Comparative Gut Microbiome Differences between High and Low Aortic Arch Calcification Score in Patients with Chronic Diseases
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
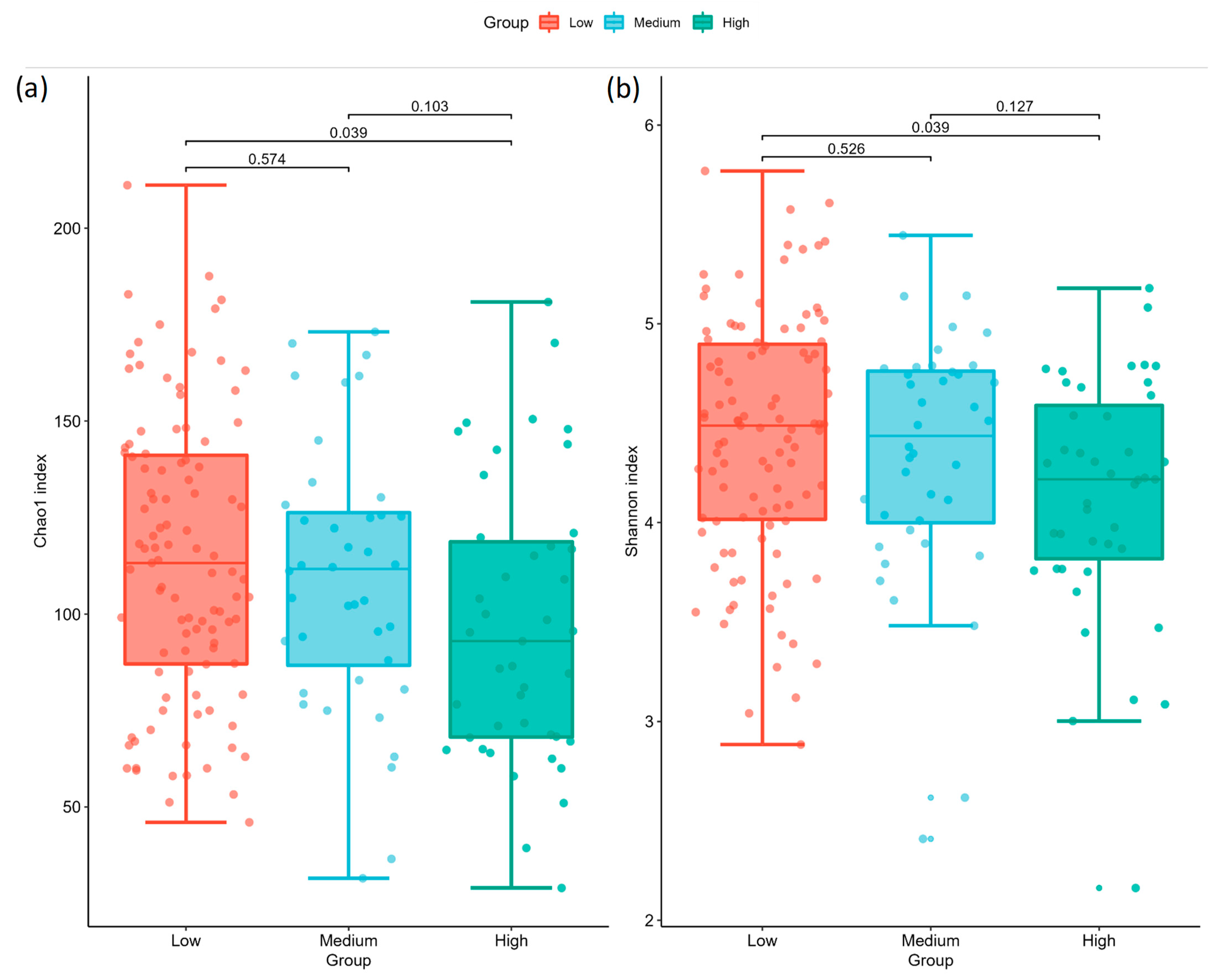
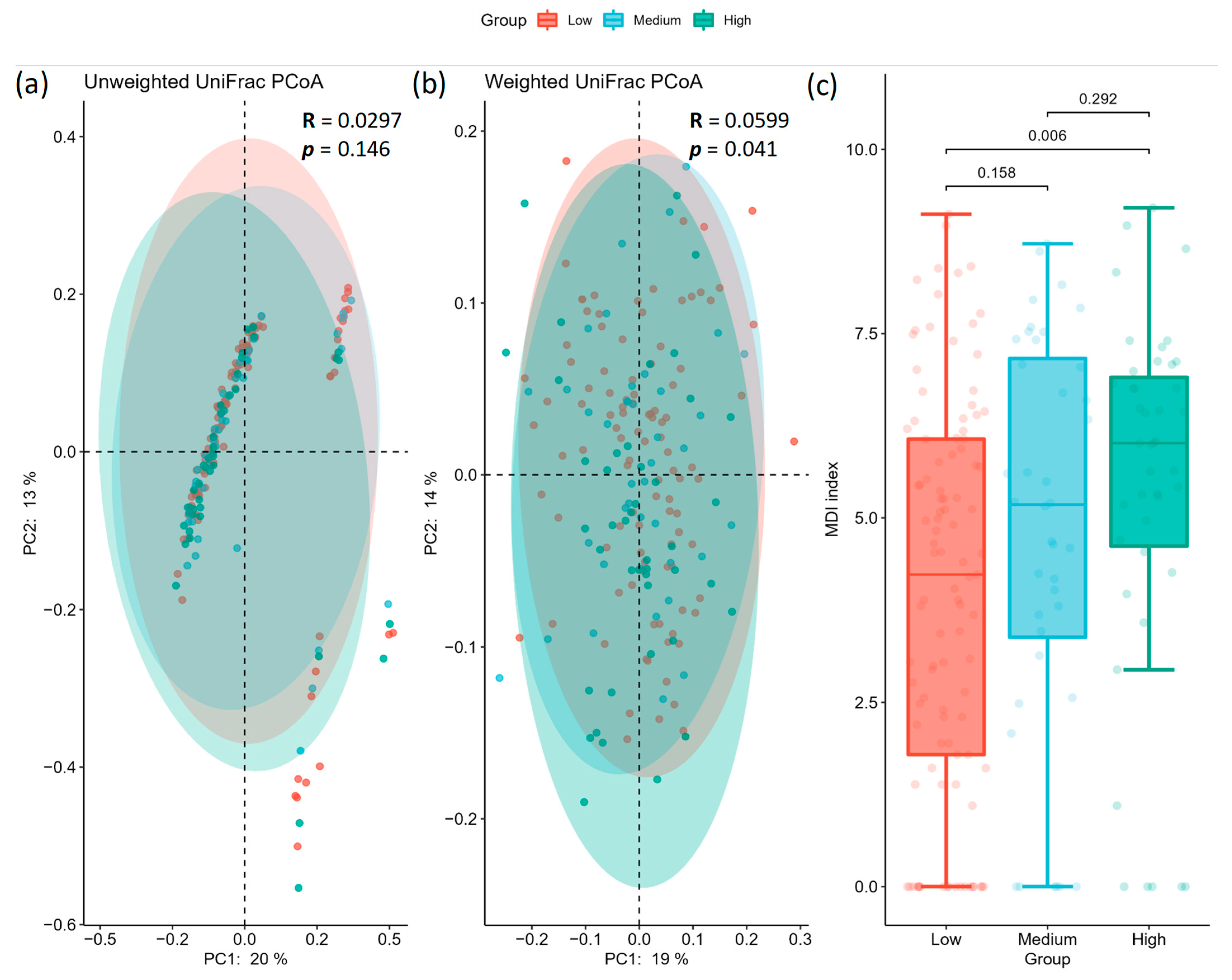
2.2. Gut Microbiota Profile Differs among Different AoAC Severity
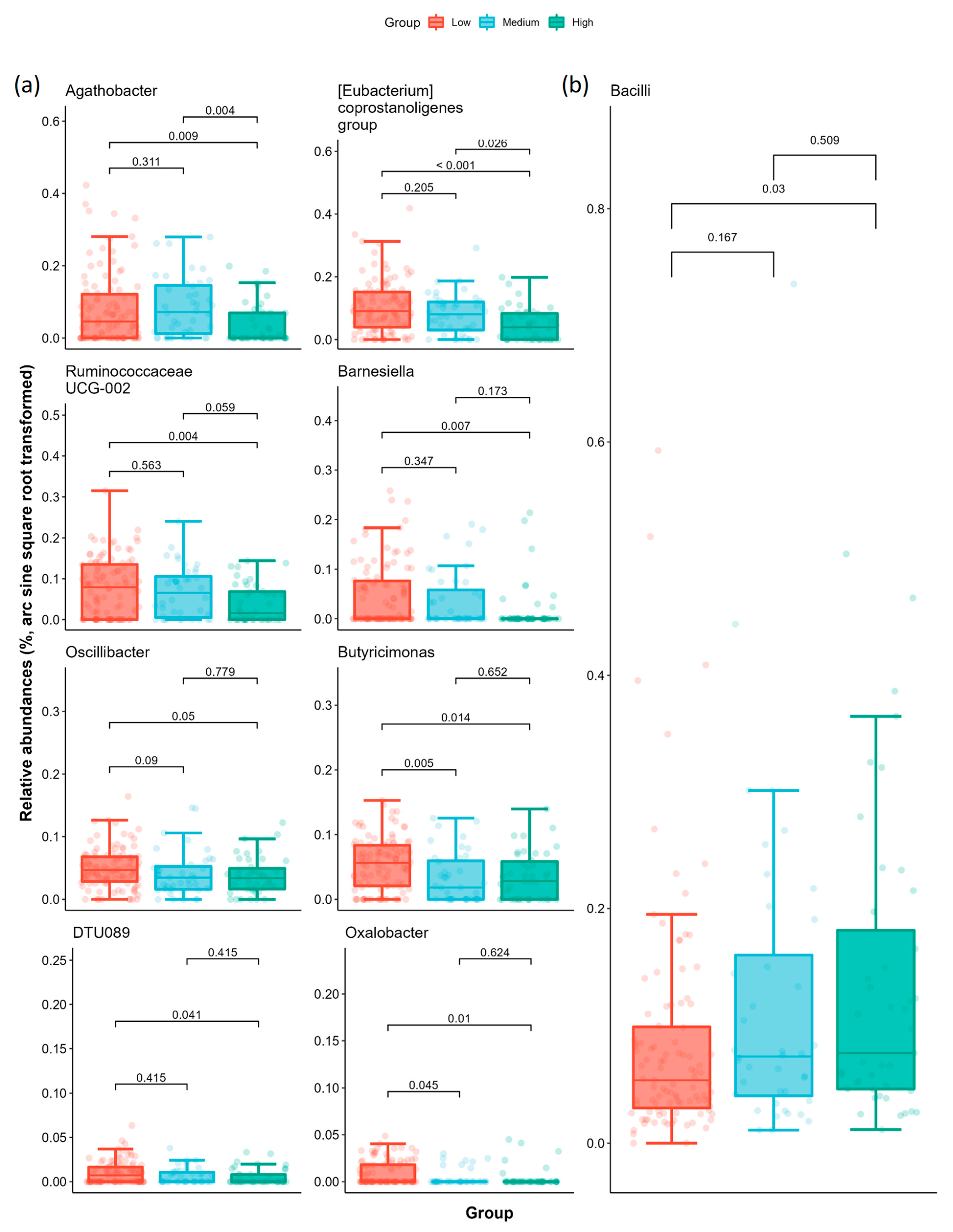
2.3. Specific Microbial Taxa Are Associated with Different AoAC Severity
3. Discussion
4. Materials and Methods
4.1. Study Participants
4.2. Demographic, Medical, and Laboratory Data
4.3. Evaluation of AoAC by Chest Radiography
4.4. Fecal Sample Collection and Bacterial 16S rRNA Amplicon Sequencing and Processing
4.5. Statistical and Bioinformatics Analyses of the Microbiota
4.6. Functional Annotation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Disthabanchong, S.; Boongird, S. Role of different imaging modalities of vascular calcification in predicting outcomes in chronic kidney disease. World J. Nephrol. 2017, 6, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Osborne, M.T.; Tung, B.; Li, M.; Li, Y. Imaging Cardiovascular Calcification. J. Am. Heart Assoc. 2018, 7, e008564. [Google Scholar] [CrossRef]
- Bellasi, A.; Raggi, P. Techniques and technologies to assess vascular calcification. Semin. Dial 2007, 20, 129–133. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Feng, G.; Fan, T.; Jiang, H.; Wang, Z. Advances in CT Techniques in Vascular Calcification. Front. Cardiovasc. Med. 2021, 8, 716822. [Google Scholar] [CrossRef]
- Smith-Bindman, R.; Lipson, J.; Marcus, R.; Kim, K.P.; Mahesh, M.; Gould, R.; Berrington de Gonzalez, A.; Miglioretti, D.L. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch. Intern. Med. 2009, 169, 2078–2086. [Google Scholar] [CrossRef]
- Ogawa, T.; Ishida, H.; Matsuda, N.; Fujiu, A.; Matsuda, A.; Ito, K.; Ando, Y.; Nitta, K. Simple evaluation of aortic arch calcification by chest radiography in hemodialysis patients. Hemodial Int. 2009, 13, 301–306. [Google Scholar] [CrossRef]
- Adar, A.; Erkan, H.; Gokdeniz, T.; Karadeniz, A.; Cavusoglu, I.G.; Onalan, O. Aortic arch calcification is strongly associated with coronary artery calcification. Vasa 2015, 44, 106–114. [Google Scholar] [CrossRef]
- Iijima, K.; Hashimoto, H.; Hashimoto, M.; Son, B.K.; Ota, H.; Ogawa, S.; Eto, M.; Akishita, M.; Ouchi, Y. Aortic arch calcification detectable on chest X-ray is a strong independent predictor of cardiovascular events beyond traditional risk factors. Atherosclerosis 2010, 210, 137–144. [Google Scholar] [CrossRef]
- Ma, X.; Hou, F.; Tian, J.; Zhou, Z.; Ma, Y.; Cheng, Y.; Du, Y.; Shen, H.; Hu, B.; Wang, Z.; et al. Aortic Arch Calcification Is a Strong Predictor of the Severity of Coronary Artery Disease in Patients with Acute Coronary Syndrome. Biomed. Res. Int. 2019, 2019, 7659239. [Google Scholar] [CrossRef]
- Li, L.C.; Lee, Y.T.; Lee, Y.W.; Chou, C.A.; Lee, C.T. Aortic arch calcification predicts the renal function progression in patients with stage 3 to 5 chronic kidney disease. Biomed. Res. Int. 2015, 2015, 131263. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Teh, M.; Huang, J.C.; Wu, P.Y.; Chen, C.Y.; Tsai, Y.C.; Chiu, Y.W.; Chang, J.M.; Chen, H.C. Increased Aortic Arch Calcification and Cardiomegaly is Associated with Rapid Renal Progression and Increased Cardiovascular Mortality in Chronic Kidney Disease. Sci. Rep. 2019, 9, 5354. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, S.; Li, H.; Yang, J.; Wu, H. Aortic arch calcification and risk of cardiovascular or all-cause and mortality in dialysis patients: A meta-analysis. Sci. Rep. 2016, 6, 35375. [Google Scholar] [CrossRef]
- Wu, C.F.; Lee, Y.F.; Lee, W.J.; Su, C.T.; Lee, L.J.; Wu, K.D.; Chen, P.C.; Kao, T.W. Severe aortic arch calcification predicts mortality in patients undergoing peritoneal dialysis. J. Med. Assoc. 2017, 116, 366–372. [Google Scholar] [CrossRef]
- Ogawa, T.; Ishida, H.; Akamatsu, M.; Matsuda, N.; Fujiu, A.; Ito, K.; Ando, Y.; Nitta, K. Progression of aortic arch calcification and all-cause and cardiovascular mortality in chronic hemodialysis patients. Int. Urol. Nephrol. 2010, 42, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.B.; Zhang, W.S.; Jiang, C.Q.; Liu, X.Y.; Jin, Y.L.; Lam, T.H.; Cheng, K.K.; Xu, L. Aortic arch calcification and risk of all-cause mortality and cardiovascular disease: The Guangzhou Biobank Cohort Study. Lancet Reg. Health West Pac. 2022, 23, 100460. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The Human Microbiota in Health and Disease. Engineering 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Malard, F.; Dore, J.; Gaugler, B.; Mohty, M. Introduction to host microbiome symbiosis in health and disease. Mucosal Immunol. 2021, 14, 547–554. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Sniffen, S.; McGill Percy, K.C.; Pallaval, V.B.; Chidipi, B. Gut Dysbiosis and Immune System in Atherosclerotic Cardiovascular Disease (ACVD). Microorganisms 2022, 10, 108. [Google Scholar] [CrossRef]
- Rajendiran, E.; Ramadass, B.; Ramprasath, V. Understanding connections and roles of gut microbiome in cardiovascular diseases. Can. J. Microbiol. 2021, 67, 101–111. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Fak, F.; Nookaew, I.; Tremaroli, V.; Fagerberg, B.; Petranovic, D.; Backhed, F.; Nielsen, J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 2012, 3, 1245. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, X.; Hu, X.; Niu, H.; Tian, R.; Wang, H.; Pang, H.; Jiang, L.; Qiu, B.; Chen, X.; et al. Alterations in the gut microbiome and metabolism with coronary artery disease severity. Microbiome 2019, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Lindskog Jonsson, A.; Hallenius, F.F.; Akrami, R.; Johansson, E.; Wester, P.; Arnerlov, C.; Backhed, F.; Bergstrom, G. Bacterial profile in human atherosclerotic plaques. Atherosclerosis 2017, 263, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Ziganshina, E.E.; Sharifullina, D.M.; Lozhkin, A.P.; Khayrullin, R.N.; Ignatyev, I.M.; Ziganshin, A.M. Bacterial Communities Associated with Atherosclerotic Plaques from Russian Individuals with Atherosclerosis. PLoS ONE 2016, 11, e0164836. [Google Scholar] [CrossRef]
- Mitra, S.; Drautz-Moses, D.I.; Alhede, M.; Maw, M.T.; Liu, Y.; Purbojati, R.W.; Yap, Z.H.; Kushwaha, K.K.; Gheorghe, A.G.; Bjarnsholt, T.; et al. In silico analyses of metagenomes from human atherosclerotic plaque samples. Microbiome 2015, 3, 38. [Google Scholar] [CrossRef]
- Koren, O.; Spor, A.; Felin, J.; Fak, F.; Stombaugh, J.; Tremaroli, V.; Behre, C.J.; Knight, R.; Fagerberg, B.; Ley, R.E.; et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4592–4598. [Google Scholar] [CrossRef]
- Kashtanova, D.A.; Tkacheva, O.N.; Doudinskaya, E.N.; Strazhesko, I.D.; Kotovskaya, Y.V.; Popenko, A.S.; Tyakht, A.V.; Alexeev, D.G. Gut Microbiota in Patients with Different Metabolic Statuses: Moscow Study. Microorganisms 2018, 6, 98. [Google Scholar] [CrossRef]
- Menni, C.; Lin, C.; Cecelja, M.; Mangino, M.; Matey-Hernandez, M.L.; Keehn, L.; Mohney, R.P.; Steves, C.J.; Spector, T.D.; Kuo, C.F.; et al. Gut microbial diversity is associated with lower arterial stiffness in women. Eur. Heart J. 2018, 39, 2390–2397. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, A.L.; Backhed, F. Role of gut microbiota in atherosclerosis. Nat. Rev. Cardiol. 2017, 14, 79–87. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Lau, K.; Srivatsav, V.; Rizwan, A.; Nashed, A.; Liu, R.; Shen, R.; Akhtar, M. Bridging the Gap between Gut Microbial Dysbiosis and Cardiovascular Diseases. Nutrients 2017, 9, 859. [Google Scholar] [CrossRef] [PubMed]
- Duttaroy, A.K. Role of Gut Microbiota and Their Metabolites on Atherosclerosis, Hypertension and Human Blood Platelet Function: A Review. Nutrients 2021, 13, 144. [Google Scholar] [CrossRef]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Ohira, H.; Tsutsui, W.; Fujioka, Y. Are Short Chain Fatty Acids in Gut Microbiota Defensive Players for Inflammation and Atherosclerosis? J Atheroscler. Thromb. 2017, 24, 660–672. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Manichanh, C.; Rigottier-Gois, L.; Bonnaud, E.; Gloux, K.; Pelletier, E.; Frangeul, L.; Nalin, R.; Jarrin, C.; Chardon, P.; Marteau, P.; et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut 2006, 55, 205–211. [Google Scholar] [CrossRef]
- Sun, S.; Lulla, A.; Sioda, M.; Winglee, K.; Wu, M.C.; Jacobs, D.R., Jr.; Shikany, J.M.; Lloyd-Jones, D.M.; Launer, L.J.; Fodor, A.A.; et al. Gut Microbiota Composition and Blood Pressure. Hypertension 2019, 73, 998–1006. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Correa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef]
- Nogal, A.; Valdes, A.M.; Menni, C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes 2021, 13, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J., Jr.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G.; et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yi, C.X.; Katiraei, S.; Kooijman, S.; Zhou, E.; Chung, C.K.; Gao, Y.; van den Heuvel, J.K.; Meijer, O.C.; Berbee, J.F.P.; et al. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut 2018, 67, 1269–1279. [Google Scholar] [CrossRef]
- Yadav, H.; Lee, J.H.; Lloyd, J.; Walter, P.; Rane, S.G. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J. Biol. Chem. 2013, 288, 25088–25097. [Google Scholar] [CrossRef]
- Gao, F.; Lv, Y.W.; Long, J.; Chen, J.M.; He, J.M.; Ruan, X.Z.; Zhu, H.B. Butyrate Improves the Metabolic Disorder and Gut Microbiome Dysbiosis in Mice Induced by a High-Fat Diet. Front. Pharmacol. 2019, 10, 1040. [Google Scholar] [CrossRef]
- Zhang, J.M.; Sun, Y.S.; Zhao, L.Q.; Chen, T.T.; Fan, M.N.; Jiao, H.C.; Zhao, J.P.; Wang, X.J.; Li, F.C.; Li, H.F.; et al. SCFAs-Induced GLP-1 Secretion Links the Regulation of Gut Microbiome on Hepatic Lipogenesis in Chickens. Front. Microbiol. 2019, 10, 2176. [Google Scholar] [CrossRef]
- Meehan, C.J.; Beiko, R.G. A phylogenomic view of ecological specialization in the Lachnospiraceae, a family of digestive tract-associated bacteria. Genome Biol. Evol. 2014, 6, 703–713. [Google Scholar] [CrossRef]
- Pinna, N.K.; Anjana, R.M.; Saxena, S.; Dutta, A.; Gnanaprakash, V.; Rameshkumar, G.; Aswath, S.; Raghavan, S.; Rani, C.S.S.; Radha, V.; et al. Trans-ethnic gut microbial signatures of prediabetic subjects from India and Denmark. Genome Med. 2021, 13, 36. [Google Scholar] [CrossRef]
- Verhaar, B.J.H.; Prodan, A.; Nieuwdorp, M.; Muller, M. Gut Microbiota in Hypertension and Atherosclerosis: A Review. Nutrients 2020, 12, 2982. [Google Scholar] [CrossRef]
- He, Y.; Wu, W.; Wu, S.; Zheng, H.M.; Li, P.; Sheng, H.F.; Chen, M.X.; Chen, Z.H.; Ji, G.Y.; Zheng, Z.D.; et al. Linking gut microbiota, metabolic syndrome and economic status based on a population-level analysis. Microbiome 2018, 6, 172. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhuo, L.B.; He, Y.; Fu, Y.; Shen, L.; Xu, F.; Gou, W.; Miao, Z.; Shuai, M.; Liang, Y.; et al. The gut microbiota-bile acid axis links the positive association between chronic insomnia and cardiometabolic diseases. Nat. Commun. 2022, 13, 3002. [Google Scholar] [CrossRef]
- Farsijani, S.; Cauley, J.A.; Peddada, S.D.; Langsetmo, L.; Shikany, J.M.; Orwoll, E.S.; Ensrud, K.E.; Cawthon, P.M.; Newman, A.B. Relationship Between Dietary Protein Intake and Gut Microbiome Composition in Community-Dwelling Older Men: Findings from the MrOS Study. J. Nutr. 2022, 152, 2877–2887. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, N.; Wang, X.; Chi, Y.; Zhang, Y.; Qiu, X.; Hu, Y.; Li, J.; Liu, Y. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci. Rep. 2015, 5, 8096. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef]
- Liu, X.; Tong, X.; Zou, Y.; Lin, X.; Zhao, H.; Tian, L.; Jie, Z.; Wang, Q.; Zhang, Z.; Lu, H.; et al. Mendelian randomization analyses support causal relationships between blood metabolites and the gut microbiome. Nat. Genet. 2022, 54, 52–61. [Google Scholar] [CrossRef]
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology 2013, 57, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, Y.; Kim, J.; An, J.; Lee, S.; Kong, H.; Song, Y.; Lee, C.K.; Kim, K. Modulation of the gut microbiota by metformin improves metabolic profiles in aged obese mice. Gut Microbes 2018, 9, 155–165. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; An, J.; Song, Y.; Lee, C.K.; Kim, K.; Kong, H. Alterations in Gut Microbiota by Statin Therapy and Possible Intermediate Effects on Hyperglycemia and Hyperlipidemia. Front. Microbiol. 2019, 10, 1947. [Google Scholar] [CrossRef]
- Lee, H.; An, J.; Kim, J.; Choi, D.; Song, Y.; Lee, C.K.; Kong, H.; Kim, S.B.; Kim, K. A Novel Bacterium, Butyricimonas virosa, Preventing HFD-Induced Diabetes and Metabolic Disorders in Mice via GLP-1 Receptor. Front. Microbiol. 2022, 13, 858192. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Indias, I.; Sanchez-Alcoholado, L.; Garcia-Fuentes, E.; Cardona, F.; Queipo-Ortuno, M.I.; Tinahones, F.J. Insulin resistance is associated with specific gut microbiota in appendix samples from morbidly obese patients. Am. J. Transl. Res. 2016, 8, 5672–5684. [Google Scholar] [PubMed]
- Gong, J.; Shen, Y.; Zhang, H.; Cao, M.; Guo, M.; He, J.; Zhang, B.; Xiao, C. Gut Microbiota Characteristics of People with Obesity by Meta-Analysis of Existing Datasets. Nutrients 2022, 14, 2993. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lordan, C.; Ross, R.P.; Cotter, P.D. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes 2020, 12, 1802866. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Buhman, K.K.; Hartman, P.A.; Beitz, D.C. Hypocholesterolemic effect of Eubacterium coprostanoligenes ATCC 51222 in rabbits. Lett. Appl. Microbiol. 1995, 20, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Kenny, D.J.; Plichta, D.R.; Shungin, D.; Koppel, N.; Hall, A.B.; Fu, B.; Vasan, R.S.; Shaw, S.Y.; Vlamakis, H.; Balskus, E.P.; et al. Cholesterol Metabolism by Uncultured Human Gut Bacteria Influences Host Cholesterol Level. Cell Host Microbe 2020, 28, 245–257.e246. [Google Scholar] [CrossRef]
- Matthan, N.R.; Pencina, M.; LaRocque, J.M.; Jacques, P.F.; D’Agostino, R.B.; Schaefer, E.J.; Lichtenstein, A.H. Alterations in cholesterol absorption/synthesis markers characterize Framingham offspring study participants with CHD. J. Lipid Res. 2009, 50, 1927–1935. [Google Scholar] [CrossRef]
- Veiga, P.; Juste, C.; Lepercq, P.; Saunier, K.; Beguet, F.; Gerard, P. Correlation between faecal microbial community structure and cholesterol-to-coprostanol conversion in the human gut. FEMS Microbiol. Lett. 2005, 242, 81–86. [Google Scholar] [CrossRef]
- Lupp, C.; Robertson, M.L.; Wickham, M.E.; Sekirov, I.; Champion, O.L.; Gaynor, E.C.; Finlay, B.B. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2007, 2, 204. [Google Scholar] [CrossRef]
- Ubeda, C.; Bucci, V.; Caballero, S.; Djukovic, A.; Toussaint, N.C.; Equinda, M.; Lipuma, L.; Ling, L.; Gobourne, A.; No, D.; et al. Intestinal microbiota containing Barnesiella species cures vancomycin-resistant Enterococcus faecium colonization. Infect. Immun. 2013, 81, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Presley, L.L.; Wei, B.; Braun, J.; Borneman, J. Bacteria associated with immunoregulatory cells in mice. Appl. Environ. Microbiol. 2010, 76, 936–941. [Google Scholar] [CrossRef]
- Kaufman, D.W.; Kelly, J.P.; Curhan, G.C.; Anderson, T.E.; Dretler, S.P.; Preminger, G.M.; Cave, D.R. Oxalobacter formigenes may reduce the risk of calcium oxalate kidney stones. J. Am. Soc. Nephrol. 2008, 19, 1197–1203. [Google Scholar] [CrossRef]
- Daniel, S.L.; Moradi, L.; Paiste, H.; Wood, K.D.; Assimos, D.G.; Holmes, R.P.; Nazzal, L.; Hatch, M.; Knight, J. Forty Years of Oxalobacter formigenes, a Gutsy Oxalate-Degrading Specialist. Appl. Environ. Microbiol. 2021, 87, e0054421. [Google Scholar] [CrossRef]
- Matsui, Y.; Rittling, S.R.; Okamoto, H.; Inobe, M.; Jia, N.; Shimizu, T.; Akino, M.; Sugawara, T.; Morimoto, J.; Kimura, C.; et al. Osteopontin deficiency attenuates atherosclerosis in female apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1029–1034. [Google Scholar] [CrossRef]
- Asokan, D.; Kalaiselvi, P.; Varalakshmi, P. Modulatory effect of the 23-kD calcium oxalate monohydrate binding protein on calcium oxalate stone formation during oxalate stress. Nephron Physiol. 2004, 97, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Willis, A.D. Rarefaction, alpha diversity, and statistics. Front. Microbiol. 2019, 10, 2407. [Google Scholar] [CrossRef] [PubMed]
- Colwell, R.K.; Mao, C.X.; Chang, J. Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 2004, 85, 2717–2727. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois: Urbana, IL, USA, 1949; Volume 117. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A New Phylogenetic Method for Comparing Microbial Communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The Treatment-Naive Microbiome in New-Onset Crohn’s Disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Abdi, H. Holm’s sequential Bonferroni procedure. Encycl. Res. Des. 2010, 1, 1–8. [Google Scholar]
- Mallick, H.; Rahnavard, A.; McIver, L.J.; Ma, S.; Zhang, Y.; Nguyen, L.H.; Tickle, T.L.; Weingart, G.; Ren, B.; Schwager, E.H.; et al. Multivariable association discovery in population-scale meta-omics studies. PLOS Comput. Biol. 2021, 17, e1009442. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2007, 36, D480–D484. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Low AoAC Group AoAC ≤ 3 (N = 103) | Medium AoAC Group 3 < AoAC ≤ 6 (N = 40) | High AoAC Group AoAC > 6 (N = 43) | p |
|---|---|---|---|---|
| AoAC score | 1.17 ± 1.20 | 4.75 ± 0.58 * | 8.51 ± 2.06 *† | < 0.001 |
| Age (year) | 61.56 ± 9.54 | 69.93 ± 7.98 * | 71.63 ± 9.31 *† | < 0.001 |
| Male (%) | 66.99% | 67.50% | 51.16% | 0.164 |
| BMI | 26.65 ± 4.83 | 26.38 ± 4.12 | 26.13 ± 3.22 | 0.802 |
| SBP (mmHg) | 134.91 ± 17.30 | 137.03 ± 17.55 | 141.30 ± 21.51 | 0.163 |
| DBP (mmHg) | 73.55 ± 12.98 | 68.53 ± 9.58 | 67.33 ± 12.37 * | 0.007 |
| Comorbidities | ||||
| Diabetes mellitus | 85.44% | 65.00% * | 83.72% | 0.017 |
| Hypertension | 68.93% | 75.00% | 90.70% * | 0.021 |
| Chronic kidney disease | 40.78% | 57.50% | 60.47% | 0.045 |
| Cardiovascular disease | 6.80% | 15.00% | 9.30% | 0.314 |
| Cerebrovascular disease | 0.97% | 5% | 2.33% | 0.332 |
| Medications | ||||
| ACEI/ARB | 66.02% | 62.50% | 67.44% | 0.887 |
| Beta-blocker | 14.56% | 25.00% | 34.88% * | 0.020 |
| CCB | 44.66% | 45.00% | 69.77% * | 0.016 |
| Diuretic | 21.36% | 30.00% | 30.23% | 0.397 |
| Statins | 66.02% | 67.50% | 72.09% | 0.777 |
| Fibrate | 4.85% | 10.00% | 0% | 0.106 |
| OHA | 78.64% | 62.50% | 81.40% | 0.081 |
| Insulin | 16.50% | 17.50% | 16.28% | 0.987 |
| Clinical laboratory data | ||||
| Hemoglobin (g/dL) | 13.48 ± 1.92 | 12.27 ± 2.20 * | 12.46 ± 2.24 * | 0.001 |
| Albumin (g/dL) | 4.17 ± 0.24 | 4.12 ± 0.23 | 4.11 ± 0.24 | 0.269 |
| HbA1c (%) | 7.18 ± 1.22 | 6.58 ± 1.02 * | 6.99 ± 1.10 | 0.020 |
| eGFR (mL/min/1.73 m2) | 56.19 ± 22.55 | 43.54 ± 24.72 * | 44.50 ± 22.02 * | 0.002 |
| Triglycerides (mg/dL) | 122.42 (108.07–136.77) | 143.25 (113.93–172.57) | 131.98 (104.60–159.35) | 0.377 |
| Total cholesterol (mg/dL) | 162.46 ± 33.74 | 164.80 ± 44.80 | 165.42 ± 34.79 | 0.884 |
| HDL-cholesterol (mg/dL) | 46.58 ± 10.12 | 43.26 ± 9.56 | 48.53 ± 11.45 | 0.064 |
| LDL-cholesterol (mg/dL) | 86.07 ± 28.16 | 83.93 ± 34.51 | 81.37 ± 21.21 | 0.651 |
| Total Calcium (mg/dL) | 9.17 ± 0.37 | 9.07 ± 0.39 | 9.17 ± 0.47 | 0.359 |
| Phosphorous (mg/dL) | 3.51 ± 0.52 | 3.76 ± 0.81 | 3.68 ± 0.68 | 0.063 |
| (a) Chao1 | ||||
| Group 1 | Group 2 | H | p | Adjusted p |
| Low | Medium | 0.316048 | 0.573992 | 0.573992 |
| Medium | High | 3.322329 | 0.068345 | 0.102517 |
| Low | High | 6.179246 | 0.012926 | 0.038777 |
| (b) Shannon | ||||
| Group 1 | Group 2 | H | p | Adjusted p |
| Low | Medium | 0.402124 | 0.525994 | 0.525994 |
| Medium | High | 2.966860 | 0.084987 | 0.127480 |
| Low | High | 6.189636 | 0.012850 | 0.038550 |
| (a) unweighted | ||||
| Group 1 | Group 2 | R | p | Adjusted p |
| Low | Medium | 0.029929 | 0.232 | 0.278 |
| Medium | High | 0.004101 | 0.278 | 0.278 |
| Low | High | 0.034003 | 0.168 | 0.278 |
| (b) weighted | ||||
| Group 1 | Group 2 | R | p | Adjusted p |
| Low | Medium | 0.022670 | 0.283 | 0.4245 |
| Medium | High | −0.003829 | 0.544 | 0.5440 |
| Low | High | 0.105367 | 0.006 | 0.0180 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.-H.; Peng, P.; Hung, W.-C.; Wu, P.-H.; Kao, C.-Y.; Wu, P.-Y.; Huang, J.-C.; Hung, C.-H.; Su, H.-M.; Chen, S.-C.; et al. Comparative Gut Microbiome Differences between High and Low Aortic Arch Calcification Score in Patients with Chronic Diseases. Int. J. Mol. Sci. 2023, 24, 5673. https://doi.org/10.3390/ijms24065673
Liu Y-H, Peng P, Hung W-C, Wu P-H, Kao C-Y, Wu P-Y, Huang J-C, Hung C-H, Su H-M, Chen S-C, et al. Comparative Gut Microbiome Differences between High and Low Aortic Arch Calcification Score in Patients with Chronic Diseases. International Journal of Molecular Sciences. 2023; 24(6):5673. https://doi.org/10.3390/ijms24065673
Chicago/Turabian StyleLiu, Yi-Hsueh, Po Peng, Wei-Chun Hung, Ping-Hsun Wu, Cheng-Yuan Kao, Pei-Yu Wu, Jiun-Chi Huang, Chih-Hsing Hung, Ho-Ming Su, Szu-Chia Chen, and et al. 2023. "Comparative Gut Microbiome Differences between High and Low Aortic Arch Calcification Score in Patients with Chronic Diseases" International Journal of Molecular Sciences 24, no. 6: 5673. https://doi.org/10.3390/ijms24065673
APA StyleLiu, Y.-H., Peng, P., Hung, W.-C., Wu, P.-H., Kao, C.-Y., Wu, P.-Y., Huang, J.-C., Hung, C.-H., Su, H.-M., Chen, S.-C., & Kuo, C.-H. (2023). Comparative Gut Microbiome Differences between High and Low Aortic Arch Calcification Score in Patients with Chronic Diseases. International Journal of Molecular Sciences, 24(6), 5673. https://doi.org/10.3390/ijms24065673






