Antibiotics and Bacterial Resistance—A Short Story of an Endless Arms Race
Abstract
1. Introduction
2. Mechanisms of Antibiotic Actions
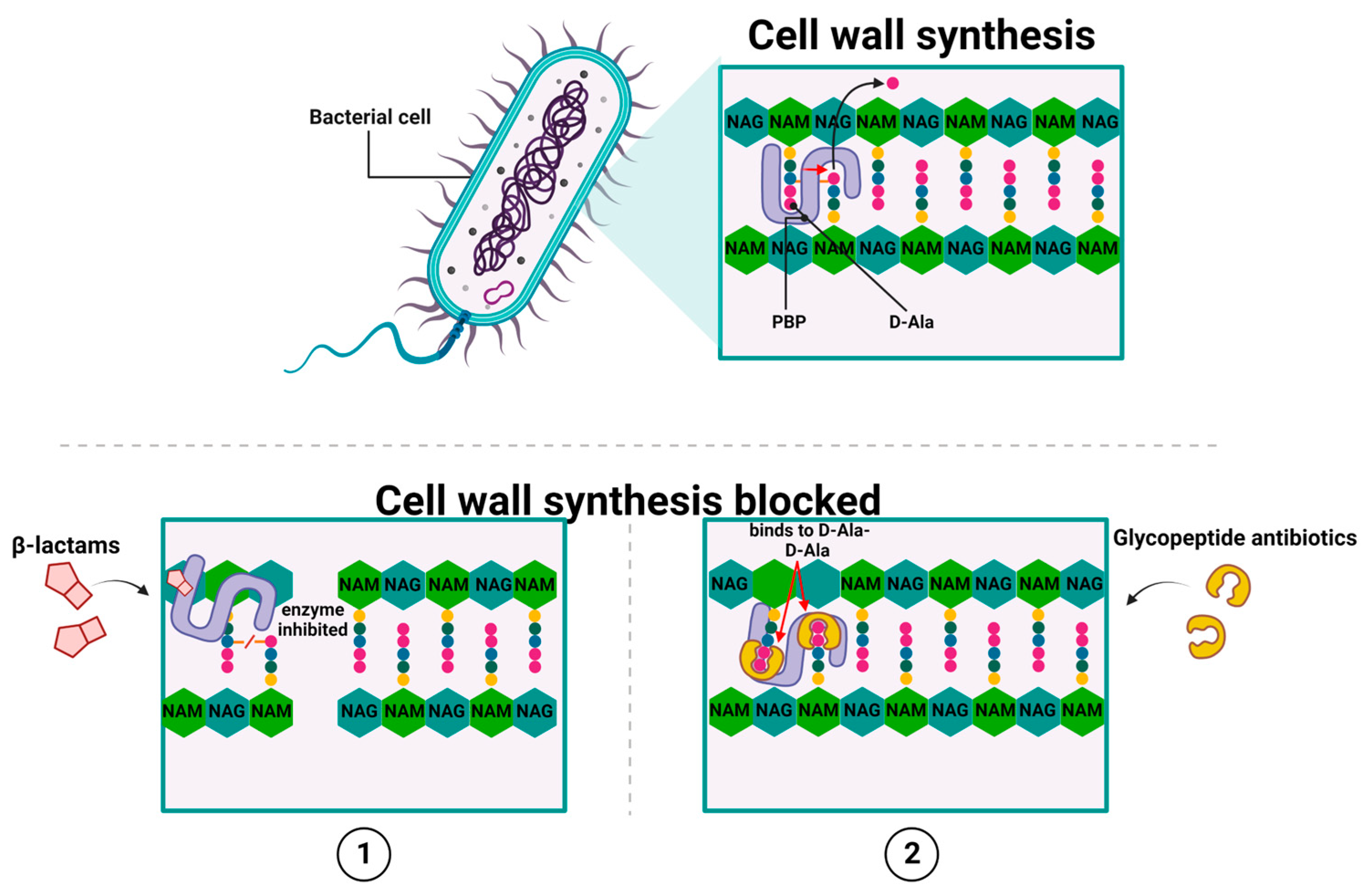
2.1. Antibiotics That Inhibit Cell Wall Synthesis
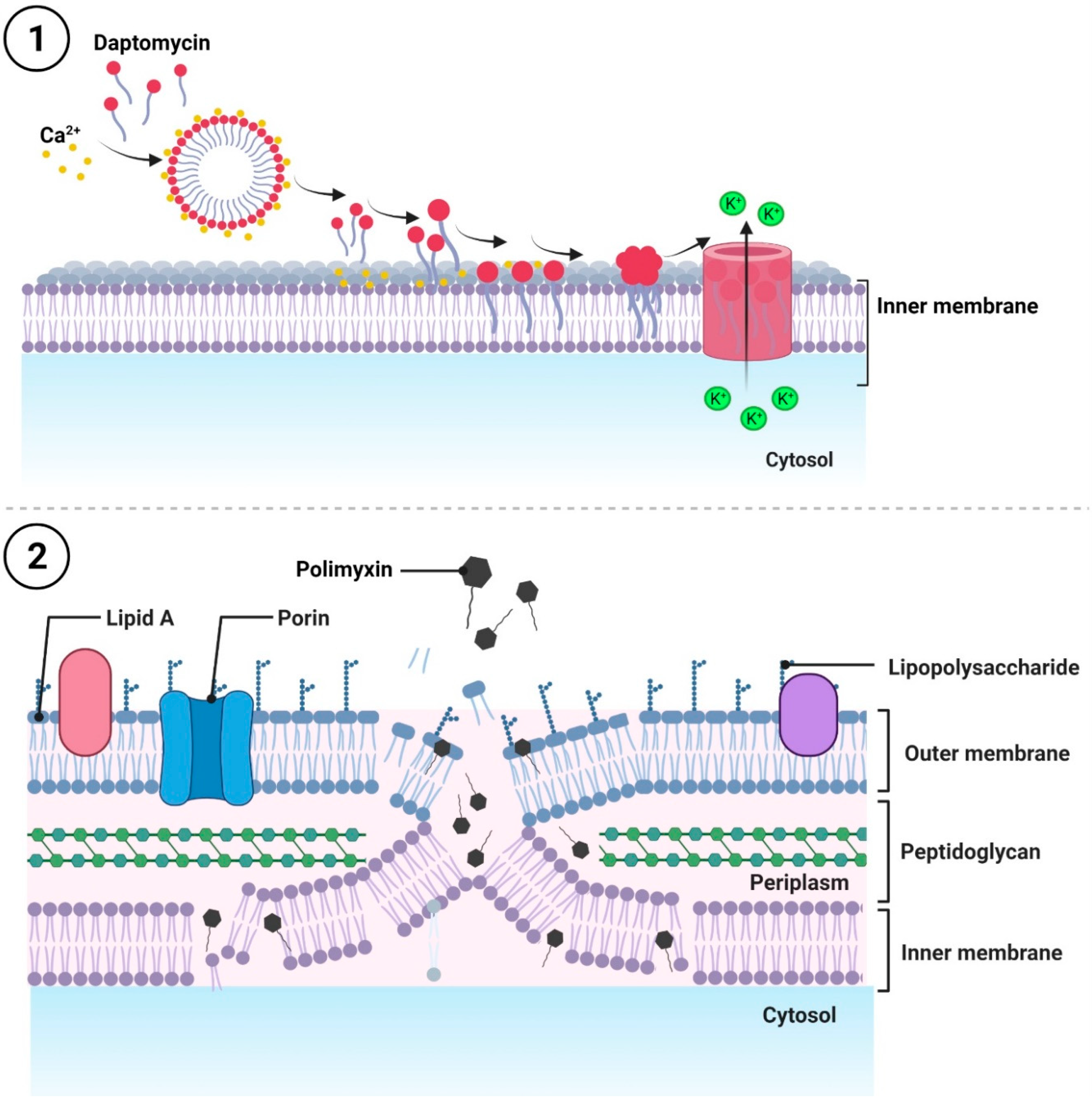
2.2. Antibiotics That Disrupt the Integrity of the Cell Membrane
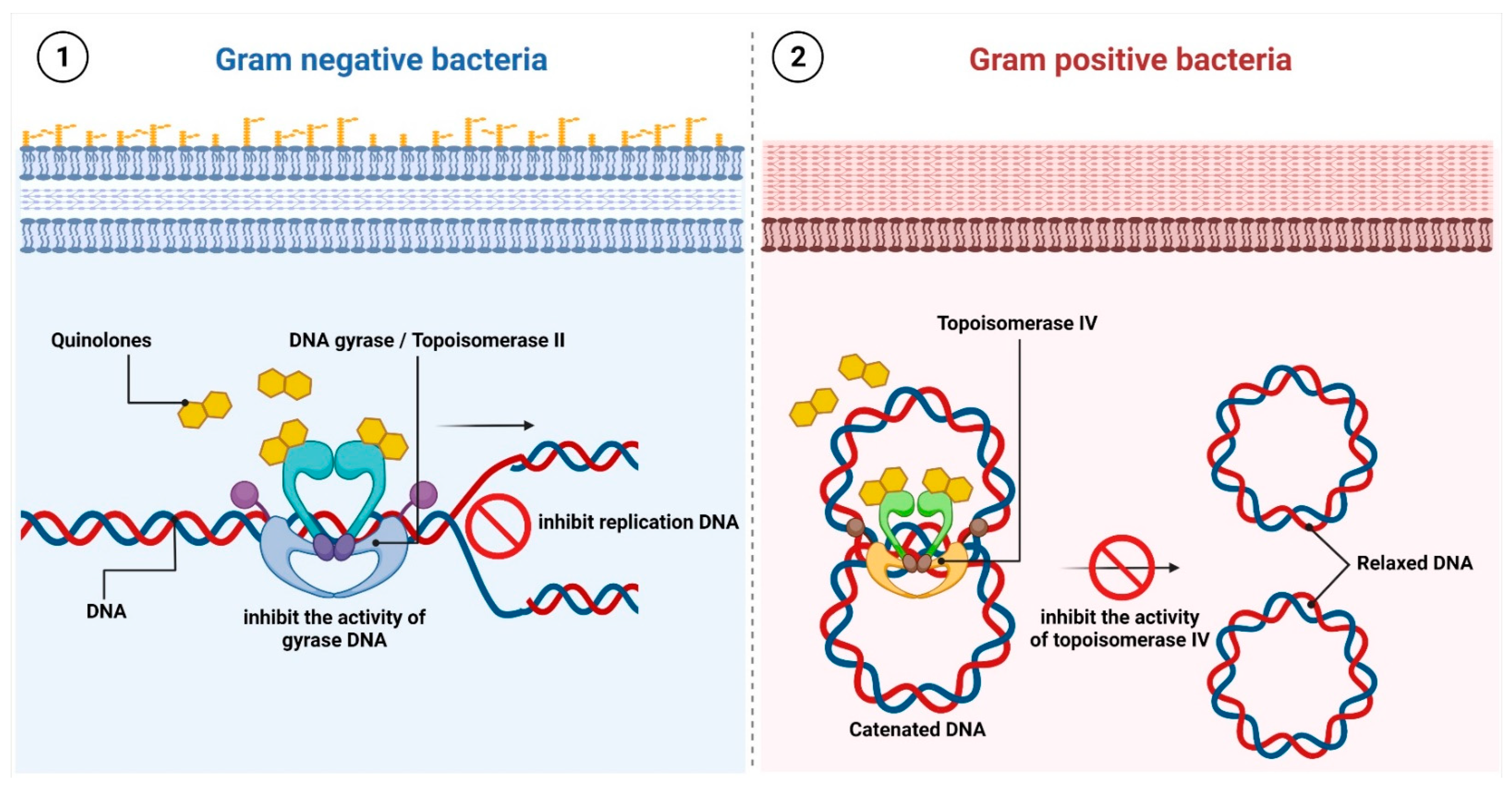
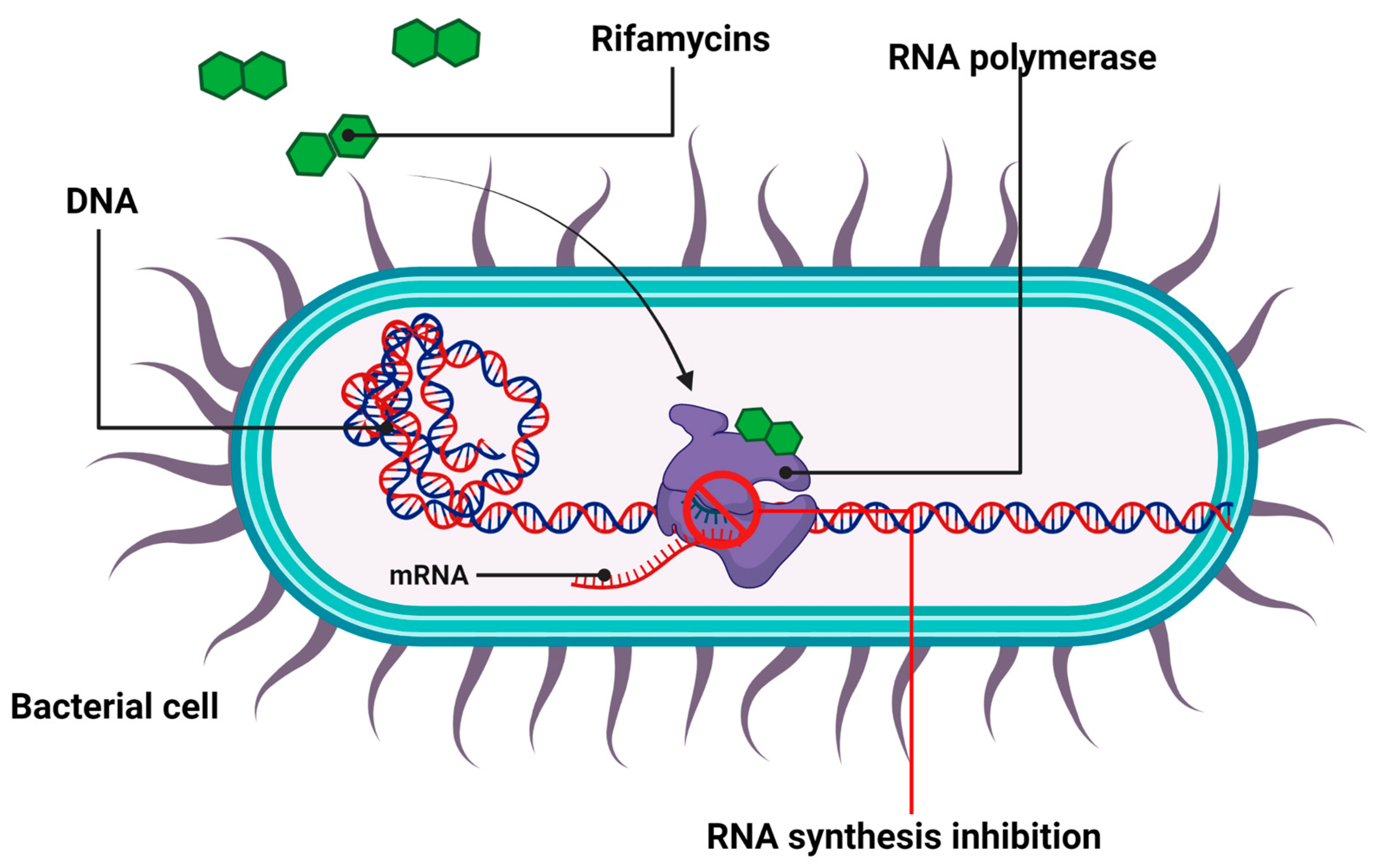
2.3. Antibiotics That Inhibit Nucleic Acid Synthesis
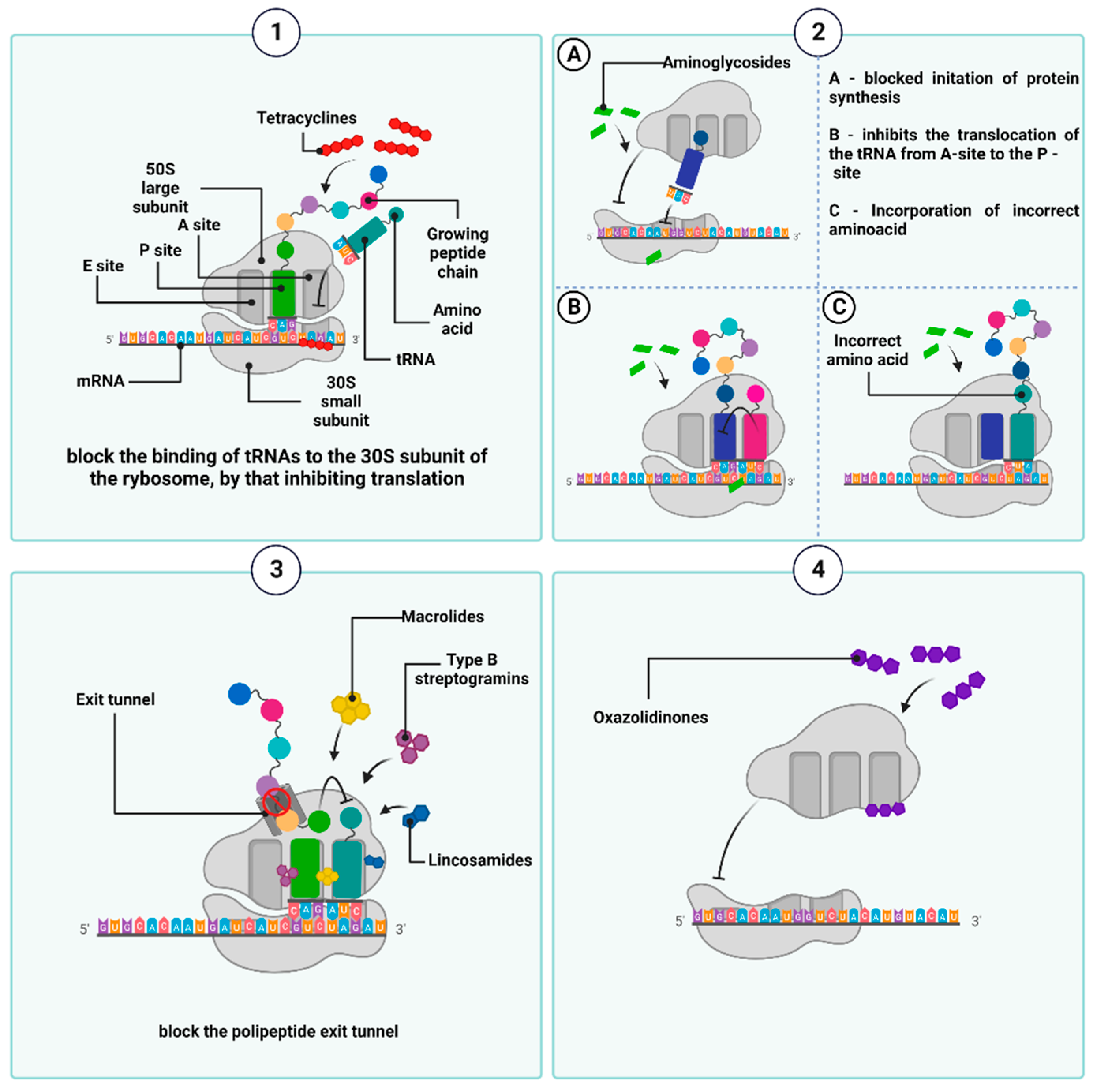
2.4. Antibiotics That Inhibit Protein Synthesis
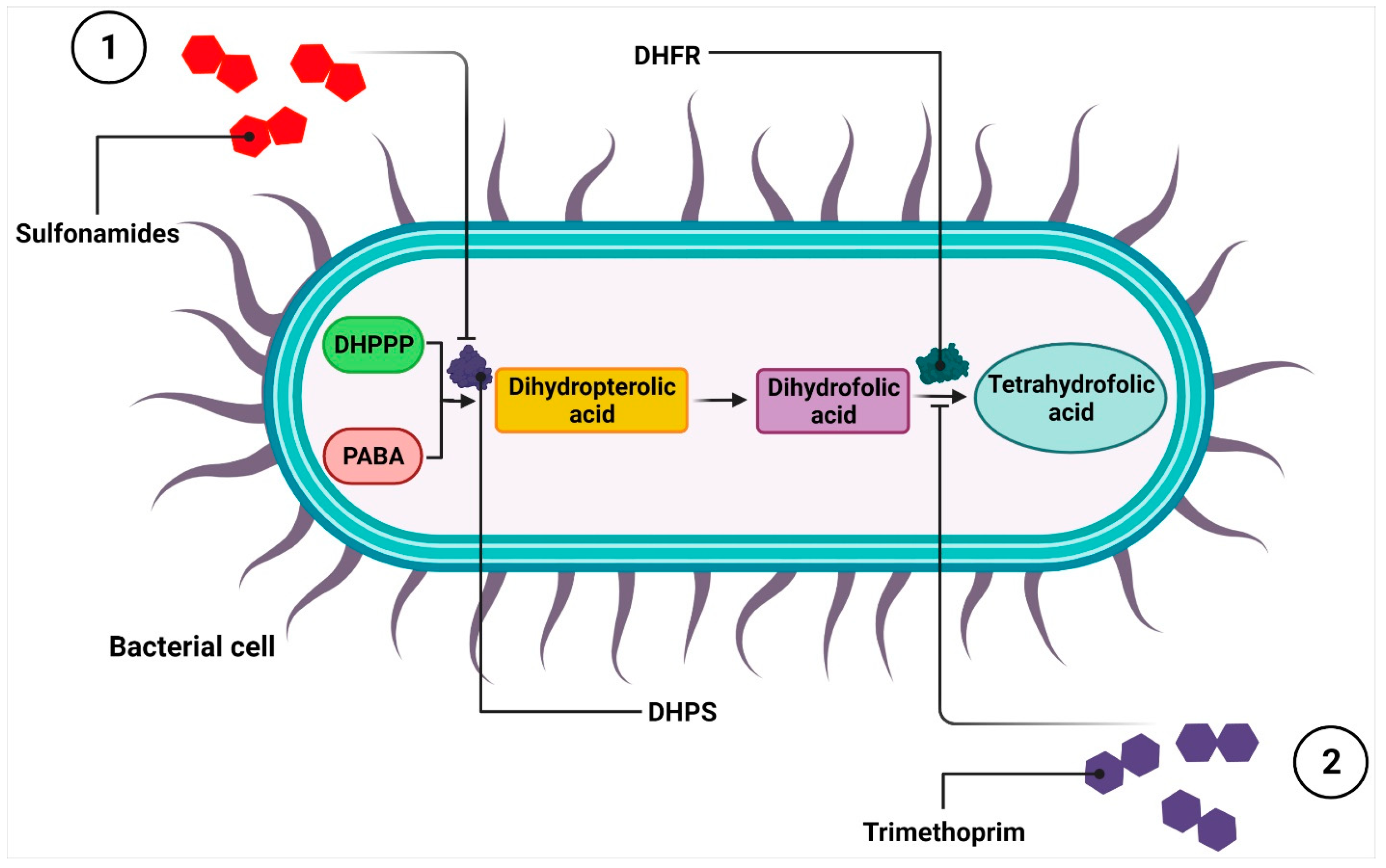
2.5. Antimicrobial Substances That Interfere with Metabolic Pathways
3. Mechanisms of Antibiotic Resistance
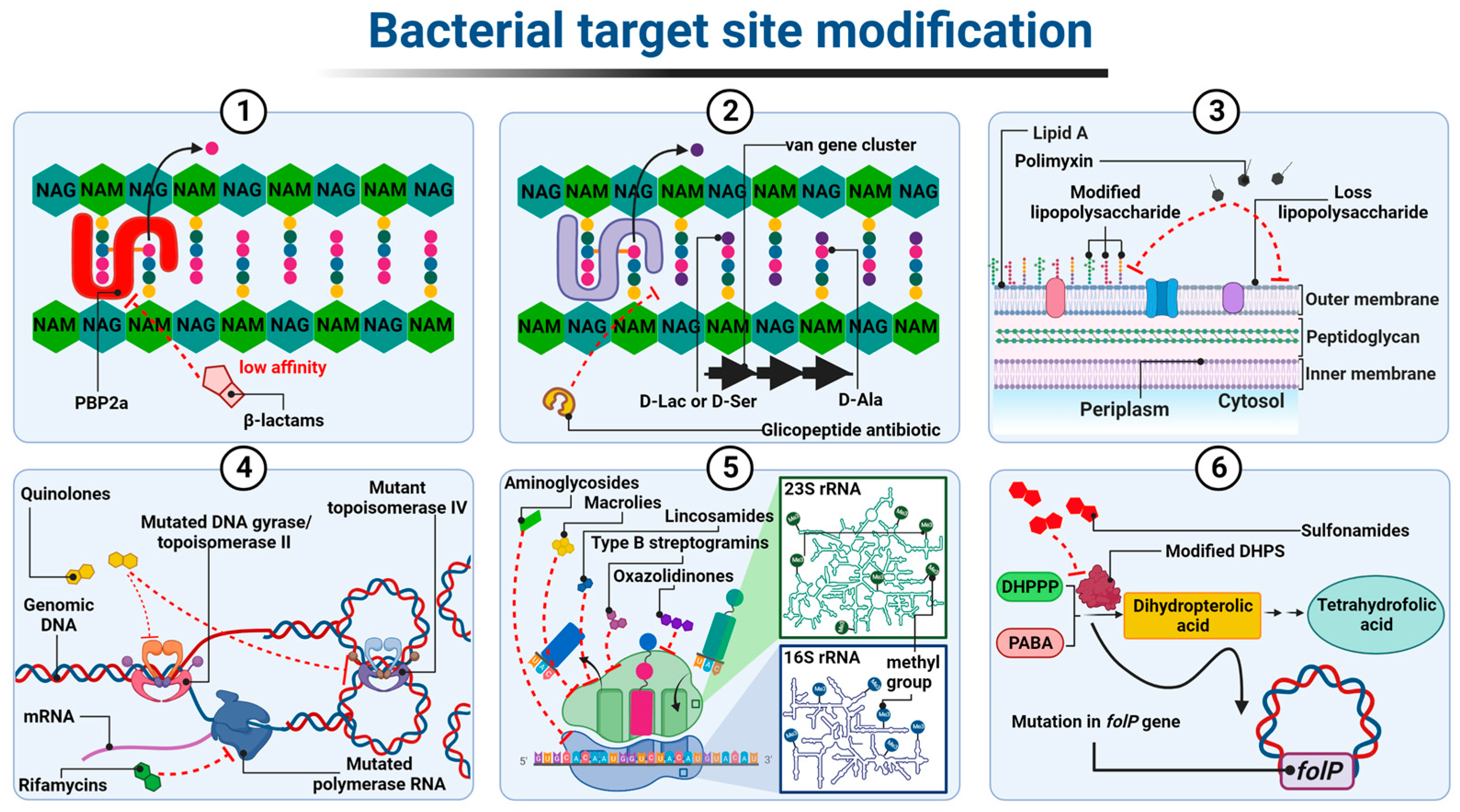
3.1. Modification of the Antibiotic Target Site
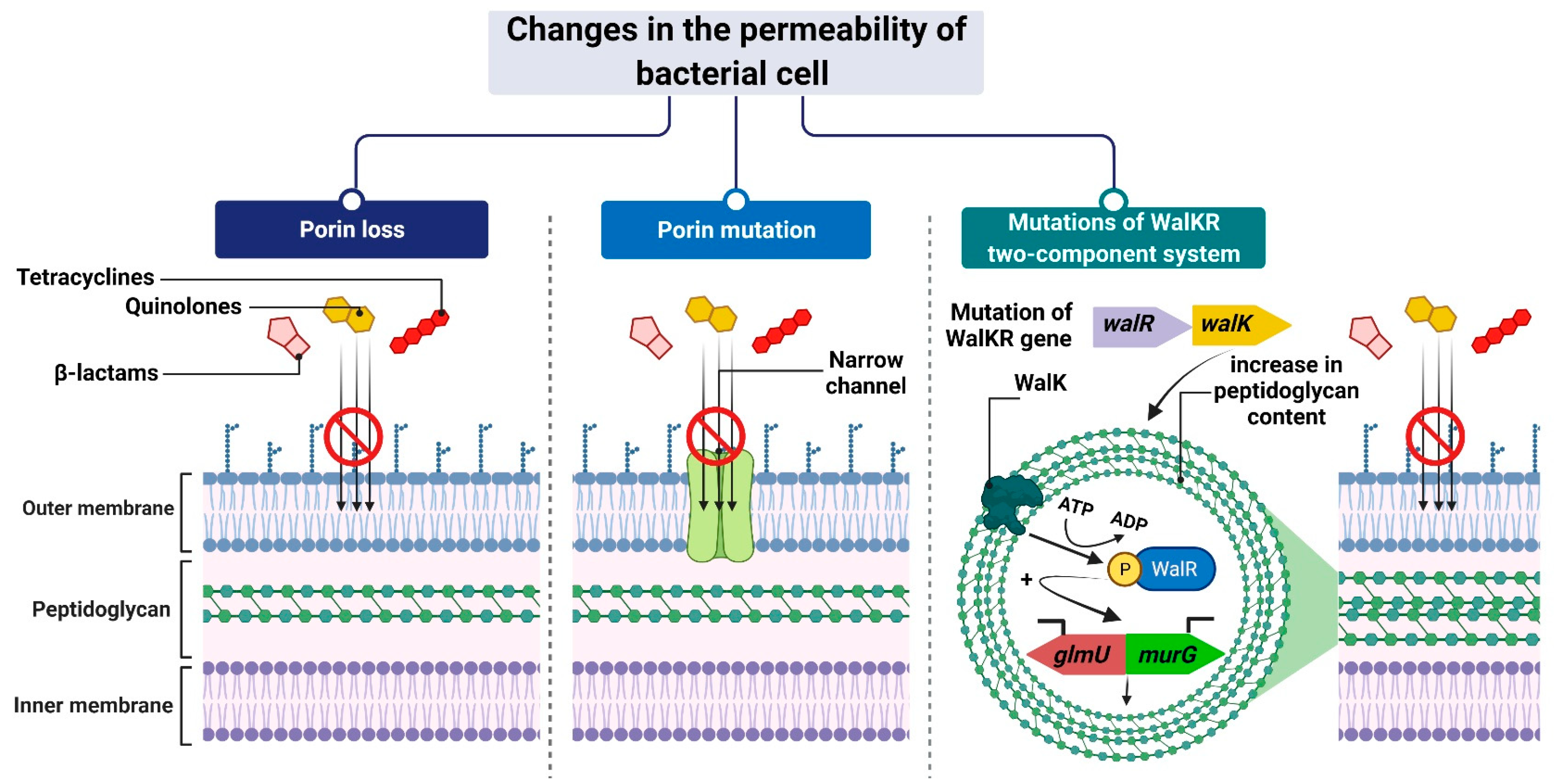
3.2. Changes in the Permeability of a Bacterial Cell
3.3. Active Pumping of the Antibiotic out of the Cell
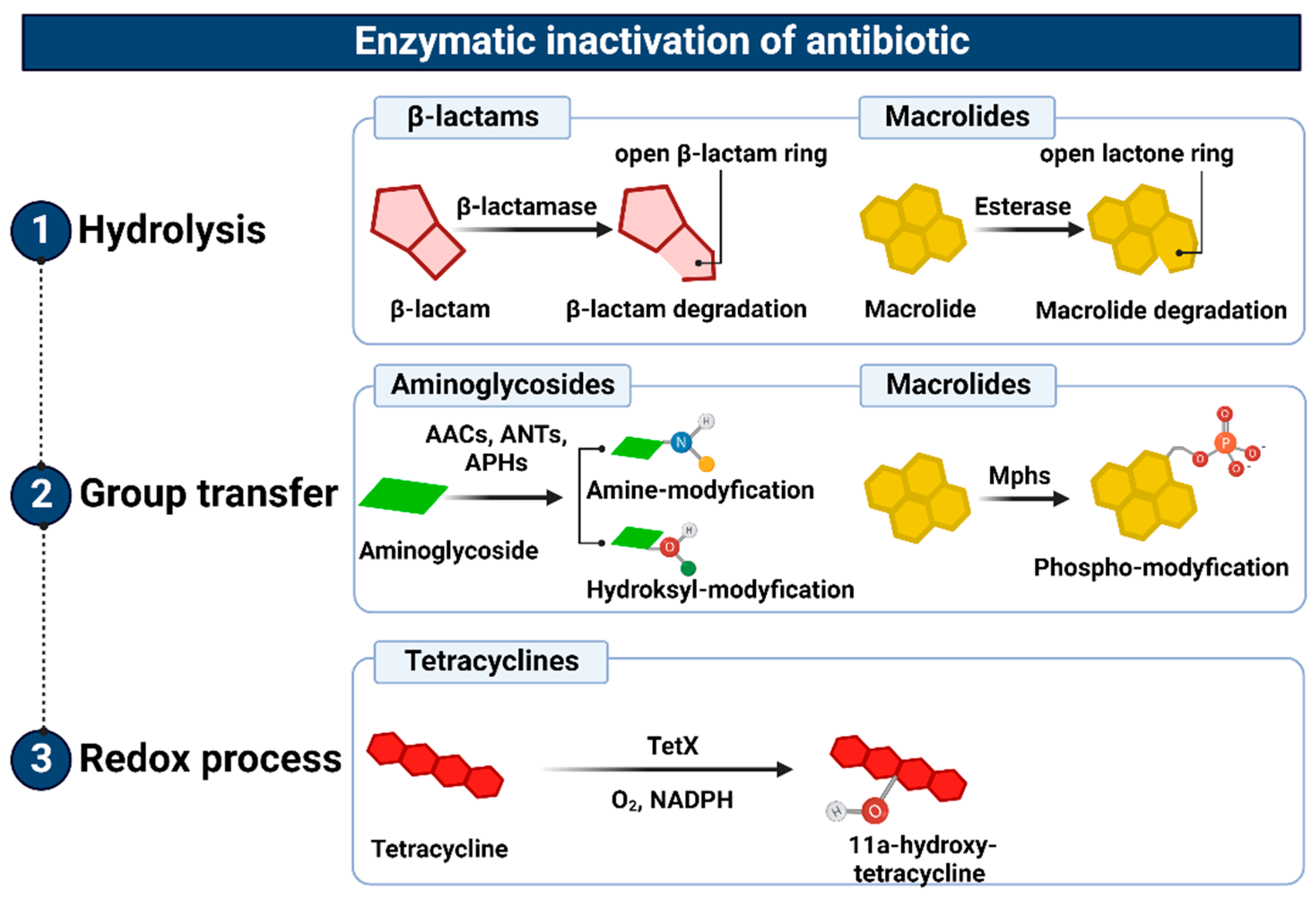
3.4. Enzymatic Inactivation
4. At the Dawn of the Post-Antibiotic Era?
4.1. Natural Born Killers Contra Natural Born Protectors
4.2. Nanotechnology in the Service of the Antibiotic R&D
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAC | aminoglycoside acetyltransferases |
| ALIS | amikacin liposome inhalation suspension |
| ANT | nucleotidyltransferases |
| aPDT | antibacterial photodynamic therapy |
| APH | aminoglycoside O-phosphotransferase |
| aPTD | antibacterial photodynamic therapy |
| DHFR | dihydrofolate reductase |
| DHPPP | 6- hydroxymethyl-7,8-dihydropterin-pyrophosphate |
| DHPS | dihydropteroate synthase |
| ESBLs | extended-spectrum β-lactamases |
| ETEC | enterotoxigenic Escherichia coli |
| LPS | lipopolysaccharide |
| MAC | Mycobacterium avium complex |
| MBLs | metallo-β-lactamases |
| MLSB group | macrolides, lincosamides, and streptogramines |
| MNMs | molecular nanomachines |
| MRSA | methicillin-resistant Staphylococcus aureus |
| NAG | N-acetylglucosamine |
| NAM | N-acetylmuramic acid |
| NPET | nascent polypeptide exit tunnel in the large subunit ribosome |
| NPs | nanoparticles |
| OMVs | outer membrane vesicles |
| PABA | para-aminobenzoic acid |
| PAS | bacteriophage-antibiotic synergy |
| PBPs | penicillin-binding proteins |
| RBPs | receptor-binding proteins |
| RNAP | DNA-dependent multisubunit RNA polymerase |
| SBLs | serine β-lactamases |
References
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; Salamat, M.K.F.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M.; Albericio, F. Antibiotic Resistance: From the Bench to Patients. Antibiotics 2019, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014; Available online: https://apps.who.int/iris/handle/10665/112642 (accessed on 19 December 2021).
- Lack of New Antibiotics Threatens Global Efforts to Contain Drug-Resistant Infections. Available online: https://www.who.int/news/item/17-01-2020-lack-of-new-antibiotics-threatens-global-efforts-to-contain-drug-resistant-infections (accessed on 19 December 2021).
- CDC’s Antibiotic Resistance Threats in the United States (2019 AR Threats Report). 2019. Available online: https://www.cdc.gov/drugresistance/biggest-threats.html (accessed on 19 December 2021).
- Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations/the Review on Antimicrobial Resistance chaired by Jim O’Neill. | Wellcome Collection. Available online: https://wellcomecollection.org/works/rdpck35v (accessed on 19 December 2021).
- Terreni, M.; Taccani, M.; Pregnolato, M. New Antibiotics for Multidrug-Resistant Bacterial Strains: Latest Research Developments and Future Perspectives. Molecules 2021, 26, 2671. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, E.; Bax, H.I.; Verkaik, N.J.; van Westreenen, M. An Update on Eight “New” Antibiotics against Multidrug-Resistant Gram-Negative Bacteria. J. Clin. Med. 2021, 10, 1068. [Google Scholar] [CrossRef]
- Andrei, S.; Droc, G.; Stefan, G. FDA approved antibacterial drugs: 2018-2019. Discoveries 2019, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Clardy, J.; Fischbach, M.A.; Currie, C.R. The natural history of antibiotics. Curr. Biol. 2009, 19, R437–R441. [Google Scholar] [CrossRef]
- Leisner, J.J. The Diverse Search for Synthetic, Semisynthetic and Natural Product Antibiotics From the 1940s and Up to 1960 Exemplified by a Small Pharmaceutical Player. Front. Microbiol. 2020, 11, 976. [Google Scholar] [CrossRef]
- Uluseker, C.; Kaster, K.M.; Thorsen, K.; Basiry, D.; Shobana, S.; Jain, M.; Kumar, G.; Kommedal, R.; Pala-Ozkok, I. A Review on Occurrence and Spread of Antibiotic Resistance in Wastewaters and in Wastewater Treatment Plants: Mechanisms and Perspectives. Front. Microbiol. 2021, 12, 717809. [Google Scholar] [CrossRef]
- Sarkar, P.; Yarlagadda, V.; Ghosh, C.; Haldar, J. A review on cell wall synthesis inhibitors with an emphasis on glycopeptide antibiotics. MedChemComm 2017, 8, 516–533. [Google Scholar] [CrossRef]
- Levine, D.P. Vancomycin: A History. Clin. Infect. Dis. 2006, 42, S5–S12. [Google Scholar] [CrossRef]
- Binda, E.; Marinelli, F.; Marcone, G.L. Old and New Glycopeptide Antibiotics: Action and Resistance. Antibiotics 2014, 3, 572–594. [Google Scholar] [CrossRef] [PubMed]
- Kahne, D.; Leimkuhler, C.; Lu, A.W.; Walsh, C. Glycopeptide and Lipoglycopeptide Antibiotics. Chem. Rev. 2005, 105, 425–448. [Google Scholar] [CrossRef] [PubMed]
- Marschall, E.; Cryle, M.J.; Tailhades, J. Biological, chemical, and biochemical strategies for modifying glycopeptide antibiotics. J. Biol. Chem. 2019, 294, 18769–18783. [Google Scholar] [CrossRef] [PubMed]
- James, R.C.; Pierce, J.G.; Okano, A.; Xie, J.; Boger, D.L. Redesign of Glycopeptide Antibiotics: Back to the Future. ACS Chem. Biol. 2012, 7, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Scheffers, D.-J.; Pinho, M.G. Bacterial Cell Wall Synthesis: New Insights from Localization Studies. Microbiol. Mol. Biol. Rev. 2005, 69, 585–607. [Google Scholar] [CrossRef]
- Abraham, E.P. An Enzyme from Bacteria able to Destroy Penicillin. Nature 1940, 146, 837. [Google Scholar] [CrossRef]
- Wise, E.M.; Park, J.T. Penicillin: Its basic site of action as an inhibitor of a peptide cross-linking reaction in cell wall mucopeptide synthesis. Proc. Natl. Acad. Sci. USA 1965, 54, 75–81. [Google Scholar] [CrossRef]
- Tipper, D.J.; Strominger, J.L. Mechanism of action of penicillins: A proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc. Natl. Acad. Sci. USA 1965, 54, 1133–1141. [Google Scholar] [CrossRef]
- Sauvage, E.; Kerff, F.; Terrak, M.; Ayala, J.A.; Charlier, P. The penicillin-binding proteins: Structure and role in peptidoglycan biosynthesis. FEMS Microbiol. Rev. 2008, 32, 234–258. [Google Scholar] [CrossRef]
- Frère, J.-M.; Duez, C.; Ghuysen, J.-M.; Vandekerkhove, J. Occurrence of a serine residue in the penicillin-binding site of the exocellular DD-carboxy-peptidase-transpeptidase fromStreptomycesR61. FEBS Lett. 1976, 70, 257–260. [Google Scholar] [CrossRef]
- Massova, I.; Mobashery, S. Kinship and Diversification of Bacterial Penicillin-Binding Proteins and β-Lactamases. Antimicrob. Agents Chemother. 1998, 42, 1–17. [Google Scholar] [CrossRef]
- Edoo, Z.; Arthur, M.; Hugonnet, J.-E. Reversible inactivation of a peptidoglycan transpeptidase by a β-lactam antibiotic mediated by β-lactam-ring recyclization in the enzyme active site. Sci. Rep. 2017, 7, 9136. [Google Scholar] [CrossRef]
- Bush, K. Past and present perspectives on β-lactamases. Antimicrob. Agents Chemother. 2018, 62, e01076-18. [Google Scholar] [CrossRef]
- Krajewska, J.; Laudy, A. Nowości w lekach przeciwbakteryjnych zarejestrowanych przez europejską agencję leków—odpowiedź na raport who z 2017 r. O globalnym problemie wielolekooporności. Postepy Mikrobiologii 2022, 60, 249–264. [Google Scholar] [CrossRef]
- Sato, T.; Yamawaki, K. Cefiderocol: Discovery, Chemistry, and In Vivo Profiles of a Novel Siderophore Cephalosporin. Clin. Infect. Dis. 2019, 69 (Suppl. 7), S538–S543. [Google Scholar] [CrossRef] [PubMed]
- Hebeisen, P.; Heinze-Krauss, I.; Angehrn, P.; Hohl, P.; Page, M.G.; Then, R.L. In vitro and in vivo properties of Ro 63-9141, a novel broad-spectrum cephalosporin with activity against methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 2001, 45, 825–836. [Google Scholar] [CrossRef]
- Lovering, A.; Danel, F.; Page, M.; Strynadka, N. Mechanism of action of ceftobiprole: Structural basis for anti-MRSA activity, Poster P-1586. In Proceedings of the 16th European congress of clinical microbiology and infectious diseases (ECCMID), Nice, France, 1–4 April 2006. [Google Scholar]
- Kisgen, J.; Whitney, D. Ceftobiprole, a Broad-Spectrum Cephalosporin With Activity against Methicillin-Resistant Staphylococcus aureus (MRSA). P T 2008, 33, 631–641. [Google Scholar]
- Allen, N.E.; LeTourneau, D.L.; Hobbs, J.N. Molecular interactions of a semisynthetic glycopeptide antibiotic with D-alanyl-D-alanine and D-alanyl-D-lactate residues. Antimicrob. Agents Chemother. 1997, 41, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhou, H.; Olademehin, O.P.; Kim, S.J.; Tao, P. Insights into Key Interactions between Vancomycin and Bacterial Cell Wall Structures. ACS Omega 2018, 3, 37–45. [Google Scholar] [CrossRef]
- Hubbard, B.K.; Walsh, C.T. Vancomycin Assembly: Nature’s Way. Angew. Chem. Int. Ed. 2003, 42, 730–765. [Google Scholar] [CrossRef]
- Zeng, D.; Debabov, D.; Hartsell, T.L.; Cano, R.J.; Adams, S.; Schuyler, J.A.; McMillan, R.; Pace, J.L. Approved Glycopeptide Antibacterial Drugs: Mechanism of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a026989. [Google Scholar] [CrossRef] [PubMed]
- Kövér, K.E.; Szilágyi, L.; Batta, G.; Uhrín, D.; Jiménez-Barbero, J. Biomolecular Recognition by Oligosaccharides and Glycopeptides: The NMR Point of View. Chem. Biol. 2010, 9, 197–246. [Google Scholar] [CrossRef]
- Malin, J.J.; de Leeuw, E. Therapeutic compounds targeting Lipid II for antibacterial purposes. Infect. Drug Resist. 2019, 12, 2613–2625. [Google Scholar] [CrossRef]
- Epand, R.M.; Walker, C.; Epand, R.F.; Magarvey, N.A. Molecular mechanisms of membrane targeting antibiotics. Biochim. Biophys. Acta (BBA)-Biomembr. 2016, 1858, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Baltz, R.H.; Brian, P.; Miao, V.; Wrigley, S.K. Combinatorial biosynthesis of lipopeptide antibiotics in Streptomyces roseosporus. J. Ind. Microbiol. Biotechnol. 2005, 33, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Tonge, P.J. Inhibitors of FabI, an Enzyme Drug Target in the Bacterial Fatty Acid Biosynthesis Pathway. Accounts Chem. Res. 2008, 41, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.D.; Palmer, M. The action mechanism of daptomycin. Bioorganic Med. Chem. 2016, 24, 6253–6268. [Google Scholar] [CrossRef]
- Huang, H.W. DAPTOMYCIN, its membrane-active mechanism vs. that of other antimicrobial peptides. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183395. [Google Scholar] [CrossRef]
- Kelesidis, T. The Interplay between Daptomycin and the Immune System. Front. Immunol. 2014, 5, 52. [Google Scholar] [CrossRef]
- Steenbergen, J.N.; Alder, J.; Thorne, G.M.; Tally, F.P. Daptomycin: A lipopeptide antibiotic for the treatment of serious Gram-positive infections. J. Antimicrob. Chemother. 2005, 55, 283–288. [Google Scholar] [CrossRef]
- Baltz, R.H. Daptomycin: Mechanisms of action and resistance, and biosynthetic engineering. Curr. Opin. Chem. Biol. 2009, 13, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.A.; Perlmutter, N.G.; Shapiro, H.M. Correlation of Daptomycin Bactericidal Activity and Membrane Depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 2003, 47, 2538–2544. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Wenzel, M.; Strahl, H.; Grein, F.; Saaki, T.N.V.; Kohl, B.; Siersma, T.; Bandow, J.E.; Sahl, H.-G.; Schneider, T.; et al. Daptomycin inhibits cell envelope synthesis by interfering with fluid membrane microdomains. Proc. Natl. Acad. Sci. USA 2016, 113, E7077–E7086. [Google Scholar] [CrossRef] [PubMed]
- Maget-Dana, R.; Lakey, J.H.; Ptak, M. A comparative monomolecular film study of antibiotic A21978C homologues of various lipid chain length. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1988, 962, 201–207. [Google Scholar] [CrossRef]
- Velkov, T.; Roberts, K.D.; Thompson, P.E.; Li, J. Polymyxins: A new hope in combating Gram-negative superbugs? Futur. Med. Chem. 2016, 8, 1017–1025. [Google Scholar] [CrossRef]
- Benedict, R.G.; Langlykke, A.F. Antibiotic activity of Bacillus polymyxa. J. Bacteriol. 1947, 54, 24. [Google Scholar]
- Orwa, J.A.; Govaeris, C.; Busson, R.; Roets, E.; VAN Schepdael, A.; Hoogmartens, J. Isolation and Structural Characterization of Colistin Components. J. Antibiot. 2001, 54, 595–599. [Google Scholar] [CrossRef]
- Komura, S.; Kurahashi, K. Partial purification and properties of L-2,4-diaminobutyric acid activating enzyme from a polymyxin E producing organism. J. Biochem. 1979, 86, 1013–1021. [Google Scholar] [CrossRef]
- Katz, E.; Demain, A.L. The peptide antibiotics of Bacillus: Chemistry, biogenesis, and possible functions. Bacteriol. Rev. 1977, 41, 449–474. [Google Scholar] [CrossRef]
- Huang, E.; Yousef, A.E. The lipopeptide antibiotic paenibacterin binds to the bacterial outer membrane and exerts bactericidal activity through cytoplasmic membrane damage. Appl. Environ. Microbiol. 2014, 80, 2700–2704. [Google Scholar] [CrossRef]
- Poirel, L.; Jayol, A.; Nordmann, P. Polymyxins: Antibacterial Activity, Susceptibility Testing, and Resistance Mechanisms Encoded by Plasmids or Chromosomes. Clin. Microbiol. Rev. 2017, 30, 557–596. [Google Scholar] [CrossRef] [PubMed]
- Storm, D.R.; Rosenthal, K.S.; Swanson, P.E. Polymyxin and Related Peptide Antibiotics. Annu. Rev. Biochem. 1977, 46, 723–763. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Meng, Q.; Cheng, D.; Fu, J.; Luo, Q.; Liu, Y.; Yu, Z. Mechanisms of bactericidal action and resistance of polymyxins for Gram-positive bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 3771–3780. [Google Scholar] [CrossRef]
- Moubareck, C.A. Polymyxins and Bacterial Membranes: A Review of Antibacterial Activity and Mechanisms of Resistance. Membranes 2020, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Velkov, T.; Thompson, P.E.; Nation, R.L.; Li, J. Structure−Activity Relationships of Polymyxin Antibiotics. J. Med. Chem. 2010, 53, 1898–1916. [Google Scholar] [CrossRef] [PubMed]
- Clausell, A.; Rabanal, F.; Garcia-Subirats, M.; Alsina, M.A.; Cajal, Y. Membrane Association and Contact Formation by a Synthetic Analogue of Polymyxin B and Its Fluorescent Derivatives. J. Phys. Chem. B 2006, 110, 4465–4471. [Google Scholar] [CrossRef]
- Trimble, M.J.; Mlynárčik, P.; Kolář, M.; Hancock, R.E. Polymyxin: Alternative Mechanisms of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025288. [Google Scholar] [CrossRef]
- Li, Z.; Velkov, T. Polymyxins: Mode of Action. Adv. Exp. Med. Biol. 2019, 1145, 37–54. [Google Scholar] [CrossRef]
- Bisacchi, G.S. Origins of the Quinolone Class of Antibacterials: An Expanded “Discovery Story”. J. Med. Chem. 2015, 58, 4874–4882. [Google Scholar] [CrossRef]
- Bush, N.; Diez-Santos, I.; Abbott, L.; Maxwell, A. Quinolones: Mechanism, Lethality and Their Contributions to Antibiotic Resistance. Molecules 2020, 25, 5662. [Google Scholar] [CrossRef]
- Anderson, V.; Osheroff, V.E.A.A.N. Type II Topoisomerases as Targets for Quinolone Antibacterials Turning Dr. Jekyll into Mr. Hyde. Curr. Pharm. Des. 2001, 7, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Hao, H.; Dai, M.; Liu, Z.; Yuan, Z. Antibacterial action of quinolones: From target to network. Eur. J. Med. Chem. 2013, 66, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Champoux, J.J. DNA Topoisomerases: Structure, Function, and Mechanism. Annu. Rev. Biochem. 2001, 70, 369–413. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.; Poeta, P.; Hébraud, M.; Capelo, J.L.; Igrejas, G. Mechanisms of quinolone action and resistance: Where do we stand? J. Med. Microbiol. 2017, 66, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Aldred, K.J.; Kerns, R.J.; Osheroff, N. Mechanism of Quinolone Action and Resistance. Biochemistry 2014, 53, 1565–1574. [Google Scholar] [CrossRef]
- Drlica, K.; Malik, M.; Kerns, R.J.; Zhao, X. Quinolone-Mediated Bacterial Death. Antimicrob. Agents Chemother. 2008, 52, 385–392. [Google Scholar] [CrossRef]
- Hawkey, P.M. Mechanisms of quinolone action and microbial response. J. Antimicrob. Chemother. 2003, 51, 29–35. [Google Scholar] [CrossRef]
- Pohlhaus, J.R.; Kreuzer, K.N. Norfloxacin-induced DNA gyrase cleavage complexes block Escherichia coli replication forks, causing double-stranded breaks in vivo. Mol. Microbiol. 2005, 56, 1416–1429. [Google Scholar] [CrossRef]
- Phillips, I. Clinical uses and control of rifampicin and clindamycin. J. Clin. Pathol. 1971, 24, 410–418. [Google Scholar] [CrossRef]
- Wehrli, W. Ansamycins chemistry, biosynthesis and biological activity. Med. Chem. 1977, 72, 21–49. [Google Scholar] [CrossRef]
- Kluepfel, D.; Lancini, G.C.; Sartori, G. Metabolism of Barbital by Streptomyces mediterranei. Appl. Microbiol. 1965, 13, 600–604. [Google Scholar] [CrossRef]
- Zhao, W.; Zhong, Y.; Yuan, H.; Wang, J.; Zheng, H.; Wang, Y.; Cen, X.; Xu, F.; Bai, J.; Han, X.; et al. Complete genome sequence of the rifamycin SV-producing Amycolatopsis mediterranei U32 revealed its genetic characteristics in phylogeny and metabolism. Cell Res. 2010, 20, 1096–1108. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, D.M. Rifamycins, Alone and in Combination. Cold Spring Harb. Perspect. Med. 2016, 6, a027011. [Google Scholar] [CrossRef]
- Ho, M.X.; Hudson, B.P.; Das, K.; Arnold, E.; Ebright, R.H. Structures of RNA polymerase–antibiotic complexes. Curr. Opin. Struct. Biol. 2009, 19, 715–723. [Google Scholar] [CrossRef]
- Floss, H.G.; Yu, T.-W. RifamycinMode of Action, Resistance, and Biosynthesis. Chem. Rev. 2005, 105, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Mosaei, H.; Molodtsov, V.; Kepplinger, B.; Harbottle, J.; Moon, C.W.; Jeeves, R.E.; Ceccaroni, L.; Shin, Y.; Morton-Laing, S.; Marrs, E.C.L.; et al. Mode of Action of Kanglemycin A, an Ansamycin Natural Product that Is Active against Rifampicin-Resistant Mycobacterium tuberculosis. Mol. Cell 2018, 72, 263–274.e5. [Google Scholar] [CrossRef]
- Kumar, K.; Chatterji, D. Differential inhibition of abortive transcription initiation at different promoters catalysed byE. coliRNA polymerase Effect of rifampicin on purine or pyramidine-initiated phosphodiester synthesis. FEBS Lett. 1992, 306, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Talaue, M.; Liu, S.; Degen, D.; Ebright, R.Y.; Sineva, E.; Chakraborty, A.; Druzhinin, S.Y.; Chatterjee, S.; Mukhopadhyay, J.; et al. New target for inhibition of bacterial RNA polymerase: ‘switch region’. Curr. Opin. Microbiol. 2011, 14, 532–543. [Google Scholar] [CrossRef]
- Reid, P.; Speyer, J. Rifampicin Inhibition of Ribonucleic Acid and Protein Synthesis in Normal and Ethylenediaminetetraacetic Acid-Treated Escherichia coli. J. Bacteriol. 1970, 104, 376–389. [Google Scholar] [CrossRef]
- Arenz, S.; Wilson, D. Bacterial Protein Synthesis as a Target for Antibiotic Inhibition. Cold Spring Harb. Perspect. Med. 2016, 6, a025361. [Google Scholar] [CrossRef]
- Duggar, B.M. Aureomycin: A product of the continuing search for new antibiotics. Ann. N. Y. Acad. Sci. 1948, 51, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.L.; Levy, S.B. The history of the tetracyclines. Ann. N. Y. Acad. Sci. 2011, 1241, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I.; Hawkey, P.M.; Hinton, M. Tetracyclines, molecular and clinical aspects. J. Antimicrob. Chemother. 1992, 29, 245–277. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I.; Roberts, M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [PubMed]
- Chukwudi, C.U. rRNA Binding Sites and the Molecular Mechanism of Action of the Tetracyclines. Antimicrob. Agents Chemother. 2016, 60, 4433–4441. [Google Scholar] [CrossRef]
- Nguyen, F.; Starosta, A.L.; Arenz, S.; Sohmen, D.; Dönhöfer, A.; Wilson, D.N. Tetracycline antibiotics and resistance mechanisms. Biol. Chem. 2014, 395, 559–575. [Google Scholar] [CrossRef]
- Giuliodori, A.M.; Spurio, R.; Milon, P.; Fabbretti, A. Antibiotics Targeting the 30S Ribosomal Subunit: A Lesson from Nature to Find and Develop New Drugs. Curr. Top. Med. Chem. 2019, 18, 2080–2096. [Google Scholar] [CrossRef]
- Scott, L.J. Eravacycline: A Review in Complicated Intra-Abdominal Infections. Drugs 2019, 79, 315–324. [Google Scholar] [CrossRef]
- Grossman, T.H.; Starosta, A.L.; Fyfe, C.; O’Brien, W.; Rothstein, D.M.; Mikolajka, A.; Wilson, D.N.; Sutcliffe, J.A. Target- and resistance-based mechanistic studies with TP-434, a novel fluorocycline antibiotic. Antimicrob. Agents Chemother. 2012, 56, 2559–2564. [Google Scholar] [CrossRef]
- Solomkin, J.; Evans, D.; Slepavicius, A.; Lee, P.; Marsh, A.; Tsai, L.; Sutcliffe, J.A.; Horn, P. Assessing the Efficacy and Safety of Eravacycline vs Ertapenem in Complicated Intra-abdominal Infections in the Investigating Gram-Negative Infections Treated With Eravacycline (IGNITE 1) Trial: A Randomized Clinical Trial. JAMA Surg. 2017, 152, 224–232. [Google Scholar] [CrossRef]
- Batool, Z.; Lomakin, I.B.; Polikanov, Y.S.; Bunick, C.G. Sarecycline interferes with tRNA accommodation and tethers mRNA to the 70S ribosome. Proc. Natl. Acad. Sci. USA 2020, 117, 20530–20537. [Google Scholar] [CrossRef] [PubMed]
- Durães, F.; Sousa, E. Omadacycline: A Newly Approved Antibacterial from the Class of Tetracyclines. Pharmaceuticals 2019, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Burgos, R.M.; Rodvold, K.A. Omadacycline: A novel aminomethylcycline. Infect. Drug Resist. 2019, 12, 1895–1915. [Google Scholar] [CrossRef] [PubMed]
- Durante-Mangoni, E.; Grammatikos, A.; Utili, R.; Falagas, M.E. Do we still need the aminoglycosides? Int. J. Antimicrob. Agents 2009, 33, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Mingeot-Leclercq, M.-P.; Glupczynski, Y.; Tulkens, P.M. Aminoglycosides: Activity and Resistance. Antimicrob. Agents Chemother. 1999, 43, 727–737. [Google Scholar] [CrossRef]
- Vakulenko, S.B.; Mobashery, S. Versatility of Aminoglycosides and Prospects for Their Future. Clin. Microbiol. Rev. 2003, 16, 430–450. [Google Scholar] [CrossRef]
- Shakil, S.; Khan, R.; Zarrilli, R.; Khan, A.U. Aminoglycosides versus bacteria—A description of the action, resistance mechanism, and nosocomial battleground. J. Biomed. Sci. 2007, 15, 5–14. [Google Scholar] [CrossRef]
- Davis, B.D.; Chen, L.L.; Tai, P.C. Misread protein creates membrane channels: An essential step in the bactericidal action of aminoglycosides. Proc. Natl. Acad. Sci. USA 1986, 83, 6164–6168. [Google Scholar] [CrossRef]
- Wirmer, J.; Westhof, E. Molecular Contacts Between Antibiotics and the 30S Ribosomal Particle. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2006; pp. 180–202. Volume 415. [Google Scholar]
- Zhanel, G.G.; Hoban, D.J.; Harding, G.K. The Postantibiotic Effect: A Review of in Vitro and in Vivo Data. Dicp 1991, 25, 153–163. [Google Scholar] [CrossRef]
- Alfieri, A.; Di Franco, S.; Donatiello, V.; Maffei, V.; Fittipaldi, C.; Fiore, M.; Coppolino, F.; Sansone, P.; Pace, M.C.; Passavanti, M.B. Plazomicin against Multidrug-Resistant Bacteria: A Scoping Review. Life 2022, 12, 1949. [Google Scholar] [CrossRef]
- Golkar, T.; Bassenden, A.V.; Maiti, K.; Arya, D.P.; Schmeing, T.M.; Berghuis, A.M. Structural basis for plazomicin antibiotic action and resistance. Commun. Biol. 2021, 729. [Google Scholar] [CrossRef]
- Tsui, W.H.; Yim, G.; Wang, H.H.; McClure, J.E.; Surette, M.G.; Davies, J. Dual Effects of MLS Antibiotics: Transcriptional Modulation and Interactions on the Ribosome. Chem. Biol. 2004, 11, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Pyörälä, S.; Baptiste, K.E.; Catry, B.; van Duijkeren, E.; Greko, C.; Moreno, M.A.; Pomba, M.C.M.F.; Rantala, M.; Ružauskas, M.; Sanders, P.; et al. Macrolides and lincosamides in cattle and pigs: Use and development of antimicrobial resistance. Vet. J. 2014, 200, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Douthwaite, S.; Hansen, L.H.; Mauvais, P. Macrolide-ketolide inhibition of MLS-resistant ribosomes is improved by alternative drug interaction with domain II of 23S rRNA. Mol. Microbiol. 2000, 36, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Eyal, Z.; Matzov, D.; Krupkin, M.; Wekselman, I.; Paukner, S.; Zimmerman, E.; Rozenberg, H.; Bashan, A.; Yonath, A. Structural insights into species-specific features of the ribosome from the pathogen Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2015, 112, E5805–E5814. [Google Scholar] [CrossRef] [PubMed]
- Tenson, T.; Lovmar, M.; Ehrenberg, M. The Mechanism of Action of Macrolides, Lincosamides and Streptogramin B Reveals the Nascent Peptide Exit Path in the Ribosome. J. Mol. Biol. 2003, 330, 1005–1014. [Google Scholar] [CrossRef]
- Champney, W.S.; Burdine, R. Macrolide antibiotics inhibit 50S ribosomal subunit assembly in Bacillus subtilis and Staphylococcus aureus. Antimicrob. Agents Chemother. 1995, 39, 2141–2144. [Google Scholar] [CrossRef]
- Slee, A.M.; Wuonola, M.A.; McRipley, R.J.; Zajac, I.; Zawada, M.J.; Bartholomew, P.T.; Gregory, W.A.; Forbes, M. Oxazolidinones, a new class of synthetic antibacterial agents: In vitro and in vivo activities of DuP 105 and DuP 721. Antimicrob. Agents Chemother. 1987, 31, 1791–1797. [Google Scholar] [CrossRef]
- Rybak, J.M.; Roberts, K. Tedizolid Phosphate: A Next-Generation Oxazolidinone. Infect. Dis. Ther. 2015, 4, 1–14. [Google Scholar] [CrossRef]
- Roger, C.; Roberts, J.; Muller, L. Clinical Pharmacokinetics and Pharmacodynamics of Oxazolidinones. Clin. Pharmacokinet. 2018, 57, 559–575. [Google Scholar] [CrossRef]
- Dutronc, H.; Bocquentin, F.; Galperine, T.; Lafarie-Castet, S.; Dupon, M. Le linézolide, premier antibiotique de la famille des oxazolidinones. MÉDecine Et Mal. Infect. 2005, 35, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, J.A.; Kanyo, Z.F.; Wang, D.; Franceschi, F.J.; Moore, P.B.; Steitz, T.A.; Duffy, E.M. Crystal Structure of the Oxazolidinone Antibiotic Linezolid Bound to the 50S Ribosomal Subunit. J. Med. Chem. 2008, 51, 3353–3356. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.N.; Schluenzen, F.; Harms, J.M.; Starosta, A.L.; Connell, S.R.; Fucini, P. The oxazolidinone antibiotics perturb the ribosomal peptidyl-transferase center and effect tRNA positioning. Proc. Natl. Acad. Sci. USA 2008, 105, 13339–13344. [Google Scholar] [CrossRef]
- Shaw, K.J.; Barbachyn, M.R. The oxazolidinones: Past, present, and future. Ann. N. Y. Acad. Sci. 2011, 1241, 48–70. [Google Scholar] [CrossRef]
- Bozdogan, B.; Appelbaum, P.C. Oxazolidinones: Activity, mode of action, and mechanism of resistance. Int. J. Antimicrob. Agents 2004, 23, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Meng, J.; Yuan, H.; Zhong, D.; Yu, J.; Cao, G.; Liu, X.; Guo, B.; Chen, Y.; Li, Y.; et al. Pharmacokinetics and Disposition of Contezolid in Humans: Resolution of a Disproportionate Human Metabolite for Clinical Development. Antimicrob. Agents Chemother. 2021, 65, 00409–00421. [Google Scholar] [CrossRef] [PubMed]
- Gordeev, M.F.; Yuan, Z.Y. New potent antibacterial oxazolidinone (MRX-I) with an improved class safety profile. J. Med. Chem. 2014, 57, 4487–4497. [Google Scholar] [CrossRef]
- Covvey, J.R.; Guarascio, A.J. Clinical use of lefamulin: A first-in-class semisynthetic pleuromutilin antibiotic. J. Intern. Med. 2022, 291, 51–63. [Google Scholar] [CrossRef]
- Sköld, O. Sulfonamides and Trimethoprim. In Antimicrobial Drug Resistance: Mechanisms of Drug Resistance; Mayers, D.L., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 259–269. [Google Scholar] [CrossRef]
- Connor, E.E. Sulfonamide antibiotics. Prim. Care Updat. OB/GYNS 1998, 5, 32–35. [Google Scholar] [CrossRef]
- King, C.H.; Shlaes, D.M.; Dul, M.J. Infection caused by thymidine-requiring, trimethoprim-resistant bacteria. J. Clin. Microbiol. 1983, 18, 79–83. [Google Scholar] [CrossRef]
- Wood, W.B. Studies on the antibacterial action of the sulfonamide drugs. J. Exp. Med. 1942, 75, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Nunes, O.C.; Manaia, C.M.; Kolvenbach, B.A.; Corvini, P.F.-X. Living with sulfonamides: A diverse range of mechanisms observed in bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 10389–10408. [Google Scholar] [CrossRef] [PubMed]
- Gaynes, R. The Discovery of Penicillin—New Insights After More Than 75 Years of Clinical Use. Emerg. Infect. Dis. 2017, 23, 849–853. [Google Scholar] [CrossRef]
- Greenwood, D. CHAPTER 29-Sulfonamides. In Antibiotic and Chemotherapy, 9th ed.; Finch, R.G., Greenwood, D., Norrby, S.R., Whitley, R.J., Eds.; W.B. Saunders: London, UK, 2010; pp. 337–343. [Google Scholar] [CrossRef]
- Giles, A.; Foushee, J.; Lantz, E.; Gumina, G. Sulfonamide Allergies. Pharmacy 2019, 7, 132. [Google Scholar] [CrossRef] [PubMed]
- Kalkut, G. Sulfonamides and trimethoprim. Cancer Investig. 1998, 16, 612–615. [Google Scholar] [CrossRef]
- Fermér, C.; Swedberg, G. Adaptation to sulfonamide resistance in Neisseria meningitidis may have required compensatory changes to retain enzyme function: Kinetic analysis of dihydropteroate synthases from N. meningitidis expressed in a knockout mutant of Escherichia coli. J. Bacteriol. 1997, 179, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Maskell, J.P.; Sefton, A.M.; Hall, L.M. Mechanism of sulfonamide resistance in clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 1997, 41, 2121–2126. [Google Scholar] [CrossRef] [PubMed]
- Fermer, C.; Kristiansen, B.-E.; Sköld, O.; Swedberg, G. Sulfonamide resistance in Neisseria meningitidis as defined by site-directed mutagenesis could have its origin in other species. J. Bacteriol. 1995, 177, 4669–4675. [Google Scholar] [CrossRef]
- Achari, A.; Somers, D.O.; Champness, J.N.; Bryant, P.; Rosemond, J.; Stammers, D.K. Crystal structure of the anti-bacterial sulfonamide drug target dihydropteroate synthase. Nat. Struct. Biol. 1997, 4, 490–497. [Google Scholar] [CrossRef]
- Sköld, O. Sulfonamide resistance: Mechanisms and trends. Drug Resist. Updat. 2000, 3, 155–160. [Google Scholar] [CrossRef]
- Eyler, R.F.; Shvets, K. Clinical Pharmacology of Antibiotics. Clin. J. Am. Soc. Nephrol. 2019, 14, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Noall, E.W.P.; Sewards, H.F.G.; Waterworth, P.M. Successful Treatment of a Case of Proteus Septicaemia. BMJ 1962, 2, 1101–1102. [Google Scholar] [CrossRef] [PubMed]
- Huovinen, P.; Sundström, L.; Swedberg, G.; Sköld, O. Trimethoprim and sulfonamide resistance. Antimicrob. Agents Chemother. 1995, 39, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Then, R.L.; Angehrn, P. Low Trimethoprim Susceptibility of Anaerobic Bacteria Due to Insensitive Dihydrofolate Reductases. Antimicrob. Agents Chemother. 1979, 15, 1–6. [Google Scholar] [CrossRef]
- Adrian, P.V.; Klugman, K.P. Mutations in the dihydrofolate reductase gene of trimethoprim-resistant isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 1997, 41, 2406–2413. [Google Scholar] [CrossRef]
- Cox, G.; Wright, G.D. Intrinsic antibiotic resistance: Mechanisms, origins, challenges and solutions. Int. J. Med. Microbiol. 2013, 303, 287–292. [Google Scholar] [CrossRef]
- Woodford, N.; Ellington, M.J. The emergence of antibiotic resistance by mutation. Clin. Microbiol. Infect. 2007, 13, 5–18. [Google Scholar] [CrossRef]
- Gómez-Gómez, C.; Blanco-Picazo, P.; Brown-Jaque, M.; Quirós, P.; Rodriguez-Rubio, L.; Cerdà-Cuellar, M.; Muniesa, M. Infectious phage particles packaging antibiotic resistance genes found in meat products and chicken feces. Sci. Rep. 2019, 9, 13281. [Google Scholar] [CrossRef]
- Peterson, E.; Kaur, P. Antibiotic Resistance Mechanisms in Bacteria: Relationships Between Resistance Determinants of Antibiotic Producers, Environmental Bacteria, and Clinical Pathogens. Front. Microbiol. 2018, 9, 2928. [Google Scholar] [CrossRef]
- Hawkey, P.M. The origins and molecular basis of antibiotic resistance. BMJ 1998, 317, 657–660. [Google Scholar] [CrossRef]
- Hackbarth, C.J.; Kocagoz, T.; Kocagoz, S.; Chambers, H.F. Point mutations in Staphylococcus aureus PBP 2 gene affect penicillin-binding kinetics and are associated with resistance. Antimicrob. Agents Chemother. 1995, 39, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Pinho, M.G.; de Lencastre, H.; Tomasz, A. An acquired and a native penicillin-binding protein cooperate in building the cell wall of drug-resistant staphylococci. Proc. Natl. Acad. Sci. USA 2001, 98, 10886–10891. [Google Scholar] [CrossRef] [PubMed]
- Raze, D.; Dardenne, O.; Hallut, S.; Martinez-Bueno, M.; Coyette, J.; Ghuysen, J.-M. The Gene Encoding the Low-Affinity Penicillin-Binding Protein 3r in Enterococcus hirae S185R Is Borne on a Plasmid Carrying Other Antibiotic Resistance Determinants. Antimicrob. Agents Chemother. 1998, 42, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Harimaya, A.; Koizumi, J.-I.; Yamazaki, N.; Himi, T.; Yokota, S.-I.; Sato, K.; Fujii, N. Alterations of pbp1a, pbp2b, and pbp2x in Streptococcus pneumoniae isolates from children with otolaryngological infectious disease in the Sapporo district of Japan. J. Infect. Chemother. 2006, 12, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Matsuhashi, M.; Song, M.D.; Ishino, F.; Wachi, M.; Doi, M.; Inoue, M.; Ubukata, K.; Yamashita, N.; Konno, M. Molecular cloning of the gene of a penicillin-binding protein supposed to cause high resistance to beta-lactam antibiotics in Staphylococcus aureus. J. Bacteriol. 1986, 167, 975–980. [Google Scholar] [CrossRef]
- Paterson, G.K.; Harrison, E.M.; Holmes, M.A. The emergence of mecC methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2013, 22, 42–47. [Google Scholar] [CrossRef]
- Becker, K.; van Alen, S.; Idelevich, E.A.; Schleimer, N.; Seggewiß, J.; Mellmann, A.; Kaspar, U.; Peters, G. Plasmid-Encoded Transferable mecB-Mediated Methicillin Resistance in Staphylococcus aureus. Emerg. Infect. Dis. 2018, 24, 242–248. [Google Scholar] [CrossRef]
- Schwendener, S.; Cotting, K.; Perreten, V. Novel methicillin resistance gene mecD in clinical Macrococcus caseolyticus strains from bovine and canine sources. Sci. Rep. 2017, 7, srep43797. [Google Scholar] [CrossRef]
- Cooper, M.; Fiorini, M.T.; Abell, C.; Williams, D.H. Binding of vancomycin group antibiotics to d -alanine and d -lactate presenting self-assembled monolayers. Bioorganic Med. Chem. 2000, 8, 2609–2616. [Google Scholar] [CrossRef]
- Reynolds, P.E.; Courvalin, P. Vancomycin Resistance in Enterococci Due to Synthesis of Precursors Terminating in d -Alanyl- d -Serine. Antimicrob. Agents Chemother. 2005, 49, 21–25. [Google Scholar] [CrossRef]
- Ge, M.; Chen, Z.; Russell, H.; Onishi, H.R.; Kohler, J.; Silver, L.L.; Kerns, R.; Fukuzawa, S.; Thompson, C.; Kahne, D. Vancomycin Derivatives That Inhibit Peptidoglycan Biosynthesis Without Binding d -Ala- d -Ala. Science 1999, 284, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.O.; Baptiste, K.E. Vancomycin-Resistant Enterococci: A Review of Antimicrobial Resistance Mechanisms and Perspectives of Human and Animal Health. Microb. Drug Resist. 2018, 24, 590–606. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.B. Mechanisms of Resistance and Clinical Relevance of Resistance to β-Lactams, Glycopeptides, and Fluoroquinolones. Mayo Clin. Proc. 2012, 87, 198–208. [Google Scholar] [CrossRef]
- Gagnon, S.; Lévesque, S.; Lefebvre, B.; Bourgault, A.-M.; Labbé, A.-C.; Roger, M. vanA-containing Enterococcus faecium susceptible to vancomycin and teicoplanin because of major nucleotide deletions in Tn1546. J. Antimicrob. Chemother. 2011, 66, 2758–2762. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.M.; Krause, K.M.; Lewis, S.R.; Blais, J.; Benton, B.M.; Mammen, M.; Humphrey, P.P.; Kinana, A.; Janc, J.W. Specificity of Induction of the vanA and vanB Operons in Vancomycin-Resistant Enterococci by Telavancin. Antimicrob. Agents Chemother. 2010, 54, 2814–2818. [Google Scholar] [CrossRef]
- Moffatt, J.H.; Harper, M.; Boyce, J.D. Mechanisms of Polymyxin Resistance. In Polymyxin Antibiotics: From Laboratory Bench to Bedside; Springer: Berlin/Heidelberg, Germany, 2019; pp. 55–71. [Google Scholar] [CrossRef]
- Hamad, M.A.; Di Lorenzo, F.; Molinaro, A.; Valvano, M.A. Aminoarabinose is essential for lipopolysaccharide export and intrinsic antimicrobial peptide resistance in Burkholderia cenocepacia. Mol. Microbiol. 2012, 85, 962–974. [Google Scholar] [CrossRef]
- Stock, I. Natural Antibiotic Susceptibility of Proteus spp., with Special Reference to P. Mirabilis and P. penneri Strains. J. Chemother. 2003, 15, 12–26. [Google Scholar] [CrossRef]
- Hase, S.; Hofstad, T.; Rietschel, E.T. Chemical structure of the lipid A component of lipopolysaccharides from Fusobacterium nucleatum. J. Bacteriol. 1977, 129, 9–14. [Google Scholar] [CrossRef]
- Johnson, L.; Horsman, S.R.; Charron-Mazenod, L.; Turnbull, A.L.; Mulcahy, H.; Surette, M.G.; Lewenza, S. Extracellular DNA-induced antimicrobial peptide resistance in Salmonella enterica serovar Typhimurium. BMC Microbiol. 2013, 13, 115. [Google Scholar] [CrossRef]
- Cheng, H.-Y.; Chen, Y.-F.; Peng, H.-L. Molecular characterization of the PhoPQ-PmrD-PmrAB mediated pathway regulating polymyxin B resistance in Klebsiella pneumoniae CG43. J. Biomed. Sci. 2010, 17, 60. [Google Scholar] [CrossRef]
- Francis, V.I.; Stevenson, E.C.; Porter, S.L. Two-component systems required for virulence in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2017, 364, fnx104. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, C.; Song, J.; Velkov, T.; Wang, L.; Zhu, Y.; Li, J. Regulating polymyxin resistance in Gram-negative bacteria: Roles of two-component systems PhoPQ and PmrAB. Futur. Microbiol. 2020, 15, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Marceau, M.; Sebbane, F.; Ewann, F.; Collyn, F.; Lindner, B.; Campos, M.A.; Bengoechea, J.-A.; Simonet, M. The pmrF polymyxin-resistance operon of Yersinia pseudotuberculosis is upregulated by the PhoP-PhoQ two-component system but not by PmrA-PmrB, and is not required for virulence. Microbiology 2004, 150, 3947–3957. [Google Scholar] [CrossRef]
- Gooderham, W.J.; Hancock, R.E.W. Regulation of virulence and antibiotic resistance by two-component regulatory systems in Pseudomonas aeruginosa. FEMS Microbiol. Rev. 2009, 33, 279–294. [Google Scholar] [CrossRef]
- Olaitan, A.; Morand, S.; Rolain, J.-M. Mechanisms of polymyxin resistance: Acquired and intrinsic resistance in bacteria. Front. Microbiol. 2014, 5, 643. [Google Scholar] [CrossRef]
- Moffatt, J.H.; Harper, M.; Harrison, P.; Hale, J.D.; Vinogradov, E.; Seemann, T.; Henry, R.; Crane, B.; St. Michael, F.; Cox, A.D.; et al. Colistin Resistance in Acinetobacter baumannii Is Mediated by Complete Loss of Lipopolysaccharide Production. Antimicrob. Agents Chemother. 2010, 54, 4971–4977. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.C.; Jacoby, G.A. Mechanisms of drug resistance: Quinolone resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 12–31. [Google Scholar] [CrossRef]
- Jacoby, G.A. Mechanisms of Resistance to Quinolones. Clin. Infect. Dis. 2005, 41, S120–S126. [Google Scholar] [CrossRef]
- Hooper, D.C.; Jacoby, G.A. Topoisomerase Inhibitors: Fluoroquinolone Mechanisms of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025320. [Google Scholar] [CrossRef]
- Ruiz, J. Mechanisms of resistance to quinolones: Target alterations, decreased accumulation and DNA gyrase protection. J. Antimicrob. Chemother. 2003, 51, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Wasyl, D.; Hoszowski, A.; Zając, M. Prevalence and characterisation of quinolone resistance mechanisms in Salmonella spp. Vet. Microbiol. 2014, 171, 307–314. [Google Scholar] [CrossRef]
- Eliopoulos, G.M. Quinolone Resistance Mechanisms in Pneumococci. Clin. Infect. Dis. 2004, 38, S346–S349. [Google Scholar] [CrossRef]
- Schmitz, F.J.; Hofmann, B.; Hansen, B.; Scheuring, S.; Lückefahr, M.; Klootwijk, M.; Verhoef, J.; Fluit, A.; Heinz, H.P.; Köhrer, K.; et al. Relationship between ciprofloxacin, ofloxacin, levofloxacin, sparfloxacin and moxifloxacin (BAY 12-8039) MICs and mutations in grlA, grlB, gyrA and gyrB in 116 unrelated clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 1998, 41, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Phelan, J.E.; Khan, M.T.; Ali, S.; Qasim, M.; Napier, G.; Campino, S.; Ahmad, S.; Cabral-Marques, O.; Zhang, S.; et al. Characterization of rifampicin-resistant Mycobacterium tuberculosis in Khyber Pakhtunkhwa, Pakistan. Sci. Rep. 2021, 11, 14194. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.A.; Korzheva, N.; Mustaev, A.; Murakami, K.; Nair, S.; Goldfarb, A.; Darst, S.A. Structural Mechanism for Rifampicin Inhibition of Bacterial RNA Polymerase. Cell 2001, 104, 901–912. [Google Scholar] [CrossRef]
- Koch, A.; Mizrahi, V.; Warner, D.F. The impact of drug resistance on Mycobacterium tuberculosis physiology: What can we learn from rifampicin? Emerg. Microbes Infect. 2014, 3, 1–11. [Google Scholar] [CrossRef]
- Zaw, M.T.; Emran, N.A.; Lin, Z. Mutations inside rifampicin-resistance determining region of rpoB gene associated with rifampicin-resistance in Mycobacterium tuberculosis. J. Infect. Public Health 2018, 11, 605–610. [Google Scholar] [CrossRef]
- Padayachee, T.; Klugman, K.P. Molecular Basis of Rifampin Resistance in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 1999, 43, 2361–2365. [Google Scholar] [CrossRef]
- Goldstein, B.P. Resistance to rifampicin: A review. J. Antibiot. 2014, 67, 625–630. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Tai, C.-H.; Li, C.-R.; Lin, C.-F.; Shi, Z.-Y. Resistance profiles and rpoB gene mutations of Mycobacterium tuberculosis isolates in Taiwan. J. Microbiol. Immunol. Infect. 2013, 46, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Weisblum, B. Erythromycin resistance by ribosome modification. Antimicrob. Agents Chemother. 1995, 39, 577–585. [Google Scholar] [CrossRef]
- Schroeder, M.R.; Stephens, D.S. Macrolide Resistance in Streptococcus pneumoniae. Front. Cell. Infect. Microbiol. 2016, 6, 98. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, R. Mechanisms of Resistance to Macrolides and Lincosamides: Nature of the Resistance Elements and Their Clinical Implications. Clin. Infect. Dis. 2002, 34, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Daoud, Z.; Kourani, M.; Saab, R.; Nader, M.A.; Hajjar, M. Resistance of Streptococcus pneumoniae isolated from Lebanese patients between 2005 and 2009. Rev. Esp. Quimioter. Publ. Soc. Esp. Quimioter. 2011, 24, 84–90. [Google Scholar]
- Portillo, A.; Ruiz-Larrea, F.; Zarazaga, M.; Alonso, A.; Martinez, J.L.; Torres, C. Macrolide Resistance Genes in Enterococcus spp. Antimicrob. Agents Chemother. 2000, 44, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Westh, H.; Hougaard, D.M.; Vuust, J.; Rosdahl, V.T. erm genes in erythromycin-resistant Staphylococcus aureus and coagulase-negative staphylococci. APMIS Acta Pathol. Microbiol. Immunol. Scand. 1995, 103, 225–232. [Google Scholar] [CrossRef]
- De Leener, E.; Martel, A.; Decostere, A.; Haesebrouck, F. Distribution of the erm(B) Gene, tet racycline Resistance Genes, and Tn 1545-like Transposons in Macrolide- and Lincosamide-Resistant Enterococci from Pigs and Humans. Microb. Drug Resist. 2004, 10, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Huber, L.; Giguère, S.; Slovis, N.M.; Álvarez-Narváez, S.; Hart, K.A.; Greiter, M.; Morris, E.R.A.; Cohen, N.D. The novel and transferable erm (51) gene confers macrolides, lincosamides and streptogramins B (MLSB) resistance to clonal Rhodococcus equi in the environment. Environ. Microbiol. 2020, 22, 2858–2869. [Google Scholar] [CrossRef] [PubMed]
- Wipf, J.R.K.; Schwendener, S.; Perreten, V. The Novel Macrolide-Lincosamide-Streptogramin B Resistance Gene erm (44) Is Associated with a Prophage in Staphylococcus xylosus. Antimicrob. Agents Chemother. 2014, 58, 6133–6138. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Yin, Y.; Li, S.; Zhang, Y.; Wang, Q.; Wang, H. Molecular characteristics of oxazolidinone resistance in enterococci from a multicenter study in China. BMC Microbiol. 2019, 19, 162. [Google Scholar] [CrossRef]
- Long, K.S.; Poehlsgaard, J.; Kehrenberg, C.; Schwarz, S.; Vester, B. The Cfr rRNA Methyltransferase Confers Resistance to Phenicols, Lincosamides, Oxazolidinones, Pleuromutilins, and Streptogramin A Antibiotics. Antimicrob. Agents Chemother. 2006, 50, 2500–2505. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y.; Wachino, J.-I.; Arakawa, Y. Aminoglycoside Resistance. Infect. Dis. Clin. N. Am. 2016, 30, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Zaman, S.; Fitzpatrick, M.; Lindahl, L.; Zengel, J. Novel mutations in ribosomal proteins L4 and L22 that confer erythromycin resistance in Escherichia coli. Mol. Microbiol. 2007, 66, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, N.; Richter, A.A.; Ehrenberg, M.; Dahlberg, A.E.; Kurland, C.G. Ribosomal RNA and protein mutants resistant to spectinomycin. EMBO J. 1990, 9, 735–739. [Google Scholar] [CrossRef]
- Kondo, J. A Structural Basis for the Antibiotic Resistance Conferred by an A1408G Mutation in 16S rRNA and for the Antiprotozoal Activity of Aminoglycosides. Angew. Chem. Int. Ed. 2011, 51, 465–468. [Google Scholar] [CrossRef]
- Garneau-Tsodikova, S.; Labby, K.J. Mechanisms of resistance to aminoglycoside antibiotics: Overview and perspectives. MedChemComm 2015, 7, 11–27. [Google Scholar] [CrossRef]
- Delcour, A.H. Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2009, 1794, 808–816. [Google Scholar] [CrossRef]
- Yarlagadda, V.; Manjunath, G.B.; Sarkar, P.; Akkapeddi, P.; Paramanandham, K.; Shome, B.R.; Ravikumar, R.; Haldar, J. Glycopeptide Antibiotic To Overcome the Intrinsic Resistance of Gram-Negative Bacteria. ACS Infect. Dis. 2015, 2, 132–139. [Google Scholar] [CrossRef]
- Konovalova, A.; Kahne, D.E.; Silhavy, T.J. Outer Membrane Biogenesis. Annu. Rev. Microbiol. 2017, 71, 539–556. [Google Scholar] [CrossRef]
- Papp-Wallace, K.M.; Endimiani, A.; Taracila, M.A.; Bonomo, R.A. Carbapenems: Past, Present, and Future. Antimicrob. Agents Chemother. 2011, 55, 4943–4960. [Google Scholar] [CrossRef]
- Pagès, J.-M.; James, C.E.; Winterhalter, M. The porin and the permeating antibiotic: A selective diffusion barrier in Gram-negative bacteria. Nat. Rev. Microbiol. 2008, 6, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Molecular Basis of Bacterial Outer Membrane Permeability Revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Schweizer, H.P. Bacterial resistance to antibiotics: Active efflux and reduced uptake. Adv. Drug Deliv. Rev. 2005, 57, 1486–1513. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Lubián, A.F.; Ma, L.; Lin, R.C.; Iredell, J.R. Characterizing the role of porin mutations in susceptibility of beta lactamase producing Klebsiella pneumoniae isolates to ceftaroline and ceftaroline-avibactam. Int. J. Infect. Dis. 2020, 93, 252–257. [Google Scholar] [CrossRef]
- Doménech-Sánchez, A.; Hernández-Allés, S.; Martínez-Martínez, L.; Benedí, V.J.; Albertí, S. Identification and Characterization of a New Porin Gene of Klebsiella pneumoniae: Its Role in β-Lactam Antibiotic Resistance. J. Bacteriol. 1999, 181, 2726–2732. [Google Scholar] [CrossRef]
- Khalifa, S.M.; El-Aziz, A.M.A.; Hassan, R.; Abdelmegeed, E.S. β-lactam resistance associated with β-lactamase production and porin alteration in clinical isolates of E. coli and K. pneumoniae. PLoS ONE 2021, 16, e0251594. [Google Scholar] [CrossRef] [PubMed]
- El Amin, N.; Giske, C.G.; Jalal, S.; Keijser, B.; Kronvall, G.; Wretlind, B. Carbapenem resistance mechanisms in Pseudomonas aeruginosa: Alterations of porin OprD and efflux proteins do not fully explain resistance patterns observed in clinical isolates. Apmis 2005, 113, 187–196. [Google Scholar] [CrossRef]
- Studemeister, A.E.; Quinn, J.P. Selective imipenem resistance in Pseudomonas aeruginosa associated with diminished outer membrane permeability. Antimicrob. Agents Chemother. 1988, 32, 1267–1268. [Google Scholar] [CrossRef]
- Takada, H.; Yoshikawa, H. Essentiality and function of WalK/WalR two-component system: The past, present, and future of research. Biosci. Biotechnol. Biochem. 2018, 82, 741–751. [Google Scholar] [CrossRef]
- Peng, H.; Hu, Q.; Shang, W.; Yuan, J.; Zhang, X.; Liu, H.; Zheng, Y.; Hu, Z.; Yang, Y.; Tan, L.; et al. WalK(S221P), a naturally occurring mutation, confers vancomycin resistance in VISA strain XN108. J. Antimicrob. Chemother. 2016, 72, 1006–1013. [Google Scholar] [CrossRef]
- Howden, B.P.; McEvoy, C.R.E.; Allen, D.L.; Chua, K.; Gao, W.; Harrison, P.; Bell, J.; Coombs, G.; Bennett-Wood, V.; Porter, J.L.; et al. Evolution of Multidrug Resistance during Staphylococcus aureus Infection Involves Mutation of the Essential Two Component Regulator WalKR. PLoS Pathog. 2011, 7, e1002359. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; Hancock, R.E.W. Adaptive and Mutational Resistance: Role of Porins and Efflux Pumps in Drug Resistance. Clin. Microbiol. Rev. 2013, 26, 163. [Google Scholar] [CrossRef]
- Kornelsen, V.; Kumar, A. Update on Multidrug Resistance Efflux Pumps in Acinetobacter spp. Antimicrob. Agents Chemother. 2021, 65, e00514-21. [Google Scholar] [CrossRef] [PubMed]
- Aron, Z.; Opperman, T.J. The hydrophobic trap-the Achilles heel of RND efflux pumps. Res. Microbiol. 2018, 169, 393–400. [Google Scholar] [CrossRef]
- Srinivasan, V.B.; Rajamohan, G.; Gebreyes, W.A. Role of AbeS, a Novel Efflux Pump of the SMR Family of Transporters, in Resistance to Antimicrobial Agents in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2009, 53, 5312–5316. [Google Scholar] [CrossRef]
- Hassan, K.A.; Liu, Q.; Henderson, P.J.F.; Paulsen, I.T. Homologs of the Acinetobacter baumannii AceI Transporter Represent a New Family of Bacterial Multidrug Efflux Systems. Mbio 2015, 6, e01982-14. [Google Scholar] [CrossRef] [PubMed]
- Bolla, J.R.; Howes, A.C.; Fiorentino, F.; Robinson, C.V. Assembly and regulation of the chlorhexidine-specific efflux pump AceI. Proc. Natl. Acad. Sci. USA 2020, 117, 17011–17018. [Google Scholar] [CrossRef] [PubMed]
- Kabra, R.; Chauhan, N.; Kumar, A.; Ingale, P.; Singh, S. Efflux pumps and antimicrobial resistance: Paradoxical components in systems genomics. Prog. Biophys. Mol. Biol. 2018, 141, 15–24. [Google Scholar] [CrossRef]
- Rouquette-Loughlin, C.; Dunham, S.A.; Kuhn, M.; Balthazar, J.T.; Shafer, W.M. The NorM Efflux Pump of Neisseria gonorrhoeae and Neisseria meningitidis Recognizes Antimicrobial Cationic Compounds. J. Bacteriol. 2003, 185, 1101–1106. [Google Scholar] [CrossRef]
- Hürlimann, L.M.; Hohl, M.; Seeger, M.A. Split tasks of asymmetric nucleotide-binding sites in the heterodimeric ABC exporter EfrCD. FEBS J. 2017, 284, 1672–1687. [Google Scholar] [CrossRef]
- Greene, N.P.; Kaplan, E.; Crow, A.; Koronakis, V. Antibiotic Resistance Mediated by the MacB ABC Transporter Family: A Structural and Functional Perspective. Front. Microbiol. 2018, 9, 950. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Z.; Plésiat, P.; Nikaido, H. The Challenge of Efflux-Mediated Antibiotic Resistance in Gram-Negative Bacteria. Clin. Microbiol. Rev. 2015, 28, 337–418. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Goli, H.R.; Nahaei, M.R.; Rezaee, M.A.; Hasani, A.; Kafil, H.S.; Aghazadeh, M.; Nikbakht, M.; Khalili, Y. Role of MexAB-OprM and MexXY-OprM efflux pumps and class 1 integrons in resistance to antibiotics in burn and Intensive Care Unit isolates of Pseudomonas aeruginosa. J. Infect. Public Health 2018, 11, 364–372. [Google Scholar] [CrossRef]
- Beggs, G.A.; Brennan, R.G.; Arshad, M. MarR family proteins are important regulators of clinically relevant antibiotic resistance. Protein Sci. 2019, 29, 647–653. [Google Scholar] [CrossRef]
- Varela, M.; Kumar, S. Molecular mechanisms of bacterial resistance to antimicrobial agents. Chemotherapy 2013, 14, 522–534. [Google Scholar]
- Drawz, S.M.; Bonomo, R.A. Three Decades of β-Lactamase Inhibitors. Clin. Microbiol. Rev. 2010, 23, 160–201. [Google Scholar] [CrossRef]
- Bonomo, R.A. β-Lactamases: A Focus on Current Challenges. Cold Spring Harb. Perspect. Med. 2017, 7, a025239. [Google Scholar] [CrossRef]
- Bush, K.; Jacoby, G.A. Updated Functional Classification of β-Lactamases. Antimicrob. Agents Chemother. 2010, 54, 969–976. [Google Scholar] [CrossRef]
- Sawa, T.; Kooguchi, K.; Moriyama, K. Molecular diversity of extended-spectrum β-lactamases and carbapenemases, and antimicrobial resistance. J. Intensiv. Care 2020, 8, 13. [Google Scholar] [CrossRef]
- Bradford, P.A. Extended-Spectrum β-Lactamases in the 21st Century: Characterization, Epidemiology, and Detection of This Important Resistance Threat. Clin. Microbiol. Rev. 2001, 14, 933–951. [Google Scholar] [CrossRef] [PubMed]
- Yong, D.; Lee, K.; Yum, J.H.; Shin, H.B.; Rossolini, G.M.; Chong, Y. Imipenem-EDTA Disk Method for Differentiation of Metallo-β-Lactamase-Producing Clinical Isolates of Pseudomonas spp. and Acinetobacter spp. J. Clin. Microbiol. 2002, 40, 3798–3801. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, G.A. AmpC β-Lactamases. Clin. Microbiol. Rev. 2009, 22, 161–182. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Jacoby, G.A.; Medeiros, A.A. A functional classification scheme for beta-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 1995, 39, 1211–1233. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.A. Imipenem/Cilastatin/Relebactam: A Review in Gram-Negative Bacterial Infections. Drugs 2021, 81, 377–388. [Google Scholar] [CrossRef]
- Golkar, T.; Zieliński, M.; Berghuis, A.M. Look and Outlook on Enzyme-Mediated Macrolide Resistance. Front. Microbiol. 2018, 9, 1942. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, M.; Park, J.; Sleno, B.; Berghuis, A.M. Structural and functional insights into esterase-mediated macrolide resistance. Nat. Commun. 2021, 12, 1732. [Google Scholar] [CrossRef]
- Xing, L.; Yu, H.; Qi, J.; Jiang, P.; Sun, B.; Cui, J.; Ou, C.; Chang, W.; Hu, Q. ErmF and ereD Are Responsible for Erythromycin Resistance in Riemerella anatipestifer. PLoS ONE 2015, 10, e0131078. [Google Scholar] [CrossRef]
- Ramirez, M.S.; Tolmasky, M.E. Aminoglycoside modifying enzymes. Drug Resist. Updat. 2010, 13, 151–171. [Google Scholar] [CrossRef]
- Jaimee, G.; Halami, P.M. Emerging resistance to aminoglycosides in lactic acid bacteria of food origin—An impending menace. Appl. Microbiol. Biotechnol. 2015, 100, 1137–1151. [Google Scholar] [CrossRef]
- Disney, M.D. Studying Modification of Aminoglycoside Antibiotics by Resistance-Causing Enzymes via Microarray. Breast Cancer 2011, 808, 303–320. [Google Scholar] [CrossRef]
- Jana, S.; Deb, J.K. Molecular understanding of aminoglycoside action and resistance. Appl. Microbiol. Biotechnol. 2006, 70, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Labby, K.J.; Garneau-Tsodikova, S. Strategies to overcome the action of aminoglycoside-modifying enzymes for treating resistant bacterial infections. Futur. Med. Chem. 2013, 5, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.S.; Spencer, J.P. Aminoglycosides: A practical review. Am. Fam. Physician 1998, 58, 1811. [Google Scholar] [PubMed]
- Yang, W.; Moore, I.F.; Koteva, K.P.; Bareich, D.C.; Hughes, D.W.; Wright, G.D. TetX Is a Flavin-dependent Monooxygenase Conferring Resistance to Tetracycline Antibiotics. J. Biol. Chem. 2004, 279, 52346–52352. [Google Scholar] [CrossRef]
- Markley, J.L.; Wencewicz, T.A. Tetracycline-Inactivating Enzymes. Front. Microbiol. 2018, 9, 1058. [Google Scholar] [CrossRef]
- Ye, J.; Chen, X. Current Promising Strategies against Antibiotic-Resistant Bacterial Infections. Antibiotics 2022, 12, 67. [Google Scholar] [CrossRef]
- Isaev, A.B.; Musharova, O.S.; Severinov, K.V. Microbial Arsenal of Antiviral Defenses—Part I. Biochemistry 2021, 86, 319–337. [Google Scholar] [CrossRef]
- Ryan, E.M.; Alkawareek, M.Y.; Donnelly, R.F.; Gilmore, B.F. Synergistic phage-antibiotic combinations for the control of Escherichia coli biofilms in vitro. FEMS Immunol. Med. Microbiol. 2012, 65, 395–398. [Google Scholar] [CrossRef]
- Comeau, A.M.; Tétart, F.; Trojet, S.N.; Prère, M.-F.; Krisch, H.M. Phage-Antibiotic Synergy (PAS): β-Lactam and Quinolone Antibiotics Stimulate Virulent Phage Growth. PLoS ONE 2007, 2, e799. [Google Scholar] [CrossRef]
- Tagliaferri, T.L.; Jansen, M.; Horz, H.-P. Fighting Pathogenic Bacteria on Two Fronts: Phages and Antibiotics as Combined Strategy. Front. Cell. Infect. Microbiol. 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Yacoby, I.; Shamis, M.; Bar, H.; Shabat, D.; Benhar, I. Targeting Antibacterial Agents by Using Drug-Carrying Filamentous Bacteriophages. Antimicrob. Agents Chemother. 2006, 50, 2087–2097. [Google Scholar] [CrossRef] [PubMed]
- Vaks, L.; Benhar, I. In vivo characteristics of targeted drug-carrying filamentous bacteriophage nanomedicines. J. Nanobiotechnol. 2011, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Panwar, R.B.; Sequeira, R.P.; Clarke, T.B. Microbiota-mediated protection against antibiotic-resistant pathogens. Genes Immun. 2021, 22, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal. Transduct. Target 2022, 7, 022–00974. [Google Scholar] [CrossRef]
- Matzaras, R.; Nikopoulou, A.; Protonotariou, E.; Christaki, E. Gut Microbiota Modulation and Prevention of Dysbiosis as an Alternative Approach to Antimicrobial Resistance: A Narrative Review. Yale J. Biol. Med. 2022, 95, 479–494. [Google Scholar]
- Garcia-Gutierrez, E.; Mayer, M.J.; Cotter, P.D.; Narbad, A. Gut microbiota as a source of novel antimicrobials. Gut Microbes 2018, 10, 1455790. [Google Scholar] [CrossRef]
- Forslund, K.; Sunagawa, S.; Kultima, J.R.; Mende, D.R.; Arumugam, M.; Typas, A.; Bork, P. Country-specific antibiotic use practices impact the human gut resistome. Genome Res. 2013, 23, 1163–1169. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, X.; Qin, J.; Lu, N.; Cheng, G.; Wu, N.; Pan, Y.; Li, J.; Zhu, L.; Wang, X.; et al. Metagenome-wide analysis of antibiotic resistance genes in a large cohort of human gut microbiota. Nat. Commun. 2013, 4, 2151. [Google Scholar] [CrossRef]
- Stracy, M.; Snitser, O.; Yelin, I.; Amer, Y.; Parizade, M.; Katz, R.; Rimler, G.; Wolf, T.; Herzel, E.; Koren, G.; et al. Minimizing treatment-induced emergence of antibiotic resistance in bacterial infections. Science 2022, 375, 889–894. [Google Scholar] [CrossRef]
- Zipperer, A.; Konnerth, M.C.; Laux, C.; Berscheid, A.; Janek, D.; Weidenmaier, C.; Burian, M.; Schilling, N.A.; Slavetinsky, C.; Marschal, M.; et al. Human commensals producing a novel antibiotic impair pathogen colonization. Nature 2016, 535, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, N.; Jha, D.; Roy, I.; Kumar, P.; Gaurav, S.S.; Marimuthu, K.; Ng, O.-T.; Lakshminarayanan, R.; Verma, N.K.; Gautam, H.K. Nanobiotics against antimicrobial resistance: Harnessing the power of nanoscale materials and technologies. J. Nanobiotechnol. 2022, 20, 375. [Google Scholar] [CrossRef] [PubMed]
- Rosini, R.; Nicchi, S.; Pizza, M.; Rappuoli, R. Vaccines Against Antimicrobial Resistance. Front. Immunol. 2020, 11, 01048. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Srinivas, P.; Pogue, J.M. Cefiderocol: A Novel Agent for the Management of Multidrug-Resistant Gram-Negative Organisms. Infect. Dis. Ther. 2020, 9, 17–40. [Google Scholar] [CrossRef] [PubMed]
- Karlowsky, J.A.; Steenbergen, J.; Zhanel, G.G. Microbiology and Preclinical Review of Omadacycline. Clin. Infect. Dis. 2019, 69, S6–S15. [Google Scholar] [CrossRef]
- Cohen, D.T.; Zhang, C.; Fadzen, C.M.; Mijalis, A.J.; Hie, L.; Johnson, K.D.; Shriver, Z.; Plante, O.; Miller, S.J.; Buchwald, S.L.; et al. A chemoselective strategy for late-stage functionalization of complex small molecules with polypeptides and proteins. Nat. Chem. 2018, 11, 78–85. [Google Scholar] [CrossRef]
- Newman, D. Old and Modern Antibiotic Structures with Potential for Today’s Infections. ADMET DMPK 2022, 10, 131–146. [Google Scholar] [CrossRef]
- Flint, A.J.; Davis, A.P. Vancomycin mimicry: Towards new supramolecular antibiotics. Org. Biomol. Chem. 2022, 20, 7694–7712. [Google Scholar] [CrossRef]
- Figazzolo, C.; Bonhomme, F.; Saidjalolov, S.; Ethève-Quelquejeu, M.; Hollenstein, M. Enzymatic Synthesis of Vancomycin-Modified DNA. Molecules 2022, 27, 8927. [Google Scholar] [CrossRef]
- Yang, J.H.; Wright, S.N.; Hamblin, M.; McCloskey, D.; Alcantar, M.A.; Schrübbers, L.; Lopatkin, A.J.; Satish, S.; Nili, A.; Palsson, B.O.; et al. A White-Box Machine Learning Approach for Revealing Antibiotic Mechanisms of Action. Cell 2019, 177, 1649–1661.e9. [Google Scholar] [CrossRef]
- Aksamit, T.; Wu, J.; Hassan, M.; Achter, E.; Chatterjee, A. Impact of initiation of amikacin liposome inhalation suspension on hospitalizations and other healthcare resource utilization measures: A retrospective cohort study in real-world settings. BMC Pulm. Med. 2022, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-antibacterial-drug-treat-serious-lung-disease-using-novel-pathway-spur-innovation (accessed on 1 February 2023).
- Cipolla, D.; Blanchard, J.; Gonda, I. Development of Liposomal Ciprofloxacin to Treat Lung Infections. Pharmaceutics 2016, 8, 6. [Google Scholar] [CrossRef]
- Micoli, F.; Bagnoli, F.; Rappuoli, R.; Serruto, D. The role of vaccines in combatting antimicrobial resistance. Nat. Rev. Genet. 2021, 19, 287–302. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Q.; Li, W.; Yuan, M.; Zhou, J.; Hua, L.; Chen, Y.; Ye, C.; Ma, Y. Development of novel nanoantibiotics using an outer membrane vesicle-based drug efflux mechanism. J. Control. Release 2019, 317, 1–22. [Google Scholar] [CrossRef]
- Lee, N.-Y.; Ko, W.-C.; Hsueh, P.-R. Nanoparticles in the Treatment of Infections Caused by Multidrug-Resistant Organisms. Front. Pharmacol. 2019, 10, 1153. [Google Scholar] [CrossRef]
- Galbadage, T.; Liu, D.; Alemany, L.B.; Pal, R.; Tour, J.M.; Gunasekera, R.S.; Cirillo, J.D. Molecular Nanomachines Disrupt Bacterial Cell Wall, Increasing Sensitivity of Extensively Drug-Resistant Klebsiella pneumoniae to Meropenem. ACS Nano 2019, 13, 14377–14387. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Deng, W.; Mo, M.; Luo, D.; Liu, H.; Jiang, Y.; Chen, W.; Xu, C. Stimuli-Responsive Nanoplatform-Assisted Photodynamic Therapy Against Bacterial Infections. Front. Med. 2021, 8, 729300. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-H.; Yu, C.-C.; Chang, K.-C.; Tseng, Y.-H.; Li, P.-J.; Hsia, S.-M.; Chiu, K.-C.; Shieh, T.-M. Synergistic Effect of Combination of a Temoporfin-Based Photodynamic Therapy with Potassium Iodide or Antibacterial Agents on Oral Disease Pathogens In Vitro. Pharmaceuticals 2022, 15, 488. [Google Scholar] [CrossRef]
- Bouley, R.; Kumarasiri, M.; Peng, Z.; Otero, L.H.; Song, W.; Suckow, M.A.; Schroeder, V.A.; Wolter, W.R.; Lastochkin, E.; Antunes, N.T.; et al. Discovery of Antibiotic (E)-3-(3-Carboxyphenyl)-2-(4-cyanostyryl)quinazolin-4(3H)-one. J. Am. Chem. Soc. 2015, 137, 1738–1741. [Google Scholar] [CrossRef]
- Janardhanan, J.; Bouley, R.; Martínez-Caballero, S.; Peng, Z.; Batuecas-Mordillo, M.; Meisel, J.E.; Ding, D.; Schroeder, V.A.; Wolter, W.R.; Mahasenan, K.V.; et al. The Quinazolinone Allosteric Inhibitor of PBP 2a Synergizes with Piperacillin and Tazobactam against Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2019, 63, e02637-18. [Google Scholar] [CrossRef]
- Perry, J.; Waglechner, N.; Wright, G. The Prehistory of Antibiotic Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025197. [Google Scholar] [CrossRef] [PubMed]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- Plackett, B. Why big pharma has abandoned antibiotics. Nature 2020, 586, S50–S52. [Google Scholar] [CrossRef]
- Kållberg, C.; Blix, H.S.; Laxminarayan, R. Challenges in Antibiotic R&D Calling for a Global Strategy Considering Both Short- and Long-Term Solutions. ACS Infect. Dis. 2019, 5, 1265–1268. [Google Scholar] [CrossRef] [PubMed]
- Klug, D.M.; Idiris, F.I.M.; Blaskovich, M.A.T.; von Delft, F.; Dowson, C.G.; Kirchhelle, C.; Roberts, A.P.; Singer, A.C.; Todd, M.H. There is no market for new antibiotics: This allows an open approach to research and development. Wellcome Open Res. 2021, 6, 146. [Google Scholar] [CrossRef]
- Available online: https://www.amractionfund.com (accessed on 10 February 2023).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baran, A.; Kwiatkowska, A.; Potocki, L. Antibiotics and Bacterial Resistance—A Short Story of an Endless Arms Race. Int. J. Mol. Sci. 2023, 24, 5777. https://doi.org/10.3390/ijms24065777
Baran A, Kwiatkowska A, Potocki L. Antibiotics and Bacterial Resistance—A Short Story of an Endless Arms Race. International Journal of Molecular Sciences. 2023; 24(6):5777. https://doi.org/10.3390/ijms24065777
Chicago/Turabian StyleBaran, Aleksandra, Aleksandra Kwiatkowska, and Leszek Potocki. 2023. "Antibiotics and Bacterial Resistance—A Short Story of an Endless Arms Race" International Journal of Molecular Sciences 24, no. 6: 5777. https://doi.org/10.3390/ijms24065777
APA StyleBaran, A., Kwiatkowska, A., & Potocki, L. (2023). Antibiotics and Bacterial Resistance—A Short Story of an Endless Arms Race. International Journal of Molecular Sciences, 24(6), 5777. https://doi.org/10.3390/ijms24065777






