Corneal Reconstruction with EGFP-Labelled Limbal Mesenchymal Stem Cells in a Rabbit Model of Limbal Stem Cell Deficiency
Abstract
1. Introduction
2. Results
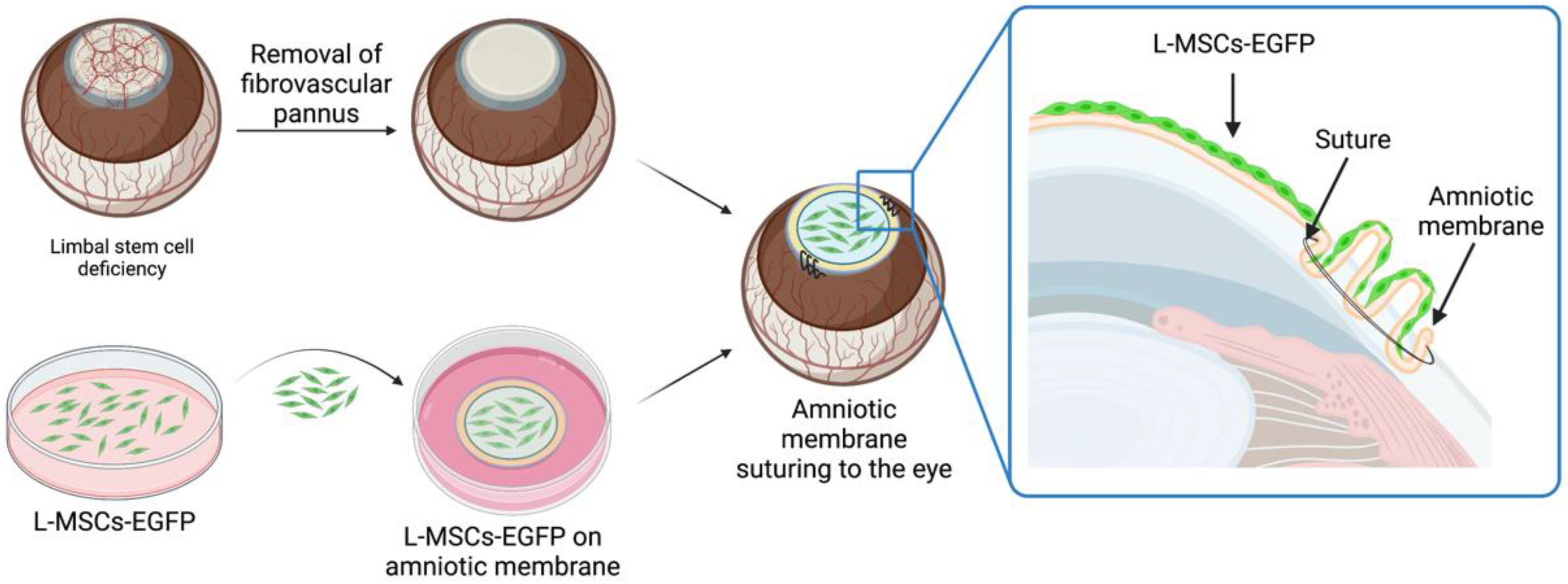
2.1. Corneal Reconstruction with L-MSCs-EGFP in the Rabbit Model of Total LSCD
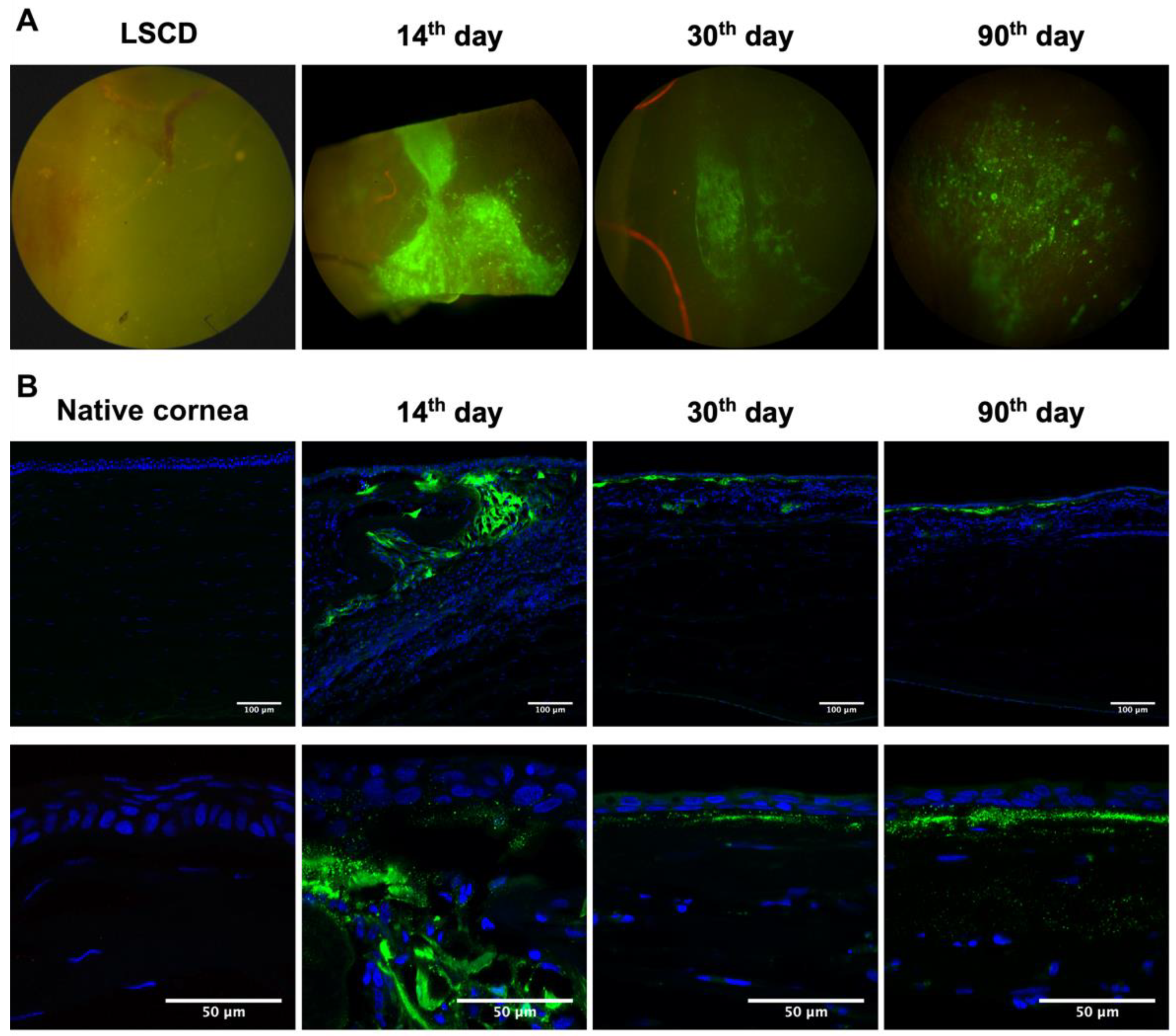
2.2. Epithelialization Process during Corneal Reconstruction
2.3. Vascularization and Inflammation Processes during Corneal Reconstruction
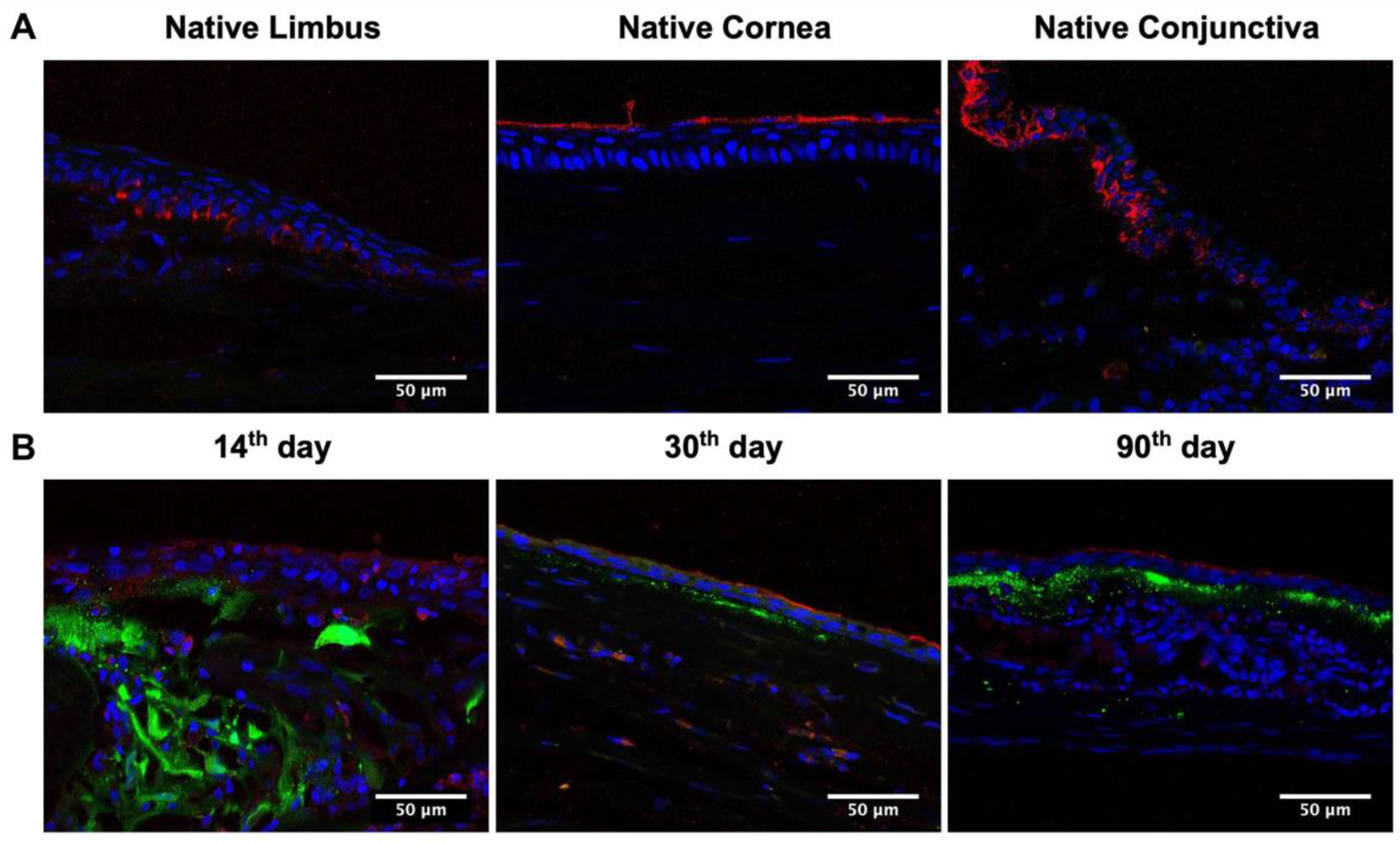
2.4. L-MSCs-EGFP Localization in Corneal Tissue after Transplantation
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Animals
4.3. Corneal Graft Preparation
4.4. Rabbit Limbal Stem Cell Deficiency Model and Graft Transplantation
4.5. Cornea Regeneration Assessment/Graft Transplantation Assessment
4.6. Histological Analysis
4.7. Analysis of Localization of EGFP-Labeled Cells within Rabbit Cornea
4.8. Immunohistochemistry
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Girolamo, N. Stem cells of the human cornea. Br. Med. Bull. 2011, 100, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Daniels, J.T.; Harris, A.R.; Mason, C. Corneal epithelial stem cells in health and disease. Stem Cell Rev. 2006, 2, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Figueira, E.C.; di Girolamo, N.; Coroneo, M.T.; Wakefield, D. The phenotype of limbal epithelial stem cells. Investig. Ophthalmol. Vis. Sci. 2007, 48, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Funderburgh, M.L.; Mann, M.M.; SundarRaj, N.; Funderburgh, J.L. Multipotent stem cells in human corneal stroma. Stem Cells 2005, 23, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Polisetty, N.; Fatima, A.; Madhira, S.L.; Sangwan, V.S.; Vemuganti, G.K. Mesenchymal cells from limbal stroma of human eye. Mol. Vis. 2008, 14, 431–442. [Google Scholar]
- Guo, P.; Sun, H.; Zhang, Y.; Tighe, S.; Chen, S.; Su, C.W.; Liu, Y.; Zhao, H.; Hu, M.; Zhu, Y. Limbal niche cells are a potent resource of adult mesenchymal progenitors. J. Cell. Mol. Med. 2018, 22, 3315–3322. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, K.; Sun, Y.; Gao, X.; Li, Y.; Chen, Z.; Wu, X. Reconstruction of Functional Ocular Surface by Acellular Porcine Cornea Matrix Scaffold and Limbal Stem Cells Derived from Human Embryonic Stem Cells. Tissue Eng. Part A 2013, 19, 2412–2425. [Google Scholar] [CrossRef]
- Deng, S.X.; Borderie, V.; Chan, C.C.; Dana, R.; Figueiredo, F.C.; Gomes, J.A.P.; Pellegrini, G.; Shimmura, S.; Kruse, F.E.; The International Limbal Stem Cell Deficiency Working Group. Global Consensus on Definition, Classification, Diagnosis, and Staging of Limbal Stem Cell Deficiency. Cornea 2019, 38, 364–375. [Google Scholar] [CrossRef]
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef]
- Boulton, M.; Albon, J.; Grant, M.B. Stem Cells in the Eye, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780123983589. [Google Scholar]
- Shukla, S.; Shanbhag, S.S.; Tavakkoli, F.; Varma, S.; Singh, V.; Basu, S. Limbal Epithelial and Mesenchymal Stem Cell Therapy for Corneal Regeneration. Curr. Eye Res. 2020, 45, 265–277. [Google Scholar] [CrossRef]
- Nieto-Nicolau, N.; Martín-Antonio, B.; Müller-Sánchez, C.; Casaroli-Marano, R.P. In vitro potential of human mesenchymal stem cells for corneal epithelial regeneration. Regen. Med. 2020, 15, 1409–1426. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Peng, H.; Lavker, R.M. Emerging Therapeutic Strategies for Limbal Stem Cell Deficiency. J. Ophthalmol. 2018, 2018, 7894647. [Google Scholar] [CrossRef] [PubMed]
- Agorogiannis, G.I.; Alexaki, V.-I.; Castana, O.; Kymionis, G.D. Topical application of autologous adipose-derived mesenchymal stem cells (MSCs) for persistent sterile corneal epithelial defect. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 250, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.Á.P.; Monteiro, B.G.; Melo, G.B.; Smith, R.L.; da Silva, M.C.P.; Lizier, N.F.; Kerkis, A.; Cerruti, H.; Kerkis, I. Corneal reconstruction with tissue-engineered cell sheets composed of human immature dental pulp stem cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xu, Y.; Xiao, Z.; Yang, W.; Zhang, C.; Song, E.; Du, Y.; Li, L. Reconstruction of Chemically Burned Rat Corneal Surface by Bone Marrow-Derived Human Mesenchymal Stem Cells. Stem Cells 2005, 24, 315–321. [Google Scholar] [CrossRef]
- Reinshagen, H.; Auw-Haedrich, C.; Sorg, R.V.; Boehringer, D.; Eberwein, P.; Schwartzkopff, J.; Sundmacher, R.; Reinhard, T. Corneal surface reconstruction using adult mesenchymal stem cells in experimental limbal stem cell deficiency in rabbits. Acta Ophthalmol. 2011, 89, 741–748. [Google Scholar] [CrossRef]
- Holan, V.; Trosan, P.; Cejka, C.; Javorkova, E.; Zajicova, A.; Hermankova, B.; Chudickova, M.; Cejkova, J. A Comparative Study of the Therapeutic Potential of Mesenchymal Stem Cells and Limbal Epithelial Stem Cells for Ocular Surface Reconstruction. Stem Cells Transl. Med. 2015, 4, 1052–1063. [Google Scholar] [CrossRef]
- Katikireddy, K.R.; Dana, R.; Jurkunas, U.V. Differentiation potential of limbal fibroblasts and bone marrow mesenchymal stem cells to corneal epithelial cells. Stem Cells 2014, 32, 717–729. [Google Scholar] [CrossRef]
- Funderburgh, M.L.; Du, Y.; Mann, M.M.; SundarRaj, N.; Funderburgh, J.L. PAX6 expression identifies progenitor cells for corneal keratocytes. FASEB J. 2005, 19, 1371–1373. [Google Scholar] [CrossRef]
- Shaharuddin, B.; Osei-Bempong, C.; Ahmad, S.; Rooney, P.; Ali, S.; Oldershaw, R.; Meeson, A. Human limbal mesenchymal stem cells express ABCB5 and can grow on amniotic membrane. Regen. Med. 2016, 11, 273–286. [Google Scholar] [CrossRef]
- Khorolskaya, J.I.; Perepletchikova, D.A.; Kachkin, D.V.; Zhurenkov, K.E.; Alexander-sinkler, E.I.; Ivanova, J.S.; Mikhailova, N.A.; Blinova, M.I. Derivation and Characterization of EGFP-Labeled Rabbit Limbal Mesenchymal Stem Cells and Their Potential for Research in Regenerative Ophthalmology. Biomedicines 2021, 9, 1134. [Google Scholar] [CrossRef] [PubMed]
- Dravida, S.; Pal, R.; Khanna, A.; Tipnis, S.P.; Ravindran, G.; Khan, F. The transdifferentiation potential of limbal fibroblast-like cells. Dev. Brain Res. 2005, 160, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Bray, L.J.; Heazlewood, C.F.; Munster, D.J.; Hutmacher, D.W.; Atkinson, K.; Harkin, D.G. Immunosuppressive properties of mesenchymal stromal cell cultures derived from the limbus of human and rabbit corneas. Cytotherapy 2014, 16, 64–73. [Google Scholar] [CrossRef]
- Hamrah, P.; Sahin, A. Limbus and corneal epithelium. In Ocular Surface Disease: Cornea, Conjunctiva and Tear Film; Elsevier: Amsterdam, The Netherlands, 2013; pp. 29–33. ISBN 9781455728763. [Google Scholar]
- Jiang, T.-S.; Cai, L.; Ji, W.-Y.; Hui, Y.-N.; Wang, Y.-S.; Hu, D.; Zhu, J. Reconstruction of the corneal epithelium with induced marrow mesenchymal stem cells in rats. Mol. Vis. 2010, 16, 1304–1316. [Google Scholar]
- Nieto-Miguel, T.; Galindo, S.; Reinoso, R.; Corell, A.; Martino, M.; Pérez-Simón, J.A.; Calonge, M. In vitro simulation of corneal epithelium microenvironment induces a corneal epithelial-like cell phenotype from human adipose tissue mesenchymal stem cells. Curr. Eye Res. 2013, 38, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Abarca, L.I.; Hernández-Galilea, E.; Lorenzo, R.; Herrero, C.; Velasco, A.; Carrancio, S.; Caballero-Velázquez, T.; Rodríguez-Barbosa, J.I.; Parrilla, M.; Cañizo, C.D.; et al. Human Bone Marrow Stromal Cells Differentiate Into Corneal Tissue and Prevent Ocular Graft-Versus-Host Disease in Mice. Cell Transplant. 2015, 24, 2423–2433. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, B.; Shenoy, S.J.; Mohan, S.; Anil Kumar, P.R.; Kumary, T.V. Bioengineered corneal epithelial cell sheet from mesenchymal stem cells—A functional alternative to limbal stem cells for ocular surface reconstruction. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1033–1045. [Google Scholar] [CrossRef]
- Calonge, M.; Pérez, I.; Galindo, S.; Nieto-Miguel, T.; López-Paniagua, M.; Fernández, I.; Alberca, M.; García-Sancho, J.; Sánchez, A.; Herreras, J.M. A proof-of-concept clinical trial using mesenchymal stem cells for the treatment of corneal epithelial stem cell deficiency. Transl. Res. 2019, 206, 18–40. [Google Scholar] [CrossRef]
- Piñero, G.; Usach, V.; Soto, P.A.; Monje, P.V.; Setton-Avruj, P. EGFP transgene: A useful tool to track transplanted bone marrow mononuclear cell contribution to peripheral remyelination. Transgenic Res. 2018, 27, 135–153. [Google Scholar] [CrossRef]
- Arnhold, S.; Absenger, Y.; Klein, H.; Addicks, K.; Schraermeyer, U. Transplantation of bone marrow-derived mesenchymal stem cells rescue photoreceptor cells in the dystrophic retina of the rhodopsin knockout mouse. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 245, 414–422. [Google Scholar] [CrossRef]
- Nakamura, T.; Inatomi, T.; Sotozono, C.; Koizumi, N.; Kinoshita, S. Ocular surface reconstruction using stem cell and tissue engineering. Prog. Retin. Eye Res. 2016, 51, 187–207. [Google Scholar] [CrossRef]
- Malhotra, C.; Jain, A.K. Human amniotic membrane transplantation: Different modalities of its use in ophthalmology. World J. Transplant. 2014, 4, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Guérin, L.P.; Le-Bel, G.; Desjardins, P.; Couture, C.; Gillard, E.; Boisselier, É.; Bazin, R.; Germain, L.; Guérin, S.L. The Human Tissue-Engineered Cornea (hTEC): Recent Progress. Int. J. Mol. Sci. 2021, 22, 1291. [Google Scholar] [CrossRef]
- Zhurenkov, K.E.; Alexander-Sinkler, E.I.; Gavrilyik, I.O.; Yartseva, N.M.; Aleksandrova, S.A.; Mashel, T.V.; Khorolskaya, J.I.; Blinova, M.I.; Kulikov, A.N.; Churashov, S.V.; et al. Labial Mucosa Stem Cells: Isolation, Characterization, and Their Potential for Corneal Epithelial Reconstruction. Investig. Opthalmol. Vis. Sci. 2022, 63, 16. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Shimmura, S.; Kawakita, T.; Miyashita, H.; Den, S.; Shimazaki, J.; Tsubota, K. Cytokeratin 15 Can Be Used to Identify the Limbal Phenotype in Normal and Diseased Ocular Surfaces. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4780–4786. [Google Scholar] [CrossRef]
- Merjava, S.; Neuwirth, A.; Tanzerova, M.; Jirsova, K. The spectrum of cytokeratins expressed in the adult human cornea, limbus and perilimbal conjunctiva. Histol. Histopathol. 2011, 26, 323–331. [Google Scholar] [CrossRef]
- Di Girolamo, N.; Park, M. Cell identity changes in ocular surface Epithelia. Prog. Retin. Eye Res. 2022, 101148. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, F.; Goh, T.W.; Setiawan, M.; Yam, G.H.F.; Mehta, J.S. Cellular therapy of corneal epithelial defect by adipose mesenchymal stem cell-derived epithelial progenitors. Stem Cell Res. Ther. 2020, 11, 14. [Google Scholar] [CrossRef]
- Walkden, A. Amniotic Membrane Transplantation in Ophthalmology: An Updated Perspective. Clin. Ophthalmol. 2020, 14, 2057–2072. [Google Scholar] [CrossRef]
- Holland, E.J.; Mogilishetty, G.; Skeens, H.M.; Hair, D.B.; Neff, K.D.; Biber, J.M.; Chan, C.C. Systemic immunosuppression in ocular surface stem cell transplantation: Results of a 10-year experience. Cornea 2012, 31, 655–661. [Google Scholar] [CrossRef]
- Aleksandrova, O.I.; Okolov, I.N.; Khorolskaya, Y.I.; Panova, I.E.; Blinova, M.I. Influence of non-steroidal anti-inflammatory eye drops on the epithelium cells of the cornea and conjunctiva in vitro. Oftalmologiya 2017, 15, 251–259. [Google Scholar] [CrossRef]
- Aleksandrova, O.I.; Okolov, I.N.; Khorolskaya, J.I.; Mikhailova, N.A.; Darvish, D.M.; Blinova, M.I. In vitro cytotoxicity screening as a criterion for the rational selection of tear substitutes. In Cytotoxicity—Definition, Identification, and Cytotoxic Compounds; IntechOpen: London, UK, 2019; pp. 1–12. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khorolskaya, J.I.; Perepletchikova, D.A.; Zhurenkov, K.E.; Kachkin, D.V.; Rubel, A.A.; Blinova, M.I.; Mikhailova, N.A. Corneal Reconstruction with EGFP-Labelled Limbal Mesenchymal Stem Cells in a Rabbit Model of Limbal Stem Cell Deficiency. Int. J. Mol. Sci. 2023, 24, 5431. https://doi.org/10.3390/ijms24065431
Khorolskaya JI, Perepletchikova DA, Zhurenkov KE, Kachkin DV, Rubel AA, Blinova MI, Mikhailova NA. Corneal Reconstruction with EGFP-Labelled Limbal Mesenchymal Stem Cells in a Rabbit Model of Limbal Stem Cell Deficiency. International Journal of Molecular Sciences. 2023; 24(6):5431. https://doi.org/10.3390/ijms24065431
Chicago/Turabian StyleKhorolskaya, Julia I., Daria A. Perepletchikova, Kirill E. Zhurenkov, Daniel V. Kachkin, Aleksandr A. Rubel, Miralda I. Blinova, and Natalia A. Mikhailova. 2023. "Corneal Reconstruction with EGFP-Labelled Limbal Mesenchymal Stem Cells in a Rabbit Model of Limbal Stem Cell Deficiency" International Journal of Molecular Sciences 24, no. 6: 5431. https://doi.org/10.3390/ijms24065431
APA StyleKhorolskaya, J. I., Perepletchikova, D. A., Zhurenkov, K. E., Kachkin, D. V., Rubel, A. A., Blinova, M. I., & Mikhailova, N. A. (2023). Corneal Reconstruction with EGFP-Labelled Limbal Mesenchymal Stem Cells in a Rabbit Model of Limbal Stem Cell Deficiency. International Journal of Molecular Sciences, 24(6), 5431. https://doi.org/10.3390/ijms24065431








