Short-Term Effects of Human versus Bovine Sialylated Milk Oligosaccharide Microinjection on Zebrafish Larvae Survival, Locomotor Behavior and Gene Expression
Abstract
1. Introduction
2. Results
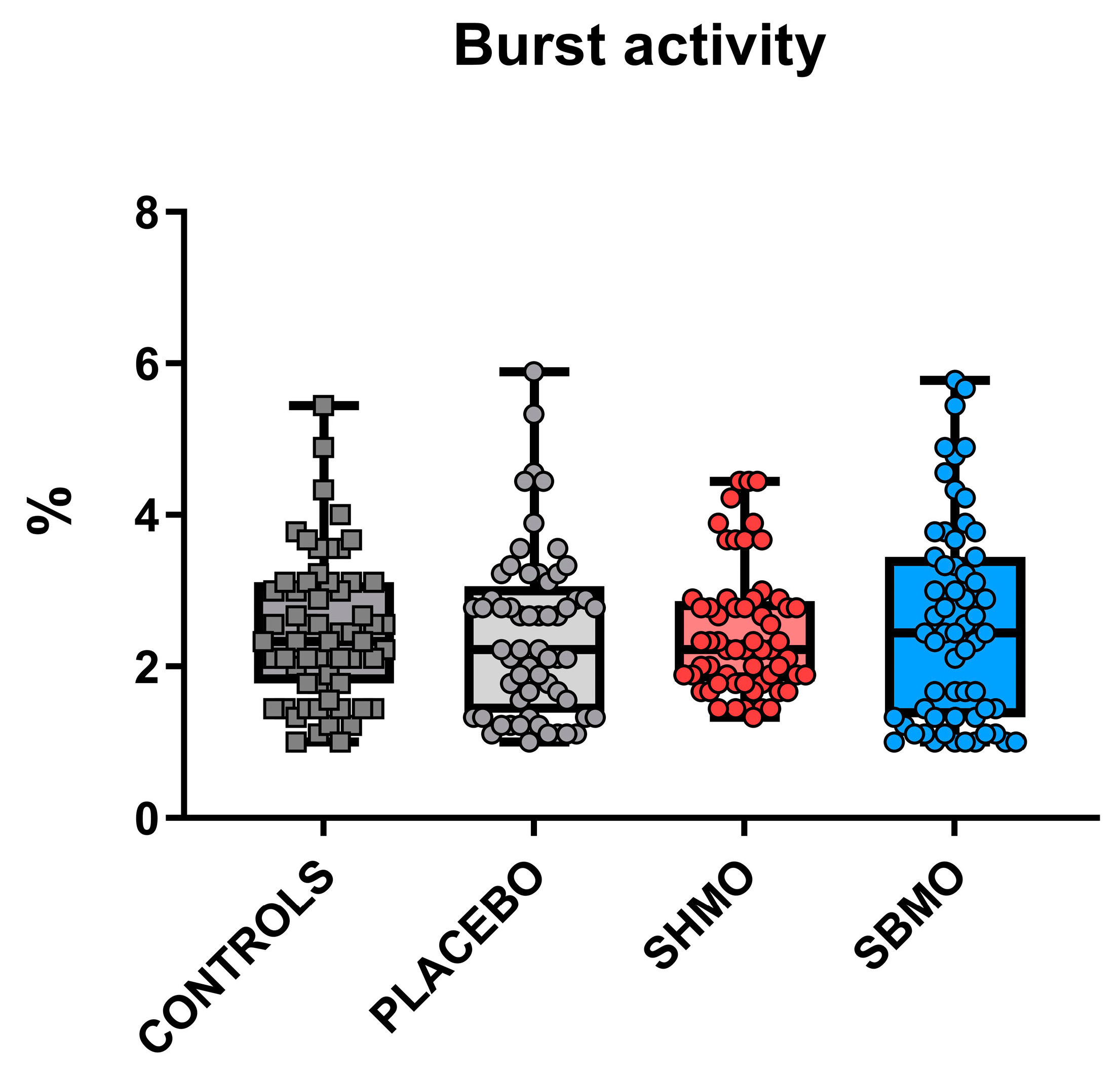
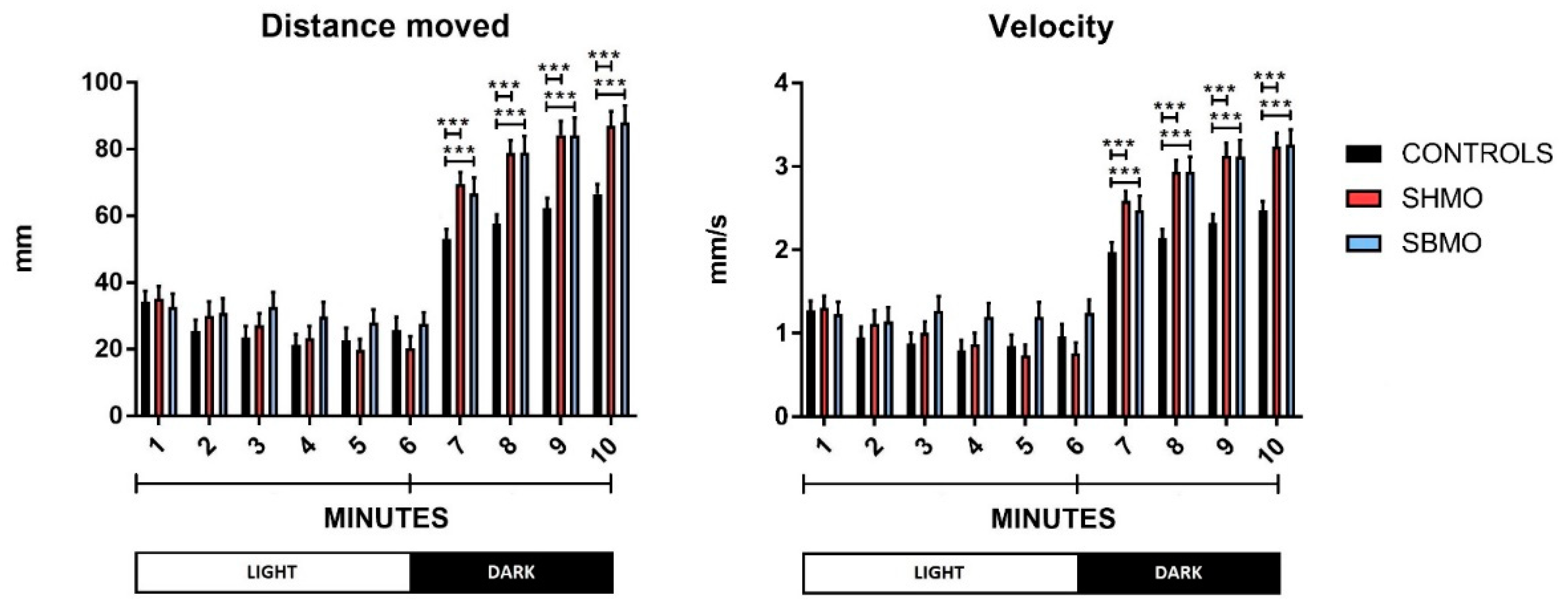
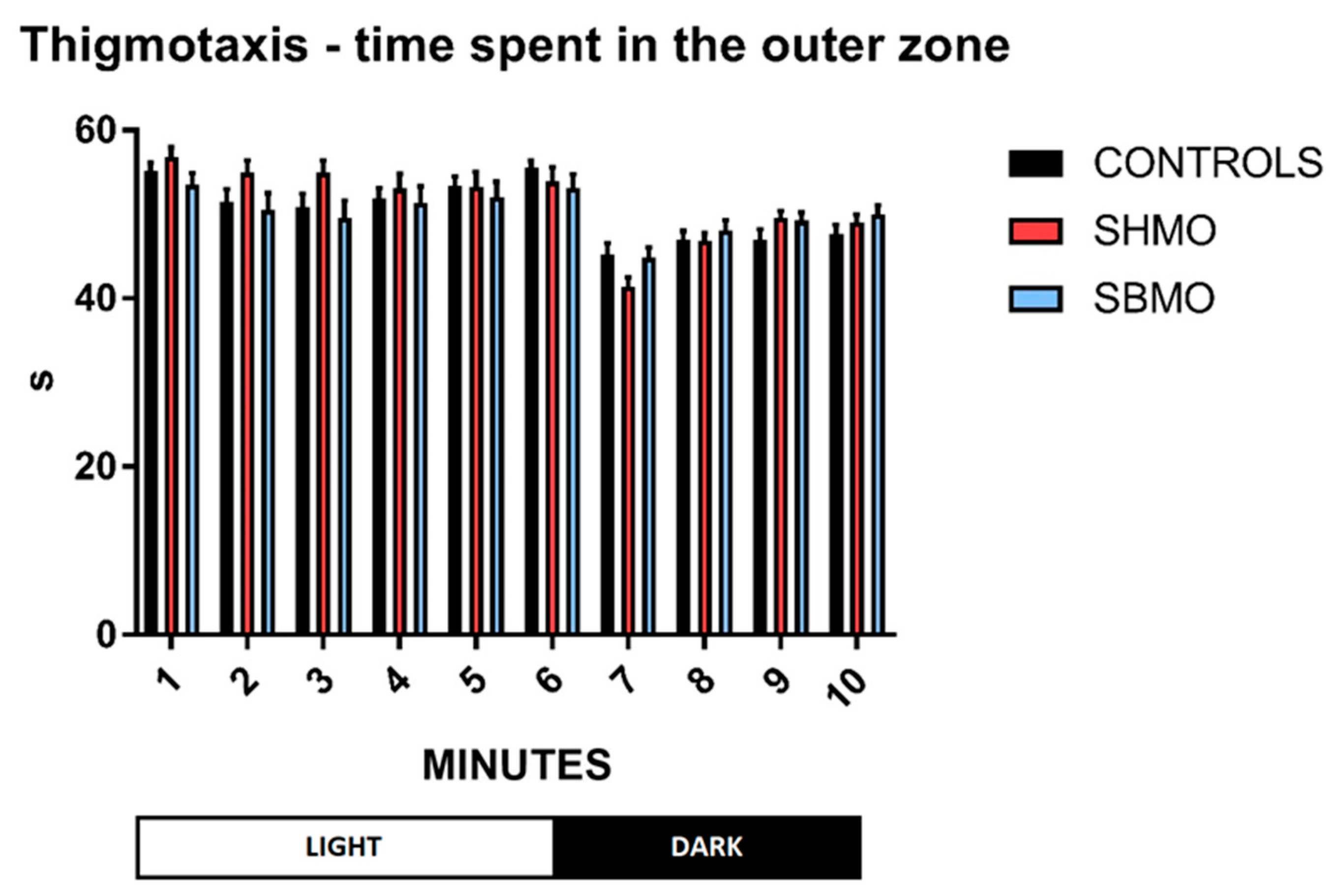
2.1. Locomotor and Thigmotactic Behavior
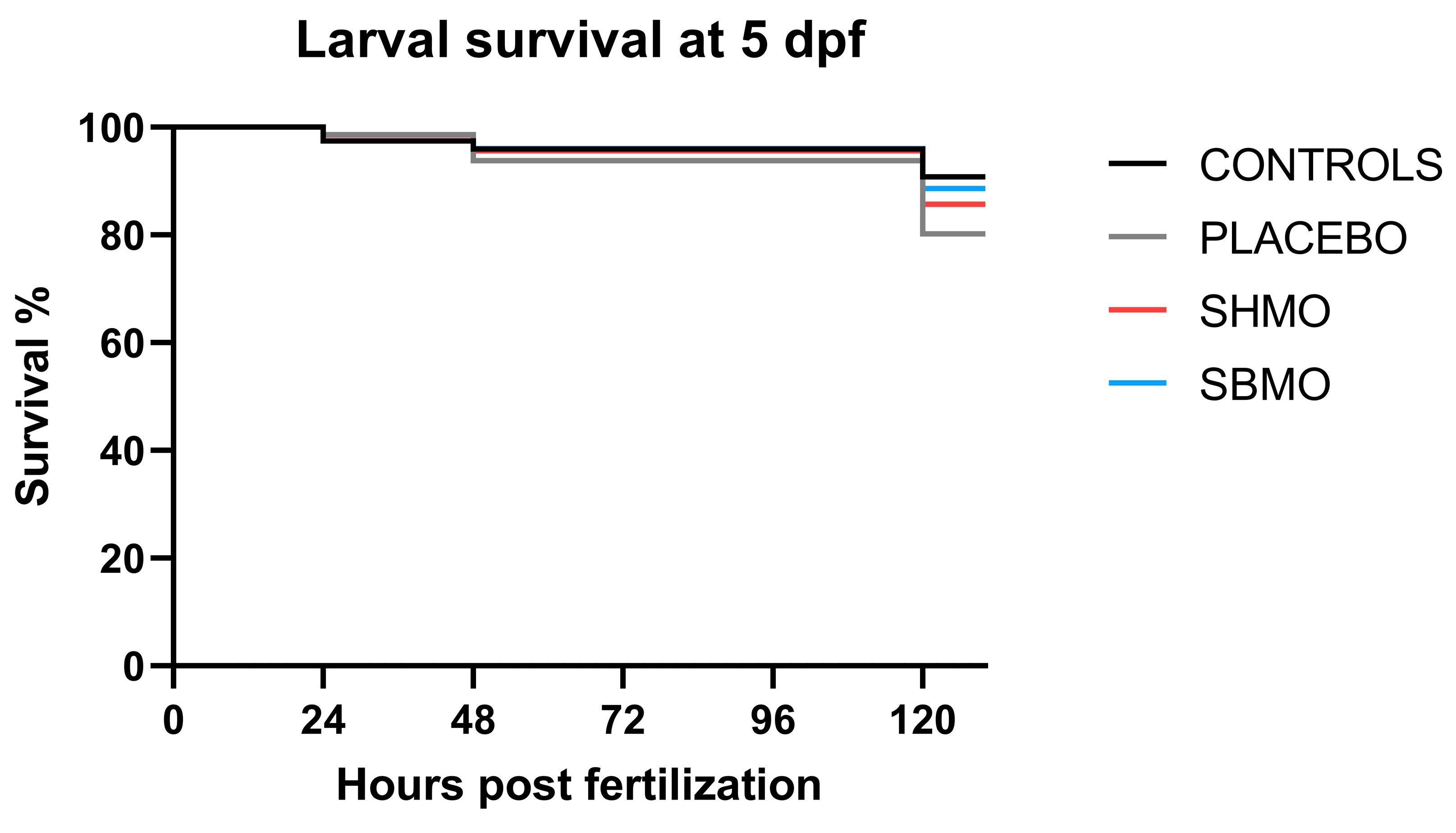
2.2. Larval Survival Rate
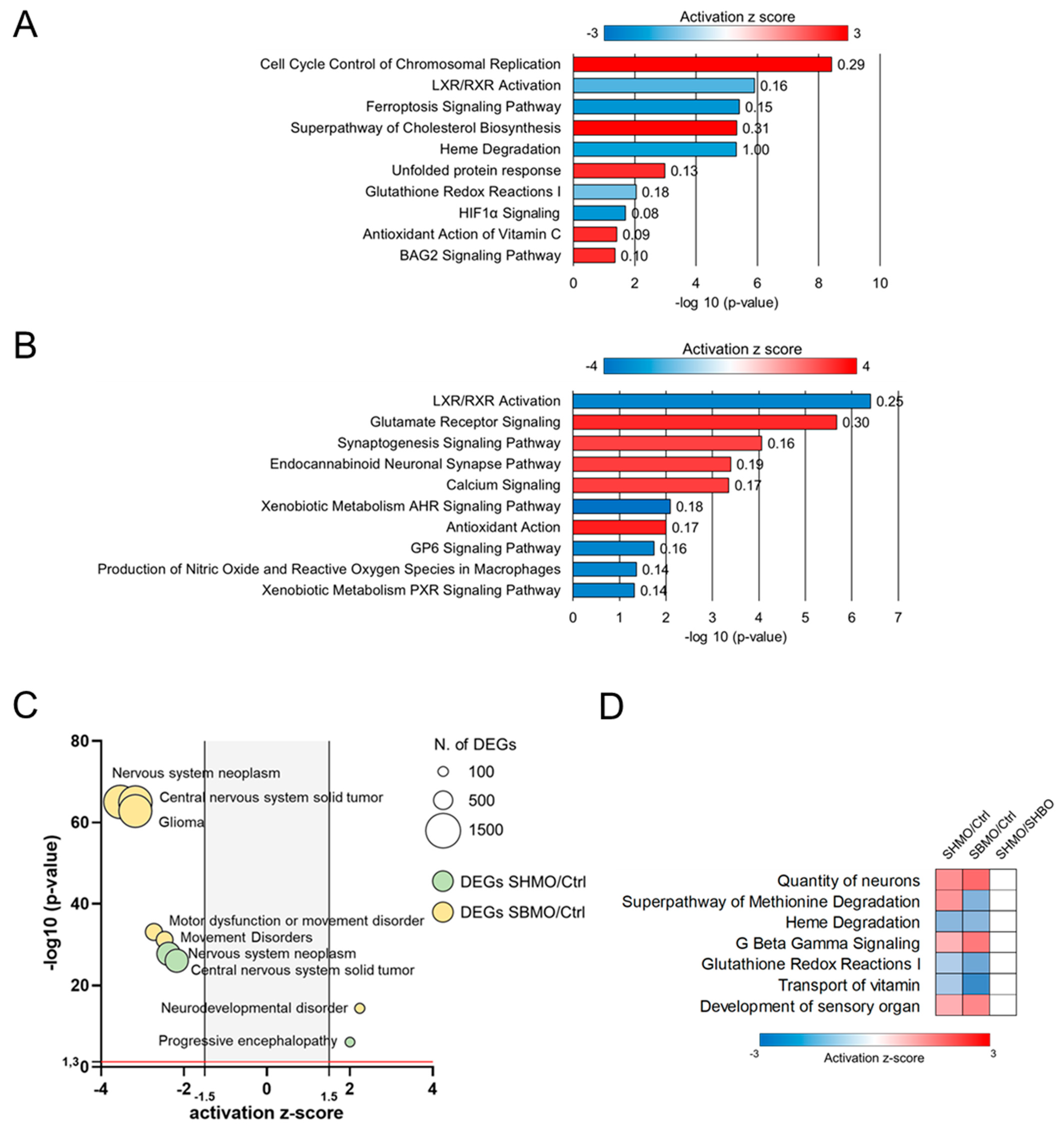
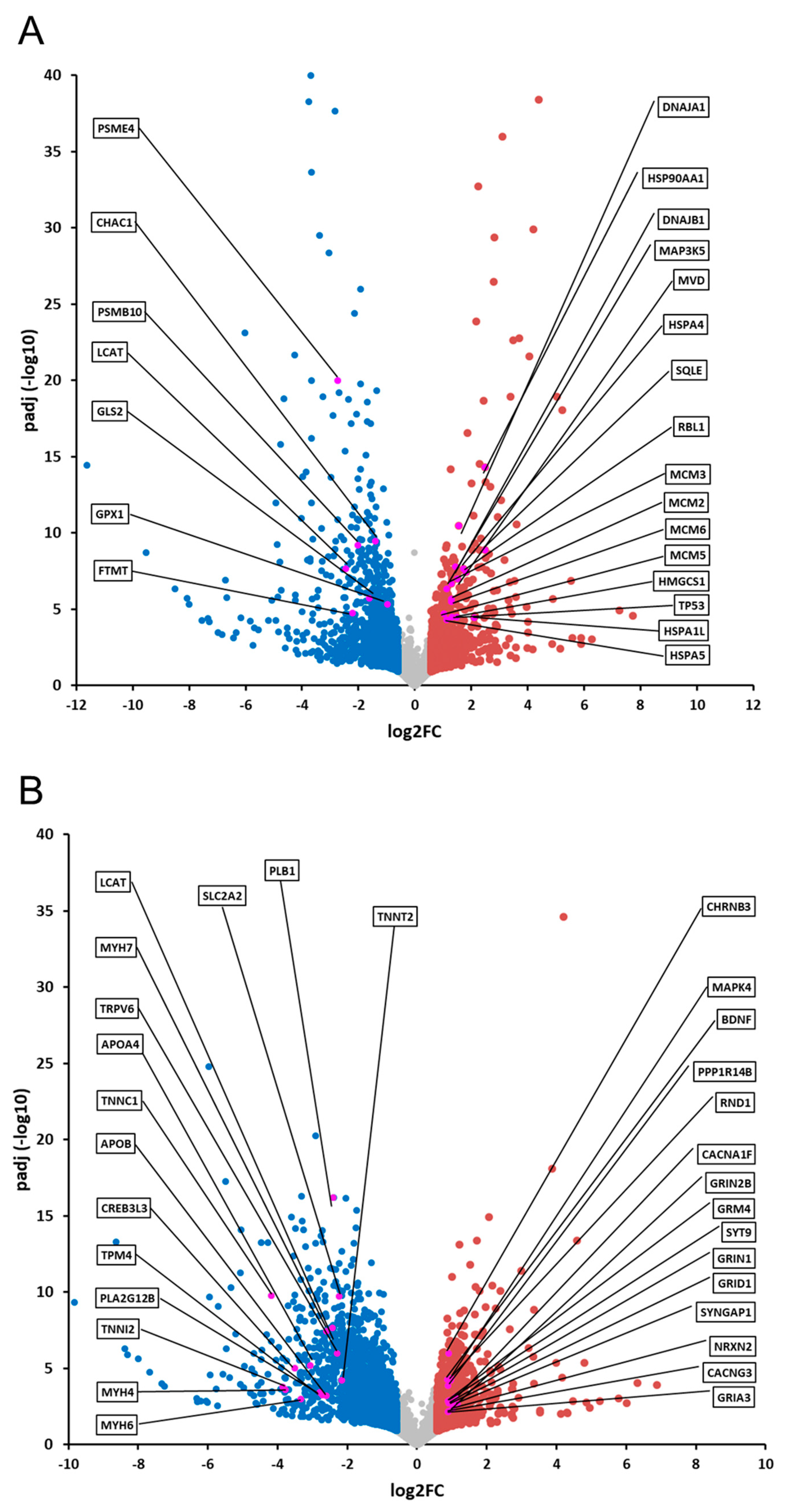
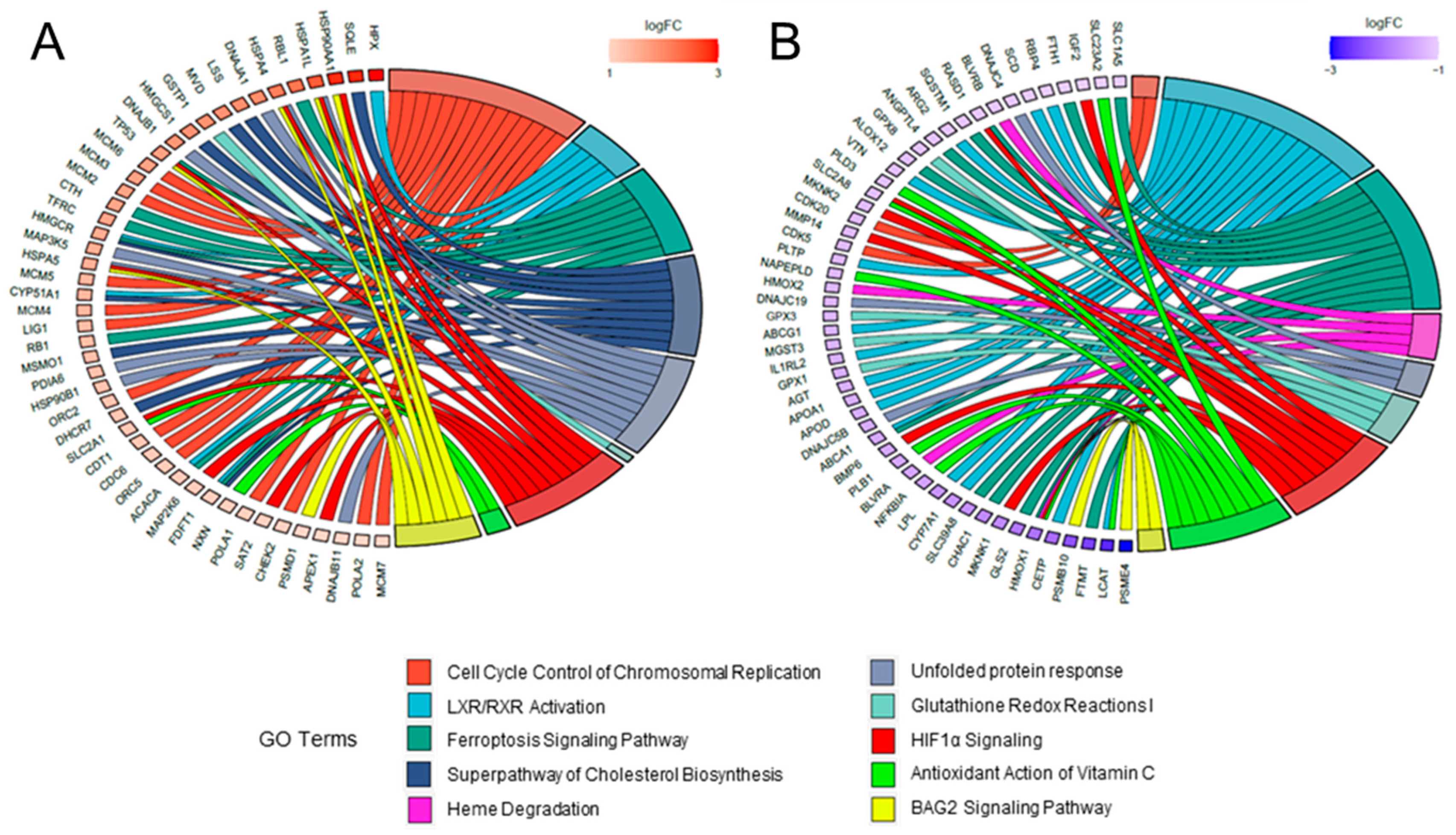
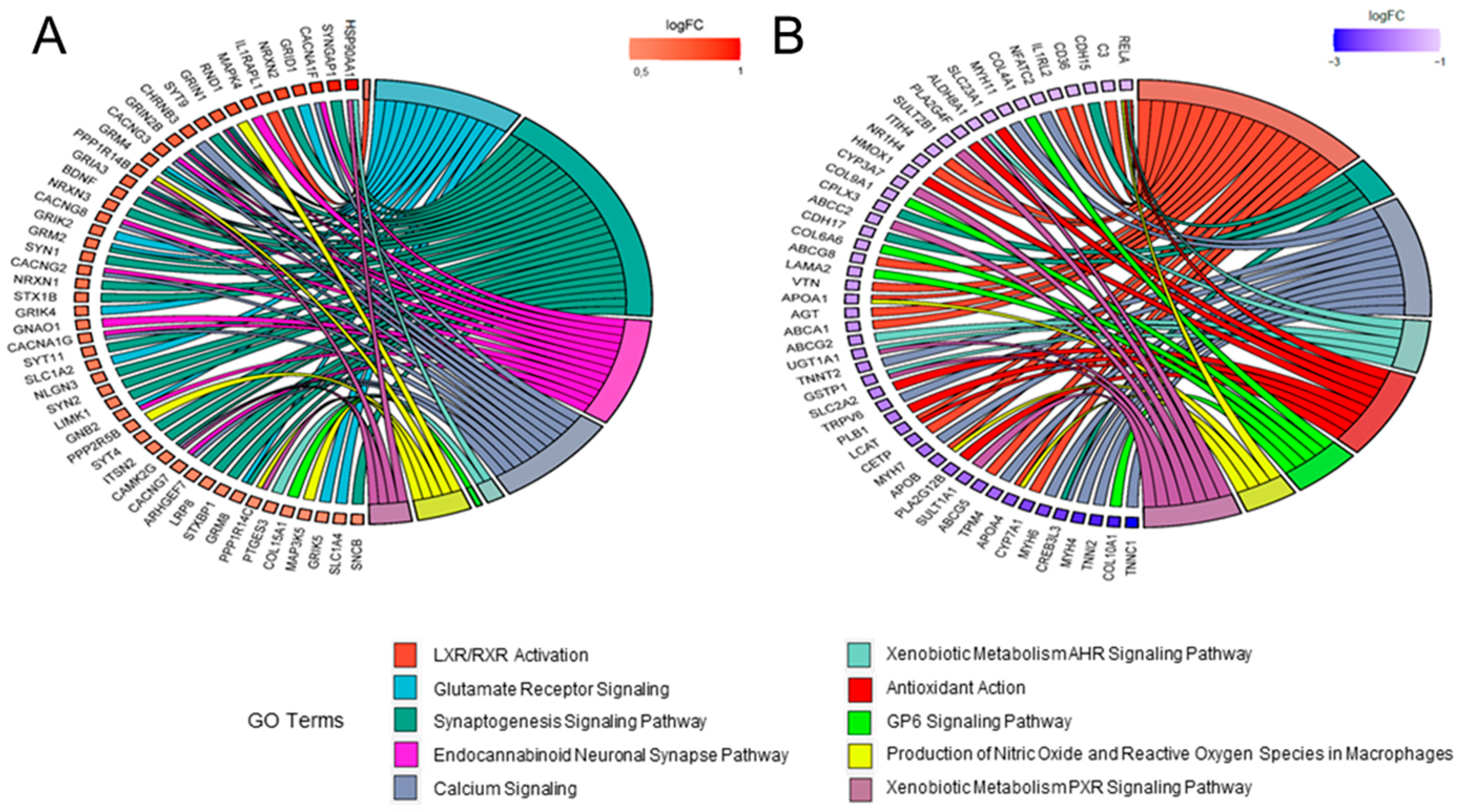
2.3. Comparative Analysis and Bioinformatic Categorization of Differentially Expressed Genes
3. Discussion
4. Materials and Methods
4.1. Zebrafish Care and Maintenance
4.2. Milk Oligosaccharide Purification and Analysis
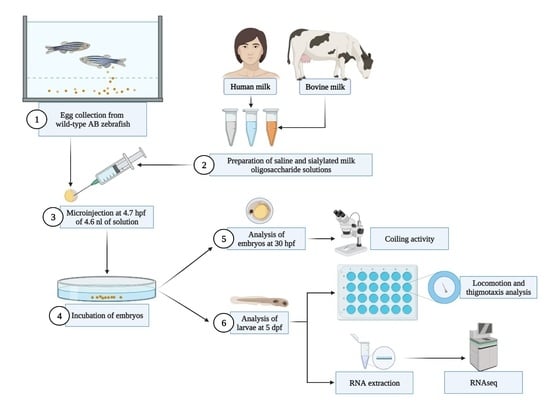
4.3. Microinjection of SMOs
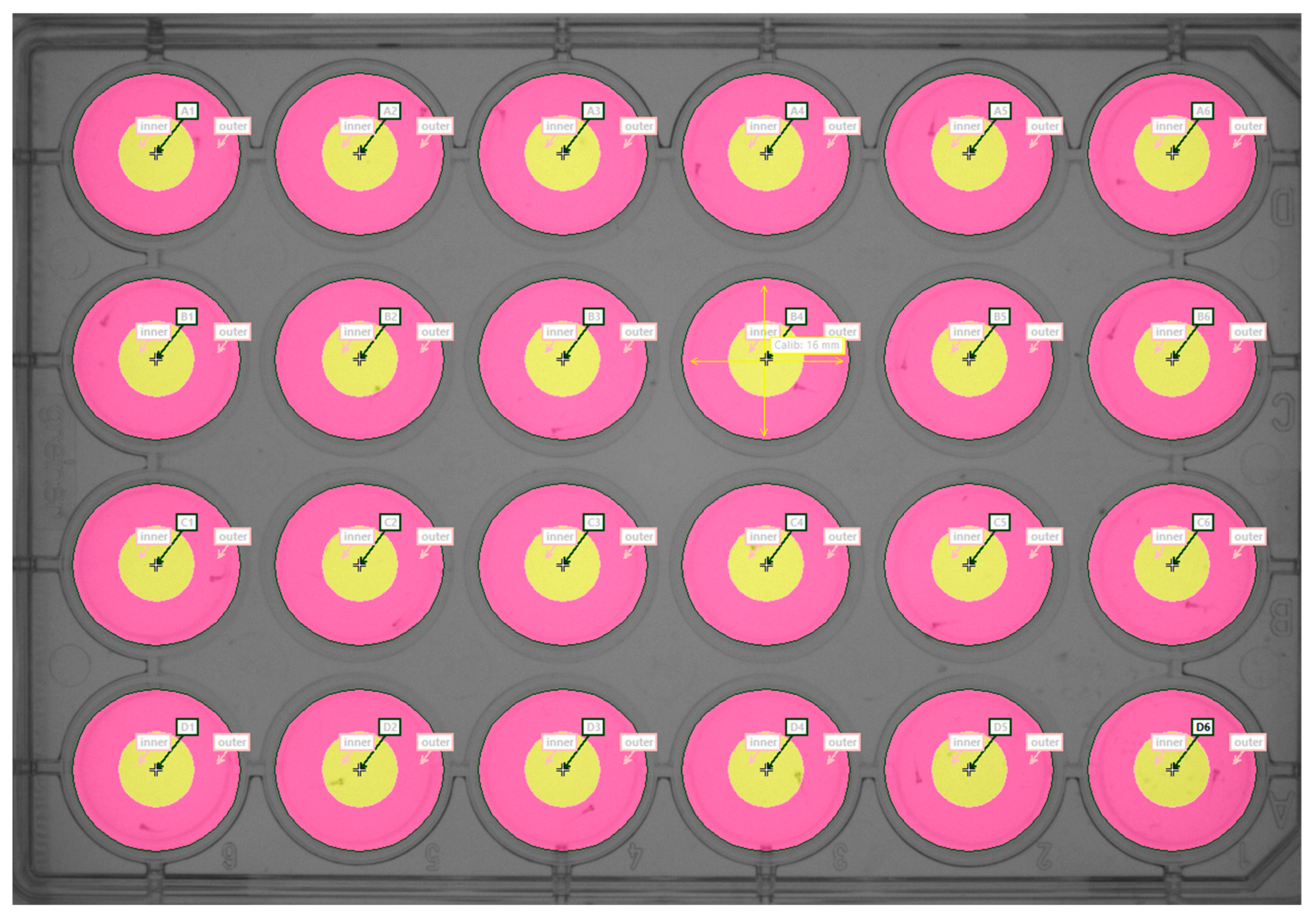
4.4. Locomotion Analysis
4.5. Behavioral Endpoints
- Burst activity during 30 s of the test procedure, expressed as %.
- General locomotor activity: this was measured as the total distance moved (mm) and velocity (mm/s) of movement over the whole area of the well during each minute of the test procedure, and under different conditions (lights ON vs. lights OFF).
- Thigmotaxis: this was presented as time spent (s) in the outer zone of the well during each minute of the test procedure, and under different conditions (lights ON vs. lights OFF).
4.6. RNA-Seq Analysis
4.7. Bioinformatic Analysis and Categorization of Transcriptomic Data
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walker, A. Breast milk as the gold standard for protective nutrients. J. Pediatr. 2010, 156, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.C. Milk nutritional composition and its role in human health. Nutrition 2014, 30, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Doherty, A.M.; Lodge, C.J.; Dharmage, S.C.; Dai, X.; Bode, L.; Lowe, A.J. Human milk oligosaccharides and associations with immune-mediated disease and infection in childhood: A systematic review. Front. Pediatr. 2018, 6, 1–8. [Google Scholar] [CrossRef]
- Wang, J.; Lei, B.; Yan, J.; Li, J.; Zhou, X.; Ren, F.; Guo, H. Donkey milk oligosaccharides influence the growth-related characteristics of intestinal cells and induce G2/M growth arrest via the p38 pathway in HT-29 cells. Food Funct. 2019, 10, 4823–4833. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Ding, J.; Jin, G.; Yu, D.; Yu, L.; Long, Z.; Guo, Z.; Chai, W.; Liang, X. Profiling of sialylated oligosaccharides in mammalian milk using online solid phase extraction-hydrophilic interaction chromatography coupled with negative-ion electrospray mass spectrometry. Anal. Chem. 2018, 90, 3174–3182. [Google Scholar] [CrossRef] [PubMed]
- Smilowitz, J.T.; Lebrilla, C.B.; Mills, D.A.; German, J.B.; Freeman, S.L. Breast milk oligosaccharides: Structure-function relationships in the neonate. Annu. Rev. Nutr. 2014, 34, 143–169. [Google Scholar] [CrossRef]
- Monti, L.; Cattaneo, T.M.P.; Orlandi, M.; Curadi, M.C. Capillary electrophoresis of sialylated oligosaccharides in milk from different species. J. Chromatogr. A 2015, 1409, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; van’t Land, B.; Engen, P.A.; Naqib, A.; Green, S.J.; Nato, A.; Leusink-Muis, T.; Garssen, J.; Keshavarzian, A.; Stahl, B.; et al. Human milk oligosaccharides protect against the development of autoimmune diabetes in NOD-mice. Sci. Rep. 2018, 8, 3829. [Google Scholar] [CrossRef] [PubMed]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef]
- Oliveira, D.L.; Wilbey, R.A.; Grandison, A.S.; Roseiro, L.B. Milk oligosaccharides: A review. Int. J. Dairy Technol. 2015, 68, 305–321. [Google Scholar] [CrossRef]
- Sischo, W.M.; Short, D.M.; Geissler, M.; Bunyatratchata, A.; Barile, D. Comparative composition, diversity, and abundance of oligosaccharides in early lactation milk from commercial dairy and beef cows. J. Dairy Sci. 2017, 100, 3883–3892. [Google Scholar] [CrossRef]
- Albrecht, S.; Lane, J.; Mariño, K.; Busadah, K.A.; Carrington, S.D.; Hickey, R.M.; Rudd, P.M. A comparative study of free oligosaccharides in the milk of domestic animals. Br. J. Nutr. 2014, 111, 1313–1328. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. Molecular mechanism underlying sialic acid as an essential nutrient for brain development and cognition. Adv. Nutr. 2012, 3, 465S–472S. [Google Scholar] [CrossRef]
- Licitra, R.; Li, J.; Liang, X.; Altomonte, I.; Salari, F.; Yan, J.; Martini, M. Profile and content of sialylated oligosaccharides in donkey milk at early lactation. LWT 2019, 115, 108437. [Google Scholar] [CrossRef]
- Sakai, F.; Ikeuchi, Y.; Urashima, T.; Fujihara, M.; Ohtsuki, K.; Yanahira, S. Effects of feeding sialyllactose and galactosylated N-acetylneuraminic acid on swimming learning ability and brain lipid composition in adult rats. J. Appl. Glycosci. 2006, 53, 249–254. [Google Scholar] [CrossRef]
- Pisa, E.; Martire, A.; Chiodi, V.; Traversa, A.; Caputo, V.; Hauser, J.; Macrì, S. Exposure to 3’sialyllactose-poor milk during lactation impairs cognitive capabilities in adulthood. Nutrients 2021, 13, 4191. [Google Scholar] [CrossRef]
- Wang, B.; Brand-Miller, J. The role and potential of sialic acid in human nutrition. Eur. J. Clin. Nutr. 2003, 57, 1351–1369. [Google Scholar] [CrossRef] [PubMed]
- Rocha, F.; Dias, J.; Engrola, S.; Gavaia, P.; Geurden, I.; Dinis, M.T.; Panserat, S. Glucose overload in yolk has little effect on the long-term modulation of carbohydrate metabolic genes in zebrafish (Danio rerio). J. Exp. Biol. 2014, 217, 1139–1149. [Google Scholar] [CrossRef]
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; França, G.V.A.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 01024. [Google Scholar] [CrossRef]
- Wang, B. Sialic acid is an essential nutrient for brain development and cognition. Annu. Rev. Nutr. 2009, 29, 177–222. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, E.B.; Fischl, B.R.; Quinn, B.T.; Chong, W.K.; Gadian, D.G.; Lucas, A. Impact of breast milk on intelligence quotient, brain size, and white matter development. Pediatr. Res. 2010, 67, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Schauer, R. Chemistry, metabolism, and biological functions of sialic acids. Adv. Carbohydr. Chem. Biochem. 1982, 40, 131–234. [Google Scholar] [CrossRef]
- Bruggencate, S.J.; Bovee-Oudenhoven, I.M.; Feitsma, A.L.; Hoffen, E.; Schoterman, M.H. Functional role and mechanisms of sialyllactose and other sialylated milk oligosaccharides. Nutr. Rev. 2014, 72, 377–389. [Google Scholar] [CrossRef]
- Maglic, D.; Petcharat, L.; Chaiyasap, P.; Bishop, K.; Sood, R.; Zerfas, P.; Gahl, W.; Huizing, M.; Malicdan, M. Gne myopathy zebrafish models with impaired sialylation. Neurology 2015, 84, S34.001. Available online: https://n.neurology.org/content/84/14_Supplement/S34.001.short (accessed on 11 February 2023).
- van Karnebeek, C.D.; Bonafé, L.; Wen, X.Y.; Tarailo-Graovac, M.; Balzano, S.; Royer-Bertrand, B.; Ashikov, A.; Garavelli, L.; Mammi, I.; Turolla, L.; et al. NANS-mediated synthesis of sialic acid is required for brain and skeletal development. Nat. Genet. 2016, 48, 777–784. [Google Scholar] [CrossRef]
- Ranjan, A.K.; Rathore, R.S.; Deepak, D.; Khare, A.; Sahai, R.; Srivastava, V.M.L. Immunostimulant fractions of novel hexa and heptasaccharide from donkey’s milk. Asian, J. Org. Chem. 2016, 1, 55–60. [Google Scholar] [CrossRef]
- Tarr, A.J.; Galley, J.D.; Fisher, S.E.; Chichlowski, M.; Berg, B.M.; Bailey, M.T. The prebiotics 3′sialyllactose and 6′sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: Evidence for effects on the gut-brain axis. Brain Behav. Immun. 2015, 50, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Obelitz-Ryom, K.; Bering, S.B.; Overgaard, S.H.; Eskildsen, S.F.; Ringgaard, S.; Olesen, J.L.; Skovgaard, K.; Pankratova, S.; Wang, B.; Brunse, A.; et al. Bovine milk oligosaccharides with sialyllactose improves cognition in preterm pigs. Nutrients 2019, 11, 1335. [Google Scholar] [CrossRef]
- Oliveros, E.; Vázquez, E.; Barranco, A.; Ramírez, M.; Gruart, A.; Delgado-García, J.M.; Buck, R.; Rueda, R.; Martín, M.J. Sialic acid and sialylated oligosaccharide supplementation during lactation improves learning and memory in rats. Nutrients 2018, 10, 1519. [Google Scholar] [CrossRef] [PubMed]
- Hauser, J.; Pisa, E.; Arias Vásquez, A.; Tomasi, F.; Traversa, A.; Chiodi, V.; Martin, F.P.; Sprenger, N.; Lukjancenko, O.; Zollinger, A.; et al. Sialylated human milk oligosaccharides program cognitive development through a non-genomic transmission mode. Mol. Psychiatry 2021, 26, 2854–2871. [Google Scholar] [CrossRef] [PubMed]
- Panula, P.; Sallinen, V.; Sundvik, M.; Kolehmainen, J.; Torkko, V.; Tiittula, A.; Moshnyakov, M.; Podlasz, P. Modulatory neurotransmitter systems and behavior: Towards zebrafish models of neurodegenerative diseases. Zebrafish 2006, 3, 235–247. [Google Scholar] [CrossRef]
- Bandmann, O.; Burton, E.A. Genetic zebrafish models of neurodegenerative diseases. Neurobiol. Dis. 2010, 40, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Cheresiz, S.V.; Volgin, A.D.; Kokorina Evsyukova, A.; Bashirzade, A.A.O.; Demin, K.A.; de Abreu, M.S.; Amstislavskaya, T.G.; Kalueff, A.V. Understanding neurobehavioral genetics of zebrafish. J. Neurogenet. 2020, 34, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Licitra, R.; Marchese, M.; Brogi, L.; Fronte, B.; Pitto, L.; Santorelli, F.M. Nutraceutical screening in a zebrafish model of muscular dystrophy: Gingerol as a possible food aid. Nutrients 2021, 13, 998. [Google Scholar] [CrossRef] [PubMed]
- Brogi, L.; Marchese, M.; Cellerino, A.; Licitra, R.; Naef, V.; Mero, S.; Bibbiani, C.; Fronte, B. β-glucans as dietary supplement to improve locomotion and mitochondrial respiration in a model of Duchenne muscular dystrophy. Nutrients 2021, 13, 1619. [Google Scholar] [CrossRef]
- Ogi, A.; Licitra, R.; Naef, V.; Marchese, M.; Fronte, B.; Gazzano, A.; Santorelli, F.M. Social preference tests in zebrafish: A systematic review. Front. Vet. Sci. 2021, 7, 590057. [Google Scholar] [CrossRef]
- Licitra, R.; Marchese, M.; Naef, V.; Ogi, A.; Martinelli, M.; Kiferle, C.; Fronte, B.; Santorelli, F.M. A review on the bioactivity of cannabinoids on zebrafish models: Emphasis on neurodevelopment. Biomedicines 2022, 10, 1820. [Google Scholar] [CrossRef]
- Licitra, R.; Martinelli, M.; Petrocchi Jasinski, L.; Marchese, M.; Kiferle, C.; Fronte, B. In vivo evaluation of Cannabis sativa full extract on zebrafish larvae development, locomotion behavior and gene expression. Pharmaceuticals 2021, 14, 1224. [Google Scholar] [CrossRef]
- Demin, K.A.; Taranov, A.S.; Ilyin, N.P.; Lakstygal, A.M.; Volgin, A.D.; de Abreu, M.S.; Strekalova, T.; Kalueff, A.V. Understanding neurobehavioral effects of acute and chronic stress in zebrafish. Stress 2021, 24, 1–18. [Google Scholar] [CrossRef]
- Audira, G.; Sampurna, B.P.; Juniardi, S.; Liang, S.-T.; Lai, Y.-H.; Hsiao, C.D. A simple setup to perform 3D locomotion tracking in zebrafish by using a single camera. Inventions 2018, 3, 11. [Google Scholar] [CrossRef]
- Ogungbemi, A.; Leuthold, D.; Scholz, S.; Küster, E. Hypo- or hyperactivity of zebrafish embryos provoked by neuroactive substances: A review on how experimental parameters impact the predictability of behavior changes. Environ. Sci. Eur. 2019, 31, 88. [Google Scholar] [CrossRef]
- Tuz-Sasik, M.U.; Boije, H.; Manuel, R. Characterization of locomotor phenotypes in zebrafish larvae requires testing under both light and dark conditions. PLoS ONE 2022, 17, e0266491. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, M.; Jahan, M.; Ghorashi, S.A.; Wang, B. Current perspective of sialylated milk oligosaccharides in mammalian milk: Implications for brain and gut health of newborns. Foods 2021, 10, 473. [Google Scholar] [CrossRef] [PubMed]
- Saint-Amant, L.; Drapeau, P. Time course of the development of motor behaviors in the zebrafish embryo. J. Neurobiol. 1998, 37, 622–632. [Google Scholar] [CrossRef]
- de Oliveira, A.A.S.; Brigante, T.A.V.; Oliveira, D.P. Tail coiling assay in zebrafish (Danio rerio) embryos: Stage of development, promising positive control candidates, and selection of an appropriate organic solvent for screening of developmental neurotoxicity (DNT). Water 2021, 13, 119. [Google Scholar] [CrossRef]
- Janik, M.; Kleinhans, F.W.; Hagedorn, M. Overcoming a permeability barrier by microinjecting cryoprotectants into zebrafish embryos (Brachydanio rerio). Cryobiology 2000, 41, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Burton, C.E.; Zhou, Y.; Bai, Q.; Burton, E.A. Spectral properties of the zebrafish visual motor response. Neurosci. Lett. 2017, 646, 62–67. [Google Scholar] [CrossRef]
- Shao, E.; Bai, Q.; Zhou, Y.; Burton, E.A. Quantitative responses of adult zebrafish to changes in ambient illumination. Zebrafish 2017, 14, 508–516. [Google Scholar] [CrossRef]
- Ganzen, L.; Venkatraman, P.; Pang, C.P.; Leung, Y.F.; Zhang, M. Utilizing zebrafish visual behaviors in drug screening for retinal degeneration. Int. J. Mol. Sci. 2017, 18, 1185. [Google Scholar] [CrossRef]
- Basnet, R.M.; Zizioli, D.; Taweedet, S.; Finazzi, D.; Memo, M. Zebrafish larvae as a behavioral model in neuropharmacology. Biomedicines 2019, 7, 23. [Google Scholar] [CrossRef]
- Carty, D.R.; Thornton, C.; Gledhill, J.H.; Willett, K.L. Developmental effects of cannabidiol and Δ9-tetrahydrocannabinol in zebrafish. Toxicol. Sci. 2018, 162, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Kirla, K.T.; Groh, K.J.; Steuer, A.E.; Poetzsch, M.; Banote, R.K.; Stadnicka-Michalak, J.; Eggen, R.I.L.; Schirmer, K.; Kraemer, T. Zebrafish larvae are insensitive to stimulation by cocaine: Importance of exposure route and toxicokinetics. Toxicol. Sci. 2016, 154, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Ellis, L.D.; Berrue, F.; Morash, M.; Achenbach, J.C.; Hill, J.; McDougall, J.J. Comparison of cannabinoids with known analgesics using a novel high throughput zebrafish larval model of nociception. Behav. Brain Res. 2018, 337, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Schnörr, S.J.; Steenbergen, P.J.; Richardson, M.K.; Champagne, D.L. Measuring thigmotaxis in larval zebrafish. Behav. Brain Res. 2012, 228, 367–374. [Google Scholar] [CrossRef]
- Díaz-Villanueva, J.F.; Díaz-Molina, R.; García-González, V. Protein Folding and Mechanisms of Proteostasis. Int. J. Mol. Sci. 2015, 16, 17193–17230. [Google Scholar] [CrossRef]
- Murao, N.; Nishitoh, H. Role of the unfolded protein response in the development of central nervous system. J. Biochem. 2017, 162, 155–162. [Google Scholar] [CrossRef]
- Schmit, M.; Bielinsky, A.K. Congenital diseases of DNA replication: Clinical phenotypes and molecular mechanisms. Int. J. Mol. Sci. 2021, 22, 911. [Google Scholar] [CrossRef]
- Carvalhal, S.; Bader, I.; Rooimans, M.A.; Oostra, A.B.; Balk, J.A.; Feichtinger, R.G.; Beichler, C.; Speicher, M.R.; van Hagen, J.M.; Waisfisz, Q.; et al. Biallelic BUB1 mutations cause microcephaly, developmental delay, and variable effects on cohesion and chromosome segregation. Sci. Adv. 2022, 8, eabk0114. [Google Scholar] [CrossRef]
- Simmons, A.J.; Park, R.; Sterling, N.A.; Jang, M.H.; van Deursen, J.; Yen, T.J.; Cho, S.H.; Kim, S. Nearly complete deletion of BubR1 causes microcephaly through shortened mitosis and massive cell death. Hum. Mol. Genet. 2019, 28, 1822–1836. [Google Scholar] [CrossRef]
- Damiani, D.; Goffinet, A.M.; Alberts, A.; Tissir, F. Lack of Diaph3 relaxes the spindle checkpoint causing the loss of neural progenitors. Nat. Commun. 2016, 7, 13509. [Google Scholar] [CrossRef] [PubMed]
- Knickmeyer, R.C.; Gouttard, S.; Kang, C.; Evans, D.; Wilber, K.; Smith, J.K.; Hamer, R.M.; Lin, W.; Gerig, G.; Gilmore, J.H. A structural MRI study of human brain development from birth to 2 years. J. Neurosci. 2008, 28, 12176–12182. [Google Scholar] [CrossRef] [PubMed]
- Alexander-Bloch, A.F.; Shou, H.; Liu, S.; Satterthwaite, T.D.; Glahn, D.C.; Shinohara, R.T.; Vandekar, S.N.; Raznahan, A. On testing for spatial correspondence between maps of human brain structure and function. NeuroImage 2018, 178, 540–551. [Google Scholar] [CrossRef]
- Ball, G.; Boardman, J.P.; Rueckert, D.; Aljabar, P.; Arichi, T.; Merchant, N.; Gousias, I.S.; Edwards, A.D.; Counsell, S.J. The effect of preterm birth on thalamic and cortical development. Cereb. Cortex 2012, 22, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Mech, A.M.; Merteroglu, M.; Sealy, I.M.; Teh, M.T.; White, R.J.; Havelange, W.; Brennan, C.H.; Busch-Nentwich, E.M. Behavioral and Gene Regulatory Responses to Developmental Drug Exposures in Zebrafish. Front. Psychiatry 2022, 12, 795175. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio Rerio), 4th ed.; University of Oregon Press: Eugene, OR, USA, 2000; Available online: https://zfin.org/zf_info/zfbook/zfbk.html (accessed on 21 December 2021).
- Li, J.; Bi, Y.; Zheng, Y.; Cao, C.; Yu, L.; Yang, Z.; Chai, W.; Yan, J.; Lai, J.; Liang, X. Development of high-throughput UPLC-MS/MS using multiple reaction monitoring for quantitation of complex human milk oligosaccharides and application to large population survey of secretor status and Lewis blood group. Food Chem. 2022, 397, 133750. [Google Scholar] [CrossRef]
- Schubert, S.; Keddig, N.; Hanel, R.; Kammann, U. Microinjection into zebrafish embryos (Danio rerio)—A useful tool in aquatic toxicity testing? Environ. Sci. Eur. 2014, 26, 32. [Google Scholar] [CrossRef]
- Iacomino, M.; Doliana, R.; Marchese, M.; Capuano, A.; Striano, P.; Spessotto, P.; Bosisio, G.; Iodice, R.; Manganelli, F.; Lanteri, P.; et al. Distal motor neuropathy associated with novel EMILIN1 mutation. Neurobiol. Dis. 2020, 137, 104757. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Licitra, R.; Naef, V.; Marchese, M.; Damiani, D.; Ogi, A.; Doccini, S.; Fronte, B.; Yan, J.; Santorelli, F.M. Short-Term Effects of Human versus Bovine Sialylated Milk Oligosaccharide Microinjection on Zebrafish Larvae Survival, Locomotor Behavior and Gene Expression. Int. J. Mol. Sci. 2023, 24, 5456. https://doi.org/10.3390/ijms24065456
Licitra R, Naef V, Marchese M, Damiani D, Ogi A, Doccini S, Fronte B, Yan J, Santorelli FM. Short-Term Effects of Human versus Bovine Sialylated Milk Oligosaccharide Microinjection on Zebrafish Larvae Survival, Locomotor Behavior and Gene Expression. International Journal of Molecular Sciences. 2023; 24(6):5456. https://doi.org/10.3390/ijms24065456
Chicago/Turabian StyleLicitra, Rosario, Valentina Naef, Maria Marchese, Devid Damiani, Asahi Ogi, Stefano Doccini, Baldassare Fronte, Jingyu Yan, and Filippo M. Santorelli. 2023. "Short-Term Effects of Human versus Bovine Sialylated Milk Oligosaccharide Microinjection on Zebrafish Larvae Survival, Locomotor Behavior and Gene Expression" International Journal of Molecular Sciences 24, no. 6: 5456. https://doi.org/10.3390/ijms24065456
APA StyleLicitra, R., Naef, V., Marchese, M., Damiani, D., Ogi, A., Doccini, S., Fronte, B., Yan, J., & Santorelli, F. M. (2023). Short-Term Effects of Human versus Bovine Sialylated Milk Oligosaccharide Microinjection on Zebrafish Larvae Survival, Locomotor Behavior and Gene Expression. International Journal of Molecular Sciences, 24(6), 5456. https://doi.org/10.3390/ijms24065456