Retrospective Analysis of Surgical Outcomes on Axial Length Elongation in Eyes with Posterior and Combined Persistent Fetal Vasculature
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
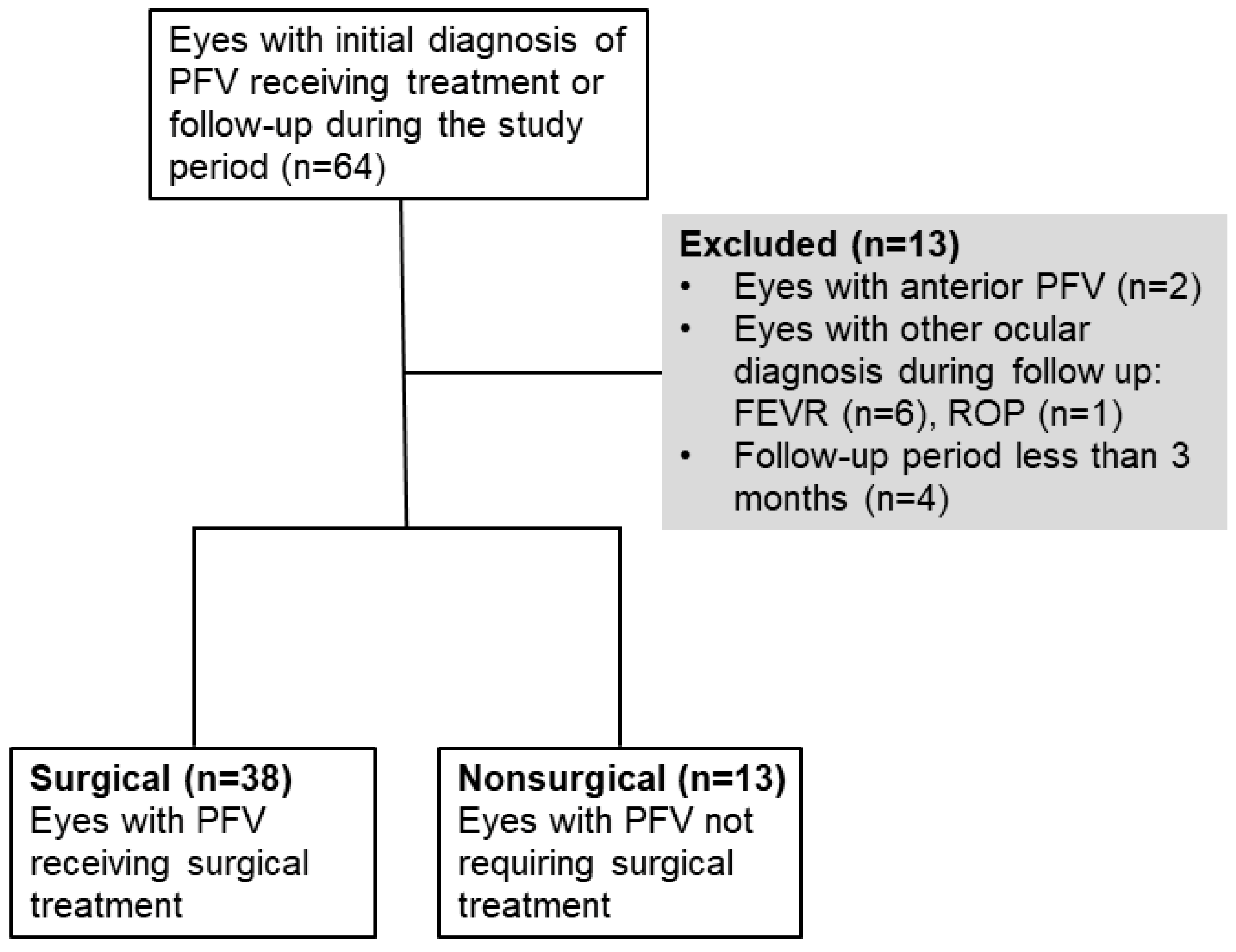
4.1. Study Population
4.2. Ocular Examination and Grouping
4.3. Surgical Procedures
4.4. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Anterior chamber |
| AL | Axial length |
| BCVA | Best-corrected visual acuity |
| IOL | Intraocular lens |
| IOP | Intraocular pressure |
| PFV | Persistent fetal vasculature |
| PPL | Plicata lensectomy |
| PPV | Plicata vitrectomy |
References
- Reese, A.B. Persistent hyperplastic primary vitreous. Am. J. Ophthalmol. 1955, 59, 271–295. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.F. Persistent fetal vasculature (pfv): An integrated interpretation of signs and symptoms associated with persistent hyperplastic primary vitreous (phpv). Liv edward jackson memorial lecture. Am. J. Ophthalmol. 1997, 124, 587–626. [Google Scholar] [CrossRef] [PubMed]
- Prakhunhungsit, S.; Berrocal, A.M. Diagnostic and management strategies in patients with persistent fetal vasculature: Current insights. Clin. Ophthalmol. 2020, 14, 4325–4335. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xiao, H.; Ding, X. Persistent fetal vasculature. Asia Pac. J. Ophthalmol. 2019, 8, 86–95. [Google Scholar] [CrossRef]
- Hu, A.; Pei, X.; Ding, X.; Li, J.; Li, Y.; Liu, F.; Li, Z.; Zhan, Z.; Lu, L. Combined persistent fetal vasculature: A classification based on high-resolution b-mode ultrasound and color doppler imaging. Ophthalmology 2016, 123, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Vasavada, A.R.; Vasavada, S.A.; Bobrova, N.; Praveen, M.R.; Shah, S.K.; Vasavada, V.A.; Pardo, A.J.; Raj, S.M.; Trivedi, R.H. Outcomes of pediatric cataract surgery in anterior persistent fetal vasculature. J. Cataract Refract. Surg. 2012, 38, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Dass, A.B.; Trese, M.T. Surgical results of persistent hyperplastic primary vitreous. Ophthalmology 1999, 106, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Pollard, Z.F. Persistent hyperplastic primary vitreous: Diagnosis, treatment and results. Trans. Am. Ophthalmol. Soc. 1997, 95, 487–549. [Google Scholar] [CrossRef] [PubMed]
- Karacorlu, M.; Hocaoglu, M.; Sayman Muslubas, I.; Arf, S.; Ersoz, M.G.; Uysal, O. Functional and anatomical outcomes following surgical management of persistent fetal vasculature: A single-center experience of 44 cases. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Hunt, A.; Rowe, N.; Lam, A.; Martin, F. Outcomes in persistent hyperplastic primary vitreous. Br. J. Ophthalmol. 2005, 89, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Sisk, R.A.; Berrocal, A.M.; Feuer, W.J.; Murray, T.G. Visual and anatomic outcomes with or without surgery in persistent fetal vasculature. Ophthalmology 2010, 117, 2178–2183.e2. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Lu, H.; Li, S.F.; Jiao, Y.H.; Lin, N.; Liu, N.P. Outcomes of small gauge pars plicata vitrectomy for patients with persistent fetal vasculature: A report of 105 cases. Int. J. Ophthalmol. 2017, 10, 1851–1856. [Google Scholar] [PubMed]
- Yeh, C.T.; Chen, K.J.; Liu, L.; Wang, N.K.; Hwang, Y.S.; Chao, A.N.; Chen, T.L.; Lai, C.C.; Wu, W.C. Visual and anatomical outcomes with vitrectomy in posterior or combined persistent fetal vasculature in an asian population. Ophthalmic Surg. Lasers Imaging Retin. 2019, 50, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Khandwala, N.; Besirli, C.; Bohnsack, B.L. Outcomes and surgical management of persistent fetal vasculature. BMJ Open Ophthalmol. 2021, 6, e000656. [Google Scholar] [CrossRef] [PubMed]
- Tartarella, M.B.; Takahagi, R.U.; Braga, A.P.; Fortes Filho, J.B. Persistent fetal vasculature: Ocular features, management of cataract and outcomes. Arq. Bras. Oftalmol. 2013, 76, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Cerón, O.; Lou, P.L.; Kroll, A.J.; Walton, D.S. The vitreo-retinal manifestations of persistent hyperplasic primary vitreous (phpv) and their management. Int. Ophthalmol. Clin. 2008, 48, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Anteby, I.; Cohen, E.; Karshai, I.; BenEzra, D. Unilateral persistent hyperplastic primary vitreous: Course and outcome. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2002, 6, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Bosjolie, A.; Ferrone, P. Visual outcome in early vitrectomy for posterior persistent fetal vasculature associated with traction retinal detachment. Retina 2015, 35, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Chen, H.; Wang, J.H.; Chen, W.; Wang, Q.W.; Chen, J.J.; Lin, X.S.; Lin, Z.L.; Lin, D.R.; Lin, H.T.; et al. Ocular development in children with unilateral congenital cataract and persistent fetal vasculature. Int. J. Ophthalmol. 2022, 15, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Bonsel, K.; Feltgen, N.; Burau, H.; Hansen, L.; Bach, M. Visual acuities “hand motion” and “counting fingers” can be quantified with the freiburg visual acuity test. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1236–1240. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Surgical (n = 38) | Nonsurgical (n = 13) | p Value |
|---|---|---|---|
| Types of PFV, n (%) | <0.001 * | ||
| Posterior | 2 (5.3) | 9 (69.2) | |
| Combined | 36 (94.7) | 4 (30.8) | |
| Initial status, n (%) | |||
| Cornea opacity | 11 (28.9) | 1 (7.7) | 0.151 * |
| AC collapse | 19 (50.0) | 1 (7.7) | 0.008 * |
| Cataract | 36 (94.7) | 4 (30.8) | <0.001* |
| Retinal detachment | 21 (55.3) | 1 (7.7) | 0.003 * |
| Stalk | 37 (97.4) | 12 (92.3) | 0.449 * |
| Macular dragging | 36 (94.7) | 11 (84.6) | 0.266 * |
| Axial length, mm | 18.3 ± 3.0 | 20.9 ± 1.4 | 0.009 † |
| Missing or unmeasurable | 15 | 2 | |
| Horizontal corneal diameter, mm | 10.2 ± 1.1 | 11.0 ± 0.8 | 0.046 † |
| Missing or unmeasurable | 2 | 1 | |
| Vertical corneal diameter, mm | 10.1 ± 0.8 | 10.6 ± 0.5 | 0.172 † |
| Missing or unmeasurable | 2 | 4 |
| Lesion Eyes (n = 51) | Normal Eyes (n = 37) | p Value * | |
|---|---|---|---|
| Axial length, mm | 19.1 ± 2.9 | 20.4 ± 1.7 | 0.036 |
| Missing or unmeasurable | 17 | 7 | |
| Horizontal corneal diameter, mm | 10.4 ± 1.1 | 11.6 ± 0.7 | <0.001 |
| Missing or unmeasurable | 3 | 3 | |
| Vertical corneal diameter, mm | 10.2 ± 0.8 | 11.3 ± 0.8 | <0.001 |
| Missing or unmeasurable | 6 | 6 | |
| IOP, mmHg | 11.0 ± 4.8 | 12.0 ± 3.9 | 0.167 |
| Missing or unmeasurable | 6 | 7 |
| Characteristics | Surgical (n = 38) | Nonsurgical (n = 13) | p Value |
|---|---|---|---|
| Final visual acuity of lesion eyes | 0.041 * | ||
| Favourable vision (CF and better than CF) | 10 (26.3) | 9 (69.2) | |
| Poor vision (worse than CF) | 17 (44.7) | 3 (23.1) | |
| Missing or unmeasurable | 11 (28.9) | 1 (7.7) | |
| Final status, n (%) | |||
| Glaucoma | 1 (2.6) | 0 (0.0) | 1.00 * |
| Phthisis bulbi | 5 (13.2) | 0 (0.0) | 0.311 * |
| Corneal opacity | 14 (36.8) | 4 (30.8) | 0.750 * |
| Lens | <0.001 * | ||
| phakia | 7 (18.4) | 13 (100.0) | |
| Aphakia | 20 (52.6) | 0 (0.0) | |
| Pseudophakia | 11 (28.9) | 0 (0.0) | |
| Fundus | 0.007 * | ||
| No retinal detachment | 16 (42.1) | 12 (92.3) | |
| Retinal detachment | 16 (42.1) | 1 (7.7) | |
| No view | 6 (15.8) | 0 (0.0) | |
| Final axial length, mm | 20.3 ± 3.6 | 22.0 ± 1.0 | 0.446 † |
| Missing or unmeasurable | 23 | 4 | |
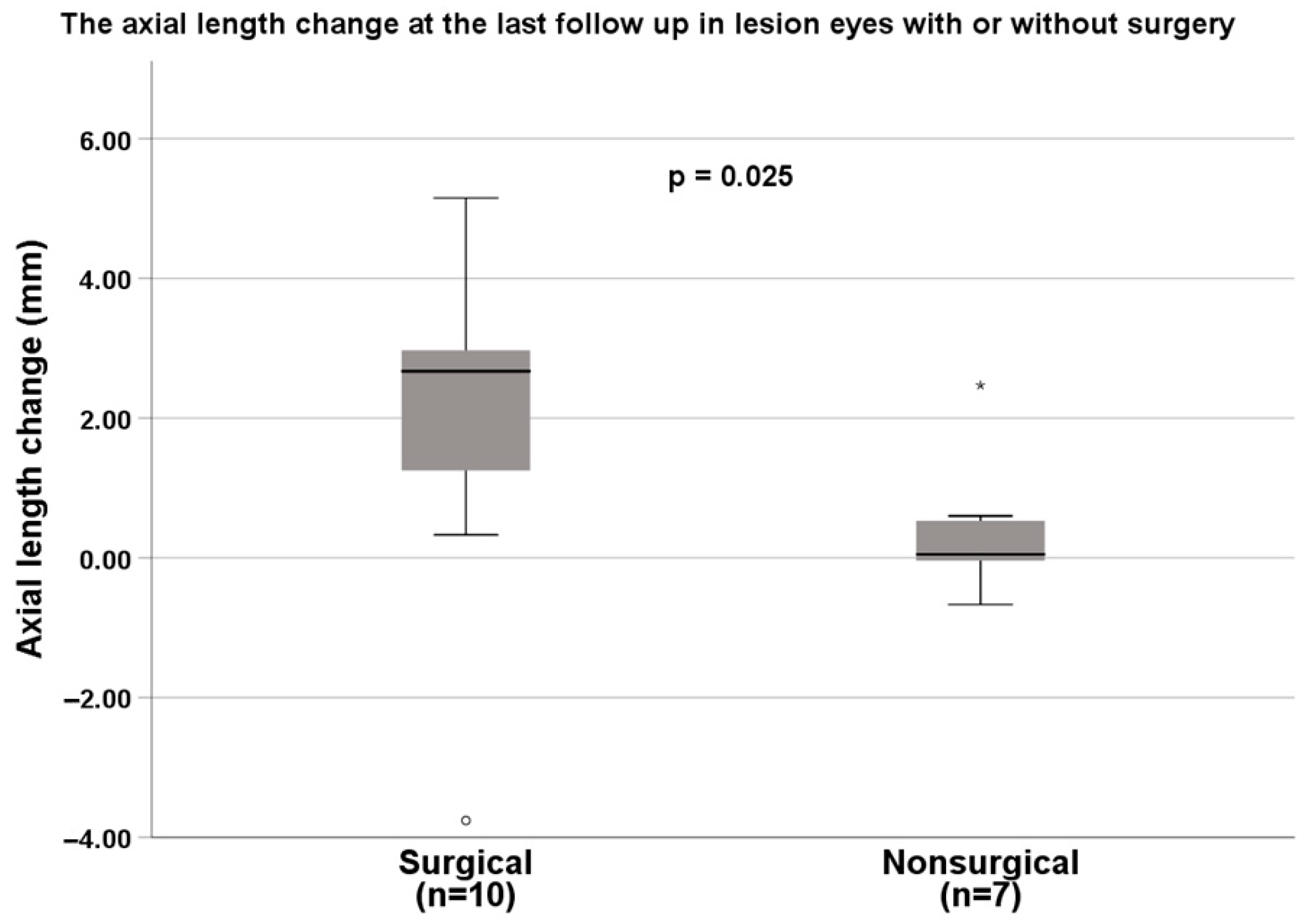
| Axial length increase, mm | 2.0 ± 2.4 | 0.4 ± 1.0 | 0.025 † |
| Missing or unmeasurable | 28 | 6 |
| Factors | Poor Vision (Worse than CF) (n = 20) | Unadjusted OR (95% CI) | p Value | Adjusted OR * (95% CI) | p Value |
|---|---|---|---|---|---|
| Corneal opacity, n (%) | 0.299 | 0.493 | |||
| Yes | 6 (30.0) | 2.286 (0.480–10.883) | 1.758 (0.350–8.819) | ||
| No | 14 (70.0) | 1.000 | 1.000 | ||
| AC collapse, n (%) | 0.004 | 0.006 | |||
| Yes | 12 (60.0) | 12.750 (2.291–70.967) | 34.256 (2.750–426.766) | ||
| No | 8 (40.0) | 1.000 | 1.000 | ||
| Cataract, n (%) | 0.128 | 0.335 | |||
| Yes | 17 (85.0) | 3.306 (0.708–15.438) | 2.334(0.416–13.087) | ||
| No | 3 (15.0) | 1.000 | 1.000 | ||
| Retinal detachment, n (%) | 0.002 | 0.002 | |||
| Yes | 14 (70.0) | 12.444 (2.614–59.251) | 26.409 (3.473–200.837) | ||
| No | 6 (30.0) | 1.000 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.-C.; Lai, C.-H.; Kang, E.Y.-C.; Chen, K.-J.; Wang, N.-K.; Liu, L.; Hwang, Y.-S.; Lai, C.-C.; Wu, W.-C. Retrospective Analysis of Surgical Outcomes on Axial Length Elongation in Eyes with Posterior and Combined Persistent Fetal Vasculature. Int. J. Mol. Sci. 2023, 24, 5836. https://doi.org/10.3390/ijms24065836
Huang H-C, Lai C-H, Kang EY-C, Chen K-J, Wang N-K, Liu L, Hwang Y-S, Lai C-C, Wu W-C. Retrospective Analysis of Surgical Outcomes on Axial Length Elongation in Eyes with Posterior and Combined Persistent Fetal Vasculature. International Journal of Molecular Sciences. 2023; 24(6):5836. https://doi.org/10.3390/ijms24065836
Chicago/Turabian StyleHuang, Heng-Chiao, Chien-Hsiung Lai, Eugene Yu-Chuan Kang, Kuan-Jen Chen, Nan-Kai Wang, Laura Liu, Yih-Shiou Hwang, Chi-Chun Lai, and Wei-Chi Wu. 2023. "Retrospective Analysis of Surgical Outcomes on Axial Length Elongation in Eyes with Posterior and Combined Persistent Fetal Vasculature" International Journal of Molecular Sciences 24, no. 6: 5836. https://doi.org/10.3390/ijms24065836
APA StyleHuang, H.-C., Lai, C.-H., Kang, E. Y.-C., Chen, K.-J., Wang, N.-K., Liu, L., Hwang, Y.-S., Lai, C.-C., & Wu, W.-C. (2023). Retrospective Analysis of Surgical Outcomes on Axial Length Elongation in Eyes with Posterior and Combined Persistent Fetal Vasculature. International Journal of Molecular Sciences, 24(6), 5836. https://doi.org/10.3390/ijms24065836










