Exploring the Role of a Novel Interleukin-17 Homolog from Invertebrate Marine Mussel Mytilus coruscus in Innate Immune Response: Is Negative Regulation by Mc-Novel_miR_145 the Key?
Abstract
1. Introduction
2. Results
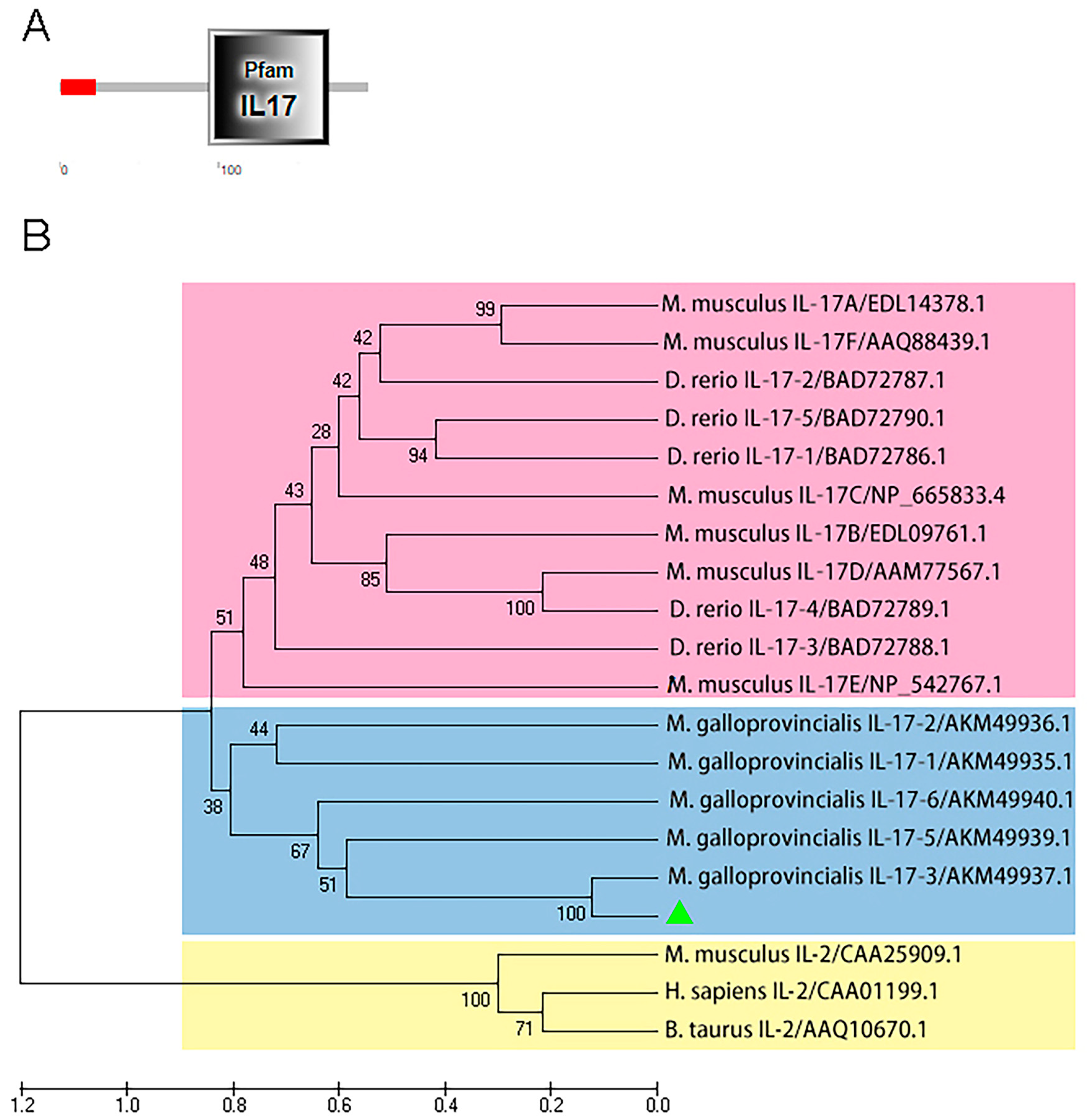
2.1. Characterization of McIL-17-3
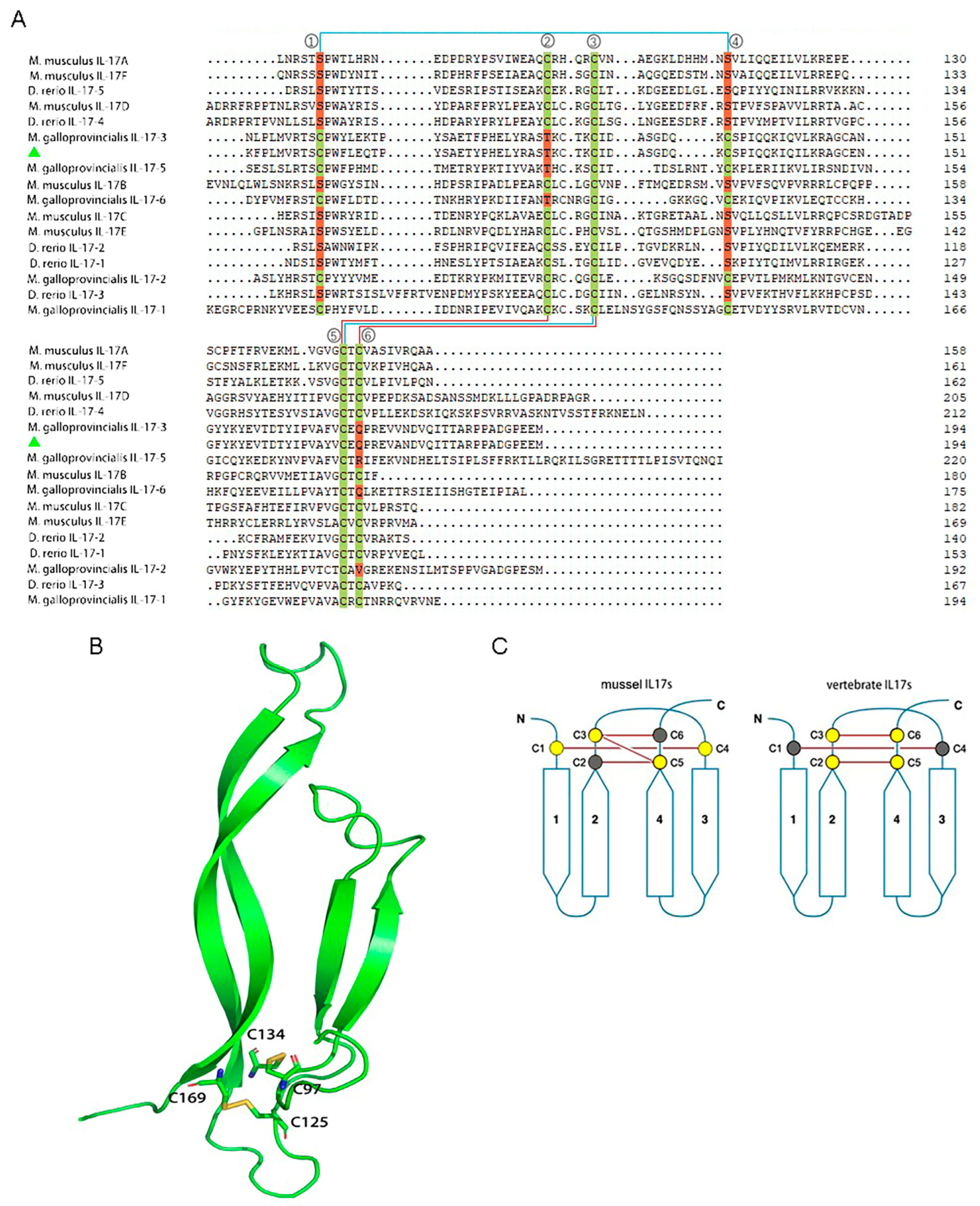
2.2. Multiple Alignment and Tertiary Structure Prediction
2.3. Transcriptional Expression of McIL-17-3
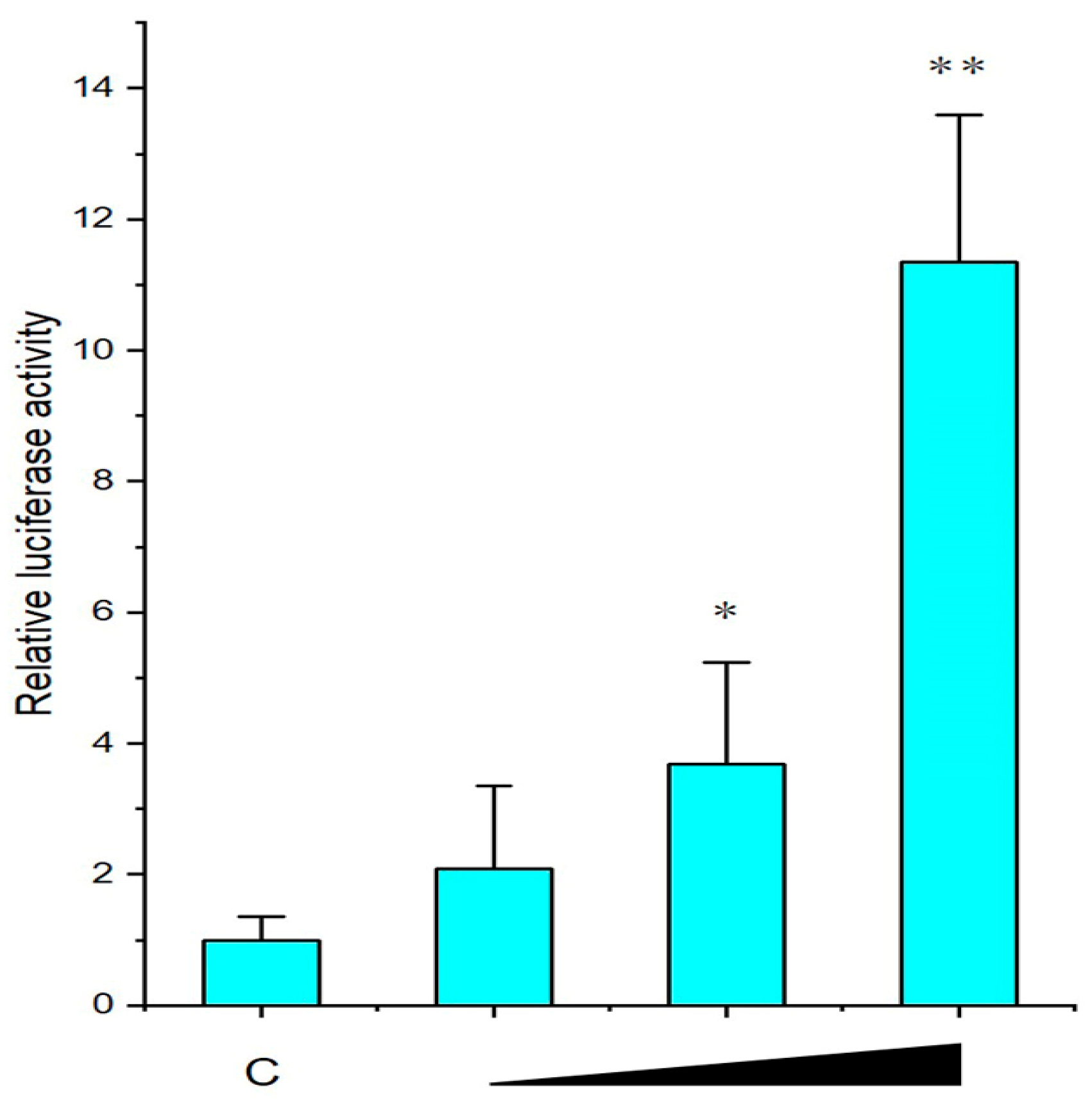
2.4. The Activation of Downstream by McIL-17-3
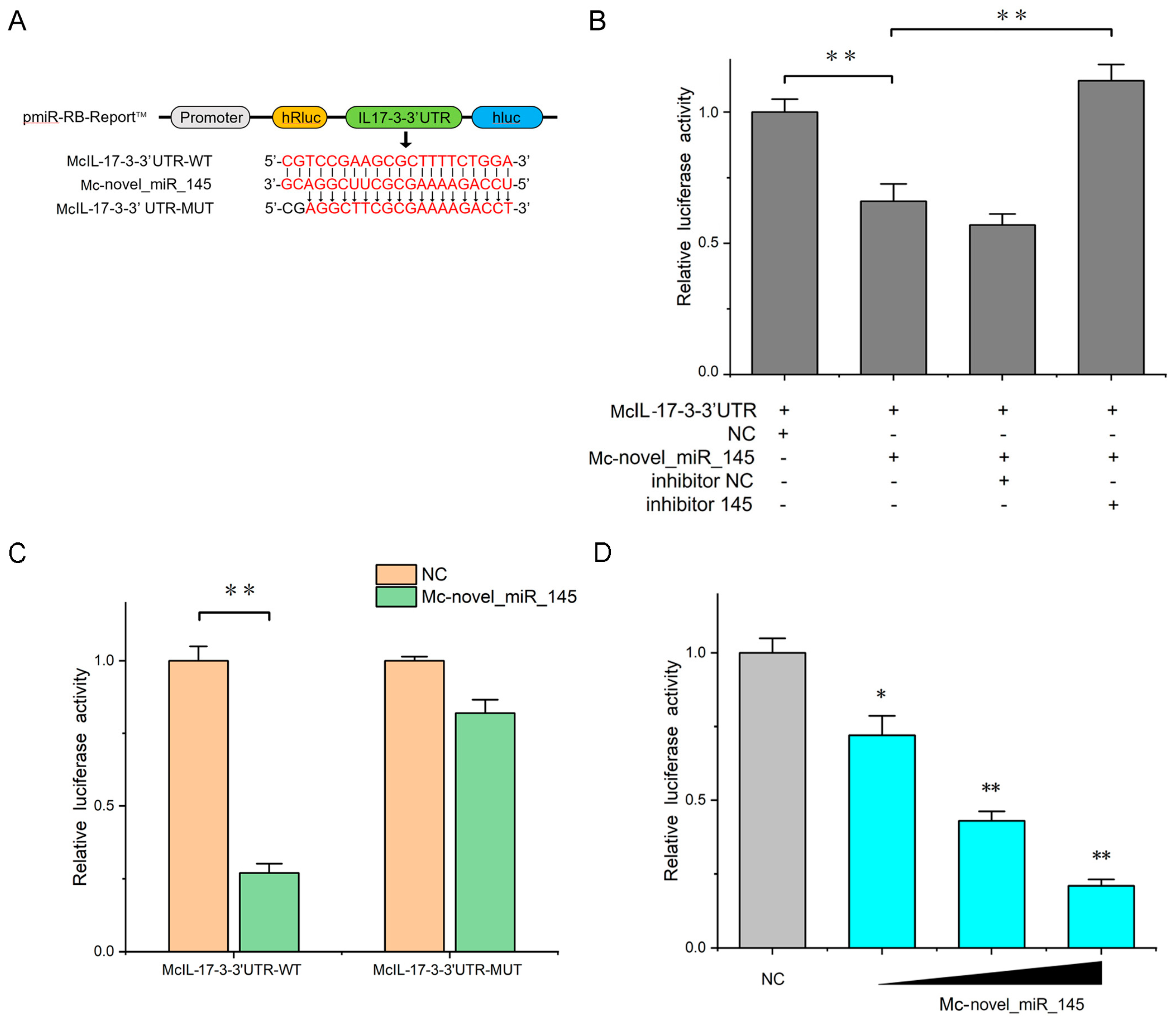
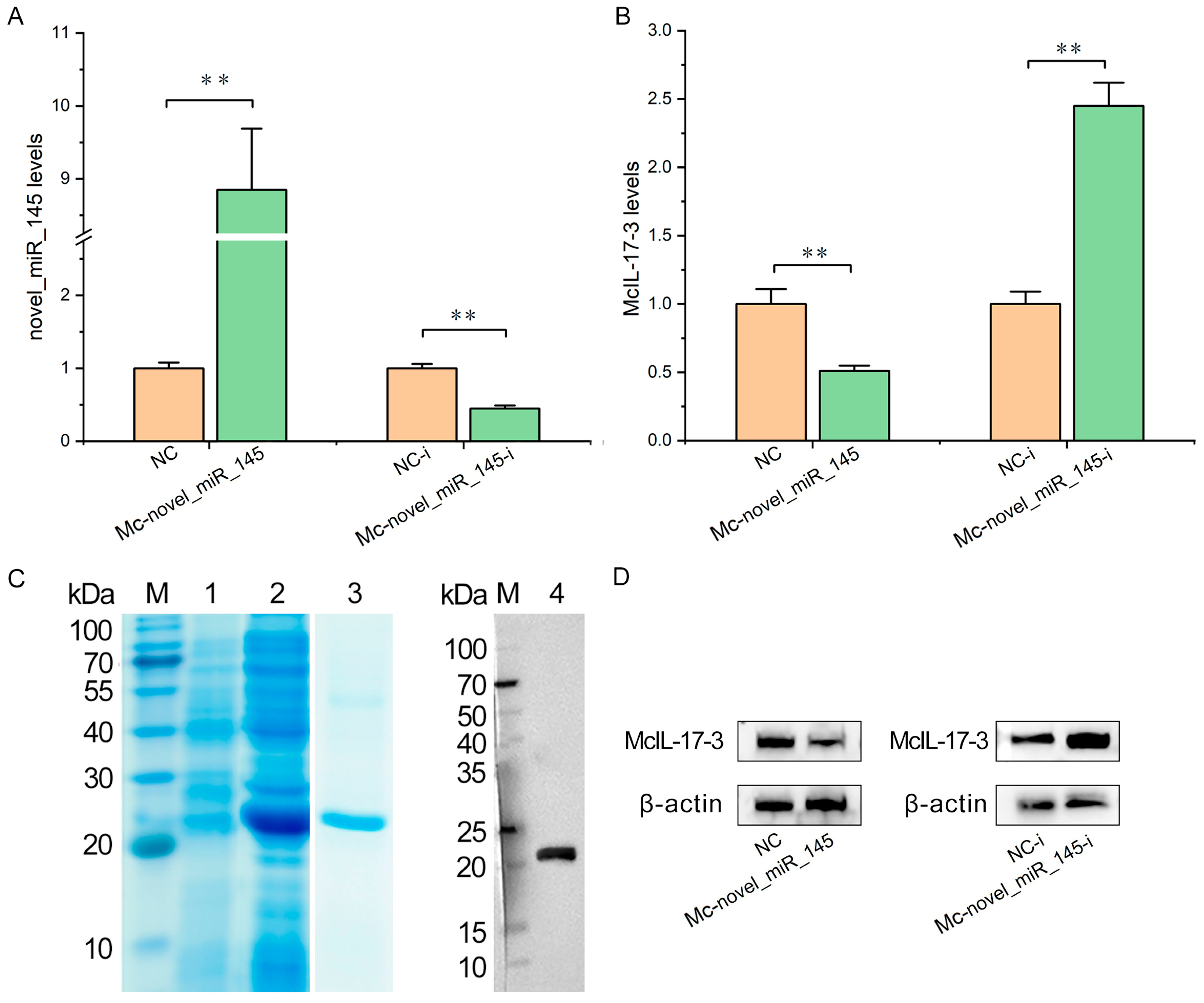
2.5. Confirmation of McIL-17-3 as a Target Gene of Mc-novel_miR_145
2.6. Mc-novel_miR_145 Negatively Regulates the Expression of McIL-17-3
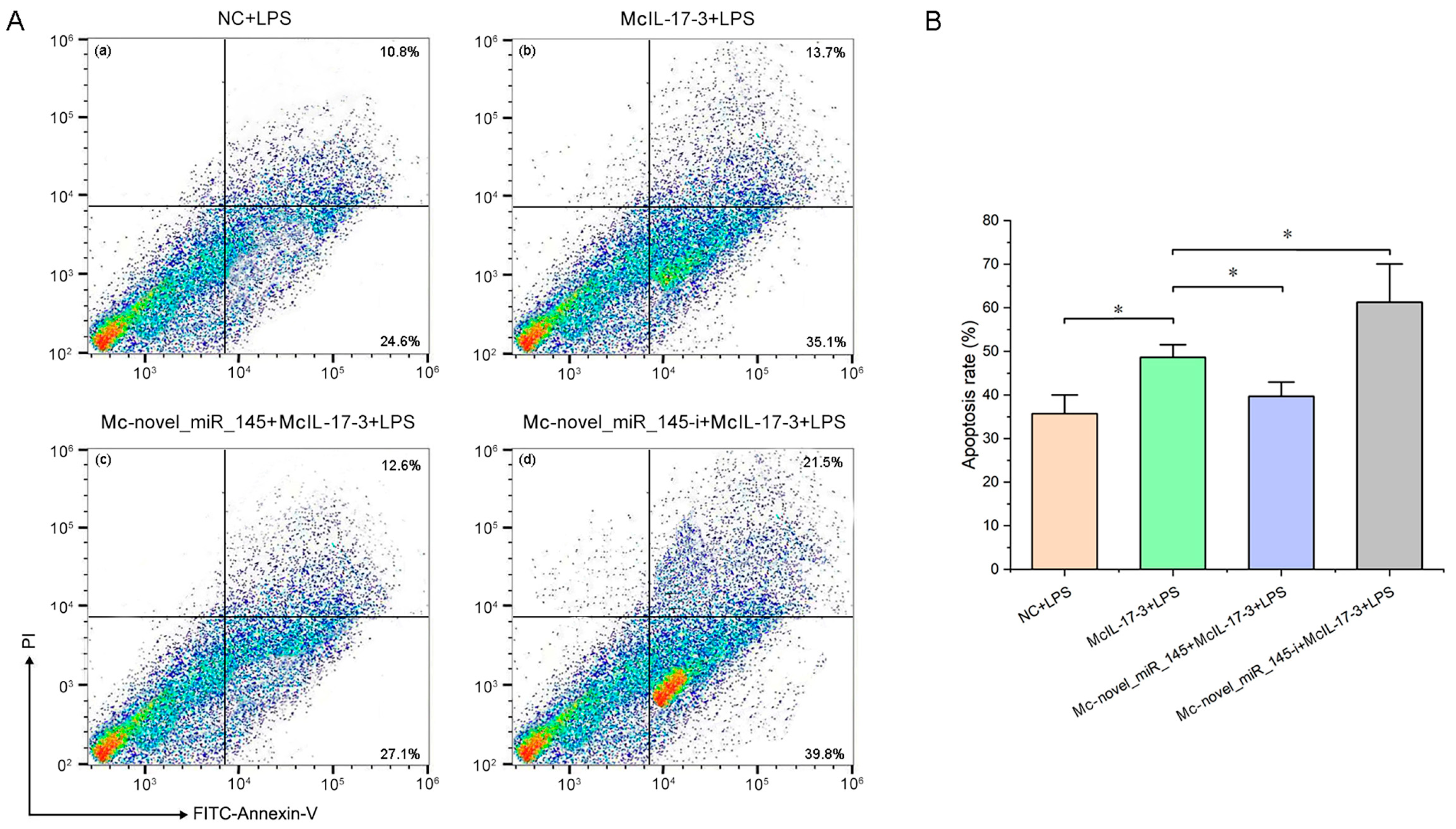
2.7. Apoptosis of Hemocytes
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Artificial Seawater
4.3. Animals
4.4. McIL-17-3 cDNA Identification
4.5. Quantitative Real-Time PCR Assays
4.6. Cell Culture
4.7. Synthesis of miRNA Mimic and Inhibitor
4.8. Luciferase Reporter Analysis
4.9. Recombinant Expression, Purification and the Antiserum Preparation
4.10. Western Blotting
4.11. Apoptosis
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medzhitov, R.; Janeway, C., Jr. Innate immunity. N. Engl. J. Med. 2000, 343, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Lacy, P.; Stow, J.L. Cytokine release from innate immune cells: Association with diverse membrane trafficking pathways. Blood J. Am. Soc. Hematol. 2011, 118, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Proinflammatory cytokines. Chest 2000, 118, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Rauta, P.R.; Nayak, B.; Das, S. Immune system and immune responses in fish and their role in comparative immunity study: A model for higher organisms. Immunol. Lett. 2012, 148, 23–33. [Google Scholar] [CrossRef]
- Hymowitz, S.G.; Filvaroff, E.H.; Yin, J.; Lee, J.; Cai, L.; Risser, P.; Maruoka, M.; Mao, W.; Foster, J.; Kelley, R.F. IL-17s adopt a cystine knot fold: Structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. EMBO J. 2001, 20, 5332–5341. [Google Scholar] [CrossRef]
- Buckley, K.M.; Ho, E.C.H.; Hibino, T.; Schrankel, C.S.; Schuh, N.W.; Wang, G.Z.; Rast, J.P. IL17 factors are early regulators in the gut epithelium during inflammatory response to Vibrio in the sea urchin larva. eLife 2017, 6, e23481. [Google Scholar] [CrossRef]
- Cua, D.J.; Tato, C.M. Innate IL-17-producing cells: The sentinels of the immune system. Nat. Rev. Immunol. 2010, 10, 479–489. [Google Scholar] [CrossRef]
- Mills, K.H. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2022, 10, 479–489. [Google Scholar] [CrossRef]
- Hibino, T.; Loza-Coll, M.; Messier, C.; Majeske, A.J.; Cohen, A.H.; Terwilliger, D.P.; Buckley, K.M.; Brockton, V.; Nair, S.V.; Berney, K. The immune gene repertoire encoded in the purple sea urchin genome. Dev. Biol. 2006, 300, 349–365. [Google Scholar] [CrossRef]
- Albertin, C.B.; Simakov, O.; Mitros, T.; Wang, Z.Y.; Pungor, J.R.; Edsinger-Gonzales, E.; Brenner, S.; Ragsdale, C.W.; Rokhsar, D.S. The octopus genome and the evolution of cephalopod neural and morphological novelties. Nature 2015, 524, 220–224. [Google Scholar] [CrossRef]
- Saco, A.; Rey-Campos, M.; Rosani, U.; Novoa, B.; Figueras, A. The evolution and diversity of interleukin-17 highlight an expansion in marine invertebrates and its conserved role in mucosal immunity. Front. Immunol. 2021, 12, 692997. [Google Scholar] [CrossRef] [PubMed]
- Gerdol, M.; Moreira, R.; Cruz, F.; Gómez-Garrido, J.; Vlasova, A.; Rosani, U.; Venier, P.; Naranjo-Ortiz, M.A.; Murgarella, M.; Greco, S. Massive gene presence-absence variation shapes an open pan-genome in the Mediterranean mussel. Genome Biol. 2020, 21, 1–21. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, S.; Feng, C.; Zhan, W.; Zheng, Z.; Wang, Q.; Deng, Y.; Jiao, Y.; Du, X. Evolution and function analysis of interleukin-17 gene from Pinctada fucata martensii. Fish Shellfish Immunol. 2019, 88, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xiao, G.; Hu, G.; Chen, R.; Li, M.; Teng, S. Interleukin-17D mediates Vibrio harveyi infection-related changes in Tegillarca granosa though activation of activator protein 1 in vivo. Aquaculture 2023, 566, 739178. [Google Scholar] [CrossRef]
- Lv, X.; Sun, J.; Li, Y.; Yang, W.; Wang, L.; Leng, J.; Yan, X.; Guo, Z.; Yang, Q.; Wang, L. CgIL17-5 regulates the mRNA expressions of immune effectors through inducing the phosphorylation of CgMAPKs and the nuclear translocation of CgRel and CgAP-1 in the Pacific oyster Crassostrea gigas. Dev. Comp. Immunol. 2022, 127, 104263. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, J.; Wu, Z.; Lian, X.; Han, S.; Huang, S.; Yang, C.; Wang, L.; Song, L. AP-1 regulates the expression of IL17-4 and IL17-5 in the pacific oyster Crassostrea gigas. Fish Shellfish Immunol. 2020, 97, 554–563. [Google Scholar] [CrossRef]
- Wu, S.-Z.; Huang, X.-D.; Li, Q.; He, M.-X. Interleukin-17 in pearl oyster (Pinctada fucata): Molecular cloning and functional characterization. Fish Shellfish Immunol. 2013, 34, 1050–1056. [Google Scholar] [CrossRef]
- Xin, L.; Zhang, H.; Zhang, R.; Li, H.; Wang, W.; Wang, L.; Wang, H.; Qiu, L.; Song, L. CgIL17-5, an ancient inflammatory cytokine in Crassostrea gigas exhibiting the heterogeneity functions compared with vertebrate interleukin17 molecules. Dev. Comp. Immunol. 2015, 53, 339–348. [Google Scholar] [CrossRef]
- Denli, A.M.; Tops, B.B.; Plasterk, R.H.; Ketting, R.F.; Hannon, G.J. Processing of primary microRNAs by the Microprocessor complex. Nature 2004, 432, 231–235. [Google Scholar] [CrossRef]
- Khan, D.; Ansar Ahmed, S. Regulation of IL-17 in autoimmune diseases by transcriptional factors and microRNAs. Front. Genet. 2015, 6, 236. [Google Scholar] [CrossRef]
- Martín-Gómez, L.; Villalba, A.; Kerkhoven, R.H.; Abollo, E. Role of microRNAs in the immunity process of the flat oyster Ostrea edulis against bonamiosis. Infect. Genet. Evol. 2014, 27, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, X.; Feng, Y.; Huang, W.; Wang, W.; Li, L.; Fang, X.; Que, H.; Zhang, G. Identification of conserved and novel microRNAs in the Pacific oyster Crassostrea gigas by deep sequencing. PLoS ONE 2014, 9, e104371. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, L.; Song, L.; Liu, R.; Zhang, H.; Huang, M.; Chen, H. The identification and characteristics of immune-related microRNAs in haemocytes of oyster Crassostrea gigas. PLoS ONE 2014, 9, e88397. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Aceves, M.A.; Cohen, A.; Smith, Y.; Faggio, C. A potential microRNA regulation of immune-related genes in invertebrate haemocytes. Sci. Total Environ. 2018, 621, 302–307. [Google Scholar] [CrossRef]
- Walker, S.E.; Spencer, G.E.; Necakov, A.; Carlone, R.L. Identification and characterization of microRNAs during retinoic acid-induced regeneration of a molluscan central nervous system. Int. J. Mol. Sci. 2018, 19, 2741. [Google Scholar] [CrossRef] [PubMed]
- Abo-Al-Ela, H.G.; Faggio, C. MicroRNA-mediated stress response in bivalve species. Ecotoxicol. Environ. Saf. 2021, 208, 111442. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Yoshitake, K.; Asaduzzaman, M.; Kinoshita, S.; Watabe, S.; Asakawa, S. Discovery and functional understanding of MiRNAs in molluscs: A genome-wide profiling approach. RNA Biol. 2021, 18, 1702–1715. [Google Scholar] [CrossRef]
- Rosani, U.; Bortoletto, E.; Bai, C.-M.; Novoa, B.; Figueras, A.; Venier, P.; Fromm, B. Digging into bivalve miRNAomes: Between conservation and innovation. Philos. Trans. R. Soc. B 2021, 376, 20200165. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, T.; Li, L.; Tu, K.; Yu, T.; Wu, B.; Zhou, L.; Tian, J.; Liu, Z. MicroRNA expression signature in the striated and smooth adductor muscles of Yesso scallop Patinopecten yessoensis. Genomics 2022, 114, 110409. [Google Scholar] [CrossRef]
- Tian, R.; Zheng, Z.; Huang, R.; Jiao, Y.; Du, X. miR-29a participated in nacre formation and immune response by targeting Y2R in Pinctada martensii. Int. J. Mol. Sci. 2015, 16, 29436–29445. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, Z.; Wang, H.; Wang, L.; Wang, W.; Liu, R.; Qiu, L.; Song, L. An invertebrate-specific and immune-responsive microRNA augments oyster haemocyte phagocytosis by targeting CgIκB2. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, Z.; Wang, L.; Wang, H.; Liu, R.; Zhang, H.; Song, L. An invertebrate-specific miRNA targeted the ancient cholinergic neuroendocrine system of oyster. Open Biol. 2016, 6, 160059. [Google Scholar] [CrossRef]
- Chen, H.; Xin, L.; Song, X.; Wang, L.; Wang, W.; Liu, Z.; Zhang, H.; Wang, L.; Zhou, Z.; Qiu, L. A norepinephrine-responsive miRNA directly promotes CgHSP90AA1 expression in oyster haemocytes during desiccation. Fish Shellfish Immunol. 2017, 64, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Li, J.; Wang, W.; Li, J.; Zhao, Q.; Li, M.; Wang, L.; Song, L. A calmodulin targeted by miRNA scaffold659_26519 regulates IL-17 expression in the early immune response of oyster Crassostrea gigas. Dev. Comp. Immunol. 2021, 124, 104180. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Li, C.; Zhang, P.; Wang, Z.; Zhang, W.; Jin, C.-H. miR-200 modulates coelomocytes antibacterial activities and LPS priming via targeting Tollip in Apostichopus japonicus. Fish Shellfish Immunol. 2015, 45, 431–436. [Google Scholar] [CrossRef]
- Li, C.; Zhao, M.; Zhang, C.; Zhang, W.; Zhao, X.; Duan, X.; Xu, W. miR210 modulates respiratory burst in Apostichopus japonicus coelomocytes via targeting Toll-like receptor. Dev. Comp. Immunol. 2016, 65, 377–381. [Google Scholar] [CrossRef]
- Lv, M.; Chen, H.; Shao, Y.; Li, C.; Zhang, W.; Zhao, X.; Jin, C.; Xiong, J. miR-92a regulates coelomocytes apoptosis in sea cucumber Apostichopus japonicus via targeting Aj14-3-3ζ in vivo. Fish Shellfish Immunol. 2017, 69, 211–217. [Google Scholar] [CrossRef]
- Shao, Y.; Li, C.; Xu, W.; Zhang, P.; Zhang, W.; Zhao, X. miR-31 links lipid metabolism and cell apoptosis in bacteria-challenged Apostichopus japonicus via targeting CTRP9. Front. Immunol. 2017, 8, 263. [Google Scholar] [CrossRef]
- Guo, M.; Wang, Y.; Fu, X.; Tao, W.; Li, C. circRNA1149 from Apostichopus japonicus suppresses coelomocyte apoptosis act as miR-92a sponge to regulate Bax expression in response to Vibrio splendidus infection. Aquaculture 2023, 562, 738812. [Google Scholar] [CrossRef]
- Zuo, H.; Weng, K.; Luo, M.; Yang, L.; Weng, S.; He, J.; Xu, X. A MicroRNA-1–Mediated Inhibition of the NF-κB Pathway by the JAK-STAT Pathway in the Invertebrate Litopenaeus vannamei. J. Immunol. 2020, 204, 2918–2930. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Z.; Guo, B.; Zhang, X.; Liao, Z.; Qi, P.; Yan, X. Integrated analysis of miRNAome and transcriptome reveals miRNA-mRNA network regulation in Vibrio alginolyticus infected thick shell mussel Mytilus coruscus. Mol. Immunol. 2021, 132, 217–226. [Google Scholar] [CrossRef] [PubMed]
- de Morales, J.M.G.R.; Puig, L.; Daudén, E.; Cañete, J.D.; Pablos, J.L.; Martín, A.O.; Juanatey, C.G.; Adán, A.; Montalbán, X.; Borruel, N. Critical role of interleukin (IL)-17 in inflammatory and immune disorders: An updated review of the evidence focusing in controversies. Autoimmun. Rev. 2020, 19, 102429. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Adachi, M.; Oda, N.; Kokubu, F.; Huang, S.-K. IL-17 cytokine family. J. Allergy Clin. Immunol. 2004, 114, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Starnes, T.; Broxmeyer, H.E.; Robertson, M.J.; Hromas, R. Cutting edge: IL-17D, a novel member of the IL-17 family, stimulates cytokine production and inhibits hemopoiesis. J. Immunol. 2002, 169, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Gaffen, S.L.; Kramer, J.M.; Jeffrey, J.Y.; Shen, F. The IL-17 cytokine family. Vitam. Horm. 2006, 74, 255–282. [Google Scholar] [PubMed]
- Roberts, S.; Gueguen, Y.; de Lorgeril, J.; Goetz, F. Rapid accumulation of an interleukin 17 homolog transcript in Crassostrea gigas hemocytes following bacterial exposure. Dev. Comp. Immunol. 2008, 32, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Zhang, Y.; Xiang, Z.; Tong, Y.; Qu, F.; Yu, Z. Genomic characterization and expression analysis of five novel IL-17 genes in the Pacific oyster, Crassostrea gigas. Fish Shellfish Immunol. 2014, 40, 455–465. [Google Scholar] [CrossRef]
- Gaffen, S.L. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 2009, 9, 556–567. [Google Scholar] [CrossRef]
- Hata, K.; Andoh, A.; Shimada, M.; Fujino, S.; Bamba, S.; Araki, Y.; Okuno, T.; Fujiyama, Y.; Bamba, T. IL-17 stimulates inflammatory responses via NF-κB and MAP kinase pathways in human colonic myofibroblasts. Am. J. Physiol. -Gastrointest. Liver Physiol. 2002, 282, G1035–G1044. [Google Scholar] [CrossRef]
- Niimoto, T.; Nakasa, T.; Ishikawa, M.; Okuhara, A.; Izumi, B.; Deie, M.; Suzuki, O.; Adachi, N.; Ochi, M. MicroRNA-146a expresses in interleukin-17 producing T cells in rheumatoid arthritis patients. BMC Musculoskelet. Disord. 2010, 11, 1–11. [Google Scholar] [CrossRef]
- Srivastava, A.; Nikamo, P.; Lohcharoenkal, W.; Li, D.; Meisgen, F.; Landén, N.X.; Ståhle, M.; Pivarcsi, A.; Sonkoly, E. MicroRNA-146a suppresses IL-17–mediated skin inflammation and is genetically associated with psoriasis. J. Allergy Clin. Immunol. 2017, 139, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Cheng, Y.; Kim, J.G. microRNA-146a downregulates IL-17 and IL-35 and inhibits proliferation of human periodontal ligament stem cells. J. Cell. Biochem. 2019, 120, 13861–13866. [Google Scholar] [CrossRef] [PubMed]
- Podsiad, A.; Standiford, T.J.; Ballinger, M.N.; Eakin, R.; Park, P.; Kunkel, S.L.; Moore, B.B.; Bhan, U. MicroRNA-155 regulates host immune response to postviral bacterial pneumonia via IL-23/IL-17 pathway. Am. J. Physiol. -Lung Cell. Mol. Physiol. 2016, 310, L465–L475. [Google Scholar] [CrossRef]
- Xaus, J.; Comalada, M.; Valledor, A.F.; Lloberas, J.; López-Soriano, F.; Argilés, J.M.; Bogdan, C.; Celada, A. LPS induces apoptosis in macrophages mostly through the autocrine production of TNF-α. Blood J. Am. Soc. Hematol. 2000, 95, 3823–3831. [Google Scholar]
- Bannerman, D.D.; Goldblum, S.E. Mechanisms of bacterial lipopolysaccharide-induced endothelial apoptosis. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2003, 284, L899–L914. [Google Scholar] [CrossRef]
- Brass, D.M.; Hollingsworth, J.W.; Cinque, M.; Li, Z.; Potts, E.; Toloza, E.; Foster, W.M.; Schwartz, D.A. Chronic LPS inhalation causes emphysema-like changes in mouse lung that are associated with apoptosis. Am. J. Respir. Cell Mol. Biol. 2008, 39, 584–590. [Google Scholar] [CrossRef]
- Dragon, S.; Saffar, A.S.; Shan, L.; Gounni, A.S. IL-17 attenuates the anti-apoptotic effects of GM-CSF in human neutrophils. Mol. Immunol. 2008, 45, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Wang, Q.; Guo, C.; Wang, X.; Cao, X.; Shi, Y.; Gao, F.; Ma, C.; Zhang, L. IL-17 induces apoptosis of vascular endothelial cells—A potential mechanism for human acute coronary syndrome. Clin. Immunol. 2011, 141, 152–160. [Google Scholar] [CrossRef]
- Chang, Y.; Al-Alwan, L.; Audusseau, S.; Chouiali, F.; Carlevaro-Fita, J.; Iwakura, Y.; Baglole, C.J.; Eidelman, D.H.; Hamid, Q. Genetic deletion of IL-17A reduces cigarette smoke-induced inflammation and alveolar type II cell apoptosis. Am. J. Physiol. -Lung Cell. Mol. Physiol. 2014, 306, L132–L143. [Google Scholar] [CrossRef]
- Hou, W.; Jin, Y.-H.; Kang, H.S.; Kim, B.S. Interleukin-6 (IL-6) and IL-17 synergistically promote viral persistence by inhibiting cellular apoptosis and cytotoxic T cell function. J. Virol. 2014, 88, 8479–8489. [Google Scholar] [CrossRef]
- Wang, Q.; Li, D.; Han, Y.; Ding, X.; Xu, T.; Tang, B. MicroRNA-146 protects A549 and H1975 cells from LPS-induced apoptosis and inflammation injury. J. Biosci. 2017, 42, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Z.; Yuan, B.; Dong, Y.; Zhang, L.; Zeng, Z. MicroRNA-146a-5p attenuates irradiation-induced and LPS-induced hepatic stellate cell activation and hepatocyte apoptosis through inhibition of TLR4 pathway. Cell Death Dis. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, L.; Zhao, Q.; Wu, Z.; Kong, L. MicroRNA-93 inhibits chondrocyte apoptosis and inflammation in osteoarthritis by targeting the TLR4/NF-κB signaling pathway. Int. J. Mol. Med. 2019, 43, 779–790. [Google Scholar] [CrossRef]
- Wan, G.; An, Y.; Tao, J.; Wang, Y.; Zhou, Q.; Yang, R.; Liang, Q. MicroRNA-129-5p alleviates spinal cord injury in mice via suppressing the apoptosis and inflammatory response through HMGB1/TLR4/NF-κB pathway. Biosci. Rep. 2020, 40, BSR20193315. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.-F.; Fang, J.; Wu, X.-N.; Yu, C.-H. MicroRNA-203 accelerates apoptosis in LPS-stimulated alveolar epithelial cells by targeting PIK3CA. Biochem. Biophys. Res. Commun. 2014, 450, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Tang, Z. The Nrf2 molecule trigger antioxidant defense against acute benzo(a)pyrene exposure in the thick shell mussel Mytilus coruscus. Aquat Toxicol 2020, 226, 105554. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]

| Primer | Sequences (5′–3′) | Usage |
|---|---|---|
| U6 | CTCGCTTCGGCAGCACA | Internal reference for miRNAs qPCR |
| AACGCTTCACGAATTTGCGT | ||
| β-actin | ATGAAACCACCTACAACAGT | Internal reference for qPCR |
| TAGACCCACCAATCCAGACG | ||
| McIL-17-3 | TGCTCATTTGGTAGATCACGGA | For McIL-17-3 qPCR |
| GCACTGTATGGCGTTTGCTC | ||
| Mc-Novel_miR_145-F | ACACTCCAGCTGGGUCCAGAAAAGCGCUU | For Mc-novel_miR_145 qPCR |
| McIL-17-3-F | AAGGATCCATGTATTTTATCAATATACTTA | For pEGFP-McIL-17-3 plasmid construction |
| McIL-17-3-R | CCGCTCGAGTTCTTCTGGTCCATCAGCTGGA | |
| McIL-17-3-YF | CACGAATTCATGTATTTTATCAATATACTTA | For pET32a-McIL-17-3 plasmid construction |
| McIL-17-3-YR | GACGGATCCTTCTTCTGGTCCATCAGCTGGA | |
| McIL-17-3-3′UTR-WT-F | CCGCTCGAGTATGTGAACAGCCAAGAGAAGTCGCAAATGATGTA | For McIL-17-3-3′UTR-WT plasmid construction |
| McIL-17-3-3′UTR-WT-R | GCGGCCGCAAACAGAATACAAAAAACCCTTTATATGGCAAGTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Qiu, L.; Si, X.; Zhang, X.; Guo, B.; Liao, Z.; Yan, X.; Qi, P. Exploring the Role of a Novel Interleukin-17 Homolog from Invertebrate Marine Mussel Mytilus coruscus in Innate Immune Response: Is Negative Regulation by Mc-Novel_miR_145 the Key? Int. J. Mol. Sci. 2023, 24, 5928. https://doi.org/10.3390/ijms24065928
Chen X, Qiu L, Si X, Zhang X, Guo B, Liao Z, Yan X, Qi P. Exploring the Role of a Novel Interleukin-17 Homolog from Invertebrate Marine Mussel Mytilus coruscus in Innate Immune Response: Is Negative Regulation by Mc-Novel_miR_145 the Key? International Journal of Molecular Sciences. 2023; 24(6):5928. https://doi.org/10.3390/ijms24065928
Chicago/Turabian StyleChen, Xinglu, Longmei Qiu, Xirui Si, Xiaolin Zhang, Baoying Guo, Zhi Liao, Xiaojun Yan, and Pengzhi Qi. 2023. "Exploring the Role of a Novel Interleukin-17 Homolog from Invertebrate Marine Mussel Mytilus coruscus in Innate Immune Response: Is Negative Regulation by Mc-Novel_miR_145 the Key?" International Journal of Molecular Sciences 24, no. 6: 5928. https://doi.org/10.3390/ijms24065928
APA StyleChen, X., Qiu, L., Si, X., Zhang, X., Guo, B., Liao, Z., Yan, X., & Qi, P. (2023). Exploring the Role of a Novel Interleukin-17 Homolog from Invertebrate Marine Mussel Mytilus coruscus in Innate Immune Response: Is Negative Regulation by Mc-Novel_miR_145 the Key? International Journal of Molecular Sciences, 24(6), 5928. https://doi.org/10.3390/ijms24065928





