Infection of Trichinella spiralis Affects the Reproductive Capacity of ICR/CD-1 Male Mice by Reducing the Urine Pheromone Contents and Sperm Quality
Abstract
1. Introduction
2. Results
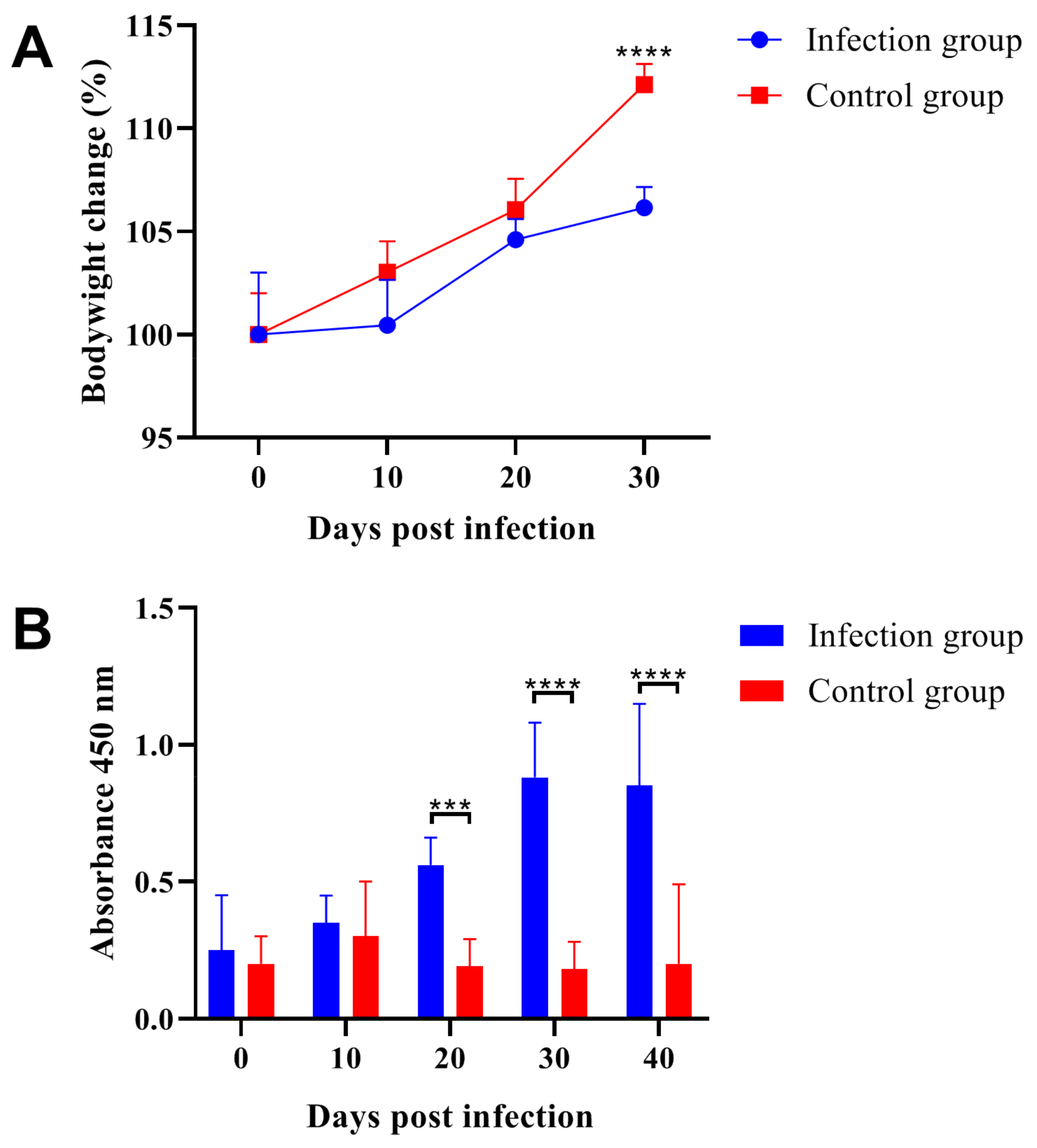
2.1. Parasitic Infection Decreased Body Weight and Induced Specific Antibody Production
2.2. Trichinella Spiralis Infection Decreased the Attractiveness of Male Mouse Urine to Female Mice
2.3. Trichinella Spiralis Infection Significantly Decreased Pheromone Content in the Male Mice Urine
2.4. Effects of Trichinella Spiralis Infection on Reproduction in Male Mice
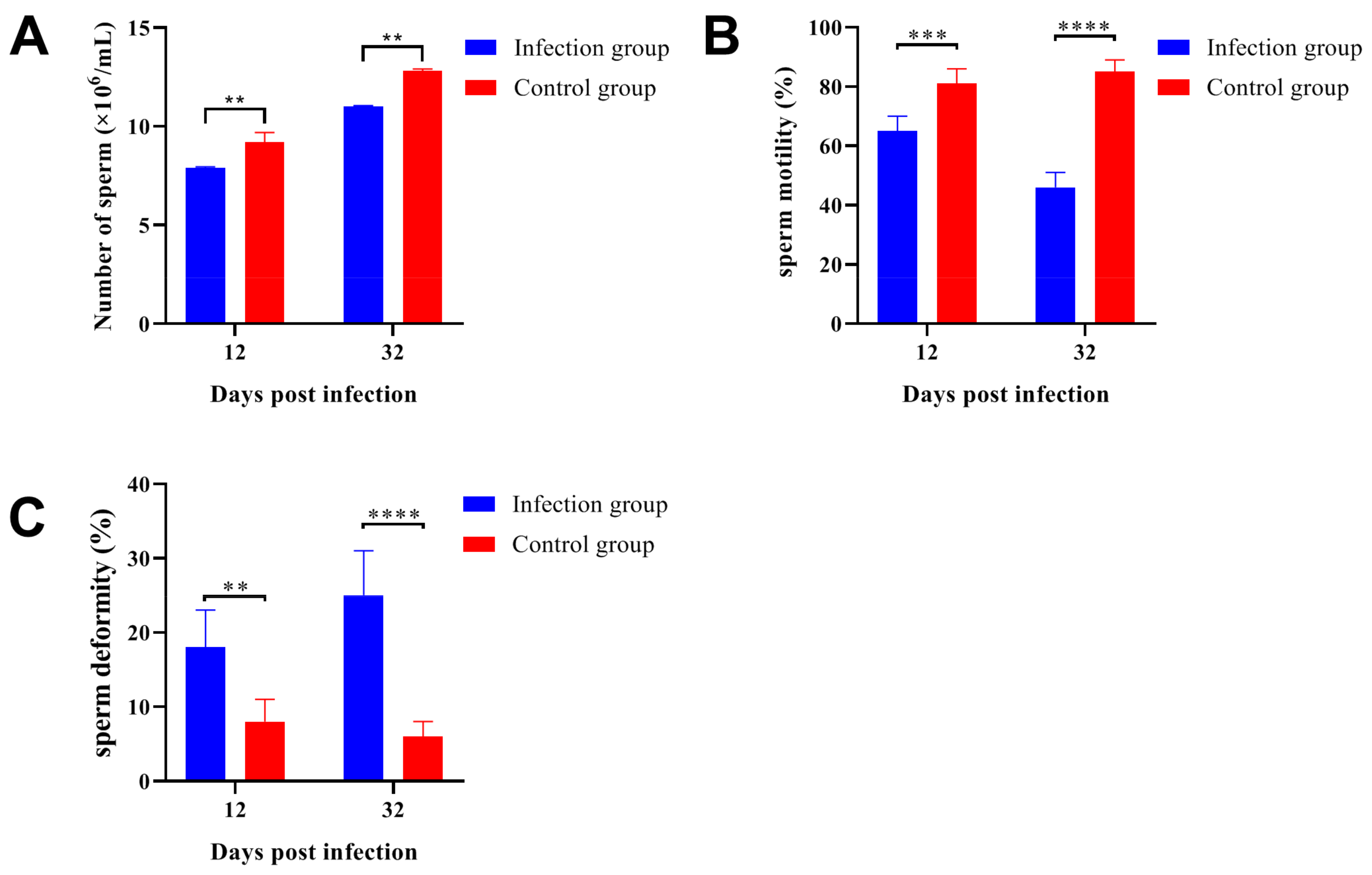
2.5. Parasitic Infection Decreased the Sperm Quality in ICR/CD-1 Male Mice
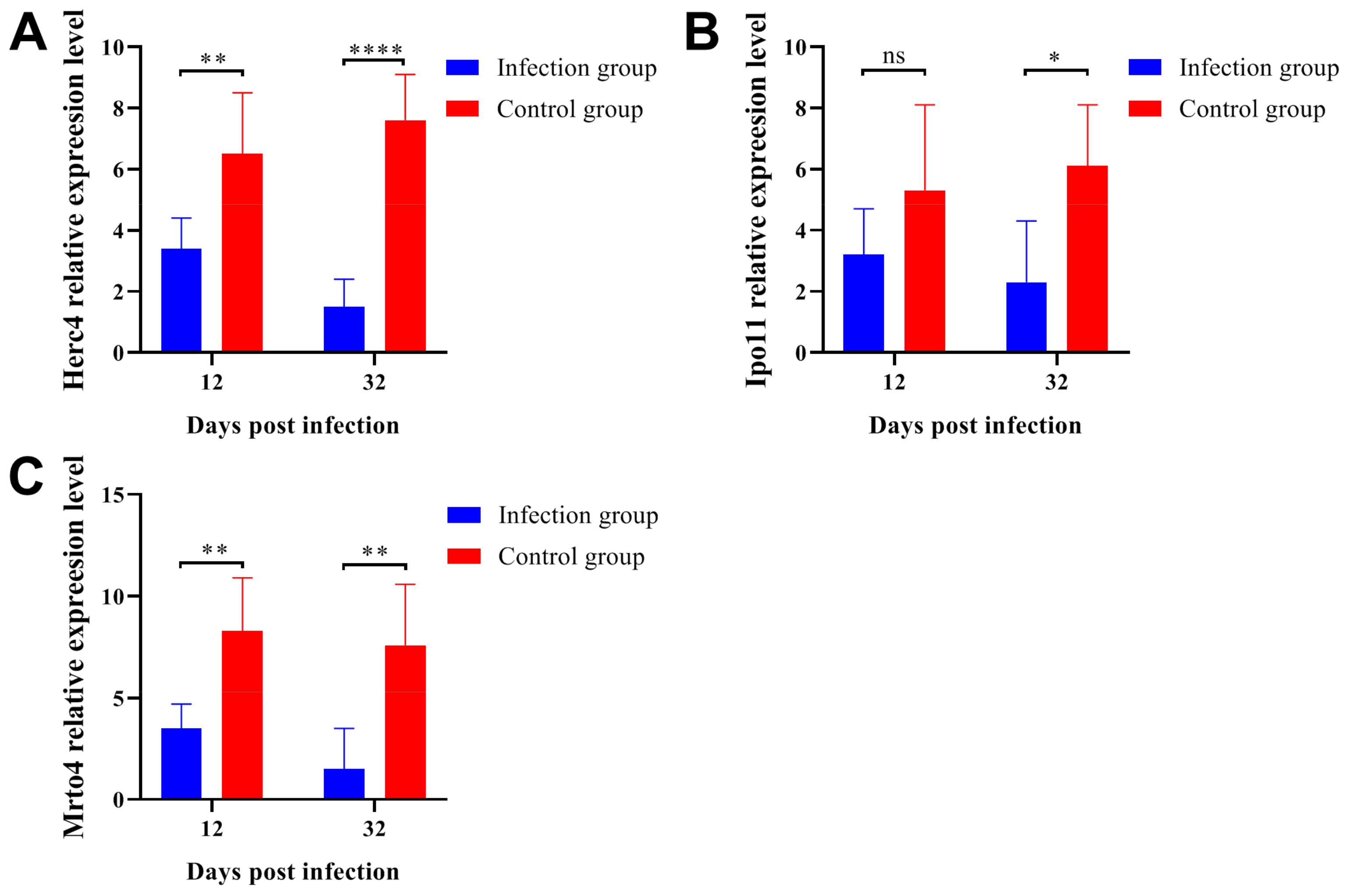
2.6. Parasitic Infection Downregulated the Gene Expression Levels Related to Spermatogenesis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Parasite Separation and Mice Infection
4.3. Preparation of Excretory-Secretory Antigen
4.4. Enzyme Linked Immunosorbent Assay (ELISA)
4.5. Mice Mating Experiment
4.6. Urine Collection
4.7. Behavioral Tests
4.8. GC-MS Analysis
4.9. Sperm Count and Sperm Quality Assessment
4.10. Quantitative Real-Time RT-PCR (qRT-PCR)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kheirandish, R.; Azizi, S.; Nourollahifard, S.; Imani, M.; Kermani, R.S.; Hassanzadeh, S. Histopathologic and histomorphometric evaluation of Dipetalonema evansi infection in camel testicular tissue. J. Parasit. Dis. 2021, 45, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Sekoni, V.O.; Rekwot, P.I.; Bawa, E.K. The effects of trypanosomosis on sperm morphology in Zebu x Friesian crossbred bulls. Trop. Anim. Health Prod. 2004, 36, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Dvorakova-Hortova, K.; Sidlova, A.; Ded, L.; Hladovcova, D.; Vieweg, M.; Weidner, W.; Steger, K.; Stopka, P.; Paradowska-Dogan, A. Toxoplasma gondii decreases the reproductive fitness in mice. PLoS ONE 2014, 9, e96770. [Google Scholar] [CrossRef]
- Betancur Hurtado, O.J.; Jimenez Castro, P.D.; Giraldo-Rios, C. Reproductive failures associated with Trypanosoma (Duttonella) vivax. Vet. Parasitol. 2016, 229, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, B.Y.; Liu, M.M.; Zhao, S.W.; Xie, S.Z.; Wang, H.; Zhang, S.; Xuan, X.N.; Jia, L.J. Reproductive injury in male BALB/c mice infected with Neospora caninum. Parasit. Vectors 2021, 14, 158. [Google Scholar] [CrossRef] [PubMed]
- Ehman, K.D.; Scott, M.E. Female mice mate preferentially with non-parasitized males. Parasitology 2002, 125, 461–466. [Google Scholar] [CrossRef]
- Gore, A.C.; Wersinger, S.R.; Rissman, E.F. Effects of female pheromones on gonadotropin-releasing hormone gene expression and luteinizing hormone release in male wild-type and oestrogen receptor-alpha knockout mice. J. Neuroendocrinol. 2000, 12, 1200–1204. [Google Scholar] [CrossRef]
- Curno, O.; Reader, T.; McElligott, A.G.; Behnke, J.M.; Barnard, C.J. Infection before pregnancy affects immunity and response to social challenge in the next generation. Philos. Trans. R Soc. Lond B Biol. Sci. 2011, 366, 3364–3374. [Google Scholar] [CrossRef]
- Penn, D.; Potts, W.K. Chemical signals and parasite-mediated sexual selection. Trends. Ecol. Evol. 1998, 13, 391–396. [Google Scholar] [CrossRef]
- Timm, D.E.; Baker, L.J.; Mueller, H.; Zidek, L.; Novotny, M.V. Structural basis of pheromone binding to mouse major urinary protein (MUP-I). Protein Sci. A Publ. Protein Soc. 2001, 10, 997–1004. [Google Scholar] [CrossRef]
- Novotny, M.V.; Jemiolo, B.; Wiesler, D.; Ma, W.; Harvey, S.; Xu, F.; Xie, T.-M.; Carmack, M. A unique urinary constituent, 6-hydroxy-6-methyl-3-heptanone, is a pheromone that accelerates puberty in female mice. Chem. Biol. 1999, 6, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Penn, D.J.; Zala, S.M.; Luzynski, K.C. Regulation of Sexually Dimorphic Expression of Major Urinary Proteins. Front. Physiol. 2022, 13, 822073. [Google Scholar] [CrossRef] [PubMed]
- Novotny, M.V.; Ma, W.; Wiesler, D.; Zídek, L. Positive identification of the puberty-accelerating pheromone of the house mouse: The volatile ligands associating with the major urinary protein. Proc. Biol. Sci. 1999, 266, 2017–2022. [Google Scholar] [CrossRef] [PubMed]
- Mucignat-Caretta, C.; Cavaggioni, A.; Caretta, A. Male urinary chemosignals differentially affect aggressive behavior in male mice. J. Chem. Ecol. 2004, 30, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Janotova, K.; Stopka, P. The level of major urinary proteins is socially regulated in wild Mus musculus musculus. J. Chem. Ecol. 2011, 37, 647–656. [Google Scholar] [CrossRef]
- Nelson, A.C.; Cauceglia, J.W.; Merkley, S.D.; Youngson, N.A.; Oler, A.J.; Nelson, R.J.; Cairns, B.R.; Whitelaw, E.; Potts, W.K. Reintroducing domesticated wild mice to sociality induces adaptive transgenerational effects on MUP expression. Proc. Natl. Acad. Sci. USA 2013, 110, 19848–19853. [Google Scholar] [CrossRef]
- Oldstone, M.B.A.; Ware, B.C.; Davidson, A.; Prescott, M.C.; Beynon, R.J.; Hurst, J.L. Lymphocytic Choriomeningitis Virus Alters the Expression of Male Mouse Scent Proteins. Viruses 2021, 13, 1180. [Google Scholar] [CrossRef]
- Lopes, P.C.; König, B. Choosing a healthy mate: Sexually attractive traits as reliable indicators of current disease status in house mice. Anim. Behav. 2016, 111, 119–126. [Google Scholar] [CrossRef]
- Charkoftaki, G.; Wang, Y.; McAndrews, M.; Bruford, E.A.; Thompson, D.C.; Vasiliou, V.; Nebert, D.W. Update on the human and mouse lipocalin (LCN) gene family, including evidence the mouse Mup cluster is result of an “evolutionary bloom”. Hum. Genom. 2019, 13, 11. [Google Scholar] [CrossRef]
- Beynon, R.J.; Hurst, J.L. Multiple roles of major urinary proteins in the house mouse, Mus domesticus. Biochem. Soc. Trans. 2003, 31, 142–146. [Google Scholar] [CrossRef]
- Ding, J.; Liu, X.; Bai, X.; Wang, Y.; Li, J.; Wang, C.; Li, S.; Liu, M.; Wang, X. Trichinella spiralis: Inflammation modulator. J. Helminthol. 2020, 94, e193. [Google Scholar] [CrossRef] [PubMed]
- Murrell, K.D.; Pozio, E. Worldwide occurrence and impact of human trichinellosis, 1986–2009. Emerg. Infect. Dis. 2011, 17, 2194–2202. [Google Scholar] [CrossRef] [PubMed]
- Stroffolini, G.; Rossi, L.; Lupia, T.; Faraoni, S.; Paltrinieri, G.; Lipani, F.; Calcagno, A.; Bonora, S.; Di Perri, G.; Calleri, G. Trichinella britovi outbreak in Piedmont, North-West Italy, 2019–2020: Clinical and epidemiological insights in the one health perspective. Travel Med. Infect. Dis. 2022, 47, 102308. [Google Scholar] [CrossRef] [PubMed]
- Ilic, N.; Vasilev, S.; Gruden-Movsesijan, A.; Gnjatovic, M.; Sofronic-Milosavljevic, L.; Mitic, I. Long lasting immunity in trichinellosis—Insight from a small study group. J. Helminthol. 2022, 96, e35. [Google Scholar] [CrossRef] [PubMed]
- Cuperlovic, K.; Djordjevic, M.; Pavlovic, S. Re-emergence of trichinellosis in southeastern Europe due to political and economic changes. Vet. Parasitol. 2005, 132, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Bekish, V.Y.; Durnev, A.D. Damage to cell DNA in the bone marrow and testes of mice with experimental trichinosis. Bull. Exp. Biol. Med. 2004, 138, 284–287. [Google Scholar] [CrossRef]
- Grzelak, S.; Stachyra, A.; Stefaniak, J.; Mrówka, K.; Moskwa, B.; Bień-Kalinowska, J. Immunoproteomic analysis of Trichinella spiralis and Trichinella britovi excretory-secretory muscle larvae proteins recognized by sera from humans infected with Trichinella. PLoS ONE 2020, 15, e0241918. [Google Scholar] [CrossRef] [PubMed]
- Srey, M.T.; Taccogna, A.; Oksov, Y.; Lustigman, S.; Tai, P.Y.; Acord, J.; Selkirk, M.E.; Lamb, T.J.; Guiliano, D.B. Vaccination with novel low-molecular weight proteins secreted from Trichinella spiralis inhibits establishment of infection. PLoS Negl. Trop. Dis. 2020, 14, e0008842. [Google Scholar] [CrossRef]
- Bruschi, F.; Gruden-Movesijan, A.; Pinto, B.; Ilic, N.; Sofronic-Milosavljevic, L. Trichinella spiralis excretory-secretory products downregulate MMP-9 in Dark Agouti rats affected by experimental autoimmune encephalomyelitis. Exp. Parasitol. 2021, 225, 108112. [Google Scholar] [CrossRef]
- Huang, D.; Li, J.; He, L.Q. Influence of Tripterygium wilfordii on the expression of spermiogenesis related genes Herc4, Ipo11 and Mrto4 in mice. Yi Chuan 2009, 31, 941–946. [Google Scholar] [CrossRef]
- Rodriguez, C.I.; Stewart, C.L. Disruption of the ubiquitin ligase HERC4 causes defects in spermatozoon maturation and impaired fertility. Dev. Biol. 2007, 312, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Meijer, H.A.; Bushell, M.; Hill, K.; Gant, T.W.; Willis, A.E.; Jones, P.; de Moor, C.H. A novel method for poly(A) fractionation reveals a large population of mRNAs with a short poly(A) tail in mammalian cells. Nucleic Acids Res. 2007, 35, e132. [Google Scholar] [CrossRef] [PubMed]
- Yamada, D.; Yoshida, M.; Williams, Y.N.; Fukami, T.; Kikuchi, S.; Masuda, M.; Maruyama, T.; Ohta, T.; Nakae, D.; Maekawa, A.; et al. Disruption of spermatogenic cell adhesion and male infertility in mice lacking TSLC1/IGSF4, an immunoglobulin superfamily cell adhesion molecule. Mol. Cell. Biol. 2006, 26, 3610–3624. [Google Scholar] [CrossRef] [PubMed]
- Stowers, L.; Kuo, T.H. Mammalian pheromones: Emerging properties and mechanisms of detection. Curr. Opin. Neurobiol. 2015, 34, 103–109. [Google Scholar] [CrossRef]
- Hurst, J.L. Female recognition and assessment of males through scent. Behav. Brain Res. 2009, 200, 295–303. [Google Scholar] [CrossRef]
- Wyatt, T.D. Proteins and peptides as pheromone signals and chemical signatures. Anim. Behav. 2014, 97, 273–280. [Google Scholar] [CrossRef]
- Zala, S.M.; Potts, W.K.; Penn, D.J. Scent-marking displays provide honest signals of health and infection. Behav. Ecol. 2004, 15, 338–344. [Google Scholar] [CrossRef]
- Kavaliers, M.; Choleris, E.; Agmo, A.; Pfaff, D.W. Olfactory-mediated parasite recognition and avoidance: Linking genes to behavior. Horm. Behav. 2004, 46, 272–283. [Google Scholar] [CrossRef]
- Klein, S.L.; Gamble, H.R.; Nelson, R.J. Role of steroid hormones in Trichinella spiralis infection among voles. Am. J. Physiol. 1999, 277, R1362–R1367. [Google Scholar] [CrossRef]
- Kavaliers, M.; Choleris, E.; Pfaff, D.W. Recognition and avoidance of the odors of parasitized conspecifics and predators: Differential genomic correlates. Neurosci. Biobehav. Rev 2005, 29, 1347–1359. [Google Scholar] [CrossRef]
- Takamoto, M.; Wang, Z.X.; Watanabe, N.; Sugane, K. The measurement of parasite antigen-specific IgE levels using anti-IgE monoclonal antibodies and biotinylated antigens. Parasitol. Res. 2001, 87, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Reiterova, K.; Antolova, D.; Hurnikova, Z. Humoral immune response of mice infected with low doses of Trichinella spiralis muscle larvae. Vet. Parasitol. 2009, 159, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, J.N.; Gordon, A.R.; Wise, P.; Frasnelli, J. Individual differences in the chemical senses: Is there a common sensitivity? Chem. Senses 2012, 37, 371–378. [Google Scholar] [CrossRef]
- Hurst, J.L.; Beynon, R.J. Scent wars: The chemobiology of competitive signalling in mice. Bioessays 2004, 26, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Hurst, J.L.; Payne, C.E.; Nevison, C.M.; Marie, A.D.; Humphries, R.E.; Robertson, D.H.; Cavaggioni, A.; Beynon, R.J. Individual recognition in mice mediated by major urinary proteins. Nature 2001, 414, 631–634. [Google Scholar] [CrossRef]
- Wolff, J.O. Social biology of rodents. Integr. Zool. 2007, 2, 193–204. [Google Scholar] [CrossRef]
- Kristan, D.M. Effects of intestinal nematodes during lactation: Consequences for host morphology, physiology and offspring mass. J. Exp. Biol. 2002, 205, 3955–3965. [Google Scholar] [CrossRef]
- Lee, J.Y.; Rahman, F.U.; Kim, E.K.; Cho, S.M.; Kim, H.R.; Lee, K.; Lee, C.S.; Yoon, W.K.; Moon, O.S.; Seo, Y.W.; et al. Importin-11 is Essential for Normal Embryonic Development in Mice. Int. J. Med. Sci. 2020, 17, 815–823. [Google Scholar] [CrossRef]
- Diaz, L.; Zambrano, E.; Flores, M.E.; Contreras, M.; Crispin, J.C.; Aleman, G.; Bravo, C.; Armenta, A.; Valdes, V.J.; Tovar, A.; et al. Ethical Considerations in Animal Research: The Principle of 3R’s. Rev. Investig. Clin. 2020, 73, 199–209. [Google Scholar] [CrossRef]
- Wu, J.; Liao, Y.; Li, D.; Zhu, Z.; Zhang, L.; Wu, Z.; He, P.; Wang, L. Extracellular vesicles derived from Trichinella Spiralis larvae promote the polarization of macrophages to M2b type and inhibit the activation of fibroblasts. Front. Immunol. 2022, 13, 974332. [Google Scholar] [CrossRef]
- Fabre, M.V.; Beiting, D.P.; Bliss, S.K.; Appleton, J.A. Immunity to Trichinella spiralis muscle infection. Vet. Parasitol. 2009, 159, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Tang, B.; Liu, X.; Bai, X.; Wang, Y.; Li, S.; Li, J.; Liu, M.; Wang, X. Excretory-secretory product of Trichinella spiralis inhibits tumor cell growth by regulating the immune response and inducing apoptosis. Acta Trop. 2022, 225, 106172. [Google Scholar] [CrossRef] [PubMed]
- Ríos-López, A.L.; Hernández-Bello, R.; González, G.M.; Sánchez-González, A. Trichinella spiralis excretory-secretory antigens selectively inhibit the release of extracellular traps from neutrophils without affecting their additional antimicrobial functions. Cell. Immunol. 2022, 382, 104630. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Shu, J.; Jin, J.; Shu, J.; Feng, H.; Chen, J.; He, Y. Development of a Multi-Epitope Vaccine for Mycoplasma hyopneumoniae and Evaluation of Its Immune Responses in Mice and Piglets. Int. J. Mol. Sci. 2022, 23, 7899. [Google Scholar] [CrossRef]
- Lai, S.C.; Vasilieva, N.; Johnston, R.E. Odors providing sexual information in Djungarian hamsters: Evidence for an across-odor code. Horm. Behav. 1996, 30, 26–36. [Google Scholar] [CrossRef]
- Singer, A.G.; Beauchamp, G.K.; Yamazaki, K. Volatile signals of the major histocompatibility complex in male mouse urine. Proc. Natl. Acad. Sci USA 1997, 94, 2210–2214. [Google Scholar] [CrossRef]
- Zhang, J.X.; Sun, L.; Novotny, M. Mice respond differently to urine and its major volatile constituents from male and female ferrets. J. Chem. Ecol. 2007, 33, 603–612. [Google Scholar] [CrossRef]

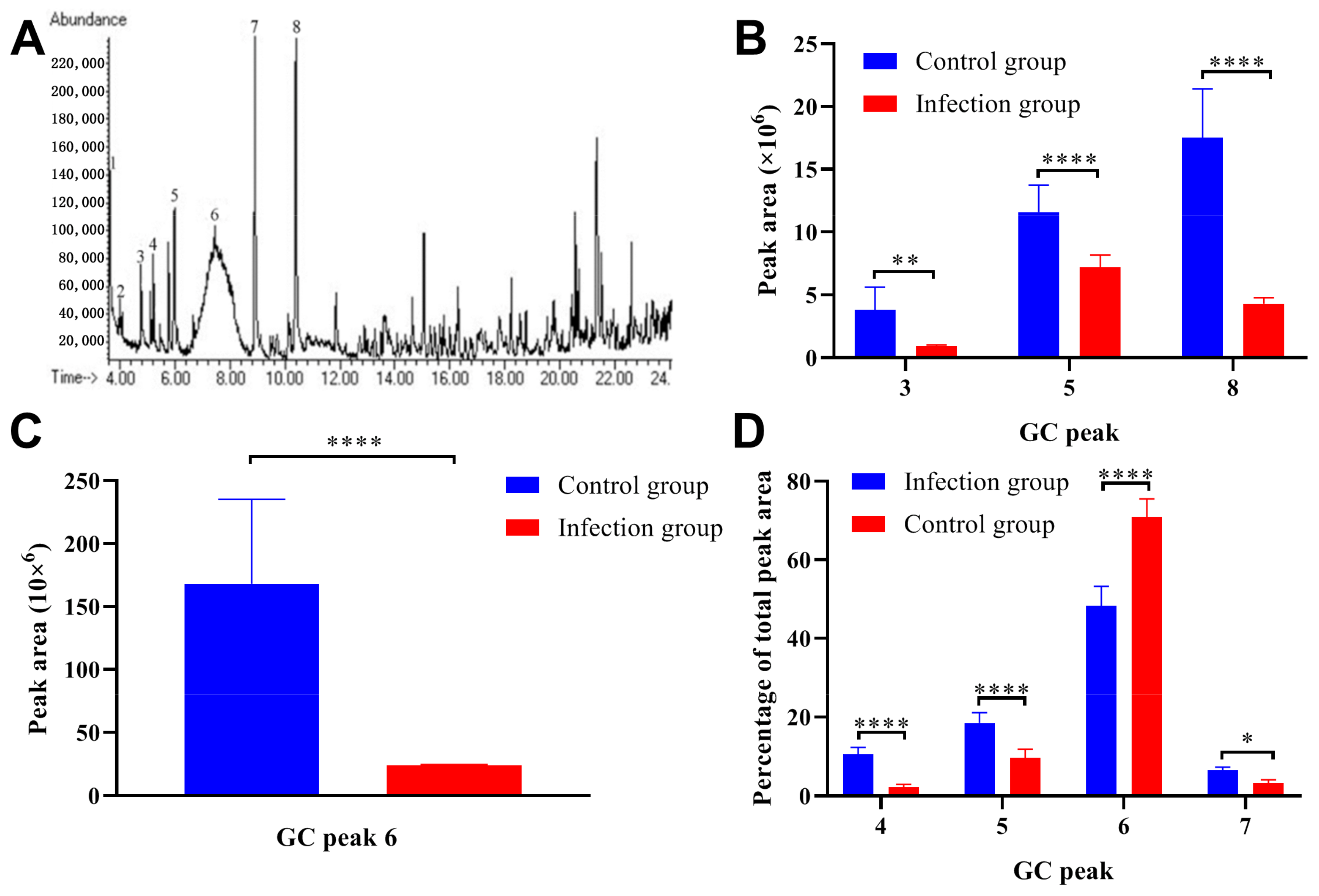

| GC Peak | Name | Peak Area | Percentage of Total Peak Area | ||
|---|---|---|---|---|---|
| Control Group | Infection Group | Control Group | Infection Group | ||
| 1 | E-β-farnesene | 3.91 × 105 ± 5.12 × 104 | 2.38 × 105 ± 3.11 × 104 | 0.39 ± 0.11 | 0.70 ± 0.17 |
| 2 | 2-Heptanone | 6.76 × 105 ± 9.70 × 104 | 4.88 × 105 ± 5.58 × 104 | 0.71 ± 0.24 | 1.33 ± 0.21 |
| 3 | Z-7-tetradecen-1-ol | 3.80 × 106 ± 1.81 × 106 | 9.36 × 105 ± 7.68 × 104 | 1.84 ± 0.39 | 2.69 ± 0.47 |
| 4 | 1-Tetradecanol | 2.35 × 106 ± 3.41 × 105 | 4.12 × 106 ± 7.28 × 105 | 2.22 ± 0.65 | 10.5 ± 1.80 |
| 5 | Dimethyl sulfone | 1.16 × 107 ± 2.13 × 106 | 7.20 × 106 ± 9.82 × 105 | 9.62 ± 2.20 | 18.5 ± 2.63 |
| 6 | 6-Hydroxy-6-methyl-3-heptanone | 1.68 × 108 ± 6.74 × 107 | 2.44 × 107 ± 6.05 × 105 | 70.9 ± 4.60 | 48.3 ± 4.94 |
| 7 | R,R-3,4-dehydro-exo-brevicomin | 6.53 × 106 ± 2.64 × 106 | 3.24 × 106 ± 8.14 × 105 | 3.26 ± 0.84 | 6.54 ± 0.79 |
| 8 | (S)-2-sec-butyl-4,5-dihydrothiazole | 1.75 × 107 ± 3.90 × 106 | 4.30 × 106 ± 4.80 × 105 | 11.00 ± 1.30 | 11.4 ± 1.58 |
| Gene Name | Primer Sequence (5′-3′) | Length (bp) |
|---|---|---|
| Herc4 | 5′-GCCTATGGAGTGTTGGCAGA-3′ | 99 |
| 5′-GACTGCATCTGTCTGGAGCA-3′ | ||
| Mrto4 | 5′-ACCTGATAGAAGAGCTTCGGA-3′ | 256 |
| 5′-TCCTTCGTGCGGTTGGTAAA-3′ | ||
| Ipo11 | 5′-CACACCAGAGCTGCTTCGTA-3′ | 860 |
| 5′-TTTCCATGAGGGACTGGAAG-3′ | ||
| β-actin | 5′-TCCAGCCTTCCTTCTTGGGT-3′ | 111 |
| 5′-GCACTGTGTTGGCATAGAGGT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Zhang, T.; Hu, B.; Han, S.; Xiang, C.; Yuan, G.; He, H. Infection of Trichinella spiralis Affects the Reproductive Capacity of ICR/CD-1 Male Mice by Reducing the Urine Pheromone Contents and Sperm Quality. Int. J. Mol. Sci. 2023, 24, 5731. https://doi.org/10.3390/ijms24065731
Li G, Zhang T, Hu B, Han S, Xiang C, Yuan G, He H. Infection of Trichinella spiralis Affects the Reproductive Capacity of ICR/CD-1 Male Mice by Reducing the Urine Pheromone Contents and Sperm Quality. International Journal of Molecular Sciences. 2023; 24(6):5731. https://doi.org/10.3390/ijms24065731
Chicago/Turabian StyleLi, Gaojian, Tao Zhang, Bin Hu, Shuyi Han, Chen Xiang, Guohui Yuan, and Hongxuan He. 2023. "Infection of Trichinella spiralis Affects the Reproductive Capacity of ICR/CD-1 Male Mice by Reducing the Urine Pheromone Contents and Sperm Quality" International Journal of Molecular Sciences 24, no. 6: 5731. https://doi.org/10.3390/ijms24065731
APA StyleLi, G., Zhang, T., Hu, B., Han, S., Xiang, C., Yuan, G., & He, H. (2023). Infection of Trichinella spiralis Affects the Reproductive Capacity of ICR/CD-1 Male Mice by Reducing the Urine Pheromone Contents and Sperm Quality. International Journal of Molecular Sciences, 24(6), 5731. https://doi.org/10.3390/ijms24065731






