A Kaleidoscope of Keratin Gene Expression and the Mosaic of Its Regulatory Mechanisms
Abstract
1. Introduction
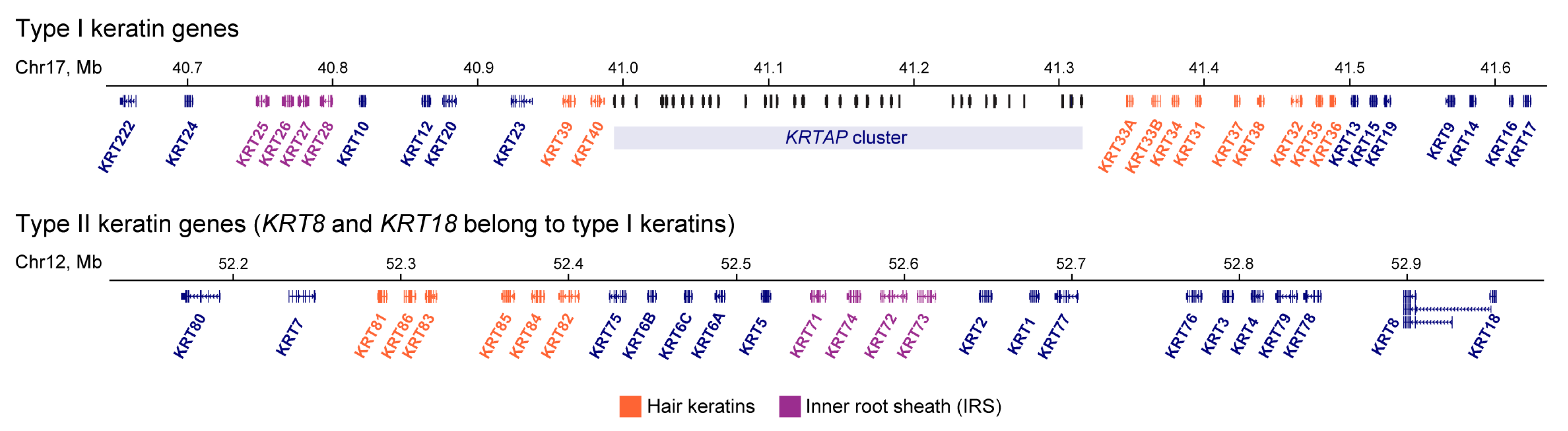
2. Keratin Expression Pattern

3. Regulation of Keratin Gene Transcription
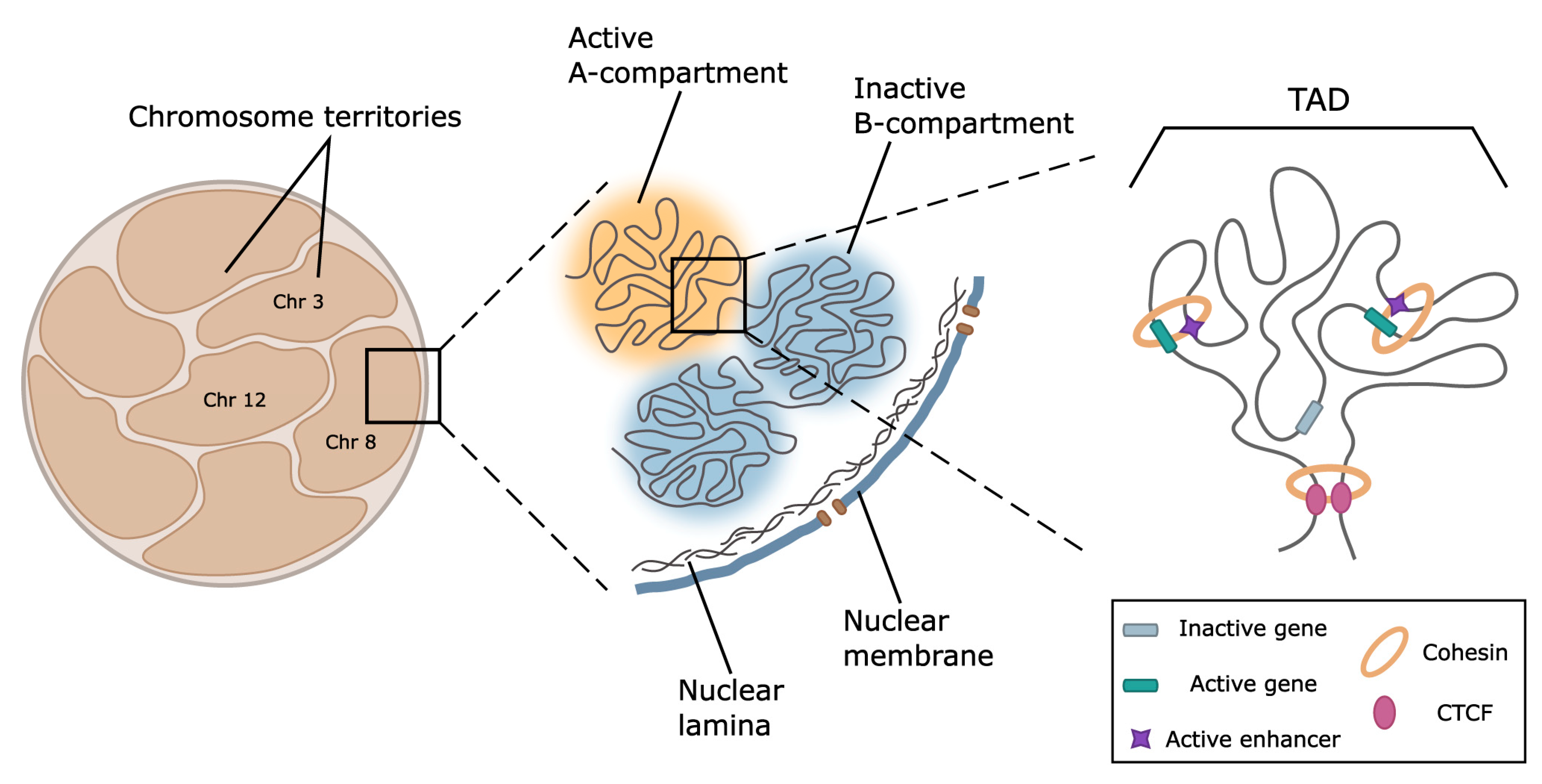
4. Chromatin Spatial Organization as a Regulator of Keratin Gene Expression
5. Conclusions and Outlooks
- (i)
- Reconstruction of the molecular cascades activating/switching keratin expression: from the incoming extracellular signal to activation of distinct keratin promoters.
- (ii)
- Disclosure of mechanisms preventing aberrant activation of keratin genes within complex regulatory landscapes of the keratin gene loci.
- (iii)
- Identification of mutations in regulatory regions associated with diseases caused by aberrant keratin expression.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CL | Companion layer |
| CTCF | CCCTC-binding factor |
| EDC | epidermal differentiation complex |
| FISH | fluorescence in situ hybridization |
| HS | DNase I hypersensitive site |
| IRS | inner root sheath |
| KLF | Kruppel-like factors |
| ORS | outer root sheath |
| TAD | topologically associated domain |
| TF | transcription factor |
| TSS | transcription start site. |
References
- Schweizer, J.; Bowden, P.E.; Coulombe, P.A.; Langbein, L.; Lane, E.B.; Magin, T.M.; Maltais, L.; Omary, M.B.; Parry, D.A.D.; Rogers, M.A.; et al. New Consensus Nomenclature for Mammalian Keratins. J. Cell Biol. 2006, 174, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Moll, R.; Divo, M.; Langbein, L. The Human Keratins: Biology and Pathology. Histochem. Cell Biol. 2008, 129, 705–733. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.T.; Coulombe, P.A.; Kwan, R.; Omary, M.B. Types I and II Keratin Intermediate Filaments. Cold Spring Harb. Perspect. Biol. 2018, 10, a018275. [Google Scholar] [CrossRef]
- Roth, W.; Hatzfeld, M.; Friedrich, M.; Thiering, S.; Magin, T.M. Keratin Function and Regulation in Tissue Homeostasis and Pathogenesis. Biomol. Concepts 2012, 3, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.; Thompson, B.; Fisk, J.N.; Nebert, D.W.; Bruford, E.A.; Vasiliou, V.; Bunick, C.G. Update of the Keratin Gene Family: Evolution, Tissue-Specific Expression Patterns, and Relevance to Clinical Disorders. Hum. Genom. 2022, 16, 1. [Google Scholar] [CrossRef]
- Ehrlich, F.; Fischer, H.; Langbein, L.; Praetzel-Wunder, S.; Ebner, B.; Figlak, K.; Weissenbacher, A.; Sipos, W.; Tschachler, E.; Eckhart, L. Differential Evolution of the Epidermal Keratin Cytoskeleton in Terrestrial and Aquatic Mammals. Mol. Biol. Evol. 2019, 36, 328–340. [Google Scholar] [CrossRef]
- Razin, S.V.; Ioudinkova, E.S.; Kantidze, O.L.; Iarovaia, O.V. Co-Regulated Genes and Gene Clusters. Genes 2021, 12, 907. [Google Scholar] [CrossRef]
- Iarovaia, O.V.; Kovina, A.P.; Petrova, N.V.; Razin, S.V.; Ioudinkova, E.S.; Vassetzky, Y.S.; Ulianov, S.V. Genetic and Epigenetic Mechanisms of β-Globin Gene Switching. Biochem. Biokhimiia 2018, 83, 381–392. [Google Scholar] [CrossRef]
- Montavon, T.; Soshnikova, N. Hox Gene Regulation and Timing in Embryogenesis. Semin. Cell Dev. Biol. 2014, 34, 76–84. [Google Scholar] [CrossRef]
- Wu, Q.; Jia, Z. Wiring the Brain by Clustered Protocadherin Neural Codes. Neurosci. Bull. 2021, 37, 117–131. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Dai, H.-Q.; Hu, H.; Alt, F.W. The Role of Chromatin Loop Extrusion in Antibody Diversification. Nat. Rev. Immunol. 2022, 22, 550–566. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.; Hur, W.; Ku, N.-O. Roles of Keratins in Intestine. Int. J. Mol. Sci. 2022, 23, 8051. [Google Scholar] [CrossRef] [PubMed]
- Cole, B.B.; Smith, R.W.; Jenkins, K.M.; Graham, B.B.; Reynolds, P.R.; Reynolds, S.D. Tracheal Basal Cells: A Facultative Progenitor Cell Pool. Am. J. Pathol. 2010, 177, 362–376. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Gao, Z.; Zhang, L.; Chen, L.; Wu, Z.; Liu, Q.; Wang, S.; Zhou, N.; Chai, T.C.; et al. Single-Cell Transcriptomes of Mouse Bladder Urothelium Uncover Novel Cell Type Markers and Urothelial Differentiation Characteristics. Cell Prolif. 2021, 54, e13007. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.; Johnson, C.; Redmond, C.J.; Nair, R.R.; Coulombe, P.A. Revisiting the Significance of Keratin Expression in Complex Epithelia. J. Cell Sci. 2022, 135, jcs260594. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.Y.G.; Alvarez, Y.D.; Gasnier, M.; Wang, Y.; Tetlak, P.; Bissiere, S.; Wang, H.; Biro, M.; Plachta, N. Keratins Are Asymmetrically Inherited Fate Determinants in the Mammalian Embryo. Nature 2020, 585, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Assou, S.; Boumela, I.; Haouzi, D.; Monzo, C.; Dechaud, H.; Kadoch, I.-J.; Hamamah, S. Transcriptome Analysis during Human Trophectoderm Specification Suggests New Roles of Metabolic and Epigenetic Genes. PLoS ONE 2012, 7, e39306. [Google Scholar] [CrossRef]
- Meinhardt, G.; Haider, S.; Kunihs, V.; Saleh, L.; Pollheimer, J.; Fiala, C.; Hetey, S.; Feher, Z.; Szilagyi, A.; Than, N.G.; et al. Pivotal Role of the Transcriptional Co-Activator YAP in Trophoblast Stemness of the Developing Human Placenta. Proc. Natl. Acad. Sci. USA 2020, 117, 13562–13570. [Google Scholar] [CrossRef]
- Nishioka, N.; Inoue, K.; Adachi, K.; Kiyonari, H.; Ota, M.; Ralston, A.; Yabuta, N.; Hirahara, S.; Stephenson, R.O.; Ogonuki, N.; et al. The Hippo Signaling Pathway Components Lats and Yap Pattern Tead4 Activity to Distinguish Mouse Trophectoderm from Inner Cell Mass. Dev. Cell 2009, 16, 398–410. [Google Scholar] [CrossRef]
- Wu, G.; Gentile, L.; Fuchikami, T.; Sutter, J.; Psathaki, K.; Esteves, T.C.; Araúzo-Bravo, M.J.; Ortmeier, C.; Verberk, G.; Abe, K.; et al. Initiation of Trophectoderm Lineage Specification in Mouse Embryos Is Independent of Cdx2. Dev. Camb. Engl. 2010, 137, 4159–4169. [Google Scholar] [CrossRef]
- Bateman, A.C.; Hübscher, S.G. Cytokeratin Expression as an Aid to Diagnosis in Medical Liver Biopsies. Histopathology 2010, 56, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Desmet, V.J.; van Eyken, P.; Sciot, R. Cytokeratins for Probing Cell Lineage Relationships in Developing Liver. Hepatology 1990, 12, 1249–1251. [Google Scholar] [CrossRef] [PubMed]
- Guldiken, N.; Ensari, G.K.; Lahiri, P.; Couchy, G.; Preisinger, C.; Liedtke, C.; Zimmermann, H.W.; Ziol, M.; Boor, P.; Zucman-Rossi, J.; et al. Keratin 23 Is a Stress-Inducible Marker of Mouse and Human Ductular Reaction in Liver Disease. J. Hepatol. 2016, 65, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Hakoda, T.; Yamamoto, K.; Terada, R.; Okano, N.; Shimada, N.; Suzuki, T.; Mizuno, M.; Shiratori, Y. A Crucial Role of Hepatocyte Nuclear Factor-4 Expression in the Differentiation of Human Ductular Hepatocytes. Lab. Investig. J. Tech. Methods Pathol. 2003, 83, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Wang, W.; Ninomiya, T.; Nagano, H.; Ohta, K.; Itoh, H. Liver Enriched Transcription Factors and Differentiation of Hepatocellular Carcinoma. Mol. Pathol. 1999, 52, 19–24. [Google Scholar] [CrossRef]
- Rhee, H.; Kim, H.-Y.; Choi, J.-H.; Woo, H.G.; Yoo, J.E.; Nahm, J.H.; Choi, J.-S.; Park, Y.N. Keratin 19 Expression in Hepatocellular Carcinoma Is Regulated by Fibroblast-Derived HGF via a MET-ERK1/2-AP1 and SP1 Axis. Cancer Res. 2018, 78, 1619–1631. [Google Scholar] [CrossRef]
- Rhodes, K.; Oshima, R.G. A Regulatory Element of the Human Keratin 18 Gene with AP-1-Dependent Promoter Activity*. J. Biol. Chem. 1998, 273, 26534–26542. [Google Scholar] [CrossRef]
- Toivola, D.M.; Baribault, H.; Magin, T.; Michie, S.A.; Omary, M.B. Simple Epithelial Keratins Are Dispensable for Cytoprotection in Two Pancreatitis Models. Am. J. Physiol.-Gastrointest. Liver Physiol. 2000, 279, G1343–G1354. [Google Scholar] [CrossRef]
- Brembeck, F.H.; Rustgi, A.K. The Tissue-Dependent Keratin 19 Gene Transcription Is Regulated by GKLF/KLF4 and Sp1*. J. Biol. Chem. 2000, 275, 28230–28239. [Google Scholar] [CrossRef]
- Deramaudt, T.B.; Sachdeva, M.M.; Wescott, M.P.; Chen, Y.; Stoffers, D.A.; Rustgi, A.K. The PDX1 Homeodomain Transcription Factor Negatively Regulates the Pancreatic Ductal Cell-Specific Keratin 19 Promoter*. J. Biol. Chem. 2006, 281, 38385–38395. [Google Scholar] [CrossRef]
- Diaferia, G.R.; Balestrieri, C.; Prosperini, E.; Nicoli, P.; Spaggiari, P.; Zerbi, A.; Natoli, G. Dissection of Transcriptional and Cis-Regulatory Control of Differentiation in Human Pancreatic Cancer. EMBO J. 2016, 35, 595–617. [Google Scholar] [CrossRef]
- Mauda-Havakuk, M.; Litichever, N.; Chernichovski, E.; Nakar, O.; Winkler, E.; Mazkereth, R.; Orenstein, A.; Bar-Meir, E.; Ravassard, P.; Meivar-Levy, I.; et al. Ectopic PDX-1 Expression Directly Reprograms Human Keratinocytes along Pancreatic Insulin-Producing Cells Fate. PLoS ONE 2011, 6, e26298. [Google Scholar] [CrossRef] [PubMed]
- Pujal, J.; Huch, M.; José, A.; Abasolo, I.; Rodolosse, A.; Duch, A.; Sánchez-Palazón, L.; Smith, F.J.D.; McLean, W.H.I.; Fillat, C.; et al. Keratin 7 Promoter Selectively Targets Transgene Expression to Normal and Neoplastic Pancreatic Ductal Cells in Vitro and in Vivo. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2009, 23, 1366–1375. [Google Scholar] [CrossRef]
- Benoit, Y.D.; Paré, F.; Francoeur, C.; Jean, D.; Tremblay, E.; Boudreau, F.; Escaffit, F.; Beaulieu, J.-F. Cooperation between HNF-1α, Cdx2, and GATA-4 in Initiating an Enterocytic Differentiation Program in a Normal Human Intestinal Epithelial Progenitor Cell Line. Am. J. Physiol.-Gastrointest. Liver Physiol. 2010, 298, G504–G517. [Google Scholar] [CrossRef]
- Chan, C.W.M.; Wong, N.A.; Liu, Y.; Bicknell, D.; Turley, H.; Hollins, L.; Miller, C.J.; Wilding, J.L.; Bodmer, W.F. Gastrointestinal Differentiation Marker Cytokeratin 20 Is Regulated by Homeobox Gene CDX1. Proc. Natl. Acad. Sci. USA 2009, 106, 1936–1941. [Google Scholar] [CrossRef]
- Chen, L.; Luo, S.; Dupre, A.; Vasoya, R.P.; Parthasarathy, A.; Aita, R.; Malhotra, R.; Hur, J.; Toke, N.H.; Chiles, E.; et al. The Nuclear Receptor HNF4 Drives a Brush Border Gene Program Conserved across Murine Intestine, Kidney, and Embryonic Yolk Sac. Nat. Commun. 2021, 12, 2886. [Google Scholar] [CrossRef]
- Hrudka, J.; Matěj, R.; Nikov, A.; Tomyak, I.; Fišerová, H.; Jelínková, K.; Waldauf, P. Loss of SATB2 Expression Correlates with Cytokeratin 7 and PD-L1 Tumor Cell Positivity and Aggressiveness in Colorectal Cancer. Sci. Rep. 2022, 12, 19152. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Seo, M.-K.; Yoo, S.-Y.; Cho, N.-Y.; Kwak, Y.; Lee, K.; Kim, J.H.; Kang, G.H. Comprehensive Clinicopathologic, Molecular, and Immunologic Characterization of Colorectal Carcinomas with Loss of Three Intestinal Markers, CDX2, SATB2, and KRT20. Virchows Arch. Int. J. Pathol. 2022, 480, 543–555. [Google Scholar] [CrossRef]
- Sree, U.D.; Prayaga, A.K.; Reddy, V.V.R.; Rukmanghadha, N.; Chowhan, A.K.; Phaneendra, B.V. Differential Expression of CK7, CK20, CDX2 in Intestinal and Pancreatobiliary Types of Preriampullary Carcinoma. Indian J. Pathol. Microbiol. 2022, 65, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Polari, L.; Alam, C.M.; Nyström, J.H.; Heikkilä, T.; Tayyab, M.; Baghestani, S.; Toivola, D.M. Keratin Intermediate Filaments in the Colon: Guardians of Epithelial Homeostasis. Int. J. Biochem. Cell Biol. 2020, 129, 105878. [Google Scholar] [CrossRef]
- Kumaran, G.K.; Hanukoglu, I. Identification and Classification of Epithelial Cells in Nephron Segments by Actin Cytoskeleton Patterns. Febs J. 2020, 287, 1176–1194. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, Y.; Yong, J.; Cui, Y.; Wang, R.; Wen, L.; Qiao, J.; Tang, F. Dissecting the Global Dynamic Molecular Profiles of Human Fetal Kidney Development by Single-Cell RNA Sequencing. Cell Rep. 2018, 24, 3554–3567.e3. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Ku, N.-O. Intermediate Filaments of the Lung. Histochem. Cell Biol. 2013, 140, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Little, D.R.; Gerner-Mauro, K.N.; Flodby, P.; Crandall, E.D.; Borok, Z.; Akiyama, H.; Kimura, S.; Ostrin, E.J.; Chen, J. Transcriptional Control of Lung Alveolar Type 1 Cell Development and Maintenance by NK Homeobox 2-1. Proc. Natl. Acad. Sci. USA 2019, 116, 20545–20555. [Google Scholar] [CrossRef]
- Strunz, M.; Simon, L.M.; Ansari, M.; Kathiriya, J.J.; Angelidis, I.; Mayr, C.H.; Tsidiridis, G.; Lange, M.; Mattner, L.F.; Yee, M.; et al. Alveolar Regeneration through a Krt8+ Transitional Stem Cell State That Persists in Human Lung Fibrosis. Nat. Commun. 2020, 11, 3559. [Google Scholar] [CrossRef] [PubMed]
- Mattey, D.L.; Nixon, N.; Wynn-Jones, C.; Dawes, P.T. Demonstration of Cytokeratin in Endothelial Cells of the Synovial Microvasculature in Situ and in Vitro. Br. J. Rheumatol. 1993, 32, 676–682. [Google Scholar] [CrossRef]
- Miettinen, M.; Fetsch, J.F. Distribution of Keratins in Normal Endothelial Cells and a Spectrum of Vascular Tumors: Implications in Tumor Diagnosis. Hum. Pathol. 2000, 31, 1062–1067. [Google Scholar] [CrossRef]
- Chi, J.-T.; Chang, H.Y.; Haraldsen, G.; Jahnsen, F.L.; Troyanskaya, O.G.; Chang, D.S.; Wang, Z.; Rockson, S.G.; van de Rijn, M.; Botstein, D.; et al. Endothelial Cell Diversity Revealed by Global Expression Profiling. Proc. Natl. Acad. Sci. USA 2003, 100, 10623–10628. [Google Scholar] [CrossRef]
- Watson, J.K.; Rulands, S.; Wilkinson, A.C.; Wuidart, A.; Ousset, M.; Van Keymeulen, A.; Göttgens, B.; Blanpain, C.; Simons, B.D.; Rawlins, E.L. Clonal Dynamics Reveal Two Distinct Populations of Basal Cells in Slow-Turnover Airway Epithelium. Cell Rep. 2015, 12, 90–101. [Google Scholar] [CrossRef]
- Rock, J.R.; Onaitis, M.W.; Rawlins, E.L.; Lu, Y.; Clark, C.P.; Xue, Y.; Randell, S.H.; Hogan, B.L.M. Basal Cells as Stem Cells of the Mouse Trachea and Human Airway Epithelium. Proc. Natl. Acad. Sci. USA 2009, 106, 12771–12775. [Google Scholar] [CrossRef]
- Hong, K.U.; Reynolds, S.D.; Watkins, S.; Fuchs, E.; Stripp, B.R. In Vivo Differentiation Potential of Tracheal Basal Cells: Evidence for Multipotent and Unipotent Subpopulations. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2004, 286, L643–L649. [Google Scholar] [CrossRef] [PubMed]
- Schoch, K.G.; Lori, A.; Burns, K.A.; Eldred, T.; Olsen, J.C.; Randell, S.H. A Subset of Mouse Tracheal Epithelial Basal Cells Generates Large Colonies in Vitro. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L631–L642. [Google Scholar] [CrossRef] [PubMed]
- Bischof, J.M.; Ott, C.J.; Leir, S.-H.; Gosalia, N.; Song, L.; London, D.; Furey, T.S.; Cotton, C.U.; Crawford, G.E.; Harris, A. A Genome-Wide Analysis of Open Chromatin in Human Tracheal Epithelial Cells Reveals Novel Candidate Regulatory Elements for Lung Function. Thorax 2012, 67, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Chow, Y.-H.; Plumb, J.; Wen, Y.; Steer, B.M.; Lu, Z.; Buchwald, M.; Hu, J. Targeting Transgene Expression to Airway Epithelia and Submucosal Glands, Prominent Sites of Human CFTR Expression. Mol. Ther. 2000, 2, 359–367. [Google Scholar] [CrossRef]
- Eenjes, E.; Buscop-van Kempen, M.; Boerema-de Munck, A.; Edel, G.G.; Benthem, F.; de Kreij-de Bruin, L.; Schnater, M.; Tibboel, D.; Collins, J.; Rottier, R.J. SOX21 Modulates SOX2-Initiated Differentiation of Epithelial Cells in the Extrapulmonary Airways. eLife 2021, 10, e57325. [Google Scholar] [CrossRef]
- Ghosh, M.; Brechbuhl, H.M.; Smith, R.W.; Li, B.; Hicks, D.A.; Titchner, T.; Runkle, C.M.; Reynolds, S.D. Context-Dependent Differentiation of Multipotential Keratin 14–Expressing Tracheal Basal Cells. Am. J. Respir. Cell Mol. Biol. 2011, 45, 403–410. [Google Scholar] [CrossRef]
- Mollaoglu, G.; Jones, A.; Wait, S.J.; Mukhopadhyay, A.; Jeong, S.; Arya, R.; Camolotto, S.A.; Mosbruger, T.L.; Stubben, C.J.; Conley, C.J.; et al. The Lineage-Defining Transcription Factors SOX2 and NKX2-1 Determine Lung Cancer Cell Fate and Shape the Tumor Immune Microenvironment. Immunity 2018, 49, 764–779.e9. [Google Scholar] [CrossRef]
- Paranjapye, A.; NandyMazumdar, M.; Browne, J.A.; Leir, S.-H.; Harris, A. Krüppel-like Factor 5 Regulates Wound Repair and the Innate Immune Response in Human Airway Epithelial Cells. J. Biol. Chem. 2021, 297, 100932. [Google Scholar] [CrossRef]
- Alonso, A.; Ikinger, U.; Kartenbeck, J. Staining Patterns of Keratins in the Human Urinary Tract. Histol. Histopathol. 2009, 24, 1425–1437. [Google Scholar] [CrossRef]
- Hustler, A.; Eardley, I.; Hinley, J.; Pearson, J.; Wezel, F.; Radvanyi, F.; Baker, S.C.; Southgate, J. Differential Transcription Factor Expression by Human Epithelial Cells of Buccal and Urothelial Derivation. Exp. Cell Res. 2018, 369, 284–294. [Google Scholar] [CrossRef]
- Papafotiou, G.; Paraskevopoulou, V.; Vasilaki, E.; Kanaki, Z.; Paschalidis, N.; Klinakis, A. KRT14 Marks a Subpopulation of Bladder Basal Cells with Pivotal Role in Regeneration and Tumorigenesis. Nat. Commun. 2016, 7, 11914. [Google Scholar] [CrossRef] [PubMed]
- Kamasako, T.; Kaga, K.; Inoue, K.; Hariyama, M.; Yamanishi, T. Supervised Machine Learning Algorithm Identified KRT20, BATF and TP63 as Biologically Relevant Biomarkers for Bladder Biopsy Specimens from Interstitial Cystitis/Bladder Pain Syndrome Patients. Int. J. Urol. 2022, 29, 406–412. [Google Scholar] [CrossRef]
- Guo, C.C.; Bondaruk, J.; Yao, H.; Wang, Z.; Zhang, L.; Lee, S.; Lee, J.-G.; Cogdell, D.; Zhang, M.; Yang, G.; et al. Assessment of Luminal and Basal Phenotypes in Bladder Cancer. Sci. Rep. 2020, 10, 9743. [Google Scholar] [CrossRef] [PubMed]
- Boecker, W.; van Horn, L.; Stenman, G.; Stürken, C.; Schumacher, U.; Loening, T.; Liesenfeld, L.; Korsching, E.; Gläser, D.; Tiemann, K.; et al. Spatially Correlated Phenotyping Reveals K5-Positive Luminal Progenitor Cells and P63-K5/14-Positive Stem Cell-like Cells in Human Breast Epithelium. Lab. Investig. 2018, 98, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Asselin-Labat, M.-L.; Sutherland, K.D.; Barker, H.; Thomas, R.; Shackleton, M.; Forrest, N.C.; Hartley, L.; Robb, L.; Grosveld, F.G.; van der Wees, J.; et al. Gata-3 Is an Essential Regulator of Mammary-Gland Morphogenesis and Luminal-Cell Differentiation. Nat. Cell Biol. 2007, 9, 201–209. [Google Scholar] [CrossRef]
- Chakrabarti, R.; Wei, Y.; Hwang, J.; Hang, X.; Blanco, M.A.; Choudhury, A.; Tiede, B.; Romano, R.-A.; DeCoste, C.; Mercatali, L.; et al. ΔNp63 Promotes Stem Cell Activity in Mammary Gland Development and Basal-like Breast Cancer by Enhancing Fzd7 Expression and Wnt Signaling. Nat. Cell Biol. 2014, 16, 1004–1013. [Google Scholar] [CrossRef]
- LaMarca, H.L.; Visbal, A.P.; Creighton, C.J.; Liu, H.; Zhang, Y.; Behbod, F.; Rosen, J.M. C/EBPβ Regulates Stem Cell Activity and Specifies Luminal Cell Fate in the Mammary Gland. Stem Cells 2010, 28, 535–544. [Google Scholar] [CrossRef]
- Verma, A.; Singh, A.; Singh, M.P.; Nengroo, M.A.; Saini, K.K.; Satrusal, S.R.; Khan, M.A.; Chaturvedi, P.; Sinha, A.; Meena, S.; et al. EZH2-H3K27me3 Mediated KRT14 Upregulation Promotes TNBC Peritoneal Metastasis. Nat. Commun. 2022, 13, 7344. [Google Scholar] [CrossRef]
- Raap, M.; Gierendt, L.; Werlein, C.; Kuehnle, E.; Kreipe, H.H.; Christgen, M. Co-Expression of Transcription Factor AP-2beta (TFAP2B) and GATA3 in Human Mammary Epithelial Cells with Intense, Apicobasal Immunoreactivity for CK8/18. J. Mol. Histol. 2021, 52, 1257–1264. [Google Scholar] [CrossRef]
- Giroux, V.; Lento, A.A.; Islam, M.; Pitarresi, J.R.; Kharbanda, A.; Hamilton, K.E.; Whelan, K.A.; Long, A.; Rhoades, B.; Tang, Q.; et al. Long-Lived Keratin 15+ Esophageal Progenitor Cells Contribute to Homeostasis and Regeneration. J. Clin. Investig. 2017, 127, 2378–2391. [Google Scholar] [CrossRef]
- Tetreault, M.-P.; Yang, Y.; Travis, J.; Yu, Q.-C.; Klein-Szanto, A.; Tobias, J.W.; Katz, J.P. Esophageal Squamous Cell Dysplasia and Delayed Differentiation with Deletion of Klf4 in Murine Esophagus. Gastroenterology 2010, 139, 171–181.e9. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.D.; Opitz, O.G.; Okano, J.; Rustgi, A.K. Transactivation of the Human Keratin 4 and Epstein-Barr Virus ED-L2 Promoters by Gut-Enriched Krüppel-like Factor. J. Biol. Chem. 1998, 273, 10747–10754. [Google Scholar] [CrossRef] [PubMed]
- Brembeck, F.H.; Opitz, O.G.; Libermann, T.A.; Rustgi, A.K. Dual Function of the Epithelial Specific Ets Transcription Factor, ELF3, in Modulating Differentiation. Oncogene 2000, 19, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- DeWard, A.D.; Cramer, J.; Lagasse, E. Cellular Heterogeneity in the Mouse Esophagus Implicates the Presence of a Nonquiescent Epithelial Stem Cell Population. Cell Rep. 2014, 9, 701–711. [Google Scholar] [CrossRef]
- Que, J.; Okubo, T.; Goldenring, J.R.; Nam, K.-T.; Kurotani, R.; Morrisey, E.E.; Taranova, O.; Pevny, L.H.; Hogan, B.L.M. Multiple Dose-Dependent Roles for Sox2 in the Patterning and Differentiation of Anterior Foregut Endoderm. Dev. Camb. Engl. 2007, 134, 2521–2531. [Google Scholar] [CrossRef]
- Stavniichuk, R.; DeLaForest, A.; Thompson, C.A.; Miller, J.; Souza, R.F.; Battle, M.A. GATA4 Blocks Squamous Epithelial Cell Gene Expression in Human Esophageal Squamous Cells. Sci. Rep. 2021, 11, 3206. [Google Scholar] [CrossRef]
- Yu, W.-Y.; Slack, J.M.W.; Tosh, D. Conversion of Columnar to Stratified Squamous Epithelium in the Developing Mouse Oesophagus. Dev. Biol. 2005, 284, 157–170. [Google Scholar] [CrossRef]
- Whelan, K.A.; Muir, A.B.; Nakagawa, H. Esophageal 3D Culture Systems as Modeling Tools in Esophageal Epithelial Pathobiology and Personalized Medicine. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 461–478. [Google Scholar] [CrossRef]
- Sankoda, N.; Tanabe, W.; Tanaka, A.; Shibata, H.; Woltjen, K.; Chiba, T.; Haga, H.; Sakai, Y.; Mandai, M.; Yamamoto, T.; et al. Epithelial Expression of Gata4 and Sox2 Regulates Specification of the Squamous–Columnar Junction via MAPK/ERK Signaling in Mice. Nat. Commun. 2021, 12, 560. [Google Scholar] [CrossRef]
- Attico, E.; Galaverni, G.; Bianchi, E.; Losi, L.; Manfredini, R.; Lambiase, A.; Rama, P.; Pellegrini, G. SOX2 Is a Univocal Marker for Human Oral Mucosa Epithelium Useful in Post-COMET Patient Characterization. Int. J. Mol. Sci. 2022, 23, 5785. [Google Scholar] [CrossRef]
- Iglesias-Bartolome, R.; Uchiyama, A.; Molinolo, A.A.; Abusleme, L.; Brooks, S.R.; Callejas-Valera, J.L.; Edwards, D.; Doci, C.; Asselin-Labat, M.-L.; Onaitis, M.W.; et al. Transcriptional Signature Primes Human Oral Mucosa for Rapid Wound Healing. Sci. Transl. Med. 2018, 10, eaap8798. [Google Scholar] [CrossRef]
- Katada, R.; Tanaka, J.; Takamatsu, K.; Hata, K.; Yasuhara, R.; Ohnuma, S.; Takakura, I.; Nishimura, R.; Shirota, T.; Mishima, K. Induction of Salivary Gland-like Cells from Epithelial Tissues Transdifferentiated from Mouse Embryonic Fibroblasts. Biochem. Biophys. Res. Commun. 2022, 586, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ebersole, J.L.; Orraca, L.; Novak, M.J.; Kirakodu, S.; Gonzalez-Martinez, J.; Gonzalez, O.A. Comparative Analysis of Gene Expression Patterns for Oral Epithelium-Related Functions with Aging. Adv. Exp. Med. Biol. 2019, 1197, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Chaloin-Dufau, C.; Pavitt, I.; Delorme, P.; Dhouailly, D. Identification of Keratins 3 and 12 in Corneal Epithelium of Vertebrates. Epithel. Cell Biol. 1993, 2, 120–125. [Google Scholar]
- Swamynathan, S.K.; Katz, J.P.; Kaestner, K.H.; Ashery-Padan, R.; Crawford, M.A.; Piatigorsky, J. Conditional Deletion of the Mouse Klf4 Gene Results in Corneal Epithelial Fragility, Stromal Edema, and Loss of Conjunctival Goblet Cells. Mol. Cell. Biol. 2007, 27, 182–194. [Google Scholar] [CrossRef]
- Chiambaretta, F.; Blanchon, L.; Rabier, B.; Kao, W.W.-Y.; Liu, J.J.; Dastugue, B.; Rigal, D.; Sapin, V. Regulation of Corneal Keratin-12 Gene Expression by the Human Krüppel-like Transcription Factor 6. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3422–3429. [Google Scholar]
- Liu, J.J.; Kao, W.W.; Wilson, S.E. Corneal Epithelium-Specific Mouse Keratin K12 Promoter. Exp. Eye Res. 1999, 68, 295–301. [Google Scholar] [CrossRef]
- Yoshida, N.; Yoshida, S.; Araie, M.; Handa, H.; Nabeshima, Y. Ets Family Transcription Factor ESE-1 Is Expressed in Corneal Epithelial Cells and Is Involved in Their Differentiation. Mech. Dev. 2000, 97, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Lupasco, T.; He, Z.; Cassagne, M.; Sagnial, T.; Brion, L.; Fournié, P.; Gain, P.; Thuret, G.; Allouche, M.; Malecaze, F.; et al. Corneal Epithelium in Keratoconus Underexpresses Active NRF2 and a Subset of Oxidative Stress-Related Genes. PLoS ONE 2022, 17, e0273807. [Google Scholar] [CrossRef]
- Li, M.; Zhu, L.; Liu, J.; Huang, H.; Guo, H.; Wang, L.; Li, L.; Gu, S.; Tan, J.; Zhong, J.; et al. Loss of FOXC1 Contributes to the Corneal Epithelial Fate Switch and Pathogenesis. Signal Transduct. Target. Ther. 2021, 6, 5. [Google Scholar] [CrossRef]
- Fujimoto, S.; Hayashi, R.; Hara, S.; Sasamoto, Y.; Harrington, J.; Tsujikawa, M.; Nishida, K. KLF4 Prevents Epithelial to Mesenchymal Transition in Human Corneal Epithelial Cells via Endogenous TGF-Β2 Suppression. Regen. Ther. 2019, 11, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.H.; Zeng, Y.M.; Lin, J.S. Dynamic Spatiotemporal Expression Pattern of Limbal Stem Cell Putative Biomarkers during Mouse Development. Exp. Eye Res. 2020, 192, 107915. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, K.; Hikichi, T.; Nakamura, T.; Nakamura, M.; Sotozono, C.; Masui, S.; Kinoshita, S. Direct Reprogramming Into Corneal Epithelial Cells Using a Transcriptional Network Comprising PAX6, OVOL2, and KLF4. Cornea 2019, 38 (Suppl. 1), S34–S41. [Google Scholar] [CrossRef] [PubMed]
- Smits, J.; Lima Cunha, D.; Jieqiong, Q.; Owen, N.; Latta, L.; Szentmáry, N.; Seitz, B.; Roux, L.; Moosajee, M.; Aberdam, D.; et al. Multi-Omics Analyses Identify Transcription Factor Interplay in Corneal Epithelial Fate Determination and Disease. bioRxiv 2022. [Google Scholar] [CrossRef]
- Zhang, L. Keratins in Skin Epidermal Development and Diseases; IntechOpen: Rijeka, Croatia, 2018; ISBN 978-1-78984-805-2. [Google Scholar]
- Romano, R.-A.; Ortt, K.; Birkaya, B.; Smalley, K.; Sinha, S. An Active Role of the ΔN Isoform of P63 in Regulating Basal Keratin Genes K5 and K14 and Directing Epidermal Cell Fate. PLoS ONE 2009, 4, e5623. [Google Scholar] [CrossRef]
- Brauweiler, A.M.; Leung, D.Y.M.; Goleva, E. The Transcription Factor P63 Is a Direct Effector of IL-4- and IL-13-Mediated Repression of Keratinocyte Differentiation. J. Investig. Dermatol. 2021, 141, 770–778. [Google Scholar] [CrossRef]
- Segre, J.A.; Bauer, C.; Fuchs, E. Klf4 Is a Transcription Factor Required for Establishing the Barrier Function of the Skin. Nat. Genet. 1999, 22, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Jaubert, J.; Cheng, J.; Segre, J.A. Ectopic Expression of Kruppel like Factor 4 (Klf4) Accelerates Formation of the Epidermal Permeability Barrier. Development 2003, 130, 2767–2777. [Google Scholar] [CrossRef]
- Chen, X.; Whitney, E.M.; Gao, S.Y.; Yang, V.W. Transcriptional Profiling of Krüppel-like Factor 4 Reveals a Function in Cell Cycle Regulation and Epithelial Differentiation. J. Mol. Biol. 2003, 326, 665–677. [Google Scholar] [CrossRef]
- Chen, Y.; Mistry, D.; Sen, G. Highly Rapid and Efficient Conversion of Human Fibroblasts to Keratinocyte-Like Cells. J. Investig. Dermatol. 2013, 134, 335–344. [Google Scholar] [CrossRef]
- Lee, D.-D.; Stojadinovic, O.; Krzyzanowska, A.; Vouthounis, C.; Blumenberg, M.; Tomic-Canic, M. Retinoid-Responsive Transcriptional Changes in Epidermal Keratinocytes. J. Cell. Physiol. 2009, 220, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Rao, L.; Freedberg, I.M.; Blumenberg, M. Transcriptional Control of K5, K6, K14, and K17 Keratin Genes by AP-1 and NF-ΚB Family Members. Gene Expr. 2018, 6, 361–370. [Google Scholar]
- Maytin, E.V.; Lin, J.C.; Krishnamurthy, R.; Batchvarova, N.; Ron, D.; Mitchell, P.J.; Habener, J.F. Keratin 10 Gene Expression during Differentiation of Mouse Epidermis Requires Transcription Factors C/EBP and AP-2. Dev. Biol. 1999, 216, 164–181. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, E.; Edamitsu, T.; Ohmori, H.; Kohu, K.; Kurokawa, M.; Kiyonari, H.; Satake, M.; Okuyama, R. Transcription Factors Runx1 and Runx3 Suppress Keratin Expression in Undifferentiated Keratinocytes. Int. J. Mol. Sci. 2022, 23, 10039. [Google Scholar] [CrossRef]
- Ohtsuki, M.; Flanagan, S.; Freedberg, I.M.; Blumenberg, M. A Cluster of Five Nuclear Proteins Regulates Keratin Gene Transcription. Gene Expr. 2018, 3, 201–213. [Google Scholar]
- Radoja, N.; Stojadinovic, O.; Waseem, A.; Tomic-Canic, M.; Milisavljevic, V.; Teebor, S.; Blumenberg, M. Thyroid Hormones and Gamma Interferon Specifically Increase K15 Keratin Gene Transcription. Mol. Cell. Biol. 2004, 24, 3168–3179. [Google Scholar] [CrossRef]
- Maytin, E.V.; Habener, J.F. Transcription Factors C/EBP Alpha, C/EBP Beta, and CHOP (Gadd153) Expressed during the Differentiation Program of Keratinocytes in Vitro and in Vivo. J. Investig. Dermatol. 1998, 110, 238–246. [Google Scholar] [CrossRef]
- Radoja, N.; Komine, M.; Jho, S.H.; Blumenberg, M.; Tomic-Canic, M. Novel Mechanism of Steroid Action in Skin through Glucocorticoid Receptor Monomers. Mol. Cell. Biol. 2000, 20, 4328–4339. [Google Scholar] [CrossRef]
- Gatti, V.; Fierro, C.; Compagnone, M.; La Banca, V.; Mauriello, A.; Montanaro, M.; Scalera, S.; De Nicola, F.; Candi, E.; Ricci, F.; et al. ΔNp63-Senataxin Circuit Controls Keratinocyte Differentiation by Promoting the Transcriptional Termination of Epidermal Genes. Proc. Natl. Acad. Sci. USA 2022, 119, e2104718119. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, P.; Liu, D.; Gao, M.; Wang, J.; Wang, X.; Liu, Y.; Zhang, X. C-Myc Upregulated by High Glucose Inhibits HaCaT Differentiation by S100A6 Transcriptional Activation. Front. Endocrinol. 2021, 12, 676403. [Google Scholar] [CrossRef]
- Dai, X.; Shiraishi, K.; Muto, J.; Utsunomiya, R.; Mori, H.; Murakami, M.; Sayama, K. Nuclear IL-33 Plays an Important Role in IL-31–Mediated Downregulation of FLG, Keratin 1, and Keratin 10 by Regulating Signal Transducer and Activator of Transcription 3 Activation in Human Keratinocytes. J. Investig. Dermatol. 2022, 142, 136–144.e3. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, K.; Ki, S.; Ukai, M.; Suzuki, Y.; Inoue, K.; Suda, W.; Matsui, T.; Ito, Y.; Honda, K.; Koseki, H.; et al. Essential Role of STAT3 Signaling in Hair Follicle Homeostasis. Front. Immunol. 2021, 12, 663177. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Ishitsuka, Y.; Inoue, S.; Nakamura, Y.; Saito, A.; Okiyama, N.; Fujisawa, Y.; Furuta, J.; Watanabe, R.; Fujimoto, M. Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) Regulates Epidermal Keratinization under Psoriatic Skin Inflammation. Am. J. Pathol. 2020, 190, 577–585. [Google Scholar] [CrossRef]
- Wu, R.; Zhang, H.; Zhao, M.; Li, J.; Hu, Y.; Fu, J.; Pi, J.; Wang, H.; Xu, Y. Nrf2 in Keratinocytes Protects against Skin Fibrosis via Regulating Epidermal Lesion and Inflammatory Response. Biochem. Pharmacol. 2020, 174, 113846. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Ma, W.; Yan, J.; Zhong, H. Evaluation of the Effects of IL-22 on the Proliferation and Differentiation of Keratinocytes in Vitro. Mol. Med. Rep. 2020, 22, 2715–2722. [Google Scholar] [CrossRef]
- Bowden, P.E. Mutations in a Keratin 6 Isomer (K6c) Cause a Type of Focal Palmoplantar Keratoderma. J. Investig. Dermatol. 2010, 130, 336–338. [Google Scholar] [CrossRef] [PubMed]
- Bernot, K.M.; Coulombe, P.A.; McGowan, K.M. Keratin 16 Expression Defines a Subset of Epithelial Cells During Skin Morphogenesis and the Hair Cycle. J. Investig. Dermatol. 2002, 119, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Dunn, S.M.; Keough, R.A.; Rogers, G.E.; Powell, B.C. Regulation of a Hair Follicle Keratin Intermediate Filament Gene Promoter. J. Cell Sci. 1998, 111, 3487–3496. [Google Scholar] [CrossRef]
- Merrill, B.J.; Gat, U.; DasGupta, R.; Fuchs, E. Tcf3 and Lef1 Regulate Lineage Differentiation of Multipotent Stem Cells in Skin. Genes Dev. 2001, 15, 1688–1705. [Google Scholar] [CrossRef]
- Gat, U.; DasGupta, R.; Degenstein, L.; Fuchs, E. De Novo Hair Follicle Morphogenesis and Hair Tumors in Mice Expressing a Truncated β-Catenin in Skin. Cell 1998, 95, 605–614. [Google Scholar] [CrossRef]
- DasGupta, R.; Fuchs, E. Multiple Roles for Activated LEF/TCF Transcription Complexes during Hair Follicle Development and Differentiation. Development 1999, 126, 4557–4568. [Google Scholar] [CrossRef] [PubMed]
- Jave-Suarez, L.F.; Winter, H.; Langbein, L.; Rogers, M.A.; Schweizer, J. HOXC13 Is Involved in the Regulation of Human Hair Keratin Gene Expression*. J. Biol. Chem. 2002, 277, 3718–3726. [Google Scholar] [CrossRef]
- Kaufman, C.K.; Zhou, P.; Pasolli, H.A.; Rendl, M.; Bolotin, D.; Lim, K.-C.; Dai, X.; Alegre, M.-L.; Fuchs, E. GATA-3: An Unexpected Regulator of Cell Lineage Determination in Skin. Genes Dev. 2003, 17, 2108–2122. [Google Scholar] [CrossRef] [PubMed]
- Vidal, V.P.I.; Chaboissier, M.-C.; Lützkendorf, S.; Cotsarelis, G.; Mill, P.; Hui, C.-C.; Ortonne, N.; Ortonne, J.-P.; Schedl, A. Sox9 Is Essential for Outer Root Sheath Differentiation and the Formation of the Hair Stem Cell Compartment. Curr. Biol. 2005, 15, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Zhao, J.; Du, L.; Chen, Z.; Zhang, L.; Liu, X.; Cheng, J.; Yan, Y.; Zhang, C.; Li, H. The Combination of Hair Follicle-Specific Marker LHX2 and Co-Expressed Marker Can Distinguish between Sweat Gland Placodes and Hair Placodes in Rat. J. Mol. Histol. 2021, 52, 1225–1232. [Google Scholar] [CrossRef]
- Mokry, J.; Pisal, R. Development and Maintenance of Epidermal Stem Cells in Skin Adnexa. Int. J. Mol. Sci. 2020, 21, 9736. [Google Scholar] [CrossRef]
- Wang, J.; He, J.; Zhu, M.; Han, Y.; Yang, R.; Liu, H.; Xu, X.; Chen, X. Cellular Heterogeneity and Plasticity of Skin Epithelial Cells in Wound Healing and Tumorigenesis. Stem Cell Rev. Rep. 2022, 18, 1912–1925. [Google Scholar] [CrossRef]
- Perrin, C. Expression of Follicular Sheath Keratins in the Normal Nail With Special Reference to the Morphological Analysis of the Distal Nail Unit. Am. J. Dermatopathol. 2007, 29, 543. [Google Scholar] [CrossRef]
- Cai, J.; Ma, L. Msx2 and Foxn1 Regulate Nail Homeostasis. Genes 2011, 49, 449–459. [Google Scholar] [CrossRef]
- Liu, M.; Li, F.; Wang, X.; Liu, Z.; Wong, H.S.; Zhou, Y.; Wang, D. Expression Patterns of Hair-Related Keratins and Epithelial Keratins in Onychopapilloma: The Significance of Clarifying the Origin of Onychopapilloma. Front. Med. 2022, 9, 1059624. [Google Scholar] [CrossRef]
- Rice, R.H.; Xia, Y.; Alvarado, R.J.; Phinney, B.S. Proteomic Analysis of Human Nail Plate. Available online: https://pubs.acs.org/doi/pdf/10.1021/pr1009349 (accessed on 26 December 2022).
- Komine, M.; Freedberg, I.M.; Blumenberg, M. Regulation of Epidermal Expression of Keratin K17 in Inflammatory Skin Diseases. J. Investig. Dermatol. 1996, 107, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Peng, H.; Li, R.; Xu, X.; Peng, J. BarH-like Homeobox 2 Represses the Transcription of Keratin 16 and Affects Ras Signaling Pathway to Suppress Nasopharyngeal Carcinoma Progression. Bioengineered 2022, 13, 3122–3136. [Google Scholar] [CrossRef] [PubMed]
- Mikami, Y.; Fujii, S.; Nagata, K.; Wada, H.; Hasegawa, K.; Abe, M.; Yoshimoto, R.U.; Kawano, S.; Nakamura, S.; Kiyoshima, T. GLI-Mediated Keratin 17 Expression Promotes Tumor Cell Growth through the Anti-Apoptotic Function in Oral Squamous Cell Carcinomas. J. Cancer Res. Clin. Oncol. 2017, 143, 1381–1393. [Google Scholar] [CrossRef]
- Navarro, J.M.; Casatorres, J.; Jorcano, J.L. Elements Controlling the Expression and Induction of the Skin Hyperproliferation-Associated Keratin K6 (∗). J. Biol. Chem. 1995, 270, 21362–21367. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-N.; Chang, W.-C. Induction of Disease-Associated Keratin 16 Gene Expression by Epidermal Growth Factor Is Regulated through Cooperation of Transcription Factors Sp1 and c-Jun. J. Biol. Chem. 2003, 278, 45848–45857. [Google Scholar] [CrossRef]
- Yang, L.; Fan, X.; Cui, T.; Dang, E.; Wang, G. Nrf2 Promotes Keratinocyte Proliferation in Psoriasis through Up-Regulation of Keratin 6, Keratin 16, and Keratin 17. J. Investig. Dermatol. 2017, 137, 2168–2176. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, M.; Zhang, L. Keratin 6, 16 and 17—Critical Barrier Alarmin Molecules in Skin Wounds and Psoriasis. Cells 2019, 8, 807. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, S.; Wang, G. Keratin 17 in Disease Pathogenesis: From Cancer to Dermatoses. J. Pathol. 2019, 247, 158–165. [Google Scholar] [CrossRef]
- Lombardo, G.; Melzi, G.; Indino, S.; Piazza, S.; Sangiovanni, E.; Baruffaldi Preis, F.; Marabini, L.; Donetti, E. Keratin 17 as a Marker of UVB-Induced Stress in Human Epidermis and Modulation by Vitis Vinifera Extract. Cells Tissues Organs 2022, 211, 611–627. [Google Scholar] [CrossRef]
- Cornaghi, L.; Gagliano, N.; Preis, F.W.B.; Prignano, F.; Donetti, E. Inside-out and Outside-in Organotypic Normal Human Skin Culture: JAK-STAT Pathway Is Activated after pro-Inflammatory Psoriatic Cytokine Exposure. Tissue Cell 2022, 74, 101675. [Google Scholar] [CrossRef]
- Dai, X.; Utsunomiya, R.; Shiraishi, K.; Mori, H.; Muto, J.; Murakami, M.; Sayama, K. Nuclear IL-33 Plays an Important Role in the Suppression of FLG, LOR, Keratin 1, and Keratin 10 by IL-4 and IL-13 in Human Keratinocytes. J. Investig. Dermatol. 2021, 141, 2646–2655.e6. [Google Scholar] [CrossRef]
- Bai, X.; Yu, C.; Yang, L.; Luo, Y.; Zhi, D.; Wang, G.; Dang, E. Anti-Psoriatic Properties of Paeoniflorin: Suppression of the NF-KappaB Pathway and Keratin 17. Eur. J. Dermatol. EJD 2020, 30, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Hesse, M.; Peters, B.; Magin, T.M. Type II Keratins Precede Type I Keratins during Early Embryonic Development. Eur. J. Cell Biol. 2005, 84, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Sakellariou, S.; Michaelides, C.; Voulgaris, T.; Vlachogiannakos, J.; Manesis, E.; Tiniakos, D.G.; Delladetsima, I. Keratin 7 Expression in Hepatic Cholestatic Diseases. Virchows Arch. 2021, 479, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Menz, A.; Weitbrecht, T.; Gorbokon, N.; Büscheck, F.; Luebke, A.M.; Kluth, M.; Hube-Magg, C.; Hinsch, A.; Höflmayer, D.; Weidemann, S.; et al. Diagnostic and Prognostic Impact of Cytokeratin 18 Expression in Human Tumors: A Tissue Microarray Study on 11,952 Tumors. Mol. Med. Camb. Mass 2021, 27, 16. [Google Scholar] [CrossRef]
- Bader, B.L.; Jahn, L.; Franke, W.W. Low Level Expression of Cytokeratins 8, 18 and 19 in Vascular Smooth Muscle Cells of Human Umbilical Cord and in Cultured Cells Derived Therefrom, with an Analysis of the Chromosomal Locus Containing the Cytokeratin 19 Gene. Eur. J. Cell Biol. 1988, 47, 300–319. [Google Scholar]
- Muriel, J.M.; O’Neill, A.; Kerr, J.P.; Kleinhans-Welte, E.; Lovering, R.M.; Bloch, R.J. Keratin 18 Is an Integral Part of the Intermediate Filament Network in Murine Skeletal Muscle. Am. J. Physiol.-Cell Physiol. 2020, 318, C215–C224. [Google Scholar] [CrossRef]
- Shah, S.; Love, J.; O’Neill, A.; Lovering, R.; Bloch, R. Influences of Desmin and Keratin 19 on Passive Biomechanical Properties of Mouse Skeletal Muscle. J. Biomed. Biotechnol. 2012, 2012, 704061. [Google Scholar] [CrossRef]
- Kuruc, N.; Franke, W.W. Transient Coexpression of Desmin and Cytokeratins 8 and 18 in Developing Myocardial Cells of Some Vertebrate Species. Differ. Res. Biol. Divers. 1988, 38, 177–193. [Google Scholar] [CrossRef]
- Ievlev, V.; Lynch, T.J.; Freischlag, K.W.; Gries, C.B.; Shah, A.; Pai, A.C.; Ahlers, B.A.; Park, S.; Engelhardt, J.F.; Parekh, K.R. Krt14 and Krt15 Differentially Regulate Regenerative Properties and Differentiation Potential of Airway Basal Cells. JCI Insight 2023, 8, e162041. [Google Scholar] [CrossRef]
- Liu, Y.; Lyle, S.; Yang, Z.; Cotsarelis, G. Keratin 15 Promoter Targets Putative Epithelial Stem Cells in the Hair Follicle Bulge. J. Investig. Dermatol. 2003, 121, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.W.; Selvarajah, S.; Franey, S.; Wills, K.A.; Berkovitz, B.K. Interspecies Variations in Oral Epithelial Cytokeratin Expression. J. Anat. 1998, 193 Pt 2, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Tomakidi, P.; Breitkreutz, D.; Fusenig, N.E.; Zöller, J.; Kohl, A.; Komposch, G. Establishment of Oral Mucosa Phenotype in Vitro in Correlation to Epithelial Anchorage. Cell Tissue Res. 1998, 292, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Paladini, R.D.; Takahashi, K.; Bravo, N.S.; Coulombe, P.A. Onset of Re-Epithelialization after Skin Injury Correlates with a Reorganization of Keratin Filaments in Wound Edge Keratinocytes: Defining a Potential Role for Keratin 16. J. Cell Biol. 1996, 132, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, S.; Liu, H.B.; Parent, C.A.; Coulombe, P.A. Keratin 6 Regulates Collective Keratinocyte Migration by Altering Cell–Cell and Cell–Matrix Adhesion. J. Cell Biol. 2018, 217, 4314–4330. [Google Scholar] [CrossRef]
- Pang, B.; Zhu, Z.; Xiao, C.; Luo, Y.; Fang, H.; Bai, Y.; Sun, Z.; Ma, J.; Dang, E.; Wang, G. Keratin 17 Is Required for Lipid Metabolism in Keratinocytes and Benefits Epidermal Permeability Barrier Homeostasis. Front. Cell Dev. Biol. 2021, 9, 779257. [Google Scholar] [CrossRef]
- Sakamoto, K.; Aragaki, T.; Morita, K.; Kawachi, H.; Kayamori, K.; Nakanishi, S.; Omura, K.; Miki, Y.; Okada, N.; Katsube, K.; et al. Down-Regulation of Keratin 4 and Keratin 13 Expression in Oral Squamous Cell Carcinoma and Epithelial Dysplasia: A Clue for Histopathogenesis. Histopathology 2011, 58, 531–542. [Google Scholar] [CrossRef]
- Waseem, A.; Alam, Y.; Lalli, A.; Dogan, B.; Tidman, N.; Purkis, P.; Jackson, S.; Machesney, M.; Leigh, I.M. Keratin 15 Expression in Stratified Epithelia: Downregulation in Activated Keratinocytes. J. Investig. Dermatol. 1999, 112, 362–369. [Google Scholar] [CrossRef]
- Bloor, B.K.; Tidman, N.; Leigh, I.M.; Odell, E.; Dogan, B.; Wollina, U.; Ghali, L.; Waseem, A. Expression of Keratin K2e in Cutaneous and Oral Lesions: Association with Keratinocyte Activation, Proliferation, and Keratinization. Am. J. Pathol. 2003, 162, 963–975. [Google Scholar] [CrossRef]
- Totsuka, A.; Omori-Miyake, M.; Kawashima, M.; Yagi, J.; Tsunemi, Y. Expression of Keratin 1, Keratin 10, Desmoglein 1 and Desmocollin 1 in the Epidermis: Possible Downregulation by Interleukin-4 and Interleukin-13 in Atopic Dermatitis. Eur. J. Dermatol. EJD 2017, 27, 247–253. [Google Scholar] [CrossRef]
- Perrin, C.; Langbein, L.; Schweizer, J. Expression of Hair Keratins in the Adult Nail Unit: An Immunohistochemical Analysis of the Onychogenesis in the Proximal Nail Fold, Matrix and Nail Bed. Br. J. Dermatol. 2004, 151, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Lessard, J.C.; Coulombe, P.A. Keratin 16-Null Mice Develop Palmoplantar Keratoderma, a Hallmark Feature of Pachyonychia Congenita and Related Disorders. J. Investig. Dermatol. 2012, 132, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Langbein, L.; Schweizer, J. Keratins of the Human Hair Follicle11This Article Is Dedicated with Gratitude to Werner W. Franke on the Occasion of His 65th Birthday. His Pioneering Work on Epithelial and Hair Keratins Has Been Pivotal to Our Own Investigations in This Field. In International Review of Cytology; Academic Press: Cambridge, MA, USA, 2005; Volume 243, pp. 1–78. [Google Scholar]
- Chovatiya, G.; Ghuwalewala, S.; Walter, L.D.; Cosgrove, B.D.; Tumbar, T. High-Resolution Single-Cell Transcriptomics Reveals Heterogeneity of Self-Renewing Hair Follicle Stem Cells. Exp. Dermatol. 2021, 30, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Yu, Z.; Song, Y.; Zhang, Y.; Du, J.; Su, Y.; Ma, X. Hair Follicle Bulge-Derived Stem Cells Promote Tissue Regeneration during Skin Expansion. Biomed. Pharmacother. 2020, 132, 110805. [Google Scholar] [CrossRef]
- Langbein, L.; Yoshida, H.; Praetzel-Wunder, S.; Parry, D.; Schweizer, J. The Keratins of the Human Beard Hair Medulla: The Riddle in the Middle. J. Investig. Dermatol. 2009, 130, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, J.; Langbein, L.; Rogers, M.A.; Winter, H. Hair Follicle-Specific Keratins and Their Diseases. Exp. Cell Res. 2007, 313, 2010–2020. [Google Scholar] [CrossRef] [PubMed]
- Winter, H.; Langbein, L.; Praetzel, S.; Jacobs, M.; Rogers, M.A.; Leigh, I.M.; Tidman, N.; Schweizer, J. A Novel Human Type II Cytokeratin, K6hf, Specifically Expressed in the Companion Layer of the Hair Follicle. J. Investig. Dermatol. 1998, 111, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Langbein, L.; Eckhart, L.; Rogers, M.A.; Praetzel-Wunder, S.; Schweizer, J. Against the Rules: Human Keratin K80: Two Functional Alternative Splice Variants, K80 and K80.1, with Special Cellular Localization in a Wide Range of Epithelia. J. Biol. Chem. 2010, 285, 36909–36921. [Google Scholar] [CrossRef]
- Nocelli, C.; Cappelli, K.; Capomaccio, S.; Pascucci, L.; Mercati, F.; Pazzaglia, I.; Mecocci, S.; Antonini, M.; Renieri, C. Shedding Light on Cashmere Goat Hair Follicle Biology: From Morphology Analyses to Transcriptomic Landascape. BMC Genom. 2020, 21, 458. [Google Scholar] [CrossRef]
- Armstrong, C.; Cassimeris, L.; Da Silva Santos, C.; Micoogullari, Y.; Wagner, B.; Babasyan, S.; Brooks, S.; Galantino-Homer, H. The Expression of Equine Keratins K42 and K124 Is Restricted to the Hoof Epidermal Lamellae of Equus Caballus. PLoS ONE 2019, 14, e0219234. [Google Scholar] [CrossRef]
- Kröger, C.; Vijayaraj, P.; Reuter, U.; Windoffer, R.; Simmons, D.; Heukamp, L.; Leube, R.; Magin, T.M. Placental Vasculogenesis Is Regulated by Keratin-Mediated Hyperoxia in Murine Decidual Tissues. Am. J. Pathol. 2011, 178, 1578–1590. [Google Scholar] [CrossRef]
- Baribault, H.; Price, J.; Miyai, K.; Oshima, R.G. Mid-Gestational Lethality in Mice Lacking Keratin 8. Genes Dev. 1993, 7, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Magin, T.M.; Schröder, R.; Leitgeb, S.; Wanninger, F.; Zatloukal, K.; Grund, C.; Melton, D.W. Lessons from Keratin 18 Knockout Mice: Formation of Novel Keratin Filaments, Secondary Loss of Keratin 7 and Accumulation of Liver-Specific Keratin 8-Positive Aggregates. J. Cell Biol. 1998, 140, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Stenvall, C.-G.A.; Tayyab, M.; Grönroos, T.J.; Ilomäki, M.A.; Viiri, K.; Ridge, K.M.; Polari, L.; Toivola, D.M. Targeted Deletion of Keratin 8 in Intestinal Epithelial Cells Disrupts Tissue Integrity and Predisposes to Tumorigenesis in the Colon. Cell. Mol. Life Sci. 2021, 79, 10. [Google Scholar] [CrossRef] [PubMed]
- Toivola, D.M.; Nakamichi, I.; Strnad, P.; Michie, S.A.; Ghori, N.; Harada, M.; Zeh, K.; Oshima, R.G.; Baribault, H.; Omary, M.B. Keratin Overexpression Levels Correlate with the Extent of Spontaneous Pancreatic Injury. Am. J. Pathol. 2008, 172, 882–892. [Google Scholar] [CrossRef]
- Lloyd, C.; Yu, Q.C.; Cheng, J.; Turksen, K.; Degenstein, L.; Hutton, E.; Fuchs, E. The Basal Keratin Network of Stratified Squamous Epithelia: Defining K15 Function in the Absence of K14. J. Cell Biol. 1995, 129, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.; Kirfel, J.; Büssow, H.; Vidal, M.; Magin, T.M. Complete Cytolysis and Neonatal Lethality in Keratin 5 Knockout Mice Reveal Its Fundamental Role in Skin Integrity and in Epidermolysis Bullosa Simplex. Mol. Biol. Cell 2001, 12, 1775–1789. [Google Scholar] [CrossRef]
- Cockburn, K.; Annusver, K.; Gonzalez, D.G.; Ganesan, S.; May, D.P.; Mesa, K.R.; Kawaguchi, K.; Kasper, M.; Greco, V. Gradual Differentiation Uncoupled from Cell Cycle Exit Generates Heterogeneity in the Epidermal Stem Cell Layer. Nat. Cell Biol. 2022, 24, 1692–1700. [Google Scholar] [CrossRef]
- Morgan, H.J.; Benketah, A.; Olivero, C.; Rees, E.; Ziaj, S.; Mukhtar, A.; Lanfredini, S.; Patel, G.K. Hair Follicle Differentiation-Specific Keratin Expression in Human Basal Cell Carcinoma. Clin. Exp. Dermatol. 2020, 45, 417–425. [Google Scholar] [CrossRef]
- Gu, L.-H.; Coulombe, P.A. Keratin Expression Provides Novel Insight into the Morphogenesis and Function of the Companion Layer in Hair Follicles. J. Investig. Dermatol. 2007, 127, 1061–1073. [Google Scholar] [CrossRef]
- Mesler, A.L.; Veniaminova, N.A.; Lull, M.V.; Wong, S.Y. Hair Follicle Terminal Differentiation Is Orchestrated by Distinct Early and Late Matrix Progenitors. Cell Rep. 2017, 19, 809–821. [Google Scholar] [CrossRef]
- Leask, A.; Rosenberg, M.; Vassar, R.; Fuchs, E. Regulation of a Human Epidermal Keratin Gene: Sequences and Nuclear Factors Involved in Keratinocyte-Specific Transcription. Genes Dev. 1990, 4, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Degenstein, L.; Copenhaver, C.; Fuchs, E. Defining the Regulatory Factors Required for Epidermal Gene Expression. Mol. Cell. Biol. 2000, 20, 2543–2555. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Fuchs, E. Identification and Dissection of an Enhancer Controlling Epithelial Gene Expression in Skin. Proc. Natl. Acad. Sci. USA 2001, 98, 2455–2460. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, C.K.; Sinha, S.; Bolotin, D.; Fan, J.; Fuchs, E. Dissection of a Complex Enhancer Element: Maintenance of Keratinocyte Specificity but Loss of Differentiation Specificity. Mol. Cell. Biol. 2002, 22, 4293–4308. [Google Scholar] [CrossRef]
- Takahashi, K.; Coulombe, P.A. Defining a Region of the Human Keratin 6a Gene That Confers Inducible Expression in Stratified Epithelia of Transgenic Mice. J. Biol. Chem. 1997, 272, 11979–11985. [Google Scholar] [CrossRef]
- Rothnagel, J.A.; Greenhalgh, D.A.; Gagne, T.A.; Longley, M.A.; Roop, D.R. Identification of a Calcium-Inducible, Epidermal-Specific Regulatory Element in the 3′-Flanking Region of the Human Keratin 1 Gene. J. Investig. Dermatol. 1993, 101, 506–513. [Google Scholar] [CrossRef]
- Hu, L.; Gudas, L.J. Activation of Keratin 19 Gene Expression by a 3′ Enhancer Containing an AP1 Site. J. Biol. Chem. 1994, 269, 183–191. [Google Scholar] [CrossRef]
- Neznanov, N.; Thorey, I.S.; Ceceña, G.; Oshima, R.G. Transcriptional Insulation of the Human Keratin 18 Gene in Transgenic Mice. Mol. Cell. Biol. 1993, 13, 2214–2223. [Google Scholar] [CrossRef]
- Oshima, R.G.; Abrams, L.; Kulesh, D. Activation of an Intron Enhancer within the Keratin 18 Gene by Expression of C-Fos and c-Jun in Undifferentiated F9 Embryonal Carcinoma Cells. Genes Dev. 1990, 4, 835–848. [Google Scholar] [CrossRef]
- Pankov, R.; Umezawa, A.; Maki, R.; Der, C.J.; Hauser, C.A.; Oshima, R.G. Oncogene Activation of Human Keratin 18 Transcription via the Ras Signal Transduction Pathway. Proc. Natl. Acad. Sci. USA 1994, 91, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Pankov, R.; Neznanov, N.; Umezawa, A.; Oshima, R.G. AP-1, ETS, and Transcriptional Silencers Regulate Retinoic Acid-Dependent Induction of Keratin 18 in Embryonic Cells. Mol. Cell. Biol. 1994, 14, 7744–7757. [Google Scholar] [CrossRef] [PubMed]
- Neznanov, N.; Umezawa, A.; Oshima, R.G. A Regulatory Element within a Coding Exon Modulates Keratin 18 Gene Expression in Transgenic Mice. J. Biol. Chem. 1997, 272, 27549–27557. [Google Scholar] [CrossRef] [PubMed]
- Casanova, L.; Bravo, A.; Were, F.; Ramírez, A.; Jorcano, J.J.; Vidal, M. Tissue-Specific and Efficient Expression of the Human Simple Epithelial Keratin 8 Gene in Transgenic Mice. J. Cell Sci. 1995, 108 Pt 2, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Soares, E.; Zhou, H. Master Regulatory Role of P63 in Epidermal Development and Disease. Cell. Mol. Life Sci. CMLS 2018, 75, 1179–1190. [Google Scholar] [CrossRef]
- Romano, R.-A.; Smalley, K.; Magraw, C.; Serna, V.A.; Kurita, T.; Raghavan, S.; Sinha, S. ΔNp63 Knockout Mice Reveal Its Indispensable Role as a Master Regulator of Epithelial Development and Differentiation. Dev. Camb. Engl. 2012, 139, 772–782. [Google Scholar] [CrossRef]
- Bao, X.; Rubin, A.J.; Qu, K.; Zhang, J.; Giresi, P.G.; Chang, H.Y.; Khavari, P.A. A Novel ATAC-Seq Approach Reveals Lineage-Specific Reinforcement of the Open Chromatin Landscape via Cooperation between BAF and P63. Genome Biol. 2015, 16, 284. [Google Scholar] [CrossRef]
- Kouwenhoven, E.N.; Oti, M.; Niehues, H.; van Heeringen, S.J.; Schalkwijk, J.; Stunnenberg, H.G.; van Bokhoven, H.; Zhou, H. Transcription Factor P63 Bookmarks and Regulates Dynamic Enhancers during Epidermal Differentiation. EMBO Rep. 2015, 16, 863–878. [Google Scholar] [CrossRef]
- Yu, X.; Singh, P.K.; Tabrejee, S.; Sinha, S.; Buck, M.J. ΔNp63 Is a Pioneer Factor That Binds Inaccessible Chromatin and Elicits Chromatin Remodeling. Epigenetics Chromatin 2021, 14, 20. [Google Scholar] [CrossRef]
- Lee, A.-Y. The Role of MicroRNAs in Epidermal Barrier. Int. J. Mol. Sci. 2020, 21, 5781. [Google Scholar] [CrossRef]
- Tiwari, N.; Meyer-Schaller, N.; Arnold, P.; Antoniadis, H.; Pachkov, M.; van Nimwegen, E.; Christofori, G. Klf4 Is a Transcriptional Regulator of Genes Critical for EMT, Including Jnk1 (Mapk8). PLoS ONE 2013, 8, e57329. [Google Scholar] [CrossRef]
- Mistry, D.S.; Chen, Y.; Wang, Y.; Sen, G.L. SNAI2 Controls the Undifferentiated State of Human Epidermal Progenitor Cells. Stem Cells 2014, 32, 3209–3218. [Google Scholar] [CrossRef] [PubMed]
- Hudson, L.G.; Newkirk, K.M.; Chandler, H.L.; Choi, C.; Fossey, S.L.; Parent, A.E.; Kusewitt, D.F. Cutaneous Wound Reepithelialization Is Compromised in Mice Lacking Functional Slug (Snai2). J. Dermatol. Sci. 2009, 56, 19–26. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Misra, S.; Chaudhuri, G. Negative Regulation of the Expressions of Cytokeratins 8 and 19 by SLUG Repressor Protein in Human Breast Cells. Biochem. Biophys. Res. Commun. 2005, 329, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Houschyar, K.S.; Borrelli, M.R.; Tapking, C.; Popp, D.; Puladi, B.; Ooms, M.; Chelliah, M.P.; Rein, S.; Pförringer, D.; Thor, D.; et al. Molecular Mechanisms of Hair Growth and Regeneration: Current Understanding and Novel Paradigms. Dermatology 2020, 236, 271–280. [Google Scholar] [CrossRef] [PubMed]
- van Genderen, C.; Okamura, R.M.; Fariñas, I.; Quo, R.G.; Parslow, T.G.; Bruhn, L.; Grosschedl, R. Development of Several Organs That Require Inductive Epithelial-Mesenchymal Interactions Is Impaired in LEF-1-Deficient Mice. Genes Dev. 1994, 8, 2691–2703. [Google Scholar] [CrossRef]
- Su, Y.; Wen, J.; Zhu, J.; Xie, Z.; Liu, C.; Ma, C.; Zhang, Q.; Xu, X.; Wu, X. Pre-Aggregation of Scalp Progenitor Dermal and Epidermal Stem Cells Activates the WNT Pathway and Promotes Hair Follicle Formation in in Vitro and in Vivo Systems. Stem Cell Res. Ther. 2019, 10, 403. [Google Scholar] [CrossRef]
- Sharma, R.; Choi, K.-J.; Quan, M.D.; Sharma, S.; Sankaran, B.; Park, H.; LaGrone, A.; Kim, J.J.; MacKenzie, K.R.; Ferreon, A.C.M.; et al. Liquid Condensation of Reprogramming Factor KLF4 with DNA Provides a Mechanism for Chromatin Organization. Nat. Commun. 2021, 12, 5579. [Google Scholar] [CrossRef]
- Qu, J.; Yi, G.; Zhou, H. P63 Cooperates with CTCF to Modulate Chromatin Architecture in Skin Keratinocytes. Epigenetics Chromatin 2019, 12, 31. [Google Scholar] [CrossRef]
- Cremer, T.; Cremer, M. Chromosome Territories. Cold Spring Harb. Perspect. Biol. 2010, 2, a003889. [Google Scholar] [CrossRef]
- Lieberman-Aiden, E.; van Berkum, N.L.; Williams, L.; Imakaev, M.; Ragoczy, T.; Telling, A.; Amit, I.; Lajoie, B.R.; Sabo, P.J.; Dorschner, M.O.; et al. Comprehensive Mapping of Long-Range Interactions Reveals Folding Principles of the Human Genome. Science 2009, 326, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Schoenfelder, S.; Sexton, T.; Chakalova, L.; Cope, N.F.; Horton, A.; Andrews, S.; Kurukuti, S.; Mitchell, J.A.; Umlauf, D.; Dimitrova, D.S.; et al. Preferential Associations between Co-Regulated Genes Reveal a Transcriptional Interactome in Erythroid Cells. Nat. Genet. 2010, 42, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Monahan, K.; Horta, A.; Lomvardas, S. LHX2- and LDB1-Mediated Trans Interactions Regulate Olfactory Receptor Choice. Nature 2019, 565, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Razin, S.V.; Gavrilov, A.A. The Role of Liquid-Liquid Phase Separation in the Compartmentalization of Cell Nucleus and Spatial Genome Organization. Biochem. Biokhimiia 2020, 85, 643–650. [Google Scholar] [CrossRef]
- Lu, H.; Yu, D.; Hansen, A.S.; Ganguly, S.; Liu, R.; Heckert, A.; Darzacq, X.; Zhou, Q. Phase-Separation Mechanism for C-Terminal Hyperphosphorylation of RNA Polymerase II. Nature 2018, 558, 318–323. [Google Scholar] [CrossRef]
- Hansen, J.C.; Maeshima, K.; Hendzel, M.J. The Solid and Liquid States of Chromatin. Epigenetics Chromatin 2021, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.R.; Selvaraj, S.; Yue, F.; Kim, A.; Li, Y.; Shen, Y.; Hu, M.; Liu, J.S.; Ren, B. Topological Domains in Mammalian Genomes Identified by Analysis of Chromatin Interactions. Nature 2012, 485, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Sexton, T.; Yaffe, E.; Kenigsberg, E.; Bantignies, F.; Leblanc, B.; Hoichman, M.; Parrinello, H.; Tanay, A.; Cavalli, G. Three-Dimensional Folding and Functional Organization Principles of the Drosophila Genome. Cell 2012, 148, 458–472. [Google Scholar] [CrossRef]
- Brandão, H.B.; Paul, P.; van den Berg, A.A.; Rudner, D.Z.; Wang, X.; Mirny, L.A. RNA Polymerases as Moving Barriers to Condensin Loop Extrusion. Proc. Natl. Acad. Sci. USA 2019, 116, 20489–20499. [Google Scholar] [CrossRef]
- Rao, S.S.P.; Huntley, M.H.; Durand, N.C.; Stamenova, E.K.; Bochkov, I.D.; Robinson, J.T.; Sanborn, A.L.; Machol, I.; Omer, A.D.; Lander, E.S.; et al. A 3D Map of the Human Genome at Kilobase Resolution Reveals Principles of Chromatin Looping. Cell 2014, 159, 1665–1680. [Google Scholar] [CrossRef]
- Nora, E.P.; Goloborodko, A.; Valton, A.-L.; Gibcus, J.H.; Uebersohn, A.; Abdennur, N.; Dekker, J.; Mirny, L.A.; Bruneau, B.G. Targeted Degradation of CTCF Decouples Local Insulation of Chromosome Domains from Genomic Compartmentalization. Cell 2017, 169, 930–944.e22. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.P.; Huang, S.-C.; Glenn St Hilaire, B.; Engreitz, J.M.; Perez, E.M.; Kieffer-Kwon, K.-R.; Sanborn, A.L.; Johnstone, S.E.; Bascom, G.D.; Bochkov, I.D.; et al. Cohesin Loss Eliminates All Loop Domains. Cell 2017, 171, 305–320.e24. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.R.; Jung, I.; Selvaraj, S.; Shen, Y.; Antosiewicz-Bourget, J.E.; Lee, A.Y.; Ye, Z.; Kim, A.; Rajagopal, N.; Xie, W.; et al. Chromatin Architecture Reorganization during Stem Cell Differentiation. Nature 2015, 518, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Pope, B.D.; Ryba, T.; Dileep, V.; Yue, F.; Wu, W.; Denas, O.; Vera, D.L.; Wang, Y.; Hansen, R.S.; Canfield, T.K.; et al. Topologically Associating Domains Are Stable Units of Replication-Timing Regulation. Nature 2014, 515, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Nora, E.P.; Lajoie, B.R.; Schulz, E.G.; Giorgetti, L.; Okamoto, I.; Servant, N.; Piolot, T.; van Berkum, N.L.; Meisig, J.; Sedat, J.; et al. Spatial Partitioning of the Regulatory Landscape of the X-Inactivation Centre. Nature 2012, 485, 381–385. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, Q.; Canzio, D.; Shou, J.; Li, J.; Gorkin, D.U.; Jung, I.; Wu, H.; Zhai, Y.; Tang, Y.; et al. CRISPR Inversion of CTCF Sites Alters Genome Topology and Enhancer/Promoter Function. Cell 2015, 162, 900–910. [Google Scholar] [CrossRef]
- Sehgal, N.; Seifert, B.; Ding, H.; Chen, Z.; Stojkovic, B.; Bhattacharya, S.; Xu, J.; Berezney, R. Reorganization of the Interchromosomal Network during Keratinocyte Differentiation. Chromosoma 2016, 125, 389–403. [Google Scholar] [CrossRef]
- Cavazza, A.; Miccio, A.; Romano, O.; Petiti, L.; Malagoli Tagliazucchi, G.; Peano, C.; Severgnini, M.; Rizzi, E.; De Bellis, G.; Bicciato, S.; et al. Dynamic Transcriptional and Epigenetic Regulation of Human Epidermal Keratinocyte Differentiation. Stem Cell Rep. 2016, 6, 618–632. [Google Scholar] [CrossRef]
- Rubin, A.J.; Barajas, B.C.; Furlan-Magaril, M.; Lopez-Pajares, V.; Mumbach, M.R.; Howard, I.; Kim, D.S.; Boxer, L.D.; Cairns, J.; Spivakov, M.; et al. Lineage-Specific Dynamic and Pre-Established Enhancer-Promoter Contacts Cooperate in Terminal Differentiation. Nat. Genet. 2017, 49, 1522–1528. [Google Scholar] [CrossRef]
- Poterlowicz, K.; Yarker, J.L.; Malashchuk, I.; Lajoie, B.R.; Mardaryev, A.N.; Gdula, M.R.; Sharov, A.A.; Kohwi-Shigematsu, T.; Botchkarev, V.A.; Fessing, M.Y. 5C Analysis of the Epidermal Differentiation Complex Locus Reveals Distinct Chromatin Interaction Networks between Gene-Rich and Gene-Poor TADs in Skin Epithelial Cells. PLoS Genet. 2017, 13, e1006966. [Google Scholar] [CrossRef]
- Chen, G.-D.; Fatima, I.; Xu, Q.; Rozhkova, E.; Fessing, M.Y.; Mardaryev, A.N.; Sharov, A.A.; Xu, G.-L.; Botchkarev, V.A. DNA Dioxygenases Tet2/3 Regulate Gene Promoter Accessibility and Chromatin Topology in Lineage-Specific Loci to Control Epithelial Differentiation. Sci. Adv. 2023, 9, eabo7605. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.S.; Wu, H.; Ji, X.; Stelzer, Y.; Wu, X.; Czauderna, S.; Shu, J.; Dadon, D.; Young, R.A.; Jaenisch, R. Editing DNA Methylation in the Mammalian Genome. Cell 2016, 167, 233–247.e17. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-C.; Wu, P.; Lin, G.-W.; Chen, C.-K.; Yeh, C.-Y.; Tsai, S.; Yan, J.; Jiang, T.-X.; Lai, Y.-C.; Huang, D.; et al. Folding Keratin Gene Clusters during Skin Regional Specification. Dev. Cell 2020, 53, 561–576.e9. [Google Scholar] [CrossRef]
- Abaci, H.E.; Coffman, A.; Doucet, Y.; Chen, J.; Jacków, J.; Wang, E.; Guo, Z.; Shin, J.U.; Jahoda, C.A.; Christiano, A.M. Tissue Engineering of Human Hair Follicles Using a Biomimetic Developmental Approach. Nat. Commun. 2018, 9, 5301. [Google Scholar] [CrossRef]
- Ji, S.; Zhu, Z.; Sun, X.; Fu, X. Functional Hair Follicle Regeneration: An Updated Review. Signal Transduct. Target. Ther. 2021, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Itami, S.; Tarutani, M.; Hosokawa, K.; Miura, H.; Yoshikawa, K. Regulation of Keratin 9 in Nonpalmoplantar Keratinocytes by Palmoplantar Fibroblasts Through Epithelial–Mesenchymal Interactions. J. Investig. Dermatol. 1999, 112, 483–488. [Google Scholar] [CrossRef]
- Yang, X.; Moldovan, N.; Zhao, Q.; Mi, S.; Zhou, Z.; Chen, D.; Gao, Z.; Tong, D.; Dou, Z. Reconstruction of Damaged Cornea by Autologous Transplantation of Epidermal Adult Stem Cells. Mol. Vis. 2008, 14, 1064–1070. [Google Scholar]
- Rogovaya, O.S.; Fayzulin, A.K.; Vasiliev, A.V.; Kononov, A.V.; Terskikh, V.V. Reconstruction of Rabbit Urethral Epithelium with Skin Keratinocytes. Acta Nat. 2015, 7, 70–77. [Google Scholar] [CrossRef]
- Yao, S.; Huang, H.-Y.; Han, X.; Ye, Y.; Qin, Z.; Zhao, G.; Li, F.; Hu, G.; Hu, L.; Ji, H. Keratin 14-High Subpopulation Mediates Lung Cancer Metastasis Potentially through Gkn1 Upregulation. Oncogene 2019, 38, 6354–6369. [Google Scholar] [CrossRef]
- Ogunnigbagbe, O.; Bunick, C.G.; Kaur, K. Keratin 1 as a Cell-Surface Receptor in Cancer. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188664. [Google Scholar] [CrossRef]
- Hu, W.-Y.; Hu, D.-P.; Xie, L.; Nonn, L.; Lu, R.; Abern, M.; Shioda, T.; Prins, G.S. Keratin Profiling by Single-Cell RNA-Sequencing Identifies Human Prostate Stem Cell Lineage Hierarchy and Cancer Stem-Like Cells. Int. J. Mol. Sci. 2021, 22, 8109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, G.; Yan, W.; Jiang, H. MiR-4268 Suppresses Gastric Cancer Genesis through Inhibiting Keratin 80. Cell Cycle Georget. Tex 2022, 21, 2051–2064. [Google Scholar] [CrossRef]
- Baraks, G.; Tseng, R.; Pan, C.-H.; Kasliwal, S.; Leiton, C.V.; Shroyer, K.R.; Escobar-Hoyos, L.F. Dissecting the Oncogenic Roles of Keratin 17 in the Hallmarks of Cancer. Cancer Res. 2022, 82, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Roa-Peña, L.; Babu, S.; Leiton, C.V.; Wu, M.; Taboada, S.; Akalin, A.; Buscaglia, J.; Escobar-Hoyos, L.F.; Shroyer, K.R. Keratin 17 Testing in Pancreatic Cancer Needle Aspiration Biopsies Predicts Survival. Cancer Cytopathol. 2021, 129, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lozar, T.; Golfinos, A.E.; Lee, D.; Gronski, E.; Ward-Shaw, E.; Hayes, M.; Bruce, J.Y.; Kimple, R.J.; Hu, R.; et al. Stress Keratin 17 Expression in Head and Neck Cancer Contributes to Immune Evasion and Resistance to Immune-Checkpoint Blockade. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, 28, 2953–2968. [Google Scholar] [CrossRef]
- Ouyang, S.; Kang, W.-M. Research Advances in the Role of Keratins in Gastrointestinal Cancer. Chin. Med. Sci. J. 2022, 37, 73–78. [Google Scholar] [CrossRef]
- Sequeira, I.; Neves, J.F.; Carrero, D.; Peng, Q.; Palasz, N.; Liakath-Ali, K.; Lord, G.M.; Morgan, P.R.; Lombardi, G.; Watt, F.M. Immunomodulatory Role of Keratin 76 in Oral and Gastric Cancer. Nat. Commun. 2018, 9, 3437. [Google Scholar] [CrossRef]
- Roa-Peña, L.; Leiton, C.V.; Babu, S.; Pan, C.-H.; Vanner, E.A.; Akalin, A.; Bandovic, J.; Moffitt, R.A.; Shroyer, K.R.; Escobar-Hoyos, L.F. Keratin 17 Identifies the Most Lethal Molecular Subtype of Pancreatic Cancer. Sci. Rep. 2019, 9, 11239. [Google Scholar] [CrossRef]
- Zhu, R.-J.; Zhou, J.; Liang, P.-Q.; Xiang, X.-X.; Ran, J.; Xie, T.-A.; Guo, X.-G. Accuracy of Cytokeratin 19 Fragment in the Diagnosis of Bladder Cancer. Biomark. Med. 2022, 16, 197–216. [Google Scholar] [CrossRef]
- Hung, C.-S.; Wang, Y.-C.; Guo, J.-W.; Yang, R.-N.; Lee, C.-L.; Shen, M.-H.; Huang, C.-C.; Huang, C.-J.; Yang, J.-Y.; Liu, C.-Y. Expression Pattern of Placenta Specific 8 and Keratin 20 in Different Types of Gastrointestinal Cancer. Mol. Med. Rep. 2020, 21, 659–666. [Google Scholar] [CrossRef]
- Kim, J.; Villadsen, R. The Expression Pattern of Epidermal Differentiation Marker Keratin 10 in the Normal Human Breast and Breast Cancer Cells. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2020, 68, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Wang, J.; Song, H.M.; Deng, B.; Li, J.G. KRT15 Overexpression Predicts Poor Prognosis in Colorectal Cancer. Neoplasma 2020, 67, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.D.K.; Babu, S.; Roa-Peña, L.; Hou, W.; Akalin, A.; Escobar-Hoyos, L.F.; Shroyer, K.R. Keratin 17 Is a Negative Prognostic Biomarker in High-Grade Endometrial Carcinomas. Hum. Pathol. 2019, 94, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.E.; Torbenson, M.S.; Wu, T.-T.; Chandan, V.S. Aberrant Keratin Expression Is Common in Primary Hepatic Malignant Vascular Tumors: A Potential Diagnostic Pitfall. Ann. Diagn. Pathol. 2020, 49, 151589. [Google Scholar] [CrossRef]
- Werner, S.; Keller, L.; Pantel, K. Epithelial Keratins: Biology and Implications as Diagnostic Markers for Liquid Biopsies. Mol. Aspects Med. 2020, 72, 100817. [Google Scholar] [CrossRef]
- Zhang, Z.; Tu, K.; Liu, F.; Liang, M.; Yu, K.; Wang, Y.; Luo, Y.; Yang, B.; Qin, Y.; He, D.; et al. FoxM1 Promotes the Migration of Ovarian Cancer Cell through KRT5 and KRT7. Gene 2020, 757, 144947. [Google Scholar] [CrossRef]
- Ji, R.; Ji, Y.; Ma, L.; Ge, S.; Chen, J.; Wu, S.; Huang, T.; Sheng, Y.; Wang, L.; Yi, N.; et al. Keratin 17 Upregulation Promotes Cell Metastasis and Angiogenesis in Colon Adenocarcinoma. Bioengineered 2021, 12, 12598–12611. [Google Scholar] [CrossRef]
- Liu, L.; Sun, L.; Zheng, J.; Cui, L. Berberine Modulates Keratin 17 to Inhibit Cervical Cancer Cell Viability and Metastasis. J. Recept. Signal Transduct. Res. 2021, 41, 521–531. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, W.; Zhang, M.; Wang, X.; Peng, S.; Zhang, R. KRT6A Promotes EMT and Cancer Stem Cell Transformation in Lung Adenocarcinoma. Technol. Cancer Res. Treat. 2020, 19, 1533033820921248. [Google Scholar] [CrossRef]
- Ren, M.; Gao, Y.; Chen, Q.; Zhao, H.; Zhao, X.; Yue, W. The Overexpression of Keratin 23 Promotes Migration of Ovarian Cancer via Epithelial-Mesenchymal Transition. BioMed Res. Int. 2020, 2020, 8218735. [Google Scholar] [CrossRef]
- Tsai, F.-J.; Lai, M.-T.; Cheng, J.; Chao, S.C.-C.; Korla, P.K.; Chen, H.-J.; Lin, C.-M.; Tsai, M.-H.; Hua, C.-H.; Jan, C.-I.; et al. Novel K6-K14 Keratin Fusion Enhances Cancer Stemness and Aggressiveness in Oral Squamous Cell Carcinoma. Oncogene 2019, 38, 5113–5126. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.-B.; Yang, X.-P.; Zhou, P.-X.; Yang, X.-A.; Yin, B. High Expression of Keratin 6C Is Associated with Poor Prognosis and Accelerates Cancer Proliferation and Migration by Modulating Epithelial-Mesenchymal Transition in Lung Adenocarcinoma. Genes Genom. 2020, 42, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.-C.; Parajuli, K.R.; Han, S.I. Keratin 6, Induced by Chronic Cisplatin Exposure, Confers Chemoresistance in Human Gastric Carcinoma Cells. Oncol. Rep. 2019, 42, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yu, S.; Ye, S.; Shen, Z.; Gao, L.; Han, Z.; Zhang, P.; Luo, F.; Chen, S.; Kang, M. Keratin 17 Activates AKT Signalling and Induces Epithelial-Mesenchymal Transition in Oesophageal Squamous Cell Carcinoma. J. Proteom. 2020, 211, 103557. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Q.; Deng, Z.; Chen, X.; Liu, H.; Tao, Y.; Wang, X.; Lin, S.; Liu, N. KRT17 Confers Paclitaxel-Induced Resistance and Migration to Cervical Cancer Cells. Life Sci. 2019, 224, 255–262. [Google Scholar] [CrossRef]
- Liu, J.; Liu, L.; Cao, L.; Wen, Q. Keratin 17 Promotes Lung Adenocarcinoma Progression by Enhancing Cell Proliferation and Invasion. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 4782–4790. [Google Scholar] [CrossRef]
- Zieman, A.G.; Poll, B.G.; Ma, J.; Coulombe, P.A. Altered Keratinocyte Differentiation Is an Early Driver of Keratin Mutation-Based Palmoplantar Keratoderma. Hum. Mol. Genet. 2019, 28, 2255–2270. [Google Scholar] [CrossRef]
- Spörrer, M.; Prochnicki, A.; Tölle, R.C.; Nyström, A.; Esser, P.R.; Homberg, M.; Athanasiou, I.; Zingkou, E.; Schilling, A.; Gerum, R.; et al. Treatment of Keratinocytes with 4-Phenylbutyrate in Epidermolysis Bullosa: Lessons for Therapies in Keratin Disorders. eBioMedicine 2019, 44, 502–515. [Google Scholar] [CrossRef]
- Westin, M.; Rekabdar, E.; Blomstrand, L.; Klintberg, P.; Jontell, M.; Robledo-Sierra, J. Mutations in the Genes for Keratin-4 and Keratin-13 in Swedish Patients with White Sponge Nevus. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2018, 47, 152–157. [Google Scholar] [CrossRef]
- Mathews, J.; Hansen, C.D.; Chandrashekar, L. Homozygous Dominant Missense Mutation in Keratin 6b Leading to Severe Pachyonychia Congenita. Clin. Exp. Dermatol. 2021, 46, 410–412. [Google Scholar] [CrossRef]
- Komine, M. Regulation of Expression of Keratins and Their Pathogenic Roles in Keratinopathies; IntechOpen: Rijeka, Croatia, 2018; ISBN 978-1-78984-805-2. [Google Scholar]
- Logli, E.; Marzuolo, E.; D’Agostino, M.; Conti, L.A.; Lena, A.M.; Diociaiuti, A.; Dellambra, E.; Has, C.; Cianfanelli, V.; Zambruno, G.; et al. Proteasome-Mediated Degradation of Keratins 7, 8, 17 and 18 by Mutant KLHL24 in a Foetal Keratinocyte Model: Novel Insight in Congenital Skin Defects and Fragility of Epidermolysis Bullosa Simplex with Cardiomyopathy. Hum. Mol. Genet. 2022, 31, 1308–1324. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.-Y.; Chou, C.-C. Molecular Origin of the Effects of Mutation on the Structure and Mechanical Properties of Human Epithelial Keratin K5/K14. J. Mech. Behav. Biomed. Mater. 2021, 124, 104798. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Yao, L.; Zhang, X.; Gu, Y.; Yu, H.; Yao, Z.; Zhang, J.; Li, M. Damaged Keratin Filament Network Caused by KRT5 Mutations in Localized Recessive Epidermolysis Bullosa Simplex. Front. Genet. 2021, 12, 736610. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S.; Deguchi, S.; Magin, T.M. Disease-Associated Keratin Mutations Reduce Traction Forces and Compromise Adhesion and Collective Migration. J. Cell Sci. 2020, 133, jcs243956. [Google Scholar] [CrossRef]
- Pânzaru, M.-C.; Caba, L.; Florea, L.; Braha, E.E.; Gorduza, E.V. Epidermolysis Bullosa—A Different Genetic Approach in Correlation with Genetic Heterogeneity. Diagnostics 2022, 12, 1325. [Google Scholar] [CrossRef]
- Evtushenko, N.A.; Beilin, A.K.; Kosykh, A.V.; Vorotelyak, E.A.; Gurskaya, N.G. Keratins as an Inflammation Trigger Point in Epidermolysis Bullosa Simplex. Int. J. Mol. Sci. 2021, 22, 12446. [Google Scholar] [CrossRef]
- Spaunhurst, K.M.; Hogendorf, A.M.; Smith, F.J.D.; Lingala, B.; Schwartz, M.E.; Cywinska-Bernas, A.; Zeman, K.J.; Tang, J.Y. Pachyonychia Congenita Patients with Mutations in KRT6A Have More Extensive Disease Compared with Patients Who Have Mutations in KRT16. Br. J. Dermatol. 2012, 166, 875–878. [Google Scholar] [CrossRef]
- Wu, T.T.; Eldirany, S.A.; Bunick, C.G.; Teng, J.M.C. Genotype-Structurotype-Phenotype Correlations in Patients with Pachyonychia Congenita. J. Investig. Dermatol. 2021, 141, 2876–2884.e4. [Google Scholar] [CrossRef]
- Vodo, D.; Sarig, O.; Peled, A.; Samuelov, L.; Malchin, N.; Grafi-Cohen, M.; Sprecher, E. Recessive Epidermolytic Ichthyosis Results from Loss of Keratin 10 Expression, Regardless of the Mutation Location. Clin. Exp. Dermatol. 2018, 43, 187–190. [Google Scholar] [CrossRef]
- Smith, F.J.D.; Kreuser-Genis, I.M.; Jury, C.S.; Wilson, N.J.; Terron-Kwiatowski, A.; Zamiri, M. Novel and Recurrent Mutations in Keratin 1 Cause Epidermolytic Ichthyosis and Palmoplantar Keratoderma. Clin. Exp. Dermatol. 2019, 44, 528–534. [Google Scholar] [CrossRef]
- Suzuki, Y.; Takeichi, T.; Tanahashi, K.; Muro, Y.; Suga, Y.; Ogi, T.; Akiyama, M. Deep Phenotyping of Superficial Epidermolytic Ichthyosis Due to a Recurrent Mutation in KRT2. Int. J. Mol. Sci. 2022, 23, 7791. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wu, Y.; Li, M.; Gong, X.; Zhong, B. Keratin 8 Mutations Were Associated With Susceptibility to Chronic Hepatitis B and Related Progression. J. Infect. Dis. 2020, 221, 464–473. [Google Scholar] [CrossRef]
- Yi, H.; Yoon, H.-N.; Kim, S.; Ku, N.-O. The Role of Keratins in the Digestive System: Lessons from Transgenic Mouse Models. Histochem. Cell Biol. 2018, 150, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.-R.; Chen, X.-L.; Tang, Y.-X.; Zhang, J.-Y.; Gao, X.; Ke, H.-P.; Lin, Z.-Y.; Zhang, X.-N. CRISPR/Cas9-Mediated Treatment Ameliorates the Phenotype of the Epidermolytic Palmoplantar Keratoderma-like Mouse. Mol. Ther.-Nucleic Acids 2018, 12, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Koller, U.; Bauer, J.W. Gene Replacement Therapies for Genodermatoses: A Status Quo. Front. Genet. 2021, 12, 515. [Google Scholar] [CrossRef]
- Claringbould, A.; Zaugg, J.B. Enhancers in Disease: Molecular Basis and Emerging Treatment Strategies. Trends Mol. Med. 2021, 27, 1060–1073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Shi, J.; Zhu, S.; Lan, Y.; Xu, L.; Yuan, H.; Liao, G.; Liu, X.; Zhang, Y.; Xiao, Y.; et al. DiseaseEnhancer: A Resource of Human Disease-Associated Enhancer Catalog. Nucleic Acids Res. 2018, 46, D78–D84. [Google Scholar] [CrossRef] [PubMed]
- Karnuta, J.M.; Scacheri, P.C. Enhancers: Bridging the Gap between Gene Control and Human Disease. Hum. Mol. Genet. 2018, 27, R219–R227. [Google Scholar] [CrossRef]
- Kircher, M.; Xiong, C.; Martin, B.; Schubach, M.; Inoue, F.; Bell, R.J.A.; Costello, J.F.; Shendure, J.; Ahituv, N. Saturation Mutagenesis of Twenty Disease-Associated Regulatory Elements at Single Base-Pair Resolution. Nat. Commun. 2019, 10, 3583. [Google Scholar] [CrossRef]
- Maurya, S.S. Role of Enhancers in Development and Diseases. Epigenomes 2021, 5, 21. [Google Scholar] [CrossRef]
- Tena, J.J.; Santos-Pereira, J.M. Topologically Associating Domains and Regulatory Landscapes in Development, Evolution and Disease. Front. Cell Dev. Biol. 2021, 9, 702787. [Google Scholar] [CrossRef] [PubMed]
- Luchnik, A.N. Chromosome Instability Induced by Mutations in TAD Anchors Leads to Tumors. Genome Instab. Dis. 2021, 2, 309–329. [Google Scholar] [CrossRef]
- Bruneau, B.G.; Nora, E.P. Chromatin Domains Go on Repeat in Disease. Cell 2018, 175, 38–40. [Google Scholar] [CrossRef] [PubMed]
| Tissues (Organs) and Keratins | Transcription Factors | Links |
|---|---|---|
| Simple Epithelia Keratin Complex | ||
| Trophectoderm: K8/18, 7, 19 | CDX2, TEAD4, BAF155, AP-1, Ets | [16,17,18,19,20] |
| Liver: Precursors: K8/18, 19 Hepatocytes: K8/18 Bile ducts: K8/18, 7, 19, 23 | HNF-1, HNF-4alpha, HNF-3beta, C/EBPalpha, AP-1, SP1 | [21,22,23,24,25,26,27] |
| Pancreas: Acinar cells: K8/18, 19, 20 Ducts: K8/18, 7, 19, 20 | KLF4, KLF5, PDX1, MEIS1a, PBX1b, ELF3, Sp1, AP-2 | [28,29,30,31,32,33] |
| Intestine: K8/18, 7, 19, 20, 21 | CDX1, HNF-1α, HNF-4α, Cdx2, GATA-4 AP1, SATB2 | [12,27,34,35,36,37,38,39,40] |
| Kidney (Nephron): K8/18, 7, 19 | HNF-4α, SIX1 | [36,41,42] |
| Lungs (alveoli): K8/18, 7, 19 | Nkx2-1 | [43,44,45] |
| Endothelia: K18, 7 | Hey2 | [46,47,48] |
| Pseudostratified Epithelia Complex | ||
| Lungs: Bronchioles: K8/18, 19, minor: K5, 6, 7, 15, 17 Trachea & bronchi: K5, 15, 8/18, 6a, 19, minor: K14 | p63, SOX2, SOX21, KLF5, CFTR, ESE-2 FOXA1/A2 | [13,43,45,49,50,51,52,53,54,55,56,57,58] |
| Urinary system: Renal pelvis: K8/18, 7, 19 Bladder: K8/18, 5, 4, 7, 19, 20, 13 | p63, PPARγ1, GATA3, FOXA1 | [14,59,60,61,62,63] |
| Mammary gland (ducts): K5/14, 8/18, 19 | p63, ER, FOXA1, GATA3, ZNF217, C/EBPβ, SP1 | [64,65,66,67,68,69] |
| Stratified Epithelia Complex | ||
| Esophagus: Basal layer: K5/14, 15. Differentiated layers: K4/13 | p63, SOX2, KLF4, KLF5, GATA4, ESE-1 | [70,71,72,73,74,75,76,77,78,79] |
| Oral mucosa: Basal layer: K5/14, 15. Differentiated layers: K4/13 | p63, SOX2, PITX1, AP-2α, c-MYC, GRHL2 | [60,80,81,82,83] |
| Cornea: Basal layer: K5/14, 12 Differentiated layers: K3*/12 | p63, KLF4, KLF6, PAX6, FOXC1, ESE-1, RUNX1 | [84,85,86,87,88,89,90,91,92,93,94] |
| Skin epidermis: Basal layer: K5/14, 15, 19 Differentiated layers: K1/10, 2, 9, 6 a,b,c*/16, 17 | p63, KLF4, RUNX1, RUNX3, NF-κB, AP1, AP2, SP1, C/EBP, T3R, RAR, CHOP, GR, SETX, C-MYC, STAT3, NRF2, C/EBPα | [95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117] |
| Hair Follicle Keratin Complex | ||
| Hair follicle: ORS: K5/14, 15, 6 a,b,c*/16, 17, 19 Companion layer: K6 a,b,c,*/16, 17, 75, 79 IRS: K25-28/71-74 Hair shaft: K31-40/81-86 | LEF1, TCF3, SP1, AP2-like, NF1-like, NF-κB, LHX2, STAT3, SOX9 (ORS), HOXC13 (hair shaft), GATA3 (IRS) | [113,118,119,120,121,122,123,124,125,126,127,128] |
| Nail:Matrix: K5/14, 1/10, 6 a,b,c*/16, 17, 31, 34, 85, 86 Nail bed: K6 a,b,c*/16, 75 | GATA3, MSX2, FOXN1, PAI2 | [117,124,129,130,131,132] |
| Stress-Induced Keratin Complex | ||
| K6 a,b,c*/16,17 | p53, STAT1, Gli1/2, Nrf, BARX2, AP1/2, NF-κB, SP1, GR, RAR, RUNX1 | [103,106,109,133,134,135,136,137,138,139,140,141,142,143,144] |
| Keratins: Type I, Type II | TFs: Kruppel-like, p53 family, SOX family. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalabusheva, E.P.; Shtompel, A.S.; Rippa, A.L.; Ulianov, S.V.; Razin, S.V.; Vorotelyak, E.A. A Kaleidoscope of Keratin Gene Expression and the Mosaic of Its Regulatory Mechanisms. Int. J. Mol. Sci. 2023, 24, 5603. https://doi.org/10.3390/ijms24065603
Kalabusheva EP, Shtompel AS, Rippa AL, Ulianov SV, Razin SV, Vorotelyak EA. A Kaleidoscope of Keratin Gene Expression and the Mosaic of Its Regulatory Mechanisms. International Journal of Molecular Sciences. 2023; 24(6):5603. https://doi.org/10.3390/ijms24065603
Chicago/Turabian StyleKalabusheva, Ekaterina P., Anastasia S. Shtompel, Alexandra L. Rippa, Sergey V. Ulianov, Sergey V. Razin, and Ekaterina A. Vorotelyak. 2023. "A Kaleidoscope of Keratin Gene Expression and the Mosaic of Its Regulatory Mechanisms" International Journal of Molecular Sciences 24, no. 6: 5603. https://doi.org/10.3390/ijms24065603
APA StyleKalabusheva, E. P., Shtompel, A. S., Rippa, A. L., Ulianov, S. V., Razin, S. V., & Vorotelyak, E. A. (2023). A Kaleidoscope of Keratin Gene Expression and the Mosaic of Its Regulatory Mechanisms. International Journal of Molecular Sciences, 24(6), 5603. https://doi.org/10.3390/ijms24065603






