Modification of the TRP Channel TRPA1 as a Relevant Factor in Migraine-Related Intracranial Hypersensitivity
Abstract
1. Introduction
1.1. Migraine
Migraine-Associated Hypersensitivity
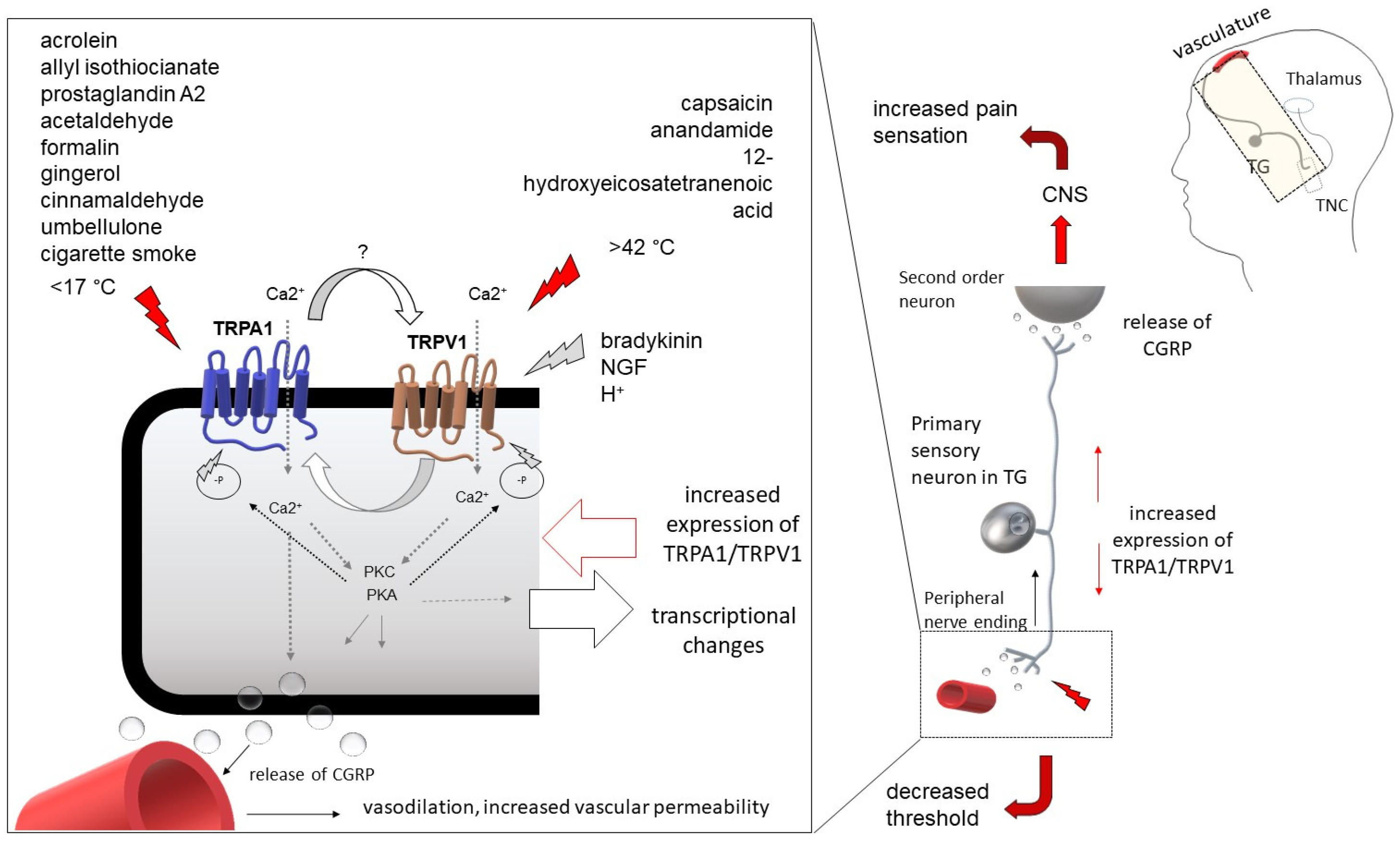
1.2. Transient Receptor Potential Ankyrin 1 (TRPA1)
Emphasized Role of TRPA1 in Hypersensitivity Reactions
1.3. TRPA1 in Migraine-Related Research
1.3.1. The Presence of TRPA1 in the Trigeminal System
1.3.2. Relevance of TRPA1 in Migraine-Like Pain
1.4. Possible Cooperation between TRPA1 and TRPV1
1.5. Possible Therapeutic Interventions
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burch, R.C.; Buse, D.C.; Lipton, R.B. Migraine: Epidemiology, Burden, and Comorbidity. Neurol. Clin. 2019, 37, 631–649. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.; Cappa, K.G.; Smith, C.R. Common migraine: Criteria for diagnosis. Headache 1988, 28, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Han, S.M.; Kim, K.M.; Cho, S.-J.; Yang, K.I.; Kim, D.; Yun, C.-H.; Chu, M.K. Prevalence and characteristics of cutaneous allodynia in probable migraine. Sci. Rep. 2021, 11, 2467. [Google Scholar] [CrossRef]
- Ray, J.C.; Cheema, S.; Foster, E.; Gunasekera, L.; Mehta, D.; Corcoran, S.J.; Matharu, M.S.; Hutton, E.J. Autonomic symptoms in migraine: Results of a prospective longitudinal study. Front. Neurol. 2022, 13, 1036798. [Google Scholar] [CrossRef]
- Luda, E.; Bo, E.; Sicuro, L.; Comitangelo, R.; Campana, M. Sustained visual aura: A totally new variation of migraine. Headache 1991, 31, 582–583. [Google Scholar] [CrossRef]
- Peatfield, R.C.; Gawel, M.J.; Rose, F.C. Asymmetry of the aura and pain in migraine. J. Neurol. Neurosurg. Psychiatry 1981, 44, 846–848. [Google Scholar] [CrossRef]
- Russell, M.B.; Rasmussen, B.K.; Thorvaldsen, P.; Olesen, J. Prevalence and sex-ratio of the subtypes of migraine. Int. J. Epidemiol. 1995, 24, 612–618. [Google Scholar] [CrossRef]
- Rasmussen, B.K.; Olesen, J. Migraine with aura and migraine without aura: An epidemiological study. Cephalalgia Int. J. Headache 1992, 12, 221–228; discussion 186. [Google Scholar] [CrossRef] [PubMed]
- Nappi, G.; Agnoli, A.; Manzoni, G.C.; Nattero, G.; Sicuteri, F. Classification and diagnostic criteria for primary headache disorders (Ad Hoc Committee IHS, 1988). Funct. Neurol. 1989, 4, 65–71. [Google Scholar] [PubMed]
- Lipton, R.B.; Goadsby, P.; Silberstein, S.D. Classification and epidemiology of headache. Clin. Cornerstone 1999, 1, 1–10. [Google Scholar] [CrossRef]
- Feindel, W.; Penfield, W.; McNaughton, F. The tentorial nerves and Iocalization of intracranial pain in man. Neurology 1960, 10, 555–563. [Google Scholar] [CrossRef]
- Moskowitz, M.A. The neurobiology of vascular head pain. Ann. Neurol. 1984, 16, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, M.A.; Buzzi, M.G. Neuroeffector functions of sensory fibres: Implications for headache mechanisms and drug actions. J. Neurol. 1991, 238 (Suppl. 1), S18–S22. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, T.; Sakai, F.; Suzuki, K.; Igarashi, H.; Suzuki, N. Implication of augmented vasogenic leakage in the mechanism of persistent aura in sporadic hemiplegic migraine. Cephalalgia Int. J. Headache 2006, 26, 332–335. [Google Scholar] [CrossRef]
- Albrecht, D.S.; Mainero, C.; Ichijo, E.; Ward, N.; Granziera, C.; Zürcher, N.R.; Akeju, O.; Bonnier, G.; Price, J.; Hooker, J.M.; et al. Imaging of neuroinflammation in migraine with aura: A [11C]PBR28 PET/MRI study. Neurology 2019, 92, e2038–e2050. [Google Scholar] [CrossRef]
- Messlinger, K. What is a nociceptor? Anaesthesist 1997, 46, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Strassman, A.M.; Raymond, S.A.; Burstein, R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature 1996, 384, 560–564. [Google Scholar] [CrossRef]
- Kunkler, P.E.; Zhang, L.; Pellman, J.J.; Oxford, G.S.; Hurley, J.H. Sensitization of the Trigeminovascular System following Environmental Irritant Exposure. Cephalalgia Int. J. Headache 2015, 35, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Rosen, T.A.; Tominaga, M.; Brake, A.J.; Julius, D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 1999, 398, 436–441. [Google Scholar] [CrossRef]
- Lee, H.; Iida, T.; Mizuno, A.; Suzuki, M.; Caterina, M.J. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Siemens, J.; Glazer, J.M.; Tsuruda, P.R.; Basbaum, A.I.; Stucky, C.L.; Jordt, S.-E.; Julius, D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007, 448, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Montell, C. The TRP superfamily of cation channels. Sci. STKE Signal Transduct. Knowl. Environ. 2005, 2005, re3. [Google Scholar] [CrossRef] [PubMed]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like Channel Expressed in Nociceptive Neurons, Is Activated by Cold Temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef]
- Karashima, Y.; Talavera, K.; Everaerts, W.; Janssens, A.; Kwan, K.Y.; Vennekens, R.; Nilius, B.; Voets, T. TRPA1 acts as a cold sensor in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 1273–1278. [Google Scholar] [CrossRef]
- Kobayashi, K.; Fukuoka, T.; Obata, K.; Yamanaka, H.; Dai, Y.; Tokunaga, A.; Noguchi, K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J. Comp. Neurol. 2005, 493, 596–606. [Google Scholar] [CrossRef]
- Jordt, S.-E.; Bautista, D.M.; Chuang, H.-H.; McKemy, D.D.; Zygmunt, P.M.; Högestätt, E.D.; Meng, I.D.; Julius, D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004, 427, 260–265. [Google Scholar] [CrossRef]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41, 849–857. [Google Scholar] [CrossRef]
- Babes, A.; Zorzon, D.; Reid, G. Two populations of cold-sensitive neurons in rat dorsal root ganglia and their modulation by nerve growth factor. Eur. J. Neurosci. 2004, 20, 2276–2282. [Google Scholar] [CrossRef]
- Andersen, H.H.; Lo Vecchio, S.; Gazerani, P.; Arendt-Nielsen, L. Dose-response study of topical allyl isothiocyanate (mustard oil) as a human surrogate model of pain, hyperalgesia, and neurogenic inflammation. Pain 2017, 158, 1723–1732. [Google Scholar] [CrossRef]
- Kremeyer, B.; Lopera, F.; Cox, J.J.; Momin, A.; Rugiero, F.; Marsh, S.; Woods, C.G.; Jones, N.G.; Paterson, K.J.; Fricker, F.R.; et al. A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron 2010, 66, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Weyer-Menkhoff, I.; Pinter, A.; Schlierbach, H.; Schänzer, A.; Lötsch, J. Epidermal expression of human TRPM8, but not of TRPA1 ion channels, is associated with sensory responses to local skin cooling. Pain 2019, 160, 2699–2709. [Google Scholar] [CrossRef] [PubMed]
- Obata, K.; Katsura, H.; Mizushima, T.; Yamanaka, H.; Kobayashi, K.; Dai, Y.; Fukuoka, T.; Tokunaga, A.; Tominaga, M.; Noguchi, K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J. Clin. Investig. 2005, 115, 2393–2401. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Lu, J.; Li, J. TRPA1 mediates amplified sympathetic responsiveness to activation of metabolically sensitive muscle afferents in rats with femoral artery occlusion. Front. Physiol. 2015, 6, 249. [Google Scholar] [CrossRef]
- Kimball, E.S.; Prouty, S.P.; Pavlick, K.P.; Wallace, N.H.; Schneider, C.R.; Hornby, P.J. Stimulation of neuronal receptors, neuropeptides and cytokines during experimental oil of mustard colitis. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2007, 19, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.; Birrell, M.A.; Dubuis, E.; Maher, S.A.; Belvisi, M.G. Transient receptor potential channels mediate the tussive response to prostaglandin E2 and bradykinin. Thorax 2012, 67, 891–900. [Google Scholar] [CrossRef]
- Bang, S.; Kim, K.Y.; Yoo, S.; Kim, Y.G.; Hwang, S.W. Transient receptor potential A1 mediates acetaldehyde-evoked pain sensation. Eur. J. Neurosci. 2007, 26, 2516–2523. [Google Scholar] [CrossRef]
- Bautista, D.M.; Jordt, S.-E.; Nikai, T.; Tsuruda, P.R.; Read, A.J.; Poblete, J.; Yamoah, E.N.; Basbaum, A.I.; Julius, D. TRPA1 Mediates the Inflammatory Actions of Environmental Irritants and Proalgesic Agents. Cell 2006, 124, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Al-Shamlan, F.; El-Hashim, A.Z. Bradykinin sensitizes the cough reflex via a B2 receptor dependent activation of TRPV1 and TRPA1 channels through metabolites of cyclooxygenase and 12-lipoxygenase. Respir. Res. 2019, 20, 110. [Google Scholar] [CrossRef]
- Katsura, H.; Obata, K.; Mizushima, T.; Yamanaka, H.; Kobayashi, K.; Dai, Y.; Fukuoka, T.; Tokunaga, A.; Sakagami, M.; Noguchi, K. Antisense knock down of TRPA1, but not TRPM8, alleviates cold hyperalgesia after spinal nerve ligation in rats. Exp. Neurol. 2006, 200, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Tsagareli, M.G.; Nozadze, I.; Tsiklauri, N.; Carstens, M.I.; Gurtskaia, G.; Carstens, E. Thermal Hyperalgesia and Mechanical Allodynia Elicited by Histamine and Non-histaminergic Itch Mediators: Respective Involvement of TRPV1 and TRPA1. Neuroscience 2020, 449, 35–45. [Google Scholar] [CrossRef]
- Miyano, K.; Shiraishi, S.; Minami, K.; Sudo, Y.; Suzuki, M.; Yokoyama, T.; Terawaki, K.; Nonaka, M.; Murata, H.; Higami, Y.; et al. Carboplatin Enhances the Activity of Human Transient Receptor Potential Ankyrin 1 through the Cyclic AMP-Protein Kinase A-A-Kinase Anchoring Protein (AKAP) Pathways. Int. J. Mol. Sci. 2019, 20, 3271. [Google Scholar] [CrossRef] [PubMed]
- Marcotti, A.; Fernández-Trillo, J.; González, A.; Vizcaíno-Escoto, M.; Ros-Arlanzón, P.; Romero, L.; Vela, J.M.; Gomis, A.; Viana, F.; de la Peña, E. TRPA1 modulation by Sigma-1 receptor prevents oxaliplatin-induced painful peripheral neuropathy. Brain J. Neurol. 2023, 146, 475–491. [Google Scholar] [CrossRef]
- Wang, S.; Qi, S.; Kogure, Y.; Kanda, H.; Tian, L.; Yamamoto, S.; Noguchi, K.; Dai, Y. The ubiquitin E3 ligase Nedd4-2 relieves mechanical allodynia through the ubiquitination of TRPA1 channel in db/db mice. Eur. J. Neurosci. 2021, 53, 1691–1704. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.M.; Itson-Zoske, B.; Cai, Y.; Qiu, C.; Pan, B.; Stucky, C.L.; Hogan, Q.H.; Yu, H. Satellite glial cells in sensory ganglia express functional transient receptor potential ankyrin 1 that is sensitized in neuropathic and inflammatory pain. Mol. Pain 2020, 16, 1744806920925425. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Chen, Y.-J.; Chen, B.; Ru, X.; Wang, J.; Lin, J.; Tang, X.; Chen, W.; Hu, R.; Li, W.; et al. Knockout of transient receptor potential ankyrin 1 (TRPA1) modulates the glial phenotype and alleviates perihematomal neuroinflammation after intracerebral hemorrhage in mice via MAPK/NF-κB signaling. Neuroreport 2023, 34, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Demartini, C.; Greco, R.; Magni, G.; Zanaboni, A.M.; Riboldi, B.; Francavilla, M.; Nativi, C.; Ceruti, S.; Tassorelli, C. Modulation of Glia Activation by TRPA1 Antagonism in Preclinical Models of Migraine. Int. J. Mol. Sci. 2022, 23, 14085. [Google Scholar] [CrossRef]
- Kunkler, P.E.; Ballard, C.J.; Oxford, G.S.; Hurley, J.H. TRPA1 receptors mediate environmental irritant-induced meningeal vasodilatation. Pain 2011, 152, 38–44. [Google Scholar] [CrossRef]
- Nagata, K.; Duggan, A.; Kumar, G.; García-Añoveros, J. Nociceptor and Hair Cell Transducer Properties of TRPA1, a Channel for Pain and Hearing. J. Neurosci. 2005, 25, 4052–4061. [Google Scholar] [CrossRef]
- Edelmayer, R.M.; Le, L.N.; Yan, J.; Wei, X.; Nassini, R.; Materazzi, S.; Preti, D.; Appendino, G.; Geppetti, P.; Dodick, D.W.; et al. Activation of TRPA1 on dural afferents: A potential mechanism of headache pain. Pain 2012, 153, 1949–1958. [Google Scholar] [CrossRef]
- Huang, D.; Li, S.; Dhaka, A.; Story, G.M.; Cao, Y.-Q. Expression of the transient receptor potential channels TRPV1, TRPA1 and TRPM8 in mouse trigeminal primary afferent neurons innervating the dura. Mol. Pain 2012, 8, 66. [Google Scholar] [CrossRef]
- MacDonald, N.J.; Butters, L.; O’Shaughnessy, D.J.; Riddell, A.J.; Rubin, P.C. A comparison of the effects of human alpha calcitonin gene-related peptide and glyceryl trinitrate on regional blood velocity in man. Br. J. Clin. Pharmacol. 1989, 28, 257–261. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Edvinsson, L. The trigeminovascular system and migraine: Studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann. Neurol. 1993, 33, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L.; Goadsby, P.J. Neuropeptides in migraine and cluster headache. Cephalalgia Int. J. Headache 1994, 14, 320–327. [Google Scholar] [CrossRef]
- Fischer, M.J.M.; Leffler, A.; Niedermirtl, F.; Kistner, K.; Eberhardt, M.; Reeh, P.W.; Nau, C. The General Anesthetic Propofol Excites Nociceptors by Activating TRPV1 and TRPA1 Rather than GABAA Receptors. J. Biol. Chem. 2010, 285, 34781–34792. [Google Scholar] [CrossRef] [PubMed]
- Pozsgai, G.; Hajna, Z.; Bagoly, T.; Boros, M.; Kemény, Á.; Materazzi, S.; Nassini, R.; Helyes, Z.; Szolcsányi, J.; Pintér, E. The role of transient receptor potential ankyrin 1 (TRPA1) receptor activation in hydrogen-sulphide-induced CGRP-release and vasodilation. Eur. J. Pharmacol. 2012, 689, 56–64. [Google Scholar] [CrossRef]
- Hansted, A.K.; Bhatt, D.K.; Olesen, J.; Jensen, L.J.; Jansen-Olesen, I. Effect of TRPA1 activator allyl isothiocyanate (AITC) on rat dural and pial arteries. Pharmacol. Rep. PR 2019, 71, 565–572. [Google Scholar] [CrossRef]
- Wang, X.-L.; Cui, L.-W.; Liu, Z.; Gao, Y.-M.; Wang, S.; Li, H.; Liu, H.-X.; Yu, L.-J. Effects of TRPA1 activation and inhibition on TRPA1 and CGRP expression in dorsal root ganglion neurons. Neural Regen. Res. 2019, 14, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Iversen, H.K. Experimental headache in humans. Cephalalgia Int. J. Headache 1995, 15, 281–287. [Google Scholar] [CrossRef]
- Anderson, P.J.; Lau, G.S.; Taylor, W.R.; Critchley, J.A. Acute effects of the potent lacrimator o-chlorobenzylidene malononitrile (CS) tear gas. Hum. Exp. Toxicol. 1996, 15, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Kelman, L. The triggers or precipitants of the acute migraine attack. Cephalalgia Int. J. Headache 2007, 27, 394–402. [Google Scholar] [CrossRef]
- Rozen, T.D. Cluster headache as the result of secondhand cigarette smoke exposure during childhood. Headache 2010, 50, 130–132. [Google Scholar] [CrossRef]
- Brône, B.; Peeters, P.J.; Marrannes, R.; Mercken, M.; Nuydens, R.; Meert, T.; Gijsen, H.J.M. Tear gasses CN, CR, and CS are potent activators of the human TRPA1 receptor. Toxicol. Appl. Pharmacol. 2008, 231, 150–156. [Google Scholar] [CrossRef]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M.; et al. TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Dubin, A.E.; Petrus, M.J.; Patapoutian, A. TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PLoS ONE 2009, 4, e7596. [Google Scholar] [CrossRef]
- Trevisani, M.; Siemens, J.; Materazzi, S.; Bautista, D.M.; Nassini, R.; Campi, B.; Imamachi, N.; Andrè, E.; Patacchini, R.; Cottrell, G.S.; et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. USA 2007, 104, 13519–13524. [Google Scholar] [CrossRef]
- Tunis, M.M.; Wolff, H.G. The hemodynamic analysis of cranial artery pulse wave contours in vascular headache of the migraine type. Trans. Am. Neurol. Assoc. 1952, 56, 22–25. [Google Scholar]
- Kobari, M.; Meyer, J.S.; Ichijo, M.; Imai, A.; Oravez, W.T. Hyperperfusion of cerebral cortex, thalamus and basal ganglia during spontaneously occurring migraine headaches. Headache 1989, 29, 282–289. [Google Scholar] [CrossRef]
- May, A.; Büchel, C.; Turner, R.; Goadsby, P.J. Magnetic resonance angiography in facial and other pain: Neurovascular mechanisms of trigeminal sensation. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2001, 21, 1171–1176. [Google Scholar] [CrossRef]
- Nassini, R.; Materazzi, S.; Vriens, J.; Prenen, J.; Benemei, S.; De Siena, G.; la Marca, G.; Andrè, E.; Preti, D.; Avonto, C.; et al. The “headache tree” via umbellulone and TRPA1 activates the trigeminovascular system. Brain J. Neurol. 2012, 135, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, Y.; Xu, Y.; Ma, D.; Wang, M. The Transient Receptor Potential Ankyrin Type 1 Plays a Critical Role in Cortical Spreading Depression. Neuroscience 2018, 382, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kunkler, P.E.; Knopp, K.L.; Oxford, G.S.; Hurley, J.H. Role of intraganglionic transmission in the trigeminovascular pathway. Mol. Pain 2019, 15, 1744806919836570. [Google Scholar] [CrossRef]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.B.; Gray, J.; Gunthorpe, M.J.; Hatcher, J.P.; Davey, P.T.; Overend, P.; Harries, M.H.; Latcham, J.; Clapham, C.; Atkinson, K.; et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 2000, 405, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.L.H.; Facer, P.; Davis, J.B.; Smith, G.D.; Egerton, J.; Bountra, C.; Williams, N.S.; Anand, P. Sensory fibres expressing capsaicin receptor TRPV1 in patients with rectal hypersensitivity and faecal urgency. Lancet Lond. Engl. 2003, 361, 385–391. [Google Scholar] [CrossRef]
- Ogawa, N.; Kurokawa, T.; Fujiwara, K.; Polat, O.K.; Badr, H.; Takahashi, N.; Mori, Y. Functional and Structural Divergence in Human TRPV1 Channel Subunits by Oxidative Cysteine Modification. J. Biol. Chem. 2016, 291, 4197–4210. [Google Scholar] [CrossRef]
- Kumar, R.; Hazan, A.; Geron, M.; Steinberg, R.; Livni, L.; Matzner, H.; Priel, A. Activation of transient receptor potential vanilloid 1 by lipoxygenase metabolites depends on PKC phosphorylation. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 1238–1247. [Google Scholar] [CrossRef]
- Robilotto, G.L.; Mohapatra, D.P.; Shepherd, A.J.; Mickle, A.D. Role of Src kinase in regulating protein kinase C mediated phosphorylation of TRPV1. Eur. J. Pain Lond. Engl. 2022, 26, 1967–1978. [Google Scholar] [CrossRef]
- Minke, B.; Cook, B. TRP Channel Proteins and Signal Transduction. Physiol. Rev. 2002, 82, 429–472. [Google Scholar] [CrossRef] [PubMed]
- Salas, M.M.; Hargreaves, K.M.; Akopian, A.N. TRPA1-mediated responses in trigeminal sensory neurons: Interaction between TRPA1 and TRPV1. Eur. J. Neurosci. 2009, 29, 1568–1578. [Google Scholar] [CrossRef]
- Fischer, M.J.M.; Balasuriya, D.; Jeggle, P.; Goetze, T.A.; McNaughton, P.A.; Reeh, P.W.; Edwardson, J.M. Direct evidence for functional TRPV1/TRPA1 heteromers. Pflüg. Arch.-Eur. J. Physiol. 2014, 466, 2229–2241. [Google Scholar] [CrossRef]
- Clapham, D.E. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.-J.; Patel, K.N.; Jeske, N.A.; Bierbower, S.M.; Zou, W.; Tiwari, V.; Zheng, Q.; Tang, Z.; Mo, G.C.H.; Wang, Y.; et al. Tmem100 Is a Regulator of TRPA1-TRPV1 Complex and Contributes to Persistent Pain. Neuron 2015, 85, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Montero, E.; Rodríguez-Muñoz, M.; Ruiz-Cantero, M.D.C.; Cobos, E.J.; Sánchez-Blázquez, P.; Garzón-Niño, J. Calmodulin Supports TRPA1 Channel Association with Opioid Receptors and Glutamate NMDA Receptors in the Nervous Tissue. Int. J. Mol. Sci. 2020, 22, 229. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.R.; Crown, E.D.; Moore, E.L.; Liang, H.A.; Choong, K.-C.; Dima, S.; Henze, D.A.; Kane, S.A.; Urban, M.O. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol. Pain 2008, 4, 48. [Google Scholar] [CrossRef]
- Hu, H.; Tian, J.; Zhu, Y.; Wang, C.; Xiao, R.; Herz, J.M.; Wood, J.D.; Zhu, M.X. Activation of TRPA1 channels by fenamate nonsteroidal anti-inflammatory drugs. Pflug. Arch. 2010, 459, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Joshi, S.K.; DiDomenico, S.; Perner, R.J.; Mikusa, J.P.; Gauvin, D.M.; Segreti, J.A.; Han, P.; Zhang, X.-F.; Niforatos, W.; et al. Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain 2011, 152, 1165–1172. [Google Scholar] [CrossRef]
- Mesch, S.; Walter, D.; Laux-Biehlmann, A.; Basting, D.; Flanagan, S.; Miyatake Ondozabal, H.; Bäurle, S.; Pearson, C.; Jenkins, J.; Elves, P.; et al. Discovery of BAY-390, a Selective CNS Penetrant Chemical Probe as Transient Receptor Potential Ankyrin 1 (TRPA1) Antagonist. J. Med. Chem. 2023, 66, 1583–1600. [Google Scholar] [CrossRef] [PubMed]
- Li Puma, S.; Landini, L.; Macedo, S.J.; Seravalli, V.; Marone, I.M.; Coppi, E.; Patacchini, R.; Geppetti, P.; Materazzi, S.; Nassini, R.; et al. TRPA1 mediates the antinociceptive properties of the constituent of Crocus sativus L., safranal. J. Cell. Mol. Med. 2019, 23, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Bressan, E.; Touska, F.; Vetter, I.; Kistner, K.; Kichko, T.I.; Teixeira, N.B.; Picolo, G.; Cury, Y.; Lewis, R.J.; Fischer, M.J.M.; et al. Crotalphine desensitizes TRPA1 ion channels to alleviate inflammatory hyperalgesia. Pain 2016, 157, 2504–2516. [Google Scholar] [CrossRef]
- Heber, S.; Gold-Binder, M.; Ciotu, C.I.; Witek, M.; Ninidze, N.; Kress, H.-G.; Fischer, M.J.M. A Human TRPA1-Specific Pain Model. J. Neurosci. Off. J. Soc. Neurosci. 2019, 39, 3845–3855. [Google Scholar] [CrossRef]
- Materazzi, S.; Benemei, S.; Fusi, C.; Gualdani, R.; De Siena, G.; Vastani, N.; Andersson, D.A.; Trevisan, G.; Moncelli, M.R.; Wei, X.; et al. Parthenolide inhibits nociception and neurogenic vasodilatation in the trigeminovascular system by targeting the TRPA1 channel. Pain 2013, 154, 2750–2758. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Pollastro, F.; Prenen, J.; Zhu, Z.; Appendino, G.; Nilius, B. Ligustilide: A novel TRPA1 modulator. Pflug. Arch. 2011, 462, 841–849. [Google Scholar] [CrossRef]
- Lipton, R.B.; Göbel, H.; Einhäupl, K.M.; Wilks, K.; Mauskop, A. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology 2004, 63, 2240–2244. [Google Scholar] [CrossRef] [PubMed]
- Benemei, S.; De Logu, F.; Li Puma, S.; Marone, I.M.; Coppi, E.; Ugolini, F.; Liedtke, W.; Pollastro, F.; Appendino, G.; Geppetti, P.; et al. The anti-migraine component of butterbur extracts, isopetasin, desensitizes peptidergic nociceptors by acting on TRPA1 cation channel. Br. J. Pharmacol. 2017, 174, 2897–2911. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, A.; Jalava, N.; Bratty, R.; Pertovaara, A. TRPA1 Antagonists for Pain Relief. Pharmaceuticals 2018, 11, 117. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masood, T.; Lakatos, S.; Rosta, J. Modification of the TRP Channel TRPA1 as a Relevant Factor in Migraine-Related Intracranial Hypersensitivity. Int. J. Mol. Sci. 2023, 24, 5375. https://doi.org/10.3390/ijms24065375
Masood T, Lakatos S, Rosta J. Modification of the TRP Channel TRPA1 as a Relevant Factor in Migraine-Related Intracranial Hypersensitivity. International Journal of Molecular Sciences. 2023; 24(6):5375. https://doi.org/10.3390/ijms24065375
Chicago/Turabian StyleMasood, Thannoon, Szandra Lakatos, and Judit Rosta. 2023. "Modification of the TRP Channel TRPA1 as a Relevant Factor in Migraine-Related Intracranial Hypersensitivity" International Journal of Molecular Sciences 24, no. 6: 5375. https://doi.org/10.3390/ijms24065375
APA StyleMasood, T., Lakatos, S., & Rosta, J. (2023). Modification of the TRP Channel TRPA1 as a Relevant Factor in Migraine-Related Intracranial Hypersensitivity. International Journal of Molecular Sciences, 24(6), 5375. https://doi.org/10.3390/ijms24065375




