Short-Term Decreasing and Increasing Dietary BCAA Have Similar, but Not Identical Effects on Lipid and Glucose Metabolism in Lean Mice
Abstract
1. Introduction
2. Results
2.1. Different Dietary BCAA Levels Influenced the Ratio of Muscle and WAT in Lean Mice
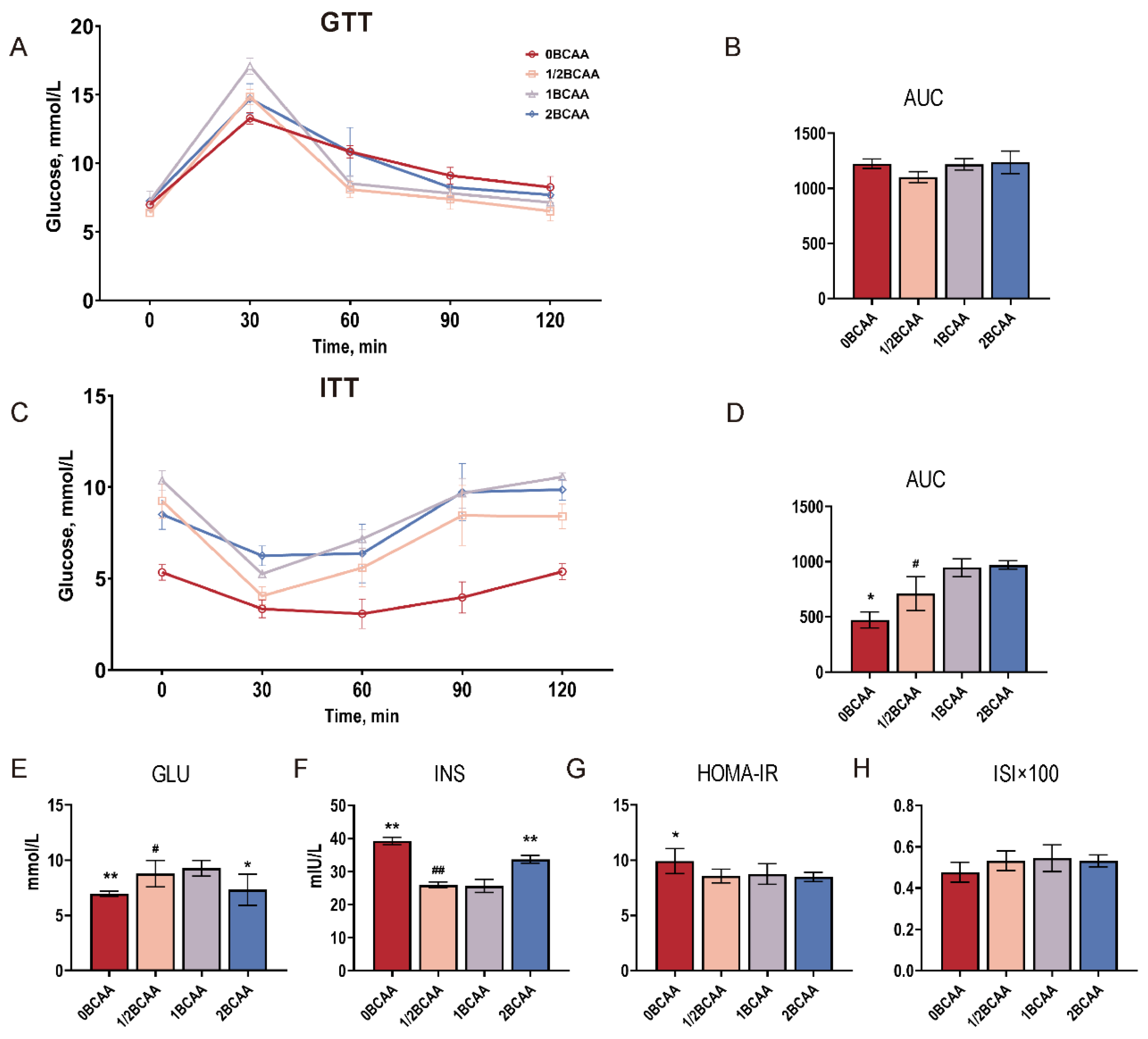
2.2. Complete Deprivation of Dietary BCAA Impaired Glucose Homeostasis
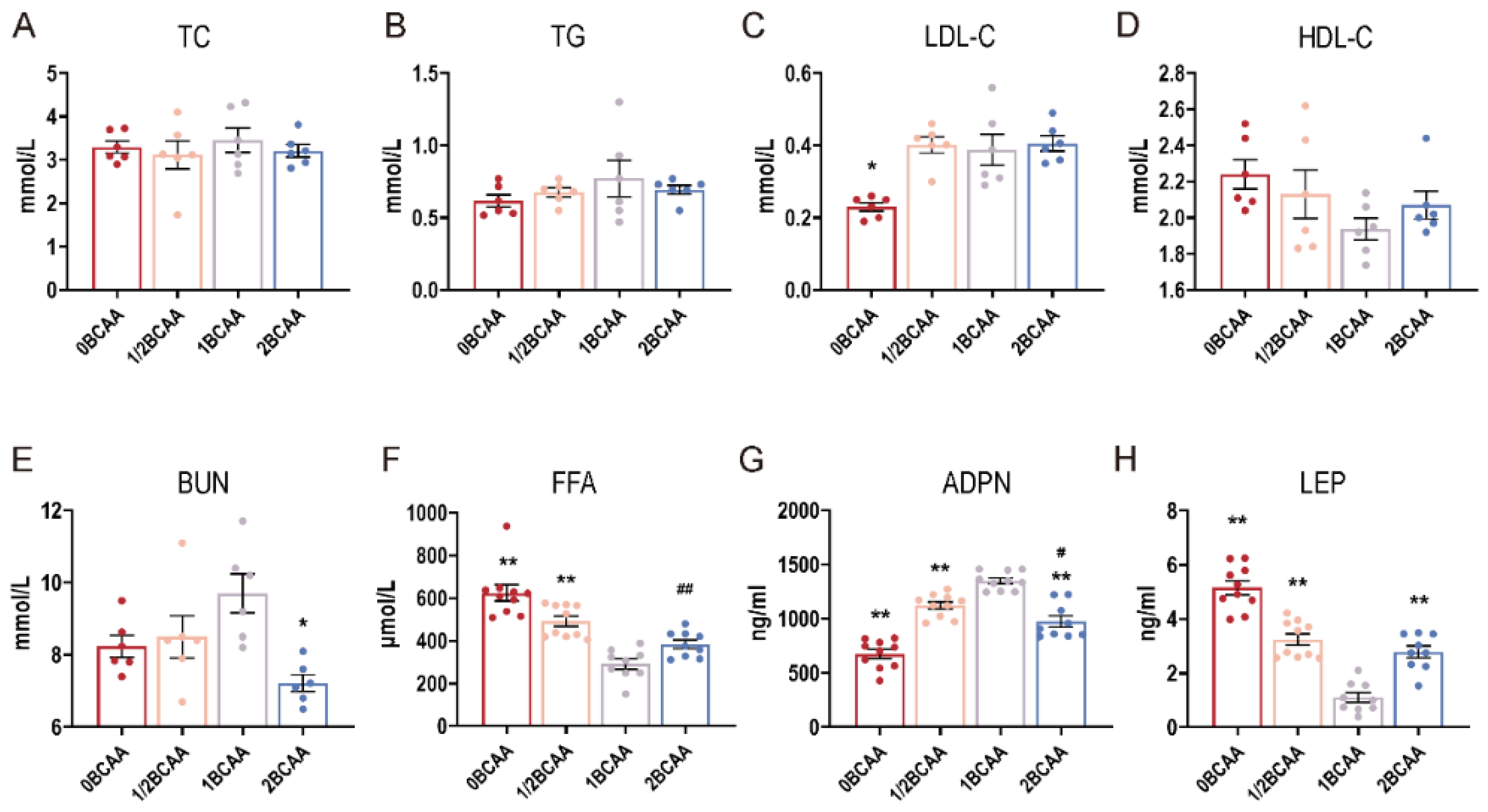
2.3. Different Dietary BCAA Levels Altered the Serum Indexes in Lean Mice
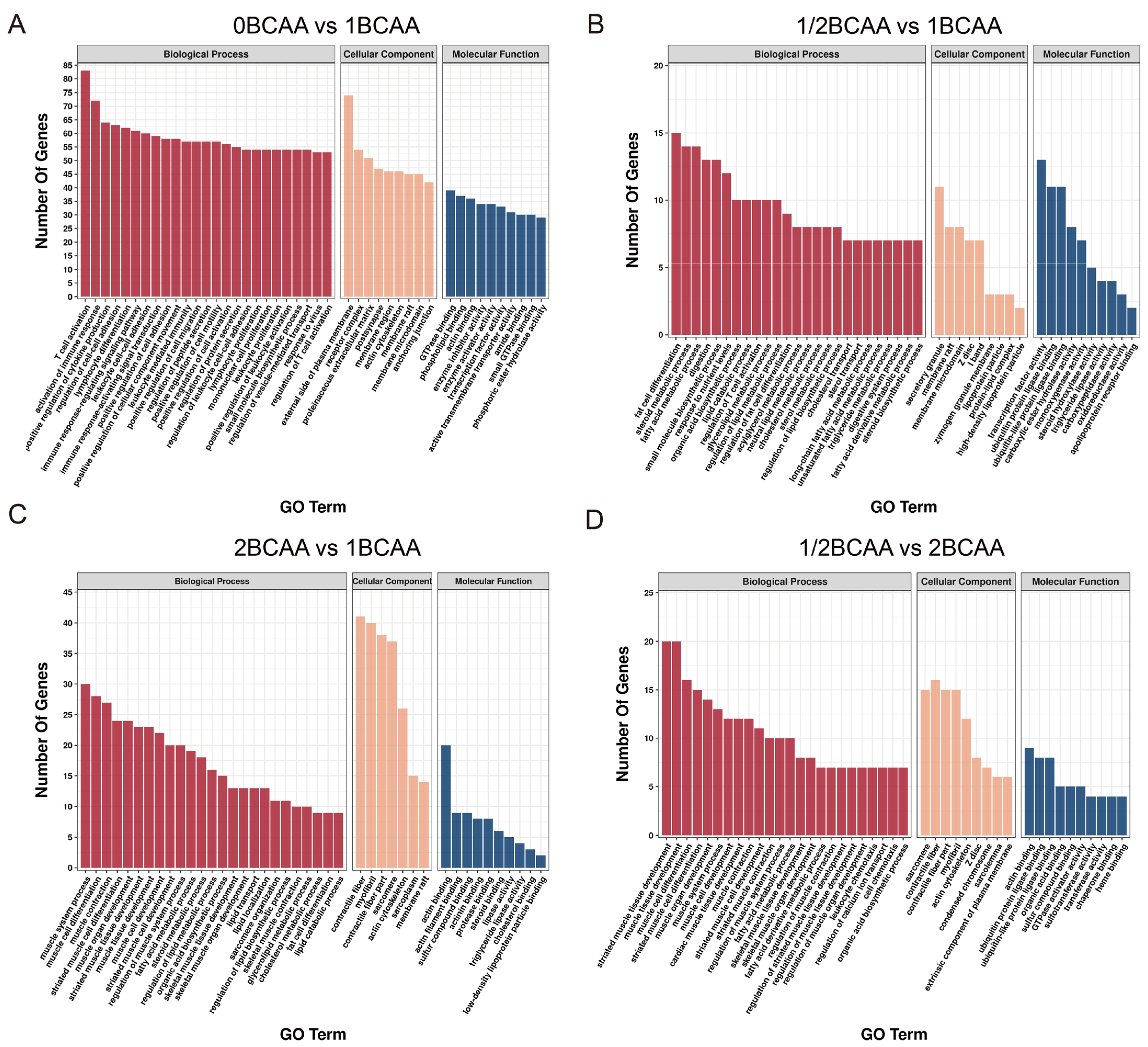
2.4. Different Dietary BCAA Levels Have Different Effects on Metabolic Genes
3. Discussion
4. Materials and Methods
4.1. Animals and Diets
4.2. Glucose Tolerance Test (GTT) and Insulin Tolerance Test (ITT)
4.3. Sample Collection
4.4. Histological Analysis
4.5. Serum Parameter Determination
4.6. Transcriptomic Analysis and Data Processing
4.7. Quantitative Real-Time PCR Analysis
4.8. Western Blot Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miyamoto, J.; Igarashi, M.; Watanabe, K.; Karaki, S.-I.; Mukouyama, H.; Kishino, S.; Li, X.; Ichimura, A.; Irie, J.; Sugimoto, Y.; et al. Gut microbiota confers host resistance to obesity by metabolizing dietary polyunsaturated fatty acids. Nat. Commun. 2019, 10, 4007. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Functional amino acids in nutrition and health. Amino Acids 2013, 45, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhou, X.; Sun, Y.; Hu, L.; Zhu, J.; Shao, C.; Meng, Q.; Shan, A. Threonine, but Not Lysine and Methionine, Reduces Fat Accumulation by Regulating Lipid Metabolism in Obese Mice. J. Agric. Food Chem. 2020, 68, 4876–4883. [Google Scholar] [CrossRef]
- Ma, Q.; Chen, J.; Zhou, X.; Hu, L.; Sun, Y.; Wang, Z.; Yue, Z.; Shan, A. Dietary supplementation with aromatic amino acids decreased triglycerides and alleviated hepatic steatosis by stimulating bile acid synthesis in mice. Food Funct. 2020, 12, 267–277. [Google Scholar] [CrossRef]
- McCormack, S.E.; Shaham, O.; McCarthy, M.A.; Deik, A.A.; Wang, T.J.; Gerszten, R.E.; Clish, C.B.; Mootha, V.K.; Grinspoon, S.K.; Fleischman, A. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr. Obes. 2012, 8, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, M.; Guénard, F.; Garneau, V.; Allam-Ndoul, B.; Lemieux, S.; Pérusse, L.; Vohl, M.-C. Associations Between Dietary Protein Sources, Plasma BCAA and Short-Chain Acylcarnitine Levels in Adults. Nutrients 2019, 11, 173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, K.; LeBlanc, R.E.; Loh, D.; Schwartz, G.J.; Yu, Y.-H. Increasing Dietary Leucine Intake Reduces Diet-Induced Obesity and Improves Glucose and Cholesterol Metabolism in Mice via Multimechanisms. Diabetes 2007, 56, 1647–1654. [Google Scholar] [CrossRef]
- Eller, L.K.; Saha, D.C.; Shearer, J.; Reimer, R.A. Dietary leucine improves whole-body insulin sensitivity independent of body fat in diet-induced obese Sprague–Dawley rats. J. Nutr. Biochem. 2013, 24, 1285–1294. [Google Scholar] [CrossRef]
- Ma, Q.; Zhou, X.; Hu, L.; Chen, J.; Zhu, J.; Shan, A. Leucine and isoleucine have similar effects on reducing lipid accumulation, improving insulin sensitivity and increasing the browning of WAT in high-fat diet-induced obese mice. Food Funct. 2020, 11, 2279–2290. [Google Scholar] [CrossRef]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.; Shah, S.; Arlotto, M.; Slentz, C.; et al. A Branchedchain Amino Acid-Related Metabolic Signature that Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab. 2009, 9, 565–566. [Google Scholar] [CrossRef]
- Fontana, L.; Cummings, N.E.; Apelo, S.I.A.; Neuman, J.C.; Kasza, I.; Schmidt, B.A.; Cava, E.; Spelta, F.; Tosti, V.; Syed, F.A.; et al. Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health. Cell Rep. 2016, 16, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Richardson, N.E.; Green, C.L.; Spicer, A.B.; Murphy, M.E.; Flores, V.; Jang, C.; Kasza, I.; Nikodemova, M.; Wakai, M.H.; et al. The Adverse Metabolic Effects of Branched-Chain Amino Acids Are Mediated by Isoleucine and Valine. Cell Metab. 2021, 33, 905–922.e6. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, J.; Sun, B.; Wang, Z.; Zhu, J.; Yue, Z.; Zhang, Y.; Shan, A.; Ma, Q.; Wang, J. Leucine, but not isoleucine or valine, affects serum lipid profiles and browning of WAT in mice. Food Funct. 2021, 12, 6712–6724. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Zhao, F.; Liu, W.; Lv, R.; Khine, W.W.T.; Han, J.; Sun, Z.; Lee, Y.-K.; Zhang, H. Probiotic-directed modulation of gut microbiota is basal microbiome dependent. Gut Microbes 2020, 12, 1736974. [Google Scholar] [CrossRef]
- Nishimura, J.; Masaki, T.; Arakawa, M.; Seike, M.; Yoshimatsu, H. Isoleucine Prevents the Accumulation of Tissue Triglycerides and Upregulates the Expression of Pparalpha and Uncoupling Protein in Diet-Induced Obese Mice. J. Nutr. 2010, 140, 496–500. [Google Scholar] [CrossRef]
- Kamei, Y.; Hatazawa, Y.; Uchitomi, R.; Yoshimura, R.; Miura, S. Regulation of Skeletal Muscle Function by Amino Acids. Nutrients 2020, 12, 261. [Google Scholar] [CrossRef]
- O’Rielly, R.; Li, H.; Lim, S.M.; Yazbeck, R.; Kritas, S.; Ullrich, S.S.; Feinle-Bisset, C.; Heilbronn, L.; Page, A.J. The Effect of Isoleucine Supplementation on Body Weight Gain and Blood Glucose Response in Lean and Obese Mice. Nutrients 2020, 12, 2446. [Google Scholar] [CrossRef]
- Binder, E.; Bermúdez-Silva, F.J.; André, C.; Elie, M.; Romero-Zerbo, S.Y.; Leste-Lasserre, T.; Belluomo, L.; Duchampt, A.; Clark, S.; Aubert, A.; et al. Leucine Supplementation Protects from Insulin Resistance by Regulating Adiposity Levels. PLoS ONE 2013, 8, e74705. [Google Scholar] [CrossRef]
- Magne, H.; Savary-Auzeloux, I.; Rémond, D.; Dardevet, D. Nutritional strategies to counteract muscle atrophy caused by disuse and to improve recovery. Nutr. Res. Rev. 2013, 26, 149–165. [Google Scholar] [CrossRef]
- Buettner, C.; Muse, E.D.; Cheng, A.; Chen, L.; Scherer, T.; Pocai, A.; Su, K.; Cheng, B.; Li, X.; Harvey-White, J.; et al. Leptin Controls Adipose Tissue Lipogenesis Via Central, Stat3-Independent Mechanisms. Nat. Med. 2008, 14, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Noguchi, K.; Shigemura, N.; Jyotaki, M.; Takahashi, I.; Margolskee, R.F.; Ninomiya, Y. Leptin Suppresses Mouse Taste Cell Responses to Sweet Compounds. Diabetes 2015, 64, 3751–3762. [Google Scholar] [CrossRef]
- Yoneshiro, T.; Wang, Q.; Tajima, K.; Matsushita, M.; Maki, H.; Igarashi, K.; Dai, Z.; White, P.J.; McGarrah, R.W.; Ilkayeva, O.R.; et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 2019, 572, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Maida, A.; Chan, J.S.; Sjøberg, K.A.; Zota, A.; Schmoll, D.; Kiens, B.; Herzig, S.; Rose, A.J. Repletion of branched chain amino acids reverses mTORC1 signaling but not improved metabolism during dietary protein dilution. Mol. Metab. 2017, 6, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Vijayakumar, A.; White, P.J.; Xu, Y.; Ilkayeva, O.; Lynch, C.J.; Newgard, C.B.; Kahn, B.B. BCAA Supplementation in Mice with Diet-induced Obesity Alters the Metabolome without Impairing Glucose Homeostasis. Endocrinology 2021, 162, bqab062. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite Profiles and the Risk of Developing Diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.; Sandusky, G.; Hammond, L.; Moyers, J.; Owens, R.; et al. Fgf-21 as a Novel Metabolic Regulator. J. Clin. Investig. 2005, 115, 1627–1635. [Google Scholar] [CrossRef]
- Li, H.; Wu, G.; Fang, Q.; Zhang, M.; Hui, X.; Sheng, B.; Wu, L.; Bao, Y.; Li, P.; Xu, A.; et al. Fibroblast growth factor 21 increases insulin sensitivity through specific expansion of subcutaneous fat. Nat. Commun. 2018, 9, 272. [Google Scholar] [CrossRef]
- Barb, D.; Bril, F.; Kalavalapalli, S.; Cusi, K. Plasma Fibroblast Growth Factor 21 Is Associated with Severity of Nonalcoholic Steatohepatitis in Patients with Obesity and Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 3327–3336. [Google Scholar] [CrossRef]
- Veniant, M.M.; Hale, C.; Helmering, J.; Chen, M.M.; Stanislaus, S.; Busby, J.; Vonderfecht, S.; Xu, J.; Lloyd, D.J. FGF21 Promotes Metabolic Homeostasis via White Adipose and Leptin in Mice. PLoS ONE 2012, 7, e40164. [Google Scholar] [CrossRef]
- Yan, T.; Luo, Y.; Yan, N.; Hamada, K.; Zhao, N.; Xia, Y.; Wang, P.; Zhao, C.; Qi, D.; Yang, S. Intestinal Peroxisome Proliferator-Activated Receptor A—Fatty Acid-Binding Protein 1 Axis Modulates Nonalcoholic Steatohepatitis. Hepatology 2022, 77, 239–255. [Google Scholar] [CrossRef]
- Ali, F.; Ismail, A.; Esa, N.M.; Pei, C.P.; Kersten, S. Hepatic genome-wide expression of lipid metabolism in diet-induced obesity rats treated with cocoa polyphenols. J. Funct. Foods 2015, 17, 969–978. [Google Scholar] [CrossRef]
- Flannery, C.A.; Choe, G.H.; Cooke, K.M.; Fleming, A.G.; Radford, C.C.; Kodaman, P.H.; Jurczak, M.J.; Kibbey, R.G.; Taylor, H.S. Insulin Regulates Glycogen Synthesis in Human Endometrial Glands through Increased GYS2. J. Clin. Endocrinol. Metab. 2018, 103, 2843–2850. [Google Scholar] [CrossRef] [PubMed]
- Bramer, S.A.; Macedo, A.; Klein, C. Hexokinase 2 drives glycogen accumulation in equine endometrium at day 12 of diestrus and pregnancy. Reprod. Biol. Endocrinol. 2017, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Migocka-Patrzałek, M.; Lewicka, A.; Elias, M.; Daczewska, M. The effect of muscle glycogen phosphorylase (Pygm) knockdown on zebrafish morphology. Int. J. Biochem. Cell Biol. 2019, 118, 105658. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Patankar, J.V.; de Haan, W.; Ruddle, P.; Wijesekara, N.; Groen, A.K.; Verchere, C.B.; Singaraja, R.R.; Hayden, M.R. Loss of Cyp8b1 Improves Glucose Homeostasis by Increasing GLP-1. Diabetes 2014, 64, 1168–1179. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Bian, Y.; Luo, J.; Lu, M.; Xiong, Y.; Guo, S.-Y.; Yin, H.-Y.; Lin, X.; Li, Q.; Chang, C.C.Y.; et al. Cholesterol and fatty acids regulate cysteine ubiquitylation of ACAT2 through competitive oxidation. Nature 2017, 19, 808–819. [Google Scholar] [CrossRef]


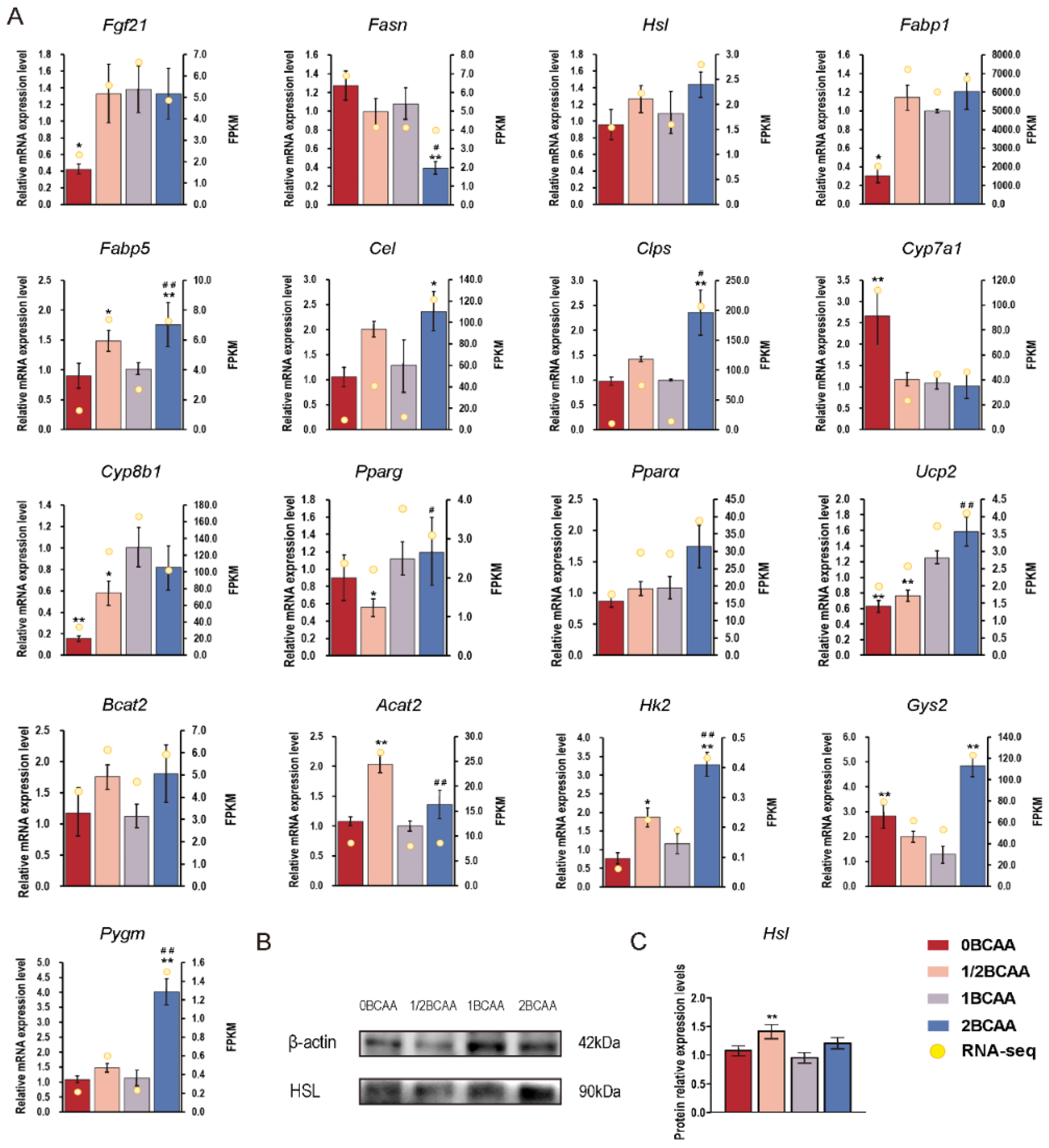
| ID | log2FoldChange | p-Value | Direction | Gene Name |
|---|---|---|---|---|
| 0 BCAA vs. 1 BCAA | ||||
| ENSMUSG00000030827 | −1.4493 | 0.002 | Down | Fgf21 |
| ENSMUSG00000054422 | −1.5147 | 0.026 | Down | Fabp1 |
| ENSMUSG00000027533 | −0.9817 | 0.011 | Down | Fabp5 |
| ENSMUSG00000028240 | 1.4359 | 0.019 | Up | Cyp7a1 |
| ENSMUSG00000050445 | −2.2162 | <0.001 | Down | Cyp8b1 |
| ENSMUSG00000000440 | −0.5740 | 0.030 | Down | Pparg |
| ENSMUSG00000022383 | −0.6585 | 0.070 | Down | Pparα |
| ENSMUSG00000033685 | −0.8187 | 0.008 | Down | Ucp2 |
| ENSMUSG00000000628 | −1.5350 | 0.014 | Down | Hk2 |
| 0.5 BCAA vs. 1 BCAA | ||||
| ENSMUSG00000027533 | 1.5888 | 0.003 | Up | Fabp5 |
| ENSMUSG00000003123 | 0.5969 | 0.006 | Up | Hsl |
| ENSMUSG00000028240 | −0.8180 | <0.001 | Down | Cyp7a1 |
| ENSMUSG00000000440 | −0.6348 | 0.011 | Down | Pparg |
| ENSMUSG00000033685 | −0.4057 | 0.052 | Down | Ucp2 |
| ENSMUSG00000024225 | 11.4522 | 0.003 | Up | Clps |
| ENSMUSG00000026818 | 12.3784 | 0.002 | Up | Cel |
| ENSMUSG00000032648 | 1.4813 | 0.031 | Up | Pygm |
| ENSMUSG00000023832 | 1.8763 | 0.005 | Up | Acat2 |
| ENSMUSG00000030826 | 0.5064 | 0.008 | Up | Bcat2 |
| 2 BCAA vs. 1 BCAA | ||||
| ENSMUSG00000027533 | 1.4697 | 0.026 | Up | Fabp5 |
| ENSMUSG00000003123 | 0.8616 | <0.001 | Up | Hsl |
| ENSMUSG00000024225 | 26.1467 | <0.001 | Up | Clps |
| ENSMUSG00000050445 | −0.6652 | 0.031 | Down | Cyp8b1 |
| ENSMUSG00000022383 | 0.4712 | 0.062 | Up | Pparα |
| ENSMUSG00000026818 | 27.1306 | <0.001 | Up | Cel |
| ENSMUSG00000032648 | 2.6947 | <0.001 | Up | Pygm |
| ENSMUSG00000030244 | 1.3039 | 0.010 | Up | Gys2 |
| ENSMUSG00000000628 | 1.2138 | 0.030 | Up | Hk2 |
| ENSMUSG00000030826 | 0.3879 | 0.063 | Up | Bcat2 |
| 0.5 BCAA vs. 2 BCAA | ||||
| ENSMUSG00000030244 | −0.9722 | 0.087 | Down | Gys2 |
| ENSMUSG00000023832 | 1.7224 | 0.010 | Up | Acat2 |
| ENSMUSG00000033685 | −0.6000 | 0.020 | Down | Ucp2 |
| ENSMUSG00000028240 | −0.9493 | 0.052 | Down | Cyp7a1 |
| Amino Acid Defined Diets | 0BCAA | 1/2BCAA | 1BCAA | 2BCAA |
|---|---|---|---|---|
| Ingredient composition, g/kg as fed | ||||
| L-Alanine | 5.7 | 5.1 | 4.6 | 3.5 |
| L-Arginine HCl | 9.5 | 8.6 | 7.7 | 5.9 |
| L-Aspartic acid | 15.0 | 13.6 | 12.2 | 9.4 |
| L-Cystine | 4.6 | 4.1 | 3.7 | 2.8 |
| L-Glutamic acid | 44.7 | 40.5 | 36.3 | 27.9 |
| Glycine | 3.9 | 3.6 | 3.2 | 2.5 |
| L-Histidine HCl, monohydrate | 5.7 | 5.1 | 4.6 | 3.5 |
| L-Lysine HCl | 16.0 | 14.5 | 13.0 | 10.0 |
| L-Methionine | 5.7 | 5.1 | 4.6 | 3.5 |
| L-Proline | 25.2 | 22.9 | 20.5 | 15.8 |
| L-Serine | 11.9 | 10.8 | 9.7 | 7.5 |
| L-Threonine | 8.2 | 7.5 | 6.7 | 5.2 |
| L-Tryptophan | 2.6 | 2.3 | 2.1 | 1.6 |
| L-Tyrosine | 11.5 | 10.4 | 9.3 | 7.1 |
| L-Phenylalanine | 10.8 | 9.8 | 8.8 | 6.8 |
| L-Isoleucine | - | 4.3 | 8.6 | 17.2 |
| L-Leucine | - | 7.7 | 15.4 | 30.8 |
| L-Valine | - | 5.0 | 10.0 | 20.0 |
| Sucrose | 304.5 | 304.5 | 304.5 | 304.5 |
| Corn Starch | 302 | 302 | 302 | 302 |
| Maltodextrin | 75 | 75 | 75 | 75 |
| Vitamin Mix | 10 | 10 | 10 | 10 |
| Mineral Mix, AIN-93 | 35 | 35 | 35 | 35 |
| Choline Bitartrate | 2.5 | 2.5 | 2.5 | 2.5 |
| Corn oil | 40 | 40 | 40 | 40 |
| Cellulose | 50 | 50 | 50 | 50 |
| % kcal from | ||||
| Protein | 18.8 | 18.8 | 18.8 | 18.8 |
| Carbohydrates | 71.8 | 71.8 | 71.8 | 71.8 |
| Fat | 9.4 | 9.4 | 9.4 | 9.4 |
| kcal/g | 3.8 | 3.8 | 3.8 | 3.8 |
| Genes | GenBank ID | Forward Sequence | Reverse Sequence |
|---|---|---|---|
| β-Actin | XM_021187106.2 | CAGGCATTGCTGACAGGATG | TGCTGATCCACATCTGCTGG |
| Fgf21 | NM_020013.4 | GTGTCAAAGCCTCTAGGTTTCTT | GGTACACATTGTAACCGTCCTC |
| Fasn | NM_001146708.1 | TGCTTGCTGGCTCACAGTTA | ATCAGTTTCACGAACCCGCC |
| Hsl | XM_029479277.1 | TGGTGACACTCGCAGAAGAC | GATGGCAGGTGTGAACTGGA |
| Fabp1 | NM_017399.5 | GTACCAATTGCAGAGCCAGG | CATGGTCTCCAGTTCGCACT |
| Fabp5 | NM_001272097.1 | TGAAAGAGCTAGGAGTAGGACTG | CTCTCGGTTTTGACCGTGATG |
| Cel | NM_009885.2 | AGGACAACACCTATGGGCAAG | CTCCTCCCCGTCATACAGGTA |
| Clps | NM_001317065.1 | GAACAGTATGCAGTGTAAGAGCA | GCAGATGCCATAGTTGGTGTTG |
| Cyp7a1 | NM_007824.3 | AAACTCCCTGTCATACCACAAAG | TTTCCATCACTTGGGTCTATGC |
| Cyp8b1 | NM_010012.3 | TGTACAGTGCTAGGAGCCCT | GCTCAGGAAGCCAGCCTTAA |
| Pparg | NM_133249.3 | TCCTGTAAAAGCCCGGAGTAT | GCTCTGGTAGGGGCAGTGA |
| Pparα | NM_001113418.1 | CCATCTGTCCTCTCTCCCCA | TTGCAGCTCCGATCACACTT |
| Ucp2 | NM_011671.5 | ATGGTTGGTTTCAAGGCCACA | CGGTATCCAGAGGGAAAGTGAT |
| Bcat2 | NM_001243052.2 | CAGCCACACTAGGACAGGTCT | CAGCCTTGTTATTCCACTCCAC |
| Acat2 | NM_009338.3 | CCCGTGGTCATCGTCTCAG | GGACAGGGCACCATTGAAGG |
| Hk2 | NM_013820.3 | TGATCGCCTGCTTATTCACGG | AACCGCCTAGAAATCTCCAGA |
| Gys2 | NM_145572.2 | GAGTGGGGAGAGAATTACTTCCT | GGGCTCACATTGTTCTACTTGA |
| Pygm | NM_011224.2 | CTTAGCCGGAGTGGAAAATGT | GTAATCTCTCGGAGTAGCCACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Sun, B.; Wang, Z.; Lv, Y.; Ma, Q. Short-Term Decreasing and Increasing Dietary BCAA Have Similar, but Not Identical Effects on Lipid and Glucose Metabolism in Lean Mice. Int. J. Mol. Sci. 2023, 24, 5401. https://doi.org/10.3390/ijms24065401
Sun Y, Sun B, Wang Z, Lv Y, Ma Q. Short-Term Decreasing and Increasing Dietary BCAA Have Similar, but Not Identical Effects on Lipid and Glucose Metabolism in Lean Mice. International Journal of Molecular Sciences. 2023; 24(6):5401. https://doi.org/10.3390/ijms24065401
Chicago/Turabian StyleSun, Yuchen, Bo Sun, Zhishen Wang, Yinfeng Lv, and Qingquan Ma. 2023. "Short-Term Decreasing and Increasing Dietary BCAA Have Similar, but Not Identical Effects on Lipid and Glucose Metabolism in Lean Mice" International Journal of Molecular Sciences 24, no. 6: 5401. https://doi.org/10.3390/ijms24065401
APA StyleSun, Y., Sun, B., Wang, Z., Lv, Y., & Ma, Q. (2023). Short-Term Decreasing and Increasing Dietary BCAA Have Similar, but Not Identical Effects on Lipid and Glucose Metabolism in Lean Mice. International Journal of Molecular Sciences, 24(6), 5401. https://doi.org/10.3390/ijms24065401






