MicroRNA miR171b Positively Regulates Resistance to Huanglongbing of Citrus
Abstract
1. Introduction
2. Results
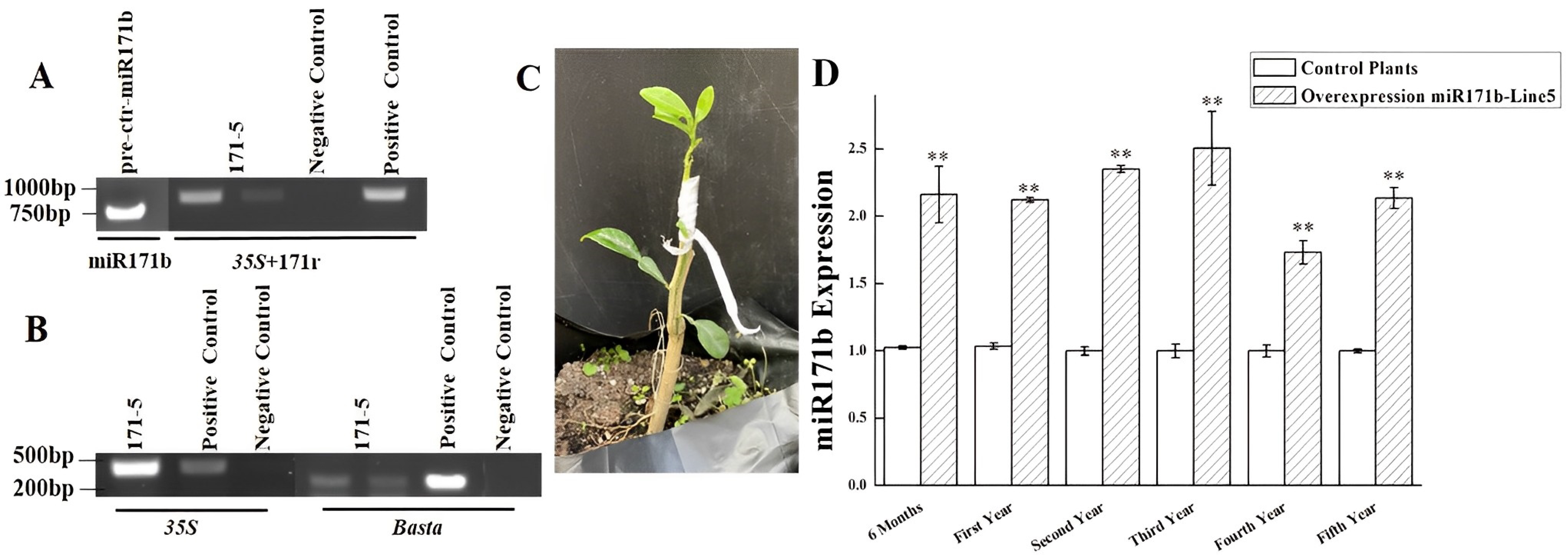
2.1. Generation of Transgenic Plants Overexpressing pre-ctr-miR171b
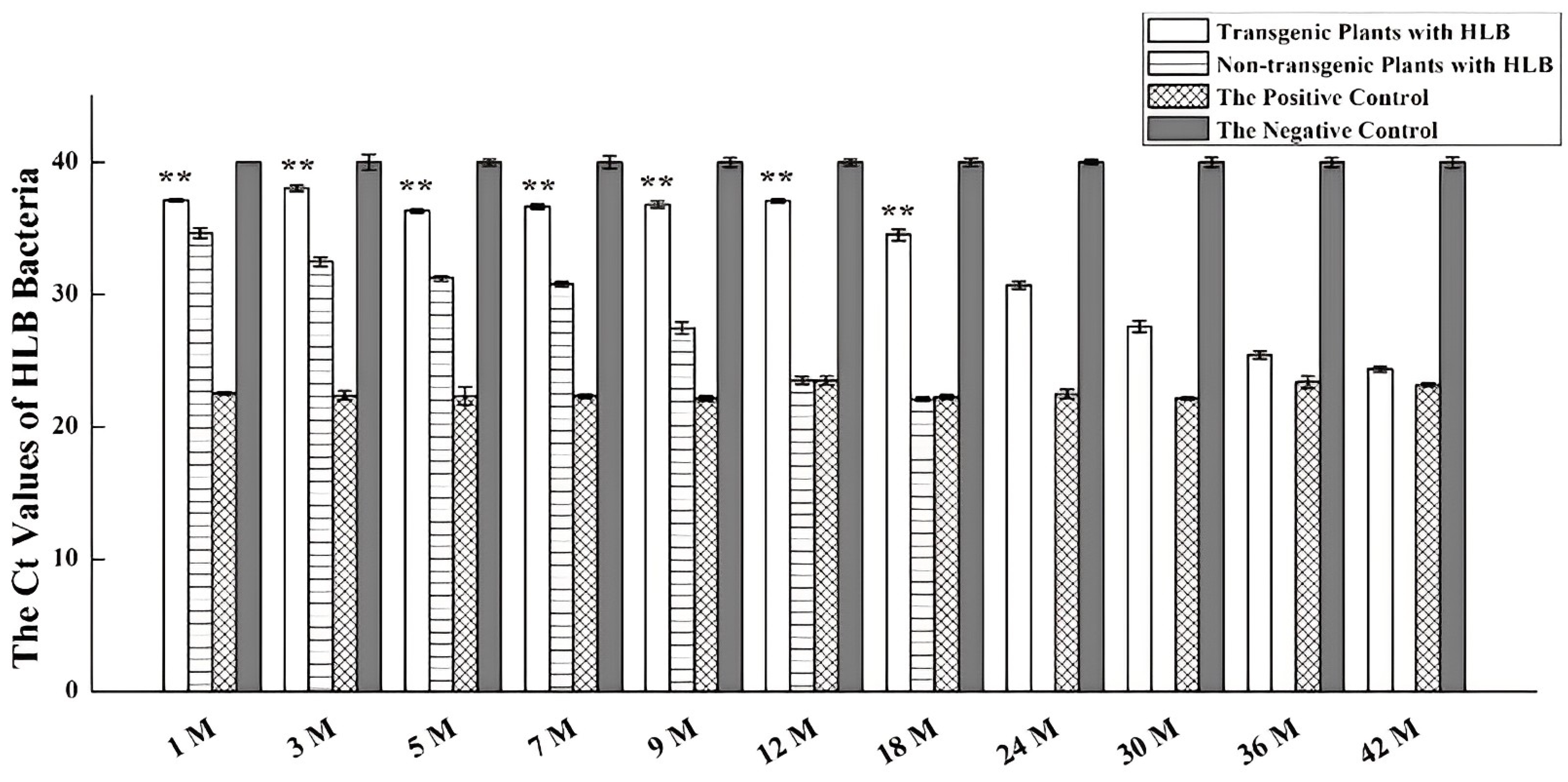
2.2. The 171-5 Asexual Progenies Show HLB-Tolerance
2.3. Transcriptome Analysis via Illumina-Based RNA Sequencing
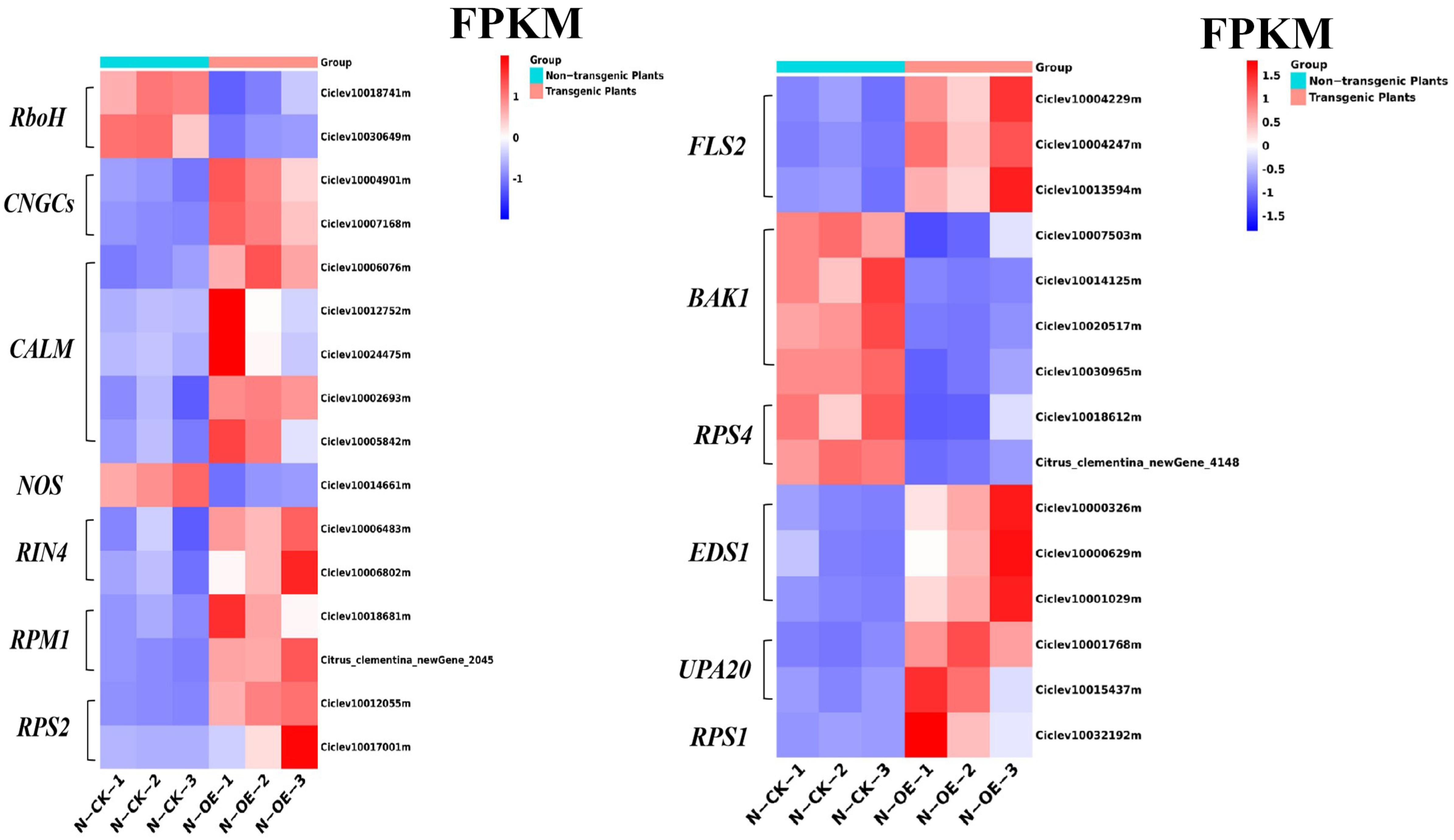
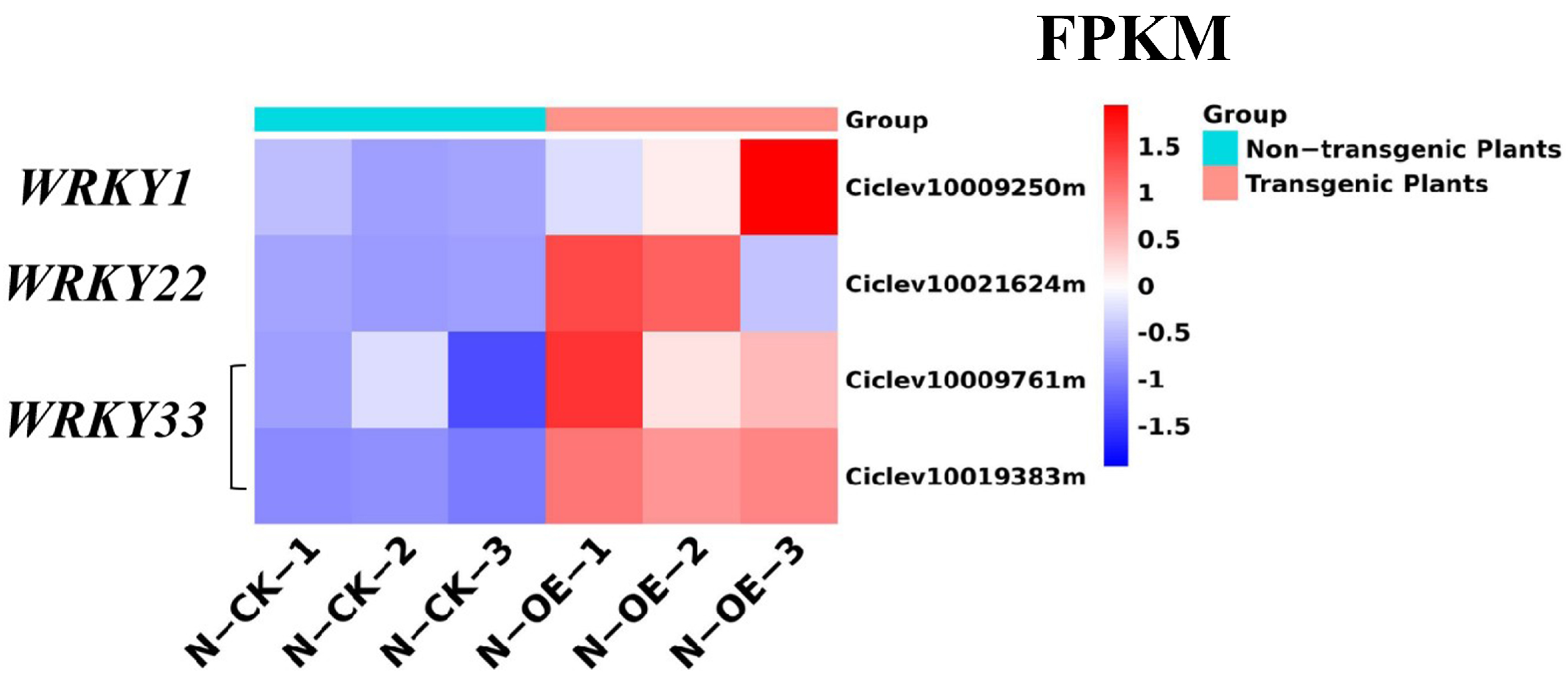
2.4. Gene Expression Validation Using qRT-PCR
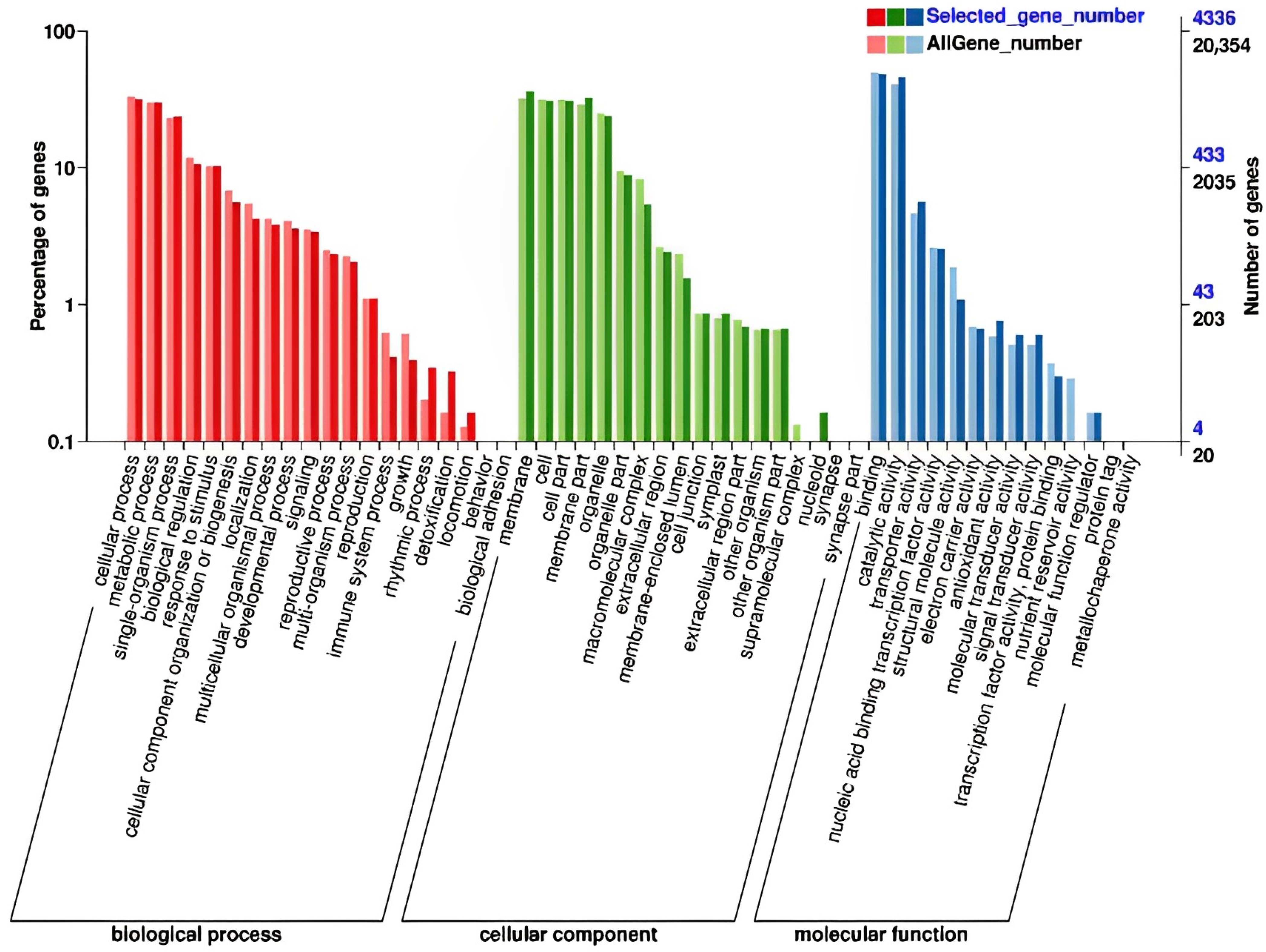
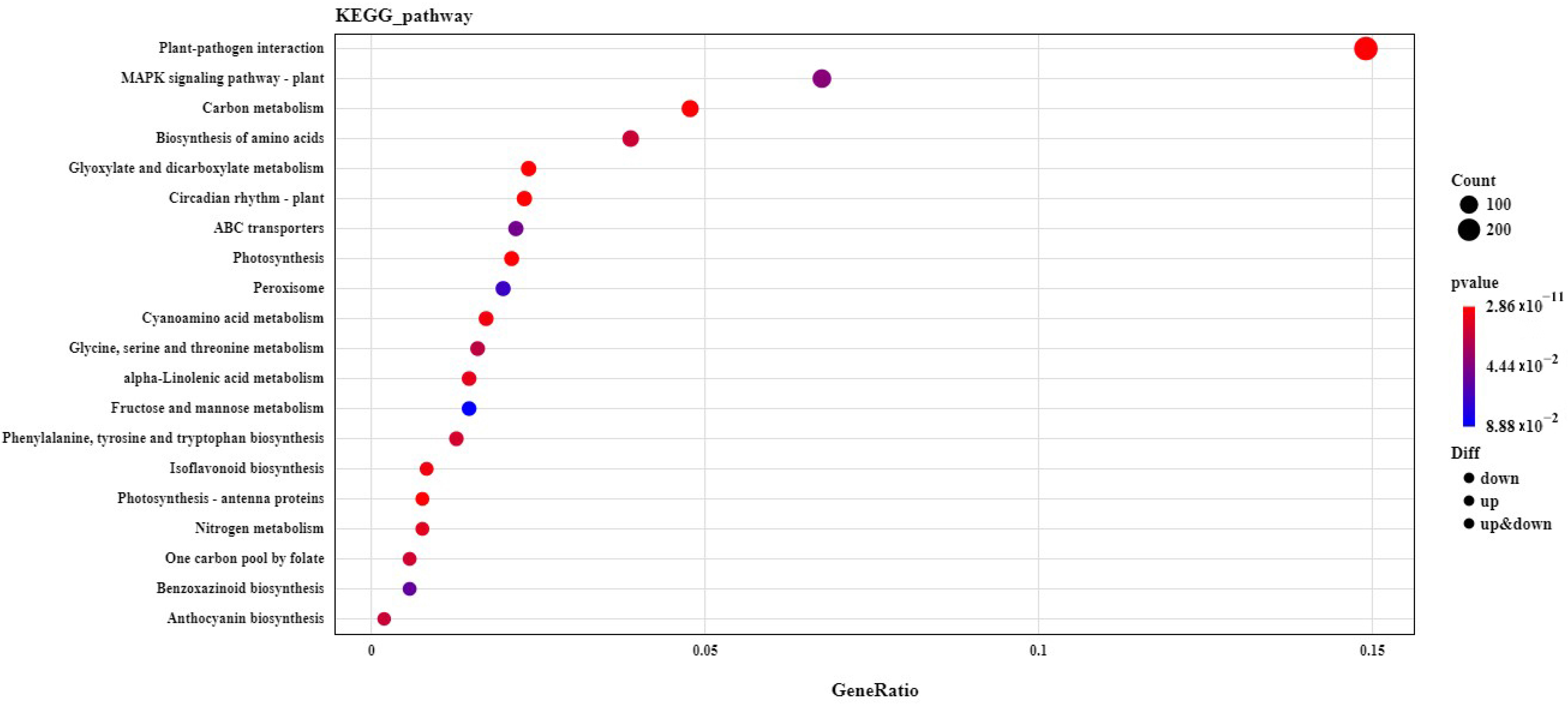
2.5. GO and KEGG Analysis
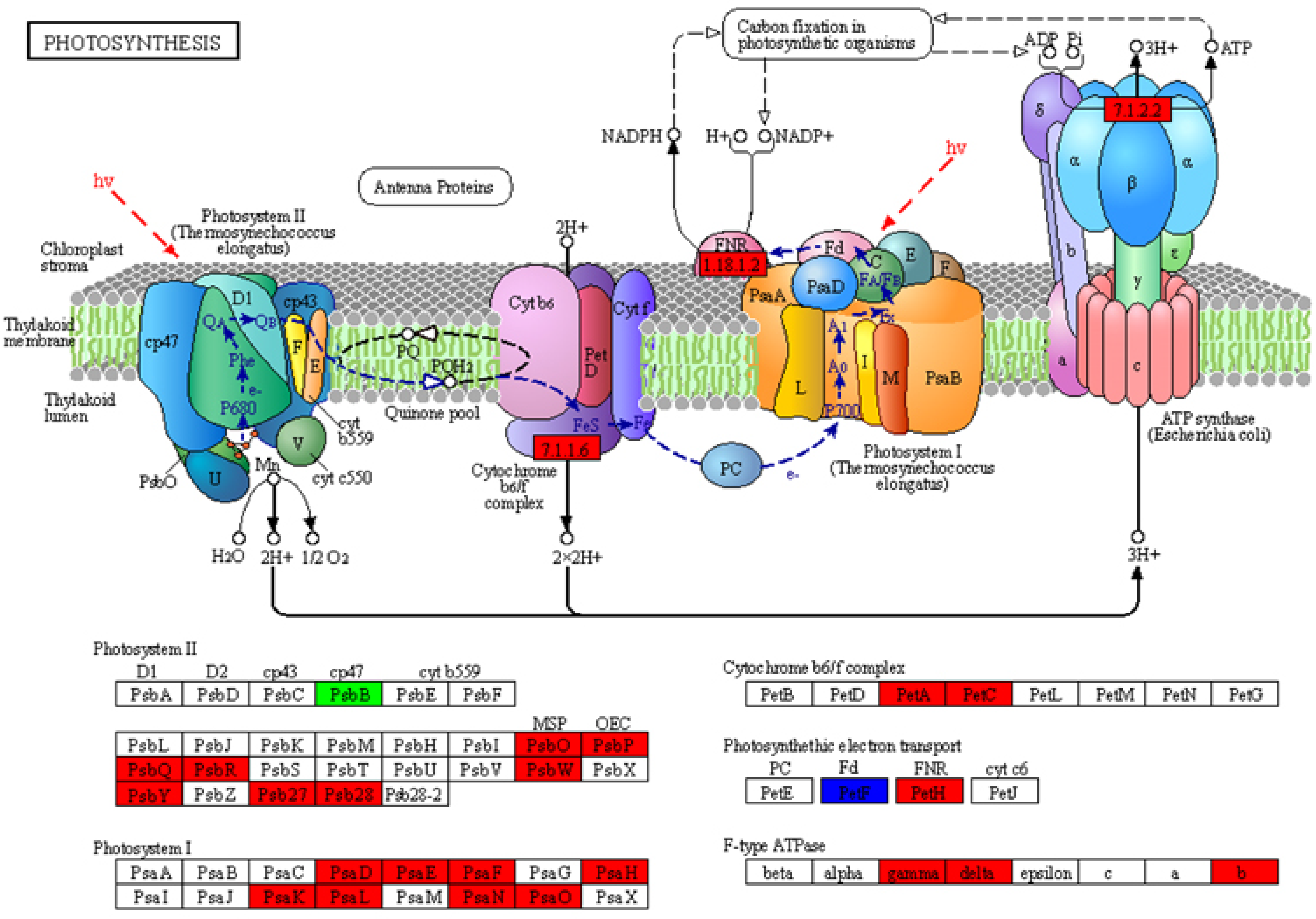
2.6. Analysis of the DEGs in the Photosynthesis Pathway
2.7. Analysis of the DEGs in the Plant–Pathogen Interaction Pathway
2.8. Analysis of the DEGs in the MAPK Pathway
2.9. Analysis of the DEGs in the Plant Hormone Signal Transduction Pathway
2.10. Predicted Targets of miRNA171b
2.11. Expression of the Genes Targeted by miR171b
3. Discussion
4. Materials and Methods
4.1. Cloning of pre-ctr-miR171b
4.2. Construction of Expression Vectors
4.3. Genetic Transformation and Identification of Transgenic Plants
4.4. Analysis of miR171b Expression Levels
4.5. Determining the Ct Values of HLB Bacteria
4.6. Plant Materials, RNA Isolation, and Quantification
4.7. RNA-Seq Experiment
4.8. Validation of DEGs in a qRT-PCR Assay
4.9. Bioinformatics Analysis of miR171b Target Genes
4.10. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Donkersley, P.; Silva, F.W.; Carvalho, C.M.; Al-Sadi, A.M.; Elliot, S.L. Biological, environmental and socioeconomic threats to citrus lime production. J. Plant Dis. Prot. 2018, 125, 339–356. [Google Scholar] [CrossRef]
- Morris, J.; Shiller, J.; Mann, R.; Smith, G.; Yen, A.; Rodoni, B. Novel ‘Candidatus Liberibacter’species identified in the Australian eggplant psyllid, Acizzia solanicola. Microb. Biotechnol. 2017, 10, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Cheng, C.; Jiang, B.; Jiang, J.; Zhang, Y.; Hu, M.; Zhong, G. Digital Gene Expression Analysis of Ponkan Mandarin (Citrus reticulata Blanco) in Response to Asia Citrus Psyllid-Vectored Huanglongbing Infection. Int. J. Mol. Sci. 2016, 17, 1063. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zhou, L.; Hall, D.G.; Li, W.; Doddapaneni, H.; Lin, H.; Gottwald, T. Complete genome sequence of citrus huanglongbing bacterium,‘Candidatus Liberibacter asiaticus’ obtained through metagenomics. Mol. Plant-Microbe Interact. 2009, 22, 1011–1020. [Google Scholar] [CrossRef]
- Narouei-Khandan, H.A.; Halbert, S.E.; Worner, S.P.; van Bruggen, A.H. Global climate suitability of citrus huanglongbing and its vector, the Asian citrus psyllid, using two correlative species distribution modeling approaches, with emphasis on the USA. Eur. J. Plant Pathol. 2016, 144, 655–670. [Google Scholar] [CrossRef]
- McRoberts, N.; Figuera, S.G.; Olkowski, S.; McGuire, B.; Luo, W.; Posny, D.; Gottwald, T. Using models to provide rapid programme support for California’s efforts to suppress Huanglongbing disease of citrus. Philos. Trans. R. Soc. B 2019, 374, 20180281. [Google Scholar] [CrossRef]
- Graham, J.; Gottwald, T.; Setamou, M. Status of huanglongbing (HLB) outbreaks in Florida, California and Texas. Trop. Plant Pathol. 2020, 45, 265–278. [Google Scholar] [CrossRef]
- Sillero, J.C.; Villegas-Fernández, A.M.; Thomas, J.; Rojas-Molina, M.M.; Emeran, A.A.; Fernández-Aparicio, M.; Rubiales, D. Faba bean breeding for disease resistance. Field Crops Res. 2010, 115, 297–307. [Google Scholar] [CrossRef]
- Georges, F.; Ray, H. Genome editing of crops: A renewed opportunity for food security. GM Crops Food 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Caruso, M.; Smith, M.W.; Froelicher, Y.; Russo, G.; Gmitter, F.G., Jr. Traditional breeding. In The Genus Citrus; Woodhead Publishing: Sawston, UK, 2020; pp. 129–148. [Google Scholar]
- Albrecht, U.; Bowman, K.D. Tolerance of the trifoliate citrus hybrid US-897 (Citrus reticulata Blanco x Poncirus trifoliate L.Raf.) to Huanglongbing. Hortscience 2011, 46, 16–22. [Google Scholar] [CrossRef]
- Albrecht, U.; Bowman, K.D. Tolerance of trifoliate citrus rootstock hybrids to Candidatus Liberibacter asiaticus. Sci. Hortic. 2012, 147, 71–80. [Google Scholar] [CrossRef]
- Ramadugu, C.; Keremane, M.L.; Halbert, S.E.; Duan, Y.P.; Roose, M.L.; Stover, E.; Lee, R.F. Long-term field evaluation reveals Huanglongbing resistance in Citrus relatives. Plant Dis. 2016, 100, 1858–1869. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Roose, M.L.; Yu, Q.; Du, D.; Yu, Y.; Zhang, Y.; Gmitter, F.G., Jr. Construction of high-density genetic maps and detection of QTLs associated with Huanglongbing tolerance in citrus. Front. Plant Sci. 2018, 9, 1694. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Zhong, Y.; Yan, H.; Yuanda, L.; Jiang, B.; Zhong, G. Exploration of susceptible genes with clustered regularly interspaced short palindromic repeats–tissue-specific knockout (CRISPR-TSKO) to enhance host resistance. Crit. Rev. Plant Sci. 2020, 39, 387–417. [Google Scholar] [CrossRef]
- Van Eck, J. Applying gene editing to tailor precise genetic modifications in plants. J. Biol. Chem. 2020, 295, 13267–13276. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Rana, T.M. Therapeutic targeting of microRNAs: Current status and future challenges. Nat. Rev. Drug Discov. 2014, 13, 622–638. [Google Scholar] [CrossRef] [PubMed]
- Budak, H.; Akpinar, B.A. Plant miRNAs: Biogenesis, organization and origins. Funct. Integr. Genom. 2015, 15, 523–531. [Google Scholar]
- Yang, Z.; Zhu, P.; Kang, H.; Liu, L.; Cao, Q.; Sun, J.; Xu, T. High-throughput deep sequencing reveals the important role that microRNAs play in the salt response in sweet potato (Ipomoea batatas L.). BMC Genom. 2020, 21, 164. [Google Scholar] [CrossRef]
- Yang, Z.; Dong, T.; Dai, X.; Wei, Y.; Fang, Y.; Zhang, L.; Xu, T. Comparative analysis of salt responsive microRNAs in two sweetpotato [Ipomoea batatas (L.) Lam.] cultivars with different salt stress resistance. Front. Plant Sci. 2022, 13, 879819. [Google Scholar]
- Li, Z.; Zhang, X.; Liu, X.; Zhao, Y.; Wang, B.; Zhang, J. miRNA alterations are important mechanism in maize adaptations to low-phosphate environments. Plant Sci. 2016, 252, 103–117. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, Z.; Zhu, C. Microarray-based analysis of cadmium-responsive microRNAs in rice (Oryza sativa). J. Exp. Bot. 2011, 62, 3563–3573. [Google Scholar] [CrossRef]
- Fang, X.; Zhao, Y.; Ma, Q.; Huang, Y.; Wang, P.; Zhang, J.; Yang, C. Identification and comparative analysis of cadmium tolerance-associated miRNAs and their targets in two soybean genotypes. PLoS ONE 2013, 8, e81471. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Fu, Y.; Sunkar, R.; Barbazuk, W.B.; Zhu, J.K.; Yu, O. Novel and nodulation-regulated microRNAs in soybean roots. BMC Genom. 2008, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Sunkar, R.; Li, Y.F.; Jagadeeswaran, G. Functions of microRNAs in plant stress responses. Trends Plant Sci. 2012, 17, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Quintero, Á.L.; Quintero, A.; Urrego, O.; Vanegas, P.; López, C. Bioinformatic identification of cassava miRNAs differentially expressed in response to infection by Xanthomonas axonopodis pv. manihotis. BMC Plant Biol. 2012, 12, 29. [Google Scholar] [CrossRef]
- Ergun, S.; Oztuzcu, S. Oncocers: ceRNA-mediated cross-talk by sponging miRNAs in oncogenic pathways. Tumor Biol. 2015, 36, 3129–3136. [Google Scholar] [CrossRef]
- Huang, W.; Xian, Z.Q.; Kang, X.; Tang, N.; Li, Z.G. Genome-wideidentification, phylogeny and expression analysis of GRAS gene family intomato. BMC Plant Biol. 2015, 15, 209. [Google Scholar] [CrossRef]
- Jatan, R.; Chauhan, P.S.; Lata, C. Pseudomonas putida modulates the expression of miRNAs and their target genes in response to drought and salt stresses in chickpea (Cicer arietinum L.). Genomics 2019, 111, 509–519. [Google Scholar] [CrossRef]
- Huang, W.; Peng, S.; Xian, Z.; Lin, D.; Hu, G.; Yang, L.; Li, Z. Overexpression of a tomato miR171 target gene SlGRAS 24 impacts multiple agronomical traits via regulating gibberellin and auxin homeostasis. Plant Biotechnol. J. 2017, 15, 472–488. [Google Scholar] [CrossRef]
- Habib, S.; Waseem, M.; Li, N.; Yang, L.; Li, Z. Overexpression of SlGRAS7 affects multiple behaviors leading to confer abiotic stresses tolerance and impacts gibberellin and auxin signaling in tomato. Int. J. Genom. 2019, 2019, 4051981. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Yang, Y.; Jia, N.; Wang, C.; Sun, H. miR171 and its target gene SCL6 contribute to embryogenic callus induction and torpedo-shaped embryo formation during somatic embryogenesis in two lily species. Plant Cell Tissue Organ Cult. 2017, 130, 591–600. [Google Scholar] [CrossRef]
- Couzigou, J.M.; Lauressergues, D.; André, O.; Gutjahr, C.; Guillotin, B.; Bécard, G.; Combier, J.P. Positive gene regulation by a natural protective miRNA enables arbuscular mycorrhizal symbiosis. Cell Host Microbe 2017, 21, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; He, C.; Yan, X.; Bai, F.; Pan, Z.; Deng, X.; Xiao, S. Small RNA profiling reveals involvement of microRNA-mediated gene regulation in response to mycorrhizal symbiosis in Poncirus trifoliata L. Raf. Tree Genet. Genomes 2018, 14, 42. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, J.; Chen, Y.; Lin, X.; Sun, C.; Zhang, B. Migration of endophytic diazotroph Azorhizobium caulinodans ORS571 inside wheat (Triticum aestivum L) and its effect on microRNAs. Funct. Integr. Genom. 2017, 17, 311–319. [Google Scholar]
- Reyes, C.A.; Ocolotobiche, E.E.; Marmisollé, F.E.; Robles Luna, G.; Borniego, M.B.; Bazzini, A.A.; García, M.L. Citrus psorosis virus 24 K protein interacts with citrus miRNA precursors, affects their processing and subsequent miRNA accumulation and target expression. Mol. Plant Pathol. 2016, 17, 317–329. [Google Scholar] [CrossRef]
- Zhong, Y.; Cheng, C.; Moniruzzaman, M.; Jiang, B.; Jiang, N.; Zhong, G. Expression of miRNAs and their target genes in roots of ‘Sanhu’tangerine (Citrus reticulata blanco cv. ‘Sanhu’) in response to Candidatus Liberibacter asiaticus infection. J. Plant Dis. Prot. 2021, 128, 407–420. [Google Scholar]
- Terrón-Camero, L.C.; Molina-Moya, E.; Sanz-Fernández, M.; Sandalio, L.M.; Romero-Puertas, M.C. Detection of reactive oxygen and nitrogen species (ROS/RNS) during hypersensitive cell death. Plant Program. Cell Death Methods Protocols 2018, 97–105. [Google Scholar]
- Zurbriggen, M.D.; Carrillo, N.; Hajirezaei, M.R. ROS signaling in the hypersensitive response: When, where and what for? Plant Signal. Behav. 2010, 5, 393–396. [Google Scholar] [CrossRef]
- Cui, H.; Kong, D.; Liu, X.; Hao, Y. SCARECROW, SCR-LIKE 23 and SHORT-ROOT control bundle sheath cell fate and function in Arabidopsis thaliana. Plant J. 2014, 78, 319–327. [Google Scholar] [CrossRef]
- Pitzschke, A.; Schikora, A.; Hirt, H. MAPK cascade signalling networks in plant defence. Curr. Opin. Plant Biol. 2009, 12, 421–426. [Google Scholar] [CrossRef]
- Boller, T.; Felix, G. A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef]
- Hwang, S.H.; Kwon, S.; Jang, J.Y.; Fang, I.L.; Lee, H.; Choi, C.; Park, S.; Ahn, I.; Bae, S.; Hwang, D.J. OsWRKY51, a rice transcription factor, functions as a positive regulator in defense response against Xanthomonas oryzae pv. oryzae. Plant Cell Rep. 2016, 35, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhou, L.; Yang, Y.; Zhang, W.; Chen, Z.; Li, X.; Hou, X. The gibberellin signaling negative regulator RGA-LIKE3 promotes seed storage protein accumulation. Plant Physiology. 2021, 185, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Fariduddin, Q.; Castroverde, C.D.M. Salicylic acid: A key regulator of redox signalling and plant immunity. Plant Physiology and Biochemistry. 2021, 168, 381–397. [Google Scholar] [CrossRef]
- Jatan, R.; Lata, C.H.A.R.U. Role of microRNAs in abiotic and biotic stress resistance in plants. Proc. Indian Natl. Sci. Acad. 2019, 13, 553–567. [Google Scholar]
- Lauressergues, D.; Delaux, P.M.; Formey, D.; Lelandais-Brie`re, C.; Fort, S.; Cottaz, S.; Be’card, G.; Niebel, A.; Roux, C.; Combier, J.P. The microRNA miR171h modulates arbuscular mycorrhizal colonization of Medicago truncatula by targeting NSP2. Plant J. 2012, 72, 512–522. [Google Scholar] [CrossRef]
- Xin, M.; Wang, Y.; Yao, Y.; Xie, C.; Peng, H.; Ni, Z.; Sun, Q. Diverse set of microRNAs are responsive to powdery mildew infection and heat stress in wheat (Triticum aestivum L.). BMC Plant Biol. 2010, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Hoang, N.T.; Yan, Z.; Tóth, K.; Meyers, B.C.; Stacey, G. Characterization of the spatial and temporal expression of two soybean miRNAs identifies SCL6 as a novel regulator of soybean nodulation. Front. Plant Sci. 2019, 10, 475. [Google Scholar] [CrossRef]
- Berger, S.; Sinha, A.K.; Roitsch, T. Plant physiology meets phytopathology: Plant primary metabolism and plant pathogen interactions. J. Exp. Bot. 2007, 58, 4019–4026. [Google Scholar] [CrossRef]
- Kanwar, P.; Jha, G. Alterations in plant sugar metabolism: Signatory of pathogen attack. Planta 2019, 249, 305–318. [Google Scholar] [CrossRef]
- Smith, J.E.; Mengesha, B.; Tang, H.; Mengiste, T.; Bluhm, B.H. Resistance to Botrytis cinerea in Solanum lycopersicoides involves widespread transcriptional reprogramming. BMC Genom. 2014, 15, 334. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, Y.; Wang, J.; Zou, F.; Jia, Y.; Shen, D.; Zhang, M. A Phytophthora capsici virulence effector associates with NPR1 and suppresses plant immune responses. Phytopathology Research. 2019, 1, 1–11. [Google Scholar] [CrossRef]
- Smirnova, O.G.; Kochetov, A.V. Promoters of plant genes responsive to pathogen invasion. Russ. J. Genet. Appl. Res. 2015, 5, 254–261. [Google Scholar] [CrossRef]
- Yang, C.; Liang, Y.; Qiu, D.; Zeng, H.; Yuan, J.; Yang, X. Lignin metabolism involves Botrytis cinerea BcGs1-induced defense response in tomato. BMC Plant Biol. 2018, 18, 103. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.S.; Liang, D.; Shuai, P.; Xia, X.L.; Yin, W.L. The salt- and drought-inducible poplar GRAS protein SCL7 confers salt and drought tolerance in Arabidopsis thaliana. J. Exp. Bot. 2010, 61, 4011–4019. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Pang, Z.; Huang, X.; Xu, J.; Pandey, S.S.; Li, J.; Wang, N. Citrus Huanglongbing is a pathogen-triggered immune disease that can be mitigated with antioxidants and gibberellin. Nat. Commun. 2022, 13, 529. [Google Scholar] [CrossRef]
- Klessig, D.F.; Choi, H.W.; Dempsey, D.M.A. Systemic acquired resistance and salicylic acid: Past, present, and future. Mol. Plant-Microbe Interact. 2018, 31, 871–888. [Google Scholar] [CrossRef]
- Kunkel, B.N.; Brooks, D.M. Cross talk between signaling pathways in pathogen defense. Curr. Opin. Plant Biol. 2002, 5, 325–331. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, X.; Li, B.; Zhang, Q.; Liang, W.; Wang, C. Salicylic acid confers enhanced resistance to Glomerella leaf spot in apple. Plant Physiology and Biochemistry. 2016, 106, 64–72. [Google Scholar] [CrossRef]
- Dutt, M.; Barthe, G.; Irey, M.; Grosser, J. Transgenic citrus expressing an Arabidopsis NPR1 gene exhibit enhanced resistance against Huanglongbing (HLB; Citrus Greening). PLoS ONE 2015, 10, e0137134. [Google Scholar] [CrossRef]
- Wang, L.; Mai, Y.X.; Zhang, Y.C.; Luo, Q.; Yang, H.Q. MicroRNA171c-targeted SCL6-II, SCL6-III, and SCL6-IV genes regulate shoot branching in Arabidopsis. Mol. Plant 2010, 3, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Branscheid, A.; Devers, E.A.; May, P.; Krajinski, F. Distribution pattern of small RNA and degradome reads provides information on miRNA gene structure and regulation. Plant Signal. Behav. 2011, 6, 1609–1611. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Ling, H.; Chen, X.; Guo, S. Genome-wide identification, phylogeny and function analysis of GRAS gene family in Dendrobium catenatum (Orchidaceae). Gene. 2019, 705, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Grimplet, J.; Agudelo-Romero, P.; Teixeira, R.T.; Martinez-Zapater, J.M.; Fortes, A.M. Structural and functional analysis of the GRAS gene family in grapevine indicates a role of GRAS proteins in the control of development and stress responses. Front. Plant Sci. 2016, 7, 353. [Google Scholar] [CrossRef]
- Navarro, L.; Bari, R.; Achard, P.; Lisón, P.; Nemri, A.; Harberd, N.P.; Jones, J.D. DELLAs control plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Curr. Biol. 2008, 18, 650–655. [Google Scholar] [CrossRef]
- Khan, Y.; Xiong, Z.; Zhang, H.; Liu, S.; Yaseen, T.; Hui, T. Expression and roles of GRAS gene family in plant growth, signal transduction, biotic and abiotic stress resistance and symbiosis formation-a review. Plant Biol. 2021, 24, 404–416. [Google Scholar] [CrossRef]
- Cheng, C.Z.; Yang, J.W.; Yan, H.B.; Bei, X.J.; Zhang, Y.Y.; Lu, Z.M.; Zhong, G.Y. Expressing p20 hairpin RNA of Citrus tristeza virus confers Citrus aurantium with tolerance/resistance against stem pitting and seedling yellow CTV strains. J. Integr. Agric. 2015, 14, 1767–1777. [Google Scholar] [CrossRef]
- Tang, F.; Chu, L.; Shu, W.; He, X.; Wang, L.; Lu, M. Selection and validation of reference genes for quantitative expression analysis of miRNAs and mRNAs in Poplar. Plant Methods. 2019, 15, 1–15. [Google Scholar] [CrossRef]
- Li, W.; Hartung, J.S.; Levy, L. Evaluation of DNA amplification methods for improved detection of “Candidatus Liberibacter species” associated with citrus huanglongbing. Plant Dis. 2007, 91, 51–58. [Google Scholar] [CrossRef]
- Morgan, J.K.; Zhou, L.; Li, W.; Shatters, R.G.; Keremane, M.; Duan, Y.P. Improved real-time PCR detection of ‘Candidatus Liberibacter asiaticus’ from citrus and psyllid hosts by targeting the intragenic tandem-repeats of its prophage genes. Mol. Cell. Probes 2012, 26, 90–98. [Google Scholar] [CrossRef]




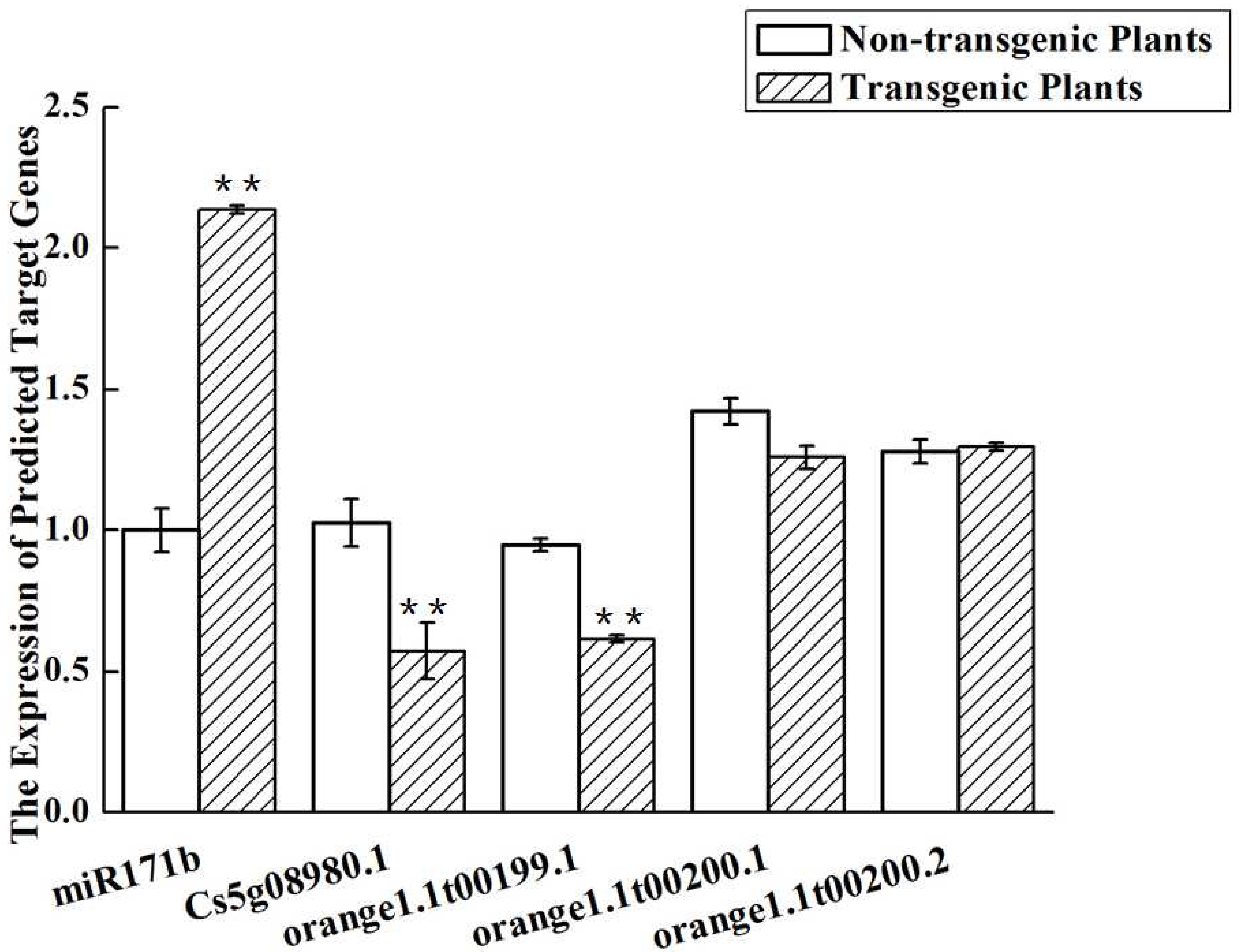
| Gene ID | Gene Info |
|---|---|
| orange1.1t00200.1 | Scarecrow transcription factor family protein; Scarecrow-like protein 6; DELLA protein RGA |
| orange1.1t00200.2 | GRAS family transcription factor containing protein; Scarecrow-like protein 6; DELLA protein RGA |
| Cs5g08980.1 | GRAS family transcription factor containing protein; Scarecrow-like protein 6 |
| orange1.1t00199.1 | GRAS family transcription factor containing protein; Scarecrow-like protein 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Y.; Zhong, Y.; Jiang, B.; Yan, H.; Ren, S.; Cheng, C. MicroRNA miR171b Positively Regulates Resistance to Huanglongbing of Citrus. Int. J. Mol. Sci. 2023, 24, 5737. https://doi.org/10.3390/ijms24065737
Lv Y, Zhong Y, Jiang B, Yan H, Ren S, Cheng C. MicroRNA miR171b Positively Regulates Resistance to Huanglongbing of Citrus. International Journal of Molecular Sciences. 2023; 24(6):5737. https://doi.org/10.3390/ijms24065737
Chicago/Turabian StyleLv, Yuanda, Yun Zhong, Bo Jiang, Huaxue Yan, Shuang Ren, and Chunzhen Cheng. 2023. "MicroRNA miR171b Positively Regulates Resistance to Huanglongbing of Citrus" International Journal of Molecular Sciences 24, no. 6: 5737. https://doi.org/10.3390/ijms24065737
APA StyleLv, Y., Zhong, Y., Jiang, B., Yan, H., Ren, S., & Cheng, C. (2023). MicroRNA miR171b Positively Regulates Resistance to Huanglongbing of Citrus. International Journal of Molecular Sciences, 24(6), 5737. https://doi.org/10.3390/ijms24065737






