A Novel Resveratrol-Induced Pathway Increases Neuron-Derived Cell Resilience against Oxidative Stress
Abstract
1. Introduction
2. Results
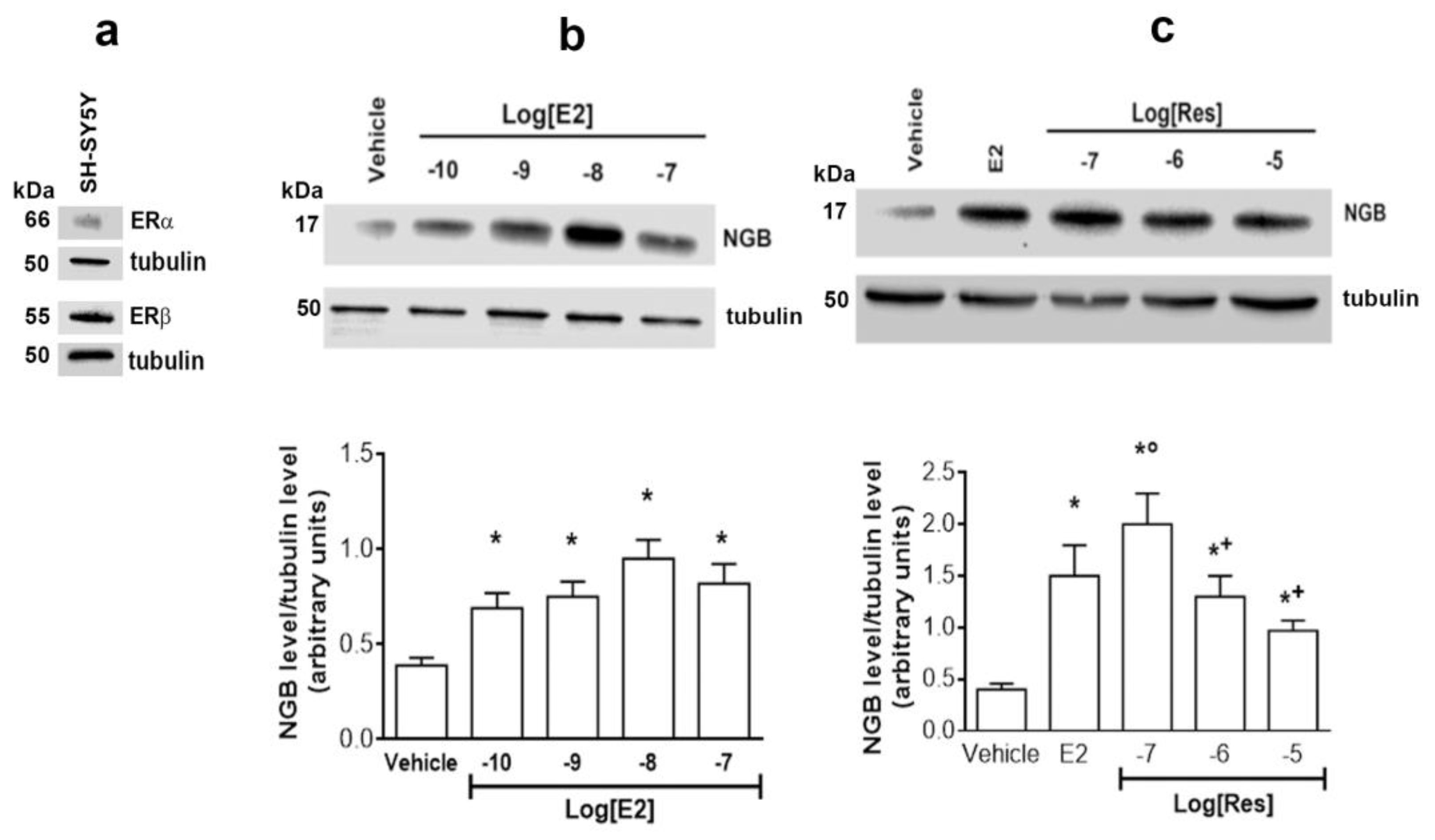
2.1. E2-Res Comparison on NGB Levels in SH-SY5Y
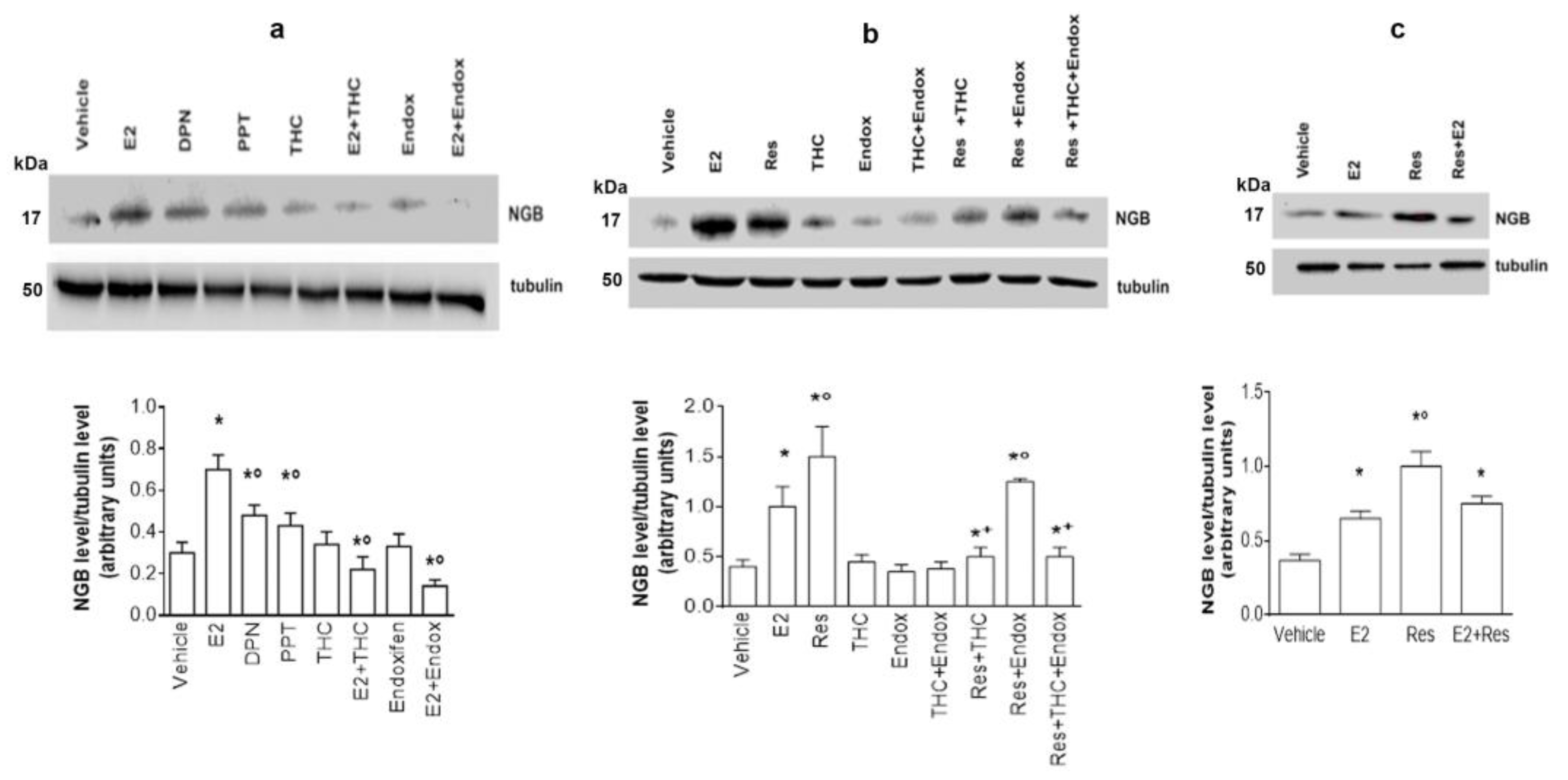
2.2. ERs Involvement in Res-Induced NGB Accumulation in SH-SY5Y
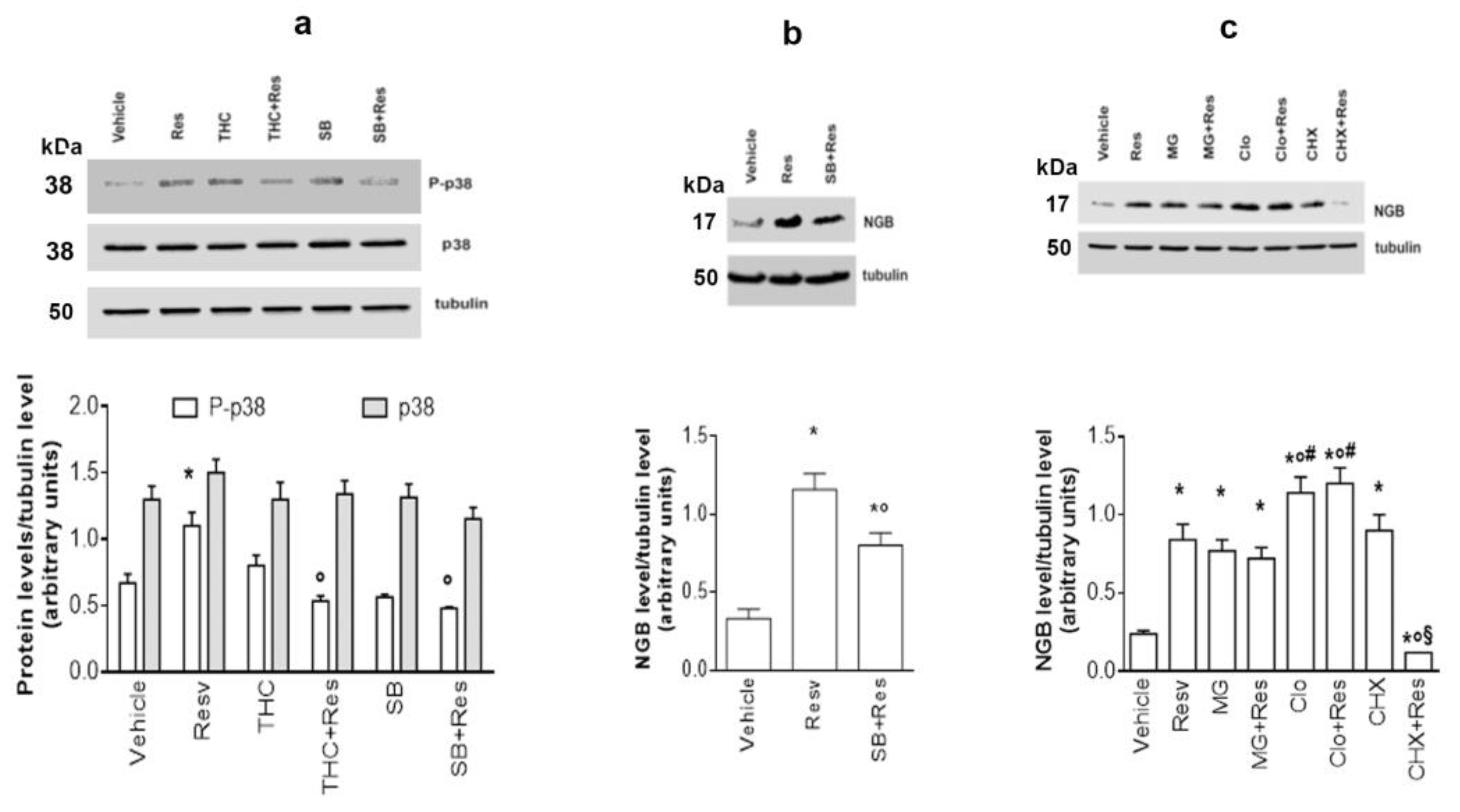
2.3. Signaling Pathways Involved in Res Effects
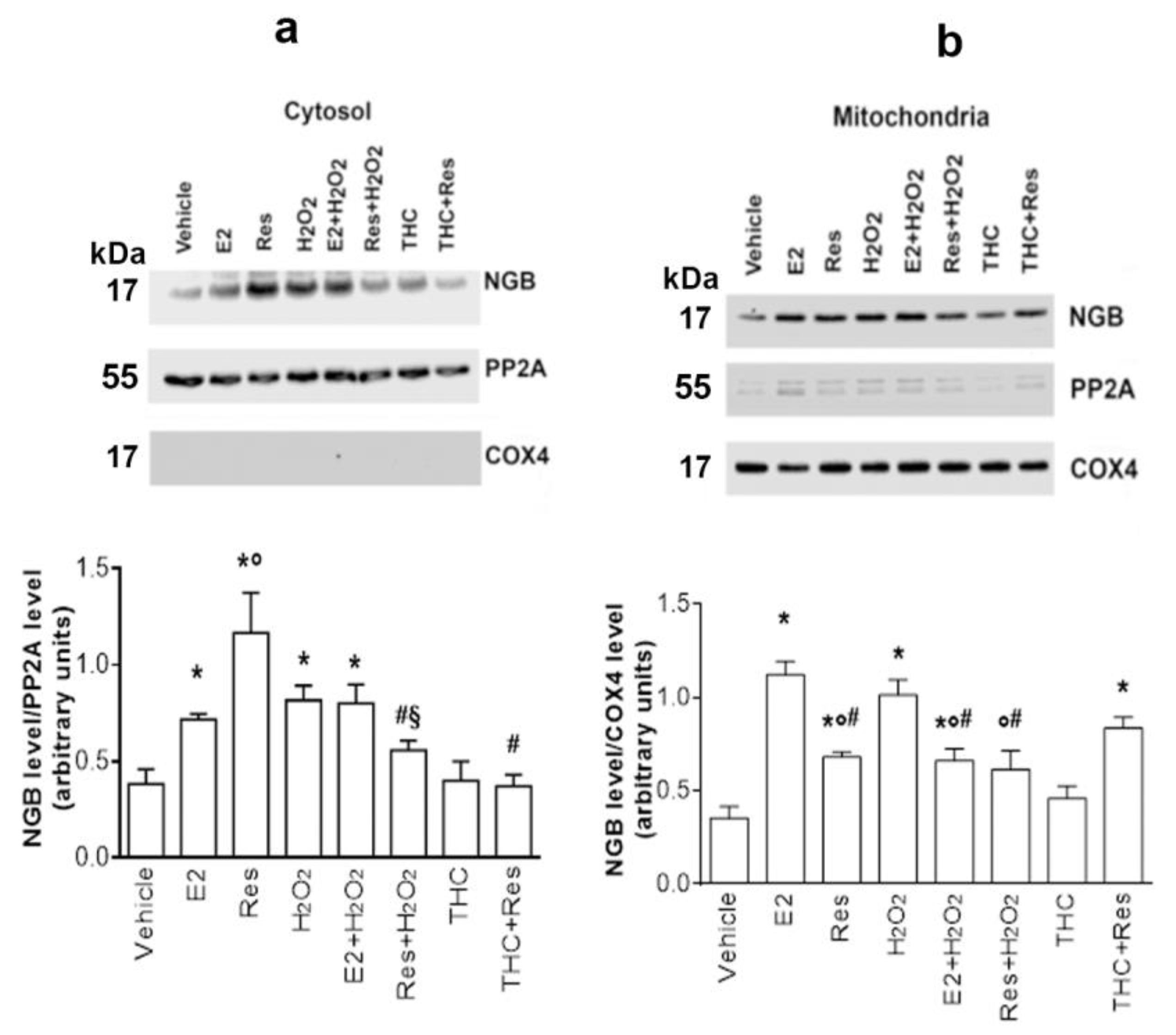
2.4. Res/ERβ Effect on NGB Localization
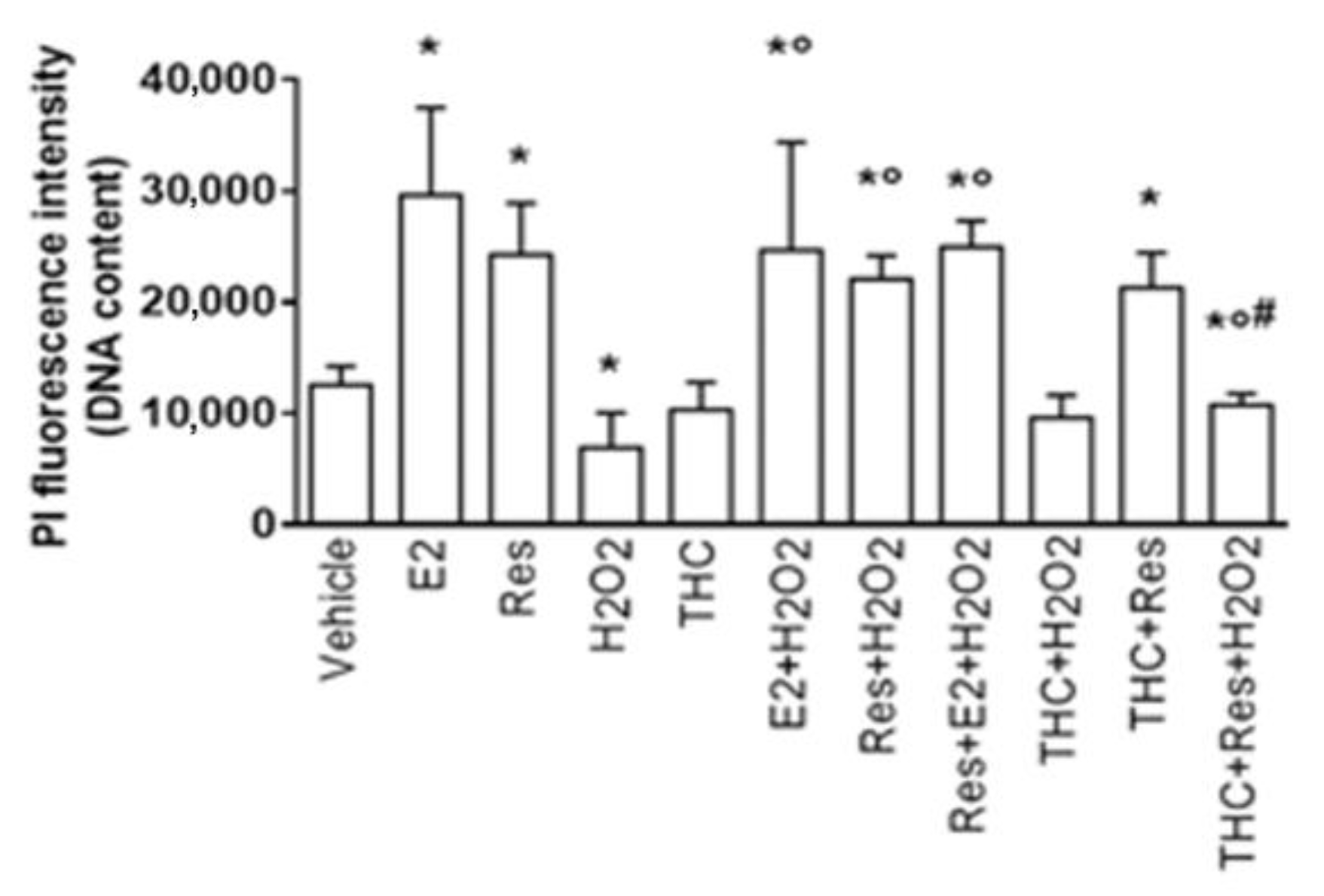
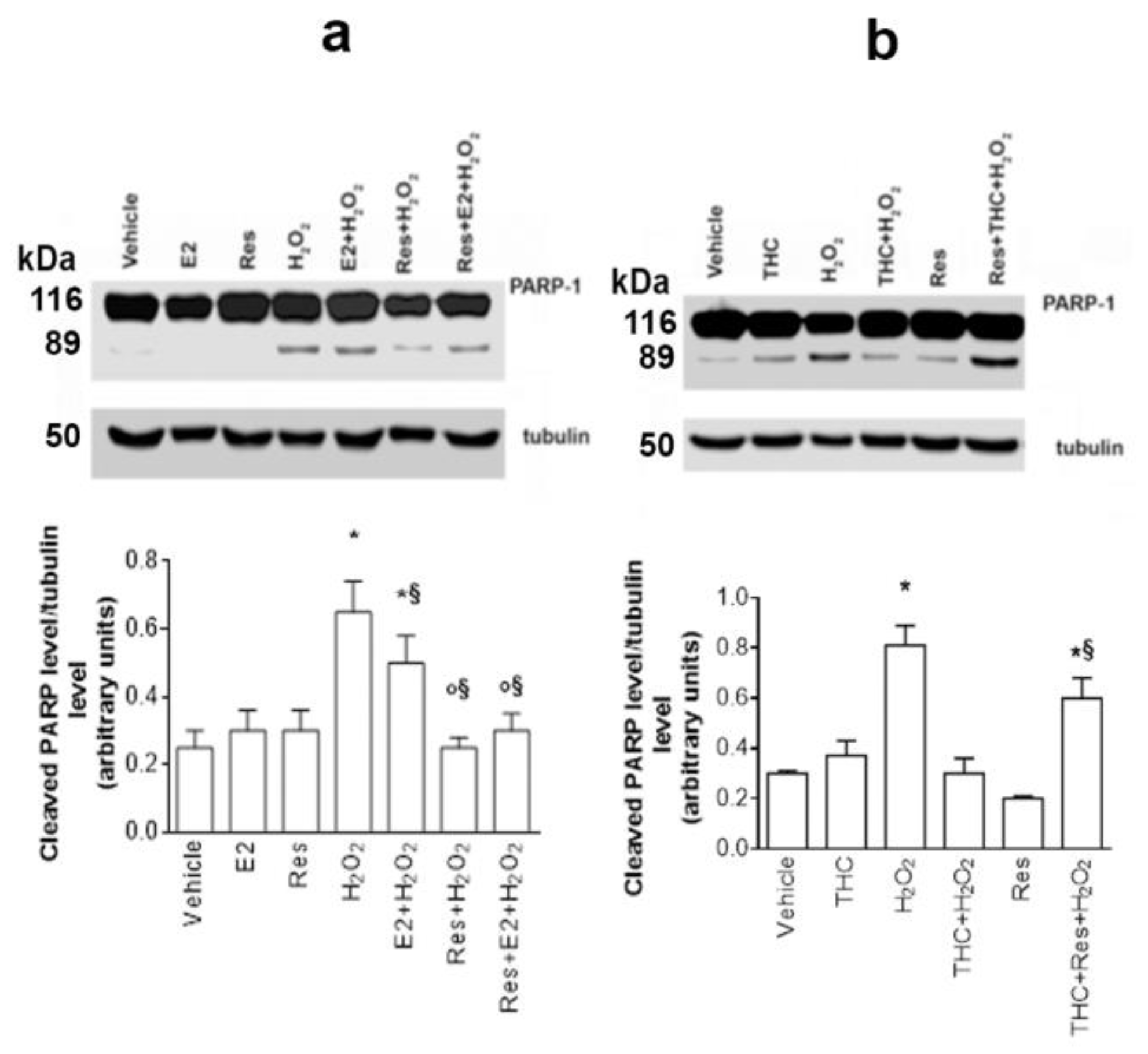
2.5. Res/ERβ Effect on Cell Resilience to Oxidative Stress
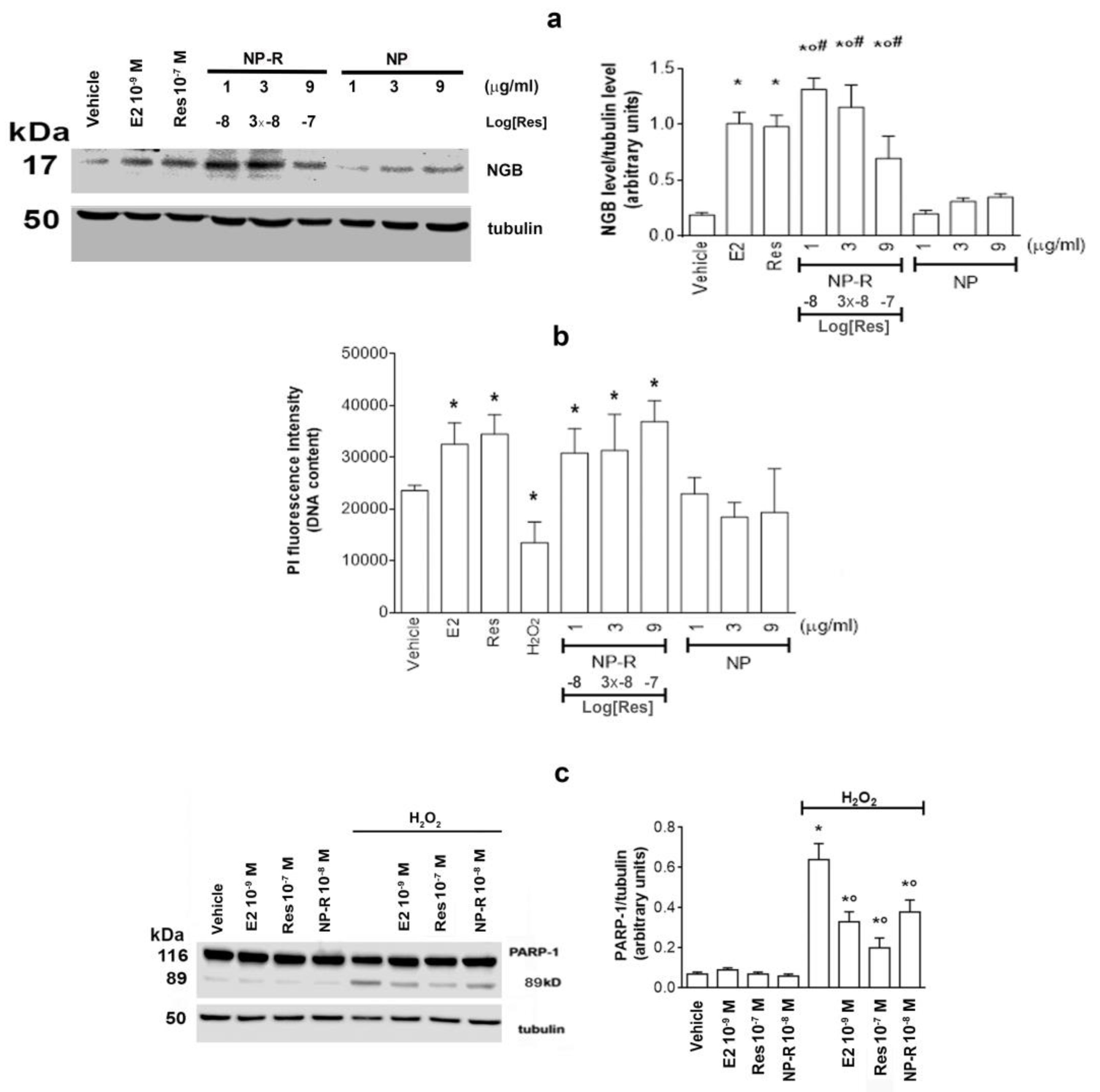
2.6. Effect of Res Conjugated with Gold Nanoparticles on NGB and on Cell Resilience to Oxidative Stress
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Western Blot Assay
4.4. Mitochondria/Cytosol Fractionation
4.5. NGB Mitochondrial Localization with Confocal Microscopy
4.6. Cellular DNA Content—Propidium Iodide (PI) Assay
4.7. Synthesis and Purification of Gold NP and NP-R
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azam, S.; Haque, M.E.; Balakrishnan, R.; Kim, I.-S.; Choi, D.-K. The Ageing Brain: Molecular and Cellular Basis of Neurodegeneration. Front. Cell Dev. Biol. 2021, 9, 683459. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wei, H. Ryanodine Receptors: A Potential Treatment Target in Various Neurodegenerative Disease. Cell. Mol. Neurobiol. 2021, 41, 1613–1624. [Google Scholar] [CrossRef]
- Brown, A.J.H.; Bradley, S.J.; Marshall, F.H.; Brown, G.A.; Bennett, K.A.; Brown, J.; Cansfield, J.E.; Cross, D.M.; de Graaf, C.; Hudson, B.D.; et al. From Structure to Clinic: Design of a Muscarinic M1 Receptor Agonist with Potential to Treatment of Alzheimer’s Disease. Cell 2021, 184, 5886–5901.e22. [Google Scholar] [CrossRef] [PubMed]
- Elmaleh, D.R.; Downey, M.A.; Kundakovic, L.; Wilkinson, J.E.; Neeman, Z.; Segal, E. New Approaches to Profile the Microbiome for Treatment of Neurodegenerative Disease. J. Alzheimers Dis. JAD 2021, 82, 1373–1401. [Google Scholar] [CrossRef] [PubMed]
- Fayazi, N.; Sheykhhasan, M.; Soleimani Asl, S.; Najafi, R. Stem Cell-Derived Exosomes: A New Strategy of Neurodegenerative Disease Treatment. Mol. Neurobiol. 2021, 58, 3494–3514. [Google Scholar] [CrossRef]
- De Marinis, E.; Fiocchetti, M.; Acconcia, F.; Ascenzi, P.; Marino, M. Neuroglobin Upregulation Induced by 17β-Estradiol Sequesters Cytocrome c in the Mitochondria Preventing H2O2-Induced Apoptosis of Neuroblastoma Cells. Cell Death Dis. 2013, 4, e508. [Google Scholar] [CrossRef]
- Fiocchetti, M.; Nuzzo, M.T.; Totta, P.; Acconcia, F.; Ascenzi, P.; Marino, M. Neuroglobin, a pro-Survival Player in Estrogen Receptor α-Positive Cancer Cells. Cell Death Dis. 2014, 5, e1449. [Google Scholar] [CrossRef]
- Nuzzo, M.T.; Fiocchetti, M.; Totta, P.; Melone, M.A.B.; Cardinale, A.; Fusco, F.R.; Gustincich, S.; Persichetti, F.; Ascenzi, P.; Marino, M. Huntingtin PolyQ Mutation Impairs the 17β-Estradiol/Neuroglobin Pathway Devoted to Neuron Survival. Mol. Neurobiol. 2017, 54, 6634–6646. [Google Scholar] [CrossRef]
- Shrikanta, A.; Kumar, A.; Govindaswamy, V. Resveratrol Content and Antioxidant Properties of Underutilized Fruits. J. Food Sci. Technol. 2015, 52, 383–390. [Google Scholar] [CrossRef]
- Tian, B.; Liu, J. Resveratrol: A Review of Plant Sources, Synthesis, Stability, Modification and Food Application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef]
- Yadav, E.; Yadav, P.; Khan, M.M.U.; Singh, H.; Verma, A. Resveratrol: A Potential Therapeutic Natural Polyphenol for Neurodegenerative Diseases Associated with Mitochondrial Dysfunction. Front. Pharmacol. 2022, 13, 922232. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhou, J.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Health Benefits and Molecular Mechanisms of Resveratrol: A Narrative Review. Foods 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Huang, N.; Xu, S.; Luo, Y.; Li, Y.; Jin, H.; Yu, C.; Shi, J.; Jin, F. Signaling Mechanisms Underlying Inhibition of Neuroinflammation by Resveratrol in Neurodegenerative Diseases. J. Nutr. Biochem. 2021, 88, 108552. [Google Scholar] [CrossRef] [PubMed]
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health Benefits of Resveratrol Administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef]
- Peng, K.; Tao, Y.; Zhang, J.; Wang, J.; Ye, F.; Dan, G.; Zhao, Y.; Cai, Y.; Zhao, J.; Wu, Q.; et al. Resveratrol Regulates Mitochondrial Biogenesis and Fission/Fusion to Attenuate Rotenone-Induced Neurotoxicity. Oxid. Med. Cell. Longev. 2016, 2016, 6705621. [Google Scholar] [CrossRef]
- Andrade, S.; Nunes, D.; Dabur, M.; Ramalho, M.J.; Pereira, M.C.; Loureiro, J.A. Therapeutic Potential of Natural Compounds in Neurodegenerative Diseases: Insights from Clinical Trials. Pharmaceutics 2023, 15, 212. [Google Scholar] [CrossRef]
- Almeida, L.; Vaz-da-Silva, M.; Falcão, A.; Soares, E.; Costa, R.; Loureiro, A.I.; Fernandes-Lopes, C.; Rocha, J.-F.; Nunes, T.; Wright, L.; et al. Pharmacokinetic and Safety Profile of Trans-Resveratrol in a Rising Multiple-Dose Study in Healthy Volunteers. Mol. Nutr. Food Res. 2009, 53 (Suppl. 1), S7–S15. [Google Scholar] [CrossRef]
- Sergides, C.; Chirilă, M.; Silvestro, L.; Pitta, D.; Pittas, A. Bioavailability and Safety Study of Resveratrol 500 Mg Tablets in Healthy Male and Female Volunteers. Exp. Ther. Med. 2016, 11, 164–170. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
- Montalesi, E.; Cipolletti, M.; Cracco, P.; Fiocchetti, M.; Marino, M. Divergent Effects of Daidzein and Its Metabolites on Estrogen-Induced Survival of Breast Cancer Cells. Cancers 2020, 12, 167. [Google Scholar] [CrossRef]
- Virgili, F.; Marino, M. Regulation of Cellular Signals from Nutritional Molecules: A Specific Role for Phytochemicals, beyond Antioxidant Activity. Free Radic. Biol. Med. 2008, 45, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Cipolletti, M.; Montalesi, E.; Nuzzo, M.T.; Fiocchetti, M.; Ascenzi, P.; Marino, M. Potentiation of Paclitaxel Effect by Resveratrol in Human Breast Cancer Cells by Counteracting the 17β-Estradiol/Estrogen Receptor α/Neuroglobin Pathway. J. Cell. Physiol. 2019, 234, 3147–3157. [Google Scholar] [CrossRef] [PubMed]
- De Marinis, E.; Ascenzi, P.; Pellegrini, M.; Galluzzo, P.; Bulzomi, P.; Arevalo, M.A.; Garcia-Segura, L.M.; Marino, M. 17β-Estradiol—A New Modulator of Neuroglobin Levels in Neurons: Role in Neuroprotection against H2O2-Induced Toxicity. Neurosignals 2010, 18, 223–235. [Google Scholar] [CrossRef]
- Fiocchetti, M.; Cipolletti, M.; Ascenzi, P.; Marino, M. Dissecting the 17β-Estradiol Pathways Necessary for Neuroglobin Anti-Apoptotic Activity in Breast Cancer. J. Cell. Physiol. 2018, 233, 5087–5103. [Google Scholar] [CrossRef] [PubMed]
- Venditti, I.; Iucci, G.; Fratoddi, I.; Cipolletti, M.; Montalesi, E.; Marino, M.; Secchi, V.; Battocchio, C. Direct Conjugation of Resveratrol on Hydrophilic Gold Nanoparticles: Structural and Cytotoxic Studies for Biomedical Applications. Nanomaterials 2020, 10, 1898. [Google Scholar] [CrossRef]
- Montalesi, E.; Cracco, P.; Acconcia, F.; Fiocchetti, M.; Iucci, G.; Battocchio, C.; Orlandini, E.; Ciccone, L.; Nencetti, S.; Muzzi, M.; et al. Resveratrol Analogs and Prodrugs Differently Affect the Survival of Breast Cancer Cells Impairing Estrogen/Estrogen Receptor α/Neuroglobin Pathway. Int. J. Mol. Sci. 2023, 24, 2148. [Google Scholar] [CrossRef]
- Renaud, S.; de Lorgeril, M. Wine, Alcohol, Platelets, and the French Paradox for Coronary Heart Disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Dobrydneva, Y.; Williams, R.L.; Blackmore, P.F. Trans-Resveratrol Inhibits Calcium Influx in Thrombin-Stimulated Human Platelets. Br. J. Pharmacol. 1999, 128, 149–157. [Google Scholar] [CrossRef]
- Lasa, A.; Schweiger, M.; Kotzbeck, P.; Churruca, I.; Simón, E.; Zechner, R.; Portillo, M. del P. Resveratrol Regulates Lipolysis via Adipose Triglyceride Lipase. J. Nutr. Biochem. 2012, 23, 379–384. [Google Scholar] [CrossRef]
- Rubiolo, J.A.; Mithieux, G.; Vega, F.V. Resveratrol Protects Primary Rat Hepatocytes against Oxidative Stress Damage: Activation of the Nrf2 Transcription Factor and Augmented Activities of Antioxidant Enzymes. Eur. J. Pharmacol. 2008, 591, 66–72. [Google Scholar] [CrossRef]
- Silva, B.R.; Silva, J.R.V. Mechanisms of Action of Non-Enzymatic Antioxidants to Control Oxidative Stress during in Vitro Follicle Growth, Oocyte Maturation, and Embryo Development. Anim. Reprod. Sci. 2022, 249, 107186. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M.; Yamabe, N.; Zhu, B.T. Resveratrol Attenuates the Anticancer Efficacy of Paclitaxel in Human Breast Cancer Cells in Vitro and in Vivo. Eur. J. Cancer 2010, 46, 1882–1891. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.G.; D’Orazio, J.A.; Pearson, K.J. Resveratrol and Cancer: Focus on in Vivo Evidence. Endocr. Relat. Cancer 2014, 21, R209–R225. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Banerjee, S.; Acosta, E.P.; Lillard, J.W.; Singh, R. Resveratrol Induces Cell Cycle Arrest and Apoptosis with Docetaxel in Prostate Cancer Cells via a P53/P21WAF1/CIP1 and P27KIP1 Pathway. Oncotarget 2017, 8, 17216–17228. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Rafiei, H.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Anti-Tumor Activity of Resveratrol against Gastric Cancer: A Review of Recent Advances with an Emphasis on Molecular Pathways. Cancer Cell Int. 2021, 21, 66. [Google Scholar] [CrossRef]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The Therapeutic Potential of Resveratrol: A Review of Clinical Trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef]
- Wang, D.; Li, S.-P.; Fu, J.-S.; Zhang, S.; Bai, L.; Guo, L. Resveratrol Defends Blood-Brain Barrier Integrity in Experimental Autoimmune Encephalomyelitis Mice. J. Neurophysiol. 2016, 116, 2173–2179. [Google Scholar] [CrossRef]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol Regulates Neuro-Inflammation and Induces Adaptive Immunity in Alzheimer’s Disease. J. Neuroinflammation 2017, 14, 1. [Google Scholar] [CrossRef]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.-L.; et al. Small Molecule Activators of Sirtuins Extend Saccharomyces Cerevisiae Lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Borra, M.T.; Smith, B.C.; Denu, J.M. Mechanism of Human SIRT1 Activation by Resveratrol. J. Biol. Chem. 2005, 280, 17187–17195. [Google Scholar] [CrossRef]
- Gomes, B.A.Q.; Silva, J.P.B.; Romeiro, C.F.R.; Dos Santos, S.M.; Rodrigues, C.A.; Gonçalves, P.R.; Sakai, J.T.; Mendes, P.F.S.; Varela, E.L.P.; Monteiro, M.C. Neuroprotective Mechanisms of Resveratrol in Alzheimer’s Disease: Role of SIRT1. Oxid. Med. Cell. Longev. 2018, 2018, 8152373. [Google Scholar] [CrossRef]
- Bowers, J.L.; Tyulmenkov, V.V.; Jernigan, S.C.; Klinge, C.M. Resveratrol Acts as a Mixed Agonist/Antagonist for Estrogen Receptors Alpha and Beta. Endocrinology 2000, 141, 3657–3667. [Google Scholar] [CrossRef]
- Goldberg, D.M.; Yan, J.; Soleas, G.J. Absorption of Three Wine-Related Polyphenols in Three Different Matrices by Healthy Subjects. Clin. Biochem. 2003, 36, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Garza, S.L.; Laveriano-Santos, E.P.; Marhuenda-Muñoz, M.; Storniolo, C.E.; Tresserra-Rimbau, A.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Health Effects of Resveratrol: Results from Human Intervention Trials. Nutrients 2018, 10, 1892. [Google Scholar] [CrossRef] [PubMed]
- Springer, M.; Moco, S. Resveratrol and Its Human Metabolites-Effects on Metabolic Health and Obesity. Nutrients 2019, 11, 143. [Google Scholar] [CrossRef]
- Adams, M.L.; Lavasanifar, A.; Kwon, G.S. Amphiphilic Block Copolymers for Drug Delivery. J. Pharm. Sci. 2003, 92, 1343–1355. [Google Scholar] [CrossRef]
- Neves, A.R.; Lúcio, M.; Martins, S.; Lima, J.L.C.; Reis, S. Novel Resveratrol Nanodelivery Systems Based on Lipid Nanoparticles to Enhance Its Oral Bioavailability. Int. J. Nanomed. 2013, 8, 177–187. [Google Scholar] [CrossRef]
- Nassir, A.M.; Shahzad, N.; Ibrahim, I.A.A.; Ahmad, I.; Md, S.; Ain, M.R. Resveratrol-Loaded PLGA Nanoparticles Mediated Programmed Cell Death in Prostate Cancer Cells. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2018, 26, 876–885. [Google Scholar] [CrossRef]
- Cipolletti, M.; Solar Fernandez, V.; Montalesi, E.; Marino, M.; Fiocchetti, M. Beyond the Antioxidant Activity of Dietary Polyphenols in Cancer: The Modulation of Estrogen Receptors (ERs) Signaling. Int. J. Mol. Sci. 2018, 19, 2624. [Google Scholar] [CrossRef]
- Ascenzi, P.; di Masi, A.; Leboffe, L.; Fiocchetti, M.; Nuzzo, M.T.; Brunori, M.; Marino, M. Neuroglobin: From Structure to Function in Health and Disease. Mol. Aspects Med. 2016, 52, 1–48. [Google Scholar] [CrossRef]
- Dai, C.L.; Shi, J.; Chen, Y.; Iqbal, K.; Liu, F.; Gong, C.X. Inhibition of Protein Synthesis Alters Protein Degradation through Activation of Protein Kinase B (Akt). J. Biol. Chem. 2013, 288, 23875–23883. [Google Scholar] [CrossRef] [PubMed]
- Boccuni, L.; Podgorschek, E.; Schmiedeberg, M.; Platanitis, E.; Traxler, P.; Fischer, P.; Schirripa, A.; Novoszel, P.; Nebreda, A.R.; Arthur, J.S.C.; et al. Stress Signaling Boosts Interferon-Induced Gene Transcription in Macrophages. Sci. Signal. 2022, 15, eabq5389. [Google Scholar] [CrossRef]
- Ishijima, T.; Nakajima, K. Changes of Signaling Molecules in the Axotomized Rat Facial Nucleus. J. Chem. Neuroanat. 2022, 126, 102179. [Google Scholar] [CrossRef]
- Zarubin, T.; Han, J. Activation and Signaling of the p38 MAP Kinase Pathway. Cell Res. 2005, 15, 11–18. [Google Scholar] [CrossRef]
- Fiocchetti, M.; Solar Fernandez, V.; Segatto, M.; Leone, S.; Cercola, P.; Massari, A.; Cavaliere, F.; Marino, M. Extracellular Neuroglobin as a Stress-Induced Factor Activating Pre-Adaptation Mechanisms against Oxidative Stress and Chemotherapy-Induced Cell Death in Breast Cancer. Cancers 2020, 12, 2451. [Google Scholar] [CrossRef] [PubMed]
- Amri, F.; Ghouili, I.; Amri, M.; Carrier, A.; Masmoudi-Kouki, O. Neuroglobin Protects Astroglial Cells from Hydrogen Peroxide-Induced Oxidative Stress and Apoptotic Cell Death. J. Neurochem. 2017, 140, 151–169. [Google Scholar] [CrossRef]
- Venturini, A.; Passalacqua, M.; Pelassa, S.; Pastorino, F.; Tedesco, M.; Cortese, K.; Gagliani, M.C.; Leo, G.; Maura, G.; Guidolin, D.; et al. Exosomes From Astrocyte Processes: Signaling to Neurons. Front. Pharmacol. 2019, 10, 1452. [Google Scholar] [CrossRef]
- Intagliata, S.; Modica, M.N.; Santagati, L.M.; Montenegro, L. Strategies to Improve Resveratrol Systemic and Topical Bioavailability: An Update. Antioxidants 2019, 8, 244. [Google Scholar] [CrossRef]
- Venditti, I.; Testa, G.; Sciubba, F.; Carlini, L.; Porcaro, F.; Meneghini, C.; Mobilio, S.; Battocchio, C.; Fratoddi, I. Hydrophilic Metal Nanoparticles Functionalized by 2-Diethylaminoethanethiol: A Close Look at the Metal–Ligand Interaction and Interface Chemical Structure. J. Phys. Chem. C 2017, 121, 8002–8013. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cracco, P.; Montalesi, E.; Parente, M.; Cipolletti, M.; Iucci, G.; Battocchio, C.; Venditti, I.; Fiocchetti, M.; Marino, M. A Novel Resveratrol-Induced Pathway Increases Neuron-Derived Cell Resilience against Oxidative Stress. Int. J. Mol. Sci. 2023, 24, 5903. https://doi.org/10.3390/ijms24065903
Cracco P, Montalesi E, Parente M, Cipolletti M, Iucci G, Battocchio C, Venditti I, Fiocchetti M, Marino M. A Novel Resveratrol-Induced Pathway Increases Neuron-Derived Cell Resilience against Oxidative Stress. International Journal of Molecular Sciences. 2023; 24(6):5903. https://doi.org/10.3390/ijms24065903
Chicago/Turabian StyleCracco, Patrizio, Emiliano Montalesi, Martina Parente, Manuela Cipolletti, Giovanna Iucci, Chiara Battocchio, Iole Venditti, Marco Fiocchetti, and Maria Marino. 2023. "A Novel Resveratrol-Induced Pathway Increases Neuron-Derived Cell Resilience against Oxidative Stress" International Journal of Molecular Sciences 24, no. 6: 5903. https://doi.org/10.3390/ijms24065903
APA StyleCracco, P., Montalesi, E., Parente, M., Cipolletti, M., Iucci, G., Battocchio, C., Venditti, I., Fiocchetti, M., & Marino, M. (2023). A Novel Resveratrol-Induced Pathway Increases Neuron-Derived Cell Resilience against Oxidative Stress. International Journal of Molecular Sciences, 24(6), 5903. https://doi.org/10.3390/ijms24065903









