Rapid and Efficient Optimization Method for a Genetic Transformation System of Medicinal Plants Erigeron breviscapus
Abstract
1. Introduction
2. Results
2.1. Selection Pressure
2.2. Pre-Culture Period
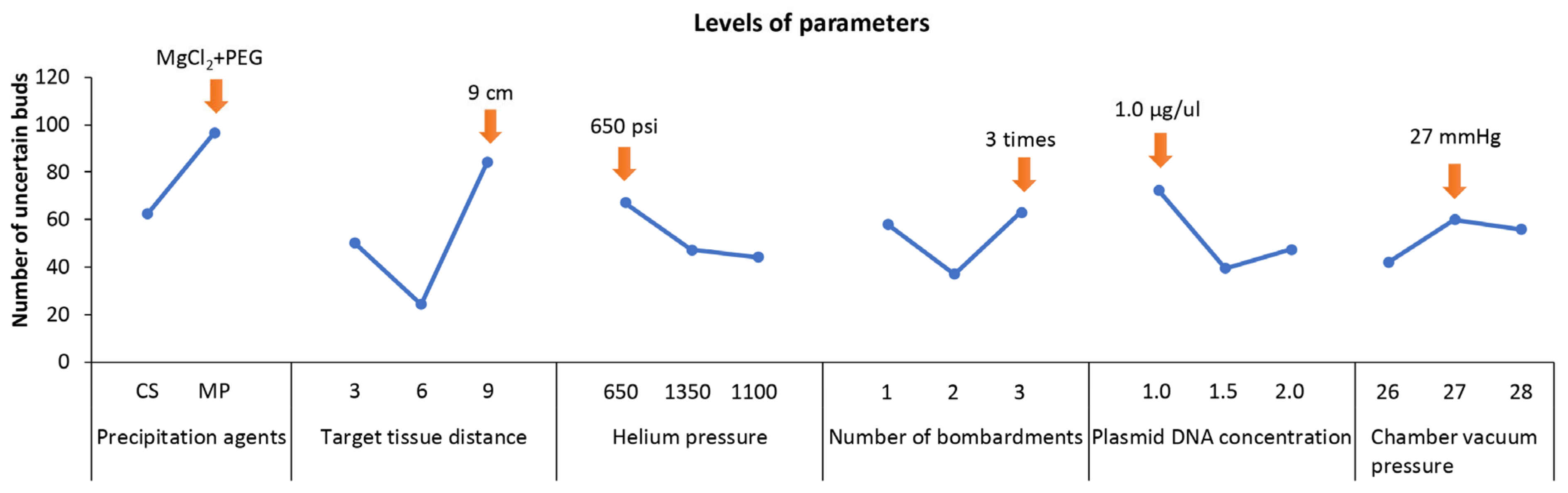
2.3. Transformation Conditions
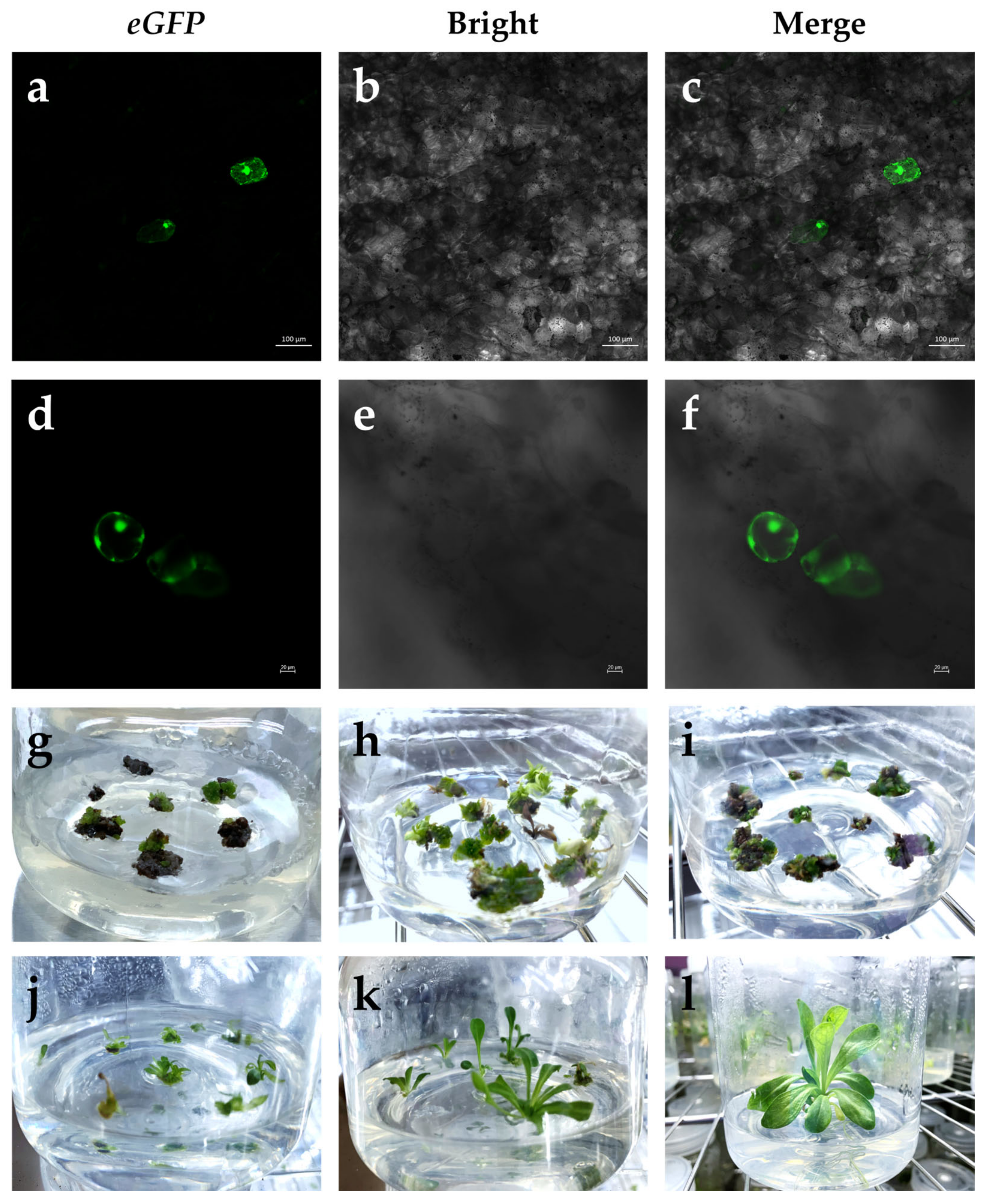
2.4. Selection and Plant Regeneration
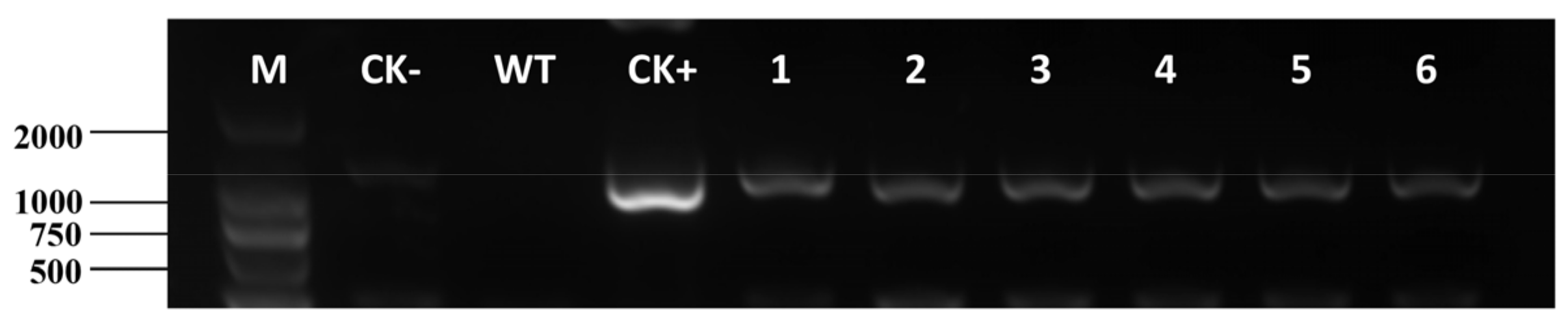
2.5. Identification of Transgenic E. breviscapus Plants
3. Discussion
4. Materials and Methods
4.1. Plant Material and Culture Conditions
4.2. Optimization of Explant Pre-Culture Time
4.3. Selection Pressure of Leaf Explants to Hygromycin B
4.4. Preparation of Gold Microprojectile
4.5. Microprojectile Bombardment
4.6. Selection and Regeneration of Transformed Plants
4.7. PCR Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MS | Murashige and Skoog |
| LSCM | Laser Scanning Confocal Microscope |
| 6-BA | 6-Benzylaminopurine |
| NAA | 1-Naphthylacetic acid |
| IBA | Indole-3-butyric acid |
| Hyg | Hygromycin B |
| CIM | Callus induction medium |
| RM | Regeneration medium |
| SEM | Shoot elongation medium |
| RIM | Root induction medium |
| CaMV | Cauliflower mosaic virus |
References
- Dong, X.; Qu, S. Erigeron breviscapus (Vant.) Hand-Mazz.: A Promising Natural Neuroprotective Agent for Alzheimer’s Disease. Front. Pharmacol. 2022, 13, 877872. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Lin, P.; Kang, Q.; Zhao, Z.L.; Wang, J.; Cheng, J.Y. Metabolism and Pharmacological Mechanisms of Active Ingredients in Erigeron breviscapus. Curr. Drug Metab. 2021, 22, 24–39. [Google Scholar] [CrossRef]
- Jiang, L.; Hu, Y.; He, X.; Lv, Q.; Wang, T.H.; Xia, Q.J. Breviscapine reduces neuronal injury caused by traumatic brain injury insult: Partly associated with suppression of interleukin-6 expression. Neural Regen. Res. 2017, 12, 90–95. [Google Scholar]
- Deng, M.; Sun, J.; Peng, L.; Huang, Y.; Jiang, W.; Wu, S.; Zhou, L.; Chung, S.K.; Cheng, X. Scutellarin acts on the AR-NOX axis to remediate oxidative stress injury in a mouse model of cerebral ischemia/reperfusion injury. Phytomedicine 2022, 103, 154214. [Google Scholar] [CrossRef] [PubMed]
- Pengyue, Z.; Tao, G.; Hongyun, H.; Liqiang, Y.; Yihao, D. Breviscapine confers a neuroprotective efficacy against transient focal cerebral ischemia by attenuating neuronal and astrocytic autophagy in the penumbra. Biomed. Pharmacother. 2017, 90, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, Q. Clinical benefits and pharmacology of scutellarin: A comprehensive review. Pharmacol. Ther. 2018, 190, 105–127. [Google Scholar] [CrossRef]
- Wang, J.; Tan, J.; Luo, J.; Huang, P.; Zhou, W.; Chen, L.; Long, L.; Zhang, L.M.; Zhu, B.; Yang, L.; et al. Enhancement of scutellarin oral delivery efficacy by vitamin B12-modified amphiphilic chitosan derivatives to treat type II diabetes induced-retinopathy. J. Nanobiotechnol. 2017, 15, 18. [Google Scholar] [CrossRef]
- Tang, G.; Li, S.; Zhang, C.; Chen, H.; Wang, N.; Feng, Y. Clinical efficacies, underlying mechanisms and molecular targets of Chinese medicines for diabetic nephropathy treatment and management. Acta Pharm Sin B. 2021, 11, 2749–2767. [Google Scholar] [CrossRef]
- Safdari, M.R.; Shakeri, F.; Mohammadi, A.; Bibak, B.; Alesheikh, P.; Jamialahmadi, T.; Sathyapalan, T.; Sahebkar, A. Role of Herbal Medicines in the Management of Brain Injury. Adv. Exp. Med. Biol. 2021, 1328, 287–305. [Google Scholar]
- Avagimyan, A.; Gvianishvili, T.; Gogiashvili, L.; Kakturskiy, L.; Sarrafzadegan, N.; Aznauryan, A. Chemotherapy, hypothyroidism and oral dysbiosis as a novel risk factor of cardiovascular pathology development. Curr. Probl. Cardiol. 2023, 48, 101051. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, J.; Zhang, G.; Ding, W.; Duan, L.; Yang, J.; Kui, L.; Cheng, X.; Ruan, J.; Fan, W. Engineering yeast for the production of breviscapine by genomic analysis and synthetic biology approaches. Nat. Commun. 2018, 9, 448. [Google Scholar] [CrossRef]
- Wang, W.; Xu, J.; Fang, H.; Li, Z.; Li, M. Advances and challenges in medicinal plant breeding. Plant Sci. 2020, 298, 110573. [Google Scholar] [CrossRef]
- Hwang, H.-H.; Yu, M.; Lai, E.-M. Agrobacterium-mediated plant transformation: Biology and applications. Arab. Book 2017, 15, e0186. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Debernardi, J.M.; Dubcovsky, J.; Gallavotti, A. Recent advances in crop transformation technologies. Nat. Plants 2022, 8, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Abd Samad, A.; Rahmat, Z. Agrobacterium-mediated transformation of rice: Constraints and possible solutions. Rice Sci. 2019, 26, 133–146. [Google Scholar] [CrossRef]
- Ahmed, R.I.; Ding, A.; Xie, M.; Kong, Y. Progress in Optimization of Agrobacterium-Mediated Transformation in Sorghum (Sorghum bicolor). Int. J. Mol. Sci. 2018, 19, 2983. [Google Scholar] [CrossRef]
- Lacroix, B.; Citovsky, V. Biolistic approach for transient gene expression studies in plants. In Biolistic DNA Delivery in Plants: Methods; Springer: Berlin/Heidelberg, Germany, 2020; pp. 125–139. [Google Scholar]
- Chandrasekaran, R.; Rajiv, P.; Abd-Elsalam, K.A. Carbon nanotubes: Plant gene delivery and genome editing. In Carbon Nanomaterials for Agri-Food and Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 279–296. [Google Scholar]
- Sood, P.; Bhattacharya, A.; Sood, A. Problems and possibilities of monocot transformation. Biol. Plant. 2011, 55, 1–15. [Google Scholar] [CrossRef]
- Van Eck, J. The Status of Setaria viridis Transformation: Agrobacterium-Mediated to Floral Dip. Front. Plant Sci. 2018, 9, 652. [Google Scholar] [CrossRef]
- Altpeter, F.; Baisakh, N.; Beachy, R.; Bock, R.; Capell, T.; Christou, P.; Daniell, H.; Datta, K.; Datta, S.; Dix, P.J. Particle bombardment and the genetic enhancement of crops: Myths and realities. Mol. Breed. 2005, 15, 305–327. [Google Scholar] [CrossRef]
- Ahmar, S.; Mahmood, T.; Fiaz, S.; Mora-Poblete, F.; Shafique, M.S.; Chattha, M.S.; Jung, K.H. Advantage of Nanotechnology-Based Genome Editing System and Its Application in Crop Improvement. Front. Plant Sci. 2021, 12, 663849. [Google Scholar] [CrossRef]
- Basso, M.F.; Arraes, F.B.M.; Grossi-de-Sa, M.; Moreira, V.J.V.; Alves-Ferreira, M.; Grossi-de-Sa, M.F. Insights Into Genetic and Molecular Elements for Transgenic Crop Development. Front. Plant Sci. 2020, 11, 509. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Z.; Gao, M.; Guo, B. Plant regeneration of Erigeron breviscapus (vant.) Hand. Mazz. and its chromatographic fingerprint analysis for quality control. Plant Cell Rep. 2008, 27, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, C.; Lin, L.; Chen, W. Callus Induction and Adventitious Shoot Regeneration from Petiole of Erigeron breviscapus. Plant Prod. Sci. 2007, 10, 343–345. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Z.; Yang, W.; Zhang, J.; Yang, M.; Jin, H. Preliminary study on anther culture of Erigeron breviscapus. Bull. Bot. Res. 2009, 29, 509–512. [Google Scholar]
- He, S.; Dong, X.; Zhang, G.; Fan, W.; Duan, S.; Shi, H.; Li, D.; Li, R.; Chen, G.; Long, G.; et al. High quality genome of Erigeron breviscapus provides a reference for herbal plants in Asteraceae. Mol. Ecol. Resour. 2021, 21, 153–169. [Google Scholar] [CrossRef]
- Qiu, L. Genetic transformation of Erigeron breviscapus induced by particle gun with a few affecting factors. Chin. Tradit. Herb. Drugs 1994, 7, 1076–1080. [Google Scholar]
- Wang, Z.-Y.; Ge, Y. Recent advances in genetic transformation of forage and turf grasses. Vitr. Cell. Dev. Biol.-Plant 2006, 42, 1–18. [Google Scholar] [CrossRef]
- Zárate, R.; Verpoorte, R. Strategies for the genetic modification of the medicinal plant Catharanthus roseus (L.) G. Don. Phytochem. Rev. 2007, 6, 475–491. [Google Scholar] [CrossRef]
- Franklin, G.; Oliveira, M.; Dias, A.C.P. Production of transgenic Hypericum perforatum plants via particle bombardment-mediated transformation of novel organogenic cell suspension cultures. Plant Sci. 2007, 172, 1193–1203. [Google Scholar] [CrossRef]
- Lai, K.-S.; Abdullah, P.; Yusoff, K.; Mahmood, M. An efficient protocol for particle bombardment-mediated transformation of Centella asiatica callus. Acta Physiol. Plant. 2011, 33, 2547–2552. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Su, P.; Yang, J.; Huang, L.; Gao, W. Genetic transformation system for woody plant Tripterygium wilfordii and its application to product natural celastrol. Front. Plant Sci. 2018, 8, 2221. [Google Scholar] [CrossRef]
- Narra, M.; Ellendula, R.; Kota, S.; Kalva, B.; Velivela, Y.; Abbagani, S. Efficient genetic transformation of Momordica charantia L. by microprojectile bombardment. 3 Biotech 2018, 8, 2. [Google Scholar] [CrossRef]
- Srinivas, K.; Muralikrishna, N.; Kumar, K.B.; Raghu, E.; Mahender, A.; Kiranmayee, K.; Yashodahara, V.; Sadanandam, A. Biolistic transformation of Scoparia dulcis L. Physiol. Mol. Biol. Plants 2016, 22, 61–68. [Google Scholar] [CrossRef]
- Folling, L.; Olesen, A. Transformation of wheat (Triticum aestivum L.) microspore-derived callus and microspores by particle bombardment. Plant Cell Rep. 2001, 20, 629–636. [Google Scholar] [CrossRef]
- Naser, I.; Yabu, Y.; Maeda, Y.; Tanaka, T. Highly Efficient Genetic Transformation Methods for the Marine Oleaginous Diatom Fistulifera solaris. Mar. Biotechnol. 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, P.; Ashwath, N.; Midmore, D.J. Effects of genotype, explant orientation, and wounding on shoot regeneration in tomato. Vitr. Cell. Dev. Biol. Plant 2005, 41, 457–464. [Google Scholar] [CrossRef]
- George, E.F. Plant Propagation by Tissue Culture; Part 1: The Technology; Exegetics Limited: Worcester, UK, 1993. [Google Scholar]
- Mazumdar, P.; Basu, A.; Paul, A.; Mahanta, C.; Sahoo, L. Age and orientation of the cotyledonary leaf explants determine the efficiency of de novo plant regeneration and Agrobacterium tumefaciens-mediated transformation in Jatropha curcas L. South Afr. J. Bot. 2010, 76, 337–344. [Google Scholar] [CrossRef]
- Rani, T.; Yadav, R.C.; Yadav, N.R.; Kumar, M. Effect of explant orientation on shoot regeneration in tomato (Lycopersicon esculentum). Indian J. Agric. Sci. 2013, 83, 514–517. [Google Scholar]
- Welander, M.; Maheswaran, G. Shoot regeneration from leaf explants of dwarfing apple rootstocks. J. Plant Physiol. 1992, 140, 223–228. [Google Scholar] [CrossRef]
- Bartish, I.; Korkhovoi, V. The composition of nutrient medium and the efficiency of shoot induction in vitro from apple leaf explants. Russ. J. Plant Physiol. 1997, 44, 381–385. [Google Scholar]
- Sanford, J.C.; Smith, F.D.; Russell, J.A. Optimizing the biolistic process for different biological applications. Method Enzymol. 1993, 217, 483–509. [Google Scholar]
- Ismagul, A.; Yang, N.; Maltseva, E.; Iskakova, G.; Mazonka, I.; Skiba, Y.; Bi, H.; Eliby, S.; Jatayev, S.; Shavrukov, Y.; et al. A biolistic method for high-throughput production of transgenic wheat plants with single gene insertions. BMC Plant Biol. 2018, 18, 135. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, C.P.; Carneiro, N.P.; Purcino, A.Á.C.; Carvalho, C.H.S.; Alves, J.D.; Carneiro, A.A. Optimization of particle bombardment parameters for the genetic transformation of Brazilian maize inbred lines. Pesqui. Agropecuária Bras. 2008, 43, 371–378. [Google Scholar] [CrossRef]
- Mahdavi, F.; Mahmood, M.; Noor, N.M. Optimization of particle bombardment parameters for DNA delivery into the male flowers of banana. Biologia 2014, 69, 888–894. [Google Scholar] [CrossRef]
- Gharanjik, S.; Moieni, A.; Mousavi, A.; Alizadeh, H. Optimization of transient expression of uidA gene in androgenic embryos of wheat (Triticum aestivum L. cv. Falat) via particle bombardment. Iran. J. Biotechnol. 2008, 6, 207–213. [Google Scholar]
- Singh, N.; Mishra, A.; Joshi, M.; Jha, B. Microprojectile bombardment mediated genetic transformation of embryo axes and plant regeneration in cumin (Cuminum cyminum L.). Plant Cell Tissue Organ Cult. 2010, 103, 1–6. [Google Scholar] [CrossRef]
- Vasudevan, R.A.; Nadimuthu, K.; Ramachandran, S. Method of High Frequency Regeneration of Sorghum. U.S. Patent 8,431,402, 30 April 2013. [Google Scholar]
- Jähne, A.; Becker, D.; Brettschneider, R.; Lörz, H. Regeneration of transgenic, microspore-derived, fertile barley. Theor. Appl. Genet. 1994, 89, 525–533. [Google Scholar] [CrossRef]
- Harwood, W.A.; Bean, S.J.; Chen, D.F.; Mullineaux, P.M.; Snape, J.W. Transformation studies in Hordeum vulgare using a highly regenerable microspore system. Euphytica 1995, 85, 113–118. [Google Scholar] [CrossRef]
- Mentewab, A.; Letellier, V.; Marque, C.; Sarrafi, A. Use of anthocyanin biosynthesis stimulatory genes as markers for the genetic transformation of haploid embryos and isolated microspores in wheat. Cereal Res. Commun. 1999, 27, 17–24. [Google Scholar] [CrossRef]
- Classic Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Aguilar-Zarate, P.; Cruz-Hernandez, M.A.; Montañez, J.C.; Belmares-Cerda, R.E.; Aguilar, C.N. Enhancement of tannase production by Lactobacillus plantarum CIR1: Validation in gas-lift bioreactor. Bioprocess Biosyst. Eng. 2014, 37, 2305–2316. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.G.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4325. [Google Scholar] [CrossRef] [PubMed]

| Antibiotic | Concentration (mg·L−1) | Callus Induction Rate (%) |
|---|---|---|
| Hyg | 0.0 | 88.61 ± 5.11 * |
| 2.5 | 43.34 ± 3.67 | |
| 5.0 | 15.93 ± 4.90 | |
| 7.5 | 0 | |
| 10.0 | 0 |
| Pre-Culture Day (d) | Transformed Callus Frequency (%) |
|---|---|
| 2 | 0 |
| 3 | 0 |
| 5 | 0 |
| 7 | 54.40 ± 17.40 * |
| 14 | 59.67 ± 12.04 |
| 21 | 0 |
| Symbol | Parameters | Levels | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| A | Precipitation agents | CaCl2 + Spd | MgCl2 + PEG | - |
| B | Target tissue distance (cm) | 3 | 6 | 9 |
| C | Helium pressure (psi) | 650 | 1350 | 1100 |
| D | Number of bombardments | 1 | 2 | 3 |
| E | Plasmid DNA concentration (μg·μL−1) | 1 | 1.5 | 2 |
| F | Chamber vacuum pressure (mmHg) | 26 | 27 | 28 |
| Parameters | K1 1 | K2 2 | K3 3 | R 4 | R’ 5 |
|---|---|---|---|---|---|
| Precipitation agents | 62 | 96 | - | 34 | 59.13 |
| Target tissue distance (cm) | 50 | 24 | 84 | 60 | 54.04 |
| Helium pressure (psi) | 67 | 47 | 44 | 23 | 20.72 |
| Number of bombardments | 58 | 37 | 63 | 26 | 23.42 |
| Plasmid DNA concentration (μg·μL−1) | 72 | 39 | 47 | 33 | 29.72 |
| Chamber vacuum pressure (mmHg) | 42 | 60 | 56 | 18 | 16.21 |
| Parameters | df a | SS b | MS c | Fd | p |
|---|---|---|---|---|---|
| Precipitation agents | 1 | 64.22 | 64.22 | 9.53 | 0.021 * |
| Target tissue distance (cm) | 2 | 301.78 | 150.89 | 22.38 | 0.002 ** |
| Helium pressure (psi) | 2 | 52.11 | 26.06 | 3.87 | 0.083 |
| Number of bombardments | 2 | 63.44 | 31.72 | 4.71 | 0.059 |
| Plasmid DNA concentration (μg·μL−1) | 2 | 98.78 | 49.39 | 7.33 | 0.025 * |
| Chamber vacuum pressure (mmHg) | 2 | 29.78 | 14.89 | 2.21 | 0.191 |
| Error | 6 | 40.44 | 6.74 | ||
| Total | 17 | 650.56 | 38.27 |
| Group | A | B | C | D | E | F | Vacant Column |
|---|---|---|---|---|---|---|---|
| Group 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Group 2 | 1 | 1 | 2 | 2 | 2 | 2 | 2 |
| Group 3 | 1 | 1 | 3 | 3 | 3 | 3 | 3 |
| Group 4 | 1 | 2 | 1 | 1 | 2 | 2 | 3 |
| Group 5 | 1 | 2 | 2 | 2 | 3 | 3 | 1 |
| Group 6 | 1 | 2 | 3 | 3 | 1 | 1 | 2 |
| Group 7 | 1 | 3 | 1 | 2 | 1 | 3 | 2 |
| Group 8 | 1 | 3 | 2 | 3 | 2 | 1 | 3 |
| Group 9 | 1 | 3 | 3 | 1 | 3 | 2 | 1 |
| Group 10 | 2 | 1 | 1 | 3 | 3 | 2 | 2 |
| Group 11 | 2 | 1 | 2 | 1 | 1 | 3 | 3 |
| Group 12 | 2 | 1 | 3 | 2 | 2 | 1 | 1 |
| Group 13 | 2 | 2 | 1 | 2 | 3 | 1 | 3 |
| Group 14 | 2 | 2 | 2 | 3 | 1 | 2 | 1 |
| Group 15 | 2 | 2 | 3 | 1 | 2 | 3 | 2 |
| Group 16 | 2 | 3 | 1 | 3 | 2 | 3 | 1 |
| Group 17 | 2 | 3 | 2 | 1 | 3 | 1 | 2 |
| Group 18 | 2 | 3 | 3 | 2 | 1 | 2 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Yu, Y.; Guo, J.; Zhang, Y.; Huang, L. Rapid and Efficient Optimization Method for a Genetic Transformation System of Medicinal Plants Erigeron breviscapus. Int. J. Mol. Sci. 2023, 24, 5611. https://doi.org/10.3390/ijms24065611
Zhao Y, Yu Y, Guo J, Zhang Y, Huang L. Rapid and Efficient Optimization Method for a Genetic Transformation System of Medicinal Plants Erigeron breviscapus. International Journal of Molecular Sciences. 2023; 24(6):5611. https://doi.org/10.3390/ijms24065611
Chicago/Turabian StyleZhao, Yujun, Yifan Yu, Juan Guo, Yifeng Zhang, and Luqi Huang. 2023. "Rapid and Efficient Optimization Method for a Genetic Transformation System of Medicinal Plants Erigeron breviscapus" International Journal of Molecular Sciences 24, no. 6: 5611. https://doi.org/10.3390/ijms24065611
APA StyleZhao, Y., Yu, Y., Guo, J., Zhang, Y., & Huang, L. (2023). Rapid and Efficient Optimization Method for a Genetic Transformation System of Medicinal Plants Erigeron breviscapus. International Journal of Molecular Sciences, 24(6), 5611. https://doi.org/10.3390/ijms24065611






