The Role of Olfactomedin 2 in the Adipose Tissue–Liver Axis and Its Implication in Obesity-Associated Nonalcoholic Fatty Liver Disease
Abstract
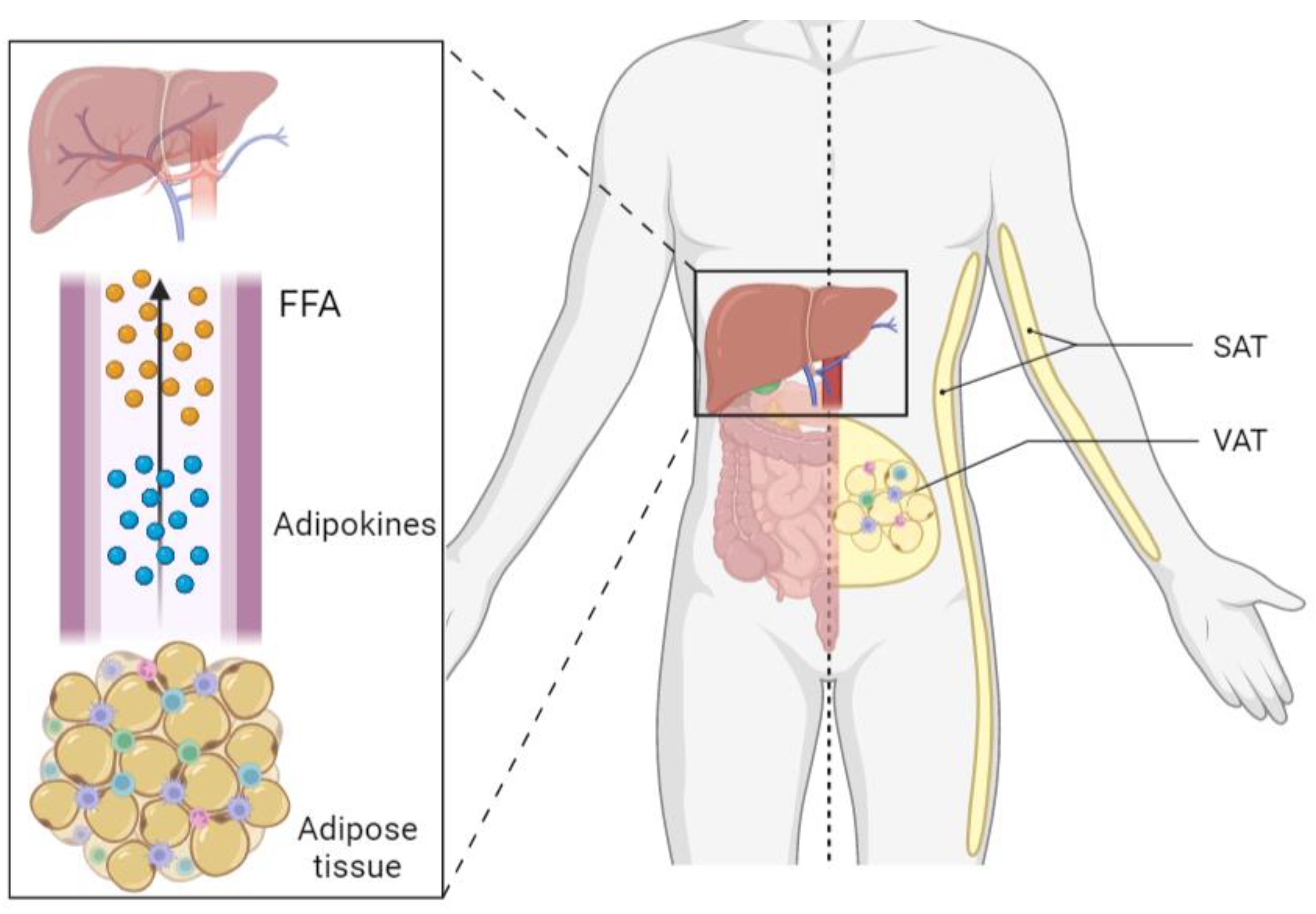
1. Introduction
2. Results
2.1. Patients’ Baseline Characteristics
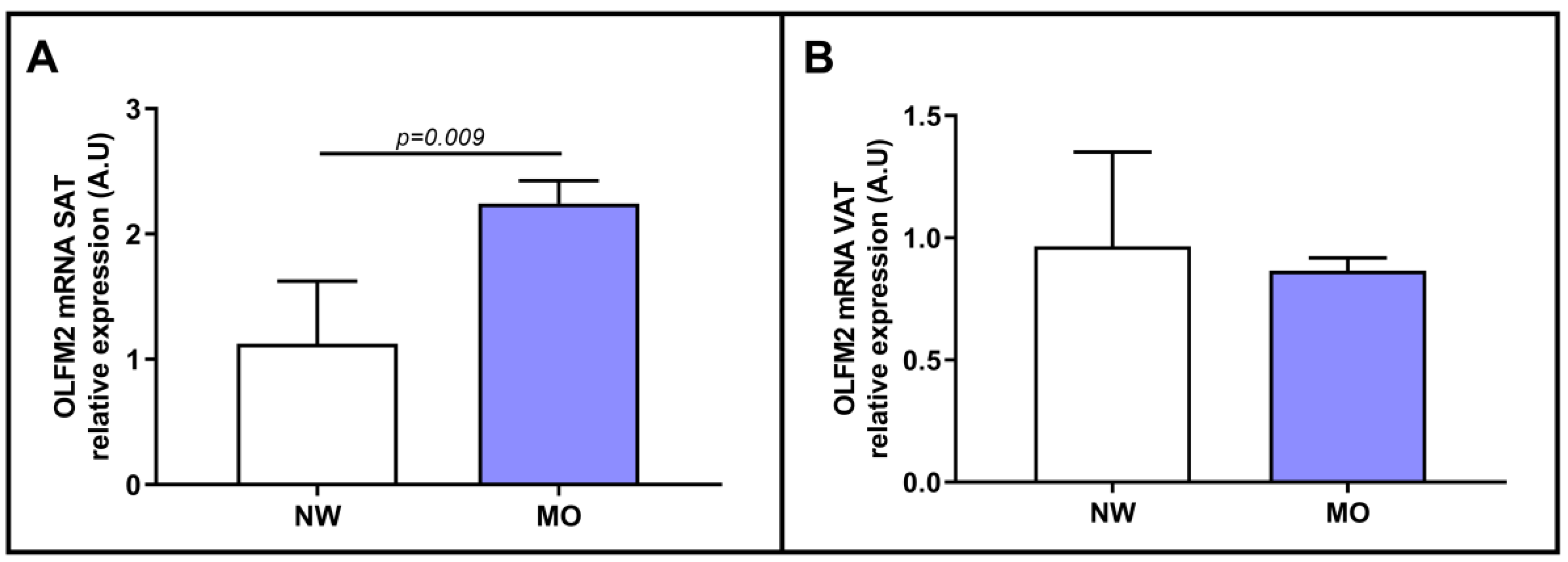
2.2. Evaluation of the Relative mRNA Expression of OLFM2 in the Adipose Tissue of Patients with NW or MO
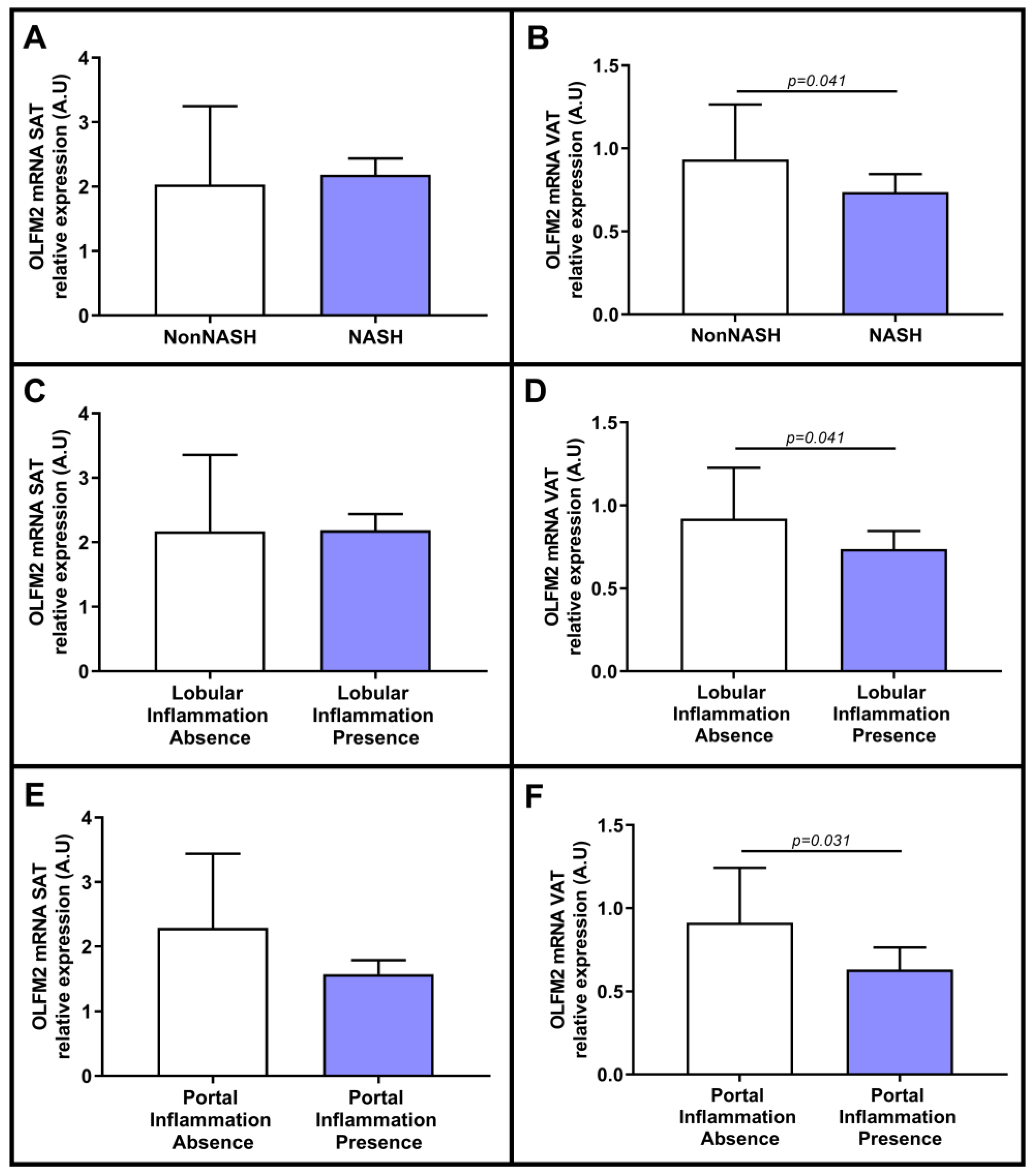
2.3. Evaluation of the Relative mRNA Expression of OLFM2 in Adipose Tissue Based on Hepatic Histology
2.4. Evaluation of Relative OLFM2 mRNA Expression in Adipose Tissues Based on Liver Steatosis Degree
2.5. Evaluation of Relative OLFM2 mRNA Expression in Adipose Tissue in the Presence of NASH and in Relation to NASH-Related Parameters
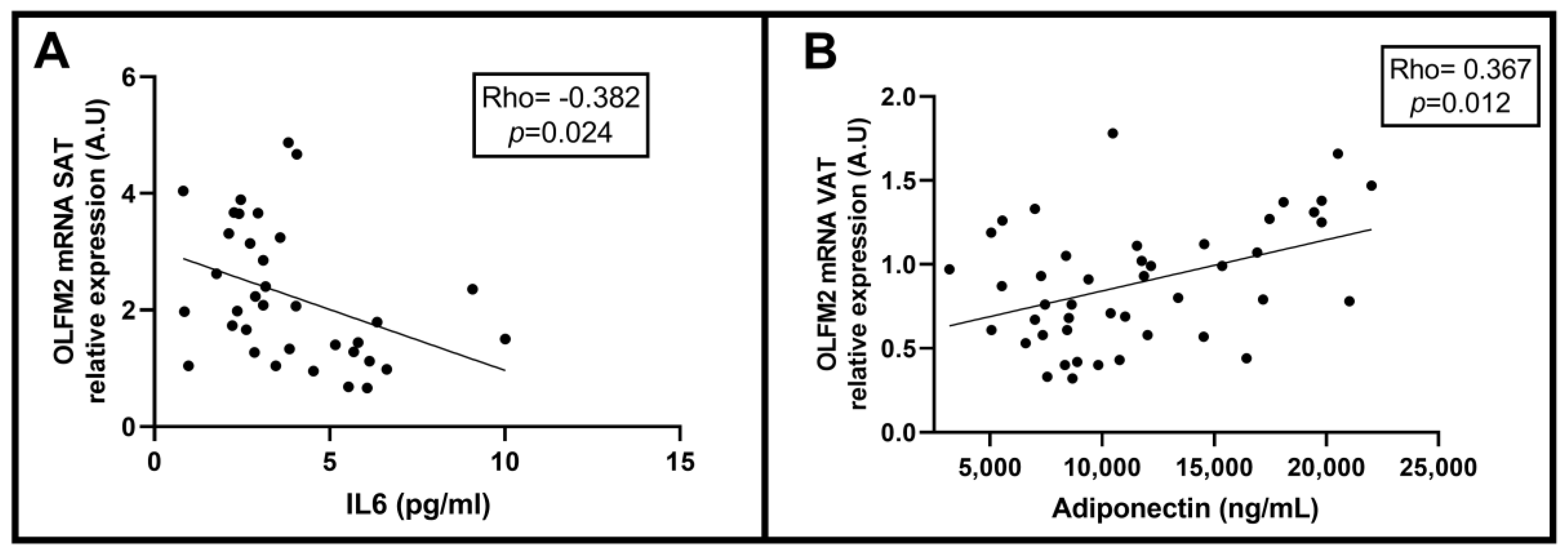
2.6. Correlation between Relative OLFM2 mRNA Expression in SAT and VAT and Clinical and Biochemical NAFLD-Related Parameters
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Sample Size
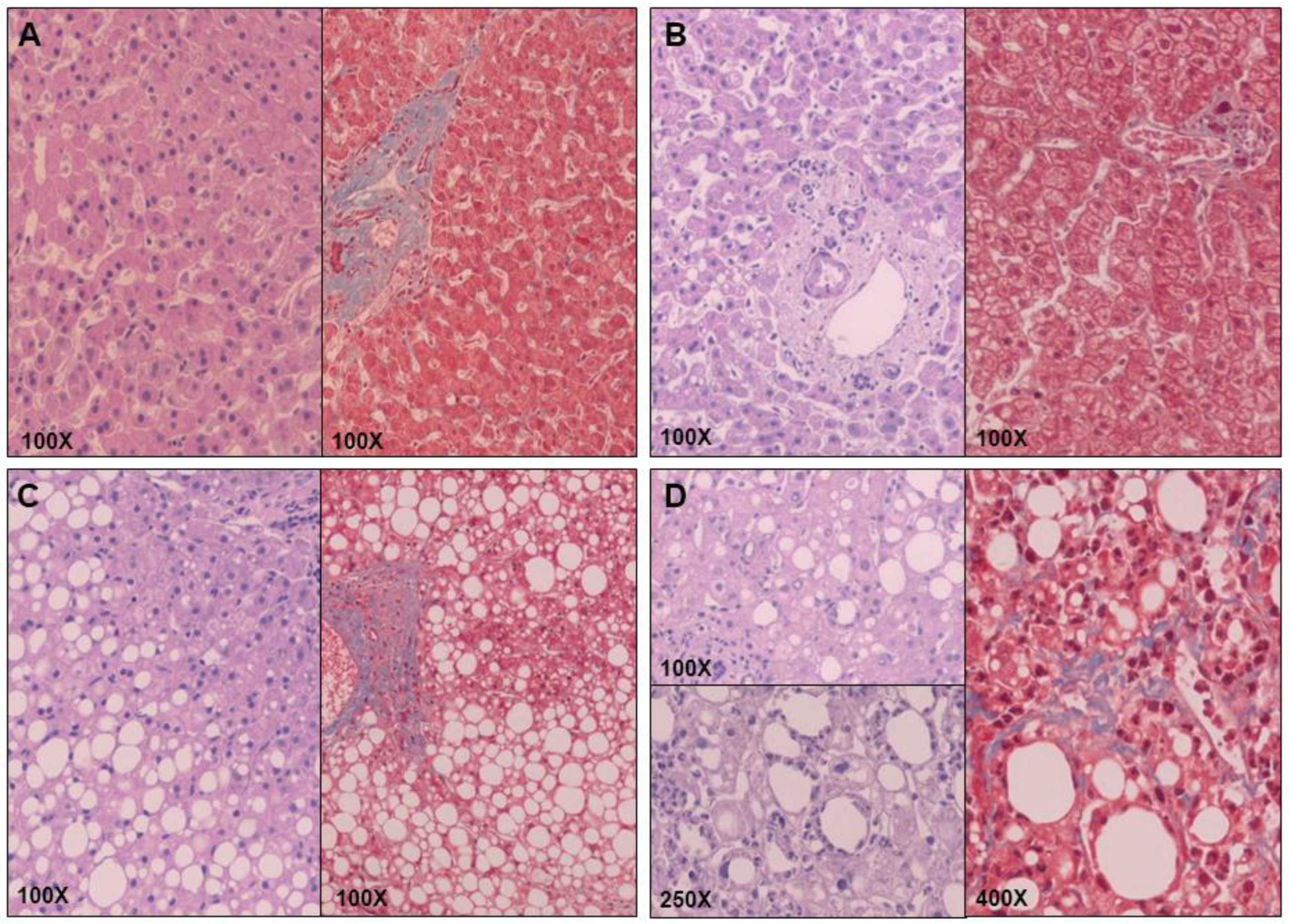
4.3. Liver Pathology
4.4. Biochemical Analyses
4.5. Gene Expression in Liver
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cotter, T.G.; Rinella, M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology 2020, 158, 1851–1864. [Google Scholar] [CrossRef]
- Cobbina, E.; Akhlaghi, F. Non-Alcoholic Fatty Liver Disease (NAFLD)—Pathogenesis, Classification, and Effect on Drug Metabolizing Enzymes and Transporters. Drug Metab. Rev. 2017, 49, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Evolution of Inflammation in Nonalcoholic Fatty Liver Disease: The Multiple Parallel Hits Hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Adolph, T.E.; Moschen, A.R. Multiple Parallel Hits Hypothesis in Nonalcoholic Fatty Liver Disease: Revisited After a Decade. Hepatology 2021, 73, 833–842. [Google Scholar] [CrossRef]
- Mittal, B. Subcutaneous Adipose Tissue & Visceral Adipose Tissue. Indian J. Med. Res. 2019, 149, 571–573. [Google Scholar] [CrossRef]
- Alligier, M.; Gabert, L.; Meugnier, E.; Lambert-Porcheron, S.; Chanseaume, E.; Pilleul, F.; Debard, C.; Sauvinet, V.; Morio, B.; Vidal-Puig, A.; et al. Visceral Fat Accumulation During Lipid Overfeeding Is Related to Subcutaneous Adipose Tissue Characteristics in Healthy Men. J. Clin. Endocrinol. Metab. 2013, 98, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remón, A.; Martínez-González, M.A.; de la Torre, R.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Inverse Association between Habitual Polyphenol Intake and Incidence of Cardiovascular Events in the PREDIMED Study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 639–647. [Google Scholar] [CrossRef]
- Spoto, B.; Di Betta, E.; Mattace-Raso, F.; Sijbrands, E.; Vilardi, A.; Parlongo, R.M.; Pizzini, P.; Pisano, A.; Vermi, W.; Testa, A.; et al. Pro- and Anti-Inflammatory Cytokine Gene Expression in Subcutaneous and Visceral Fat in Severe Obesity. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1137–1143. [Google Scholar] [CrossRef]
- Smith, C.J.; Perfetti, T.A.; Hayes, A.W.; Berry, S.C. Obesity as a Source of Endogenous Compounds Associated With Chronic Disease: A Review. Toxicol. Sci. 2020, 175, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Dufour, J.-F.; Anstee, Q.M.; Bugianesi, E.; Harrison, S.; Loomba, R.; Paradis, V.; Tilg, H.; Wong, V.W.-S.; Zelber-sagi, S. Current Therapies and New Developments in NASH. Gut 2022, 71, 2123–2134. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Nonalcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Non-Alcoholic Fatty Liver Disease: Assessment and Management; National Institute for Health and Care Excellence: London, UK, 2016; ISBN 978-1-4731-1996-3.
- Tomarev, S.I.; Nakaya, N. Olfactomedin Domain-Containing Proteins: Possible Mechanisms of Action and Functions in Normal Development and Pathology. Mol. Neurobiol. 2009, 40, 122–138. [Google Scholar] [CrossRef]
- González-García, I.; Freire-Agulleiro, Ó.; Nakaya, N.; Ortega, F.J.; Garrido-Gil, P.; Liñares-Pose, L.; Fernø, J.; Labandeira-Garcia, J.L.; Diéguez, C.; Sultana, A.; et al. Olfactomedin 2 Deficiency Protects against Diet-Induced Obesity. Metabolism 2022, 129, 155122. [Google Scholar] [CrossRef]
- Holt, R.; Ugur Iseri, S.A.; Wyatt, A.W.; Bax, D.A.; Gold Diaz, D.; Santos, C.; Broadgate, S.; Dunn, R.; Bruty, J.; Wallis, Y.; et al. Identification and Functional Characterisation of Genetic Variants in OLFM2 in Children with Developmental Eye Disorders. Hum. Genet. 2017, 136, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sun, N.-N.; Zhao, Z.-N.; He, S.-X.; Zhang, M.; Zhang, D.-D.; Yu, X.-W.; Zhang, J.-M.; Fan, Z.-G. Coinheritance of OLFM2 and SIX6 Variants in a Chinese Family with Juvenile-Onset Primary Open-Angle Glaucoma: A Case Report. WJCC 2021, 9, 697–706. [Google Scholar] [CrossRef]
- Zhang, R.; Ye, J.; Huang, H.; Du, X. Mining Featured Biomarkers Associated with Vascular Invasion in HCC by Bioinformatics Analysis with TCGA RNA Sequencing Data. Biomed. Pharmacother. 2019, 118, 109274. [Google Scholar] [CrossRef] [PubMed]
- OLFM2 Protein Expression Summary—The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000105088-OLFM2 (accessed on 7 December 2022).
- Bertran, L.; Jorba-Martin, R.; Barrientos-Riosalido, A.; Portillo-Carrasquer, M.; Aguilar, C.; Riesco, D.; Martínez, S.; Vives, M.; Sabench, F.; Castillo, D.D.; et al. New Insights of OLFM2 and OLFM4 in Gut-Liver Axis and Their Potential Involvement in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 7442. [Google Scholar] [CrossRef]
- Miles, J.M.; Jensen, M.D. Counterpoint: Visceral Adiposity Is Not Causally Related to Insulin Resistance. Diabetes Care 2005, 28, 2326–2328. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Hazlehurst, J.M.; Hull, D.; Guo, K.; Borrows, S.; Yu, J.; Gough, S.C.; Newsome, P.N.; Tomlinson, J.W. Abdominal Subcutaneous Adipose Tissue Insulin Resistance and Lipolysis in Patients with Non-Alcoholic Steatohepatitis. Diabetes Obes. Metab 2014, 16, 651–660. [Google Scholar] [CrossRef]
- Freedland, E.S. Role of a Critical Visceral Adipose Tissue Threshold (CVATT) in Metabolic Syndrome: Implications for Controlling Dietary Carbohydrates: A Review. Nutr. Metab. 2004, 1, 12. [Google Scholar] [CrossRef]
- Ronquillo, M.D.; Mellnyk, A.; Cárdenas-Rodríguez, N.; Martínez, E.; Comoto, D.A.; Carmona-Aparicio, L.; Herrera, N.E.; Lara, E.; Pereyra, A.; Floriano-Sánchez, E. Different Gene Expression Profiles in Subcutaneous & Visceral Adipose Tissues from Mexican Patients with Obesity. Indian J. Med. Res. 2019, 149, 616–626. [Google Scholar] [CrossRef]
- Pandžić Jakšić, V.; Grizelj, D. Under the Surface of Subcutaneous Adipose Tissue Biology. Acta Derm. Croat. 2016, 24, 250–260. [Google Scholar]
- du Plessis, J.; van Pelt, J.; Korf, H.; Mathieu, C.; van der Schueren, B.; Lannoo, M.; Oyen, T.; Topal, B.; Fetter, G.; Nayler, S.; et al. Association of Adipose Tissue Inflammation With Histologic Severity of Nonalcoholic Fatty Liver Disease. Gastroenterology 2015, 149, 635–648.e14. [Google Scholar] [CrossRef] [PubMed]
- Chiyanika, C.; Wong, V.W.-S.; Wong, G.L.-H.; Chan, H.L.-Y.; Hui, S.C.N.; Yeung, D.K.W.; Chu, W.C.W. Implications of Abdominal Adipose Tissue Distribution on Nonalcoholic Fatty Liver Disease and Metabolic Syndrome: A Chinese General Population Study. Clin. Transl. Gastroenterol. 2021, 12, e00300. [Google Scholar] [CrossRef]
- Item, F.; Konrad, D. Visceral Fat and Metabolic Inflammation: The Portal Theory Revisited. Obes. Rev. 2012, 13 (Suppl. 2), 30–39. [Google Scholar] [CrossRef]
- Pafili, K.; Kahl, S.; Mastrototaro, L.; Strassburger, K.; Pesta, D.; Herder, C.; Pützer, J.; Dewidar, B.; Hendlinger, M.; Granata, C.; et al. Mitochondrial Respiration Is Decreased in Visceral but Not Subcutaneous Adipose Tissue in Obese Individuals with Fatty Liver Disease. J. Hepatol. 2022, 77, 1504–1514. [Google Scholar] [CrossRef] [PubMed]
- Uciechowski, P.; Dempke, W.C.M. Interleukin-6: A Masterplayer in the Cytokine Network. Oncology 2020, 98, 131–137. [Google Scholar] [CrossRef]
- Pararasa, C.; Bailey, C.J.; Griffiths, H.R. Ageing, Adipose Tissue, Fatty Acids and Inflammation. Biogerontology 2015, 16, 235–248. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose Tissue Inflammation and Metabolic Dysfunction in Obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Finelli, C.; Tarantino, G. Is Visceral Fat Reduction Necessary to Favour Metabolic Changes in the Liver? J. Gastrointestin. Liver Dis. 2012, 21, 205–208. [Google Scholar]
- Polyzos, S.A.; Kountouras, J.; Zavos, C.; Tsiaousi, E. The Role of Adiponectin in the Pathogenesis and Treatment of Non-Alcoholic Fatty Liver Disease. Diabetes Obes. Metab. 2010, 12, 365–383. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M.; Janney, C.G.; Di Bisceglie, A.M.; Neuschwander-Tetri, B.A.; Bacon, B.R. Nonalcoholic Steatohepatitis: A Proposal for Grading and Staging The Histological Lesions. Am. J. Gastroenterol. 1999, 94, 2467–2474. [Google Scholar] [CrossRef]


| MO (n = 60) | ||||
|---|---|---|---|---|
| Variables | NW (n = 16) | NL (n = 20) | SS (n = 21) | NASH (n = 19) |
| Weight (kg) | 57.75 (52.10–62.00) | 119,00 (108.50–134.00) * | 115.40 (111.63–129.80) * | 110.50 (104.00–121.68) |
| Age (years) | 42.93 (37.06–51.93) | 42.30(35.00–52.04) | 46.01 (36.11–53.1615) | 48.41 (40.62–57.88) |
| Waist (cm) | 72.00 (70.00–84.00) | 124.20 (119.75–130.00) * | 125.00 (116.75–134.00) * | 123.50 (115.00–126.75) * |
| BMI (kg/m2) | 22.47 (21.29–24.19) | 43.62 (41.56–48.83) * | 44.94 (42.07–46.83) * | 44.54 (40.95–47.23) |
| DBP (mmHg) | 70.00 (66.00–75.00) | 63.00 (58.00–71.00) | 62.00 (59.00–73.25) | 68.00 (60.25–78.00) |
| SBP (mmHg) | 117.00 (110.00–125.00) | 120.00 (100.00–132.00) | 116.00 (108.00–127.00) | 117.00 (105.00–132.00) |
| HOMA1-IR | 1.30 (0.85–2.17) | 2.07 (1.22–3.45) | 2.44 (1.27–3.05) * | 1.90 (1.45–6.16) * |
| Glucose (mg/dL) | 83.58 (64.81–94.34) | 85.59 (77.58–92.84) | 93.09 (88.08–106.11) * $ | 93.09 (88.09–106.11) * $ |
| Insulin (mUI/L) | 7.00 (4.90–9.62) | 9.70 (5.59–16.21) | 9.80 (6.94–14.10) * | 9.54 (5.68–26.02) * |
| HbA1c (%) | 5.10 (4.70–5.40) | 5.60 (5.30–5.75) * | 5.55 (5.3–5.85) * | 5.55 (5.30–5.85) * |
| TG (mg/dL) | 86.00 (57.25–110.25) | 106.00 (93.00–136.00) * | 117.50 (84.00–165.50) * | 130.50 (99.25–187.50) * |
| Cholesterol (mg/dL) | 183.25 (163.53–209.50) | 170.00 (150.15–214.50) | 165.70 (138.75–189.50) * | 162.00 (150.50–213.25) |
| HDL-C (mg/dL) | 62.40 (48.45–73.00) | 40.20 (31.50–48.50) * | 43.50 (33.25–46.75) * | 38.00 (34.50–44.00) * |
| LDL-C (mg/dL) | 112.20 (89.90–130.00) | 108.80 (95.20–141.80) | 103.10 (77.20–124.86) | 93.40 (79.30–126.83) |
| AST (UI/L) | 20.00 (16.00–26.00) | 19.50 (15.00–36.25) | 21.00 (17.00–31.00) | 30.00 (18.00–43.50) * |
| ALT (UI/L) | 17.00 (12.50–25.00) | 21.00 (16.00–37.00) | 29.50 (22.00–35.00) * | 33.50 (18.75–41.00) * |
| GGT (UI/L) | 14.00 (10.00–31.00) | 17.00 (13.00–23.00) | 21.00 (16.25–32.75) * | 26.00 (19.75–34.00) * |
| ALP (Ul/L) | 65.00 (51.50–88.00) | 57.50 (47.75–71.75) | 73.50 (62.00–86.00) $ | 61.00 (53.25–74.50) $ # |
| Liver Histology: | ||||
| Steatosis Grade 0/1/2/3 | - | 20/0/0/0 | 0/15/6/0 | 0/3/16/0 |
| Inflammation Grade 0/1/2/3 | - | 20/0/0/0 | 21/0/0/0 | 2/18/5/0 |
| Ballooning 0/1/2 | - | 20/0/0/0 | 21/0/0/0 | 2/17/0/0 |
| Fibrosis Stage 0/1/2/3/4 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrientos-Riosalido, A.; Bertran, L.; Vilaró-Blay, M.; Aguilar, C.; Martínez, S.; Paris, M.; Sabench, F.; Riesco, D.; Binetti, J.; Castillo, D.D.; et al. The Role of Olfactomedin 2 in the Adipose Tissue–Liver Axis and Its Implication in Obesity-Associated Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2023, 24, 5221. https://doi.org/10.3390/ijms24065221
Barrientos-Riosalido A, Bertran L, Vilaró-Blay M, Aguilar C, Martínez S, Paris M, Sabench F, Riesco D, Binetti J, Castillo DD, et al. The Role of Olfactomedin 2 in the Adipose Tissue–Liver Axis and Its Implication in Obesity-Associated Nonalcoholic Fatty Liver Disease. International Journal of Molecular Sciences. 2023; 24(6):5221. https://doi.org/10.3390/ijms24065221
Chicago/Turabian StyleBarrientos-Riosalido, Andrea, Laia Bertran, Mercè Vilaró-Blay, Carmen Aguilar, Salomé Martínez, Marta Paris, Fàtima Sabench, David Riesco, Jessica Binetti, Daniel Del Castillo, and et al. 2023. "The Role of Olfactomedin 2 in the Adipose Tissue–Liver Axis and Its Implication in Obesity-Associated Nonalcoholic Fatty Liver Disease" International Journal of Molecular Sciences 24, no. 6: 5221. https://doi.org/10.3390/ijms24065221
APA StyleBarrientos-Riosalido, A., Bertran, L., Vilaró-Blay, M., Aguilar, C., Martínez, S., Paris, M., Sabench, F., Riesco, D., Binetti, J., Castillo, D. D., Richart, C., & Auguet, T. (2023). The Role of Olfactomedin 2 in the Adipose Tissue–Liver Axis and Its Implication in Obesity-Associated Nonalcoholic Fatty Liver Disease. International Journal of Molecular Sciences, 24(6), 5221. https://doi.org/10.3390/ijms24065221








