Zebrafish as a Model of Cardiac Pathology and Toxicity: Spotlight on Uremic Toxins
Abstract
1. Introduction
2. Zebrafish as a Model at the Forefront of Research
- Replacement: Zebrafish is an experimental model to avoid or replace the use of animals. This includes larvae assays, which offer a model of lower potential for pain;
- Reduction: Its use reduces animal numbers without compromising on animal welfare and is statistically significant in experimental results. Zebrafish larvae, as a first-tier model for toxicity, are used to identify the effect of different compounds with a reduction in the number of animals used in testing;
- Refine: It allows us to find new ways of minimizing the suffering and pain of animals. In this case, embryos and larvae, with external fertilization and the transparency of the body during development, represent a valid non-invasive observation of toxicities.
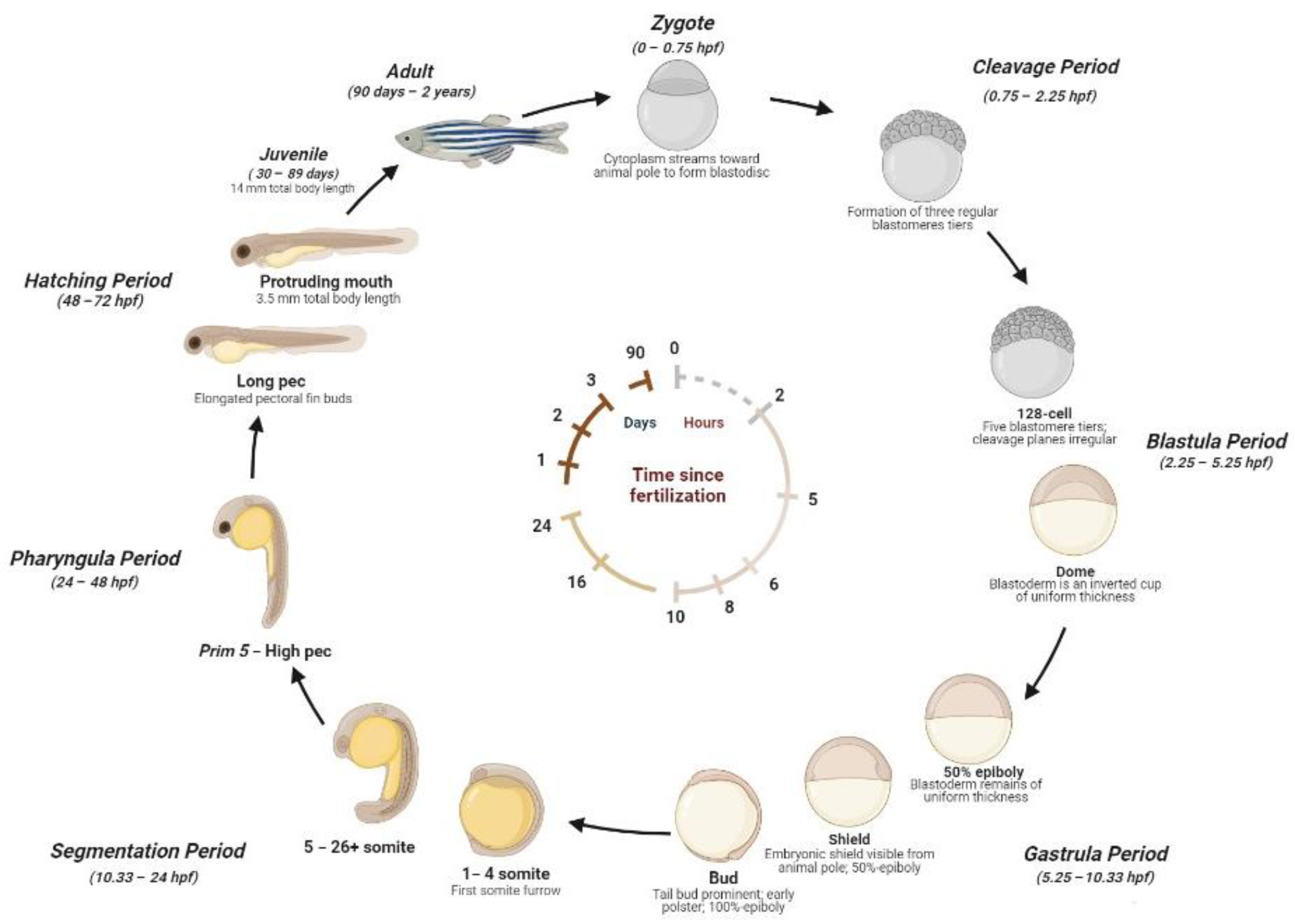
2.1. Zebrafish Life Cycle
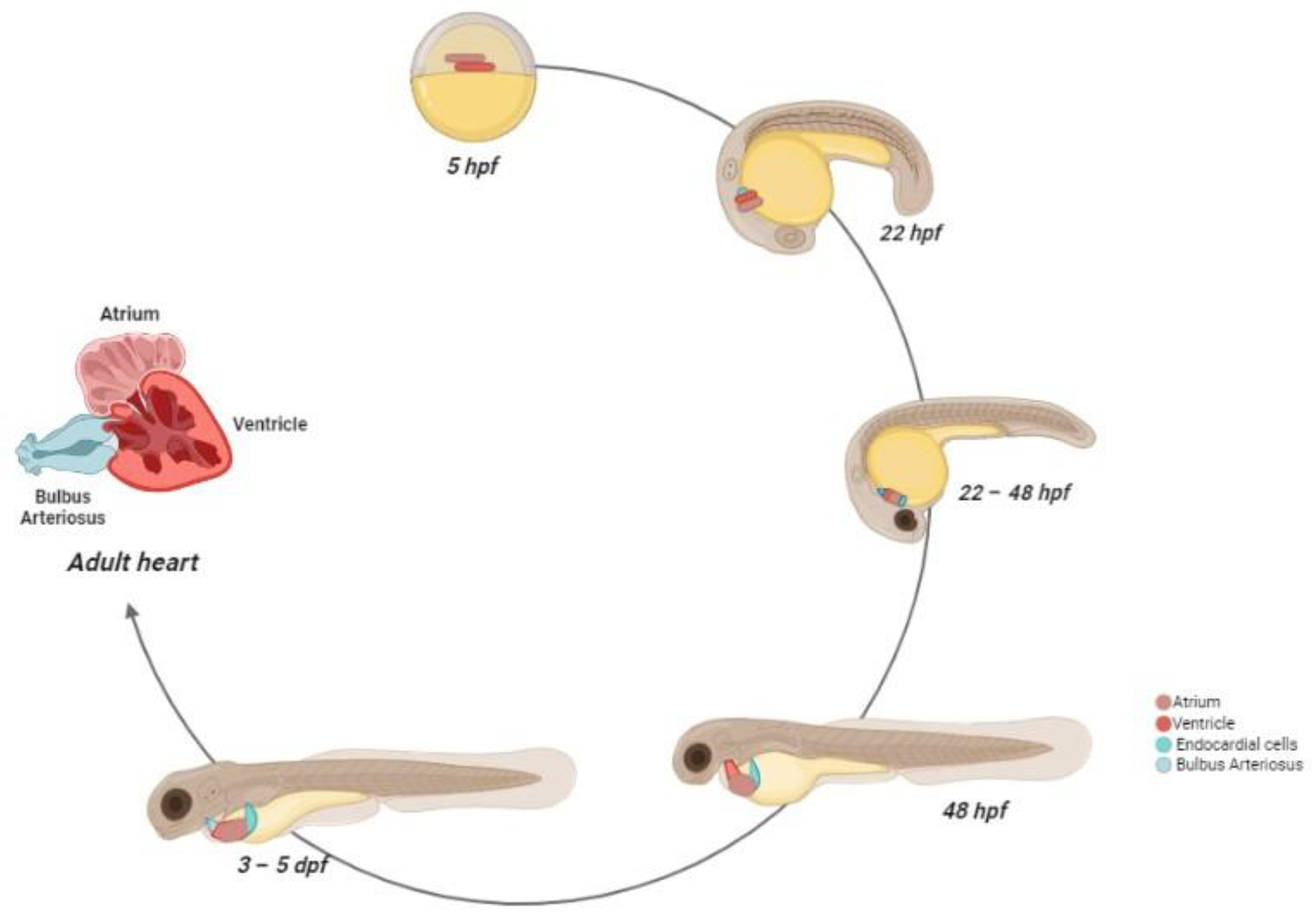
2.2. Zebrafish Cardiovascular System: Morphogenesis and Physiology
2.3. Zebrafish Vascular System: Vessels Morphogenesis and Structure
3. Zebrafish to Study Human Cardiac and Vessel Alterations
3.1. Genetic Toolbox
3.2. Genetic Cardiac Alterations
3.3. Vessel Alterations
4. Uremic Toxins
4.1. Free Water-Soluble Low-Molecular Weight Compounds
4.2. Medium Uremic Compounds
4.3. Protein-Bound Compounds
5. Zebrafish as a Model to Study Uremic Cardiotoxicity
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C. A Single Number for Advocacy and Communication-Worldwide More than 850 Million Individuals Have Kidney Diseases. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2019, 34, 1803–1805. [Google Scholar]
- Vaidya, S.R.; Aeddula, N.R. Chronic Renal Failure. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Major, R.W.; Cheng, M.R.I.; Grant, R.A.; Shantikumar, S.; Xu, G.; Oozeerally, I.; Brunskill, N.J.; Gray, L.J. Cardiovascular Disease Risk Factors in Chronic Kidney Disease: A Systematic Review and Meta-Analysis. PLoS ONE 2018, 13, e0192895. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, A.M.; Tawfik, H.M. Nontraditional Risk Factors in Chronic Kidney Disease: Correlation between Creatinine Clearance, Framingham Risk Score, Endothelial Dysfunction, and Inflammation. Egypt. J. Intern. Med. 2022, 34, 29. [Google Scholar] [CrossRef]
- Glassock, R.J. Uremic Toxins: What Are They? An Integrated Overview of Pathobiology and Classification. J. Ren. Nutr. 2008, 18, 2–6. [Google Scholar] [CrossRef]
- Falconi, C.A.; da Cruz Junho, C.V.; Fogaça-Ruiz, F.; Vernier, I.C.S.; da Cunha, R.S.; Stinghen, A.E.M.; Carneiro-Ramos, M.S. Uremic Toxins: An Alarming Danger Concerning the Cardiovascular System. Front. Physiol. 2021, 12, 686249. [Google Scholar] [CrossRef]
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R.; et al. Review on Uremic Toxins: Classification, Concentration, and Interindividual Variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef]
- Lekawanvijit, S. Cardiotoxicity of Uremic Toxins: A Driver of Cardiorenal Syndrome. Toxins 2018, 10, 352. [Google Scholar] [CrossRef]
- El Chamieh, C.; Liabeuf, S.; Massy, Z. Uremic Toxins and Cardiovascular Risk in Chronic Kidney Disease: What Have We Learned Recently beyond the Past Findings? Toxins 2022, 14, 280. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 055, 248. [Google Scholar] [CrossRef]
- Terriente, J.; Pujades, C. Use of Zebrafish Embryos for Small Molecule Screening Related to Cancer. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2013, 242, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Maddison, L.A.; Chen, W. Zebrafish as a Model for Obesity and Diabetes. Front. Cell Dev. Biol. 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, S.; Hoffman, E.J. Zebrafish: A Translational Model System for Studying Neuropsychiatric Disorders. J. Am. Acad. Child Adolesc. Psychiatry 2016, 55, 746–748. [Google Scholar] [CrossRef] [PubMed]
- Cassar, S.; Adatto, I.; Freeman, J.L.; Gamse, J.T.; Iturria, I.; Lawrence, C.; Muriana, A.; Peterson, R.T.; Van Cruchten, S.; Zon, L.I. Use of Zebrafish in Drug Discovery Toxicology. Chem. Res. Toxicol 2020, 33, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Teame, T.; Zhang, Z.; Ran, C.; Zhang, H.; Yang, Y.; Ding, Q.; Xie, M.; Gao, C.; Ye, Y.; Duan, M.; et al. The Use of Zebrafish (Danio Rerio) as Biomedical Models. Anim. Front. Rev. Mag. Anim. Agric. 2019, 9, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Parichy, D.M. Advancing Biology through a Deeper Understanding of Zebrafish Ecology and Evolution. eLife 2015, 4, e05635. [Google Scholar] [CrossRef]
- Collins, M.M.; Ahlberg, G.; Hansen, C.V.; Guenther, S.; Marín-Juez, R.; Sokol, A.M.; El-Sammak, H.; Piesker, J.; Hellsten, Y.; Olesen, M.S.; et al. Early sarcomere and metabolic defects in a zebrafish pitx2c cardiac arrhythmia model. Proc. Natl. Acad. Sci. USA 2019, 116, 24115–24121. [Google Scholar] [CrossRef]
- Müller, I.I.; Melville, D.B.; Tanwar, V.; Rybski, W.M.; Mukherjee, A.; Shoemaker, M.B.; Wang, W.D.; Schoenhard, J.A.; Roden, D.M.; Darbar, D.; et al. Functional modeling in zebrafish demonstrates that the atrial-fibrillation-associated gene GREM2 regulates cardiac laterality, cardiomyocyte differentiation and atrial rhythm. Dis. Models Mech. 2013, 2, 332–341. [Google Scholar]
- Yan, J.; Li, H.; Bu, H.; Jiao, K.; Zhang, A.X.; Le, T.; Cao, H.; Li, Y.; Ding, Y.; Xu, X. Aging-associated sinus arrest and sick sinus syndrome in adult zebrafish. PLoS ONE 2020, 5, e0232457. [Google Scholar] [CrossRef]
- Ducharme, N.A.; Reif, D.M.; Gustafsson, J.-A.; Bondesson, M. Comparison of Toxicity Values across Zebrafish Early Life Stages and Mammalian Studies: Implications for Chemical Testing. Reprod. Toxicol. 2015, 55, 3–10. [Google Scholar] [CrossRef]
- Strähle, U.; Scholz, S.; Geisler, R.; Greiner, P.; Hollert, H.; Rastegar, S.; Schumacher, A.; Selderslaghs, I.; Weiss, C.; Witters, H.; et al. Zebrafish Embryos as an Alternative to Animal Experiments--a Commentary on the Definition of the Onset of Protected Life Stages in Animal Welfare Regulations. Reprod. Toxicol. 2012, 33, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Streisinger, G.; Walker, C.; Dower, N.; Knauber, D.; Singer, F. Production of Clones of Homozygous Diploid Zebra Fish (Brachydanio Rerio). Nature 1981, 291, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.; Streisinger, G. Induction of mutations by γ-rays in pregonial germ cells of zebrafish embryos. Genetics 1983, 103, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, D.J.; Kimmel, C.B.; Westerfield, M.; Walker, C.; Streisinger, G. A Neural Degeneration Mutation That Spares Primary Neurons in the Zebrafish. Dev. Biol. 1988, 126, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Blader, P.; Strähle, U. Zebrafish Developmental Genetics and Central Nervous System. Dev. Hum. Mol. Genet. 2000, 9, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Diotel, N.; Lübke, L.; Strähle, U.; Rastegar, S. Common and Distinct Features of Adult Neurogenesis and Regeneration in the Telencephalon of Zebrafish and Mammals. Front. Neurosci. 2020, 14, 568930. [Google Scholar] [CrossRef]
- Flores, E.M.; Nguyen, A.T.; Odem, M.A.; Eisenhoffer, G.T.; Krachler, A.M. The Zebrafish as a Model for Gastrointestinal Tract-Microbe Interactions. Cell. Microbiol. 2020, 22, e13152. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.; Lu, Q.; Wang, Y.; Chen, J.-N. Zebrafish as a Model for Cardiovascular Development and Disease. Drug Discov. Today Dis. Model. 2008, 5, 135–140. [Google Scholar] [CrossRef]
- Swanhart, L.M.; Cosentino, C.C.; Diep, C.Q.; Davidson, A.J.; de Caestecker, M.; Hukriede, N.A. Zebrafish Kidney Development: Basic Science to Translational Research. Birth Defects Res. Part C Embryo Today Rev. 2011, 93, 141–156. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of Embryonic Development of the Zebrafish. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Dawid, I.B. Developmental Biology of Zebrafish. Ann. N. Y. Acad. Sci. 2004, 1038, 88–93. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, A.; Shepherd, I.T. Zebrafish Development and Genetics: Introducing Undergraduates to Developmental Biology and Genetics in a Large Introductory Laboratory Class. Zebrafish 2009, 6, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Singleman, C.; Holtzman, N.G. Growth and Maturation in the Zebrafish, Danio Rerio: A Staging Tool for Teaching and Research. Zebrafish 2014, 11, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Isogai, S.; Horiguchi, M.; Weinstein, B.M. The Vascular Anatomy of the Developing Zebrafish: An Atlas of Embryonic and Early Larval Development. Dev. Biol. 2001, 230, 278–301. [Google Scholar] [CrossRef] [PubMed]
- Stainier, D.Y.; Lee, R.K.; Fishman, M.C. Cardiovascular Development in the Zebrafish. I. Myocardial Fate Map and Heart Tube Formation. Dev. Camb. Engl. 1993, 119, 31–40. [Google Scholar] [CrossRef]
- Keegan, B.R.; Meyer, D.; Yelon, D. Organization of Cardiac Chamber Progenitors in the Zebrafish Blastula. Development 2004, 131, 3081–3091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Pas, K.E.; Ijaseun, T.; Cao, H.; Fei, P.; Lee, J. Automatic Segmentation and Cardiac Mechanics Analysis of Evolving Zebrafish Using Deep Learning. Front. Cardiovasc. Med. 2021, 8, 675291. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.R.; Samsa, L.A.; Qian, L.; Liu, J. Advances in the Study of Heart Development and Disease Using Zebrafish. J. Cardiovasc. Dev. Dis. 2016, 3, 13. [Google Scholar] [CrossRef]
- Martin, K.E.; Waxman, J.S. Atrial and Sinoatrial Node Development in the Zebrafish Heart. J. Cardiovasc. Dev. Dis. 2021, 8, 15. [Google Scholar] [CrossRef]
- Warga, R.M.; Kimmel, C.B. Cell Movements during Epiboly and Gastrulation in Zebrafish. Dev. Camb. Engl. 1990, 108, 569–580. [Google Scholar] [CrossRef]
- Milgrom-Hoffman, M.; Harrelson, Z.; Ferrara, N.; Zelzer, E.; Evans, S.M.; Tzahor, E. The heart endocardium is derived from vascular endothelial progenitors. Development 2011, 13, 4777–4787. [Google Scholar] [CrossRef] [PubMed]
- Rohr, S.; Otten, C.; Abdelilah-Seyfried, S. Asymmetric Involution of the Myocardial Field Drives Heart Tube Formation in Zebrafish. Circ. Res. 2008, 102, e12–e19. [Google Scholar] [CrossRef] [PubMed]
- Guner-Ataman, B.; Paffett-Lugassy, N.; Adams, M.S.; Nevis, K.R.; Jahangiri, L.; Obregon, P.; Kikuchi, K.; Poss, K.D.; Burns, C.E.; Burns, C.G. Zebrafish Second Heart Field Development Relies on Progenitor Specification in Anterior Lateral Plate Mesoderm and Nkx2.5 Function. Dev. Camb. Engl. 2013, 140, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Bussmann, J.; Bakkers, J.; Schulte-Merker, S. Early Endocardial Morphogenesis Requires Scl/Tal1. PLoS Genet. 2007, 3, e140. [Google Scholar] [CrossRef] [PubMed]
- de la Pompa, J.L.; Timmerman, L.A.; Takimoto, H.; Yoshida, H.; Elia, A.J.; Samper, E.; Potter, J.; Wakeham, A.; Marengere, L.; Langille, B.L.; et al. Role of the NF-ATc Transcription Factor in Morphogenesis of Cardiac Valves and Septum. Nature 1998, 392, 182–186. [Google Scholar] [CrossRef]
- Yelon, D.; Horne, S.A.; Stainier, D.Y.R. Restricted Expression of Cardiac Myosin Genes Reveals Regulated Aspects of Heart Tube Assembly in Zebrafish. Dev. Biol. 1999, 214, 23–37. [Google Scholar] [CrossRef]
- Palencia-Desai, S.; Rost, M.S.; Schumacher, J.A.; Ton, Q.V.; Craig, M.P.; Baltrunaite, K.; Koenig, A.L.; Wang, J.; Poss, K.D.; Chi, N.C.; et al. Myocardium and BMP Signaling Are Required for Endocardial Differentiation. Development 2015, 142, 2304–2315. [Google Scholar] [CrossRef]
- Chen, J.N.; van Eeden, F.J.; Warren, K.S.; Chin, A.; Nüsslein-Volhard, C.; Haffter, P.; Fishman, M.C. Left-Right Pattern of Cardiac BMP4 May Drive Asymmetry of the Heart in Zebrafish. Dev. Camb. Engl. 1997, 124, 4373–4382. [Google Scholar] [CrossRef]
- Patten, B.M. Initiation and Early Changes in the Character of the Heart Beat in Vertebrate Embryos. Physiol. Rev. 1949, 29, 31–47. [Google Scholar] [CrossRef]
- Martin, R.T.; Bartman, T. Analysis of Heart Valve Development in Larval Zebrafish. Dev. Dyn. 2009, 238, 1796–1802. [Google Scholar] [CrossRef]
- Peshkovsky, C.; Totong, R.; Yelon, D. Dependence of Cardiac Trabeculation on Neuregulin Signaling and Blood Flow in Zebrafish. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2011, 240, 446–456. [Google Scholar]
- Rasouli, S.J.; Stainier, D.Y.R. Regulation of Cardiomyocyte Behavior in Zebrafish Trabeculation by Neuregulin 2a Signaling. Nat. Commun. 2017, 8, 15281. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bressan, M.; Hassel, D.; Huisken, J.; Staudt, D.; Kikuchi, K.; Poss, K.D.; Mikawa, T.; Stainier, D.Y.R. A Dual Role for ErbB2 Signaling in Cardiac Trabeculation. Dev. Camb. Engl. 2010, 137, 3867–3875. [Google Scholar] [CrossRef] [PubMed]
- Arrenberg, A.B.; Stainier, D.Y.R.; Baier, H.; Huisken, J. Optogenetic Control of Cardiac Function. Science 2010, 330, 971–974. [Google Scholar] [CrossRef]
- Gupta, V.; Poss, K.D. Clonally Dominant Cardiomyocytes Direct Heart Morphogenesis. Nature 2012, 484, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Gancz, D.; Raftrey, B.C.; Perlmoter, G.; Marín-Juez, R.; Semo, J.; Matsuoka, R.L.; Karra, R.; Raviv, H.; Moshe, N.; Addadi, Y.; et al. Distinct Origins and Molecular Mechanisms Contribute to Lymphatic Formation during Cardiac Growth and Regeneration. eLife 2019, 8, e44153. [Google Scholar] [CrossRef]
- Hu, N.; Sedmera, D.; Yost, H.J.; Clark, E.B. Structure and Function of the Developing Zebrafish Heart. Anat. Rec. 2000, 260, 148–157. [Google Scholar] [CrossRef]
- Hoage, T.; Ding, Y.; Xu, X. Quantifying Cardiac Functions in Embryonic and Adult Zebrafish. In Cardiovascular Development; Peng, X., Antonyak, M., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 843, pp. 11–20. [Google Scholar]
- Tessadori, F.; van Weerd, J.H.; Burkhard, S.B.; Verkerk, A.O.; de Pater, E.; Boukens, B.J.; Vink, A.; Christoffels, V.M.; Bakkers, J. Identification and Functional Characterization of Cardiac Pacemaker Cells in Zebrafish. PLoS ONE 2012, 7, e47644. [Google Scholar] [CrossRef]
- Minhas, R.; Loeffler-Wirth, H.; Siddiqui, Y.H.; Obrębski, T.; Vashisht, S.; Nahia, K.A.; Paterek, A.; Brzozowska, A.; Bugajski, L.; Piwocka, K.; et al. Transcriptome Profile of the Sinoatrial Ring Reveals Conserved and Novel Genetic Programs of the Zebrafish Pacemaker. BMC Genom. 2021, 22, 715. [Google Scholar] [CrossRef] [PubMed]
- Grimes, A.C.; Stadt, H.A.; Shepherd, I.T.; Kirby, M.L. Solving an enigma: Arterial pole development in the zebrafish heart. Dev. Biol. 2006, 2, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Yost, H.J.; Clark, E.B. Cardiac morphology and blood pressure in the adult zebrafish. Anat. Rec. 2001, 264, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.V.; Monzo, K.; Cha, Y.R.; Pan, W.; Weinstein, B.M. Vascular Development in the Zebrafish. Cold Spring Harb. Perspect. Med. 2012, 2, a006684. [Google Scholar] [CrossRef] [PubMed]
- Hogan, B.; Kolodziej, P. Molecular Mechanisms Of Tubulogenesis. Nat. Rev. Genet. 2002, 3, 513–523. [Google Scholar] [CrossRef]
- Gong, B.; Li, Z.; Xiao, W.; Li, G.; Ding, S.; Meng, A.; Jia, S. Sec14l3 potentiates VEGFR2 signaling to regulate zebrafish vasculogenesis. Nat. Commun. 2019, 10, 1606. [Google Scholar] [CrossRef] [PubMed]
- Helker, C.S.; Schuermann, A.; Pollmann, C.; Chng, S.C.; Kiefer, F.; Reversade, B.; Herzog, W. The Hormonal Peptide Elabela Guides Angioblasts to the Midline during Vasculogenesis. eLife 2015, 4, e06726. [Google Scholar] [CrossRef] [PubMed]
- Lubarsky, B.; Krasnow, M.A. Tube Morphogenesis. Cell 2003, 112, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Pandya, N.M.; Dhalla, N.S.; Santani, D.D. Angiogenesis—A New Target for Future Therapy. Vascul. Pharm. 2006, 44, 265–274. [Google Scholar] [CrossRef]
- Betz, C.; Lenard, A.; Belting, H.-G.; Affolter, M. Cell Behaviors and Dynamics during Angiogenesis. Development 2016, 143, 2249–2260. [Google Scholar] [CrossRef]
- Ribatti, D.; Crivellato, E. Mast Cells, Angiogenesis, and Tumour Growth. Biochim. Biophys. Acta 2012, 1822, 2–8. [Google Scholar] [CrossRef]
- Wang, H.U.; Chen, Z.F.; Anderson, D.J. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 1998, 5, 741–753. [Google Scholar] [CrossRef]
- Zhong, T.P.; Childs, S.; Leu, J.P.; Fishman, M.C. Gridlock signalling pathway fashions the first embryonic artery. Nature 2001, 6860, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Miano, J.M.; Georger, M.A.; Rich, A.; De Mesy Bentley, K.L. Ultrastructure of Zebrafish Dorsal Aortic Cells. Zebrafish 2006, 3, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Faury, G. Function-Structure Relationship of Elastic Arteries in Evolution: From Microfibrils to Elastin and Elastic Fibres. Pathol. Biol. 2001, 49, 310–325. [Google Scholar] [CrossRef] [PubMed]
- Fritze, O.; Romero, B.; Schleicher, M.; Jacob, M.P.; Oh, D.-Y.; Starcher, B.; Schenke-Layland, K.; Bujan, J.; Stock, U.A. Age-Related Changes in the Elastic Tissue of the Human Aorta. J. Vasc. Res. 2012, 49, 77–86. [Google Scholar] [CrossRef]
- Chow, M.-J.; Turcotte, R.; Lin, C.P.; Zhang, Y. Arterial Extracellular Matrix: A Mechanobiological Study of the Contributions and Interactions of Elastin and Collagen. Biophys. J. 2014, 106, 2684–2692. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Travisano, S.; Pearson, C.A.; Lien, C.-L.; Harrison, M.R.M. The Lymphatic System in Zebrafish Heart Development, Regeneration and Disease Modeling. J. Cardiovasc. Dev. Dis. 2021, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Margiotta-Casaluci, L.; Owen, S.F.; Rand-Weaver, M.; Winter, M.J. Testing the Translational Power of the Zebrafish: An Interspecies Analysis of Responses to Cardiovascular Drugs. Front. Pharm. 2019, 16, 893. [Google Scholar] [CrossRef]
- Santoro, M.M.; Pesce, G.; Stainier, D.Y. Characterization of vascular mural cells during zebrafish development. Mech. Dev. 2009, 126, 638–649. [Google Scholar] [CrossRef]
- Narumanchi, S.; Wang, H.; Perttunen, S.; Tikkanen, I.; Lakkisto, P.; Paavola, J. Zebrafish Heart Failure Models. Front. Cell Dev. Biol. 2021, 9, 662583. [Google Scholar] [CrossRef]
- Wang, L.W.; Huttner, I.G.; Santiago, C.F.; Kesteven, S.H.; Yu, Z.Y.; Feneley, M.P.; Fatkin, D. Standardized echocardiographic assessment of cardiac function in normal adult zebrafish and heart disease models. Dis. Models Mech. 2017, 1, 63–76. [Google Scholar] [CrossRef]
- Sánchez-Iranzo, H.; Galardi-Castilla, M.; Minguillón, C.; Sanz-Morejón, A.; González-Rosa, J.M.; Felker, A.; Ernst, A.; Guzmán-Martínez, G.; Mosimann, C.; Mercader, N. Tbx5a lineage tracing shows cardiomyocyte plasticity during zebrafish heart regeneration. Nat. Commun. 2018, 9, 428. [Google Scholar] [CrossRef] [PubMed]
- Poon, K.L.; Liebling, M.; Kondrychyn, I.; Garcia-Lecea, M.; Korzh, V. Zebrafish cardiac enhancer trap lines: New tools for in vivo studies of cardiovascular development and disease. Dev. Dyn. 2010, 3, 914–926. [Google Scholar] [CrossRef] [PubMed]
- Oleykowski, C.A.; Bronson Mullins, C.R.; Godwin, A.K.; Yeung, A.T. Mutation Detection Using a Novel Plant Endonuclease. Nucleic Acids Res. 1998, 26, 4597–4602. [Google Scholar] [CrossRef] [PubMed]
- Amsterdam, A.; Hopkins, N. Mutagenesis Strategies in Zebrafish for Identifying Genes Involved in Development and Disease. Trends Genet. 2006, 22, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Shah, A.N.; Phelps, I.G.; Doherty, D.; Johnson, E.A.; Moens, C.B. Rapid Identification and Recovery of ENU-Induced Mutations with next-Generation Sequencing and Paired-End Low-Error Analysis. BMC Genom. 2015, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gays, D.; Santoro, M.M. Transgenic Zebrafish. Methods Mol. Biol. Clifton NJ 2016, 1464, 107–114. [Google Scholar]
- Bill, B.R.; Petzold, A.M.; Clark, K.J.; Schimmenti, L.A.; Ekker, S.C. A Primer for Morpholino Use in Zebrafish. Zebrafish 2009, 6, 69–77. [Google Scholar] [CrossRef]
- Eisen, J.S.; Smith, J.C. Controlling Morpholino Experiments: Don’t Stop Making Antisense. Dev. Camb. Engl. 2008, 135, 1735–1743. [Google Scholar] [CrossRef]
- Nasevicius, A.; Ekker, S.C. Effective Targeted Gene “knockdown” in Zebrafish. Nat. Genet. 2000, 26, 216–220. [Google Scholar] [CrossRef]
- Collart, C.; Verschueren, K.; Rana, A.; Smith, J.C.; Huylebroeck, D. The Novel Smad-Interacting Protein Smicl Regulates Chordin Expression in the Xenopus Embryo. Dev. Camb. Engl. 2005, 132, 4575–4586. [Google Scholar]
- Fausett, B.V.; Gumerson, J.D.; Goldman, D. The Proneural Basic Helix-Loop-Helix Gene Ascl1a Is Required for Retina Regeneration. J. Neurosci. 2008, 28, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Merker, S.; Stainier, D.Y.R. Out with the Old, in with the New: Reassessing Morpholino Knockdowns in Light of Genome Editing Technology. Development 2014, 141, 3103–3104. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.Y.; Peterson, R.T.; Yeh, J.-R.J. Methods for Targeted Mutagenesis in Zebrafish Using TALENs. Methods 2014, 69, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Xiao, A.; Tong, X.; Lin, S.; Zhang, B. Targeted Mutagenesis in Zebrafish by TALENs. Methods Mol. Biol. Clifton NJ 2016, 1338, 191–206. [Google Scholar]
- Uribe-Salazar, J.M.; Kaya, G.; Sekar, A.; Weyenberg, K.; Ingamells, C.; Dennis, M.Y. Evaluation of CRISPR Gene-Editing Tools in Zebrafish. BMC Genom. 2022, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Petree, C.; Requena, T.; Varshney, P.; Varshney, G.K. Expanding the CRISPR Toolbox in Zebrafish for Studying Development and Disease. Front. Cell Dev. Biol. 2019, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, J.; Garg, V. Genetics of congenital heart disease: A narrative review of recent advances and clinical implications. Transl. Pediatr. 2021, 9, 2366–2386. [Google Scholar] [CrossRef] [PubMed]
- Reiter, J.F.; Alexander, J.; Rodaway, A.; Yelon, D.; Patient, R.; Holder, N.; Stainier, D.Y. Gata5 is required for the development of the heart and endoderm in zebrafish. Genes Dev. 1999, 22, 2983–2995. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Yuan, X.; Racioppi, C.; Leslie, M.; Stutt, N.; Aleksandrova, A.; Christiaen, L.; Wilson, M.D.; Scott, I.C. GATA4/5/6 family transcription factors are conserved determinants of cardiac versus pharyngeal mesoderm fate. Sci. Adv. 2022, 10, eabg0834. [Google Scholar] [CrossRef]
- Schott, J.J.; Benson, D.W.; Basson, C.T.; Pease, W.; Silberbach, G.M.; Moak, J.P.; Maron, B.J.; Seidman, C.E.; Seidman, J.G. Congenital Heart Disease Caused by Mutations in the Transcription Factor NKX2-5. Science 1998, 281, 108–111. [Google Scholar] [CrossRef]
- Lu, C.-X.; Gong, H.-R.; Liu, X.-Y.; Wang, J.; Zhao, C.-M.; Huang, R.-T.; Xue, S.; Yang, Y.-Q. A Novel HAND2 Loss-of-Function Mutation Responsible for Tetralogy of Fallot. Int. J. Mol. Med. 2016, 37, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Schindler, Y.L.; Garske, K.M.; Wang, J.; Firulli, B.A.; Firulli, A.B.; Poss, K.D.; Yelon, D. Hand2 Elevates Cardiomyocyte Production during Zebrafish Heart Development and Regeneration. Dev. Camb. Engl. 2014, 141, 3112–3122. [Google Scholar] [CrossRef] [PubMed]
- Kefalos, P.; Agalou, A.; Kawakami, K.; Beis, D. Reactivation of Notch Signaling Is Required for Cardiac Valve Regeneration. Sci. Rep. 2019, 9, 16059. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Delgado, L.; Galardi-Castilla, M.; Münch, J.; Peralta, M.; Ernst, A.; González-Rosa, J.M.; Tessadori, F.; Santamaría, L.; Bakkers, J.; Vermot, J.; et al. Notch and Bmp Signaling Pathways Act Coordinately during the Formation of the Proepicardium. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2020, 249, 1455–1469. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Glen, E.; Töpf, A.; Hall, D.; O’Sullivan, J.J.; Sneddon, L.; Wren, C.; Avery, P.; Lewis, R.J.; ten Dijke, P.; et al. Nonsynonymous Variants in the SMAD6 Gene Predispose to Congenital Cardiovascular Malformation. Hum. Mutat. 2012, 33, 720–727. [Google Scholar] [CrossRef] [PubMed]
- de Pater, E.; Ciampricotti, M.; Priller, F.; Veerkamp, J.; Strate, I.; Smith, K.; Lagendijk, A.K.; Schilling, T.F.; Herzog, W.; Abdelilah-Seyfried, S.; et al. Bmp Signaling Exerts Opposite Effects on Cardiac Differentiation. Circ. Res. 2012, 110, 578–587. [Google Scholar] [CrossRef]
- Sarantis, P.; Gaitanaki, C.; Beis, D. Ventricular Remodeling of Single-Chambered Myh6-/- Adult Zebrafish Hearts Occurs via a Hyperplastic Response and Is Accompanied by Elastin Deposition in the Atrium. Cell Tissue Res. 2019, 378, 279–288. [Google Scholar] [CrossRef]
- Glenn, N.O.; McKane, M.; Kohli, V.; Wen, K.-K.; Rubenstein, P.A.; Bartman, T.; Sumanas, S. The W-Loop of Alpha-Cardiac Actin Is Critical for Heart Function and Endocardial Cushion Morphogenesis in Zebrafish. Mol. Cell. Biol. 2012, 32, 3527–3540. [Google Scholar] [CrossRef]
- Herman, D.S.; Lam, L.; Taylor, M.R.G.; Wang, L.; Teekakirikul, P.; Christodoulou, D.; Conner, L.; DePalma, S.R.; McDonough, B.; Sparks, E.; et al. Truncations of Titin Causing Dilated Cardiomyopathy. N. Engl. J. Med. 2012, 366, 619–628. [Google Scholar] [CrossRef]
- Tanaka, Y.; Hayashi, K.; Fujino, N.; Konno, T.; Tada, H.; Nakanishi, C.; Hodatsu, A.; Tsuda, T.; Nagata, Y.; Teramoto, R.; et al. Functional Analysis of KCNH2 Gene Mutations of Type 2 Long QT Syndrome in Larval Zebrafish Using Microscopy and Electrocardiography. Heart Vessel. 2019, 34, 159–166. [Google Scholar] [CrossRef]
- Samsa, L.A.; Givens, C.; Tzima, E.; Stainier, D.Y.R.; Qian, L.; Liu, J. Cardiac Contraction Activates Endocardial Notch Signaling to Modulate Chamber Maturation in Zebrafish. Development 2015, 142, 4080–4091. [Google Scholar] [CrossRef] [PubMed]
- Staudt, D.W.; Liu, J.; Thorn, K.S.; Stuurman, N.; Liebling, M.; Stainier, D.Y.R. High-Resolution Imaging of Cardiomyocyte Behavior Reveals Two Distinct Steps in Ventricular Trabeculation. Development 2014, 141, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Bu, H.; Xu, X. Modeling Inherited Cardiomyopathies in Adult Zebrafish for Precision Medicine. Front. Physiol. 2020, 11, 599244. [Google Scholar] [CrossRef] [PubMed]
- Orr, N.; Arnaout, R.; Gula, L.J.; Spears, D.A.; Leong-Sit, P.; Li, Q.; Tarhuni, W.; Reischauer, S.; Chauhan, V.S.; Borkovich, M.; et al. A Mutation in the Atrial-Specific Myosin Light Chain Gene (MYL4) Causes Familial Atrial Fibrillation. Nat. Commun. 2016, 7, 11303. [Google Scholar] [CrossRef]
- Xu, X.; Meiler, S.E.; Zhong, T.P.; Mohideen, M.; Crossley, D.A.; Burggren, W.W.; Fishman, M.C. Cardiomyopathy in zebrafish due to mutation in an alternatively spliced exon of titin. Nat. Genet. 2002, 2, 205–209. [Google Scholar] [CrossRef]
- Beqqali, A.; Monshouwer-Kloots, J.; Monteiro, R.; Welling, M.; Bakkers, J.; Ehler, E.; Verkleij, A.; Mummery, C.; Passier, R. CHAP Is a Newly Identified Z-Disc Protein Essential for Heart and Skeletal Muscle Function. J. Cell Sci. 2010, 123, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-H.; Chou, H.-C.; Chen, Y.-F.; Liu, W.; Lee, C.-C.; Liu, L.Y.-M.; Chuang, Y.-J. Development of a Rapid and Economic in Vivo Electrocardiogram Platform for Cardiovascular Drug Assay and Electrophysiology Research in Adult Zebrafish. Sci. Rep. 2018, 8, 15986. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Li, L.; Lam, Y.W.; Siu, C.W.; Cheng, S.H. Improvement of Surface ECG Recording in Adult Zebrafish Reveals That the Value of This Model Exceeds Our Expectation. Sci. Rep. 2016, 6, 25073. [Google Scholar] [CrossRef]
- Nemtsas, P.; Wettwer, E.; Christ, T.; Weidinger, G.; Ravens, U. Adult zebrafish heart as a model for human heart? An electrophysiological study. J Mol. Cell. Cardiol. 2010, 1, 161–171. [Google Scholar] [CrossRef]
- Leong, I.U.; Skinner, J.R.; Shelling, A.N.; Love, D.R. Identification and expression analysis of kcnh2 genes in the zebrafish. Biochem. Biophys. Res. Commun. 2010, 4, 817–824. [Google Scholar] [CrossRef]
- Ji, Y.; Buel, S.M.; Amack, J.D. Mutations in Zebrafish Pitx2 Model Congenital Malformations in Axenfeld-Rieger Syndrome but Do Not Disrupt Left-Right Placement of Visceral Organs. Dev. Biol. 2016, 416, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Lodder, E.M.; De Nittis, P.; Koopman, C.D.; Wiszniewski, W.; Moura de Souza, C.F.; Lahrouchi, N.; Guex, N.; Napolioni, V.; Tessadori, F.; Beekman, L.; et al. GNB5 mutations cause an autosomal-recessive multisystem syndrome with sinus bradycardia and cognitive disability. Am. J. Hum. Genet. 2016, 99, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Jeroen, B. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc. Res. 2011, 91, 279–288. [Google Scholar]
- Brunner, H.; Cockcroft, J.R.; Deanfield, J.; Donald, A.; Ferrannini, E.; Halcox, J.; Kiowski, W.; Lüscher, T.F.; Mancia, G.; Natali, A.; et al. Endothelial Function and Dysfunction. Part II: Association with Cardiovascular Risk Factors and Diseases. A Statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J. Hypertens 2005, 23, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Pahwa, R.; Jialal, I. Atherosclerosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Stoletov, K.; Fang, L.; Choi, S.-H.; Hartvigsen, K.; Hansen, L.F.; Hall, C.; Pattison, J.; Juliano, J.; Miller, E.R.; Almazan, F.; et al. Vascular Lipid Accumulation, Lipoprotein Oxidation, and Macrophage Lipid Uptake in Hypercholesterolemic Zebrafish. Circ. Res. 2009, 104, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Li, Q.; Wu, N.; Shen, Y.; Liao, W.; Yang, Y.; Dong, E.; Zhang, G.; Liu, B.; Yue, X.; et al. Chronological in Vivo Imaging Reveals Endothelial Inflammation Prior to Neutrophils Accumulation and Lipid Deposition in HCD-Fed Zebrafish. Atherosclerosis 2019, 290, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Carten, J.D.; Farber, S.A. Zebrafish Lipid Metabolism: From Mediating Early Patterning to the Metabolism of Dietary Fat and Cholesterol. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 101, pp. 111–141. ISBN 978-0-12-387036-0. [Google Scholar]
- Fang, L.; Harkewicz, R.; Hartvigsen, K.; Wiesner, P.; Choi, S.-H.; Almazan, F.; Pattison, J.; Deer, E.; Sayaphupha, T.; Dennis, E.A.; et al. Oxidized Cholesteryl Esters and Phospholipids in Zebrafish Larvae Fed a High Cholesterol Diet. J. Biol. Chem. 2010, 285, 32343–32351. [Google Scholar] [CrossRef]
- Liu, C.; Gates, K.P.; Fang, L.; Amar, M.J.; Schneider, D.; Geng, H.; Huang, W.; Kim, J.; Pattison, J.; Zhang, J.; et al. Apoc2 loss-of-function zebrafish mutant as a genetic model of hyperlipidemia. Dis. Models Mech. 2015, 8, 989–998. [Google Scholar] [CrossRef]
- Cruz-Garcia, L.; Schlegel, A. Lxr-driven enterocyte lipid droplet formation delays transport of ingested lipids. J. Lipid Res. 2014, 9, 1944–1958. [Google Scholar] [CrossRef]
- Liu, C.; Kim, Y.S.; Kim, J.; Pattison, J.; Kamaid, A.; Miller, Y.I. Modeling hypercholesterolemia and vascular lipid accumulation in LDL receptor mutant zebrafish. J. Lipid Res. 2017, 59, 391–399. [Google Scholar] [CrossRef]
- Lee, S.J.; Choi, W.; Seo, E.; Yeom, E. Association of Early Atherosclerosis with Vascular Wall Shear Stress in Hypercholesterolemic Zebrafish. PLoS ONE 2015, 10, e0142945. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, I.-K.; Jeon, J.-H. Vascular Calcification-New Insights Into Its Mechanism. Int. J. Mol. Sci. 2020, 21, 2685. [Google Scholar] [CrossRef] [PubMed]
- Villa-Bellosta, R. Vascular Calcification: Key Roles of Phosphate and Pyrophosphate. Int. J. Mol. Sci. 2021, 22, 13536. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.X.; Moe, S.M. Vascular Calcification: Pathophysiology and Risk Factors. Curr. Hypertens. Rep. 2012, 14, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Hosen, M. Zebrafish Models for Ectopic Mineralization Disorders: Practical Issues from Morpholino Design to Post-Injection Observations. Front. Genet. 2013, 4, 74. [Google Scholar] [CrossRef]
- Pasqualetti, S.; Banfi, G.; Mariotti, M. The Zebrafish Scale as Model to Study the Bone Mineralization Process. J. Mol. Histol. 2012, 43, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Sosa, M.X.; Fang, J.; Shanmukhappa, S.K.; Hubaud, A.; Fawcett, C.H.; Molind, G.J.; Tsai, T.; Capodieci, P.; Wetzel, K.; et al. AKlotho Regulates Age-Associated Vascular Calcification and Lifespan in Zebrafish. Cell Rep. 2019, 28, 2767–2776.e5. [Google Scholar] [CrossRef] [PubMed]
- Huitema, L.F.; Apschner, A.; Logister, I.; Spoorendonk, K.M.; Bussmann, J.; Hammond, C.L.; Schulte-Merker, S. Entpd5 is essential for skeletal mineralization and regulates phosphate homeostasis in zebrafish. Proc. Natl. Acad. Sci. USA 2012, 52, 21372–21377. [Google Scholar] [CrossRef]
- Sun, J.; She, P.; Liu, X.; Gao, B.; Jin, D.; Zhong, T.P. Disruption of Abcc6 Transporter in Zebrafish Causes Ocular Calcification and Cardiac Fibrosis. Int. J. Mol. Sci. 2020, 22, 278. [Google Scholar] [CrossRef]
- Ralph, D.; Nitschke, Y.; Levine, M.A.; Caffet, M.; Wurst, T.; Saeidian, A.H.; Youssefian, L.; Vahidnezhad, H.; Terry, S.F.; Rutsch, F.; et al. ENPP1 Variants in Patients with GACI and PXE Expand the Clinical and Genetic Heterogeneity of Heritable Disorders of Ectopic Calcification. PLoS Genet. 2022, 18, e1010192. [Google Scholar] [CrossRef]
- Apschner, A.; Huitema, L.F.A.; Ponsioen, B.; Peterson-Maduro, J.; Schulte-Merker, S. Zebrafish Enpp1 Mutants Exhibit Pathological Mineralization, Mimicking Features of Generalized Arterial Calcification of Infancy (GACI) and Pseudoxanthoma Elasticum (PXE). Dis. Models Mech. 2014, 7, 811–822. [Google Scholar]
- Wojtaszek, E.; Oldakowska-Jedynak, U.; Kwiatkowska, M.; Glogowski, T.; Malyszko, J. Uremic Toxins, Oxidative Stress, Atherosclerosis in Chronic Kidney Disease, and Kidney Transplantation. Oxid. Med. Cell. Longev. 2021, 2021, 6651367. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Sidor, N.A.; Tonial, N.C.; Che, A.; Urquhart, B.L. Uremic Toxins in the Progression of Chronic Kidney Disease and Cardiovascular Disease: Mechanisms and Therapeutic Targets. Toxins 2021, 13, 142. [Google Scholar] [CrossRef] [PubMed]
- Pieniazek, A.; Bernasinska-Slomczewska, J.; Gwozdzinski, L. Uremic Toxins and Their Relation with Oxidative Stress Induced in Patients with CKD. Int. J. Mol. Sci. 2021, 22, 6196. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-B.; Zhang, Y.-P.; Zhang, J.; Zhang, Y.-B. Evaluation of Vitamin C Supplementation on Kidney Function and Vascular Reactivity Following Renal Ischemic Injury in Mice. Kidney Blood Press. Res. 2016, 41, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Vila Cuenca, M.; van Bezu, J.; Beelen, R.H.J.; Vervloet, M.G.; Hordijk, P.L. Stabilization of Cell-Cell Junctions by Active Vitamin D Ameliorates Uraemia-Induced Loss of Human Endothelial Barrier Function. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2019, 34, 252–264. [Google Scholar] [CrossRef]
- Maciel, R.A.P.; Cunha, R.S.; Busato, V.; Franco, C.R.C.; Gregório, P.C.; Dolenga, C.J.R.; Nakao, L.S.; Massy, Z.A.; Boullier, A.; Pecoits-Filho, R.; et al. Uremia Impacts VE-Cadherin and ZO-1 Expression in Human Endothelial Cell-to-Cell Junctions. Toxins 2018, 10, 404. [Google Scholar] [CrossRef]
- Colombo, G.; Astori, E.; Landoni, L.; Garavaglia, M.L.; Altomare, A.; Lionetti, M.C.; Gagliano, N.; Giustarini, D.; Rossi, R.; Milzani, A.; et al. Effects of the uremic toxin indoxyl sulphate on human microvascular endothelial cells. J. Appl. Toxicol. 2022, 12, 1948–1961. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Huang, S.Y.; Wu, C.C.; Hsu, C.F. P-Cresylsulfate, the Protein-Bound Uremic Toxin, Increased Endothelial Permeability Partly Mediated by Src-Induced Phosphorylation of VE-Cadherin. Toxins 2020, 12, 62. [Google Scholar] [CrossRef]
- Stinghen, A.E.; Gonçalves, S.M.; Martines, E.G.; Nakao, L.S.; Riella, M.C.; Aita, C.A.; Pecoits-Filho, R. Increased plasma and endothelial cell expression of chemokines and adhesion molecules in chronic kidney disease. Nephron Clin. Pract. 2009, 2, c117–c126. [Google Scholar] [CrossRef]
- Glorieux, G.L.; Dhondt, A.W.; Jacobs, P.; Van Langeraert, J.; Lameire, N.H.; De Deyn, P.P.; Vanholder, R.C. In Vitro Study of the Potential Role of Guanidines in Leukocyte Functions Related to Atherogenesis and Infection. Kidney Int. 2004, 65, 2184–2192. [Google Scholar] [CrossRef] [PubMed]
- Gowda, S.; Desai, P.B.; Kulkarni, S.S.; Hull, V.V.; Math, A.A.K.; Vernekar, S.N. Markers of Renal Function Tests. N. Am. J. Med. Sci. 2010, 2, 170–173. [Google Scholar] [PubMed]
- Forman, J.P.; Choi, H.; Curhan, G.C. Uric Acid and Insulin Sensitivity and Risk of Incident Hypertension. Arch. Intern. Med. 2009, 169, 155. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Kielstein, J.T.; Schultheiss, U.T.; Sitter, T.; Titze, S.I.; Schaeffner, E.S.; McAdams-DeMarco, M.; Kronenberg, F.; Eckardt, K.-U.; Köttgen, A.; et al. Prevalence and Correlates of Gout in a Large Cohort of Patients with Chronic Kidney Disease: The German Chronic Kidney Disease (GCKD) Study. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2015, 30, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Ekundayo, O.J.; Dell’Italia, L.J.; Sanders, P.W.; Arnett, D.; Aban, I.; Love, T.E.; Filippatos, G.; Anker, S.D.; Lloyd-Jones, D.M.; Bakris, G.; et al. Association between Hyperuricemia and Incident Heart Failure among Older Adults: A Propensity-Matched Study. Int. J. Cardiol. 2010, 142, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Viet, N.N.; Gigante, B.; Lind, V.; Hammar, N.; Modig, K. Elevated Uric Acid Is Associated With New-Onset Atrial Fibrillation: Results From the Swedish AMORIS Cohort. J. Am. Heart Assoc. 2023, 3, e027089. [Google Scholar] [CrossRef]
- Kang, D.-H.; Park, S.-K.; Lee, I.-K.; Johnson, R.J. Uric Acid-Induced C-Reactive Protein Expression: Implication on Cell Proliferation and Nitric Oxide Production of Human Vascular Cells. J. Am. Soc. Nephrol. 2005, 16, 3553–3562. [Google Scholar] [CrossRef]
- Kalra, S.; Aydin, H.; Sahay, M.; Ghosh, S.; Ruder, S.; Tiwaskar, M.; Kilov, G.; Kishor, K.; Nair, T.; Makkar, V.; et al. Cardiorenal Syndrome in Type 2 Diabetes Mellitus-Rational Use of Sodium-Glucose Cotransporter-2 Inhibitors. Eur. Endocrinol. 2020, 16, 113–121. [Google Scholar] [CrossRef]
- Craciun, S.; Balskus, E.P. Microbial Conversion of Choline to Trimethylamine Requires a Glycyl Radical Enzyme. Proc. Natl. Acad. Sci. USA 2012, 109, 21307–21312. [Google Scholar] [CrossRef]
- Pelletier, C.C.; Croyal, M.; Ene, L.; Aguesse, A.; Billon-Crossouard, S.; Krempf, M.; Lemoine, S.; Guebre-Egziabher, F.; Juillard, L.; Soulage, C.O. Elevation of Trimethylamine-N-Oxide in Chronic Kidney Disease: Contribution of Decreased Glomerular Filtration Rate. Toxins 2019, 11, 635. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut Microbiota-Dependent Trimethylamine N-Oxide (TMAO) Pathway Contributes to Both Development of Renal Insufficiency and Mortality Risk in Chronic Kidney Disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Joshi, S.R. Disorders of Calcium, Phosphorus and Magnesium Metabolism. J. Assoc. Physicians India 2008, 56, 613–621. [Google Scholar] [PubMed]
- Stubbs, J.; Liu, S.; Quarles, L.D. Role of Fibroblast Growth Factor 23 in Phosphate Homeostasis and Pathogenesis of Disordered Mineral Metabolism in Chronic Kidney Disease. Semin. Dial. 2007, 20, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-L.; Huang, C.-C.; Chang, C.-C.; Chou, C.-Y.; Lin, S.-Y.; Wang, I.-K.; Hsieh, D.J.-Y.; Jong, G.-P.; Huang, C.-Y.; Wang, C.-M. Hyperphosphate-Induced Myocardial Hypertrophy through the GATA-4/NFAT-3 Signaling Pathway Is Attenuated by ERK Inhibitor Treatment. Cardiorenal Med. 2015, 5, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Cancela, A.L.; Santos, R.D.; Titan, S.M.; Goldenstein, P.T.; Rochitte, C.E.; Lemos, P.A.; dos Reis, L.M.; Graciolli, F.G.; Jorgetti, V.; Moysés, R.M. Phosphorus Is Associated with Coronary Artery Disease in Patients with Preserved Renal Function. PLoS ONE 2012, 7, e36883. [Google Scholar] [CrossRef] [PubMed]
- McGovern, A.P.; de Lusignan, S.; van Vlymen, J.; Liyanage, H.; Tomson, C.R.; Gallagher, H.; Rafiq, M.; Jones, S. Serum phosphate as a risk factor for cardiovascular events in people with and without chronic kidney disease: A large community based cohort study. PLoS ONE 2013, 9, e74996. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Ciceri, P.; Galassi, A.; Mangano, M.; Carugo, S.; Capelli, I.; Cianciolo, G. The Key Role of Phosphate on Vascular Calcification. Toxins 2019, 11, 213. [Google Scholar] [CrossRef]
- Barreto, F.C.; Stinghen, A.E.M.; de Oliveira, R.B.; Franco, A.T.B.; Moreno, A.N.; Barreto, D.V.; Pecoits-Filho, R.; Drüeke, T.B.; Massy, Z.A. The Quest for a Better Understanding of Chronic Kidney Disease Complications: An Update on Uremic Toxins. J. Bras. Nefrol. 2014, 36, 221–235. [Google Scholar] [CrossRef]
- Massy, Z.A.; Liabeuf, S. Middle-Molecule Uremic Toxins and Outcomes in Chronic Kidney Disease. In Contributions to Nephrology; Ronco, C., Ed.; S. Karger AG: Boulogne Billancourt Cedex, France, 2017; Volume 191, pp. 8–17. ISBN 978-3-318-06116-1. [Google Scholar]
- Li, L.; Dong, M.; Wang, X.-G. The Implication and Significance of Beta 2 Microglobulin: A Conservative Multifunctional Regulator. Chin. Med. J. 2016, 129, 448–455. [Google Scholar] [CrossRef]
- Shi, F.; Sun, L.; Kaptoge, S. Association of Beta-2-Microglobulin and Cardiovascular Events and Mortality: A Systematic Review and Meta-Analysis. Atherosclerosis 2021, 320, 70–78. [Google Scholar] [CrossRef]
- Faul, C.; Amaral, A.P.; Oskouei, B.; Hu, M.C.; Sloan, A.; Isakova, T.; Gutiérrez, O.M.; Aguillon-Prada, R.; Lincoln, J.; Hare, J.M.; et al. FGF23 induces left ventricular hypertrophy. J. Clin. Investig. 2011, 11, 4393–4408. [Google Scholar] [CrossRef] [PubMed]
- Akhabue, E.; Wong, M.; Mehta, R.; Isakova, T.; Wolf, M.; Yancy, C.; Gutierrez, O.M.; Carnethon, M. Fibroblast growth factor-23 and subclinical markers of cardiac dysfunction: The coronary artery risk development in young adults (CARDIA) study. Am. Heart J. 2022, 245, 10–18. [Google Scholar] [CrossRef]
- Sato, T.; Courbebaisse, M.; Ide, N.; Fan, Y.; Hanai, J.I.; Kaludjerovic, J.; Densmore, M.J.; Yuan, Q.; Toka, H.R.; Pollak, M.R.; et al. Parathyroid hormone controls paracellular Ca2+ transport in the thick ascending limb by regulating the tight-junction protein Claudin14. Proc. Natl. Acad. Sci. USA 2017, 16, E3344–E3353. [Google Scholar]
- Wang, Y.; Zhu, J.; DeLuca, H.F. The vitamin D receptor in the proximal renal tubule is a key regulator of serum 1α,25-dihydroxyvitamin D₃. Am. J. Physiol. Endocrinol. Metab. 2015, 3, E201-5. [Google Scholar] [CrossRef] [PubMed]
- Oinonen, L.; Tikkakoski, A.; Koskela, J.; Eräranta, A.; Kähönen, M.; Niemelä, O.; Mustonen, J.; Pörsti, I. Parathyroid Hormone May Play a Role in the Pathophysiology of Primary Hypertension. Endocr. Connect. 2021, 10, 54–65. [Google Scholar] [CrossRef]
- Pedersen, C.M.; Rolighed, L.; Harsløf, T.; Jensen, H.K.; Nielsen, J.C. Primary Hyperparathyroidism and Recurrent Ventricular Tachyarrhythmia in a Patient with Novel RyR2 Variant but without Structural Heart Disease. Clin. Case Rep. 2019, 7, 1907–1912. [Google Scholar] [CrossRef]
- Morsy, M.S.; Dishmon, D.A.; Garg, N.; Weber, K.T. Secondary Hyperparathyroidism in Heart Failure. Am. J. Med. Sci. 2017, 354, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Elsadany, M.; Thompson, P.D. Hyperparathyroidism Possibly Causing Increased Coronary Artery Calcification. Am. J. Cardiol. 2022, 172, 164–165. [Google Scholar] [CrossRef]
- Gao, H.; Liu, S. Role of Uremic Toxin Indoxyl Sulfate in the Progression of Cardiovascular Disease. Life Sci. 2017, 185, 23–29. [Google Scholar] [CrossRef]
- Holmar, J.; de la Puente-Secades, S.; Floege, J.; Noels, H.; Jankowski, J.; Orth-Alampour, S. Uremic Toxins Affecting Cardiovascular Calcification: A Systematic Review. Cells 2020, 9, 2428. [Google Scholar] [CrossRef]
- Carmona, A.; Guerrero, F.; Buendia, P.; Obrero, T.; Aljama, P.; Carracedo, J. Microvesicles Derived from Indoxyl Sulfate Treated Endothelial Cells Induce Endothelial Progenitor Cells Dysfunction. Front. Physiol. 2017, 8, 666. [Google Scholar] [CrossRef] [PubMed]
- Faure, V.; Dou, L.; Sabatier, F.; Cerini, C.; Sampol, J.; Berland, Y.; Brunet, P.; Dignat-George, F. Elevation of Circulating Endothelial Microparticles in Patients with Chronic Renal Failure. J. Thromb. Haemost. 2006, 4, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Koppe, L.; Pillon, N.J.; Vella, R.E.; Croze, M.L.; Pelletier, C.C.; Chambert, S.; Massy, Z.; Glorieux, G.; Vanholder, R.; Dugenet, Y.; et al. P-Cresyl Sulfate Promotes Insulin Resistance Associated with CKD. J. Am. Soc. Nephrol. 2013, 24, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Gryp, T.; Vanholder, R.; Vaneechoutte, M.; Glorieux, G. P-Cresyl Sulfate. Toxins 2017, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Poesen, R.; Claes, K.; Evenepoel, P.; de Loor, H.; Augustijns, P.; Kuypers, D.; Meijers, B. Microbiota-Derived Phenylacetylglutamine Associates with Overall Mortality and Cardiovascular Disease in Patients with CKD. J. Am. Soc. Nephrol. 2016, 27, 3479–3487. [Google Scholar] [CrossRef] [PubMed]
- Gross, P.; Massy, Z.A.; Henaut, L.; Boudot, C.; Cagnard, J.; March, C.; Kamel, S.; Drueke, T.B.; Six, I. Para-Cresyl Sulfate Acutely Impairs Vascular Reactivity and Induces Vascular Remodeling: Para-cresyl sulfate and vascular dysfunction. J. Cell. Physiol. 2015, 230, 2927–2935. [Google Scholar] [CrossRef]
- Ingrosso, D.; Cimmino, A.; Perna, A.F.; Masella, L.; De Santo, N.G.; De Bonis, M.L.; Vacca, M.; D’Esposito, M.; D’Urso, M.; Galletti, P.; et al. Folate Treatment and Unbalanced Methylation and Changes of Allelic Expression Induced by Hyperhomocysteinaemia in Patients with Uraemia. Lancet 2003, 361, 1693–1699. [Google Scholar] [CrossRef]
- Maron, B.A.; Loscalzo, J. The Treatment of Hyperhomocysteinemia. Annu. Rev. Med. 2009, 60, 39–54. [Google Scholar] [CrossRef]
- Dutta, S.; Sinha, S.; Chattopadhyay, A.; Gangopadhyay, P.K.; Mukhopadhyay, J.; Singh, M.; Mukhopadhyay, K. Cystathionine Beta-Synthase T833C/844INS68 Polymorphism: A Family-Based Study on Mentally Retarded Children. Behav. Brain Funct. BBF 2005, 1, 25. [Google Scholar] [CrossRef]
- Yakub, M.; Moti, N.; Parveen, S.; Chaudhry, B.; Azam, I.; Iqbal, M.P. Polymorphisms in MTHFR, MS and CBS Genes and Homocysteine Levels in a Pakistani Population. PLoS ONE 2012, 7, e33222. [Google Scholar] [CrossRef]
- Fowler, B. Homocystein--an independent risk factor for cardiovascular and thrombotic diseases. Ther. Umsch. Rev. Ther. 2005, 62, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, H. Homocysteine Thiolactone: Metabolic Origin and Protein Homocysteinylation in Humans. J. Nutr. 2000, 130, 377S–381S. [Google Scholar] [CrossRef] [PubMed]
- Loscalzo, J. The Oxidant Stress of Hyperhomocyst(e)Inemia. J. Clin. Investig. 1996, 98, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.F.; Ingrosso, D.; Lombardi, C.; Acanfora, F.; Satta, E.; Cesare, C.M.; Violetti, E.; Romano, M.M.; De Santo, N.G. Possible Mechanisms of Homocysteine Toxicity. Kidney Int. Suppl. 2003, 63, S137–S140. [Google Scholar] [CrossRef] [PubMed]
- Ingrosso, D.; Perna, A.F. DNA Methylation Dysfunction in Chronic Kidney Disease. Genes 2020, 11, 811. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.F.; Pane, F.; Sepe, N.; Fontanarosa, C.; Pinto, G.; Zacchia, M.; Trepiccione, F.; Anishchenko, E.; Ingrosso, D.; Pucci, P.; et al. Lanthionine and Other Relevant Sulfur Amino Acid Metabolites: Detection of Prospective Uremic Toxins in Serum by Multiple Reaction Monitoring Tandem Mass Spectrometry. Methods Mol. Biol. Clifton NJ 2019, 2007, 9–17. [Google Scholar]
- Perna, A.F.; Di Nunzio, A.; Amoresano, A.; Pane, F.; Fontanarosa, C.; Pucci, P.; Vigorito, C.; Cirillo, G.; Zacchia, M.; Trepiccione, F.; et al. Divergent Behavior of Hydrogen Sulfide Pools and of the Sulfur Metabolite Lanthionine, a Novel Uremic Toxin, in Dialysis Patients. Biochimie 2016, 126, 97–107. [Google Scholar] [CrossRef]
- Perna, A.F.; Russo, L.; D’Esposito, V.; Formisano, P.; Bruzzese, D.; Vigorito, C.; Coppola, A.; Lombari, P.; Russo, D.; Ingrosso, D. Lanthionine, a Novel Uremic Toxin, in the Vascular Calcification of Chronic Kidney Disease: The Role of Proinflammatory Cytokines. Int. J. Mol. Sci. 2021, 22, 6875. [Google Scholar] [CrossRef]
- Pahl, M.V.; Vaziri, N.D. The Chronic Kidney Disease—Colonic Axis. Semin. Dial. 2015, 28, 459–463. [Google Scholar] [CrossRef]
- Sirangelo, I.; Borriello, M.; Liccardo, M.; Scafuro, M.; Russo, P.; Iannuzzi, C. Hydroxytyrosol Selectively Affects Non-Enzymatic Glycation in Human Insulin and Protects by AGEs Cytotoxicity. Antioxid. Basel Switz. 2021, 10, 1127. [Google Scholar] [CrossRef]
- Van der Lugt, T.; Weseler, A.R.; Gebbink, W.A.; Vrolijk, M.F.; Opperhuizen, A.; Bast, A. Dietary Advanced Glycation Endproducts Induce an Inflammatory Response in Human Macrophages in Vitro. Nutrients 2018, 12, 1868. [Google Scholar] [CrossRef] [PubMed]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced Glycation End Products: Sparking the Development of Diabetic Vascular Injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhou, Z.; Liang, B.; Huang, Y.; Yang, Z.; Chen, Y.; Zhang, L.; Yan, C.; Wang, J.; Lu, L.; et al. Inhibiting Receptor of Advanced Glycation End Products Attenuates Pressure Overload-Induced Cardiac Dysfunction by Preventing Excessive Autophagy. Front. Physiol. 2018, 9, 1333. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, Z.Z.; Benslimane, F.M.; Nasrallah, G.K.; Shurbaji, S.; Younes, N.N.; Mraiche, F.; Da’as, S.I.; Yalcin, H.C. Using Zebrafish for Investigating the Molecular Mechanisms of Drug-Induced Cardiotoxicity. BioMed Res. Int. 2018, 2018, 1642684. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-J.; Xu, Y.-Q.; He, J.-H.; Yu, H.-P.; Huang, C.-J.; Gao, J.-M.; Dong, Q.-X.; Xuan, Y.-X.; Li, C.-Q. Human Cardiotoxic Drugs Delivered by Soaking and Microinjection Induce Cardiovascular Toxicity in Zebrafish. J. Appl. Toxicol. 2014, 34, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xu, X. A Swimming-Based Assay to Determine the Exercise Capacity of Adult Zebrafish Cardiomyopathy Models. Bio-Protoc 2021, 11, e4114. [Google Scholar] [CrossRef]
- Shen, Q.; Truong, L.; Simonich, M.T.; Huang, C.; Tanguay, R.L.; Dong, Q. Rapid Well-Plate Assays for Motor and Social Behaviors in Larval Zebrafish. Behav. Brain Res. 2020, 391, 112625. [Google Scholar] [CrossRef]
- Parker, T.; Libourel, P.-A.; Hetheridge, M.J.; Cumming, R.I.; Sutcliffe, T.P.; Goonesinghe, A.C.; Ball, J.S.; Owen, S.F.; Chomis, Y.; Winter, M.J. A Multi-Endpoint in Vivo Larval Zebrafish (Danio Rerio) Model for the Assessment of Integrated Cardiovascular Function. J. Pharmacol. Toxicol. Methods 2014, 69, 30–38. [Google Scholar] [CrossRef]
- Berman, N.; Lectura, M.; Thurman, J.M.; Reinecke, J.; Raff, A.C.; Melamed, M.L.; Quan, Z.; Evans, T.; Meyer, T.W.; Hostetter, T.H. A Zebrafish Model for Uremic Toxicity: Role of the Complement Pathway. Blood Purif. 2013, 35, 265–269. [Google Scholar] [CrossRef]
- Walport, M.J. Complement. First of Two Parts. N. Engl. J. Med. 2001, 344, 1058–1066. [Google Scholar] [CrossRef]
- Malik, T.H.; Cortini, A.; Carassiti, D.; Boyle, J.J.; Haskard, D.O.; Botto, M. The Alternative Pathway Is Critical for Pathogenic Complement Activation in Endotoxin- and Diet-Induced Atherosclerosis in Low-Density Lipoprotein Receptor-Deficient Mice. Circulation 2010, 122, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.; Anishchenko, E.; Vigorito, C.; Zacchia, M.; Trepiccione, F.; D’Aniello, S.; Ingrosso, D. Zebrafish, a Novel Model System to Study Uremic Toxins: The Case for the Sulfur Amino Acid Lanthionine. Int. J. Mol. Sci. 2018, 19, 1323. [Google Scholar] [CrossRef] [PubMed]
- Coppola, A.; Vigorito, C.; Lombari, P.; Martínez, Y.G.; Borriello, M.; Trepiccione, F.; Ingrosso, D.; Perna, A.F. Uremic Toxin Lanthionine Induces Endothelial Cell Mineralization In Vitro. Biomedicines 2022, 10, 444. [Google Scholar] [CrossRef] [PubMed]
- Arinze, N.V.; Yin, W.; Lotfollahzadeh, S.; Napoleon, M.A.; Richards, S.; Walker, J.A.; Belghasem, M.; Ravid, J.D.; Hassan Kamel, M.; Whelan, S.A.; et al. Tryptophan Metabolites Suppress the Wnt Pathway and Promote Adverse Limb Events in Chronic Kidney Disease. J. Clin. Investig. 2022, 132, e142260. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.W.-H.; Wu, P.-H.; Lin, Y.-T.; Chiu, C.-H.; Cheng, T.-L.; Guan, W.-H.; Lin, H.Y.-H.; Lee, K.-T.; Chen, Y.-H.; Chiu, C.-C.; et al. Zebrafish Model-Based Assessment of Indoxyl Sulfate-Induced Oxidative Stress and Its Impact on Renal and Cardiac Development. Antioxidants 2022, 11, 400. [Google Scholar] [CrossRef] [PubMed]
- Bolten, J.S.; Pratsinis, A.; Alter, C.L.; Fricker, G.; Huwyler, J. Zebrafish (Danio rerio) larva as an in vivo vertebrate model to study renal function. Am. J. Physiol. Ren. Physiol. 2022, 3, F280–F294. [Google Scholar] [CrossRef]
- Dalum, A.S.; Kraus, A.; Khan, S.; Davydova, E.; Rigaudeau, D.; Bjørgen, H.; López-Porras, A.; Griffiths, G.; Wiegertjes, G.F.; Koppang, E.O.; et al. High-Resolution, 3D Imaging of the Zebrafish Gill-Associated Lymphoid Tissue (GIALT) Reveals a Novel Lymphoid Structure, the Amphibranchial Lymphoid Tissue. Front. Immunol. 2021, 12, 769901. [Google Scholar] [CrossRef]
- Wiley, D.S.; Redfield, S.E.; Zon, L.I. Chemical screening in zebrafish for novel biological and therapeutic discovery. Methods Cell Biol. 2017, 138, 651–679. [Google Scholar]
- Van Ham, W.B.; Bossu, A.; Houtman, M.J.C.; Cornelissen, C.M.; Vos, M.A.; Van Veen, T.A.B. Determining the role of uremic toxins on cardiac electrophysiology and pro-arrhythmia during chronic kidney disease. EP Eur. 2022, 24 (Suppl. S1), euac053.600. [Google Scholar]
- Bovo, E.; Dvornikov, A.V.; Mazurek, S.R.; de Tombe, P.P.; Zima, A.V. Mechanisms of Ca2+ handling in zebrafish ventricular myocytes. Pflug. Arch. 2013, 12, 1775–1784. [Google Scholar] [CrossRef]
- Brette, F.; Luxan, G.; Cros, C.; Dixey, H.; Wilson, C.; Shiels, H.A. Characterization of isolated ventricular myocytes from adult zebrafish (Danio rerio). Biochem. Biophys. Res. Commun. 2008, 374, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Lekawanvijit, S.; Kompa, A.R.; Manabe, M.; Wang, B.H.; Langham, R.G.; Nishijima, F.; Kelly, D.J.; Krum, H. Chronic kidney disease-induced cardiac fibrosis is ameliorated by reducing circulating levels of a non-dialysable uremic toxin, indoxyl sulfate. PLoS ONE 2012, 7, e41281. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Iranzo, H.; Galardi-Castilla, M.; Sanz-Morejón, A.; González-Rosa, J.M.; Costa, R.; Ernst, A.; Sainz de Aja, J.; Langa, X.; Mercader, N. Transient fibrosis resolves via fibroblast inactivation in the regenerating zebrafish heart. Proc. Natl. Acad. Sci. USA 2018, 16, 4188–4193. [Google Scholar] [CrossRef] [PubMed]
| Massry/Chock’s Requirements |
|---|
| Toxin must be chemically identified and characterized |
| Toxin must be quantified in the biological fluids |
| Toxin in fluids must be detected at high levels in the uremia |
| Relation between the toxin levels and one or more manifestations of the uremia |
| Reduction in the level of the toxin must be correlated with an improvement in uremic effects |
| Uremic toxin levels present in the uremia must be used in vitro and in vivo models to reproduce uremic manifestation A possible pathobiological mechanism should be demonstrated to explain the link between the toxin and uremic manifestation |
| Classification | Characteristic | Example |
|---|---|---|
| Free water-soluble low-molecular weight compounds | <500 Da; removed by dialysis | Creatinine; urea; uric acid; and Trimethylamine |
| Medium compounds | >500 Da; removed by dialysis membranes with large pores | Cystatin C; Cytokines; FGF23; β2-microglobulin; and PTH |
| Protein-bound compounds | Non-dialysable | Indoxyl Sulfate; P-Cresol; Indole-2-acetate; Homocysteine; and Advanced Glycation end Products |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppola, A.; Lombari, P.; Mazzella, E.; Capolongo, G.; Simeoni, M.; Perna, A.F.; Ingrosso, D.; Borriello, M. Zebrafish as a Model of Cardiac Pathology and Toxicity: Spotlight on Uremic Toxins. Int. J. Mol. Sci. 2023, 24, 5656. https://doi.org/10.3390/ijms24065656
Coppola A, Lombari P, Mazzella E, Capolongo G, Simeoni M, Perna AF, Ingrosso D, Borriello M. Zebrafish as a Model of Cardiac Pathology and Toxicity: Spotlight on Uremic Toxins. International Journal of Molecular Sciences. 2023; 24(6):5656. https://doi.org/10.3390/ijms24065656
Chicago/Turabian StyleCoppola, Annapaola, Patrizia Lombari, Elvira Mazzella, Giovanna Capolongo, Mariadelina Simeoni, Alessandra F. Perna, Diego Ingrosso, and Margherita Borriello. 2023. "Zebrafish as a Model of Cardiac Pathology and Toxicity: Spotlight on Uremic Toxins" International Journal of Molecular Sciences 24, no. 6: 5656. https://doi.org/10.3390/ijms24065656
APA StyleCoppola, A., Lombari, P., Mazzella, E., Capolongo, G., Simeoni, M., Perna, A. F., Ingrosso, D., & Borriello, M. (2023). Zebrafish as a Model of Cardiac Pathology and Toxicity: Spotlight on Uremic Toxins. International Journal of Molecular Sciences, 24(6), 5656. https://doi.org/10.3390/ijms24065656






