Abstract
Genetic information, irrespective of cell type (normal or cancerous), is exposed to a range of harmful factors, which can lead to more than 80 different types of DNA damage. Of these, oxoG and FapyG have been identified as the most abundant in normoxic and hypoxic conditions, respectively. This article considers d[AFapyGAOXOGA]*[TCTCT] (oligo-FapyG) with clustered DNA lesions (CDLs) containing both the above types of damage at the M06-2x/6-31++G** level of theory in the condensed phase. Furthermore, the electronic properties of oligo-FapyG were analysed in both equilibrated and non-equilibrated solvation–solute interaction modes. The vertical/adiabatic ionization potential (VIP, AIP) and electron affinity (VEA, AEA) of the investigated ds-oligo were found as follows in [eV]: 5.87/5.39 and −1.41/−2.09, respectively. The optimization of the four ds-DNA spatial geometries revealed that the transFapydG was energetically privileged. Additionally, CDLs were found to have little influence on the ds-oligo structure. Furthermore, for the FapyGC base-pair isolated from the discussed ds-oligo, the ionization potential and electron affinity values were higher than those assigned to OXOGC. Finally, a comparison of the influence of FapyGC and OXOGC on charge transfer revealed that, in contrast to the OXOGC base-pair, which, as expected, acted as a radical cation/anion sink in the oligo-FapyG structure, FapyGC did not significantly affect charge transfer (electron–hole and excess–electron). The results presented below indicate that 7,8-dihydro-8-oxo-2′-deoxyguanosine plays a significant role in charge transfer through ds-DNA containing CDL and indirectly has an influence on the DNA lesion recognition and repair process. In contrast, the electronic properties obtained for 2,6-diamino-4-hydroxy-5-foramido-2′deoxypyrimidine were found to be too weak to compete with OXOG to influence charge transfer through the discussed ds-DNA containing CDL. Because increases in multi-damage site formation are observed during radio- or chemotherapy, understanding their role in the above processes can be crucial for the efficiency and safety of medical cancer treatment.
1. Introduction
Genetic information in the sequence of nucleobases of DNA is continuously exposed to harmful extra- and intra-cellular factors, both chemical and physical. These factors can lead to the formation of various types of DNA damage. To date, more than 80 different kinds of DNA lesions have been identified, including base-free sites, DNA-DNA or protein-DNA cross-links, tandem or clustered types, and sugar or base modifications [1]. From the perspective of future generations, it is highly desirable to preserve genetic information in its unchanged state. To this end, during evolution, prokaryotic and eukaryotic cells have developed DNA damage response (DDR) systems. The most common are base and nucleotide excision repair (BER, NER), mismatch repair, homologous recombination, and non-homologous end joining, all of which remove lesions in a substrate-dependent manner [2]. Disruption of these processes can lead to mutations which cause cancer and accelerate the aging process. Of all the nucleosides, 2′-deoxyguaosine is especially sensitive to harmful effects because of its particularly low oxidation potential (EOdG/dG•= 1.29 V) [3,4]. The lesion derived from guanosine has been identified as the most common. Its distribution depends on the endocellular environment (hypoxic or normoxic), the presence of a 2-deoxyribose substituent, and the ways in which hydroxyl radicals (●OH) are generated.
It should be noted that the cancer cells in close proximity to blood vases were well-oxygenated and that, as the distance increases, the condition of the microenvironment becomes hypoxic. The above oxygen gradient causes plasticity in the tumour cells and therefore increases their aggressive and metastatic phenotype. Additionally, an increase in the hypoxia-incubator factor can create an acidic environment, which results in tumour drug resistance, apoptosis/autophagy, various DNA damage, etc. For details, please refer to Shu and Simon’s recent and valuable work [5,6]. Moreover, dG can be also oxidized by other reactive oxygen and nitrogen species, free electrons, radiation, CO3●̶, etc. [7,8,9,10]. The 7,8-dihydro-8-oxo-2′-deoxyguanosine (OXOdG) has been identified as one of the most frequent dG lesions and is keenly studied because of its relative ease of measurement, chemical synthesis, and the different methods of its introduction into the oligonucleotide structure. The second most common dG lesion is 2,6-diamino-4-hydroxy-5-foramido-2′deoxypyrimidine (FapydG) [11]. In contrast to OXOdG, which is the predominant lesion formed in the presence of oxygen, FapydG is known to be the main lesion formed in hypoxic conditions [12]. However, in both cases, the shared intermediate 2-amino-8-hydroxy-1,7,9-thihydropurine-6-one (8-OHdGrad), which is formed in the presence of ●OH, has been noted. Depending on the condition of the medium, 8-OHdGrad can be converted into OXOdG by one-electron oxidation or FapydG by one-electron reduction, as shown in Figure 1. The above is justified by the high reaction constant of 8-OHdGrad with oxygen, i.e., k = 2 × 109 m−1s−1, while the constant of the imidazole ring-opening process has been found to be four magnitudes lower (k = 2 × 105 m−1s−1) [13].
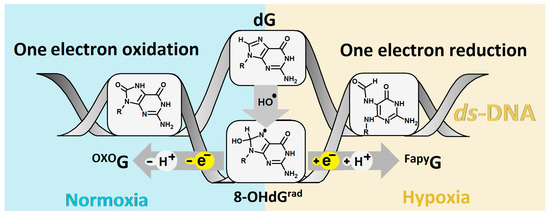
Figure 1.
Graphical representation of OXOdG and FapydG formation in ds-DNA as a product of hydroxyl radical action, depending on cellular condition.
Dizdaroglu et al. noted the level of FapydG in four different human breast cancer cell lines by GC/MS in a range of between 0.6 and 1.0 lesions per 106 nucleosides [14]. In the same studies, OXOdG was found to be between 0.07 and 0.15, depending on the cell lines. Arczewska et al. noted the frequency of FapyG in Caenorhabditis elegans (the medical and toxicological model organism) genome as 7.79 ± 0.47/106 DNA bases, while, in the case of OXOG, using the phenol extraction protocol for isolating genetic material, the frequency was 20.66 per 106 [15]. Recently, Dizdaroglu et al. noted the OXOG and FapyG levels as 14.62 ± 1.45 and 8.08 ± 0.9 per 106 DNA bases, respectively, using the high-salt DNA extraction method [11]. As discussed above, DNA damage frequency depends on several factors, such as the detection method, cell line, and isolation protocol; however, there is no doubt that the levels of FapyG and OXOG have always been at a similar level. Because of the difficulty of chemically synthesizing FapydG into DNA, the biochemical and biological roles of this lesion are limited. However, research has indicated that the methylated form of FapyG (ME-FapyG) almost completely inhibits DNA strand elongation, which suggests that it is more likely to be a lethal lesion than a mutagenic one [16]. The transversion potential G→C or G→T was noted as very low, as the polymerase inserts cytosine opposite ME-FapyG [17]. However, when FapyG was synthesized and introduced into the specified/required place of an oligonucleotide, its mutagenic potential was confirmed [18,19]. As in the case of OXOG, FapyG can form a base pair (BP) with adenine; furthermore, base misincorporation was observed to occur at the same level as for oxidized guanine [20]. Therefore, the presence of 2,6-diamino-4-hydroxy-5-foramido-2′deoxypyrimidine in the genome can lead to a mutation if it is not correctly repaired. Research has shown that FapyG is effectively removed from double-stranded (ds) DNA via formamidopyrimidine-DNA glycosylase (Fpg), which initiates the cascade of BER proteins [21]. This bifunctional glycosylase cut off FapyG more effectively when it formed a canonical base-pair with cytosine instead of adenine. Other enzymes involved in base excision repair machinery can also cleave the modification under discussion. OGG1 is active towards both FapydG and OXOdG (but not FapydA). In contrast to the above, Nei-like protein 1 (NEIL1) glycosylase removes FapyG but is inactive towards OXOG [22]. Consequently, the loss of the above proteins’ activity could be the source of GC→TA transversion. To avoid such a mutation, during evolution, organisms have developed adenosine DNA glycosylase (MutY), which removes adenine from its pair with dG, OXOdG, or FapydG [23]. Even though it is not abundant, MytY [24] can scan the Escherichia Coli genome in less than 10 min, which would almost be impossible to achieve with the application of the classical conformation of the DNA-protein switching mechanism [25]. As an alternative to the above mechanism, DNA damage site detection through electron transfer between two proteins containing [4Fe-4S] clusters was proposed by Barton et al. [26]. Lin et al. Postulated that OXOGA can accept the ejected electron and trigger MutY into action [25]. Even though this process has been investigated in the context of an OXOGA base pair, the influence of FapyG on charge transfer through the double helix and its direct comparison with 7,8-dihydro-8-oxo-2′-deoxyguanosine remains unclear. With the above in mind, this article presents, for the first time, a theoretical investigation into the influence of FapydG and OXOdG as part of a clustered DNA lesion (CDL) on the excess–electron and electron–hole migration process through double-stranded DNA. In addition, because all the biological processes take place in an aqueous environment, both non-equilibrated and equilibrated solvent models were taken into theoretical consideration.
2. Results
In this study, the short ds-oligonucleotide containing FapyG and OXOG was taken into consideration, i.e., d[A1FG2A3OG4A5]*[T1C2T3C4T5] (Figure 2), referred to throughout as oligo-FapyG. As mentioned in the introduction, both lesions are formed via an 8-OH-dG radical intermediate initiated by •OH activity [27]. Furthermore, because the imidazole ring is open, FapyG is, in fact, the pyrimidine that bonds to the 2-deoxyribose ring via the N9 atom (Figure 3). This indicates that the FapydG structure has a high level of conformational flexibility. Even though N9 lost its tertiary and aromatic character, FapydG was adopted in a ds-DNA anti-conformation instead of syn [18]. Additionally, as shown in Figure 3, four rotameric forms can be selected depending on rotation around the N7-C8 and C5-N7 linkages. For the energy calculation study, the base-pair’s geometries, formed by FapydG and dC, were isolated from the four optimized ds-pentamers containing the different rotameric forms of FapyG (Figure 3). All the ds-DNA spatial structures were fully optimized using Our Own N-layered Integrated Molecular Orbital and Molecular Mechanics (ONIOMs) method in the aqueous phase (please see the Materials and Method section). The comparison of calculated isolated base pair energies (M06-2x/6-31++G** in condensed phase) reveals the following order of stability: trans-2 (−1855.373892), cis-1 (−1855.373868), cis-2 (−1855.371179), trans-1 (−1855.367282), with all energies given in Hartree.
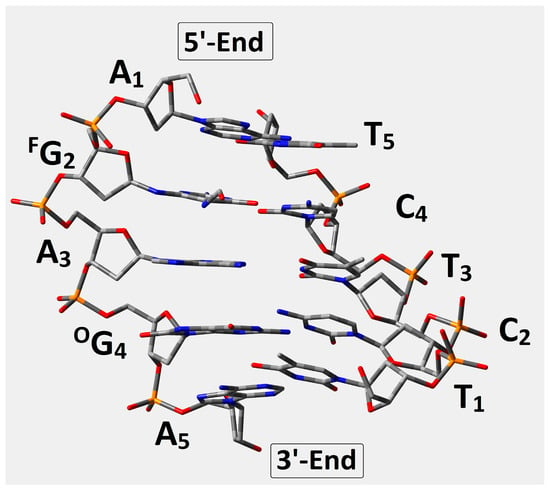
Figure 2.
Graphical representation of d[A1FG2A3OG4A5]*[T1C2T3C4T5] optimized spatial geometry at the M06-2x/D95** level of theory in the aqueous phase using the PCM solvation model. FG: 2,6-diamino-4-hydrox-5-foramido-2′deoxypyrimidine, OG:7,8-dihydro-8-oxo-2′-deoxyguanosine.
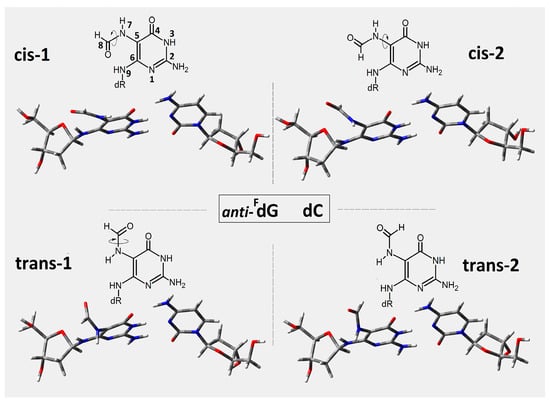
Figure 3.
The structure of four anti-FapyG:::dC rotamers isolated from initial d[A1FG2A3OG4A5]*[T1C2T3C4T5] structure optimized on the M06-2x/D95** level of theory in aqueous phase. The cis and trans forms have been assigned according to Carell et al. [18].
2.1. Electronic Properties and Geometry of oligo-FapyG
The stability of a double helix mainly depends on the following energies: the hydrogen bond (HB) energy between base pairs, staking interaction (ST) energy, and solvation shell–solute interaction energy [28]. In the latter case, which was tangentially covered in PCM, EHB and EST could be related to the HB length and the distance between the two neighbouring BPs (rise parameter), which were calculated in this study according to the standard reference frame for the description of nucleic acids [29]. As shown in Table 1, the presence of FapyG and OXOG did not change the HB length and rise parameter significantly in comparison to the corresponding native ds-oligo.

Table 1.
The structural local base-pair parameter: rise and dC1′.C1′ in [Å], λ1, and λ2 in [O] according to the standard DNA reference frame of oligo-FapyG. The hydrogen bond length in [Å] (HB-1: Ade(N1), Thy(N3) and Gua(N6), Cyt(O4); HB-2: Ade(N6), Thy(O4) and Gua(N1), Cyt(N3); HB-3: Gua(N2), Cyt(O2). F = Fapy, O = OXO.
In the case of the proposed ds-pentamer, both lesions are flanked by canonical base pairs from their 3′- and 5′-end sites. Additionally, FapydG and OXOdG did not interact together directly. Given this, the “global” vertical and adiabatic ionization potential and electronic affinity in non-equilibrated and equilibrated solvent interaction modes were calculated, as presented in Table 2. The results show that the complete ds-DNA and base-pair skeletons had the greatest differences in the case of vertical electron affinity calculated in the non-equilibrated mode (Δ = 0.25 eV), while, for the others, the difference was negligible. However, the appearance of FapyG and OXOG in the ds-DNA structure causes a vertical and adiabatic ionization potential decrease of around 0.20eV. In contrast, almost no influence on the electron affinity properties was observed (Table 2).

Table 2.
Electronic properties, in [eV], of oligo-FapyG: Vertical (VIP), adiabatic ionization potential (AIP) and vertical (VEA), adiabatic (AEA) electron affinity calculated at the M062x/6-31++G** level of theory in the aqueous phase. (a,c) complete double helix and (b,d) base-pair skeleton, NE—non-equilibrated solvent-solute interaction, EQ—equilibrated solvent–solute interaction, * data calculated for corresponding native ds-oligo [30]. Root-Mean-Square Deviation (RMSD) of atomic positions in [Å2], calculated for neutral, anionic and cationic forms of oligo-FapyG. The row data have been given in Table S1 of Supplementary Materials.
The global structural changes, forced by electron attachment or electron loss, were assigned as differences between atomic positions between neutral versus anionic or cationic forms of oligo-FapyG and expressed as RMSD in [Å2] (Table 2). As expected, the charge changes were compensated for by the flexible phosphate–sugar backbone, while the internal part containing base pairs showed almost no deviation. Furthermore, the appearance of an extra electron in the system results in an RMSD value that is about twice as high as the value for the case of a lost electron.
2.2. The Charge and Spin Distribution Oligo-FapyG Structure
It is worthwhile to investigate the influence of CDL containing two lesions formed via the same intermediate, i.e., FapyG and OXOG, on charge and spin distribution. For this proposal, the Hirschfeld methodology was applied [31]. In addition, the initial stage of the charged molecule formation, after electron adoption or ejection, was examined in light of the non-equilibrated solvent–solute interaction. The radical cation or anion migrates through stacked base pairs, and, for this reason, only the internal skeleton of ds-DNA was considered in this study. As shown in Figure 4, the radical cation (electron hole) formed (charge: 84% and spin: 90%) settles mainly on the OXOG4:::C2 base pair, with some dispersion over A3T3 and A5T1. The differences in charge and spin distribution between the non-equilibrated and equilibrated state of the vertical cation was less than 5% per the BPs mentioned. This finding is consistent with previous experimental and theoretical results [32,33]. It is important to note that no extra charge and spin was found within the FapyG2::C4 moiety. The situation becomes different when an extra electron is introduced into the oligo-FapyG structure. The initiation point of the discussed system has been described using the non-equilibrated solvent–solute interaction. The negative charge and spin are dispersed over A5T1, OG4C2, A3T3 base pairs as follows (% of charge/spin): 9/7, 70/81, and 15/11, respectively. Surprisingly, after solvent–solute equilibration, the layout discussed above changed, and the density [%] of charge/spin of the extra electron increases at A5T1 (28/26), with subsequent decreases at an OG4C2(59/66) moiety, as shown in Figure 4. However, after structure relaxation towards the adiabatic radical anion forms, the negative charge was observed almost exclusively at the OXOG4C2 moiety (spin 94% and charge 80%).
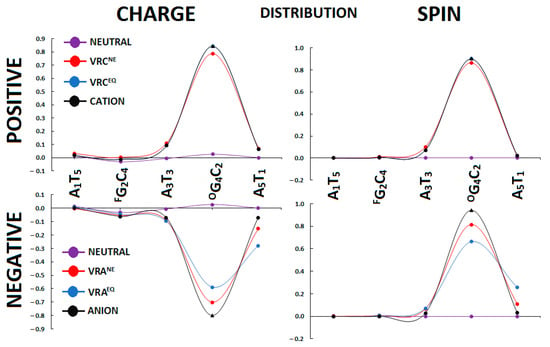
Figure 4.
Spin and charge distribution within oligo-FaoyG calculated at the M062x/6-31++G** level of theory in the condensed phase; only the stacked base pairs were taken into consideration. VRC—vertical radical cation, VRA—vertical radical anion, NE—non-equilibrated, and EQ—equilibrated solvent–solute interaction. The charge and spin distribution of vertical and adiabatic forms. The raw data have been given in Table S2. F = Fapy, O = OXO.
2.3. Electronic Properties of a Single Base-Pair Extracted from Oligo-FapyG
For this study, the spatial geometry of oligo-FapyG was optimized in its neutral, anion, and cation forms. Such a strategy allowed the calculation of each base-pair’s electronic properties, as shown in Table 3.

Table 3.
Electronic properties of isolated base pairs from oligo-FapyG: vertical (VIP), adiabatic ionization potential (AIP), and vertical (VEA) and adiabatic (AEA) electron affinity calculated at the M062x/6-31++G** level of theory in the condensed phase. The row data has been given in supplementary materials Table S3.
An analysis of the results elucidated that the OXOG4C2 base-pair adopted the lowest vertical and adiabatic ionization potentials, i.e., 5.9 and 5.56 eV, respectively. For the other base pairs, the difference between VIP and AIP was negligible, which indicates that the electron–hole can travel without nucleus movement. Moreover, the vertical ionization potential value (6.17 eV) of FapyG2C4 was found comparable to that assigned for the GC base pairs extracted from corresponding unmodified ds-oligo [30]. This was partially confirmed by an analysis of the vertical and adiabatic electron affinity of the base pairs belonging to oligo-FapyG. The OXOGC moiety demonstrated its predisposition to excess electron adoption, with the highest absolute values of VEA (1.53 eV) and AEA (1.97 eV) being noted. As expected, the FapyGC base pairs adopted a slightly lower vertical value, i.e., 1.46 eV.
2.4. The Rate Constant of Charge Transfer through oligo-FapyG
Charge transfer through ds-DNA can occur in its oxidized or reductive state, as described by Marcus theory, i.e., the charge transfer depends on the kHT (rate constant), ΔG (driving force), λ (reorganization), Ea (activation), and Vda (electron coupling) energies [34]. All these parameters for this study were calculated at the M06-2x/6-31++G** level of theory in the aqueous phase. For details, please see reference [35]. Furthermore, the influence of two different DNA lesions, FapyG and OXOG (forming CDL), on hole and electron migration through the double helix were taken into consideration in light of the Marcus theory. As shown in Table 4, the highest Ea was observed in the cases of electron–hole transfer between FapyG2C2 and next to A1T1 and A3T3. On the other hand, when FapyG becomes the bridge between the two BPs, i.e., A1T1 and A3T3, Ea adopted a negative value, which results in kHT being undetectable. The above indicates that the transfer between A1T5, A3T3, and A5T1 pairs should be unaffected and extremely fast. Additionally, the kHT value obtained for the electron–hole transfer towards OXOG was in a range of 108 to 1014 [s−1]. Moreover, the influence of FapyG on the excess electron transfer was less visible in each point of deliberation, with adopted values in a range of between 1012 and 1013[s−1]. An exception is when FapyG becomes the bridge, i.e., A1T1←A3T3, for which the activation and reorganization become negative. This indicates that FapyG accelerated the electron transfer, as opposed to OXOG, for which 4.7 × 10−13 [s−1] kHT was found.

Table 4.
Charge transfer parameters. The ΔG-driving force, λ-reorganization energy, Ea-activation energy, V12-electron coupling, and kHT-charge rate constant of permissible transfer between base pairs of oligo-FapyG, calculated at the m062x/6-31++G** level of theory in the aqueous phase and given in eV. Arrows indicate the direction of charge migration. The row data have been given in Table S4.
The above results coincide well with the assigned charge energy barrier as presented in Figure 5. The charge migration barrier can be discussed in vertical and adiabatic modes on account of the nature of the process, as described in a previous study [35]. In brief, the oligo-FapyG was initially divided into three ds-trimmers: A1FapyG2A3, FapyG2A3OXOG4, and A3OXOG4A5 (the system notation was reduced to a purine strand only). The barriers of radical cation/anion transfer can be calculated as an iterative single-step super-exchange or hole/electron hopping process, as depicted in Figure 5. The details have been given in the Supplementary Materials and Table S5. As expected, the radical cation migration from the FapyGC→AT (~0.5 eV) or from the OXOGC→AT (~1.4 eV) pairs were unprivileged (ΔGBarrier of reaction was noted as positive; see Table S2). The above indicates that OXOGC constituted a more effective electron–hole trap than FapyGC. This was confirmed by the negative ΔGBarrier value of the reverse process, i.e., AT→FapyGC (−0.49 eV) or AT→OXOGC (−1.1 eV).
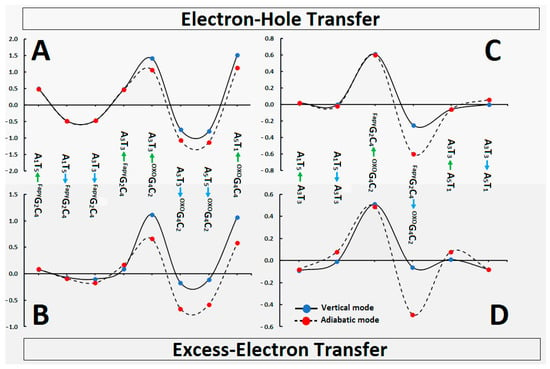
Figure 5.
In [eV], the energy barrier profile of the charge migration process through the oligo-FapyG contained CDL in vertical (black line and blue spot) and adiabatic (black dash and red spot) modes. (A,B) represented the direct transfer between neighbour base pairs, (C,D) the transfer over the single BP bridge. The numeric data has been given in Table S5.
The situation is similar to the previously discussed excess–electron transfer. As presented in Figure 5 and Table S2, the difference between the adiabatic and vertical modes around FapyGC was negligible. Moreover, the ΔG value ranged from 0.09 eV to −0.09 eV. As previously, the OXOGC pair can be recognized as a sink of the excess electron, which was confirmed by the significantly negative ΔG values of A3T3→OXOG4C2, A5T1→OXOG4C2 and FapyG2C4→OXOG4C2 as follows (vertical/adiabatic mode) in [eV]: −0.17/−0.66, −0.11/−0.58, −0.06/−0.49, respectively.
3. Discussion
The double helix contained two main structural subunits: (A) a phosphate–sugar backbone, which is highly flexible and can compensate for geometry changes forced by external factors, and (B) a rigid internal skeleton formed by the complementary aromatic nucleo-base connected by hydrogen bonds together with a stacking interaction stabilizing the ds-oligonucleotide structure [36]. Research recognizes that the latter rigid structure allows ds-DNA to act as a nanowire capable of transferring the electron–hole (radical cation) or excess electron over a long distance, possibly more than a thousand nucleosides, according to Barton et al. [37]. This is in good agreement with research showing that glycosylases such as MutY or Endo III can communicate via ds-DNA scanning in the effective detection of lesions [38]. It should be mentioned here that other proteins involved in the replication and repair of genetic material probably use a similar mechanism, many of which contain the [4Fe-4S] cluster [39].
Of all the DNA lesions, clustered DNA lesions (CDLs), defined as two or more lesions per one or two double-helix turns, pose a particularly serious challenge for DNA repair systems [40]. During CDL removal from the genome, cell “rescue” systems try to avoid a double-strand break (DSB) formation, which is potentially lethal [41]. In the vast majority of DNA damage in the genome, FapyG was yielded by a one-electron reduction process, while OXOG was yielded by one-electron oxidation. As mentioned in the introduction, these two lesions are formed preferably in conditions of hypoxia and normoxia, respectively. This suggests that they could demonstrate a different influence on charge transfer through the double helix. In the studies presented here, all the energies of d[AFapyGAOXOGA]*[TCTCT] (oligo-FapyG) with clustered DNA lesions containing both above damage types at the M06-2x/6-31++G** level of theory in the condensed phase were considered; the ds-oligo geometries in question were optimized at the M06-2x/D95**level of theory in the condensed phase. The analysis of four optimized oligo-FapyG spatial geometries contained in the FapydG structure in the two cis and two trans-rotameric forms showed the trans-2 to be energetically privileged (Figure 3). Therefore, the ds-oligo containing a trans-2 rotamer of FapyG was selected for further theoretical discussion, as depicted in Figure 2. It should be noted that, using alternative calculation methods, some research groups assigned the domination of the cis form over trans for the isolated FapydG [18,24,42,43]. The comparative structural analysis presented in this article showed that the influence of a multi-damage site including FapyG and OXOG as subunits on the discussed ds-oligo structure was noted as negligible according to the standard DNA reference frame for the analysis of structural parameters (Table 1). Moreover, the presented results are in good agreement with previous results obtained for ds-oligo containing two OXOG as a clustered damage moiety [30], which indicates that the lesion only has a negligible effect on the 3D structure of ds-DNA.
The adoption or loss of an electron by ds-DNA leads to radical cation or radical anion formation, which can migrate through a double helix for more than 200Å [44]. Most studies have discussed the electronic properties of double-stranded trimers, which are a good model for discussing single lesions, but unfortunately fail in the case of CDLs [45,46]. For these reasons, investigating the influence of the CDLs on charge transfer through a double helix, as discussed in this study, seems eminently justified.
As mentioned above, the loss or adoption of an electron can initiate electron–hole or extra electron migration through ds-DNA [47]. This process is performed using charge displacement through a π-stacked base-pair ladder. The data presented in Table 2 indicates that, during vertical anion radical formation, the involvement of the sugar-phosphate backbone appears to be minimal. This observation tallies with data obtained for the corresponding native ds-oligo [30]. Additionally, the resent research of Sevilla et al. shows that, for this process, solute–solvent interaction is crucial at the beginning point [28].
These charge and spin distribution results (Figure 4) indicate that charge transfers, through a double helix containing the discussed CDL to the destination point (OXOG2C4), should be almost unaffected by the presence of FapyG2C4. OXOG is simply preferred over FapyG as the point where the electron–hole settles in the genome. Similar to the above, the FapyG::C moiety present in the oligo-FapyG was not affected by an extra electron, which suggests that, in the electron transfer process through the double helix, 2,6-diamino-4-hydroxy-5-foramido-2′deoxypyrimidine plays a negligible role. Moreover, 94% and 80% spin and charge, respectively, were found on OXOG4C2.
The charge transfer process through ds-DNA can be discussed as single-step tunnelling, a random-walk multistep, and polaron-like hopping [48]. Each type of hole or electron migration strictly depends on the mutual positions, spatial geometries, and individual electronic properties of the base pairs. It should be pointed out here that these factors are different when they are calculated for isolated BP and BPs extracted from a ds-oligo structure, as shown in previous research [35]. An incoherent charge migration through the double helix can be observed on the hundreds of base pairs and depends on the nature of the bridge between the donor and acceptor [48]. On the other hand, at a distance of only a few BPs, tunneling paths can be noted. Even though the above mechanisms are different, the geometry and electronic properties of purine/pyrimidine moieties are crucial. OXOdG, when present in the ds-oligo structure, has been identified as a radical cation sink and terminates its movement [49]. According to the results presented above, it can be assumed that charge transfer through FapyG2C4 as a bridge should be unaffected (Table 4), similar to what was noted for a canonical BP [32,50]. In addition, the OXOGC moiety of clustered DNA damage can be predicted as the end point of the extra electron transfer. Furthermore, the electron transfer through the discussed ds-pentamer should not be affected other than by OXOGC base pairs on account of the similarity between VEA and AEA values in their cases (Table 3). Conversely, the presence of FapyG in the investigated system effectively slows down the hole transfer via a single-step tunneling process. The above yields kHT values close to zero even though the ΔG was negative (Table 4). Additionally, as shown in Figure 5 and Table S2, no differences between the vertical and adiabatic modes were noted for the charge transfer in the proximity of the FapyGC base-pair. Moreover, the hole-hopping barrier between A1T5←→A3T3 and A5T1←→A3T3 was measured as almost zero eV for both vertical and adiabatic modes (Figure 5). As above, the radical cation migration from FapyGC towards OXOGC over the AT bridge was noted as privileged.
4. Materials and Methods
The starting geometries of bi-stranded oligo-FapyG were built using BIOVIA Discovery Studio Visualizer v20.1.0.19295 software [51] and noted accordingly: d[A1FapyG2A3OXOG4A5]*d[T5C4T3C2T1]. The Cartesian coordinates (i.e.: pdb files) of discussed ds-oligo structure have been given in the supplementary materials.
The negative charges of the phosphate groups were neutralized via the addition of protons, and the other atoms were saturated using additional hydrogen atoms. The structure optimizations of oligo-FapyG were performed using the Our Own N-layered Integrated Molecular Orbital and Molecular Mechanics (ONIOMs) strategy [52]. The structures of ds-oligos were divided into high—HL (nucleobases, M06-2X/D95**) and low—LL (sugar-phosphate backbone, M06-2X/sto-3G) levels of calculation [53]. All calculations were performed in the aqueous phase. The M06-2X function with an augmented and polarized valence double-ζ basis set 6-31++G** was used for energy calculations. For all the optimized geometries, charge, and spin analyses were achieved using the Hirshfeld methodology at the M06-2X/6-31++G** level of theory [31]. The electronic properties of molecules were calculated as described previously [35,54]. The transition dipole moment of excited states and the single point calculation at the M06-2X/6-31++G** level of theory were performed using time-dependent DFT (TD-DFT) methodology [55]. Electron coupling was calculated according to the Generalized Mulliken–Hush model [56]. The solvation–solute interaction was investigated in both non-equilibrium (NE) and equilibrated (EQ) modes [57]. All the calculations of the electronic properties, i.e., VIPNE (vertical ionization potential in NE state), VIPEQ (vertical ionization potential in EQ state), AIP (adiabatic ionization potential); VEANE (vertical electron affinity in NE state), VEAEQ (vertical electron affinity in EQ state), AEA (adiabatic electron affinity), were conducted in [eV], as described previously [34]. All the above calculations were performed with the Gaussian G16 (version C.01) software package [58].
5. Conclusions
Genetic information, irrespective of cell type—normal or cancerous—is exposed to various harmful factors which can lead to more than 80 different types of DNA damage. Of these, oxoG and FapyG have been identified as the most abundant in normoxic and hypoxic conditions, respectively.
This study considered all the energies of d[AFapyGAOXOGA]*[TCTCT] (oligo-FapyG) with clustered DNA lesions containing both above damage types at the M06-2x/6-31++G** level of theory in the condensed phase; the ds-oligo geometries in question were optimized at the M06-2x/D95** level of theory in the condensed phase. The electronic properties of oligo-FapyG were analysed in equilibrated and non-equilibrated solvation–solute interaction modes.
- The vertical/adiabatic ionization potential (VIPEQ, AIP) and electron affinity (VEA, AEA) of the investigated ds-oligo were noted in [eV] as 5.87/5.39 and −1.41/−2.09, respectively;
- The analysis of four optimized oligo-FapyG spatial geometries contained in the FapydG structure in the two cis and two trans-rotameric forms showed the trans-2 to be energetically privileged. Subsequently, the influence of a multi-damage site including FapyG and OXOG as subunits on the discussed ds-oligo structure was noted as negligible according to the standard DNA reference frame for the analysis of structural parameters. Furthermore, neither the adoption of an excess electron or the loss of an electron by the oligo-FapyG forced a significant macromolecule structural distortion;
- Additionally, for the FapyGC base-pair isolated from the discussed ds-oligo, the ionization potential and electron affinity values were observed to be higher than those assigned for OXOGC;
- These results imply that FapyGC does not have a significant effect on electron–hole or excess–electron charge transfer. In parallel to this, the OXOGC base-pair becomes the radical cation/anion sink in the oligo-FapyG structure, as expected.
The results presented above indicate that 7,8-dihydro-8-oxo-2′-deoxyguanosine plays a significant role in charge transfer through ds-DNA containing CDLs and indirectly has an impact on DNA lesion recognition and repair processes. Conversely, the electronic properties obtained for 2,6-diamino-4-hydroxy-5-foramido-2′deoxypyrimidine were found to be too weak to compete with OXOG in influencing a charge transfer through the discussed ds-DNA containing CDLs.
The results presented in the article shed further light on clustered DNA damage, which might be useful for the safety of future generations. Man’s continual desire to colonize worlds beyond Earth requires a study of astronaut safety protocols and research into nutrition, among other things. In addition, as our longevity increases, so too do incidents of cancer. The slowdown and weakness of DNA damage repair machinery is also an age-dependent process. Therefore, it is vital to consider and further investigate “patient-friendly” radiotherapy treatments that use ionization radiation for killing cancer cells. New forays into radiotherapy are necessary to make the dose delivery more effective while reducing the detrimental and highly undesirable effects on healthy tissue surrounding a tumour. The discovery of a different kind of DNA damage raises the question of its influence, not only on the CDL repair, formation, and DNA replication processes, but also on charge transfer, and therefore on DNA damage recognition. Therefore, the results presented in this article show the possible role of FapydG in the “DNA recognition and repair machinery” from the perspective of multiple local damaged sites composed of different lesions, i.e., OXOdG.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24065361/s1.
Funding
This study was supported by the Medical University of Lodz (503/3-045-02/503-31-002) and in part by PL-Grid infrastructure (Prometheus, ACC Cyfronet AGH).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Evans, M.D.; Dizdaroglu, M.; Cooke, M.S. Oxidative DNA Damage and Disease: Induction, Repair and Significance; Elsevier: Amsterdam, The Netherlands, 2004; Volume 567, ISBN 1301975850. [Google Scholar]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef] [PubMed]
- Steenken, S.; Jovanovic, S.V. How easily oxidizable is DNA? One-electron reduction potentials of adenosine and guanosine radicals in aqueous solution. J. Am. Chem. Soc. 1997, 119, 617–618. [Google Scholar] [CrossRef]
- Seidel, C.A.M.; Schulz, A.; Sauer, M.H.M. Nucleobase-Specific Quenching of Fluorescent Dyes. 1. Nucleobase One-Electron Redox.pdf. Society 1996, 100, 5541–5553. [Google Scholar]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 1–15. [Google Scholar] [CrossRef]
- Keith, B.; Simon, M.C. Hypoxia-Inducible Factors, Stem Cells, and Cancer. Cell 2007, 129, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Kupan, A.; Saulière, A.; Broussy, S.; Seguy, C.; Pratviel, G.; Meunier, B. Guanine oxidation by electron transfer: One-versus two-electron oxidation mechanism. ChemBioChem 2006, 7, 125–133. [Google Scholar] [CrossRef]
- Shukla, L.I.; Adhikary, A.; Pazdro, R.; Becker, D.; Sevilla, M.D. Formation of 8-oxo-7,8-dihydroguanine-radicals in γ-irradiated DNA by multiple one-electron oxidations. Nucleic Acids Res. 2004, 32, 6565–6574. [Google Scholar] [CrossRef]
- Shafirovich, V.; Mock, S.; Kolbanovskiy, A.; Geacintov, N.E. Photochemically catalyzed generation of site-specific 8-nitroguanine adducts in DNA by the reaction of long-lived neutral guanine radicals with nitrogen dioxide. Chem. Res. Toxicol. 2002, 15, 591–597. [Google Scholar] [CrossRef]
- Shukla, P.K.; Mishra, P.C. Catalytic involvement of CO2 in the mutagenesis caused by reactions of ONOO- with guanine. J. Phys. Chem. B 2008, 112, 4779–4789. [Google Scholar] [CrossRef]
- Scanlan, L.D.; Coskun, S.H.; Jaruga, P.; Hanna, S.K.; Sims, C.M.; Almeida, J.L.; Catoe, D.; Coskun, E.; Golan, R.; Dizdaroglu, M.; et al. Measurement of Oxidatively Induced DNA Damage in Caenorhabditis elegans with High-Salt DNA Extraction and Isotope-Dilution Mass Spectrometry. Anal. Chem. 2019, 91, 12149–12155. [Google Scholar] [CrossRef]
- Greenberg, M.M. The formamidopyrimidines: Purine lesions formed in competition with 8-oxopurines from oxidative stress. Acc. Chem. Res. 2012, 45, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Candeias, S.S.L. Reaction of HO* with guanine derivatives in aqueous solution: Formation of two different redox-active OH-adduct radicals and their unimolecular transformation reactions. Properties of G(-H)*. Chem.—A Eur. J. 2000, 475–484. [Google Scholar] [CrossRef]
- Nyaga, S.G.; Jaruga, P.; Lohani, A.; Dizdaroglu, M.; Evans, M.K. Accumulation of oxidatively induced DNA damage in human breast cancer cell lines following treatment with hydrogen peroxide. Cell Cycle 2007, 6, 1471–1477. [Google Scholar] [CrossRef]
- Arczewska, K.D.; Tomazella, G.G.; Lindvall, J.M.; Kassahun, H.; Maglioni, S.; Torgovnick, A.; Henriksson, J.; Matilainen, O.; Marquis, B.J.; Nelson, B.C.; et al. Active transcriptomic and proteomic reprogramming in the C. elegans nucleotide excision repair mutant xpa-1. Nucleic Acids Res. 2013, 41, 5368–5381. [Google Scholar] [CrossRef]
- Boiteux, S.; Laval, J. Imidazole open ring 7-methylguanine: An inhibitor of DNA synthesis. Biochem. Biophys. Res. Commun. 1983, 110, 552–558. [Google Scholar] [CrossRef]
- Tudek, B.; Gra̧ziewicz, M.; Kazanova, O.; Zastawny, T.H.; Obtułowicz, T.; Laval, J. Mutagenic specificity of imidazole ring-opened 7-methylpurines in M13mp18 phage DNA. Acta Biochim. Pol. 1999, 46, 785–799. [Google Scholar] [CrossRef]
- Burgdorf, L.T.; Carell, T. Synthesis, Stability, and Conformation of the Formamidopyrimidine G DNA Lesion. Chem.—A Eur. J. 2002, 8, 293–301. [Google Scholar] [CrossRef]
- Wiederholt, C.J.; Greenberg, M.M. Fapy·dG instructs Klenow Exo- to misincorporate deoxyadenosine. J. Am. Chem. Soc. 2002, 124, 7278–7279. [Google Scholar] [CrossRef]
- Grollman, A.P.; Moriya, M. Mutagenesis by 8-oxoguanine: An enemy within. Trends Genet. 1993, 9, 246–249. [Google Scholar] [CrossRef]
- Boiteux, S.; O’Connor, T.R.; Laval, J. Formamidopyrimidine-DNA glycosylase of Escherichia coli: Cloning and sequencing of the fpg structural gene and overproduction of the protein. EMBO J. 1987, 6, 3177–3183. [Google Scholar] [CrossRef]
- Jaruga, P.; Birincioglu, M.; Rosenquist, T.A.; Dizdaroglu, M. Mouse NEIL1 protein is specific for excision of 2,6-diamino-4-hydroxy-5- formamidopyrimidine and 4,6-diamino-5-formamidopyrimidine from oxidatively damaged DNA. Biochemistry 2004, 43, 15909–15914. [Google Scholar] [CrossRef] [PubMed]
- Wiederholt, C.J.; Delaney, M.O.; Pope, M.A.; David, S.S.; Greenberg, M.M. Repair of DNA containing Fapy·dG and its β-C-nucleoside analogue by formamidopyrimidine DNA glycosylase and MutY. Biochemistry 2003, 42, 9755–9760. [Google Scholar] [CrossRef] [PubMed]
- Jena, N.R.; Mark, A.E.; Mishra, P.C. Does tautomerization of FapyG influence its mutagenicity? ChemPhysChem 2014, 15, 1779–1784. [Google Scholar] [CrossRef]
- Lin, J.C.; Singh, R.R.P.; Cox, D.L. Theoretical study of DNA damage recognition via electron transfer from the [4Fe-4S] complex of MutY. Biophys. J. 2008, 95, 3259–3268. [Google Scholar] [CrossRef]
- Boal, A.K.; Yavin, E.; Barton, J.K. DNA repair glycosylases with a [4Fe-4S] cluster: A redox cofactor for DNA-mediated charge transport? J. Inorg. Biochem. 2007, 101, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Munk, B.H.; Burrows, C.J.; Schlegel, H.B. Exploration of mechanisms for the transformation of 8-hydroxy guanine radical to FAPyG by density functional theory. Chem. Res. Toxicol. 2007, 20, 432–444. [Google Scholar] [CrossRef]
- Kumar, A.; Adhikary, A.; Sevilla, M.D.; Close, D.M. One-electron oxidation of ds(5′-GGG-3′) and ds(5′-G(8OG)G-3′) and the nature of hole distribution: A density functional theory (DFT) study. Phys. Chem. Chem. Phys. 2020, 22, 5078–5089. [Google Scholar] [CrossRef]
- Olson, W.K.; Bansal, M.; Burley, S.K.; Dickerson, R.E.; Gerstein, M.; Harvey, S.C.; Heinemann, U.; Lu, X.; Neidle, S.; Shakked, Z.; et al. N OMENCLATURE A Standard Reference Frame for the Description of Nucleic Acid Base-pair Geometry. J. Mol. Biol. 2001, 313, 229–237. [Google Scholar] [CrossRef]
- Karwowski, B.T. How Clustered DNA Damage Can Change the Electronic Properties of ds-DNA. Differences between GAG, GAOXOG, and OXOGAOXOG. Biomolecules 2023. (Accepted for publication). [Google Scholar]
- Hirshfeld, F.L. Bonded-atom fragments for describing molecular charge densities. Theor. Chim. Acta 1977, 44, 129–138. [Google Scholar] [CrossRef]
- Sanii, L.; Schuster, G.B.; June, R. V Long-Distance Charge Transport in DNA: Sequence-Dependent Radical Cation Injection Efficiency. J. Am. Chem. Soc. 2000, 122, 11545–11546. [Google Scholar] [CrossRef]
- Karwowsk, B.T. The influence of single, tandem, and clustered DNA damage on the electronic properties of the double helix: A theoretical study. Molecules 2020, 25, 3126. [Google Scholar] [CrossRef] [PubMed]
- Marcus, R.A. Electron Transfer Reactions in Chemistry: Theory and Experiment (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1993, 32, 1111–1121. [Google Scholar] [CrossRef]
- Karwowski, B.T. The AT Interstrand Cross-Link: Structure, Electronic Properties, and Influence on Charge Transfer in dsDNA. Mol. Ther.—Nucleic Acids 2018, 13, 665–685. [Google Scholar] [CrossRef] [PubMed]
- Mignon, P.; Loverix, S.; Steyaert, J.; Geerlings, P. Influence of the π-π interaction on the hydrogen bonding capacity of stacked DNA/RNA bases. Nucleic Acids Res. 2005, 33, 1779–1789. [Google Scholar] [CrossRef]
- Arnold, A.R.; Grodick, M.A.; Barton, J.K. Review DNA Charge Transport: From Chemical Principles to the Cell. Cell Chem. Biol. 2016, 23, 183–197. [Google Scholar] [CrossRef]
- Boal, A.K.; Yavin, E.; Lukianova, O.A.; O’Shea, V.L.; David, S.S.; Barton, J.K. DNA-bound redox activity of DNA repair glycosylases containing [4Fe-4S] clusters. Biochemistry 2005, 44, 8397–8407. [Google Scholar] [CrossRef]
- Cammack, R. Iron-Sulfur Proteins, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume 2, ISBN 9780123786319. [Google Scholar]
- Lomax, M.E.; Folkes, L.K.; Neill, P.O. Biological Consequences of Radiation-induced DNA Damage: Relevance to Radiotherapy Statement of Search Strategies Used and Sources of Information Why Radiation Damage is More Effective than Endogenous Damage at Killing Cells Ionising Radiation-induced Do. Clin. Oncol. 2013, 25, 578–585. [Google Scholar] [CrossRef]
- Sage, E.; Harrison, L. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis Clustered DNA lesion repair in eukaryotes: Relevance to mutagenesis and cell survival. Mutat. Res.—Fundam. Mol. Mech. Mutagen. 2011, 711, 123–133. [Google Scholar] [CrossRef]
- Jena, N.R.; Mishra, P.C. Is FapyG mutagenic?: Evidence from the DFT study. ChemPhysChem 2013, 14, 3263–3270. [Google Scholar] [CrossRef]
- Reynisson, J.; Steenken, S. DFT calculations on the electrophilic reaction with water of the guanine and adenine radical cations. A model for the situation in DNA. Phys. Chem. Chem. Phys. 2002, 4, 527–532. [Google Scholar] [CrossRef]
- Elias, B.; Shao, F.; Barton, J.K. Charge migration along the DNA duplex: Hole versus electron transport. J. Am. Chem. Soc. 2008, 130, 1152–1153. [Google Scholar] [CrossRef] [PubMed]
- Voityuk, A.A.; Jortner, J.; Bixon, M.; Rösch, N. Energetics of hole transfer in DNA. Chem. Phys. Lett. 2000, 324, 430–434. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Grozema, F.C.; Guerra, C.F.; Bickelhaupt, F.M.; Siebbeles, L.D.A. Mapping the Sites for Selective Oxidation of Guanines in DNA. J. Am. Chem. Soc. 2003, 125, 13658–13659. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Majima, T. Hole transfer kinetics of DNA. Acc. Chem. Res. 2013, 46, 2616–2625. [Google Scholar] [CrossRef]
- Lewis, F.D.; Liu, J.; Weigel, W.; Rettig, W.; Kurnikov, I.V.; Beratan, D.N. Donor-bridge-acceptor energetics determine the distance dependence of electron tunneling in DNA. Proc. Natl. Acad. Sci. USA 2002, 99, 12536–12541. [Google Scholar] [CrossRef]
- Aust, A.E.; Eveleigh, J.F. Mechanisms of DNA oxidation. Proc. Soc. Exp. Biol. Med. 1999, 222, 246–252. [Google Scholar] [CrossRef]
- Schuster, G.B.; Landman, U. The Mechanism of Long-Distance Radical Cation Transport in Duplex DNA: Ion-Gated Hopping of Polaron-Like Distortions. In Long-Range Charge Transfer in DNA I; Springer: Berlin/Heidelberg, Germany, 2012; pp. 139–161. [Google Scholar] [CrossRef]
- BIOVIA. Discovery Studio Visualizer; v16.1.0.15350; BIOVIA: San Diego, CA, USA, 2015. [Google Scholar]
- Dapprich, S.; Komáromi, I.; Byun, K.S.; Morokuma, K.; Frisch, M.J. A new ONIOM implementation in Gaussian98. Part I. The calculation of energies, gradients, vibrational frequencies and electric field derivatives. J. Mol. Struct. THEOCHEM 1999, 461–462, 1–21. [Google Scholar] [CrossRef]
- Zhao, Y.; Pu, J.; Lynch, B.J.; Truhlar, D.G. Tests of second-generation and third-generation density functionals for thermochemical kineticsElectronic supplementary information (ESI) available: Mean errors for pure and hybrid DFT methods. See http://www.rsc.org/suppdata/cp/b3/b316260e/. Phys. Chem. Chem. Phys. 2004, 6, 673. [Google Scholar] [CrossRef]
- Karwowski, B.T. Ionisation potential and electron affinity of free 5′,8-cyclopurine-2′-deoxynucleosides. DFT study in gaseous and aqueous phase. Cent. Eur. J. Chem. 2010, 8, 70–76. [Google Scholar] [CrossRef]
- Kumar, A.; Sevilla, M.D. Photoexcitation of dinucleoside radical cations: A time-dependent density functional study. J. Phys. Chem. B 2006, 110, 24181–24188. [Google Scholar] [CrossRef] [PubMed]
- Cave, R.J.; Newton, M.D. Generalization of the Mulliken-Hush treatment for the calculation of electron transfer matrix elements. Chem. Phys. Lett. 1996, 249, 15–19. [Google Scholar] [CrossRef]
- Miertus̃, S.; Tomasi, J. Approximate evaluations of the electrostatic free energy and internal energy changes in solution processes. Chem. Phys. 1982, 65, 239–245. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2019; Volume 2019, p. 2019. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).