Hyperbaric Oxygen Therapy Alleviates Paclitaxel-Induced Peripheral Neuropathy Involving Suppressing TLR4-MyD88-NF-κB Signaling Pathway
Abstract
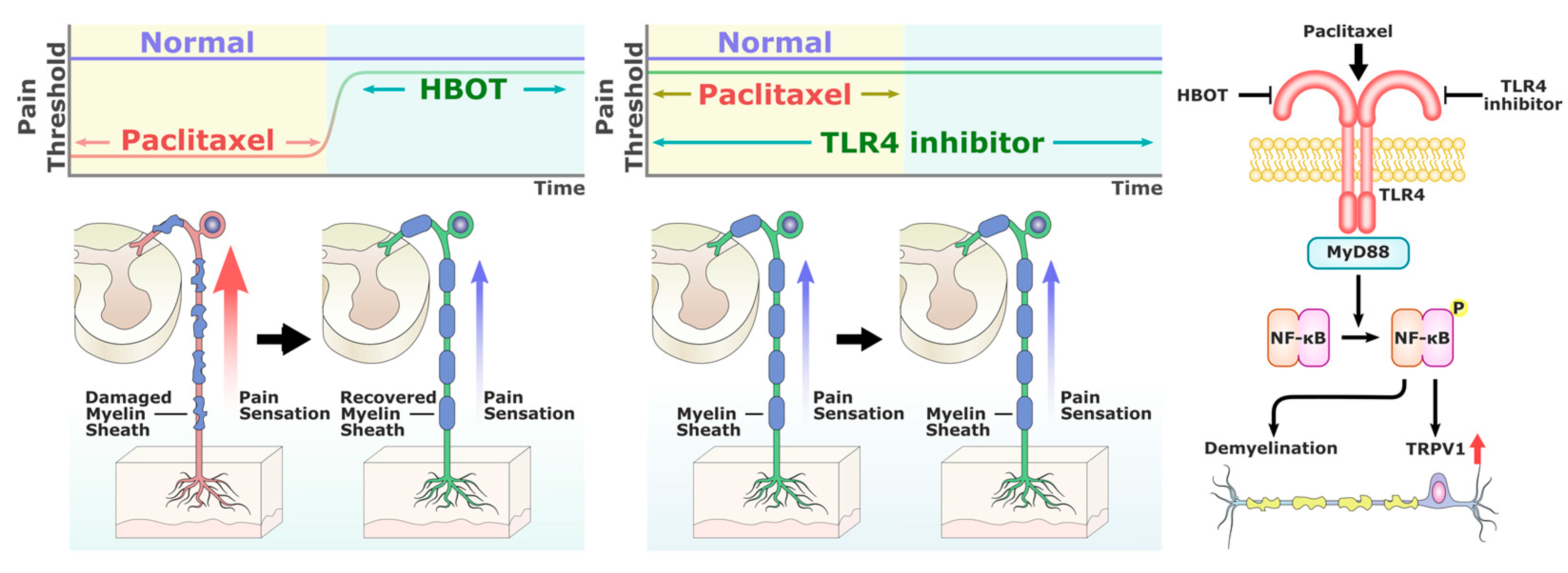
1. Introduction
2. Results
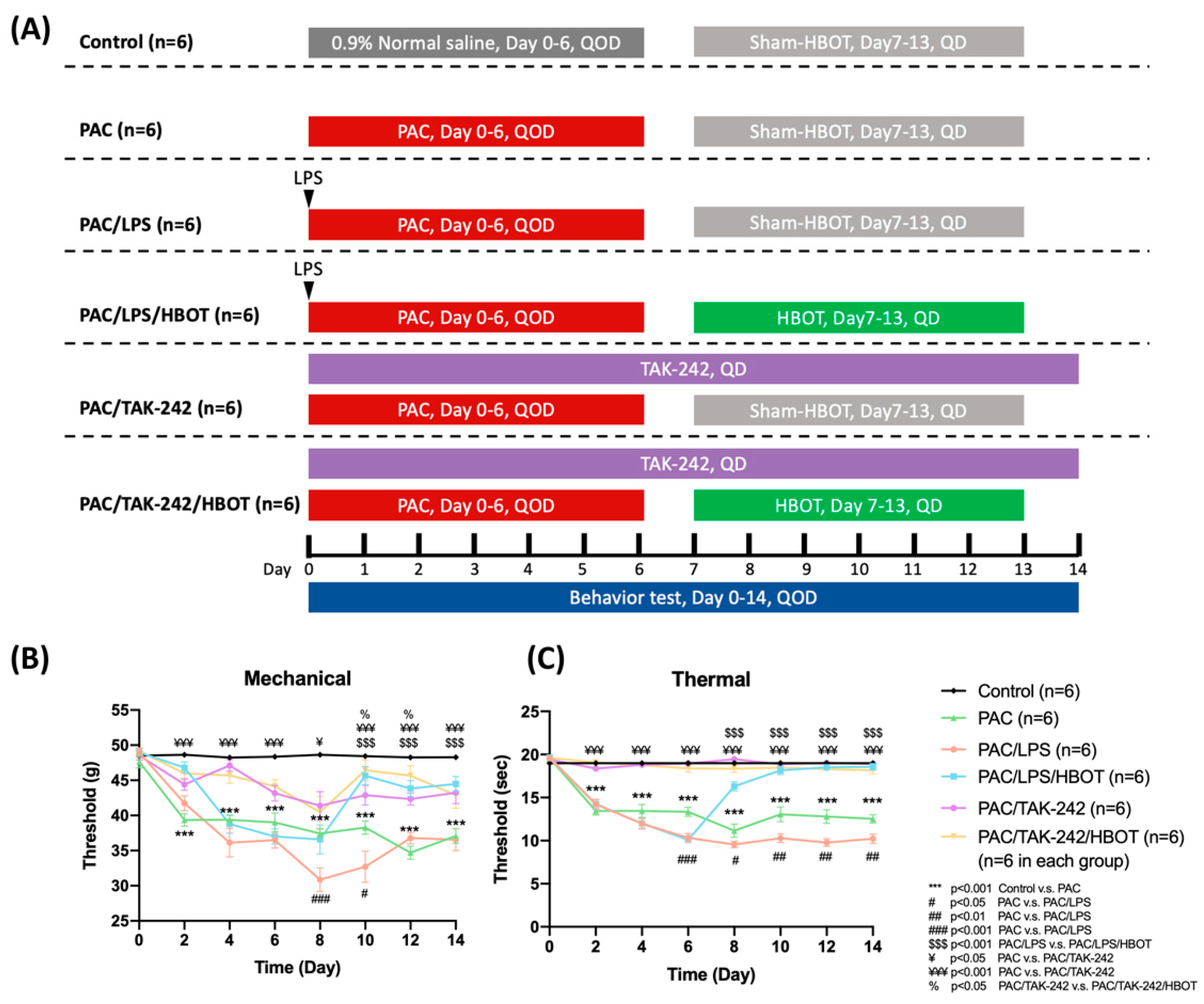
2.1. Mechanical and Thermal Withdrawal Threshold
2.2. Immunofluorescence (IF) Staining of MBP in Sciatic Nerve
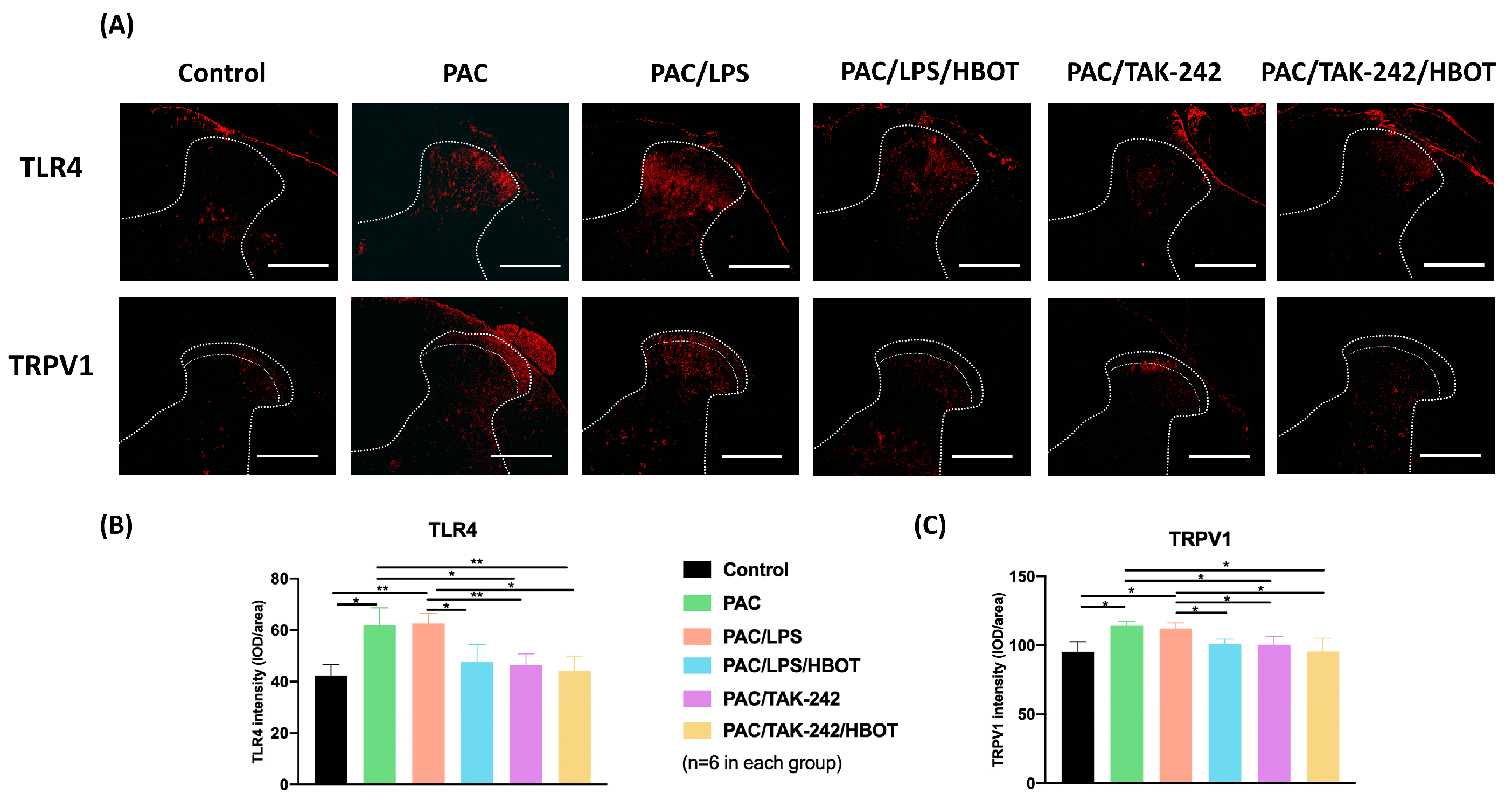
2.3. IF Staining of TLR4/TRPV1 in the Lumbar Spinal Cord Dorsal Horn
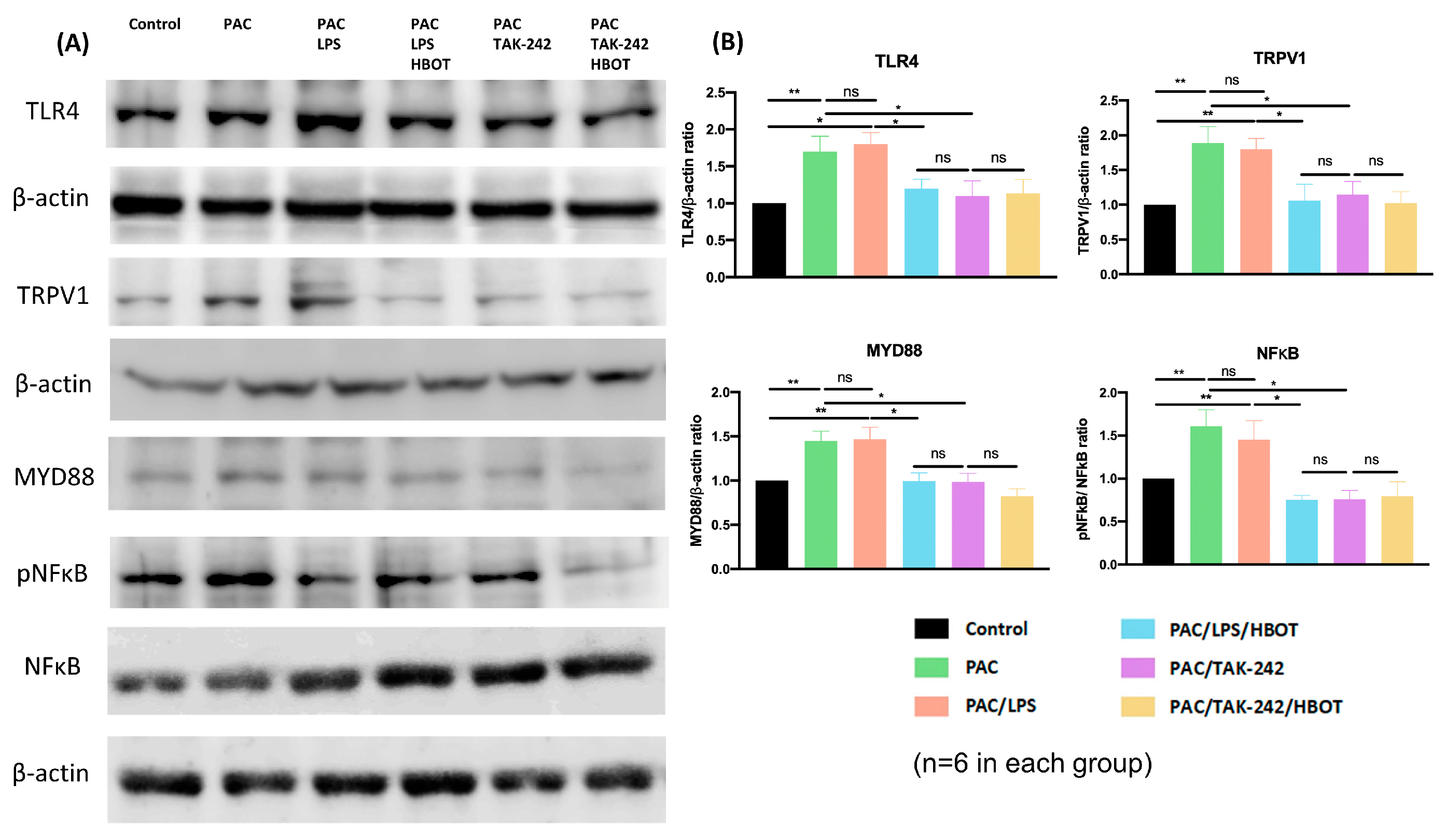
2.4. Western Blot Analysis of TLR4/MyD88/NF-κB/TRPV1 Expression of the Lumbar Spinal Cord Dorsal Horn
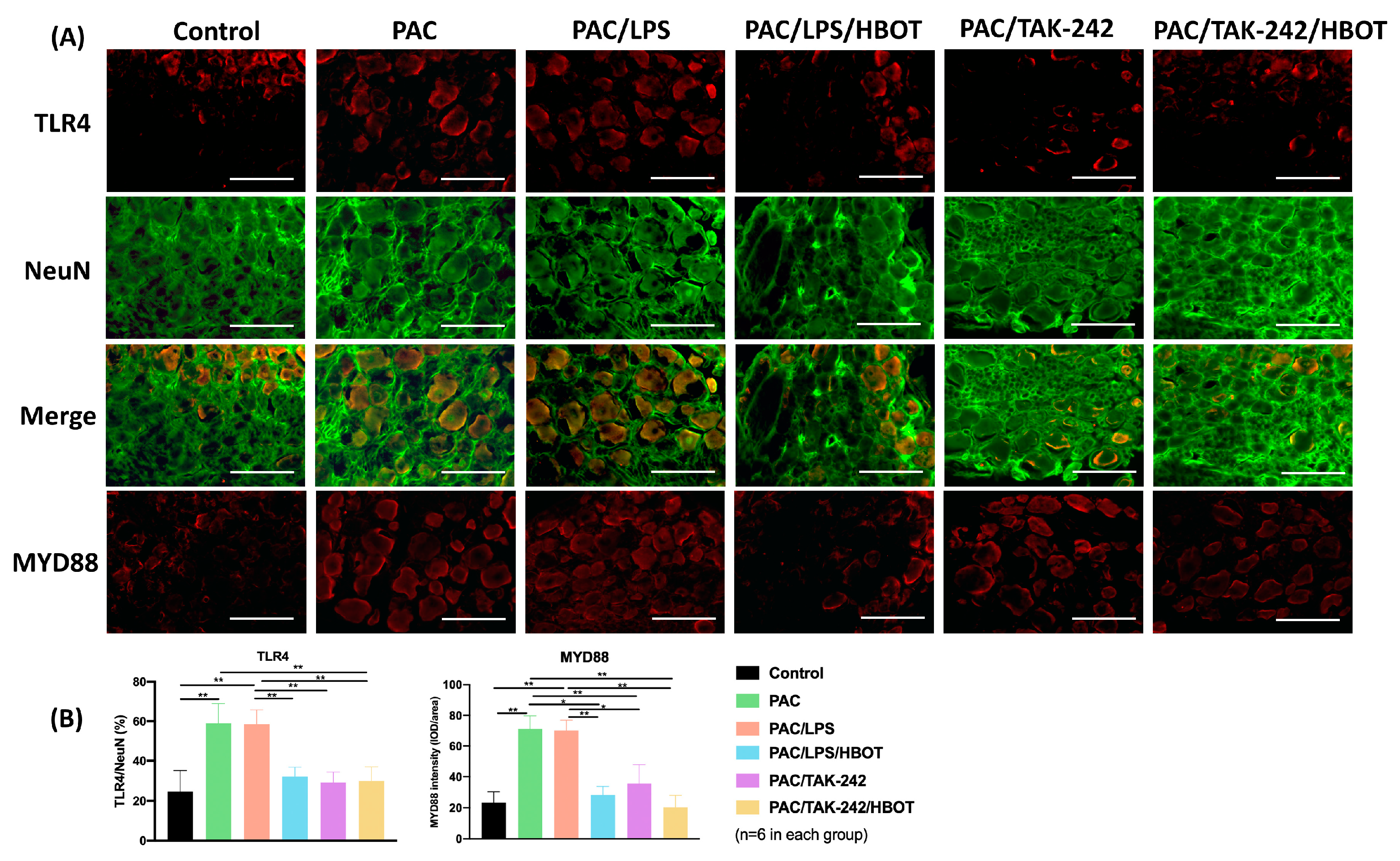
2.5. IF Staining of TLR4/MyD88 and TRPV1 in DRG
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Grouping
4.2. Chemotherapy-Induced Periphery Neuropathy (CIPN) Rat Model
4.3. Hyperbaric Oxygen Therapy (HBOT)
4.4. Assessment of Mechanical and Thermal Withdrawal Threshold
4.5. Western Blots Assay
4.6. IF Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rowinsky, E.K.; Cazenave, L.A.; Donehower, R.C. Taxol: A Novel Investigational Antimicrotubule Agent. J. Natl. Cancer Inst. 1990, 82, 1247–1259. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, F.; Domoto, R.; Nakashima, K.; Yamasoba, D.; Yamanishi, H.; Tsubota, M.; Wake, H.; Nishibori, M.; Kawabata, A. Paclitaxel-induced HMGB1 release from macrophages and its implication for peripheral neuropathy in mice: Evidence for a neuroimmune crosstalk. Neuropharmacology 2018, 141, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Maihöfner, C.; Diel, I.; Tesch, H.; Quandel, T.; Baron, R. Chemotherapy-induced peripheral neuropathy (CIPN): Current therapies and topical treatment option with high-concentration capsaicin. Support. Care Cancer 2021, 29, 4223–4238. [Google Scholar] [CrossRef]
- Li, Y.; Lustberg, M.; Hu, S. Emerging Pharmacological and Non-Pharmacological Therapeutics for Prevention and Treatment of Chemotherapy-Induced Peripheral Neuropathy. Cancers 2021, 13, 766. [Google Scholar] [CrossRef]
- Selvy, M.; Kerckhove, N.; Pereira, B.; Barreau, F.; Nguyen, D.; Busserolles, J.; Giraudet, F.; Cabrespine, A.; Chaleteix, C.; Soubrier, M.; et al. Prevalence of Chemotherapy-Induced Peripheral Neuropathy in Multiple Myeloma Patients and its Impact on Quality of Life: A Single Center Cross-Sectional Study. Front. Pharmacol. 2021, 12, 637593. [Google Scholar] [CrossRef] [PubMed]
- Flatters, S.; Dougherty, P.; Colvin, L. Clinical and preclinical perspectives on Chemotherapy-Induced Peripheral Neuropathy (CIPN): A narrative review. Br. J. Anaesth. 2017, 119, 737–749. [Google Scholar] [CrossRef]
- Ellis, A.; Bennett, D.L.H. Neuroinflammation and the generation of neuropathic pain. Br. J. Anaesth. 2013, 111, 26–37. [Google Scholar] [CrossRef]
- Vermeer, C.J.C.; Hiensch, A.E.; Cleenewerk, L.; May, A.M.; Eijkelkamp, N. Neuro-immune interactions in paclitaxel-induced peripheral neuropathy. Acta Oncol. 2021, 60, 1369–1382. [Google Scholar] [CrossRef]
- Sasamura, T.; Kuraishi, Y. Peripheral and Central Actions of Capsaicin and VR1 Receptor. Jpn. J. Pharmacol. 1999, 80, 275–280. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Boyette-Davis, J.A.; Walters, E.T.; Dougherty, P.M. Mechanisms involved in the development of chemotherapy-induced neuropathy. Pain Manag. 2015, 5, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Kalynovska, N.; Adamek, P.; Palecek, J. TRPV1 Receptors Contribute to Paclitaxel-Induced c-Fos Expression in Spinal Cord Dorsal Horn Neurons. Physiol. Res. 2017, 66, 549–552. [Google Scholar] [CrossRef]
- Li, Y.; Adamek, P.; Zhang, H.; Tatsui, C.E.; Rhines, L.D.; Mrozkova, P.; Li, Q.; Kosturakis, A.K.; Cassidy, R.M.; Harrison, D.S.; et al. The Cancer Chemotherapeutic Paclitaxel Increases Human and Rodent Sensory Neuron Responses to TRPV1 by Activation of TLR4. J. Neurosci. 2015, 35, 13487–13500. [Google Scholar] [CrossRef] [PubMed]
- Rossato, M.F.; Rigo, F.K.; Oliveira, S.M.; Guerra, G.P.; Silva, C.R.; Cunha, T.M.; Gomez, M.V.; Ferreira, J.; Trevisan, G. Participation of transient receptor potential vanilloid 1 in paclitaxel-induced acute visceral and peripheral nociception in rodents. Eur. J. Pharmacol. 2018, 828, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Bavencoffe, A.; Yang, P.; Feng, J.; Yin, S.; Qian, A.; Yu, W.; Liu, S.; Gong, X.; Cai, T.; et al. Zinc Inhibits TRPV1 to Alleviate Chemotherapy-Induced Neuropathic Pain. J. Neurosci. 2018, 38, 474–483. [Google Scholar] [CrossRef]
- Agalave, N.M.; Larsson, M.; Abdelmoaty, S.; Su, J.; Baharpoor, A.; Lundbäck, P.; Palmblad, K.; Andersson, U.; Harris, H.; Svensson, C.I. Spinal HMGB1 induces TLR4-mediated long-lasting hypersensitivity and glial activation and regulates pain-like behavior in experimental arthritis. Pain 2014, 155, 1802–1813. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Kosturakis, A.K.; Cassidy, R.M.; Zhang, H.; Kennamer-Chapman, R.M.; Jawad, A.B.; Colomand, C.M.; Harrison, D.S.; Dougherty, P.M. MAPK signaling downstream to TLR4 contributes to paclitaxel-induced peripheral neuropathy. Brain Behav. Immun. 2015, 49, 255–266. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Zhang, H.; Kosturakis, A.K.; Jawad, A.B.; Dougherty, P.M. Toll-Like Receptor 4 Signaling Contributes to Paclitaxel-Induced Peripheral Neuropathy. J. Pain 2014, 15, 712–725. [Google Scholar] [CrossRef]
- Chou, P.-R.; Lu, C.-Y.; Kan, J.-Y.; Wang, S.-H.; Lo, J.-J.; Huang, S.-H.; Wu, S.-H. Simultaneous hyperbaric oxygen therapy during systemic chemotherapy reverses chemotherapy-induced peripheral neuropathy by inhibiting TLR4 and TRPV1 activation in the central and peripheral nervous system. Support. Care Cancer 2021, 29, 6841–6850. [Google Scholar] [CrossRef]
- Assas, B.M.; Miyan, J.A.; Pennock, J.L. Cross-talk between neural and immune receptors provides a potential mechanism of homeostatic regulation in the gut mucosa. Mucosal Immunol. 2014, 7, 1283–1289. [Google Scholar] [CrossRef]
- Thom, S.R. Hyperbaric Oxygen: Its Mechanisms and Efficacy. Plast. Reconstr. Surg. 2011, 127 (Suppl. S1), 131S–141S. [Google Scholar] [CrossRef] [PubMed]
- Matera, D.V.; Smith, B.; Lam, B. Revisiting the expanded use of hyperbaric oxygen therapy for treatment of resistant migraines. Med. Gas Res. 2019, 9, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, S.; Kiralp, M.; Akin, A.; Keskin, I.; Ay, H.; Dursun, H.; Cimsit, M. A New Treatment Modality for Fibromyalgia Syndrome: Hyperbaric Oxygen Therapy. J. Int. Med. Res. 2004, 32, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Katznelson, R.; Segal, S.; Clarke, H. Successful Treatment of Lower Limb Complex Regional Pain Syndrome following Three Weeks of Hyperbaric Oxygen Therapy. Pain Res. Manag. 2016, 2016, 3458371. [Google Scholar] [CrossRef] [PubMed]
- Efrati, S.; Golan, H.; Bechor, Y.; Faran, Y.; Daphna-Tekoah, S.; Sekler, G.; Fishlev, G.; Ablin, J.N.; Bergan, J.; Volkov, O.; et al. Hyperbaric Oxygen Therapy Can Diminish Fibromyalgia Syndrome—Prospective Clinical Trial. PLoS ONE 2015, 10, e0127012. [Google Scholar] [CrossRef]
- Fischer, I.; Barak, B. Molecular and Therapeutic Aspects of Hyperbaric Oxygen Therapy in Neurological Conditions. Biomolecules 2020, 10, 1247. [Google Scholar] [CrossRef]
- Roglio, I.; Bianchi, R.; Camozzi, F.; Carozzi, V.; Cervellini, I.; Crippa, D.; Lauria, G.; Cavaletti, G.; Melcangi, R.C. Docetaxel-induced peripheral neuropathy: Protective effects of dihydroprogesterone and progesterone in an experimental model. J. Peripher. Nerv. Syst. 2009, 14, 36–44. [Google Scholar] [CrossRef]
- Kamata, Y.; Kambe, T.; Chiba, T.; Yamamoto, K.; Kawakami, K.; Abe, K.; Taguchi, K. Paclitaxel Induces Upregulation of Transient Receptor Potential Vanilloid 1 Expression in the Rat Spinal Cord. Int. J. Mol. Sci. 2020, 21, 4341. [Google Scholar] [CrossRef]
- Anderson, M.B.; Das, S.; Miller, K.E. Subcellular localization of neuronal nuclei (NeuN) antigen in size and calcitonin gene-related peptide (CGRP) populations of dorsal root ganglion (DRG) neurons during acute peripheral inflammation. Neurosci. Lett. 2021, 760, 135974. [Google Scholar] [CrossRef]
- Li, Y.; Tatsui, C.E.; Rhines, L.D.; North, R.Y.; Harrison, D.S.; Cassidy, R.M.; Johansson, C.A.; Kosturakis, A.K.; Edwards, D.D.; Zhang, H.; et al. Dorsal root ganglion neurons become hyperexcitable and increase expression of voltage-gated T-type calcium channels (Cav3.2) in paclitaxel-induced peripheral neuropathy. Pain 2017, 158, 417–429. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; de Carvalho-Barbosa, M.; Kavelaars, A.; Heijnen, C.J.; Albrecht, P.J.; Dougherty, P.M. Dorsal Root Ganglion Infiltration by Macrophages Contributes to Paclitaxel Chemotherapy-Induced Peripheral Neuropathy. J. Pain 2016, 17, 775–786. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Kao, C.-L.; Liu, C.-M. The Cancer Prevention, Anti-Inflammatory and Anti-Oxidation of Bioactive Phytochemicals Targeting the TLR4 Signaling Pathway. Int. J. Mol. Sci. 2018, 19, 2729. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Gao, Y.-J.; Ji, R.-R. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci. Bull. 2012, 28, 131–144. [Google Scholar] [CrossRef]
- Zimmer, S.M.; Liu, J.; Clayton, J.L.; Stephens, D.S.; Snyder, J.P. Paclitaxel Binding to Human and Murine MD-2. J. Biol. Chem. 2008, 283, 27916–27926. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Akashi, S.; Shimazu, R.; Yoshida, T.; Miyake, K.; Nishijima, M. Mouse Toll-like Receptor 4·MD-2 Complex Mediates Lipopolysaccharide-mimetic Signal Transduction by Taxol. J. Biol. Chem. 2000, 275, 2251–2254. [Google Scholar] [CrossRef]
- Wanderley, C.W.; Colón, D.F.; Luiz, J.P.M.; Oliveira, F.F.; Viacava, P.R.; Leite, C.A.; Pereira, J.A.; Silva, C.M.; Silva, C.R.; Silva, R.L.; et al. Paclitaxel reduces tumor growth by reprogramming tumor-associated macrophages to an M1- profile in a TLR4-dependent manner. Cancer Res. 2018, 78, 5891–5900. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.-W.; You, M.-J.; Park, H.-S.; Kim, J.W.; Kwon, M.-S. Differential effect of LPS and paclitaxel on microglial functional phenotypes and circulating cytokines: The possible role of CX3CR1 and IL-4/10 in blocking persistent inflammation. Arch. Pharmacal Res. 2019, 42, 359–368. [Google Scholar] [CrossRef]
- Abu-Ghefreh, A.A.; Masocha, W. Enhancement of antinociception by coadminstration of minocycline and a non-steroidal anti-inflammatory drug indomethacin in naïve mice and murine models of LPS-induced thermal hyperalgesia and monoarthritis. BMC Musculoskelet. Disord. 2010, 11, 276. [Google Scholar] [CrossRef]
- Shirey, K.A.; Lai, W.; Brown, L.J.; Blanco, J.C.G.; Beadenkopf, R.; Wang, Y.; Vogel, S.N.; Snyder, G.A. Select targeting of intracellular Toll-interleukin-1 receptor resistance domains for protection against influenza-induced disease. J. Endotoxin Res. 2020, 26, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, N.; Tsuchimori, N.; Matsumoto, T.; Ii, M. TAK-242 (Resatorvid), a Small-Molecule Inhibitor of Toll-Like Receptor (TLR) 4 Signaling, Binds Selectively to TLR4 and Interferes with Interactions between TLR4 and Its Adaptor Molecules. Mol. Pharmacol. 2011, 79, 34–41. [Google Scholar] [CrossRef]
- Xing, Z.; Zhen, T.; Jie, F.; Jie, Y.; Shiqi, L.; Kaiyi, Z.; Zhicui, O.Y.; Mingyan, H. Early Toll-like receptor 4 inhibition improves immune dysfunction in the hippocampus after hypoxic-ischemic brain damage. Int. J. Med. Sci. 2022, 19, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Maejima, Y.; Saito, M.; Sakamoto, K.; Horita, S.; Shimomura, K.; Inoue, S.; Kotani, J. TAK-242, a specific inhibitor of Toll-like receptor 4 signalling, prevents endotoxemia-induced skeletal muscle wasting in mice. Sci. Rep. 2020, 10, 694. [Google Scholar] [CrossRef]
- Zhang, D.; Li, H.; Li, T.; Zhou, M.; Hao, S.; Yan, H.; Yu, Z.; Li, W.; Li, K.; Hang, C. TLR4 inhibitor resatorvid provides neuroprotection in experimental traumatic brain injury: Implication in the treatment of human brain injury. Neurochem. Int. 2014, 75, 11–18. [Google Scholar] [CrossRef]
- Gottfried, I.; Schottlender, N.; Ashery, U. Hyperbaric Oxygen Treatment—From Mechanisms to Cognitive Improvement. Biomolecules 2021, 11, 1520. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Liang, F.; Jia, X.Y.; Zhao, L.; Zhou, Y.; Yang, J. Hyperbaric Oxygen Treatment Improves Hearing Level via Attenuating TLR4/NF-kappaB Mediated Inflammation in Sudden Sensorineural Hearing Loss Patients. Biomed. Environ. Sci. 2020, 33, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lu, C.; Liu, Y.; Zhou, X.; Sun, L.; Gu, Q.; Shen, G.; Guo, A. Hyperbaric Oxygen Alleviates the Inflammatory Response Induced by LPS Through Inhibition of NF-κB/MAPKs-CCL2/CXCL1 Signaling Pathway in Cultured Astrocytes. Inflammation 2018, 41, 2003–2011. [Google Scholar] [CrossRef]
- Wozniak, K.M.; Vornov, J.J.; Wu, Y.; Liu, Y.; Carozzi, V.A.; Rodriguez-Menendez, V.; Ballarini, E.; Alberti, P.; Pozzi, E.; Semperboni, S.; et al. Peripheral Neuropathy Induced by Microtubule-Targeted Chemotherapies: Insights into Acute Injury and Long-term Recovery. Cancer Res. 2018, 78, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbacher, J.C. Chemotherapy-Induced Peripheral Neuropathy. Prog. Mol. Biol. Transl. Sci. 2015, 131, 471–508. [Google Scholar] [CrossRef]
- Cook, B.M.; Wozniak, K.M.; Proctor, D.A.; Bromberg, R.B.; Wu, Y.; Slusher, B.S.; Littlefield, B.A.; Jordan, M.A.; Wilson, L.; Feinstein, S.C. Differential Morphological and Biochemical Recovery from Chemotherapy-Induced Peripheral Neuropathy Following Paclitaxel, Ixabepilone, or Eribulin Treatment in Mouse Sciatic Nerves. Neurotox. Res. 2018, 34, 677–692. [Google Scholar] [CrossRef]
- Acioglu, C.; Heary, R.F.; Elkabes, S. Roles of neuronal toll-like receptors in neuropathic pain and central nervous system injuries and diseases. Brain Behav. Immun. 2022, 102, 163–178. [Google Scholar] [CrossRef]
- Costa-Pereira, J.T.; Serrão, P.; Martins, I.; Tavares, I. Serotoninergic pain modulation from the rostral ventromedial medulla (RVM) in chemotherapy-induced neuropathy: The role of spinal 5-HT3 receptors. Eur. J. Neurosci. 2020, 51, 1756–1769. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.-Y.; Xue, M.; Wang, Y.; Huang, Z.-H.; Huang, C. Electroacupuncture Alleviates Spared Nerve Injury-Induced Neuropathic Pain And Modulates HMGB1/NF-κB Signaling Pathway In The Spinal Cord. J. Pain Res. 2019, 12, 2851–2863. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Huang, Y.; Hu, Y.; Tang, Q.; Zhong, Y. Toll-like receptor 4/nuclear factor-kappa B pathway is involved in radicular pain by encouraging spinal microglia activation and inflammatory response in a rat model of lumbar disc herniation. Korean J. Pain 2021, 34, 47–57. [Google Scholar] [CrossRef]
- Li, Y.; Yin, C.; Li, X.; Liu, B.; Wang, J.; Zheng, X.; Shao, X.; Liang, Y.; Du, J.; Fang, J.; et al. Electroacupuncture Alleviates Paclitaxel-Induced Peripheral Neuropathic Pain in Rats via Suppressing TLR4 Signaling and TRPV1 Upregulation in Sensory Neurons. Int. J. Mol. Sci. 2019, 20, 5917. [Google Scholar] [CrossRef]
- Szabo-Pardi, T.A.; Barron, L.R.; Lenert, M.E.; Burton, M.D. Sensory Neuron TLR4 mediates the development of nerve-injury induced mechanical hypersensitivity in female mice. Brain Behav. Immun. 2021, 97, 42–60. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-H.; Wu, S.-H.; Lee, S.-S.; Lin, Y.-N.; Chai, C.-Y.; Lai, C.-S.; Wang, H.-M.D. Platelet-Rich Plasma Injection in Burn Scar Areas Alleviates Neuropathic Scar Pain. Int. J. Med. Sci. 2018, 15, 238–247. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-H.; Huang, S.-H.; Hsieh, M.-C.; Lu, I.-C.; Chou, P.-R.; Tai, M.-H.; Wu, S.-H. Hyperbaric Oxygen Therapy Alleviates Paclitaxel-Induced Peripheral Neuropathy Involving Suppressing TLR4-MyD88-NF-κB Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 5379. https://doi.org/10.3390/ijms24065379
Wang S-H, Huang S-H, Hsieh M-C, Lu I-C, Chou P-R, Tai M-H, Wu S-H. Hyperbaric Oxygen Therapy Alleviates Paclitaxel-Induced Peripheral Neuropathy Involving Suppressing TLR4-MyD88-NF-κB Signaling Pathway. International Journal of Molecular Sciences. 2023; 24(6):5379. https://doi.org/10.3390/ijms24065379
Chicago/Turabian StyleWang, Shih-Hung, Shu-Hung Huang, Meng-Chien Hsieh, I-Cheng Lu, Ping-Ruey Chou, Ming-Hong Tai, and Sheng-Hua Wu. 2023. "Hyperbaric Oxygen Therapy Alleviates Paclitaxel-Induced Peripheral Neuropathy Involving Suppressing TLR4-MyD88-NF-κB Signaling Pathway" International Journal of Molecular Sciences 24, no. 6: 5379. https://doi.org/10.3390/ijms24065379
APA StyleWang, S.-H., Huang, S.-H., Hsieh, M.-C., Lu, I.-C., Chou, P.-R., Tai, M.-H., & Wu, S.-H. (2023). Hyperbaric Oxygen Therapy Alleviates Paclitaxel-Induced Peripheral Neuropathy Involving Suppressing TLR4-MyD88-NF-κB Signaling Pathway. International Journal of Molecular Sciences, 24(6), 5379. https://doi.org/10.3390/ijms24065379





