The Emerging Importance of Cirsimaritin in Type 2 Diabetes Treatment
Abstract
1. Introduction
2. Results
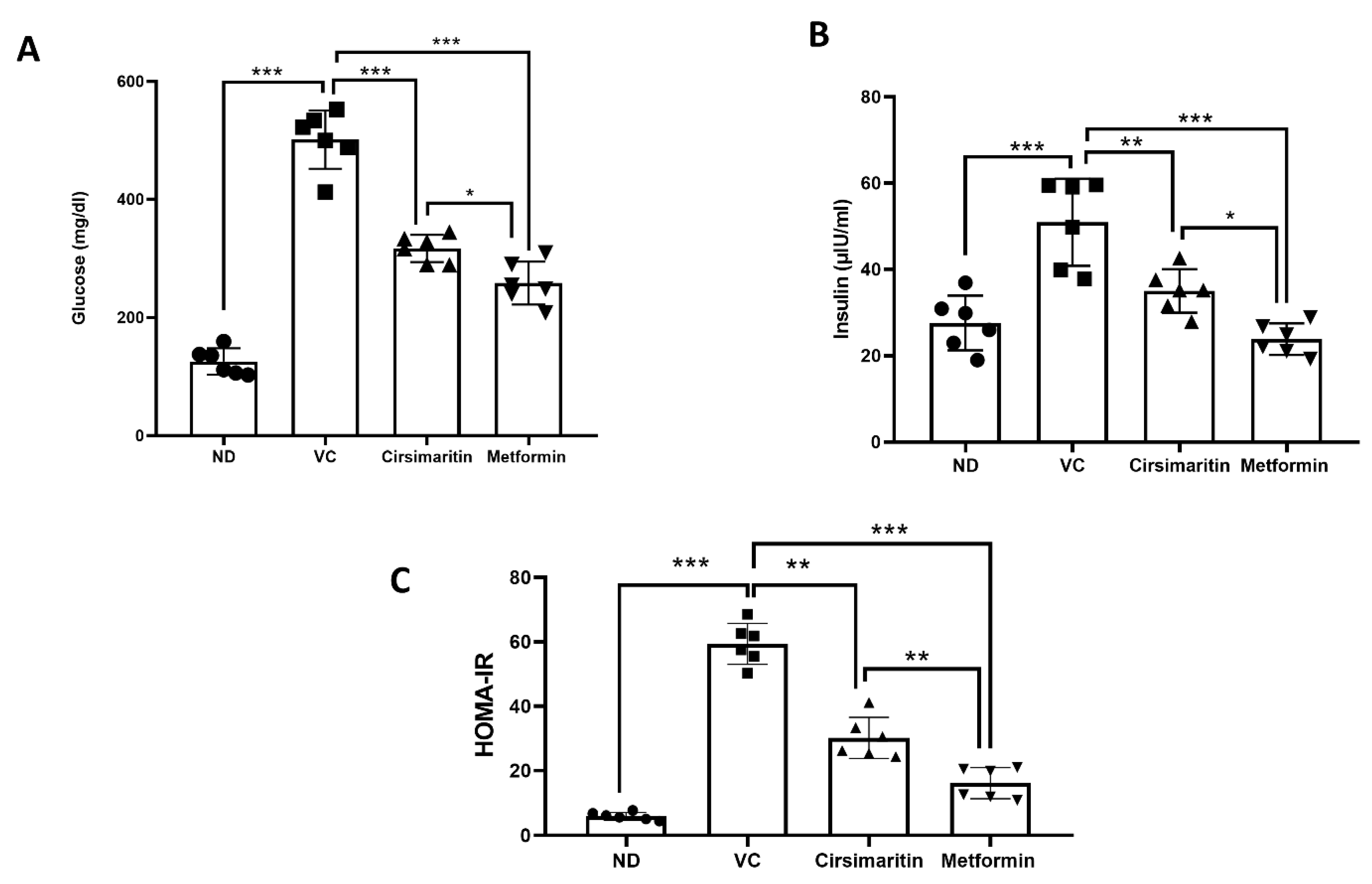
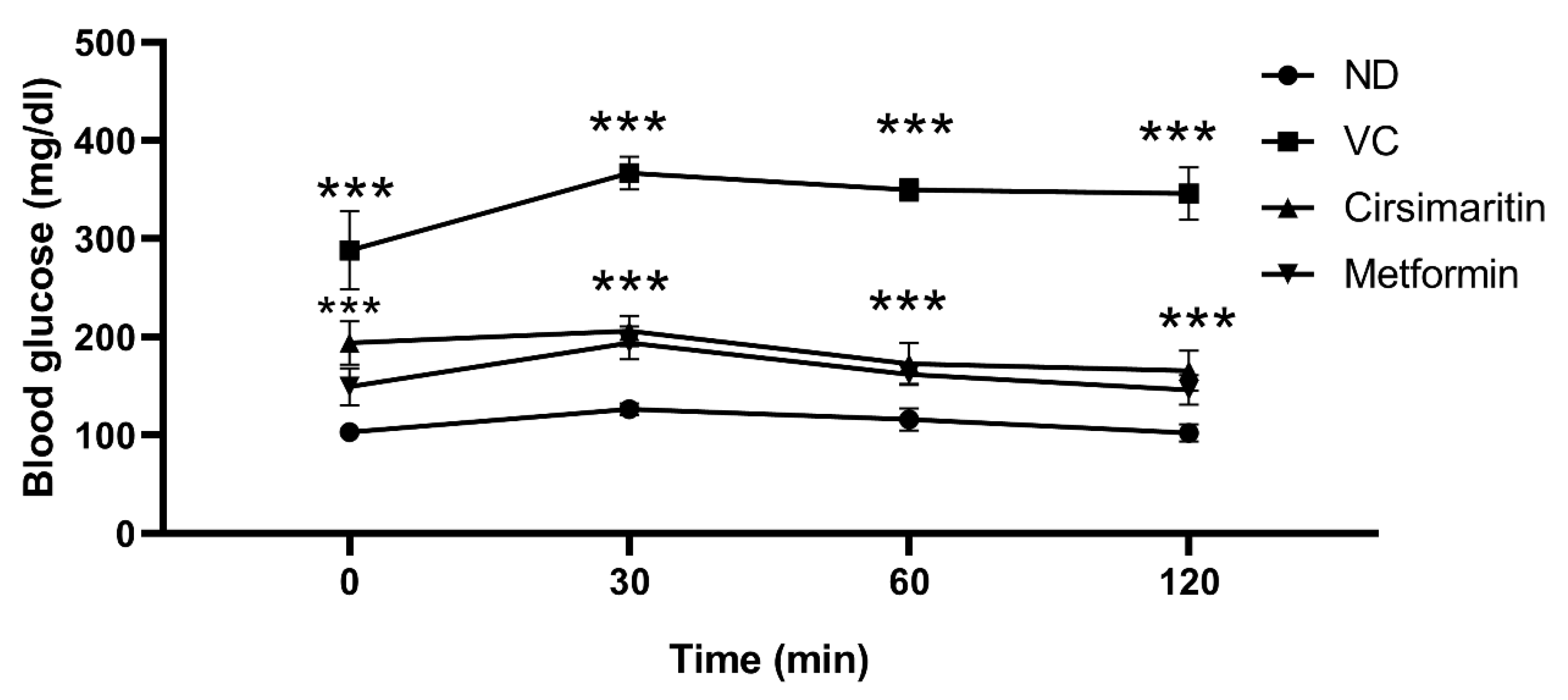
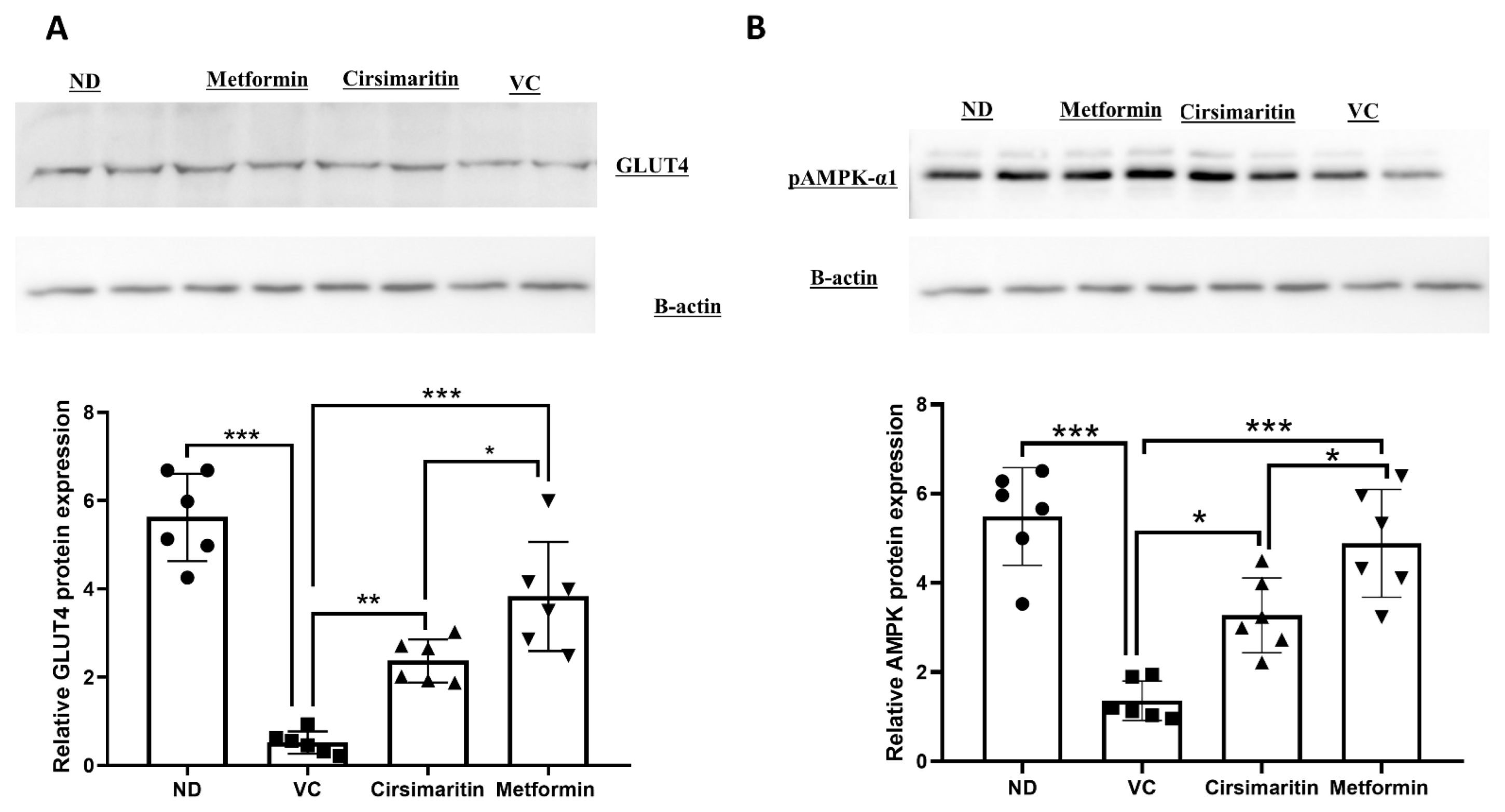
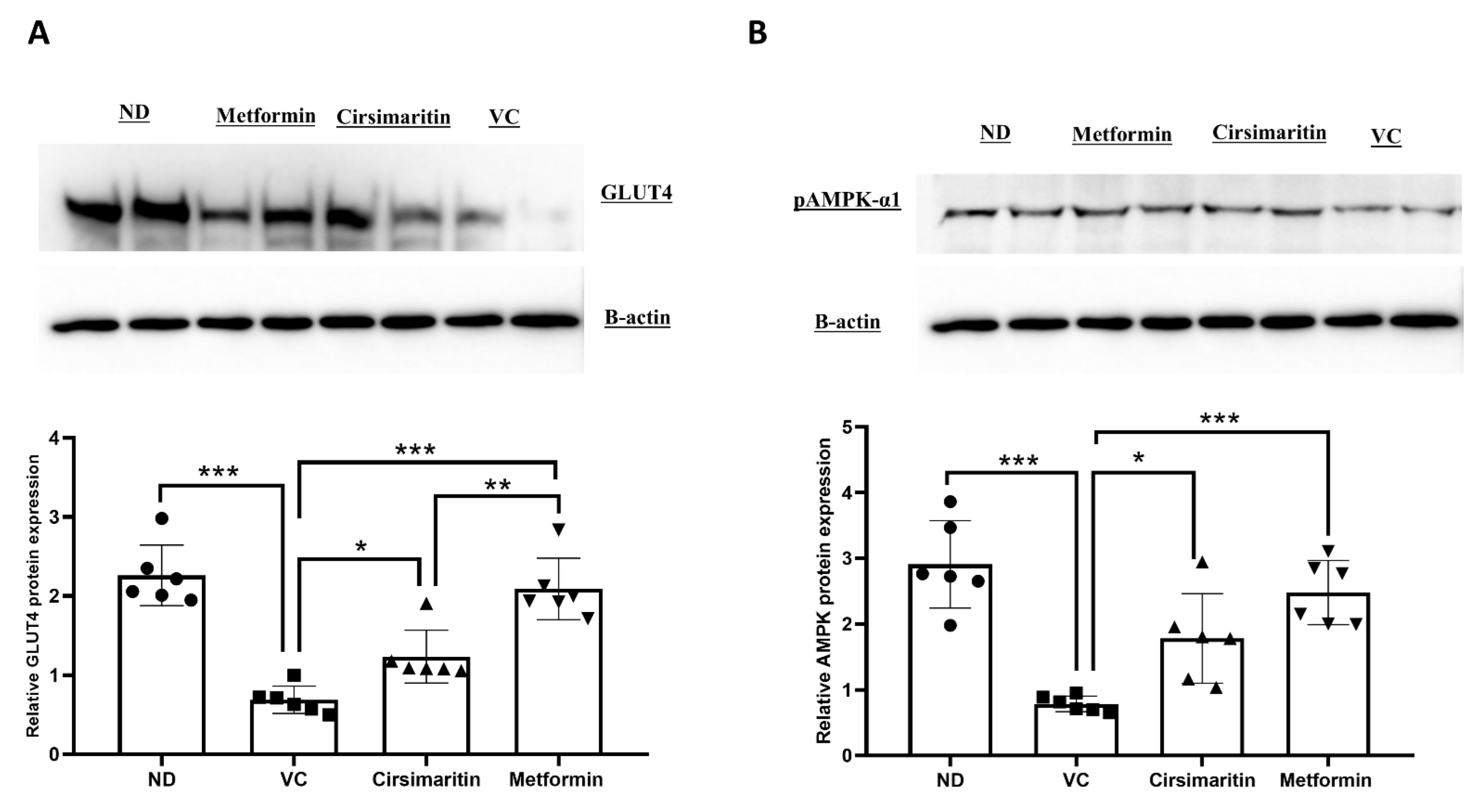
2.1. The Hypoglycemic Effect of Cirsimaritin
2.2. The Effect of Cirsimaritin on the Lipid Profile
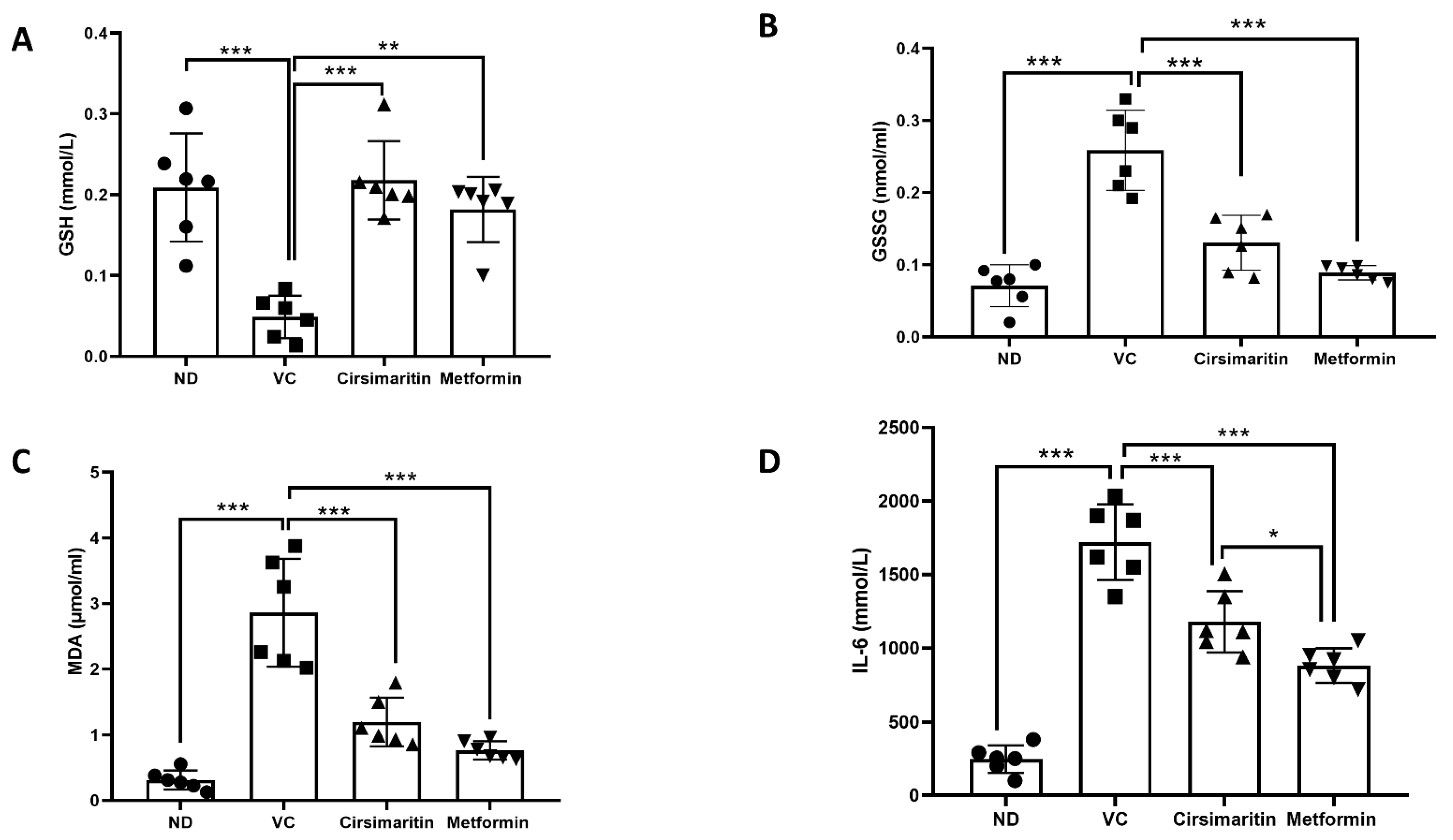
2.3. The Effects of Cirsimaritin on GSH, GSSG, MDA, and IL-6
3. Discussion
4. Materials and Methods
4.1. The Induction of T2D and Experimental Design
4.2. Biochemical Investigations
4.2.1. Measurements of Serum Glucose, Insulin, and Lipids in Rat Serum
4.2.2. Homeostasis Model Assessment of Insulin Resistance (HOMA-IR)
4.2.3. Intraperitoneal Glucose Tolerance Test
4.2.4. Measurement of Serum Glutathione (GSH), Oxidized Glutathione (GSSG), Malondialdehyde (MDA), and IL-6 Serum Concentrations
4.3. Western Blotting
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rehman, K.; Akash, M.S.H. Mechanism of Generation of Oxidative Stress and Pathophysiology of Type 2 Diabetes Mellitus: How Are They Interlinked? J. Cell. Biochem. 2017, 118, 3577–3585. [Google Scholar] [CrossRef] [PubMed]
- Stumvoll, M.; Goldstein, B.J.; Van Haeften, T.W. Type 2 Diabetes: Principles of Pathogenesis and Therapy. Lancet 2005, 365, 1333–1346. [Google Scholar] [CrossRef]
- Badawi, A.; Klip, A.; Haddad, P.; Cole, D.E.; Bailo, G.; El-Sohemy, A.; Karmali, M. Type 2 Diabetes Mellitus and Inflammation: Prospects for Biomarkers of Risk and Nutritional Intervention. Diabetes Metab. Syndr. Obes. Targets Ther. 2010, 3, 173–186. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.G.M.M.; Shaw, J. Global and Societal Implications of the Diabetes Epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M. Treating Type 2 Diabetes—Today’s Targets, Tomorrow’s Goals. Diabetes Obes. Metab. 2001, 3, 3–10. [Google Scholar] [CrossRef]
- Pickup, J.C. Inflammation and Activated Innate Immunity in the Pathogenesis of Type 2 Diabetes. Diabetes Care 2004, 27, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, I.; Pascot, A.; Prud’homme, D.; Alméras, N.; Bogaty, P.; Nadeau, A.; Bergeron, J.; Després, J.P. Elevated C-Reactive Protein. Arter. Thromb. Vasc. Biol. 2001, 21, 961–967. [Google Scholar] [CrossRef]
- Halliwell, B. The Wanderings of a Free Radical. Free Radic. Biol. Med. 2009, 46, 531–542. [Google Scholar] [CrossRef]
- Lee, Y.J.; Suh, K.S.; Choi, M.C.; Chon, S.; Oh, S.; Woo, J.T.; Kim, S.W.; Kim, J.W.; Kim, Y.S. Kaempferol Protects HIT-T15 Pancreatic Beta Cells from 2-Deoxy-D-Ribose-Induced Oxidative Damage. Phytother. Res. 2010, 24, 419–423. [Google Scholar] [CrossRef]
- Eriksson, J.W. Metabolic Stress in Insulin’s Target Cells Leads to ROS Accumulation—A Hypothetical Common Pathway Causing Insulin Resistance. FEBS Lett. 2007, 581, 3734–3742. [Google Scholar] [CrossRef] [PubMed]
- Kawahito, S.; Kitahata, H.; Oshita, S. Problems Associated with Glucose Toxicity: Role of Hyperglycemia-Induced Oxidative Stress. World J. Gastroenterol. 2009, 15, 4137–4142. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Reid, M.; Sandra Butler-Browne, G.; Llanos, P.; Palomero, J. Reactive Oxygen and Nitrogen Species (RONS) and Cytokines—Myokines Involved in Glucose Uptake and Insulin Resistance in Skeletal Muscle. Cells 2022, 11, 4008. [Google Scholar] [CrossRef]
- Chadt, A.; Al-Hasani, H. Glucose Transporters in Adipose Tissue, Liver, and Skeletal Muscle in Metabolic Health and Disease. Pflug. Arch. Eur. J. Physiol. 2020, 472, 1273–1298. [Google Scholar] [CrossRef] [PubMed]
- Alqudah, A.; Qnais, E.Y.; Wedyan, M.A.; Altaber, S.; Abudalo, R.; Gammoh, O.; Alkhateeb, H.; Bataineh, S.; Athamneh, R.Y.; Oqal, M.; et al. New Treatment for Type 2 Diabetes Mellitus Using a Novel Bipyrazole Compound. Cells 2023, 12, 267. [Google Scholar] [CrossRef]
- Alqudah, A.; Qnais, E.Y.; Wedyan, M.A.; Altaber, S.; Bseiso, Y.; Oqal, M.; AbuDalo, R.; Alrosan, K.; Alrosan, A.Z.; Bani Melhim, S.; et al. Isorhamnetin Reduces Glucose Level, Inflammation, and Oxidative Stress in High-Fat Diet/Streptozotocin Diabetic Mice Model. Molecules 2023, 28, 502. [Google Scholar] [CrossRef]
- Vlavcheski, F.; Den Hartogh, D.J.; Giacca, A.; Tsiani, E. Amelioration of High-Insulin-Induced Skeletal Muscle Cell Insulin Resistance by Resveratrol Is Linked to Activation of AMPK and Restoration of GLUT4 Translocation. Nutrients 2020, 12, 914. [Google Scholar] [CrossRef]
- Giacoman-Martínez, A.; Alarcón-Aguilar, F.J.; Zamilpa, A.; Huang, F.; Romero-Nava, R.; Román-Ramos, R.; Almanza-Pérez, J.C. α-Amyrin Induces Glut4 Translocation Mediated by AMPK and PPARδ/γ in C2c12 Myoblasts. Can. J. Physiol. Pharmacol. 2021, 99, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Dayarathne, L.A.; Ranaweera, S.S.; Natraj, P.; Rajan, P.; Lee, Y.J.; Han, C.H. The Effects of Naringenin and Naringin on the Glucose Uptake and AMPK Phosphorylation in High Glucose Treated HepG2 Cells. J. Vet. Sci. 2021, 22, e92. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, H.Y.; Shibamoto, T.; Jang, T.S.; Lee, S.C.; Shim, J.S.; Hahm, D.H.; Lee, H.J.; Lee, S.; Kang, K.S. Beneficial Effects of a Medicinal Herb, Cirsium japonicum Var. Maackii, Extract and Its Major Component, Cirsimaritin on Breast Cancer Metastasis in MDA-MB-231 Breast Cancer Cells. Bioorg. Med. Chem. Lett. 2017, 27, 3968–3973. [Google Scholar] [CrossRef]
- Pathak, G.; Singh, S.; Kumari, P.; Raza, W.; Hussain, Y.; Meena, A. Cirsimaritin, a Lung Squamous Carcinoma Cells (NCIH-520) Proliferation Inhibitor. J. Biomol. Struct. Dyn. 2020, 39, 3312–3323. [Google Scholar] [CrossRef]
- Rijo, P.; Simões, M.F.; Duarte, A.; Rodríguez, B. Isopimarane Diterpenoids from Aeollanthus rydingianus and Their Antimicrobial Activity. Phytochemistry 2009, 70, 1161–1165. [Google Scholar] [CrossRef]
- Ibañez, E.; Kubátová, A.; Señoráns, F.J.; Cavero, S.; Reglero, U.; Hawthorne, S.B. Subcritical Water Extraction of Antioxidant Compounds from Rosemary Plants. J. Agric. Food Chem. 2002, 51, 375–382. [Google Scholar] [CrossRef]
- Jipa, S.; Zaharescu, T.; Kappel, W.; Dǎneţ, A.F.; Popa, C.V.; Bumbac, M.; Gorghiu, L.M.; Maris, A.M. The Effects of γ-Irradiation on the Antioxidant Activity of Rosemary Extract. Optoelectron. Adv. Mater. Rapid Commun. 2009, 3, 1315–1320. [Google Scholar]
- Shin, M.S.; Park, J.Y.; Lee, J.; Yoo, H.H.; Hahm, D.H.; Lee, S.C.; Lee, S.; Hwang, G.S.; Jung, K.; Kang, K.S. Anti-Inflammatory Effects and Corresponding Mechanisms of Cirsimaritin Extracted from Cirsium japonicum Var. Maackii Maxim. Bioorg. Med. Chem. Lett. 2017, 27, 3076–3080. [Google Scholar] [CrossRef]
- Stefkov, G.; Kulevanova, S.; Miova, B.; Dinevska-Kjovkarovska, S.; Mølgaard, P.; Jäger, A.K.; Josefsen, K. Effects of Teucrium polium Spp. Capitatum Flavonoids on the Lipid and Carbohydrate Metabolism in Rats. Pharm. Biol. 2011, 49, 885–892. [Google Scholar] [CrossRef]
- Bower, A.M.; Real Hernandez, L.M.; Berhow, M.A.; De Mejia, E.G. Bioactive Compounds from Culinary Herbs Inhibit a Molecular Target for Type 2 Diabetes Management, Dipeptidyl Peptidase IV. J. Agric. Food Chem. 2014, 62, 6147–6158. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.H.; Lo, Y.M.; Chang, W.C.; Huang, D.W.; Wu, J.S.B.; Jhang, Y.Y.; Huang, W.C.; Ko, C.Y.; Shen, S.C. Identification of Bioactive Components from Ruellia tuberosa L. On Improving Glucose Uptake in TNF-α-Induced Insulin-Resistant Mouse FL83B Hepatocytes. Evid.-Based Complement. Altern. Med. 2020, 2020, 6644253. [Google Scholar] [CrossRef] [PubMed]
- Newell-McGloughlin, M. Nutritionally Improved Agricultural Crops. Plant Physiol. 2008, 147, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Dietary Bioactive Compounds and Their Health Implications. J. Food Sci. 2013, 78, A18–A25. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Michel, J.; Abd Rani, N.Z.; Husain, K. A Review on the Potential Use of Medicinal Plants from Asteraceae and Lamiaceae Plant Family in Cardiovascular Diseases. Front. Pharmacol. 2020, 11, 852. [Google Scholar] [CrossRef]
- Gezici, S.; Şekeroğlu, N. Current Perspectives in the Application of Medicinal Plants against Cancer: Novel Therapeutic Agents. Anticancer Agents Med. Chem. 2019, 19, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Nazarian-Samani, Z.; Sewell, R.D.E.; Lorigooini, Z.; Rafieian-Kopaei, M. Medicinal Plants with Multiple Effects on Diabetes Mellitus and Its Complications: A Systematic Review. Curr. Diab. Rep. 2018, 18, 72. [Google Scholar] [CrossRef] [PubMed]
- Tasneem, S.; Liu, B.; Li, B.; Choudhary, M.I.; Wang, W. Molecular Pharmacology of Inflammation: Medicinal Plants as Anti-Inflammatory Agents. Pharmacol. Res. 2019, 139, 126–140. [Google Scholar] [CrossRef]
- Dalmagro, A.P.; Camargo, A.; Zeni, A.L.B. Morus nigra and Its Major Phenolic, Syringic Acid, Have Antidepressant-like and Neuroprotective Effects in Mice. Metab. Brain Dis. 2017, 32, 1963–1973. [Google Scholar] [CrossRef]
- Dantas, D.M.M.; Cahu, T.B.; Oliveira, C.Y.B.; Abadie-Guedes, R.; Roberto, N.A.; Santana, W.M.; Galvez, A.O.; Guedes, R.C.A.; Bezerra, R.S. Chlorella vulgaris Functional Alcoholic Beverage: Effect on Propagation of Cortical Spreading Depression and Functional Properties. PLoS ONE 2021, 16, e0255996. [Google Scholar] [CrossRef]
- Dutta, T.; Paul, A.; Majumder, M.; Sultan, R.A.; Emran, T. Bin Pharmacological Evidence for the Use of Cissus Assamica as a Medicinal Plant in the Management of Pain and Pyrexia. Biochem. Biophys. Rep. 2020, 21, 100715. [Google Scholar] [CrossRef]
- Fowler, M.J. Microvascular and Macrovascular Complications of Diabetes. Clin. Diabetes 2008, 26, 77–82. [Google Scholar] [CrossRef]
- Herman, R.; Kravos, N.A.; Jensterle, M.; Janež, A.; Dolžan, V. Metformin and Insulin Resistance: A Review of the Underlying Mechanisms behind Changes in GLUT4-Mediated Glucose Transport. Int. J. Mol. Sci. 2022, 23, 1264. [Google Scholar] [CrossRef]
- Garneau, L.; Aguer, C. Role of Myokines in the Development of Skeletal Muscle Insulin Resistance and Related Metabolic Defects in Type 2 Diabetes. Diabetes Metab. 2019, 45, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose Tissue and Insulin Resistance in Obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef]
- Leguisamo, N.M.; Lehnen, A.M.; Machado, U.F.; Okamoto, M.M.; Markoski, M.M.; Pinto, G.H.; Schaan, B.D. GLUT4 Content Decreases along with Insulin Resistance and High Levels of Inflammatory Markers in Rats with Metabolic Syndrome. Cardiovasc. Diabetol. 2012, 11, 100. [Google Scholar] [CrossRef]
- Seböková, E.; Klimes, I.; Moss, R.; Mitková, A.; Wiersma, M.; Bohov, P. Decreased Glucose Transporter Protein (GLUT4) in Skeletal Muscle of Hypertriglyceridaemic Insulin-Resistant Rat. Physiol. Res. 1995, 44, 87–92. [Google Scholar]
- Napoli, R.; Hirshman, M.F.; Horton, E.S. Mechanisms and Time Course of Impaired Skeletal Muscle Glucose Transport Activity in Streptozocin Diabetic Rats. J. Clin. Investig. 1995, 96, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.D.; Peroni, O.; Kim, J.K.; Kim, Y.B.; Boss, O.; Hadro, E.; Minnemann, T.; Shulman, G.I.; Kahn, B.B. Adipose-Selective Targeting of the GLUT4 Gene Impairs Insulin Action in Muscle and Liver. Nature 2001, 409, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Viollet, B.; Lantier, L.; Devin-Leclerc, J.; Hebrard, S.; Amouyal, C.; Mounier, R.; Foretz, M.; Andreelli, F. Targeting the AMPK Pathway for the Treatment of Type 2 Diabetes. Front. Biosci. 2009, 9, 3380–3400. [Google Scholar] [CrossRef] [PubMed]
- Asrafuzzaman, M.; Rahman, M.M.; Mandal, M.; Marjuque, M.; Bhowmik, A.; Rokeya, B.; Hassan, Z.; Faruque, M.O. Oyster Mushroom Functions as an Anti-Hyperglycaemic through Phosphorylation of AMPK and Increased Expression of GLUT4 in Type 2 Diabetic Model Rats. J. Taibah Univ. Med. Sci. 2018, 13, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Thorens, B. GLUT2, Glucose Sensing and Glucose Homeostasis. Diabetologia 2015, 58, 221–232. [Google Scholar] [CrossRef]
- Eid, S.; Sas, K.M.; Abcouwer, S.F.; Feldman, E.L.; Gardner, T.W.; Pennathur, S.; Fort, P.E. New Insights into the Mechanisms of Diabetic Complications: Role of Lipids and Lipid Metabolism. Diabetologia 2019, 62, 1539–1549. [Google Scholar] [CrossRef]
- Athyros, V.G.; Doumas, M.; Imprialos, K.P.; Stavropoulos, K.; Georgianou, E.; Katsimardou, A.; Karagiannis, A. Diabetes and Lipid Metabolism. Hormones 2018, 17, 61–67. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Type 2 Diabetes Mellitus, Oxidative Stress and Inflammation: Examining the Links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar]
- Chikezie, P.C.; Ojiako, O.A.; Ogbuji, A.C. Oxidative Stress in Diabetes Mellitus. Int. J. Biol. Chem. 2015, 9, 92–109. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and Artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef]
- Benali, T.; Jaouadi, I.; Ghchime, R.; El Omari, N.; Harboul, K.; Hammani, K.; Rebezov, M.; Shariati, M.A.; Mubarak, M.S.; Simal-Gandara, J.; et al. The Current State of Knowledge in Biological Properties of Cirsimaritin. Antioxidants 2022, 11, 1842. [Google Scholar] [CrossRef] [PubMed]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a Link between Obesity, Metabolic Syndrome and Type 2 Diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghaithi, F.; El-Ridi, M.R.; Adeghate, E.; Amiri, M.H. Biochemical Effects of Citrullus colocynthis in Normal and Diabetic Rats. Mol. Cell. Biochem. 2004, 261, 143–149. [Google Scholar] [CrossRef] [PubMed]
- De MagalhÃes, D.A.; Kume, W.T.; Correia, F.S.; Queiroz, T.S.; Allebrandt Neto, E.W.; Dos Santos, M.P.; Kawashita, N.H.; De França, S.A. High-Fat Diet and Streptozotocin in the Induction of Type 2 Diabetes Mellitus: A New Proposal. An. Acad. Bras. Cienc. 2019, 91, e20180314. [Google Scholar] [CrossRef]
- Al-Trad, B.; Alkhateeb, H.; Alsmadi, W.; Al-Zoubi, M. Eugenol Ameliorates Insulin Resistance, Oxidative Stress and Inflammation in High Fat-Diet/Streptozotocin-Induced Diabetic Rat. Life Sci. 2019, 216, 183–188. [Google Scholar] [CrossRef]
- Abdelhalim, A.; Karim, N.; Chebib, M.; Aburjai, T.; Khan, I.; Johnston, G.A.R.; Hanrahan, J.R. Antidepressant, Anxiolytic and Antinociceptive Activities of Constituents from Rosmarinus Officinalis. J. Pharm. Pharm. Sci. 2015, 18, 448–459. [Google Scholar] [CrossRef]
- Yoon, H.; Jeon, D.J.; Park, C.E.; You, H.S.; Moon, A.E. Relationship between Homeostasis Model Assessment of Insulin Resistance and Beta Cell Function and Serum 25-Hydroxyvitamin D in Non-Diabetic Korean Adults. J. Clin. Biochem. Nutr. 2016, 59, 139–144. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqudah, A.; Athamneh, R.Y.; Qnais, E.; Gammoh, O.; Oqal, M.; AbuDalo, R.; Alshaikh, H.A.; AL-Hashimi, N.; Alqudah, M. The Emerging Importance of Cirsimaritin in Type 2 Diabetes Treatment. Int. J. Mol. Sci. 2023, 24, 5749. https://doi.org/10.3390/ijms24065749
Alqudah A, Athamneh RY, Qnais E, Gammoh O, Oqal M, AbuDalo R, Alshaikh HA, AL-Hashimi N, Alqudah M. The Emerging Importance of Cirsimaritin in Type 2 Diabetes Treatment. International Journal of Molecular Sciences. 2023; 24(6):5749. https://doi.org/10.3390/ijms24065749
Chicago/Turabian StyleAlqudah, Abdelrahim, Rabaa Y. Athamneh, Esam Qnais, Omar Gammoh, Muna Oqal, Rawan AbuDalo, Hanan Abu Alshaikh, Nabil AL-Hashimi, and Mohammad Alqudah. 2023. "The Emerging Importance of Cirsimaritin in Type 2 Diabetes Treatment" International Journal of Molecular Sciences 24, no. 6: 5749. https://doi.org/10.3390/ijms24065749
APA StyleAlqudah, A., Athamneh, R. Y., Qnais, E., Gammoh, O., Oqal, M., AbuDalo, R., Alshaikh, H. A., AL-Hashimi, N., & Alqudah, M. (2023). The Emerging Importance of Cirsimaritin in Type 2 Diabetes Treatment. International Journal of Molecular Sciences, 24(6), 5749. https://doi.org/10.3390/ijms24065749





