
The specific genetic anomalies that have been suggested to predict the neoplastic progression to EAC in BE include the loss of heterozygosity (LOH) at the loci within 17 p and 9 p, which contain the tumor suppressor genes TP53 and CKDN2A, respectively. The loss of these genes is associated with uncontrolled cell proliferation and tumor progression in multiple cancers. The alteration of CKDN2A has been found to be an initiating event in the pathogenesis of BE, and abnormalities in TP53 are strongly associated with the progression of the neoplastic changes in BE to HGD or EAC [118,119,120]. Interestingly, the LOH at these loci is a frequent occurrence in BE without dysplastic changes, and it is unknown whether they can be used to predict malignant transformation [121].
In addition to specific genetic changes, larger genomic and epigenetic modifications have been shown to play a role in the development of EAC. Aneuploidy has been shown to be predictive of the progression to high grade dysplasia and EAC from BE and the aberrant DNA methylation of cytosine bases in CG-rich sequences (CPG islands), leading to changes in the expression of tumor suppressors, including CDKN2A, which has been shown to be involved in the development of BE neoplasia and EAC [118].
5.1. Pepsin and BE/EAC
While the acidic and biliary components of refluxate have traditionally been implicated in the inflammatory processes that drive BE and EAC, recent studies highlight the pathophysiological role of non-acidic pepsin in the development of BE and EAC [122,123,124]. Pepsin and its precursor pepsinogen have been demonstrated to be present in gastric refluxate. Samuels et al. have shown that pepsin exposure to laryngeal squamous epithelium induces cancer-associated changes in these cells [124]. Other studies have demonstrated that the ectopic expression of proton pumps and/or pepsin in BE cells leads to the genetic and molecular signaling pathway changes associated with EAC [125].
5.2. Molecular Pathways
The ectopic expression of proton pumps and pepsin in a BE cell line leads to the differential expression of the genes involved in the kinetochore metaphase signaling, tumor microenvironment, and regulation of epithelial-to-mesenchymal (EMT) transition by the growth factors pathways [125]. Dysfunction of the kinetochore metaphase signaling pathway is associated with polyploidy and aneuploidy in dividing cells; Scott et al. have demonstrated the abnormal expression of kinetochore constituents in BE and EAC cells that contributes to chromosome congression failure during mitosis. The tumor microenvironment pathways contain genes important to tumorigenesis, and the EMT pathways regulate the functional transition of polarized epithelial cells to migratory mesenchymal elements, which play a role in metaplasia and metastasis [126,127].
The upstream regulators of these pathways include ERBB2, TNF, and TGFB1. The epidermal growth factor receptor tyrosine kinase ERBB2 is overexpressed in multiple cancers, including breast cancer and EAC [128,129,130]. Interestingly, higher ERBB2 expression is associated with improved survival in patients with EAC [131,132]. TNF is a ubiquitous cytokine involved in inflammation, cell survival, and cell death [133]. Abnormal TNF expression has been associated with BE and EAC [134]. TGFB1 is a secreted ligand of the TGF-B superfamily, members of which are implicated in the regulation of gene expression [135]. TGFB1 is upregulated in BE and EAC and plays a role in EMT [136,137].
5.3. Mitochondrial Changes
Transgenic BE cell lines expressing proton pumps, pepsin, or both demonstrate the degradation of mitochondrial cristae, mitochondrial malformations, and the presence of autophagosomes; these changes are consistent with the oxidative stress and aberrant mitochondrial function observed with direct exposure to pepsin [122,138]. These changes indicate alterations in the mitochondrial fusion and fission dynamics, as well as autophagy dysfunction, potentially secondary to the increased mitochondrial reactive oxygen species induced by TGFB1 and TNF [138,139,140]. Excess ROS production has been shown to promote mitophagy in the tumor microenvironment, which in turn increases the resistance to a form of non-apoptotic cell death involving iron known as ferroptosis [141,142]. The genes involved in ferroptosis have been implicated in the pathogenic progression of BE to EAC [125].
6. Surveillance
Due to the poor prognosis of EAC, the goal of the endoscopic surveillance of BE is to detect dysplasia or carcinoma at a stage when intervention may be curative. The invasion of the submucosa is considered as that stage by some due to the significantly increased risk of nodal metastasis (9–50% depending on depth of invasion) associated with the EAC invasion of the submucosa [8]. No RCT results currently exist to support the intervals for endoscopic surveillance. Barrett’s Oesophagus Surveillance versus endoscopy at need Study (BOSS) is such a study, taking place in the United Kingdom, examining 3400 BE patients randomized to two year surveillance intervals or “at-need” endoscopy [143]. A meta-analysis and systematic review examining the cohort study evidence of endoscopic surveillance found lower EAC and all-cause mortality, in addition to a higher likelihood of early-stage disease in the surveillance groups compared to those with incomplete or no surveillance. However, adjustment for lead- and length-time biases eliminated or attenuated the beneficial results [144]. Similarly, case-control studies have demonstrated no mortality benefit to surveillance [3]. Hence, the current evidence supporting a mortality benefit to surveillance is weak. Considering the variable annual cancer conversion rate between varying forms of BE dysplasia, it has been suggested that endoscopic surveillance should be performed at intervals based on the degree of dysplasia noted on the previous biopsies [3]. Additionally, the risk for the progression of BE to HGD or EAC has been found to increase with the increasing segment length on meta-analysis [145]. This has prompted guidelines from Europe, the United Kingdom, Australia, and, more recently, the United States to stratify surveillance intervals based on the length of the BE segment [3,8,9,146]. Although there is insufficient evidence to be recommended by the various guidelines, biomarkers such as TP53 have been utilized for risk stratification. In a recent study by Redston et al., p53 immunohistochemistry enabled the identification of higher-risk patients earlier and more frequently than a diagnosis of LGD or indefinite for dysplasia [147].
7. Conclusions
This review provides a summary of our current understanding of the definition, epidemiology, and risk factors of BE and EAC. The cellular and molecular changes that drive the progression of BE to EAC are explored. Despite continual advances in our grasp over these topics, several considerable challenges remain. A uniform definition of BE is needed at an international level to provide consistent and reliable data for future studies. Relevant to that is the continued debate regarding the risk of the neoplastic progression of non-goblet cell columnar epithelium. Diagnostic challenges remain for both BE and dysplasia, with no clear histologic or immunohistochemical markers for more reliable diagnoses and differentiation. Additionally, there are no established markers for the prediction of neoplastic progression, and further work is needed to explain the variable timescales in the progression from BE to EAC. Finally, additional data are needed to establish evidence-based intervals for endoscopic surveillance.
Author Contributions
Conceptualization, A.S.B., K.A.S. and N.J.; methodology, A.S.B. and K.A.S.; writing—original draft preparation, A.S.B. and K.A.S.; writing—review and editing, A.S.B., K.A.S., K.W.A. and N.J.; supervision, N.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Spechler, S.J.; Souza, R.F. Barrett’s Esophagus. N. Engl. J. Med. 2014, 371, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Naini, B.V.; Souza, R.F.; Odze, R.D. Barrett’s Esophagus: A Comprehensive and Contemporary Review for Pathologists. Am. J. Surg. Pathol. 2016, 40, e45–e66. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, N.J.; Falk, G.W.; Iyer, P.G.; Souza, R.F.; Yadlapati, R.H.; Sauer, B.G.; Wani, S. Diagnosis and Management of Barrett’s Esophagus: An Updated ACG Guideline. Am. J. Gastroenterol. 2022, 117, 559–587. [Google Scholar] [CrossRef]
- Coleman, H.G.; Xie, S.-H.; Lagergren, J. The Epidemiology of Esophageal Adenocarcinoma. Gastroenterology 2018, 154, 390–405. [Google Scholar] [CrossRef] [PubMed]
- Goldblum, J.R. Current Issues in Barrett’s Esophagus and Barrett’s-Related Dysplasia. Mod. Pathol. 2015, 28, S1–S6. [Google Scholar] [CrossRef] [PubMed]
- Odze, R. Histology of Barrett’s Metaplasia: Do Goblet Cells Matter? Dig. Dis. Sci. 2018, 63, 2042–2051. [Google Scholar] [CrossRef]
- American Gastroenterological Association; Spechler, S.J.; Sharma, P.; Souza, R.F.; Inadomi, J.M.; Shaheen, N.J. American Gastroenterological Association Medical Position Statement on the Management of Barrett’s Esophagus. Gastroenterology 2011, 140, 1084–1091. [Google Scholar] [CrossRef]
- Fitzgerald, R.C.; di Pietro, M.; Ragunath, K.; Ang, Y.; Kang, J.-Y.; Watson, P.; Trudgill, N.; Patel, P.; Kaye, P.V.; Sanders, S.; et al. British Society of Gastroenterology Guidelines on the Diagnosis and Management of Barrett’s Oesophagus. Gut 2014, 63, 7–42. [Google Scholar] [CrossRef]
- Weusten, B.; Bisschops, R.; Coron, E.; Dinis-Ribeiro, M.; Dumonceau, J.-M.; Esteban, J.-M.; Hassan, C.; Pech, O.; Repici, A.; Bergman, J.; et al. Endoscopic Management of Barrett’s Esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2017, 49, 191–198. [Google Scholar] [CrossRef]
- Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: Part I. Esophagus 2017, 14, 1–36. [Google Scholar] [CrossRef]
- Srivastava, A.; Appelman, H.; Goldsmith, J.D.; Davison, J.M.; Hart, J.; Krasinskas, A.M. The Use of Ancillary Stains in the Diagnosis of Barrett Esophagus and Barrett Esophagus–Associated Dysplasia: Recommendations from the Rodger C. Haggitt Gastrointestinal Pathology Society. Am. J. Surg. Pathol. 2017, 41, e8–e21. [Google Scholar] [CrossRef]
- Wright, C.L.; Kelly, J.K. The Use of Routine Special Stains for Upper Gastrointestinal Biopsies. Am. J. Surg. Pathol. 2006, 30, 357–361. [Google Scholar] [CrossRef]
- Paull, A.; Trier, J.S.; Dalton, M.D.; Camp, R.C.; Loeb, P.; Goyal, R.K. The Histologic Spectrum of Barrett’s Esophagus. N. Engl. J. Med. 1976, 295, 476–480. [Google Scholar] [CrossRef]
- Thompson, J.J.; Zinsser, K.R.; Enterline, H.T. Barrett’s Metaplasia and Adenocarcinoma of the Esophagus and Gastroesophageal Junction. Hum. Pathol. 1983, 14, 42–61. [Google Scholar] [CrossRef]
- Gatenby, P.A.C.; Ramus, J.R.; Caygill, C.P.J.; Shepherd, N.A.; Watson, A. Relevance of the Detection of Intestinal Metaplasia in Non-Dysplastic Columnar-Lined Oesophagus. Scand. J. Gastroenterol. 2008, 43, 524–530. [Google Scholar] [CrossRef]
- Harrison, R.; Perry, I.; Haddadin, W.; McDonald, S.; Bryan, R.; Abrams, K.; Sampliner, R.; Talley, N.J.; Moayyedi, P.; Jankowski, J.A. Detection of Intestinal Metaplasia in Barrett’s Esophagus: An Observational Comparator Study Suggests the Need for a Minimum of Eight Biopsies. Am. J. Gastroenterol. 2007, 102, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.F.; Sharma, P.; Daaboul, B.; Cherian, R.; Mayo, M.; Topalovski, M.; Weston, A.P. Yield of Intestinal Metaplasia in Patients with Suspected Short-Segment Barrett’s Esophagus (SSBE) on Repeat Endoscopy. Dig. Dis. Sci. 2002, 47, 2108–2111. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.L.; Waring, J.P.; Spechler, S.J.; Sampliner, R.E.; Doos, W.G.; Krol, W.F.; Williford, W.O. Diagnostic Inconsistencies in Barrett’s Esophagus. Gastroenterology 1994, 107, 945–949. [Google Scholar] [CrossRef]
- Chandrasoma, P.; Wijetunge, S.; DeMeester, S.; Ma, Y.; Hagen, J.; Zamis, L.; DeMeester, T. Columnar-Lined Esophagus Without Intestinal Metaplasia Has No Proven Risk of Adenocarcinoma. Am. J. Surg. Pathol. 2012, 36, 1–7. [Google Scholar] [CrossRef]
- Bhat, S.; Coleman, H.G.; Yousef, F.; Johnston, B.T.; McManus, D.T.; Gavin, A.T.; Murray, L.J. Risk of Malignant Progression in Barrett’s Esophagus Patients: Results from a Large Population-Based Study. JNCI J. Natl. Cancer Inst. 2011, 103, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, N.J.; Green, B.; Medapalli, R.K.; Mitchell, K.L.; Wei, J.T.; Schmitz, S.M.; West, L.M.; Brown, A.; Noble, M.; Sultan, S.; et al. The Perception of Cancer Risk in Patients with Prevalent Barrett’s Esophagus Enrolled in an Endoscopic Surveillance Program. Gastroenterology 2005, 129, 429–436. [Google Scholar] [CrossRef]
- Crockett, S.D.; Lippmann, Q.K.; Dellon, E.S.; Shaheen, N.J. Health-Related Quality of Life in Patients with Barrett’s Esophagus: A Systematic Review. Clin. Gastroenterol. Hepatol. 2009, 7, 613–623. [Google Scholar] [CrossRef]
- Westerhoff, M.; Hovan, L.; Lee, C.; Hart, J. Effects of Dropping the Requirement for Goblet Cells from the Diagnosis of Barrett’s Esophagus. Clin. Gastroenterol. Hepatol. 2012, 10, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- Kelty, C.J.; Gough, M.D.; Van Wyk, Q.; Stephenson, T.J.; Ackroyd, R. Barrett’s Oesophagus: Intestinal Metaplasia Is Not Essential for Cancer Risk. Scand. J. Gastroenterol. 2007, 42, 1271–1274. [Google Scholar] [CrossRef] [PubMed]
- Salimian, K.J.; Birkness-Gartman, J.; Waters, K.M. The Path(Ology) from Reflux Oesophagitis to Barrett Oesophagus to Oesophageal Adenocarcinoma. Pathology 2022, 54, 147–156. [Google Scholar] [CrossRef]
- Takubo, K.; Aida, J.; Naomoto, Y.; Sawabe, M.; Arai, T.; Shiraishi, H.; Matsuura, M.; Ell, C.; May, A.; Pech, O.; et al. Cardiac Rather than Intestinal-Type Background in Endoscopic Resection Specimens of Minute Barrett Adenocarcinoma. Hum. Pathol. 2009, 40, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Hahn, H.; Odze, R.D.; Goyal, R.K. Metaplastic Esophageal Columnar Epithelium Without Goblet Cells Shows DNA Content Abnormalities Similar to Goblet Cell–Containing Epithelium. Am. J. Gastroenterol. 2009, 104, 816–824. [Google Scholar] [CrossRef]
- Jain, S.; Dhingra, S. Pathology of Esophageal Cancer and Barrett’s Esophagus. Ann. Cardiothorac. Surg. 2017, 6, 99–109. [Google Scholar] [CrossRef]
- Rice, T.W.; Patil, D.T.; Blackstone, E.H. 8th Edition AJCC/UICC Staging of Cancers of the Esophagus and Esophagogastric Junction: Application to Clinical Practice. Ann. Cardiothorac. Surg. 2017, 6, 119–130. [Google Scholar] [CrossRef]
- Kelly, S.; Harris, K.M.; Berry, E.; Hutton, J.; Roderick, P.; Cullingworth, J.; Gathercole, L.; Smith, M.A. A Systematic Review of the Staging Performance of Endoscopic Ultrasound in Gastro-Oesophageal Carcinoma. Gut 2001, 49, 534–539. [Google Scholar] [CrossRef]
- Cameron, A.J.; Zinsmeister, A.R.; Ballard, D.J.; Carney, J.A. Prevalence of Columnar-Lined (Barrett’s) Esophagus. Gastroenterology 1990, 99, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Ronkainen, J.; Aro, P.; Storskrubb, T.; Johansson, S.; Lind, T.; Bolling–Sternevald, E.; Vieth, M.; Stolte, M.; Talley, N.J.; Agréus, L. Prevalence of Barrett’s Esophagus in the General Population: An Endoscopic Study. Gastroenterology 2005, 129, 1825–1831. [Google Scholar] [CrossRef] [PubMed]
- Zagari, R.M.; Fuccio, L.; Wallander, M.-A.; Johansson, S.; Fiocca, R.; Casanova, S.; Farahmand, B.Y.; Winchester, C.C.; Roda, E.; Bazzoli, F. Gastro-Oesophageal Reflux Symptoms, Oesophagitis and Barrett’s Oesophagus in the General Population: The Loiano-Monghidoro Study. Gut 2008, 57, 1354–1359. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, A.N.; Murad, M.H.; Buttar, N.S.; El–Serag, H.B.; Katzka, D.A.; Iyer, P.G. Central Adiposity Is Associated with Increased Risk of Esophageal Inflammation, Metaplasia, and Adenocarcinoma: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2013, 11, 1399–1412.e7. [Google Scholar] [CrossRef] [PubMed]
- Qumseya, B.J.; Bukannan, A.; Gendy, S.; Ahemd, Y.; Sultan, S.; Bain, P.; Gross, S.A.; Iyer, P.; Wani, S. Systematic Review and Meta-Analysis of Prevalence and Risk Factors for Barrett’s Esophagus. Gastrointest. Endosc. 2019, 90, 707–717.e1. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Thrift, A.P. The Epidemic of Oesophageal Carcinoma: Where Are We Now? Cancer Epidemiol. 2016, 41, 88–95. [Google Scholar] [CrossRef]
- He, H.; Chen, N.; Hou, Y.; Wang, Z.; Zhang, Y.; Zhang, G.; Fu, J. Trends in the Incidence and Survival of Patients with Esophageal Cancer: A SEER Database Analysis. Thorac. Cancer 2020, 11, 1121–1128. [Google Scholar] [CrossRef]
- Hoyo, C.; Cook, M.B.; Kamangar, F.; Freedman, N.D.; Whiteman, D.C.; Bernstein, L.; Brown, L.M.; Risch, H.A.; Ye, W.; Sharp, L.; et al. Body Mass Index in Relation to Oesophageal and Oesophagogastric Junction Adenocarcinomas: A Pooled Analysis from the International BEACON Consortium. Int. J. Epidemiol. 2012, 41, 1706–1718. [Google Scholar] [CrossRef]
- Pohl, H.; Welch, H.G. The Role of Overdiagnosis and Reclassification in the Marked Increase of Esophageal Adenocarcinoma Incidence. JNCI J. Natl. Cancer Inst. 2005, 97, 142–146. [Google Scholar] [CrossRef]
- Kong, C.Y.; Kroep, S.; Curtius, K.; Hazelton, W.D.; Jeon, J.; Meza, R.; Heberle, C.R.; Miller, M.C.; Choi, S.E.; Lansdorp-Vogelaar, I.; et al. Exploring the Recent Trend in Esophageal Adenocarcinoma Incidence and Mortality Using Comparative Simulation Modeling. Cancer Epidemiol. Biomark. Prev. 2014, 23, 997–1006. [Google Scholar] [CrossRef]
- Arnold, M.; Laversanne, M.; Brown, L.M.; Devesa, S.S.; Bray, F. Predicting the Future Burden of Esophageal Cancer by Histological Subtype: International Trends in Incidence up to 2030. Am. J. Gastroenterol. 2017, 112, 1247–1255. [Google Scholar] [CrossRef]
- Eloubeidi, M.A.; Provenzale, D. Clinical and Demographic Predictors of Barrett’s Esophagus Among Patients with Gastroesophageal Reflux Disease: A Multivariable Analysis in Veterans. J. Clin. Gastroenterol. 2001, 33, 306–309. [Google Scholar] [CrossRef]
- Campos, G.M.R. Predictive Factors of Barrett Esophagus: Multivariate Analysis of 502 Patients with Gastroesophageal Reflux Disease. Arch. Surg. 2001, 136, 1267. [Google Scholar] [CrossRef]
- Johansson, J.; Håkansson, H.-O.; Mellblom, L.; Kempas, A.; Johansson, K.-E.; Granath, F.; Nyrén, O. Risk Factors for Barrett’s Oesophagus: A Population-Based Approach. Scand. J. Gastroenterol. 2007, 42, 148–156. [Google Scholar] [CrossRef]
- Thrift, A.P.; Kramer, J.R.; Qureshi, Z.; Richardson, P.A.; El-Serag, H.B. Age at Onset of GERD Symptoms Predicts Risk of Barrett’s Esophagus. Am. J. Gastroenterol. 2013, 108, 915–922. [Google Scholar] [CrossRef]
- Greene, C.L.; Worrell, S.G.; DeMeester, T.R. Rat Reflux Model of Esophageal Cancer and Its Implication in Human Disease. Ann. Surg. 2015, 262, 910–924. [Google Scholar] [CrossRef]
- Andrici, J.; Tio, M.; Cox, M.R.; Eslick, G.D. Hiatal Hernia and the Risk of Barrett’s Esophagus: Hiatal Hernia and Barrett’s Esophagus. J. Gastroenterol. Hepatol. 2013, 28, 415–431. [Google Scholar] [CrossRef]
- Omer, Z.B.; Ananthakrishnan, A.N.; Nattinger, K.J.; Cole, E.B.; Lin, J.J.; Kong, C.Y.; Hur, C. Aspirin Protects Against Barrett’s Esophagus in a Multivariate Logistic Regression Analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 722–727. [Google Scholar] [CrossRef]
- Jonaitis, L.; Kriukas, D.; Kiudelis, G.; Kupčinskas, L. Risk Factors for Erosive Esophagitis and Barrett’s Esophagus in a High Helicobacter Pylori Prevalence Area. Medicina 2011, 47, 434. [Google Scholar] [CrossRef]
- Fischbach, L.A.; Graham, D.Y.; Kramer, J.R.; Rugge, M.; Verstovsek, G.; Parente, P.; Alsarraj, A.; Fitzgerald, S.; Shaib, Y.; Abraham, N.S.; et al. Association between Helicobacter Pylori and Barrett’s Esophagus: A Case–Control Study. Am. J. Gastroenterol. 2014, 109, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Corley, D.A.; Kubo, A.; Levin, T.R.; Block, G.; Habel, L.; Zhao, W.; Leighton, P.; Rumore, G.; Quesenberry, C.; Buffler, P.; et al. Helicobacter Pylori Infection and the Risk of Barrett’s Oesophagus: A Community-Based Study. Gut 2008, 57, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Ireland, C.J.; Thompson, S.K.; Laws, T.A.; Esterman, A. Risk Factors for Barrett’s Esophagus: A Scoping Review. Cancer Causes Control 2016, 27, 301–323. [Google Scholar] [CrossRef] [PubMed]
- Anaparthy, R.; Sharma, P. Progression of Barrett Oesophagus: Role of Endoscopic and Histological Predictors. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 525–534. [Google Scholar] [CrossRef]
- Chow, W.-H.; Finkle, W.D.; Frankl, H.; Ziel, K.; Fraumeni, F. The Relation of Gastroesophageal Reflux Disease and Its Treatment to Adenocarcinomas of the Esophagus and Gastric Cardia. Jama 1995, 274, 474–477. [Google Scholar] [CrossRef]
- Lagergren, J.; Bergström, R.; Lindgren, A.; Nyrén, O. Symptomatic Gastroesophageal Reflux as a Risk Factor for Esophageal Adenocarcinoma. N. Engl. J. Med. 1999, 340, 825–831. [Google Scholar] [CrossRef]
- Cook, M.B.; Corley, D.A.; Murray, L.J.; Liao, L.M.; Kamangar, F.; Ye, W.; Gammon, M.D.; Risch, H.A.; Casson, A.G.; Freedman, N.D.; et al. Gastroesophageal Reflux in Relation to Adenocarcinomas of the Esophagus: A Pooled Analysis from the Barrett’s and Esophageal Adenocarcinoma Consortium (BEACON). PLoS ONE 2014, 9, e103508. [Google Scholar] [CrossRef]
- Nguyen, D.M.; Richardson, P.; El–Serag, H.B. Medications (NSAIDs, Statins, Proton Pump Inhibitors) and the Risk of Esophageal Adenocarcinoma in Patients with Barrett’s Esophagus. Gastroenterology 2010, 138, 2260–2266. [Google Scholar] [CrossRef]
- Singh, S.; Garg, S.K.; Singh, P.P.; Iyer, P.G.; El-Serag, H.B. Acid-Suppressive Medications and Risk of Oesophageal Adenocarcinoma in Patients with Barrett’s Oesophagus: A Systematic Review and Meta-Analysis. Gut 2014, 63, 1229–1237. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, T.-T.; Hong, J.; Fang, J.-Y.; Xiong, H.; Meltzer, S.J. Proton Pump Inhibitors Do Not Reduce the Risk of Esophageal Adenocarcinoma in Patients with Barrett’s Esophagus: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0169691. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, C.; Wu, Y.; Chen, X.; Kailas, S.; Karadsheh, Z.; Li, G.; Guo, Z.; Yang, H.; Hu, L.; et al. Do Proton Pump Inhibitors Prevent Barrett’s Esophagus Progression to High-Grade Dysplasia and Esophageal Adenocarcinoma? An Updated Meta-Analysis. J. Cancer Res. Clin. Oncol. 2021, 147, 2681–2691. [Google Scholar] [CrossRef]
- Maret-Ouda, J.; Konings, P.; Lagergren, J.; Brusselaers, N. Antireflux Surgery and Risk of Esophageal Adenocarcinoma: A Systematic Review and Meta-Analysis. Ann. Surg. 2016, 263, 251–257. [Google Scholar] [CrossRef]
- Morris, C. Cyclooxygenase-2 Expression in the Barrett’s Metaplasia–Dysplasia–Adenocarcinoma Sequence. Am. J. Gastroenterol. 2001, 96, 990–996. [Google Scholar] [CrossRef]
- Corley, D.A.; Kerlikowske, K.; Verma, R.; Buffler, P. Protective Association of Aspirin/NSAIDs and Esophageal Cancer: A Systematic Review and Meta-Analysis. Gastroenterology 2003, 124, 47–56. [Google Scholar] [CrossRef]
- Abnet, C.C.; Freedman, N.D.; Kamangar, F.; Leitzmann, M.F.; Hollenbeck, A.R.; Schatzkin, A. Non-Steroidal Anti-Inflammatory Drugs and Risk of Gastric and Oesophageal Adenocarcinomas: Results from a Cohort Study and a Meta-Analysis. Br. J. Cancer 2009, 100, 551–557. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Price, J.F.; Fowkes, F.G.R.; Zanchetti, A.; Roncaglioni, M.C.; Tognoni, G.; Lee, R.; Belch, J.F.; Wilson, M.; Mehta, Z.; et al. Short-Term Effects of Daily Aspirin on Cancer Incidence, Mortality, and Non-Vascular Death: Analysis of the Time Course of Risks and Benefits in 51 Randomised Controlled Trials. Lancet 2012, 379, 1602–1612. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Fowkes, F.G.R.; Belch, J.F.; Ogawa, H.; Warlow, C.P.; Meade, T.W. Effect of Daily Aspirin on Long-Term Risk of Death Due to Cancer: Analysis of Individual Patient Data from Randomised Trials. Lancet 2011, 377, 31–41. [Google Scholar] [CrossRef]
- Kastelein, F.; Spaander, M.C.W.; Biermann, K.; Steyerberg, E.W.; Kuipers, E.J.; Bruno, M.J. Nonsteroidal Anti-Inflammatory Drugs and Statins Have Chemopreventative Effects in Patients with Barrett’s Esophagus. Gastroenterology 2011, 141, 2000–2008. [Google Scholar] [CrossRef]
- Alkhayyat, M.; Kumar, P.; Sanaka, K.O.; Thota, P.N. Chemoprevention in Barrett’s Esophagus and Esophageal Adenocarcinoma. Ther. Adv. Gastroenterol. 2021, 14, 17562848211033730. [Google Scholar] [CrossRef]
- Islami, F.; Kamangar, F. Helicobacter Pylori and Esophageal Cancer Risk: A Meta-Analysis. Cancer Prev. Res. 2008, 1, 329–338. [Google Scholar] [CrossRef]
- Xie, F.-J. Helicobacter Pylori Infection and Esophageal Cancer Risk: An Updated Meta-Analysis. World J. Gastroenterol. 2013, 19, 6098. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Chen, T.; Yang, X.; Huai, P.; Lu, M. Association of H Elicobacter Pylori Infection with Esophageal Adenocarcinoma and Squamous Cell Carcinoma: A Meta-Analysis: HP Infection and Esophageal Cancer. Dis. Esophagus 2014, 27, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Holleczek, B.; Schöttker, B.; Brenner, H. Helicobacter Pylori Infection, Chronic Atrophic Gastritis and Risk of Stomach and Esophagus Cancer: Results from the Prospective Population-based ESTHER Cohort Study. Int. J. Cancer 2020, 146, 2773–2783. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.B.; Kamangar, F.; Whiteman, D.C.; Freedman, N.D.; Gammon, M.D.; Bernstein, L.; Brown, L.M.; Risch, H.A.; Ye, W.; Sharp, L.; et al. Cigarette Smoking and Adenocarcinomas of the Esophagus and Esophagogastric Junction: A Pooled Analysis from the International BEACON Consortium. JNCI J. Natl. Cancer Inst. 2010, 102, 1344–1353. [Google Scholar] [CrossRef]
- Wang, Q.-L.; Xie, S.-H.; Li, W.-T.; Lagergren, J. Smoking Cessation and Risk of Esophageal Cancer by Histological Type: Systematic Review and Meta-Analysis. JNCI J. Natl. Cancer Inst. 2017, 109, djx115. [Google Scholar] [CrossRef]
- Freedman, N.D.; Murray, L.J.; Kamangar, F.; Abnet, C.C.; Cook, M.B.; Nyren, O.; Ye, W.; Wu, A.H.; Bernstein, L.; Brown, L.M.; et al. Alcohol Intake and Risk of Oesophageal Adenocarcinoma: A Pooled Analysis from the BEACON Consortium. Gut 2011, 60, 1029–1037. [Google Scholar] [CrossRef]
- Turati, F.; Tramacere, I.; La Vecchia, C.; Negri, E. A Meta-Analysis of Body Mass Index and Esophageal and Gastric Cardia Adenocarcinoma. Ann. Oncol. 2013, 24, 609–617. [Google Scholar] [CrossRef]
- Steffen, A.; Huerta, J.-M.; Weiderpass, E.; Bueno-de-Mesquita, H.B.; May, A.M.; Siersema, P.D.; Kaaks, R.; Neamat-Allah, J.; Pala, V.; Panico, S.; et al. General and Abdominal Obesity and Risk of Esophageal and Gastric Adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition: Obesity Not a Risk Factor for GCC and GNCC. Int. J. Cancer 2015, 137, 646–657. [Google Scholar] [CrossRef]
- Dawsey, S.M.; Fagundes, R.B.; Jacobson, B.C.; Kresty, L.A.; Mallery, S.R.; Paski, S.; Brandt, P.A.V.D. Diet and esophageal disease: Diet and esophageal disease. Ann. N. Y. Acad. Sci. 2014, 1325, 127–137. [Google Scholar] [CrossRef]
- O’Doherty, M.G.; Cantwell, M.M.; Murray, L.J.; Anderson, L.A.; Abnet, C.C.; on behalf of the FINBAR Study Group. Dietary fat and meat intakes and risk of reflux esophagitis, Barrett’s esophagus and esophageal adenocarcinoma. Int. J. Cancer 2011, 129, 1493–1502. [Google Scholar] [CrossRef]
- Anderson, L.A. Risk factors for Barrett’s oesophagus and oesophageal adenocarcinoma: Results from the FINBAR study. WJG 2007, 13, 1585. [Google Scholar] [CrossRef]
- Freedman, N.D.; Park, Y.; Subar, A.F.; Hollenbeck, A.R.; Leitzmann, M.F.; Schatzkin, A.; Abnet, C. Fruit and vegetable intake and esophageal cancer in a large prospective cohort study. Int. J. Cancer 2007, 121, 2753–2760. [Google Scholar] [CrossRef]
- O’Doherty, M.G.; Freedman, N.G.; Hollenbeck, A.R.; Schatzkin, A.; Murray, L.J.; Cantwell, M.M.; Abnet, C.C. Association of dietary fat intakes with risk of esophageal and gastric cancer in the NIH-AARP diet and health study. Int. J. Cancer 2012, 131, 1376–1387. [Google Scholar] [CrossRef]
- Jeurnink, S.; Büchner, F.; Bueno-De-Mesquita, H.; Siersema, P.; Boshuizen, H.; Numans, M.; Dahm, C.; Overvad, K.; Tjonneland, A.; Roswall, N.; et al. Variety in vegetable and fruit consumption and the risk of gastric and esophageal cancer in the European prospective investigation into cancer and nutrition. Int. J. Cancer 2012, 131, E963–E973. [Google Scholar] [CrossRef]
- Steevens, J.; Schouten, L.J.; Goldbohm, R.A.; van den Brandt, P.A. Vegetables and fruits consumption and risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Int. J. Cancer 2011, 129, 2681–2693. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, F.; Chen, D.; Zhang, C. Red and processed meat consumption and esophageal cancer risk: A systematic review and meta-analysis. Clin. Transl. Oncol. 2020, 22, 532–545. [Google Scholar] [CrossRef]
- Huang, W.; Han, Y.; Xu, J.; Zhu, W.; Li, Z. Red and processed meat intake and risk of esophageal adenocarcinoma: A meta-analysis of observational studies. Cancer Causes Control. 2013, 24, 193–201. [Google Scholar] [CrossRef]
- Killcoyne, S.; Fitzgerald, R.C. Evolution and Progression of Barrett’s Oesophagus to Oesophageal Cancer. Nat. Rev. Cancer 2021, 21, 731–741. [Google Scholar] [CrossRef]
- Martincorena, I.; Campbell, P.J. Somatic Mutation in Cancer and Normal Cells. Science 2015, 349, 1483–1489. [Google Scholar] [CrossRef]
- Greaves, M.; Maley, C.C. Clonal Evolution in Cancer. Nature 2012, 481, 306–313. [Google Scholar] [CrossRef]
- Wani, S.; Rubenstein, J.H.; Vieth, M.; Bergman, J. Diagnosis and Management of Low-Grade Dysplasia in Barrett’s Esophagus: Expert Review from the Clinical Practice Updates Committee of the American Gastroenterological Association. Gastroenterology 2016, 151, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Galipeau, P.C.; Paulson, T.G.; Sanchez, C.A.; Arnaudo, J.; Liu, K.; Sather, C.L.; Kostadinov, R.L.; Odze, R.D.; Kuhner, M.K.; et al. Temporal and Spatial Evolution of Somatic Chromosomal Alterations: A Case-Cohort Study of Barrett’s Esophagus. Cancer Prev. Res. 2014, 7, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Cross, W.C.; Graham, T.A.; Wright, N.A. New Paradigms in Clonal Evolution: Punctuated Equilibrium in Cancer: New Paradigms in Clonal Evolution: Punctuated Equilibrium in Cancer. J. Pathol. 2016, 240, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.K.; McManus, D.T.; Coleman, H.G.; Johnston, B.T.; Cardwell, C.R.; McMenamin, Ú.; Bannon, F.; Hicks, B.; Kennedy, G.; Gavin, A.T.; et al. Oesophageal Adenocarcinoma and Prior Diagnosis of Barrett’s Oesophagus: A Population-Based Study. Gut 2015, 64, 20–25. [Google Scholar] [CrossRef]
- Sawas, T.; Killcoyne, S.; Iyer, P.G.; Wang, K.K.; Smyrk, T.C.; Kisiel, J.B.; Qin, Y.; Ahlquist, D.A.; Rustgi, A.K.; Costa, R.J.; et al. Identification of Prognostic Phenotypes of Esophageal Adenocarcinoma in 2 Independent Cohorts. Gastroenterology 2018, 155, 1720–1728.e4. [Google Scholar] [CrossRef]
- Frederik, H.-J.; Lars, P.; Mohr, D.A.; Toft, S.H.; Peter, F.-J. Incidence of Adenocarcinoma among Patients with Barrett’s Esophagus. N. Engl. J. Med. 2011, 365, 1375–1383. [Google Scholar]
- Montgomery, E.; Bronner, M.P.; Goldblum, J.R.; Greenson, J.K.; Haber, M.M.; Hart, J.; Lamps, L.W.; Lauwers, G.Y.; Lazenby, A.J.; Lewin, D.N.; et al. Reproducibility of the Diagnosis of Dysplasia in Barrett Esophagus: A Reaffirmation. Hum. Pathol. 2001, 32, 368–378. [Google Scholar] [CrossRef]
- Ormsby, A.H. Observer Variation in the Diagnosis of Superficial Oesophageal Adenocarcinoma. Gut 2002, 51, 671–676. [Google Scholar] [CrossRef]
- Kaye, P.V.; Haider, S.A.; Ilyas, M.; James, P.D.; Soomro, I.; Faisal, W.; Catton, J.; Parsons, S.L.; Ragunath, K. Barrett’s Dysplasia and the Vienna Classification: Reproducibility, Prediction of Progression and Impact of Consensus Reporting and P53 Immunohistochemistry. Histopathology 2009, 54, 699–712. [Google Scholar] [CrossRef]
- van der Wel, M.J.; Coleman, H.G.; Bergman, J.J.G.H.M.; Jansen, M.; Meijer, S.L. Histopathologist Features Predictive of Diagnostic Concordance at Expert Level among a Large International Sample of Pathologists Diagnosing Barrett’s Dysplasia Using Digital Pathology. Gut 2020, 69, 811–822. [Google Scholar] [CrossRef]
- Takubo, K.; Vieth, M.; Aida, J.; Sawabe, M.; Kumagai, Y.; Hoshihara, Y.; Arai, T. Differences in the Definitions Used for Esophageal and Gastric Diseases in Different Countries. Digestion 2009, 80, 248–257. [Google Scholar] [CrossRef]
- Singh, S.; Manickam, P.; Amin, A.V.; Samala, N.; Schouten, L.J.; Iyer, P.G.; Desai, T.K. Incidence of Esophageal Adenocarcinoma in Barrett’s Esophagus with Low-Grade Dysplasia: A Systematic Review and Meta-Analysis. Gastrointest. Endosc. 2014, 79, 897–909.e4. [Google Scholar] [CrossRef]
- Curvers, W.L.; ten Kate, F.J.; Krishnadath, K.K.; Visser, M.; Elzer, B.; Baak, L.C.; Bohmer, C.; Mallant-Hent, R.C.; van Oijen, A.; Naber, A.H.; et al. Low-Grade Dysplasia in Barrett’s Esophagus: Overdiagnosed and Underestimated. Am. J. Gastroenterol. 2010, 105, 1523–1530. [Google Scholar] [CrossRef]
- Duits, L.C.; Phoa, K.N.; Curvers, W.L.; ten Kate, F.J.W.; Meijer, G.A.; Seldenrijk, C.A.; Offerhaus, G.J.; Visser, M.; Meijer, S.L.; Krishnadath, K.K.; et al. Barrett’s Oesophagus Patients with Low-Grade Dysplasia Can Be Accurately Risk-Stratified after Histological Review by an Expert Pathology Panel. Gut 2015, 64, 700–706. [Google Scholar] [CrossRef]
- Phoa, K.N.; van Vilsteren, F.G.I.; Weusten, B.L.A.M.; Bisschops, R.; Schoon, E.J.; Ragunath, K.; Fullarton, G.; Di Pietro, M.; Ravi, N.; Visser, M.; et al. Radiofrequency Ablation vs Endoscopic Surveillance for Patients with Barrett Esophagus and Low-Grade Dysplasia: A Randomized Clinical Trial. JAMA 2014, 311, 1209. [Google Scholar] [CrossRef]
- Shaheen, N.J.; Sampliner, R.E.; Goldblum, J.R.; Fennerty, M.B.; Hawes, R.H.; Mashimo, H.; Edmundowicz, S.A.; Infantolino, A.; Chak, A. Radiofrequency Ablation in Barrett’s Esophagus with Dysplasia. N. Engl. J. Med. 2009, 360, 2277–2288. [Google Scholar] [CrossRef]
- Spechler, S.J. Barrett Esophagus and Risk of Esophageal Cancer: A Clinical Review. JAMA 2013, 310, 627. [Google Scholar] [CrossRef]
- Srivastava, A.; Hornick, J.L.; Li, X.; Blount, P.L.; Sanchez, C.A.; Cowan, D.S.; Ayub, K.; Maley, C.C.; Reid, B.J.; Odze, R.D. Extent of Low-Grade Dysplasia Is a Risk Factor for the Development of Esophageal Adenocarcinoma in Barrett’s Esophagus. Am. J. Gastroenterol. 2007, 102, 483–493. [Google Scholar] [CrossRef]
- Gibson, M.K.; Dhaliwal, A.S.; Clemons, N.J.; Phillips, W.A.; Dvorak, K.; Tong, D.; Law, S.; Pirchi, E.D.; Räsänen, J.; Krasna, M.J.; et al. Barrett’s Esophagus: Cancer and Molecular Biology: Barrett’s Esophagus. Ann. N. Y. Acad. Sci. 2013, 1300, 296–314. [Google Scholar] [CrossRef]
- Litingtung, Y.; Lei, L.; Westphal, H.; Chiang, C. Sonic Hedgehog Is Essential to Foregut Development. Nat. Genet. 1998, 20, 58–61. [Google Scholar] [CrossRef]
- Wang, D.H.; Clemons, N.J.; Miyashita, T.; Dupuy, A.J.; Zhang, W.; Szczepny, A.; Corcoran–Schwartz, I.M.; Wilburn, D.L.; Montgomery, E.A.; Wang, J.S.; et al. Aberrant Epithelial–Mesenchymal Hedgehog Signaling Characterizes Barrett’s Metaplasia. Gastroenterology 2010, 138, 1810–1822.e2. [Google Scholar] [CrossRef] [PubMed]
- Clemons, N.J.; Wang, D.H.; Croagh, D.; Tikoo, A.; Fennell, C.M.; Murone, C.; Scott, A.M.; Watkins, D.N.; Phillips, W.A. Sox9 Drives Columnar Differentiation of Esophageal Squamous Epithelium: A Possible Role in the Pathogenesis of Barrett’s Esophagus. Am. J. Physiol.-Gastrointest. Liver Physiol. 2012, 303, G1335–G1346. [Google Scholar] [CrossRef] [PubMed]
- Milano, F.; van Baal, J.W.P.M.; Buttar, N.S.; Rygiel, A.M.; de Kort, F.; DeMars, C.J.; Rosmolen, W.D.; Bergman, J.J.G.H.M.; van Marle, J.; Wang, K.K.; et al. Bone Morphogenetic Protein 4 Expressed in Esophagitis Induces a Columnar Phenotype in Esophageal Squamous Cells. Gastroenterology 2007, 132, 2412–2421. [Google Scholar] [CrossRef] [PubMed]
- Menke, V.; van Es, J.H.; de Lau, W.; van den Born, M.; Kuipers, E.J.; Siersema, P.D.; de Bruin, R.W.F.; Kusters, J.G.; Clevers, H. Conversion of Metaplastic Barrett’s Epithelium into Post-Mitotic Goblet Cells by γ-Secretase Inhibition. Dis. Model. Mech. 2010, 3, 104–110. [Google Scholar] [CrossRef]
- Moyes, L.H.; McEwan, H.; Radulescu, S.; Pawlikowski, J.; Lamm, C.G.; Nixon, C.; Sansom, O.J.; Going, J.J.; Fullarton, G.M.; Adams, P.D. Activation of Wnt Signalling Promotes Development of Dysplasia in Barrett’s Oesophagus: Wnt Signalling in Barrett’s Oesophagus. J. Pathol. 2012, 228, 99–112. [Google Scholar] [CrossRef]
- Dvorak, K.; Payne, C.M.; Chavarria, M.; Ramsey, L.; Dvorakova, B.; Bernstein, H.; Holubec, H.; Sampliner, R.E.; Guy, N.; Condon, A.; et al. Bile Acids in Combination with Low PH Induce Oxidative Stress and Oxidative DNA Damage: Relevance to the Pathogenesis of Barrett’s Oesophagus. Gut 2007, 56, 763–771. [Google Scholar] [CrossRef]
- Dvorakova, K.; Payne, C.M.; Ramsey, L.; Holubec, H.; Sampliner, R.; Dominguez, J.; Dvorak, B.; Bernstein, H.; Bernstein, C.; Prasad, A.; et al. Increased Expression and Secretion of Interleukin-6 in Patients with Barrett’s Esophagus. Clin. Cancer Res. 2004, 10, 2020–2028. [Google Scholar] [CrossRef]
- Zhou, C.; Li, J.; Li, Q. CDKN2A Methylation in Esophageal Cancer: A Meta-Analysis. Oncotarget 2017, 8, 50071–50083. [Google Scholar] [CrossRef]
- Buas, M.F.; Levine, D.M.; Makar, K.W.; Utsugi, H.; Onstad, L.; Li, X.; Galipeau, P.C.; Shaheen, N.J.; Hardie, L.J.; Romero, Y.; et al. Integrative Post-Genome-Wide Association Analysis of CDKN2A and TP53 SNPs and Risk of Esophageal Adenocarcinoma. Carcinogenesis 2014, 35, 2740–2747. [Google Scholar] [CrossRef]
- Dulak, A.M.; Stojanov, P.; Peng, S.; Lawrence, M.S.; Fox, C.; Stewart, C.; Bandla, S.; Imamura, Y.; Schumacher, S.E.; Shefler, E.; et al. Exome and Whole-Genome Sequencing of Esophageal Adenocarcinoma Identifies Recurrent Driver Events and Mutational Complexity. Nat. Genet. 2013, 45, 478–486. [Google Scholar] [CrossRef]
- Suspiro, A.; Pereira, A.D.; Afonso, A.; Albuquerque, C.; Chaves, P.; Soares, J.; Leitao, C.N. Losses of Heterozygosity on Chromosomes 9p and 17p Are Frequent Events in Barrett’s Metaplasia Not Associated with Dysplasia or Adenocarcinoma. Am. J. Gastroenterol. 2003, 98, 728–734. [Google Scholar] [CrossRef]
- Samuels, T.L.; Altman, K.W.; Gould, J.C.; Kindel, T.; Bosler, M.; MacKinnon, A.; Hagen, C.E.; Johnston, N. Esophageal Pepsin and Proton Pump Synthesis in Barrett’s Esophagus and Esophageal Adenocarcinoma. Laryngoscope 2019, 129, 2687–2695. [Google Scholar] [CrossRef]
- Samuels, T.; Hoekzema, C.; Gould, J.; Goldblatt, M.; Frelich, M.; Bosler, M.; Lee, S.-H.; Johnston, N. Local Synthesis of Pepsin in Barrett’s Esophagus and the Role of Pepsin in Esophageal Adenocarcinoma. Ann. Otol. Rhinol. Laryngol. 2015, 124, 893–902. [Google Scholar] [CrossRef]
- Samuels, T.L.; Zimmermann, M.T.; Zeighami, A.; Demos, W.; Southwood, J.E.; Blumin, J.H.; Bock, J.M.; Johnston, N. RNA Sequencing Reveals Cancer-Associated Changes in Laryngeal Cells Exposed to Non-Acid Pepsin. Laryngoscope 2021, 131, 121–129. [Google Scholar] [CrossRef]
- Stabenau, K.A.; Samuels, T.L.; Lam, T.K.; Mathison, A.J.; Wells, C.; Altman, K.W.; Battle, M.A.; Johnston, N. Pepsinogen/Proton Pump Co-Expression in Barrett’s Esophageal Cells Induces CANCER-ASSOCIATED Changes. Laryngoscope 2023, 133, 59–69. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The Basics of Epithelial-Mesenchymal Transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Roche, J. The Epithelial-to-Mesenchymal Transition in Cancer. Cancers 2018, 10, 52. [Google Scholar] [CrossRef]
- Harari, D.; Yarden, Y. Molecular Mechanisms Underlying ErbB2/HER2 Action in Breast Cancer. Oncogene 2000, 19, 6102–6114. [Google Scholar] [CrossRef]
- Dahlberg, P.S.; Jacobson, B.A.; Dahal, G.; Fink, J.M.; Kratzke, R.A.; Maddaus, M.A.; Ferrin, L.J. ERBB2 Amplifications in Esophageal Adenocarcinoma. Ann. Thorac. Surg. 2004, 78, 1790–1800. [Google Scholar] [CrossRef]
- Dahlberg, P.S.; Ferrin, L.F.; Grindle, S.M.; Nelson, C.M.; Hoang, C.D.; Jacobson, B. Gene Expression Profiles in Esophageal Adenocarcinoma. Ann. Thorac. Surg. 2004, 77, 1008–1015. [Google Scholar] [CrossRef]
- Yoon, H.H.; Shi, Q.; Sukov, W.R.; Wiktor, A.E.; Khan, M.; Sattler, C.A.; Grothey, A.; Wu, T.-T.; Diasio, R.B.; Jenkins, R.B.; et al. Association of HER2/ErbB2 Expression and Gene Amplification with Pathologic Features and Prognosis in Esophageal Adenocarcinomas. Clin. Cancer Res. 2012, 18, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Plum, P.S.; Gebauer, F.; Krämer, M.; Alakus, H.; Berlth, F.; Chon, S.-H.; Schiffmann, L.; Zander, T.; Büttner, R.; Hölscher, A.H.; et al. HER2/Neu (ERBB2) Expression and Gene Amplification Correlates with Better Survival in Esophageal Adenocarcinoma. BMC Cancer 2019, 19, 38. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.-M. Tumor Necrosis Factor. Cancer Lett. 2013, 328, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Menke, V.; van Zoest, K.P.M.; Moons, L.M.G.; Hansen, B.; Pot, R.G.J.; Siersema, P.D.; Kusters, J.G.; Kuipers, E.J. Nco I TNF-β Gene Polymorphism and TNF Expression Are Associated with an Increased Risk of Developing Barrett’s Esophagus and Esophageal Adenocarcinoma. Scand. J. Gastroenterol. 2012, 47, 378–386. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinforma. 2016, 54, 1–33. [Google Scholar] [CrossRef]
- Taylor, C.; Loomans, H.A.; Le Bras, G.F.; Koumangoye, R.B.; Romero-Morales, A.I.; Quast, L.L.; Zaika, A.I.; El-Rifai, W.; Andl, T.; Andl, C.D. Activin a Signaling Regulates Cell Invasion and Proliferation in Esophageal Adenocarcinoma. Oncotarget 2015, 6, 34228–34244. [Google Scholar] [CrossRef]
- Blum, A.E.; Venkitachalam, S.; Ravillah, D.; Chelluboyina, A.K.; Kieber-Emmons, A.M.; Ravi, L.; Kresak, A.; Chandar, A.K.; Markowitz, S.D.; Canto, M.I.; et al. Systems Biology Analyses Show Hyperactivation of Transforming Growth Factor-β and JNK Signaling Pathways in Esophageal Cancer. Gastroenterology 2019, 156, 1761–1774. [Google Scholar] [CrossRef]
- Kamogashira, T.; Hayashi, K.; Fujimoto, C.; Iwasaki, S.; Yamasoba, T. Functionally and Morphologically Damaged Mitochondria Observed in Auditory Cells under Senescence-Inducing Stress. Npj Aging Mech. Dis. 2017, 3, 2. [Google Scholar] [CrossRef]
- Song, S.; Qiu, D.; Luo, F.; Wei, J.; Wu, M.; Wu, H.; Du, C.; Du, Y.; Ren, Y.; Chen, N.; et al. Knockdown of NLRP3 Alleviates High Glucose or TGFB1-Induced EMT in Human Renal Tubular Cells. J. Mol. Endocrinol. 2018, 61, 101–113. [Google Scholar] [CrossRef]
- Goossens, V.; Vos, K.D.; Vercammen, D.; Steemans, M.; Vancompernolle, K.; Fiers, W.; Vandenabeele, P.; Grooten, J. Redox Regulation of TNF Signaling. BioFactors 1999, 10, 145–156. [Google Scholar] [CrossRef]
- Lisanti, M.P.; Martinez-Outschoorn, U.E.; Chiavarina, B.; Pavlides, S.; Whitaker-Menezes, D.; Tsirigos, A.; Witkiewicz, A.K.; Lin, Z.; Balliet, R.M.; Howell, A.; et al. Understanding the “Lethal” Drivers of Tumor-Stroma Co-Evolution: Emerging Role(s) for Hypoxia, Oxidative Stress and Autophagy/Mitophagy in the Tumor Microenvironment. Cancer Biol. Ther. 2010, 10, 537–542. [Google Scholar] [CrossRef]
- Gao, M.; Yi, J.; Zhu, J.; Minikes, A.M.; Monian, P.; Thompson, C.B.; Jiang, X. Role of Mitochondria in Ferroptosis. Mol. Cell 2019, 73, 354–363.e3. [Google Scholar] [CrossRef]
- Old, O.; Moayyedi, P.; Love, S.; Roberts, C.; Hapeshi, J.; Foy, C.; Stokes, C.; Briggs, A.; Jankowski, J.; Barr, H.; et al. Barrett’s Oesophagus Surveillance versus Endoscopy at Need Study (BOSS): Protocol and Analysis Plan for a Multicentre Randomized Controlled Trial. J. Med. Screen. 2015, 22, 158–164. [Google Scholar] [CrossRef]
- Codipilly, D.C.; Chandar, A.K.; Singh, S.; Wani, S.; Shaheen, N.J.; Inadomi, J.M.; Chak, A.; Iyer, P.G. The Effect of Endoscopic Surveillance in Patients with Barrett’s Esophagus: A Systematic Review and Meta-Analysis. Gastroenterology 2018, 154, 2068–2086.e5. [Google Scholar] [CrossRef]
- Krishnamoorthi, R.; Singh, S.; Ragunathan, K.; Visrodia, K.; Wang, K.K.; Katzka, D.A.; Iyer, P.G. Factors Associated with Progression of Barrett’s Esophagus: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 1046–1055.e8. [Google Scholar] [CrossRef]
- Whiteman, D.C.; Appleyard, M.; Bahin, F.F.; Bobryshev, Y.V.; Bourke, M.J.; Brown, I.; Chung, A.; Clouston, A.; Dickins, E.; Emery, J.; et al. Australian Clinical Practice Guidelines for the Diagnosis and Management of Barrett’s Esophagus and Early Esophageal Adenocarcinoma: Australian Clinical Practice Guidelines for BE and EAC. J. Gastroenterol. Hepatol. 2015, 30, 804–820. [Google Scholar] [CrossRef]
- Redston, M.; Noffsinger, A.; Kim, A.; Akarca, F.G.; Rara, M.; Stapleton, D.; Nowden, L.; Lash, R.; Bass, A.J.; Stachler, M.D. Abnormal TP53 Predicts Risk of Progression in Patients with Barrett’s Esophagus Regardless of a Diagnosis of Dysplasia. Gastroenterology 2022, 162, 468–481. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).