Neuroinflammation and Oxidative Stress in the Pathogenesis of Autism Spectrum Disorder
Abstract
1. Introduction
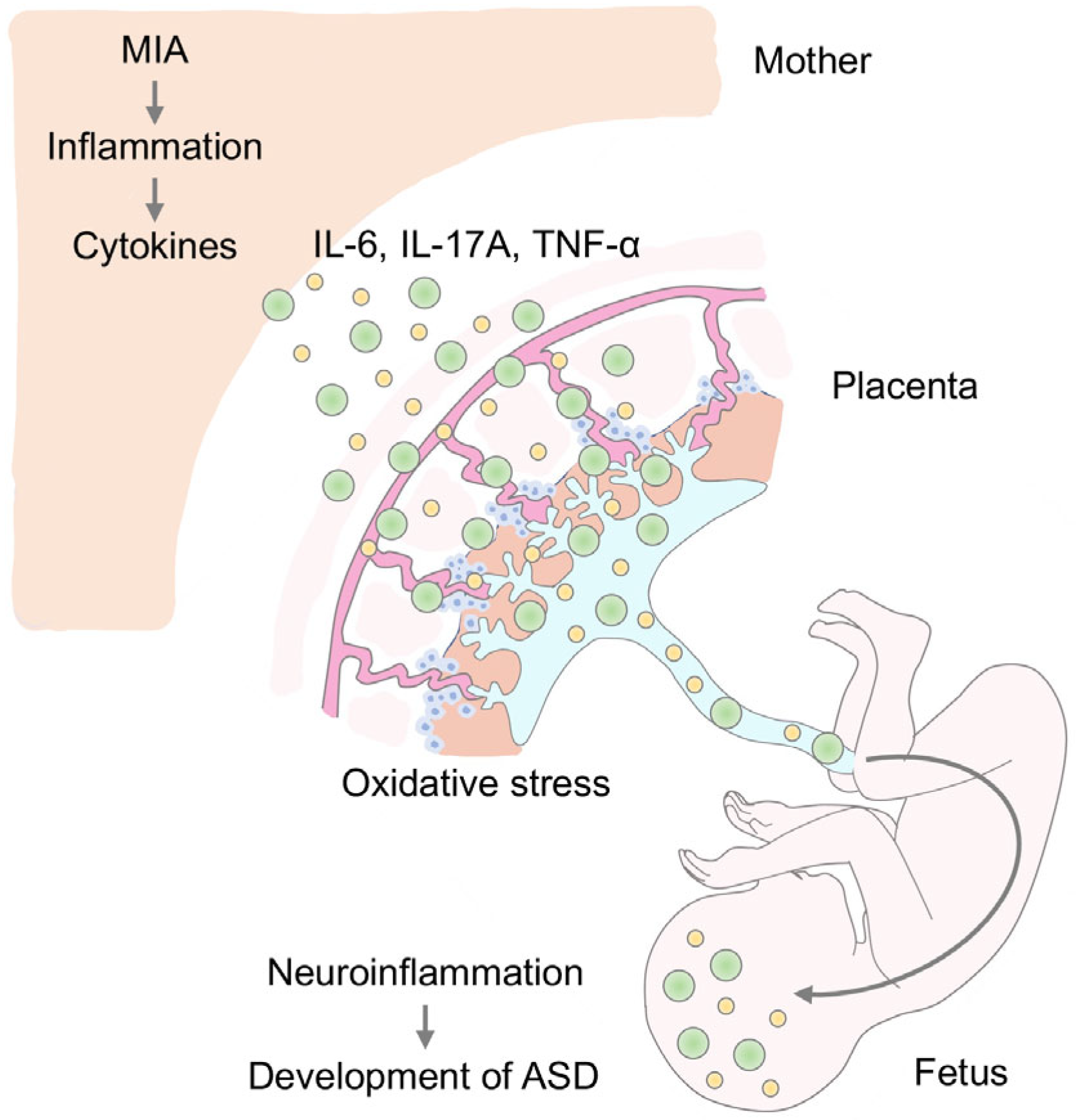
2. MIA and Neuroinflammation in ASD
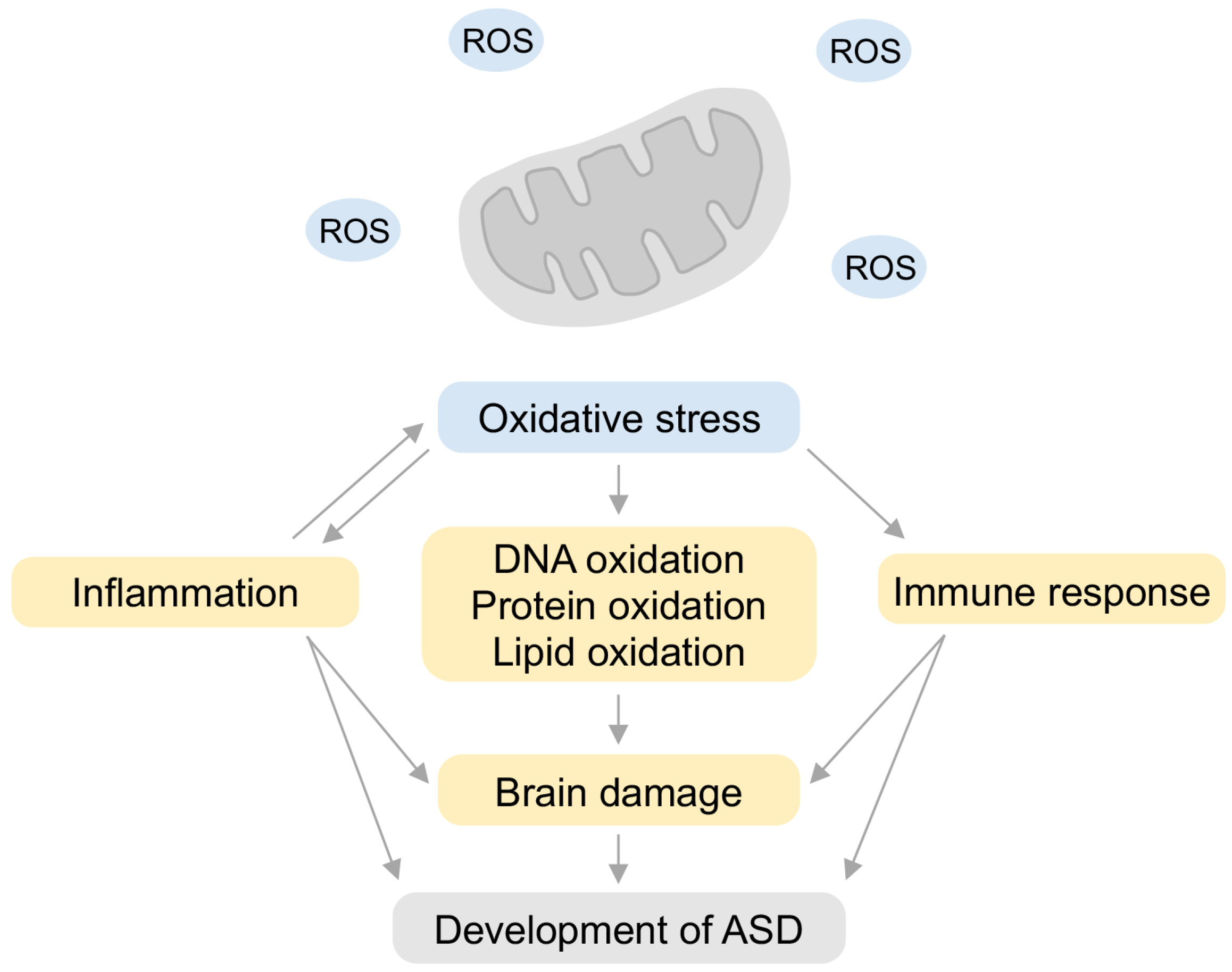
3. MIA and Oxidative Stress
4. Oxidative Stress in ASD
5. Relationship between Inflammation and Oxidative Stress
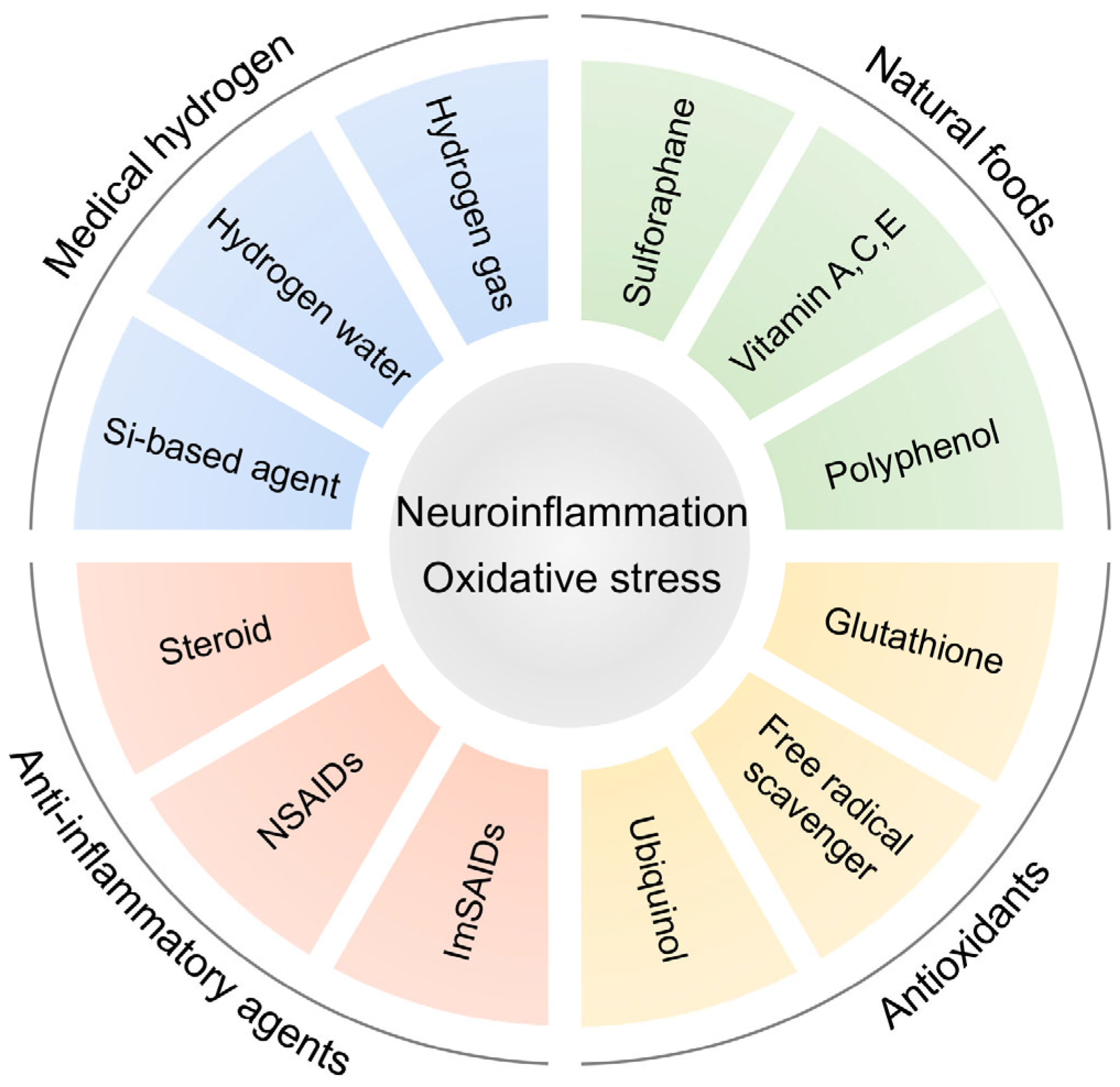
6. Anti-Inflammatory Strategy Targeting Inflammation in ASD
| Subjects | Study | Sample Size | Treatments | Results | Reference |
|---|---|---|---|---|---|
| ASD children (4–12 years) | Randomized, double-blind, placebo-controlled trial | 60 | Combination of risperidone and COX-2 inhibitor, 10 weeks | Superior to risperidone alone in treating hypersensitivity, social withdrawal, and stereotypes | [152] |
| ASD children (mean age of 8.7 ± 2.7 years) | Double-blind, placebo-controlled crossover study | 14 | ORG2766, a synthetic analog of ACTH, 4 weeks | Improved quantity and quality of social interactions | [153] |
| ASD children (4–10 years) | Prospective, open-label trial | 40 | Flavonoids (luteolin and quercetin), 26 weeks | Improvements in attention, social communication, living skills | [154] |
| ASD children (4–10 years) | Prospective, open-label trial | 38 | Flavonoids (luteolin and quercetin), 26 weeks | Improvements in social communication and living skills, decreases in IL-6 and TNF-α in the serum | [79] |
| ASD children (6–19 years) | Double-blind, placebo-controlled study | 18 | Vitamin C, 10 weeks | Improved sensorimotor behaviors | [155] |
| ASD children (3–6 years) | Prospective, open-label trial | 24 | Ubiquinol, 3 months | Improved communication | [156] |
| ASD children (3–12 years) | Randomized, parallel, placebo-controlled study | 90 | Coenzyme Q10, 3 months | Reduced oxidative stress and ASD symptoms | [157] |
| ASD individuals (13–27 years) | Double-blind, placebo-controlled trial | 29 | Sulforaphane, 18 weeks | Improved social interaction and communication | [158] |
| ASD children (3.2–10.7 years) | Randomized, placebo-controlled trial | 33 | NAC, 12 weeks | Improvement in hypersensitivity subscales | [159] |
| ASD children (3.5–16 years) | Randomized, double-blind, placebo-controlled trial | 47 | Combination of risperidone and NAC, 8 weeks | Reduced hypersensitivity subscales | [160] |
| ASD children (4–12 years) | Randomized, double-blind, placebo-controlled clinical trial | 40 | Combination of risperidone and NAC, 10 weeks | Reduced hypersensitivity | [161] |
| ASD children (mean age of 10.9 ± 3.9 years) | Pilot feasibility study | 16 | Cocoa, 4 weeks | Improvements in social communication, erratic behavior, self-regulatory behavior | [162] |
| Models | Sample Size | Treatments | Results | Reference |
|---|---|---|---|---|
| MIA mice, 20 mg/kg poly(I:C) at E12.5 | n = 25–38, analyzed at E18.5 or young adults | Anti-IL-17a antibody at E14.5 | Reduced ASD-like behaviors and morphological abnormalities in cortex | [38] |
| MIA mice, 2.5 mg/kg poly(I:C) at E12 to E16 | n = 10, analyzed at young adults | Anti-IL-6 or IL-1β antibodies at E12-E16 | Improved epileptic impairments | [144] |
| MIA mice, 20 mg/kg poly(I:C) at E12.5 | n = 7–15, analyzed at young adults | Anti-IL-6 antibody at E12.5 | Prevents PPI, LI, improved exploratory and social behavior | [146] |
| MIA mice, 20 mg/kg poly(I:C) at E12.5 | n = 15–20, analyzed at young adults | Probiotics at E0.5 to P21 | Reduced IL-6 and IL-17a, prevented ASD-like behaviors, GABAergic neurons in PFC | [150] |
| MIA mice, 1 mg/kg LPS at E15.5 and E16.0 | n = 5–12, analyzed at E16.0 | Si-based agent at E13.5 to E16.0 | Protect fetus from miscarriage, placenta from inflammation, improved expressions of Il6, Hmox1, and Ptgs | [30] |
| MIA mice, 20 mg/kg poly(I:C) at E12.5 | n = 22–31, analyzed at P7 | Si-based agent at E8.5 to P7 | Improvements in mouse vocal communication, expressions of Il6 and Ifna1 | [31] |
| VPA mice, 600 mg/kg VPA at E12.5 | n = 6–10, analyzed at young adults | HRW at E12.5 to P42 | Impairments in ASD-like behaviors, pain sensation, anxiety-like behavior, memory, IL-6 and TNF-α | [163] |
| VPA mice, 600 mg/kg VPA at E12.5 | n = 10, analyzed at young adults | Astaxanthin at P26 to P56 | Improved ASD-like behaviors, oxidative stress such as advance protein oxidation product, nitric oxide, catalase, superoxide dismutase | [164] |
| VPA rats, 600 mg/kg VPA at E12.5 | n = 7–12, analyzed at young adults | NAC at P23 for 4 weeks | Improved ASD-like behaviors, increased glutathione, reduced malondialdehyde | [165] |
| ASD model zebrafish, 600 µg/L fipronil and 600 µg/L pyriproxyfen | n = 15, analyzed at young adults | Vitamin C at P45 for 14 days | Impairments in social behaviors, lipid peroxidation, oxidative stress such as superoxide dismutase, glutathione peroxidase | [166] |
| VPA rats, 300 mg/kg VPA at P4 or 30 mg/kg VPA at P4 for 3 days | n = 7–16, analyzed at young adults | Methionine at P4 for 3 days | Improved ASD-like behaviors, expressions of antioxidant genes in PFC | [167] |
| VPA rats, 400 mg/kg VPA at P14 | n = 12, analyzed at young adults | Green tea extract at P14 to P41 | Improved ASD-like behaviors and Purkinje cells | [168] |
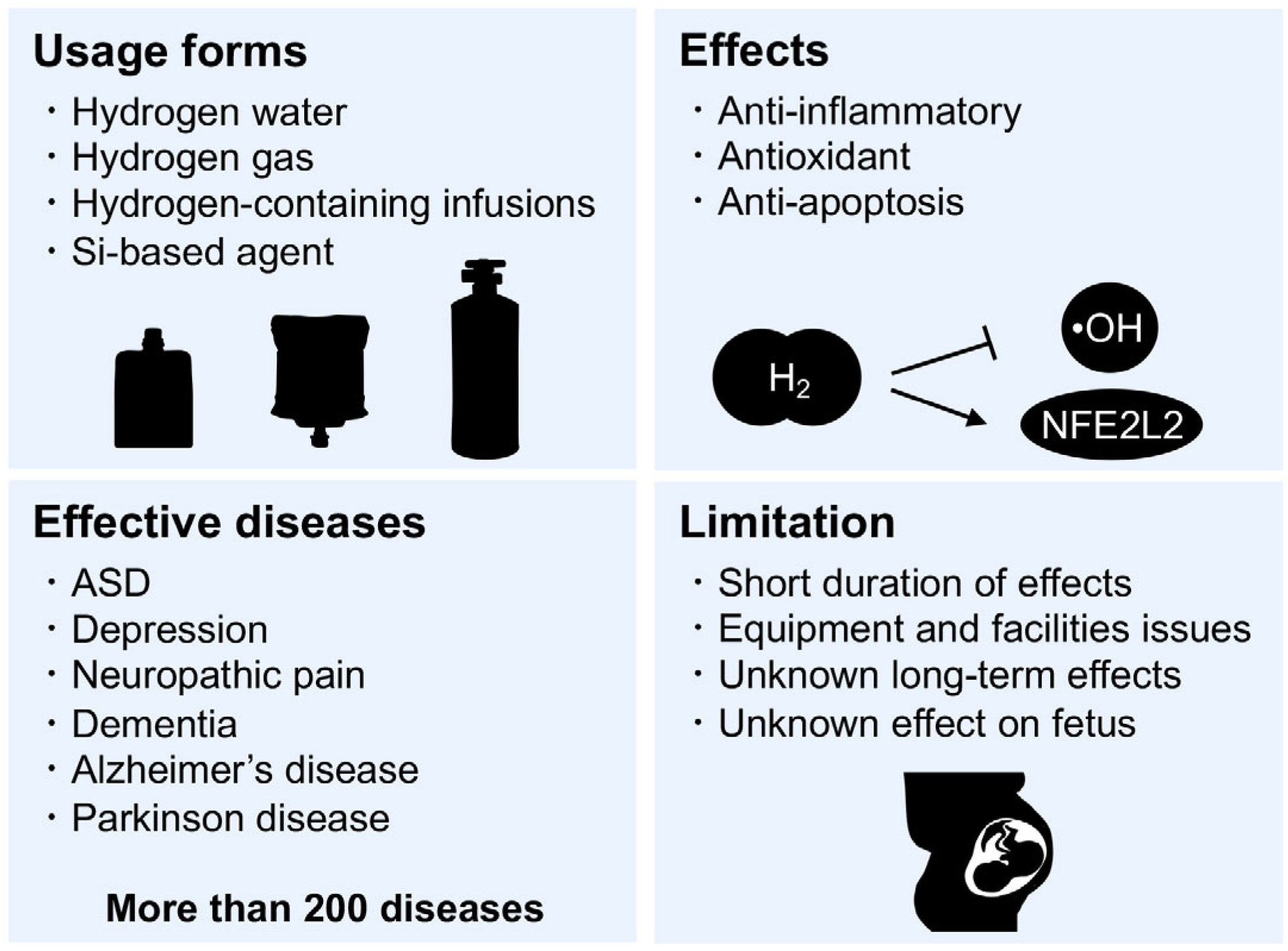
7. Hydrogen Medicine
8. Si-Based Hydrogen-Producing Agent
9. Preclinical Studies of Antioxidants in ASD Model Animals
10. Clinical Studies of Antioxidants in ASD
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism spectrum disorder. Lancet 2018, 392, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Brugha, T.S.; Charman, T.; Cusack, J.; Dumas, G.; Frazier, T.; Jones, E.J.H.; Jones, R.M.; Pickles, A.; State, M.W.; et al. Autism spectrum disorder. Nat. Rev. Dis. Primers 2020, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Maenner, M.J.; Shaw, K.A.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Esler, A.; Furnier, S.M.; Hallas, L.; Hall-Lande, J.; Hudson, A.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2018. MMWR Surveill. Summ. 2021, 70, SS-11. [Google Scholar] [CrossRef]
- Shaw, K.A.; Maenner, M.J.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Furnier, S.M.; Hughes, M.M.; Patrick, M.; Pierce, K.; Salinas, A.; et al. Early Identification of Autism Spectrum Disorder Among Children Aged 4 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2018. MMWR Surveill. Summ. 2021, 70, SS-10. [Google Scholar] [CrossRef] [PubMed]
- Fombonne, E. Epidemiology of pervasive developmental disorders. Pediatr. Res. 2009, 65, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Dawson, G.; Jones, E.J.; Merkle, K.; Venema, K.; Lowy, R.; Faja, S.; Kamara, D.; Murias, M.; Greenson, J.; Winter, J.; et al. Early behavioral intervention is associated with normalized brain activity in young children with autism. J. Am. Acad. Child. Adolesc. Psychiatry 2012, 51, 1150–1159. [Google Scholar] [CrossRef]
- Rogers, S.J.; Estes, A.; Lord, C.; Vismara, L.; Winter, J.; Fitzpatrick, A.; Guo, M.; Dawson, G. Effects of a brief Early Start Denver model (ESDM)-based parent intervention on toddlers at risk for autism spectrum disorders: A randomized controlled trial. J. Am. Acad. Child. Adolesc. Psychiatry 2012, 51, 1052–1065. [Google Scholar] [CrossRef]
- White, S.W.; Scahill, L.; Klin, A.; Koenig, K.; Volkmar, F.R. Educational placements and service use patterns of individuals with autism spectrum disorders. J. Autism Dev. Disord. 2007, 37, 1403–1412. [Google Scholar] [CrossRef]
- Meyer, U. Neurodevelopmental Resilience and Susceptibility to Maternal Immune Activation. Trends Neurosci. 2019, 42, 793–806. [Google Scholar] [CrossRef]
- Doi, M.; Usui, N.; Shimada, S. Prenatal Environment and Neurodevelopmental Disorders. Front. Endocrinol. 2022, 13, 860110. [Google Scholar] [CrossRef]
- Estes, M.L.; McAllister, A.K. Maternal immune activation: Implications for neuropsychiatric disorders. Science 2016, 353, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Bokobza, C.; Van Steenwinckel, J.; Mani, S.; Mezger, V.; Fleiss, B.; Gressens, P. Neuroinflammation in preterm babies and autism spectrum disorders. Pediatr. Res. 2019, 85, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, M.D.; Graham, A.M.; Feczko, E.; Miranda-Dominguez, O.; Rasmussen, J.M.; Nardos, R.; Entringer, S.; Wadhwa, P.D.; Buss, C.; Fair, D.A. Maternal IL-6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Nat. Neurosci. 2018, 21, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Han, V.X.; Patel, S.; Jones, H.F.; Nielsen, T.C.; Mohammad, S.S.; Hofer, M.J.; Gold, W.; Brilot, F.; Lain, S.J.; Nassar, N.; et al. Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: A systematic review. Transl. Psychiatry 2021, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Gyllenhammer, L.E.; Rasmussen, J.M.; Bertele, N.; Halbing, A.; Entringer, S.; Wadhwa, P.D.; Buss, C. Maternal Inflammation During Pregnancy and Offspring Brain Development: The Role of Mitochondria. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2022, 7, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Zawadzka, A.; Cieślik, M.; Adamczyk, A. The Role of Maternal Immune Activation in the Pathogenesis of Autism: A Review of the Evidence, Proposed Mechanisms and Implications for Treatment. Int. J. Mol. Sci. 2021, 22, 11516. [Google Scholar] [CrossRef]
- Holmström, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef]
- D’Autréaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef]
- Nathan, C.; Cunningham-Bussel, A. Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013, 13, 349–361. [Google Scholar] [CrossRef]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.Y.; Xu, L.L.; Shao, L.; Xia, R.M.; Yu, Z.H.; Ling, Z.X.; Yang, F.; Deng, M.; Ruan, B. Maternal infection during pregnancy and risk of autism spectrum disorders: A systematic review and meta-analysis. Brain Behav. Immun. 2016, 58, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Tioleco, N.; Silberman, A.E.; Stratigos, K.; Banerjee-Basu, S.; Spann, M.N.; Whitaker, A.H.; Turner, J.B. Prenatal maternal infection and risk for autism in offspring: A meta-analysis. Autism Res. 2021, 14, 1296–1316. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Flores, V.; Romero, R.; Xu, Y.; Theis, K.R.; Arenas-Hernandez, M.; Miller, D.; Peyvandipour, A.; Bhatti, G.; Galaz, J.; Gershater, M.; et al. Maternal-fetal immune responses in pregnant women infected with SARS-CoV-2. Nat. Commun. 2022, 13, 320. [Google Scholar] [CrossRef]
- Wang, R.; Wu, Z.; Huang, C.; Hashimoto, K.; Yang, L.; Yang, C. Deleterious effects of nervous system in the offspring following maternal SARS-CoV-2 infection during the COVID-19 pandemic. Transl. Psychiatry 2022, 12, 232. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Matsuda, S.; Imamura, K.; Kobayashi, H. Hydrogen generation by reaction of Si nanopowder with neutral water. J. Nanopart. Res. 2017, 19, 176. [Google Scholar] [CrossRef]
- Imamura, K.; Kobayashi, Y.; Matsuda, S.; Akai, T.; Kobayashi, H. Reaction of Si nanopowder with water investigated by FT-IR and XPS. AIP Adv. 2017, 7, 085310. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Imamura, R.; Koyama, Y.; Kondo, M.; Kobayashi, H.; Nonomura, N.; Shimada, S. Renoprotective and neuroprotective effects of enteric hydrogen generation from Si-based agent. Sci. Rep. 2020, 10, 5859. [Google Scholar] [CrossRef]
- Usui, N.; Togawa, S.; Sumi, T.; Kobayashi, Y.; Koyama, Y.; Nakamura, Y.; Kondo, M.; Shinoda, K.; Kobayashi, H.; Shimada, S. Si-Based Hydrogen-Producing Nanoagent Protects Fetuses From Miscarriage Caused by Mother-to-Child Transmission. Front. Med. Technol. 2021, 3, 665506. [Google Scholar] [CrossRef]
- Usui, N.; Matsumoto-Miyai, K.; Koyama, Y.; Kobayashi, Y.; Nakamura, Y.; Kobayashi, H.; Shimada, S. Social Communication of Maternal Immune Activation-Affected Offspring Is Improved by Si-Based Hydrogen-Producing Agent. Front. Psychiatry 2022, 13, 72302. [Google Scholar] [CrossRef] [PubMed]
- Girchenko, P.; Lahti-Pulkkinen, M.; Heinonen, K.; Reynolds, R.M.; Laivuori, H.; Lipsanen, J.; Villa, P.M.; Hämäläinen, E.; Kajantie, E.; Lahti, J.; et al. Persistently High Levels of Maternal Antenatal Inflammation Are Associated With and Mediate the Effect of Prenatal Environmental Adversities on Neurodevelopmental Delay in the Offspring. Biol. Psychiatry 2020, 87, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Dale, R.C.; Rose, D.; Heath, B.; Nordahl, C.W.; Rogers, S.; Guastella, A.J.; Ashwood, P. Maternal immune conditions are increased in males with autism spectrum disorders and are associated with behavioural and emotional but not cognitive co-morbidity. Transl. Psychiatry 2020, 10, 286. [Google Scholar] [CrossRef] [PubMed]
- Beversdorf, D.Q.; Manning, S.E.; Hillier, A.; Anderson, S.L.; Nordgren, R.E.; Walters, S.E.; Nagaraja, H.N.; Cooley, W.C.; Gaelic, S.E.; Bauman, M.L. Timing of prenatal stressors and autism. J. Autism Dev. Disord. 2005, 35, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.; Grønborg, T.K.; Sørensen, M.J.; Schendel, D.; Parner, E.T.; Pedersen, L.H.; Vestergaard, M. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 2013, 309, 1696–1703. [Google Scholar] [CrossRef]
- Han, V.X.; Patel, S.; Jones, H.F.; Dale, R.C. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat. Rev. Neurol. 2021, 17, 564–579. [Google Scholar] [CrossRef]
- Chow, K.H.; Yan, Z.; Wu, W.L. Induction of Maternal Immune Activation in Mice at Mid-gestation Stage with Viral Mimic Poly(I:C). J. Vis. Exp. 2016, 109, e53643. [Google Scholar] [CrossRef]
- Choi, G.B.; Yim, Y.S.; Wong, H.; Kim, S.; Kim, H.; Kim, S.V.; Hoeffer, C.A.; Littman, D.R.; Huh, J.R. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 2016, 351, 933–939. [Google Scholar] [CrossRef]
- Reisinger, S.; Khan, D.; Kong, E.; Berger, A.; Pollak, A.; Pollak, D.D. The poly(I:C)-induced maternal immune activation model in preclinical neuropsychiatric drug discovery. Pharmacol. Ther. 2015, 149, 213–226. [Google Scholar] [CrossRef]
- Shin Yim, Y.; Park, A.; Berrios, J.; Lafourcade, M.; Pascual, L.M.; Soares, N.; Yeon Kim, J.; Kim, S.; Kim, H.; Waisman, A.; et al. Reversing behavioural abnormalities in mice exposed to maternal inflammation. Nature 2017, 549, 482–487. [Google Scholar] [CrossRef]
- De la Torre-Ubieta, L.; Won, H.; Stein, J.L.; Geschwind, D.H. Advancing the understanding of autism disease mechanisms through genetics. Nat. Med. 2016, 22, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.D.; Yim, Y.S.; Wimmer, R.D.; Kim, H.; Ryu, C.; Welch, G.M.; Andina, M.; King, H.O.; Waisman, A.; Halassa, M.M.; et al. IL-17a promotes sociability in mouse models of neurodevelopmental disorders. Nature 2020, 577, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Grzadzinski, R.; Lord, C.; Sanders, S.J.; Werling, D.; Bal, V.H. Children with autism spectrum disorder who improve with fever: Insights from the Simons Simplex Collection. Autism Res. 2018, 11, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Curran, L.K.; Newschaffer, C.J.; Lee, L.C.; Crawford, S.O.; Johnston, M.V.; Zimmerman, A.W. Behaviors associated with fever in children with autism spectrum disorders. Pediatrics 2007, 120, e1386–e1392. [Google Scholar] [CrossRef] [PubMed]
- Matta, S.M.; Hill-Yardin, E.L.; Crack, P.J. The influence of neuroinflammation in Autism Spectrum Disorder. Brain Behav. Immun. 2019, 79, 75–90. [Google Scholar] [CrossRef]
- Sasaki, T.; Tome, S.; Takei, Y. Intraventricular IL-17A administration activates microglia and alters their localization in the mouse embryo cerebral cortex. Mol. Brain 2020, 13, 93. [Google Scholar] [CrossRef]
- Mattei, D.; Ivanov, A.; Ferrai, C.; Jordan, P.; Guneykaya, D.; Buonfiglioli, A.; Schaafsma, W.; Przanowski, P.; Deuther-Conrad, W.; Brust, P.; et al. Maternal immune activation results in complex microglial transcriptome signature in the adult offspring that is reversed by minocycline treatment. Transl. Psychiatry 2017, 7, e1120. [Google Scholar] [CrossRef] [PubMed]
- Das Sarma, J.; Ciric, B.; Marek, R.; Sadhukhan, S.; Caruso, M.L.; Shafagh, J.; Fitzgerald, D.C.; Shindler, K.S.; Rostami, A. Functional interleukin-17 receptor A is expressed in central nervous system glia and upregulated in experimental autoimmune encephalomyelitis. J. Neuroinflammation 2009, 6, 14. [Google Scholar] [CrossRef]
- Hayes, L.N.; An, K.; Carloni, E.; Li, F.; Vincent, E.; Trippaers, C.; Paranjpe, M.; Dölen, G.; Goff, L.A.; Ramos, A.; et al. Prenatal immune stress blunts microglia reactivity, impairing neurocircuitry. Nature 2022, 610, 327–334. [Google Scholar] [CrossRef]
- Lombardo, M.V.; Moon, H.M.; Su, J.; Palmer, T.D.; Courchesne, E.; Pramparo, T. Maternal immune activation dysregulation of the fetal brain transcriptome and relevance to the pathophysiology of autism spectrum disorder. Mol. Psychiatry 2018, 23, 1001–1013. [Google Scholar] [CrossRef]
- Guma, E.; Bordignon, P.D.C.; Devenyi, G.A.; Gallino, D.; Anastassiadis, C.; Cvetkovska, V.; Barry, A.D.; Snook, E.; Germann, J.; Greenwood, C.M.T.; et al. Early or Late Gestational Exposure to Maternal Immune Activation Alters Neurodevelopmental Trajectories in Mice: An Integrated Neuroimaging, Behavioral, and Transcriptional Study. Biol. Psychiatry 2021, 90, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Mueller, F.S.; Scarborough, J.; Schalbetter, S.M.; Richetto, J.; Kim, E.; Couch, A.; Yee, Y.; Lerch, J.P.; Vernon, A.C.; Weber-Stadlbauer, U.; et al. Behavioral, neuroanatomical, and molecular correlates of resilience and susceptibility to maternal immune activation. Mol. Psychiatry 2021, 26, 396–410. [Google Scholar] [CrossRef] [PubMed]
- Kalish, B.T.; Kim, E.; Finander, B.; Duffy, E.E.; Kim, H.; Gilman, C.K.; Yim, Y.S.; Tong, L.; Kaufman, R.J.; Griffith, E.C.; et al. Maternal immune activation in mice disrupts proteostasis in the fetal brain. Nat. Neurosci. 2021, 24, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.; Tsung, A.; Billiar, T. Linking oxidative stress to inflammation: Toll-like receptors. Free Radic. Biol. Med. 2010, 48, 1121–1132. [Google Scholar] [CrossRef]
- Sato, A.; Kotajima-Murakami, H.; Tanaka, M.; Katoh, Y.; Ikeda, K. Influence of Prenatal Drug Exposure, Maternal Inflammation, and Parental Aging on the Development of Autism Spectrum Disorder. Front. Psychiatry 2022, 13, 821455. [Google Scholar] [CrossRef]
- Arsenault, D.; St-Amour, I.; Cisbani, G.; Rousseau, L.S.; Cicchetti, F. The different effects of LPS and poly I:C prenatal immune challenges on the behavior, development and inflammatory responses in pregnant mice and their offspring. Brain Behav. Immun. 2014, 38, 77–90. [Google Scholar] [CrossRef]
- Braun, A.E.; Carpentier, P.A.; Babineau, B.A.; Narayan, A.R.; Kielhold, M.L.; Moon, H.M.; Shankar, A.; Su, J.; Saravanapandian, V.; Haditsch, U.; et al. “Females Are Not Just ‘Protected’ Males”: Sex-Specific Vulnerabilities in Placenta and Brain after Prenatal Immune Disruption. eNeuro 2019, 6, ENEURO.0358-19. [Google Scholar] [CrossRef]
- Carpentier, P.A.; Haditsch, U.; Braun, A.E.; Cantu, A.V.; Moon, H.M.; Price, R.O.; Anderson, M.P.; Saravanapandian, V.; Ismail, K.; Rivera, M.; et al. Stereotypical alterations in cortical patterning are associated with maternal illness-induced placental dysfunction. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 16874–16888. [Google Scholar] [CrossRef]
- Schwartzer, J.J.; Careaga, M.; Onore, C.E.; Rushakoff, J.A.; Berman, R.F.; Ashwood, P. Maternal immune activation and strain specific interactions in the development of autism-like behaviors in mice. Transl. Psychiatry 2013, 3, e240. [Google Scholar] [CrossRef]
- Xuan, I.C.; Hampson, D.R. Gender-dependent effects of maternal immune activation on the behavior of mouse offspring. PLoS ONE 2014, 9, e104433. [Google Scholar] [CrossRef]
- Haida, O.; Al Sagheer, T.; Balbous, A.; Francheteau, M.; Matas, E.; Soria, F.; Fernagut, P.O.; Jaber, M. Sex-dependent behavioral deficits and neuropathology in a maternal immune activation model of autism. Transl. Psychiatry 2019, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Lanté, F.; Meunier, J.; Guiramand, J.; Maurice, T.; Cavalier, M.; de Jesus Ferreira, M.C.; Aimar, R.; Cohen-Solal, C.; Vignes, M.; Barbanel, G. Neurodevelopmental damage after prenatal infection: Role of oxidative stress in the fetal brain. Free Radic. Biol. Med. 2007, 42, 1231–1245. [Google Scholar] [CrossRef] [PubMed]
- Howland, J.G.; Cazakoff, B.N.; Zhang, Y. Altered object-in-place recognition memory, prepulse inhibition, and locomotor activity in the offspring of rats exposed to a viral mimetic during pregnancy. Neuroscience 2012, 201, 184–198. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Tsilioni, I.; Patel, A.B.; Doyle, R. Atopic diseases and inflammation of the brain in the pathogenesis of autism spectrum disorders. Transl. Psychiatry 2016, 6, e844. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Asadi, S.; Patel, A.B. Focal brain inflammation and autism. J. Neuroinflammation 2013, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Zaretsky, M.V.; Alexander, J.M.; Byrd, W.; Bawdon, R.E. Transfer of inflammatory cytokines across the placenta. Obs. Gynecol 2004, 103, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, E.Y.; Patterson, P.H. Placental regulation of maternal-fetal interactions and brain development. Dev. Neurobiol. 2012, 72, 1317–1326. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; Patterson, P.H. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav. Immun. 2011, 25, 604–615. [Google Scholar] [CrossRef]
- Ben-Zvi, A.; Liebner, S. Developmental regulation of barrier- and non-barrier blood vessels in the CNS. J. Intern. Med. 2022, 292, 31–46. [Google Scholar] [CrossRef]
- Rubin, L.L.; Staddon, J.M. The cell biology of the blood-brain barrier. Annu. Rev. Neurosci. 1999, 22, 11–28. [Google Scholar] [CrossRef]
- Makinodan, M.; Iwata, K.; Ikawa, D.; Yamashita, Y.; Yamamuro, K.; Toritsuka, M.; Kimoto, S.; Okumura, K.; Yamauchi, T.; Yoshino, H.; et al. Tumor necrosis factor-alpha expression in peripheral blood mononuclear cells correlates with early childhood social interaction in autism spectrum disorder. Neurochem. Int. 2017, 104, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, G.; Hashimoto, K.; Iwata, Y.; Nakamura, K.; Tsujii, M.; Tsuchiya, K.J.; Sekine, Y.; Suzuki, K.; Suda, S.; Matsuzaki, H.; et al. Decreased serum levels of hepatocyte growth factor in male adults with high-functioning autism. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Hashimoto, K.; Iwata, Y.; Nakamura, K.; Tsujii, M.; Tsuchiya, K.J.; Sekine, Y.; Suda, S.; Suzuki, K.; Sugihara, G.-i.; et al. Decreased serum levels of transforming growth factor-beta1 in patients with autism. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Hashimoto, K.; Iwata, Y.; Nakamura, K.; Tsujii, M.; Tsuchiya, K.J.; Sekine, Y.; Suda, S.; Sugihara, G.; Matsuzaki, H.; et al. Decreased serum levels of epidermal growth factor in adult subjects with high-functioning autism. Biol. Psychiatry 2007, 62, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Fujita-Shimizu, A.; Suzuki, K.; Nakamura, K.; Miyachi, T.; Matsuzaki, H.; Kajizuka, M.; Shinmura, C.; Iwata, Y.; Suda, S.; Tsuchiya, K.J.; et al. Decreased serum levels of adiponectin in subjects with autism. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 455–458. [Google Scholar] [CrossRef]
- Kordulewska, N.K.; Kostyra, E.; Piskorz-Ogórek, K.; Moszyńska, M.; Cieślińska, A.; Fiedorowicz, E.; Jarmołowska, B. Serum cytokine levels in children with spectrum autism disorder: Differences in pro- and anti-inflammatory balance. J. Neuroimmunol. 2019, 337, 577066. [Google Scholar] [CrossRef]
- Ashwood, P.; Krakowiak, P.; Hertz-Picciotto, I.; Hansen, R.; Pessah, I.; Van de Water, J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav. Immun. 2011, 25, 40–45. [Google Scholar] [CrossRef]
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2005, 57, 67–81. [Google Scholar] [CrossRef]
- Tsilioni, I.; Taliou, A.; Francis, K.; Theoharides, T.C. Children with autism spectrum disorders, who improved with a luteolin-containing dietary formulation, show reduced serum levels of TNF and IL-6. Transl. Psychiatry 2015, 5, e647. [Google Scholar] [CrossRef]
- Li, X.; Chauhan, A.; Sheikh, A.M.; Patil, S.; Chauhan, V.; Li, X.M.; Ji, L.; Brown, T.; Malik, M. Elevated immune response in the brain of autistic patients. J. Neuroimmunol. 2009, 207, 111–116. [Google Scholar] [CrossRef]
- Zimmerman, A.W.; Jyonouchi, H.; Comi, A.M.; Connors, S.L.; Milstien, S.; Varsou, A.; Heyes, M.P. Cerebrospinal fluid and serum markers of inflammation in autism. Pediatr. Neurol. 2005, 33, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Krakowiak, P.; Goines, P.E.; Tancredi, D.J.; Ashwood, P.; Hansen, R.L.; Hertz-Picciotto, I.; Van de Water, J. Neonatal Cytokine Profiles Associated With Autism Spectrum Disorder. Biol. Psychiatry 2017, 81, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.; Hornik, C.D.; Bilbo, S.; Holzknecht, Z.E.; Gentry, L.; Rao, R.; Lin, S.S.; Herbert, M.R.; Nevison, C.D. The role of oxidative stress, inflammation and acetaminophen exposure from birth to early childhood in the induction of autism. J. Int. Med. Res. 2017, 45, 407–438. [Google Scholar] [CrossRef]
- Oskvig, D.B.; Elkahloun, A.G.; Johnson, K.R.; Phillips, T.M.; Herkenham, M. Maternal immune activation by LPS selectively alters specific gene expression profiles of interneuron migration and oxidative stress in the fetus without triggering a fetal immune response. Brain Behav. Immun. 2012, 26, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Tian, F.J.; Lin, Y.; Xu, W.M. Oxidative Stress: Placenta Function and Dysfunction. Am. J. Reprod. Immunol. 2016, 76, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Paintlia, M.K.; Paintlia, A.S.; Singh, A.K.; Singh, I. Attenuation of lipopolysaccharide-induced inflammatory response and phospholipids metabolism at the feto-maternal interface by N-acetyl cysteine. Pediatr. Res. 2008, 64, 334–339. [Google Scholar] [CrossRef]
- Zhang, X.; Ibi, M.; Haga, R.; Iwata, K.; Matsumoto, M.; Asaoka, N.; Liu, J.; Katsuyama, M.; Yabe-Nishimura, C. NOX1/NADPH oxidase affects the development of autism-like behaviors in a maternal immune activation model. Biochem. Biophys. Res. Commun. 2021, 534, 59–66. [Google Scholar] [CrossRef]
- Guma, E.; Bordeleau, M.; González Ibáñez, F.; Picard, K.; Snook, E.; Desrosiers-Grégoire, G.; Spring, S.; Lerch, J.P.; Nieman, B.J.; Devenyi, G.A.; et al. Differential effects of early or late exposure to prenatal maternal immune activation on mouse embryonic neurodevelopment. Proc. Natl. Acad. Sci. USA 2022, 119, e2114545119. [Google Scholar] [CrossRef]
- Mohanty, A.; Tiwari-Pandey, R.; Pandey, N.R. Mitochondria: The indispensable players in innate immunity and guardians of the inflammatory response. J. Cell Commun. Signal. 2019, 13, 303–318. [Google Scholar] [CrossRef]
- Schoenfeld, R.; Wong, A.; Silva, J.; Li, M.; Itoh, A.; Horiuchi, M.; Itoh, T.; Pleasure, D.; Cortopassi, G. Oligodendroglial differentiation induces mitochondrial genes and inhibition of mitochondrial function represses oligodendroglial differentiation. Mitochondrion 2010, 10, 143–150. [Google Scholar] [CrossRef]
- Oldendorf, W.H.; Cornford, M.E.; Brown, W.J. The large apparent work capability of the blood-brain barrier: A study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann. Neurol. 1977, 1, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.L.; Yuan, Y.; Tian, L. Microglial regional heterogeneity and its role in the brain. Mol. Psychiatry 2020, 25, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, T.; Petrelli, F.; Romanos, J.; De Oliveira Figueiredo, E.C.; Lewis, T.L., Jr.; Déglon, N.; Polleux, F.; Santello, M.; Bezzi, P. Mitochondrial biogenesis in developing astrocytes regulates astrocyte maturation and synapse formation. Cell Rep. 2021, 35, 108952. [Google Scholar] [CrossRef] [PubMed]
- Khacho, M.; Clark, A.; Svoboda, D.S.; Azzi, J.; MacLaurin, J.G.; Meghaizel, C.; Sesaki, H.; Lagace, D.C.; Germain, M.; Harper, M.E.; et al. Mitochondrial Dynamics Impacts Stem Cell Identity and Fate Decisions by Regulating a Nuclear Transcriptional Program. Cell Stem Cell 2016, 19, 232–247. [Google Scholar] [CrossRef]
- Cieślik, M.; Gassowska-Dobrowolska, M.; Zawadzka, A.; Frontczak-Baniewicz, M.; Gewartowska, M.; Dominiak, A.; Czapski, G.A.; Adamczyk, A. The Synaptic Dysregulation in Adolescent Rats Exposed to Maternal Immune Activation. Front. Mol. Neurosci. 2020, 13, 555290. [Google Scholar] [CrossRef]
- Robicsek, O.; Ene, H.M.; Karry, R.; Ytzhaki, O.; Asor, E.; McPhie, D.; Cohen, B.M.; Ben-Yehuda, R.; Weiner, I.; Ben-Shachar, D. Isolated Mitochondria Transfer Improves Neuronal Differentiation of Schizophrenia-Derived Induced Pluripotent Stem Cells and Rescues Deficits in a Rat Model of the Disorder. Schizophr. Bull. 2018, 44, 432–442. [Google Scholar] [CrossRef]
- Frustaci, A.; Neri, M.; Cesario, A.; Adams, J.B.; Domenici, E.; Dalla Bernardina, B.; Bonassi, S. Oxidative stress-related biomarkers in autism: Systematic review and meta-analyses. Free Radic. Biol. Med. 2012, 52, 2128–2141. [Google Scholar] [CrossRef]
- Bjørklund, G.; Meguid, N.A.; El-Bana, M.A.; Tinkov, A.A.; Saad, K.; Dadar, M.; Hemimi, M.; Skalny, A.V.; Hosnedlová, B.; Kizek, R.; et al. Oxidative Stress in Autism Spectrum Disorder. Mol. Neurobiol. 2020, 57, 2314–2332. [Google Scholar] [CrossRef]
- Rose, S.; Melnyk, S.; Pavliv, O.; Bai, S.; Nick, T.G.; Frye, R.E.; James, S.J. Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl. Psychiatry 2012, 2, e134. [Google Scholar] [CrossRef]
- Chauhan, A.; Chauhan, V. Oxidative stress in autism. Pathophysiol. Off. J. Int. Soc. Pathophysiol. 2006, 13, 171–181. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.; Sulaiman Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharm. 2018, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Iakovou, E.; Kourti, M. A Comprehensive Overview of the Complex Role of Oxidative Stress in Aging, The Contributing Environmental Stressors and Emerging Antioxidant Therapeutic Interventions. Front. Aging Neurosci. 2022, 14, 827900. [Google Scholar] [CrossRef] [PubMed]
- Warraich, U.E.; Hussain, F.; Kayani, H.U.R. Aging—Oxidative stress, antioxidants and computational modeling. Heliyon 2020, 6, e04107. [Google Scholar] [CrossRef]
- Morimoto, M.; Hashimoto, T.; Tsuda, Y.; Nakatsu, T.; Kitaoka, T.; Kyotani, S. Assessment of oxidative stress in autism spectrum disorder using reactive oxygen metabolites and biological antioxidant potential. PLoS ONE 2020, 15, e0233550. [Google Scholar] [CrossRef]
- Boso, M.; Emanuele, E.; Minoretti, P.; Arra, M.; Politi, P.; Ucelli di Nemi, S.; Barale, F. Alterations of circulating endogenous secretory RAGE and S100A9 levels indicating dysfunction of the AGE-RAGE axis in autism. Neurosci. Lett. 2006, 410, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Söğüt, S.; Zoroğlu, S.S.; Ozyurt, H.; Yilmaz, H.R.; Ozuğurlu, F.; Sivasli, E.; Yetkin, O.; Yanik, M.; Tutkun, H.; Savaş, H.A.; et al. Changes in nitric oxide levels and antioxidant enzyme activities may have a role in the pathophysiological mechanisms involved in autism. Clin. Chim. Acta 2003, 331, 111–117. [Google Scholar] [CrossRef]
- Chauhan, A.; Chauhan, V.; Brown, W.T.; Cohen, I. Oxidative stress in autism: Increased lipid peroxidation and reduced serum levels of ceruloplasmin and transferrin--the antioxidant proteins. Life Sci 2004, 75, 2539–2549. [Google Scholar] [CrossRef]
- Frye, R.E.; Lionnard, L.; Singh, I.; Karim, M.A.; Chajra, H.; Frechet, M.; Kissa, K.; Racine, V.; Ammanamanchi, A.; McCarty, P.J.; et al. Mitochondrial morphology is associated with respiratory chain uncoupling in autism spectrum disorder. Transl. Psychiatry 2021, 11, 527. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Mitochondrial dysfunction in autism spectrum disorders: A systematic review and meta-analysis. Mol. Psychiatry 2012, 17, 290–314. [Google Scholar] [CrossRef]
- Cheng, N.; Rho, J.M.; Masino, S.A. Metabolic Dysfunction Underlying Autism Spectrum Disorder and Potential Treatment Approaches. Front. Mol. Neurosci. 2017, 10, 34. [Google Scholar] [CrossRef]
- Chauhan, A.; Gu, F.; Essa, M.M.; Wegiel, J.; Kaur, K.; Brown, W.T.; Chauhan, V. Brain region-specific deficit in mitochondrial electron transport chain complexes in children with autism. J. Neurochem. 2011, 117, 209–220. [Google Scholar] [CrossRef] [PubMed]
- James, S.J.; Rose, S.; Melnyk, S.; Jernigan, S.; Blossom, S.; Pavliv, O.; Gaylor, D.W. Cellular and mitochondrial glutathione redox imbalance in lymphoblastoid cells derived from children with autism. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2009, 23, 2374–2383. [Google Scholar] [CrossRef] [PubMed]
- Hollis, F.; Kanellopoulos, A.K.; Bagni, C. Mitochondrial dysfunction in Autism Spectrum Disorder: Clinical features and perspectives. Curr. Opin. Neurobiol. 2017, 45, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Gutteridge, J.M.C.; Richmond, R.; Halliwell, B. Oxygen free-radicals and lipid peroxidation: Inhibition by the protein caeruloplasmin. FEBS Lett. 1980, 112, 269–272. [Google Scholar] [CrossRef]
- Loeffler, D.A.; Connor, J.R.; Juneau, P.L.; Snyder, B.S.; Kanaley, L.; DeMaggio, A.J.; Nguyen, H.; Brickman, C.M.; LeWitt, P.A. Transferrin and iron in normal, Alzheimer’s disease, and Parkinson’s disease brain regions. J. Neurochem. 1995, 65, 710–724. [Google Scholar] [CrossRef]
- Rose, S.; Melnyk, S.; Trusty, T.A.; Pavliv, O.; Seidel, L.; Li, J.; Nick, T.; James, S.J. Intracellular and extracellular redox status and free radical generation in primary immune cells from children with autism. Autism Res. Treat. 2012, 2012, 986519. [Google Scholar] [CrossRef]
- James, S.J.; Cutler, P.; Melnyk, S.; Jernigan, S.; Janak, L.; Gaylor, D.W.; Neubrander, J.A. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am. J. Clin. Nutr. 2004, 80, 1611–1617. [Google Scholar] [CrossRef]
- James, S.J.; Melnyk, S.; Fuchs, G.; Reid, T.; Jernigan, S.; Pavliv, O.; Hubanks, A.; Gaylor, D.W. Efficacy of methylcobalamin and folinic acid treatment on glutathione redox status in children with autism. Am. J. Clin. Nutr. 2009, 89, 425–430. [Google Scholar] [CrossRef]
- Sajdel-Sulkowska, E.M.; Xu, M.; Koibuchi, N. Increase in cerebellar neurotrophin-3 and oxidative stress markers in autism. Cerebellum 2009, 8, 366–372. [Google Scholar] [CrossRef]
- Melnyk, S.; Fuchs, G.J.; Schulz, E.; Lopez, M.; Kahler, S.G.; Fussell, J.J.; Bellando, J.; Pavliv, O.; Rose, S.; Seidel, L.; et al. Metabolic imbalance associated with methylation dysregulation and oxidative damage in children with autism. J. Autism Dev. Disord. 2012, 42, 367–377. [Google Scholar] [CrossRef]
- Usui, N.; Iwata, K.; Miyachi, T.; Takagai, S.; Wakusawa, K.; Nara, T.; Tsuchiya, K.J.; Matsumoto, K.; Kurita, D.; Kameno, Y.; et al. VLDL-specific increases of fatty acids in autism spectrum disorder correlate with social interaction. EBioMedicine 2020, 58, 102917. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, A.; Wakusawa, K.; Fujioka, T.; Iwata, K.; Usui, N.; Kurita, D.; Kameno, Y.; Wakuda, T.; Takagai, S.; Hirai, T.; et al. Simultaneous evaluation of antioxidative serum profiles facilitates the diagnostic screening of autism spectrum disorder in under-6-year-old children. Sci. Rep. 2020, 10, 20602. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Usui, N.; Iwata, K.; Miyachi, T.; Tsuchiya, K.J.; Xie, M.-J.; Nakamura, K.; Tsujii, M.; Sugiyama, T.; Matsuzaki, H. Increased plasma lipoprotein lipase activity in males with autism spectrum disorder. Res. Autism Spectr. Disord. 2020, 77, 101630. [Google Scholar] [CrossRef]
- Chauhan, A.; Audhya, T.; Chauhan, V. Brain region-specific glutathione redox imbalance in autism. Neurochem. Res. 2012, 37, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Geier, D.A.; Kern, J.K.; Garver, C.R.; Adams, J.B.; Audhya, T.; Geier, M.R. A prospective study of transsulfuration biomarkers in autistic disorders. Neurochem. Res. 2009, 34, 386–393. [Google Scholar] [CrossRef]
- Pangrazzi, L.; Balasco, L.; Bozzi, Y. Oxidative Stress and Immune System Dysfunction in Autism Spectrum Disorders. Int. J. Mol. Sci. 2020, 21, 3293. [Google Scholar] [CrossRef]
- Pangrazzi, L.; Balasco, L.; Bozzi, Y. Natural Antioxidants: A Novel Therapeutic Approach to Autism Spectrum Disorders? Antioxidants 2020, 9, 1186. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharm. Exp. 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Wang, L.; Wang, B.; Wu, C.; Wang, J.; Sun, M. Autism Spectrum Disorder: Neurodevelopmental Risk Factors, Biological Mechanism, and Precision Therapy. Int. J. Mol. Sci. 2023, 24, 1819. [Google Scholar] [CrossRef]
- Hardy, R.S.; Raza, K.; Cooper, M.S. Therapeutic glucocorticoids: Mechanisms of actions in rheumatic diseases. Nat. Rev. Rheumatol. 2020, 16, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Cain, D.W.; Cidlowski, J.A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Na, K.S.; Jung, H.Y.; Kim, Y.K. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 48, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.Y.; Lin, C.H.; Lane, H.Y. Molecular Basis of Late-Life Depression. Int. J. Mol. Sci. 2021, 22, 7421. [Google Scholar] [CrossRef]
- Momtazmanesh, S.; Zare-Shahabadi, A.; Rezaei, N. Cytokine Alterations in Schizophrenia: An Updated Review. Front. Psychiatry 2019, 10, 892. [Google Scholar] [CrossRef]
- Dabbah-Assadi, F.; Handel, R.; Shamir, A. What we know about the role of corticosteroids in psychiatric disorders; evidence from animal and clinical studies. J. Psychiatr. Res. 2022, 155, 363–370. [Google Scholar] [CrossRef]
- Schjerning, A.M.; McGettigan, P.; Gislason, G. Cardiovascular effects and safety of (non-aspirin) NSAIDs. Nat. Rev. Cardiol. 2020, 17, 574–584. [Google Scholar] [CrossRef]
- Ulrich, C.M.; Bigler, J.; Potter, J.D. Non-steroidal anti-inflammatory drugs for cancer prevention: Promise, perils and pharmacogenetics. Nat. Rev. Cancer 2006, 6, 130–140. [Google Scholar] [CrossRef]
- Bao, F.; John, S.M.; Chen, Y.; Mathison, R.D.; Weaver, L.C. The tripeptide phenylalanine-(D) glutamate-(D) glycine modulates leukocyte infiltration and oxidative damage in rat injured spinal cord. Neuroscience 2006, 140, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Mathison, R.D.; Befus, A.D.; Davison, J.S.; Woodman, R.C. Modulation of neutrophil function by the tripeptide feG. BMC Immunol. 2003, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Mathison, R.; Davison, J.S.; Befus, A.D. Neuroendocrine regulation of inflammation and tissue repair by submandibular gland factors. Immunol. Today 1994, 15, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Li, J.; Garbett, K.; Mirnics, K.; Patterson, P.H. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 10695–10702. [Google Scholar] [CrossRef]
- Pineda, E.; Shin, D.; You, S.J.; Auvin, S.; Sankar, R.; Mazarati, A. Maternal immune activation promotes hippocampal kindling epileptogenesis in mice. Ann. Neurol. 2013, 74, 11–19. [Google Scholar] [CrossRef]
- Ruskin, D.N.; Murphy, M.I.; Slade, S.L.; Masino, S.A. Ketogenic diet improves behaviors in a maternal immune activation model of autism spectrum disorder. PLoS ONE 2017, 12, e0171643. [Google Scholar] [CrossRef]
- Lusardi, T.A.; Akula, K.K.; Coffman, S.Q.; Ruskin, D.N.; Masino, S.A.; Boison, D. Ketogenic diet prevents epileptogenesis and disease progression in adult mice and rats. Neuropharmacology 2015, 99, 500–509. [Google Scholar] [CrossRef]
- Chen, J.Y.; Tran, C.; Hwang, L.; Deng, G.; Jung, M.E.; Faull, K.F.; Levine, M.S.; Cepeda, C. Partial Amelioration of Peripheral and Central Symptoms of Huntington’s Disease via Modulation of Lipid Metabolism. J. Huntingt. Dis. 2016, 5, 65–81. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Zhang, H.; Yu, J.; Yao, Z. Oral probiotic administration during pregnancy prevents autism-related behaviors in offspring induced by maternal immune activation via anti-inflammation in mice. Autism Res. 2019, 12, 576–588. [Google Scholar] [CrossRef]
- Hafizi, S.; Tabatabaei, D.; Lai, M.C. Review of Clinical Studies Targeting Inflammatory Pathways for Individuals With Autism. Front. Psychiatry 2019, 10, 849. [Google Scholar] [CrossRef]
- Asadabadi, M.; Mohammadi, M.R.; Ghanizadeh, A.; Modabbernia, A.; Ashrafi, M.; Hassanzadeh, E.; Forghani, S.; Akhondzadeh, S. Celecoxib as adjunctive treatment to risperidone in children with autistic disorder: A randomized, double-blind, placebo-controlled trial. Psychopharmacology 2013, 225, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Buitelaar, J.K.; van Engeland, H.; de Kogel, K.H.; de Vries, H.; van Hooff, J.A.; van Ree, J.M. The use of adrenocorticotrophic hormone (4-9) analog ORG 2766 in autistic children: Effects on the organization of behavior. Biol. Psychiatry 1992, 31, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Taliou, A.; Zintzaras, E.; Lykouras, L.; Francis, K. An open-label pilot study of a formulation containing the anti-inflammatory flavonoid luteolin and its effects on behavior in children with autism spectrum disorders. Clin. Ther. 2013, 35, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.A.K.; Hardin, J.W.; Love, B.L.; Merchant, A.T.; McDermott, S. Relationship of Nonsteroidal Anti-Inflammatory Drug Use During Pregnancy with Autism Spectrum Disorder and Intellectual Disability Among Offspring. J. Womens Health 2022, 32, 356–365. [Google Scholar] [CrossRef]
- Gvozdjáková, A.; Kucharská, J.; Ostatníková, D.; Babinská, K.; Nakládal, D.; Crane, F.L. Ubiquinol improves symptoms in children with autism. Oxid. Med. Cell. Longev. 2014, 2014, 798957. [Google Scholar] [CrossRef]
- Mousavinejad, E.; Ghaffari, M.A.; Riahi, F.; Hajmohammadi, M.; Tiznobeyk, Z.; Mousavinejad, M. Coenzyme Q(10) supplementation reduces oxidative stress and decreases antioxidant enzyme activity in children with autism spectrum disorders. Psychiatry Res. 2018, 265, 62–69. [Google Scholar] [CrossRef]
- Singh, K.; Connors, S.L.; Macklin, E.A.; Smith, K.D.; Fahey, J.W.; Talalay, P.; Zimmerman, A.W. Sulforaphane treatment of autism spectrum disorder (ASD). Proc. Natl. Acad. Sci. USA 2014, 111, 15550–15555. [Google Scholar] [CrossRef]
- Hardan, A.Y.; Fung, L.K.; Libove, R.A.; Obukhanych, T.V.; Nair, S.; Herzenberg, L.A.; Frazier, T.W.; Tirouvanziam, R. A randomized controlled pilot trial of oral N-acetylcysteine in children with autism. Biol. Psychiatry 2012, 71, 956–961. [Google Scholar] [CrossRef]
- Ghanizadeh, A.; Moghimi-Sarani, E. A randomized double blind placebo controlled clinical trial of N-Acetylcysteine added to risperidone for treating autistic disorders. BMC Psychiatry 2013, 13, 196. [Google Scholar] [CrossRef]
- Deepmala; Slattery, J.; Kumar, N.; Delhey, L.; Berk, M.; Dean, O.; Spielholz, C.; Frye, R. Clinical trials of N-acetylcysteine in psychiatry and neurology: A systematic review. Neurosci. Biobehav. Rev. 2015, 55, 294–321. [Google Scholar] [CrossRef]
- Sadek, A.; Berk, L.S.; Mainess, K.; Daher, N.S. Antioxidants and Autism: Teachers’ Perceptions of Behavioral Changes. Adv. Mind-Body Med. 2018, 32, 12–17. [Google Scholar] [PubMed]
- Rossignol, D.A.; Frye, R.E. Melatonin in autism spectrum disorders: A systematic review and meta-analysis. Dev. Med. Child. Neurol. 2011, 53, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Al-Amin, M.M.; Rahman, M.M.; Khan, F.R.; Zaman, F.; Mahmud Reza, H. Astaxanthin improves behavioral disorder and oxidative stress in prenatal valproic acid-induced mice model of autism. Behav. Brain Res. 2015, 286, 112–121. [Google Scholar] [CrossRef]
- Rushworth, G.F.; Megson, I.L. Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 2014, 141, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Méndez, R.; Rivas-Arancibia, S. Vitamin C in Health and Disease: Its Role in the Metabolism of Cells and Redox State in the Brain. Front. Physiol. 2015, 6, 397. [Google Scholar] [CrossRef]
- Ornoy, A.; Weinstein-Fudim, L.; Tfilin, M.; Ergaz, Z.; Yanai, J.; Szyf, M.; Turgeman, G. S-adenosyl methionine prevents ASD like behaviors triggered by early postnatal valproic acid exposure in very young mice. Neurotoxicol. Teratol. 2019, 71, 64–74. [Google Scholar] [CrossRef]
- Banji, D.; Banji, O.J.; Abbagoni, S.; Hayath, M.S.; Kambam, S.; Chiluka, V.L. Amelioration of behavioral aberrations and oxidative markers by green tea extract in valproate induced autism in animals. Brain Res. 2011, 1410, 141–151. [Google Scholar] [CrossRef]
- Needham, B.D.; Adame, M.D.; Serena, G.; Rose, D.R.; Preston, G.M.; Conrad, M.C.; Campbell, A.S.; Donabedian, D.H.; Fasano, A.; Ashwood, P.; et al. Plasma and Fecal Metabolite Profiles in Autism Spectrum Disorder. Biol. Psychiatry 2021, 89, 451–462. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.-i.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Ohta, S. Molecular hydrogen as a preventive and therapeutic medical gas: Initiation, development and potential of hydrogen medicine. Pharmacol. Ther. 2014, 144, 1–11. [Google Scholar] [CrossRef]
- Shigeo, O. Recent Progress toward Hydrogen Medicine: Potential of Molecular Hydrogen for Preventive and Therapeutic Applications. Curr. Pharm. Des. 2011, 17, 2241–2252. [Google Scholar] [CrossRef]
- Ohta, S. Molecular hydrogen is a novel antioxidant to efficiently reduce oxidative stress with potential for the improvement of mitochondrial diseases. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2012, 1820, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Fontanari, P.; Badier, M.; Guillot, C.; Tomei, C.; Burnet, H.; Gardette, B.; Jammes, Y. Changes in maximal performance of inspiratory and skeletal muscles during and after the 7.1-MPa Hydra 10 record human dive. Eur. J. Appl. Physiol. 2000, 81, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Abraini, J.H.; Gardette-Chauffour, M.C.; Martinez, E.; Rostain, J.C.; Lemaire, C. Psychophysiological reactions in humans during an open sea dive to 500 m with a hydrogen-helium-oxygen mixture. J. Appl. Physiol. 1994, 76, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, M.; Sobue, S.; Ito, M.; Ito, M.; Hirayama, M.; Ohno, K. Beneficial biological effects and the underlying mechanisms of molecular hydrogen—Comprehensive review of 321 original articles. Med. Gas. Res. 2015, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- LeBaron, T.W.; Kura, B.; Kalocayova, B.; Tribulova, N.; Slezak, J. A New Approach for the Prevention and Treatment of Cardiovascular Disorders. Molecular Hydrogen Significantly Reduces the Effects of Oxidative Stress. Molecules 2019, 24, 2076. [Google Scholar] [CrossRef]
- Xun, Z.M.; Zhao, Q.H.; Zhang, Y.; Ju, F.D.; He, J.; Yao, T.T.; Zhang, X.K.; Yi, Y.; Ma, S.N.; Zhao, P.X.; et al. Effects of long-term hydrogen intervention on the physiological function of rats. Sci. Rep. 2020, 10, 18509. [Google Scholar] [CrossRef]
- Usui, N.; Co, M.; Harper, M.; Rieger, M.A.; Dougherty, J.D.; Konopka, G. Sumoylation of FOXP2 Regulates Motor Function and Vocal Communication Through Purkinje Cell Development. Biol. Psychiatry 2017, 81, 220–230. [Google Scholar] [CrossRef]
- Usui, N.; Araujo, D.J.; Kulkarni, A.; Co, M.; Ellegood, J.; Harper, M.; Toriumi, K.; Lerch, J.P.; Konopka, G. Foxp1 regulation of neonatal vocalizations via cortical development. Genes Dev. 2017, 31, 2039–2055. [Google Scholar] [CrossRef]
- Lammert, C.R.; Lukens, J.R. Modeling Autism-Related Disorders in Mice with Maternal Immune Activation (MIA). Methods Mol. Biol. 2019, 1960, 227–236. [Google Scholar] [CrossRef]
- Arora, N.; Sadovsky, Y.; Dermody, T.S.; Coyne, C.B. Microbial Vertical Transmission during Human Pregnancy. Cell Host Microbe 2017, 21, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Yin, X.; Qiao, M.; Jia, Y.; Chen, D.; Shao, J.; Lebaron, T.W.; Gao, Y.; Shi, H.; Jia, B. Hydrogen-Rich Water Ameliorates Autistic-Like Behavioral Abnormalities in Valproic Acid-Treated Adolescent Mice Offspring. Front. Behav. Neurosci. 2018, 12, 170. [Google Scholar] [CrossRef] [PubMed]
- Lasram, M.M.; Dhouib, I.B.; Annabi, A.; El Fazaa, S.; Gharbi, N. A review on the possible molecular mechanism of action of N-acetylcysteine against insulin resistance and type-2 diabetes development. Clin. Biochem. 2015, 48, 1200–1208. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, W.; Zhai, Q.; Zhang, T.; Wen, X. N-acetylcysteine ameliorates repetitive/stereotypic behavior due to its antioxidant properties without activation of the canonical Wnt pathway in a valproic acid-induced rat model of autism. Mol. Med. Rep. 2017, 16, 2233–2240. [Google Scholar] [CrossRef]
- Rebouche, C.J. Ascorbic acid and carnitine biosynthesis. Am. J. Clin. Nutr. 1991, 54, 1147s–1152s. [Google Scholar] [CrossRef]
- Cobley, J.N.; McHardy, H.; Morton, J.P.; Nikolaidis, M.G.; Close, G.L. Influence of vitamin C and vitamin E on redox signaling: Implications for exercise adaptations. Free Radic. Biol. Med. 2015, 84, 65–76. [Google Scholar] [CrossRef]
- Robea, M.A.; Jijie, R.; Nicoara, M.; Plavan, G.; Ciobica, A.S.; Solcan, C.; Audira, G.; Hsiao, C.D.; Strungaru, S.A. Vitamin C Attenuates Oxidative Stress and Behavioral Abnormalities Triggered by Fipronil and Pyriproxyfen Insecticide Chronic Exposure on Zebrafish Juvenile. Antioxidants 2020, 9, 944. [Google Scholar] [CrossRef]
- Bjørklund, G.; Waly, M.I.; Al-Farsi, Y.; Saad, K.; Dadar, M.; Rahman, M.M.; Elhoufey, A.; Chirumbolo, S.; Jóźwik-Pruska, J.; Kałużna-Czaplińska, J. The Role of Vitamins in Autism Spectrum Disorder: What Do We Know? J. Mol. Neurosci. 2019, 67, 373–387. [Google Scholar] [CrossRef]
- Dolske, M.C.; Spollen, J.; McKay, S.; Lancashire, E.; Tolbert, L. A preliminary trial of ascorbic acid as supplemental therapy for autism. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1993, 17, 765–774. [Google Scholar] [CrossRef]
- Nikoo, M.; Radnia, H.; Farokhnia, M.; Mohammadi, M.R.; Akhondzadeh, S. N-acetylcysteine as an adjunctive therapy to risperidone for treatment of irritability in autism: A randomized, double-blind, placebo-controlled clinical trial of efficacy and safety. Clin. Neuropharmacol. 2015, 38, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Usui, N.; Shimada, S. Prenatal Sex Hormone Exposure Is Associated with the Development of Autism Spectrum Disorder. Int. J. Mol. Sci. 2023, 24, 2203. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.W.; Chavan, A.R.; Protopapas, S.; Maziarz, J.; Romero, R.; Wagner, G.P. Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proc. Natl. Acad. Sci. USA 2017, 114, E6566–E6575. [Google Scholar] [CrossRef] [PubMed]
- Duhig, K.; Chappell, L.C.; Shennan, A.H. Oxidative stress in pregnancy and reproduction. Obs. Med. 2016, 9, 113–116. [Google Scholar] [CrossRef]
- Hussain, T.; Murtaza, G.; Metwally, E.; Kalhoro, D.H.; Kalhoro, M.S.; Rahu, B.A.; Sahito, R.G.A.; Yin, Y.; Yang, H.; Chughtai, M.I.; et al. The Role of Oxidative Stress and Antioxidant Balance in Pregnancy. Mediat. Inflamm. 2021, 2021, 9962860. [Google Scholar] [CrossRef]
- Chiarello, D.I.; Abad, C.; Rojas, D.; Toledo, F.; Vázquez, C.M.; Mate, A.; Sobrevia, L.; Marín, R. Oxidative stress: Normal pregnancy versus preeclampsia. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165354. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usui, N.; Kobayashi, H.; Shimada, S. Neuroinflammation and Oxidative Stress in the Pathogenesis of Autism Spectrum Disorder. Int. J. Mol. Sci. 2023, 24, 5487. https://doi.org/10.3390/ijms24065487
Usui N, Kobayashi H, Shimada S. Neuroinflammation and Oxidative Stress in the Pathogenesis of Autism Spectrum Disorder. International Journal of Molecular Sciences. 2023; 24(6):5487. https://doi.org/10.3390/ijms24065487
Chicago/Turabian StyleUsui, Noriyoshi, Hikaru Kobayashi, and Shoichi Shimada. 2023. "Neuroinflammation and Oxidative Stress in the Pathogenesis of Autism Spectrum Disorder" International Journal of Molecular Sciences 24, no. 6: 5487. https://doi.org/10.3390/ijms24065487
APA StyleUsui, N., Kobayashi, H., & Shimada, S. (2023). Neuroinflammation and Oxidative Stress in the Pathogenesis of Autism Spectrum Disorder. International Journal of Molecular Sciences, 24(6), 5487. https://doi.org/10.3390/ijms24065487






