Research Progress on the Construction and Application of a Diabetic Zebrafish Model
Abstract
1. Introduction
2. Methods of Diabetic Zebrafish Model Construction
2.1. Type I Diabetes Model Construction Methods
2.1.1. Method of Surgical Resection
2.1.2. Drug Induction Method
2.1.3. Induction by Genetic Modification
2.2. Construction Methods for Type II Diabetes
2.2.1. Glucose Solution Immersion Method
2.2.2. High-Fat Food Induction Method
2.2.3. CRISPR/Cas9 Gene Knockout Method
2.2.4. Genetic Ablation Method
2.3. Evaluation Indicators of Diabetic Zebrafish Models
3. Types of Zebrafish Models of Diabetes and Their Complications
3.1. Maturity-Onset Diabetes Mellitus Model
3.2. Gestational Diabetes Mellitus Model
3.3. Diabetic Cardiovascular Disease Model
3.4. Diabetic Retinopathy Model
3.5. Neurological Complications
3.6. Diabetic Nephropathy
3.7. Diabetic Wound Model
3.8. Diabetic Immune Injury Model
4. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schuster, D.P.; Duvuuri, V. Diabetes mellitus. Clin. Podiatr. Med. Surg. 2002, 19, 79–107. [Google Scholar] [CrossRef]
- Alam, U.; Asghar, O.; Azmi, S.; Malik, R.A. General aspects of diabetes mellitus. Handb. Clin. Neurol. 2014, 126, 211–222. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Teck, J. Diabetes-Associated Comorbidities. Prim. Care Clin. Off. Pract. 2022, 49, 275–286. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2012, 36, S67–S74. [Google Scholar] [CrossRef]
- Pecoits-Filho, R.; Abensur, H.; Betônico, C.C.R.; Machado, A.D.; Parente, E.B.; Queiroz, M.; Salles, J.E.N.; Titan, S.; Vencio, S. Interactions between kidney disease and diabetes: Dangerous liaisons. Diabetol. Metab. Syndr. 2016, 8, 50. [Google Scholar] [CrossRef]
- Munshi, M.N.; Florez, H.; Huang, E.S.; Kalyani, R.R.; Mupanomunda, M.; Pandya, N.; Swift, C.S.; Taveira, T.H.; Haas, L.B. Management of Diabetes in Long-term Care and Skilled Nursing Facilities: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 308–318. [Google Scholar] [CrossRef]
- Surwit, R.S.; Schneider, M.S.; Feinglos, M.N. Stress and Diabetes Mellitus. Diabetes Care 1992, 15, 1413–1422. [Google Scholar] [CrossRef]
- Syed, F.Z. Type 1 Diabetes Mellitus. Ann. Intern. Med. 2022, 175, ITC33–ITC48. [Google Scholar] [CrossRef]
- Hau, J. Animal Models for Human Diseases; Humana Press: Jakarta, Indonesia, 2008; pp. 3–8. [Google Scholar] [CrossRef]
- Boelsterli, U.A. Animal models of human disease in drug safety assessment. J. Toxicol. Sci. 2003, 28, 109–121. [Google Scholar] [CrossRef]
- Webb, D.R. Animal models of human disease: Inflammation. Biochem. Pharmacol. 2014, 87, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, B.; Wolf, E.; Schönmann, U.; Ludwig, S. Large Animal Models of Diabetes; Springer: Berlin/Heidelberg, Germany, 2020; pp. 115–134. [Google Scholar] [CrossRef]
- Wagner, J.; Thiele, F.; Ganten, D. Transgenic Animals as Models for Human Disease. Clin. Exp. Hypertens. 1995, 17, 593–605. [Google Scholar] [CrossRef]
- Garcia, G.R.; Noyes, P.D.; Tanguay, R.L.; Garcia, G.R.; Noyes, P.D.; Tanguay, R.L. Advancements in zebrafish applications for 21st century toxicology. Pharmacol. Ther. 2016, 161, 11–21. [Google Scholar] [CrossRef]
- Bailone, R.L.; Fukushima, H.C.S.; Ventura Fernandes, B.H.; De Aguiar, L.K.; Corrêa, T.; Janke, H.; Grejo Setti, P.; Roça, R.D.O.; Borra, R.C. Zebrafish as an alternative animal model in human and animal vaccination research. Lab. Anim. Res. 2020, 36, 13. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.-Y.; Choi, T.-I.; Lee, Y.-R.; Choe, S.-K.; Kim, C.-H. Zebrafish as an animal model for biomedical research. Exp. Mol. Med. 2021, 53, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Rihel, J.; Ghosh, M. Drug Discovery and Evaluation: Pharmacological Assays; Springer International Publishing: Berlin/Heidelberg, Gernany, 2016; pp. 4071–4155. [Google Scholar] [CrossRef]
- Narumanchi, S.; Wang, H.; Perttunen, S. Zebrafish Heart Failure Models. Front. Cell Dev. Biol. 2021, 9, 662583. [Google Scholar] [CrossRef]
- Lieschke, G.J.; Currie, P.D. Animal models of human disease: Zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef]
- Xie, H.; Li, M.; Kang, Y.; Zhang, J.; Zhao, C. Zebrafish: An important model for understanding scoliosis. Cell. Mol. Life Sci. 2022, 79, 1–16. [Google Scholar] [CrossRef]
- Álvarez-Rendón, J.P.; Salceda, R.; Riesgo-Escovar, J.R. Drosophila melanogasteras a Model for Diabetes Type 2 Progression. BioMed Res. Int. 2018, 2018, 1417528. [Google Scholar] [CrossRef]
- Miao, Y.; Chen, R.; Wang, X.; Zhang, J.; Tang, W.; Zhang, Z.; Liu, Y.; Xu, Q. Drosophila melanogaster diabetes models and its usage in the research of anti-diabetes management with traditional Chinese medicines. Front. Med. 2022, 9, 953490. [Google Scholar] [CrossRef]
- Olivares, A.M.; Althoff, K.; Chen, G.F.; Wu, S.; Morrisson, M.A.; DeAngelis, M.M.; Haider, N. Animal Models of Diabetic Retinopathy. Curr. Diabetes Rep. 2017, 17, 93. [Google Scholar] [CrossRef] [PubMed]
- Acharjee, S.; Ghosh, B.; Aldhubiab, B.; Nair, A.B. Understanding Type 1 Diabetes: Etiology and Models. Can. J. Diabetes 2013, 37, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Animal Models of Type 1 Diabetes. Autoimmunity 1995, 21, 1–10. [CrossRef]
- Wolf, E.; Braun-Reichhart, C.; Streckel, E.; Renner, S. Genetically engineered pig models for diabetes research. Transgenic Res. 2013, 23, 27–38. [Google Scholar] [CrossRef]
- Zhu, H.; Yu, L.; He, Y.; Lyu, Y.; Wang, B. Microencapsulated Pig Islet Xenotransplantation as an Alternative Treatment of Diabetes. Tissue Eng. Part B Rev. 2015, 21, 474–489. [Google Scholar] [CrossRef] [PubMed]
- Bakker, J.; de la Garza, M.A. Naturally Occurring Endocrine Disorders in Non-Human Primates: A Comprehensive Review. Animals 2022, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- O’Rahilly, R.; Müller, F. Developmental Stages in Human Embryos: Revised and New Measurements. Cells Tissues Organs 2010, 192, 73–84. [Google Scholar] [CrossRef]
- Jirásek, J.E. Developmental stages of human embryos. Czechoslov. Med. 1978, 1, 156–161. [Google Scholar]
- Dyban, A.P.; Puchkov, V.F.; Samoshkina, N.A.; Khozhai, L.I.; Chebotar’, N.A.; Baranov, V.S. Laboratory Mammals: Mouse (Mus musculus), Rat (Rattus norvegicus), Rabbit (Oryctolagus cuniculus), and Golden Hamster (Cricetus auratus); Springer: New York, NY, USA; Boston, MA, USA, 1991; pp. 351–443. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Tiso, N.; Moro, E.; Argenton, F. Zebrafish pancreas development. Mol. Cell. Endocrinol. 2009, 312, 24–30. [Google Scholar] [CrossRef]
- Prince, V.E.; Anderson, R.M.; Dalgin, G. Zebrafish Pancreas Development and Regeneration. Curr. Top. Dev. Biol. 2017, 124, 235–276. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, Z.; Lin, S. Endocrine pancreas development in zebrafish. Cell Cycle 2011, 10, 3466–3472. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-A.; Lee, S.-H.; Lee, J.-H.; Kang, N.; Oh, J.-Y.; Seun-Heui, S.-H.; Ahn, G.; Ko, S.C.; Fernando, S.P.; Kim, S.-Y.; et al. A marine algal polyphenol, dieckol, attenuates blood glucose levels by Akt pathway in alloxan induced hyperglycemia zebrafish model. RSC Adv. 2016, 6, 78570–78575. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Han, T.; Yang, Y.; Jiang, Y.; Yang, M.; Xu, Y.; Harpaz, S. Effects of different dietary carbohydrate levels on growth, feed utilization and body composition of juvenile grouper Epinephelus akaara. Aquaculture 2016, 459, 143–147. [Google Scholar] [CrossRef]
- Capiotti, K.M.; Antonioli, R., Jr.; Kist, L.W.; Bogo, M.R.; Bonan, C.D.; Da Silva, R.S. Persistent impaired glucose metabolism in a zebrafish hyperglycemia model. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2014, 171, 58–65. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, C.; Yang, L.; Lu, R.; Zhao, X.; Nie, G. Spatiotemporal control of zebrafish (Danio rerio) gene expression using a light-activated CRISPR activation system. BMC Genom. 2018, 19, 246. [Google Scholar] [CrossRef]
- Zang, L.; Maddison, L.A.; Chen, W. Zebrafish as a Model for Obesity and Diabetes. Front. Cell Dev. Biol. 2018, 6, 91. [Google Scholar] [CrossRef]
- Ulloa, P.E.; Iturra, P.; Neira, R.; Araneda, C. Zebrafish as a model organism for nutrition and growth: Towards comparative studies of nutritional genomics applied to aquacultured fishes. Rev. Fish Biol. Fish. 2011, 21, 649–666. [Google Scholar] [CrossRef]
- Weil, M.; Scholz, S.; Zimmer, M.; Sacher, F.; Duis, K. Gene expression analysis in zebrafish embryos: A potential approach to predict effect concentrations in the fish early life stage test. Environ. Toxicol. Chem. 2009, 28, 1970–1978. [Google Scholar] [CrossRef]
- Li, Y.; Jia, Z.; Zhang, S.; He, X. Progress in Gene-Editing Technology of Zebrafish. Biomolecules 2021, 11, 1300. [Google Scholar] [CrossRef]
- Pourghadamyari, H.; Rezaei, M.; Basiri, M.; Tahamtani, Y.; Asgari, B.; Hassani, S.-N.; Meshkani, R.; Golmohammadi, T.; Baharvand, H. Generation of a Transgenic Zebrafish Model for Pancreatic Beta Cell Regeneration. Galen Med. J. 2019, 8, e1056. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, Y.; Jin, Y.; Xu, C.-H.; Liu, D.-J.; Xu, A.-N.; Zhang, Y.-L.; Xie, Y.-Y.; Shi, J.-Y.; Wang, L.; et al. Angiogenesis Induced By Aminoacyl-tRNA Synthetase Deficiency Is Dependent on Amino Acid Response (AAR) but Not Unfolded Protein Response (UPR) Pathways. Blood 2018, 132, 77. [Google Scholar] [CrossRef]
- Eames, S.C.; Kinkel, M.D.; Rajan, S.; Prince, V.E.; Philipson, L.H. Transgenic zebrafish model of the C43G human insulin gene mutation. J. Diabetes Investig. 2012, 4, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Roep, B.O.; Thomaidou, S.; van Tienhoven, R.; Zaldumbide, A. Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?). Nat. Rev. Endocrinol. 2020, 17, 150–161. [Google Scholar] [CrossRef]
- King, A.; Bowe, J. Animal models for diabetes: Understanding the pathogenesis and finding new treatments. Biochem. Pharmacol. 2016, 99, 1–10. [Google Scholar] [CrossRef]
- von Herrath, M.; Nepom, G.T. Animal models of human type 1 diabetes. Nat. Immunol. 2009, 10, 129–132. [Google Scholar] [CrossRef]
- Lueckgen, A. Animal models of T1D. Nat. Res. 2021 6, 128–132. [CrossRef]
- Cheţa, D. Animal Models of Type I (Insulin-Dependent) Diabetes Mellitus. J. Pediatr. Endocrinol. Metab. 1998, 11, 11–19. [Google Scholar] [CrossRef]
- Lebovitz, H.E. Insulin resistance: Definition and consequences. Exp. Clin. Endocrinol. Diabetes 2001, 109, S135–S148. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Sun, W.; Xu, G.; Guo, X.; Luo, G.; Wu, L.; Hou, Y.; Guo, X.; Zhou, J.; Xu, T.; Qin, L.; et al. Protective effects of asiatic acid in a spontaneous type 2 diabetic mouse model. Mol. Med. Rep. 2017, 16, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- James, D.E.; Stöckli, J.; Birnbaum, M.J. The aetiology and molecular landscape of insulin resistance. Nat. Rev. Mol. Cell Biol. 2021, 22, 751–771. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Austin, A. Animal Models of Type 1 and Type 2 Diabetes Mellitus; Academic Press: Cambridge, MA, USA, 2017; pp. 245–265. [Google Scholar] [CrossRef]
- Xu, F.; Wang, N.; Li, G.; Guo, W.; Yang, C.; Liu, D. Establishment and Assessment of Mice Models of Type 2 Diabetes Mellitus. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2017, 39, 324–329. [Google Scholar] [PubMed]
- Kottaisamy, C.P.D.; Raj, D.S.; Kumar, V.P.; Sankaran, U. Experimental animal models for diabetes and its related complications—A review. Lab. Anim. Res. 2021, 37, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Loots, D.T. Experimental rodent models of type 2 diabetes: A review. Methods Find. Exp. Clin. Pharmacol. 2009, 31, 249–261. [Google Scholar] [CrossRef]
- King, A.J. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef]
- Bosmia, A.N.; Tubbs, R.I.; Clapp, D.C.; Batzdorf, U.; Loukas, M.; Tubbs, R.S. Johann Conrad Brunner (1653–1727) and the first description of syringomyelia. Child’s Nerv. Syst. 2014, 30, 193–196. [Google Scholar] [CrossRef]
- Slezak, L.A.; Andersen, D.K. Pancreatic Resection: Effects on Glucose Metabolism. World J. Surg. 2001, 25, 452–460. [Google Scholar] [CrossRef]
- Scavini, M.; Dugnani, E.; Pasquale, V.; Liberati, D.; Aleotti, F.; Di Terlizzi, G.; Petrella, G.; Balzano, G.; Piemonti, L. Diabetes After Pancreatic Surgery: Novel Issues. Curr. Diabetes Rep. 2015, 15, 1–11. [Google Scholar] [CrossRef]
- Lee, C.Y.C.; Depczynski, B.; Poynten, A.; Haghighi, K.S. Diabetes-related outcomes after pancreatic surgery. ANZ J. Surg. 2020, 90, 2004–2010. [Google Scholar] [CrossRef]
- Maeda, H.; Hanazaki, K. Pancreatogenic Diabetes after Pancreatic Resection. Pancreatology 2011, 11, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-A.; Cole, G.J. Generation of Transgenic Zebrafish Expressing Green Fluorescent Protein Under Control of Zebrafish Amyloid Precursor Protein Gene Regulatory Elements. Zebrafish 2007, 4, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Bernardos, R.L.; Raymond, P.A. GFAP transgenic zebrafish. Gene Expr. Patterns 2006, 6, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Radenković, M.; Stojanović, M.; Prostran, M. Experimental diabetes induced by alloxan and streptozotocin: The current state of the art. J. Pharmacol. Toxicol. Methods 2016, 78, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, D.C.; Netto, A.O.; Iessi, I.L.; Gallego, F.Q.; Corvino, S.B.; Dallaqua, B.; Sinzato, Y.K.; Bueno, A.; Calderon, I.M.P.; Rudge, M.V.C. Streptozotocin-Induced Diabetes Models: Pathophysiological Mechanisms and Fetal Outcomes. BioMed Res. Int. 2014, 2014, 819065. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.K.; Huan, Y. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. Unit 2008, 5, 5–47. [Google Scholar] [CrossRef]
- Lenzen, S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 2007, 51, 216–226. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.-L.; Liu, K.-C.; Sheng, W.-L.; Xia, Q.; Wang, R.-C.; Chen, X.-Q.; Zhang, Y. Effects of streptozotocin on pancreatic islet β-cell apoptosis and glucose metabolism in zebrafish larvae. Fish Physiol. Biochem. 2020, 46, 1025–1038. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Adeosun, A.M.; Akinloye, O.A. Alloxan-induced diabetes, a common model for evaluating the glycemic-control potential of therapeutic compounds and plants extracts in experimental studies. Medicina 2017, 53, 365–374. [Google Scholar] [CrossRef]
- Kinkel, M.D.; Eames, S.C.; Philipson, L.H.; Prince, V. Intraperitoneal Injection into Adult Zebrafish. J. Vis. Exp. 2010, 42, e2126. [Google Scholar] [CrossRef]
- Li, M.; Maddison, L.A.; Page-McCaw, P.; Chen, W. Overnutrition induces β-cell differentiation through prolonged activation of β-cells in zebrafish larvae. Am. J. Physiol. Metab. 2014, 306, E799–E807. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-P.; Xiong, Q.; Zhang, G.-X.; Xu, A.-L. The research progress of zebrafish gene engineering. Yi Chuan Xue Bao 2004, 31, 1167–1174. [Google Scholar] [PubMed]
- Kharade, S.V.; Sanchez-Andres, J.V.; Fulton, M.G.; Shelton, E.L.; Blobaum, A.L.; Engers, D.W.; Hofmann, C.S.; Dadi, P.K.; Lantier, L.; Jacobson, D.A.; et al. Structure-Activity Relationships, Pharmacokinetics, and Pharmacodynamics of the Kir6.2/SUR1-Specific Channel Opener VU0071063. J. Pharmacol. Exp. Ther. 2019, 370, 350–359. [Google Scholar] [CrossRef]
- Haider, S.; Antcliff, J.F.; Proks, P.; Sansom, M.S.; Ashcroft, F.M. Focus on Kir6.2: A key component of the ATP-sensitive potassium channel. J. Mol. Cell. Cardiol. 2005, 38, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Korzh, V.; Gong, Z. DTA-mediated targeted ablation revealed differential interdependence of endocrine cell lineages in early development of zebrafish pancreas. Differentiation 2009, 78, 241–252. [Google Scholar] [CrossRef]
- Xiong, F.; Wei, Z.-Q.; Zhu, Z.-Y.; Sun, Y.-H. Targeted Expression in Zebrafish Primordial Germ Cells by Cre/loxP and Gal4/UAS Systems. Mar. Biotechnol. 2013, 15, 526–539. [Google Scholar] [CrossRef]
- Brenin, D.; Talamonti, M.; Iannaccone, P. Transgenic technology: An overview of approaches useful in surgical research. Surg. Oncol. 1997, 6, 99–110. [Google Scholar] [CrossRef]
- Xi, Y.; Yu, M.; Godoy, R.; Hatch, G.; Poitras, L.; Ekker, M. Transgenic zebrafish expressing green fluorescent protein in dopaminergic neurons of the ventral diencephalon. Dev. Dyn. 2011, 240, 2539–2547. [Google Scholar] [CrossRef]
- Renshaw, S.A.; Loynes, C.A.; Trushell, D.M.; Elworthy, S.; Ingham, P.W.; Whyte, M.K. A transgenic zebrafish model of neutrophilic inflammation. Blood 2006, 108, 3976–3978. [Google Scholar] [CrossRef]
- Connaughton, V.P.; Baker, C.; Fonde, L.; Gerardi, E.; Slack, C. Alternate Immersion in an External Glucose Solution Differentially Affects Blood Sugar Values in Older Versus Younger Zebrafish Adults. Zebrafish 2016, 13, 87–94. [Google Scholar] [CrossRef]
- Jurczyk, A.; Roy, N.; Bajwa, R.; Gut, P.; Lipson, K.; Yang, C.; Covassin, L.; Racki, W.J.; Rossini, A.A.; Phillips, N.; et al. Dynamic glucoregulation and mammalian-like responses to metabolic and developmental disruption in zebrafish. Gen. Comp. Endocrinol. 2011, 170, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Kishida, M.; Rahma, K.; Prasetyawan, S.; Aulanni’Am, A. Effects of D-Glucose Exposure on Motor Activity by Swimming Distance During Early Development of Zebrafish (Danio rerio). Int. J. Pharm. Clin. Res. 2017, 9, 258. [Google Scholar] [CrossRef]
- Choudhary, S.; Sinha, S.; Zhao, Y.; Banerjee, S.; Sathyanarayana, P.; Shahani, S.; Sherman, V.; Tilton, R.G.; Bajaj, M. NF-κB-Inducing Kinase (NIK) Mediates Skeletal Muscle Insulin Resistance: Blockade by Adiponectin. Endocrinology 2011, 152, 3622–3627. [Google Scholar] [CrossRef] [PubMed]
- Malle, E.K.; Zammit, N.; Walters, S.N.; Koay, Y.C.; Wu, J.; Tan, B.M.; Villanueva, J.; Brink, R.; Loudovaris, T.; Cantley, J.; et al. Nuclear factor κB–inducing kinase activation as a mechanism of pancreatic β cell failure in obesity. J. Exp. Med. 2015, 212, 1239–1254. [Google Scholar] [CrossRef]
- Zang, L.; Shimada, Y.; Nishimura, N. Development of a Novel Zebrafish Model for Type 2 Diabetes Mellitus. Sci. Rep. 2017, 7, 1461. [Google Scholar] [CrossRef]
- Vargas, R.; Vásquez, I.C. Effects of overfeeding and high-fat diet on cardiosomatic parameters and cardiac structures in young and adult zebrafish. Fish Physiol. Biochem. 2017, 43, 1761–1773. [Google Scholar] [CrossRef]
- Arias-Jayo, N.; Abecia, L.; Alonso-Sáez, L.; Ramirez-Garcia, A.; Rodriguez, A.; Pardo, M.A. High-Fat Diet Consumption Induces Microbiota Dysbiosis and Intestinal Inflammation in Zebrafish. Microb. Ecol. 2018, 76, 1089–1101. [Google Scholar] [CrossRef]
- Meguro, S.; Hosoi, S.; Hasumura, T. High-fat diet impairs cognitive function of zebrafish. Sci. Rep. 2019, 9, 17063. [Google Scholar] [CrossRef]
- Carnovali, M.; Luzi, L.; Terruzzi, I.; Banfi, G.; Mariotti, M. Metabolic and bone effects of high-fat diet in adult zebrafish. Endocrine 2018, 61, 317–326. [Google Scholar] [CrossRef]
- Navarro-Barrón, E.; Hernández, C.; Llera-Herrera, R.; García-Gasca, A.; Gómez-Gil, B. Overfeeding a High-Fat Diet Promotes Sex-Specific Alterations on the Gut Microbiota of the Zebrafish (Danio rerio). Zebrafish 2019, 16, 268–279. [Google Scholar] [CrossRef]
- Fang, L.; Liang, X.-F.; Zhou, Y.; Guo, X.-Z.; He, Y.; Yi, T.-L.; Liu, L.-W.; Yuan, X.-C.; Tao, Y.-X. Programming effects of high-carbohydrate feeding of larvae on adult glucose metabolism in zebrafish, Danio rerio. Br. J. Nutr. 2014, 111, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Picolo, V.L.; Quadros, V.A.; Canzian, J.; Grisolia, C.K.; Goulart, J.T.; Pantoja, C.; de Bem, A.F.; Rosemberg, D.B. Short-term high-fat diet induces cognitive decline, aggression, and anxiety-like behavior in adult zebrafish. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 110, 110288. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Tabuchi, M.; Suzuki, W.; Iizuka, S.; Nagata, M.; Ikeya, Y.; Takeda, S.; Shimada, T.; Aburada, M. Insulin resistance and low sympathetic nerve activity in the Tsumura Suzuki obese diabetic mouse: A new model of spontaneous type 2 diabetes mellitus and obesity. Metabolism 2006, 55, 1664–1669. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, S.; Xue, S.; Li, X.; Sun, Z.; Yang, Y.; Hu, X.; Geng, T.; Cui, H. Generation of Cas9 transgenic zebrafish and their application in establishing an ERV-deficient animal model. Biotechnol. Lett. 2018, 40, 1507–1518. [Google Scholar] [CrossRef]
- Williams, B.O.; Warman, M.L. CRISPR/CAS9 Technologies. J. Bone Miner. Res. 2017, 32, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y. Advances in CRISPR/Cas9. BioMed Res. Int. 2022, 2022, 9978571. [Google Scholar] [CrossRef]
- Isiaku, A.I.; Zhang, Z.; Pazhakh, V.; Manley, H.R.; Thompson, E.R.; Fox, L.C.; Yerneni, S.; Blombery, P.; Lieschke, G.J. Transient, flexible gene editing in zebrafish neutrophils and macrophages for determination of cell-autonomous functions. Dis. Model. Mech. 2021, 14, dmm047431. [Google Scholar] [CrossRef]
- Gao, B.; Wang, W.; Wu, H.; Chen, C.; Shen, D.; Wang, S.; Chen, W.; Zhang, L.; Chan, S.; Song, C. Changes in Skeletal Muscle and Body Weight on Sleeping Beauty Transposon-Mediated Transgenic Mice Overexpressing Pig mIGF-1. Biochem. Genet. 2018, 56, 341–355. [Google Scholar] [CrossRef]
- Maddison, L.A.; Joest, K.E.; Kammeyer, R.M.; Chen, W. Skeletal muscle insulin resistance in zebrafish induces alterations in β-cell number and glucose tolerance in an age- and diet-dependent manner. Am. J. Physiol. Metab. 2015, 308, E662–E669. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Lu, J.-W.; Huo, X.; Gong, Z. Liver-specific androgen receptor knockout attenuates early liver tumor development in zebrafish. Sci. Rep. 2019, 9, 10645. [Google Scholar] [CrossRef]
- Delgadillo-Silva, L.F.; Tsakmaki, A.; Akhtar, N.; Franklin, Z.J.; Konantz, J.; Bewick, G.A.; Ninov, N. Modelling pancreatic β-cell inflammation in zebrafish identifies the natural product wedelolactone for human islet protection. Dis. Model. Mech. 2019, 12, 185–213. [Google Scholar] [CrossRef] [PubMed]
- Wyett, G.; Gibert, Y.; Ellis, M.; Castillo, H.A.; Kaslin, J.; Aston-Mourney, K. Metformin, beta-cell development, and novel processes following beta-cell ablation in zebrafish. Endocrine 2017, 59, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Pisharath, H.; Rhee, J.M.; Swanson, M.A.; Leach, S.D.; Parsons, M.J. Targeted ablation of beta cells in the embryonic zebrafish pancreas using E. coli nitroreductase. Mech. Dev. 2007, 124, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Delaspre, F.; Beer, R.L.; Rovira, M.; Huang, W.; Wang, G.; Gee, S.; Del Carmen Vitery, M.; Wheelan, S.J.; Parsons, M.J. Centroacinar Cells Are Progenitors That Contribute to Endocrine Pancreas Regeneration. Diabetes 2015, 64, 3499–3509. [Google Scholar] [CrossRef] [PubMed]
- Keck, F.S.; Pfeiffer, E.F. The first experimental diabetes mellitus. Acta Diabetol. 1989, 26, 79–81. [Google Scholar] [CrossRef]
- Olsen, A.S.; Sarras, M.P.; Intine, R.V. Limb regeneration is impaired in an adult zebrafish model of diabetes mellitus. Wound Repair Regen. 2010, 18, 532–542. [Google Scholar] [CrossRef]
- Nam, Y.H.; Na Hong, B.; Rodriguez, I.; Ji, M.G.; Kim, K.; Kim, U.-J.; Kang, T.H. Synergistic Potentials of Coffee on Injured Pancreatic Islets and Insulin Action via KATP Channel Blocking in Zebrafish. J. Agric. Food Chem. 2015, 63, 5612–5621. [Google Scholar] [CrossRef]
- Moss, J.B.; Koustubhan, P.; Greenman, M.; Parsons, M.J.; Walter, I.; Moss, L.G. Regeneration of the Pancreas in Adult Zebrafish. Diabetes 2009, 58, 1844–1851. [Google Scholar] [CrossRef]
- Ninov, N.; Hesselson, D.; Gut, P.; Zhou, A.; Fidelin, K.; Stainier, D.Y.R. Metabolic Regulation of Cellular Plasticity in the Pancreas. Curr. Biol. 2013, 23, 1242–1250. [Google Scholar] [CrossRef]
- Curado, S.; Anderson, R.M.; Jungblut, B.; Mumm, J.; Schroeter, E.; Stainier, D. Conditional targeted cell ablation in zebrafish: A new tool for regeneration studies. Dev. Dyn. 2007, 236, 1025–1035. [Google Scholar] [CrossRef]
- Gleeson, M.; Connaughton, V.; Arneson, L.S. Induction of hyperglycaemia in zebrafish (Danio rerio) leads to morphological changes in the retina. Acta Diabetol. 2007, 44, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Wiggenhauser, L.M.; Qi, H.; Stoll, S.J.; Metzger, L.; Bennewitz, K.; Poschet, G.; Krenning, G.; Hillebrands, J.-L.; Hammes, H.-P.; Kroll, J. Activation of Retinal Angiogenesis in Hyperglycemic pdx1 −/− Zebrafish Mutants. Diabetes 2020, 69, 1020–1031. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, G.L.; Hammes, T.O.; Escobar, T.D.; Fracasso, L.B.; Forgiarini, L.F.; Da Silveira, T.R. Blood Collection for Biochemical Analysis in Adult Zebrafish. J. Vis. Exp. 2012, 63, e3865. [Google Scholar] [CrossRef]
- Polakof, S.; Panserat, S.; Soengas, J.; Moon, T.W. Glucose metabolism in fish: A review. J. Comp. Physiol. B 2012, 182, 1015–1045. [Google Scholar] [CrossRef] [PubMed]
- Eames, S.C.; Philipson, L.H.; Prince, V.E.; Kinkel, M.D. Blood Sugar Measurement in Zebrafish Reveals Dynamics of Glucose Homeostasis. Zebrafish 2010, 7, 205–213. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Q.; Liu, Y.; Bi, L.; Jin, L.; Xu, K.; Peng, R. High glucose-induced ROS-accumulation in embryo-larval stages of zebrafish leads to mitochondria-mediated apoptosis. Apoptosis 2022, 27, 509–520. [Google Scholar] [CrossRef] [PubMed]
- D’Adamo, E.; Caprio, S. Type 2 Diabetes in Youth: Epidemiology and Pathophysiology. Diabetes Care 2011, 34, S161–S165. [Google Scholar] [CrossRef]
- Cree-Green, M.; Triolo, T.M.; Nadeau, K.J. Etiology of Insulin Resistance in Youth with Type 2 Diabetes. Curr. Diabetes Rep. 2013, 13, 81–88. [Google Scholar] [CrossRef]
- Larson, K.A.; Gerber, M.M. Effects of Social Metacognitive Training for Enhancing Overt Behavior in Learning Disabled and Low Achieving Delinquents. Except. Child. 1987, 54, 201–211. [Google Scholar] [CrossRef]
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Prim. 2019, 5, 47. [Google Scholar] [CrossRef]
- Singh, A.; Castillo, H.A.; Brown, J.; Kaslin, J.; Dwyer, K.M.; Gibert, Y. High glucose levels affect retinal patterning during zebrafish embryogenesis. Sci. Rep. 2019, 9, 4121. [Google Scholar] [CrossRef] [PubMed]
- Mapanga, R.F.; Essop, M.F. Damaging effects of hyperglycemia on cardiovascular function: Spotlight on glucose metabolic pathways. Am. J. Physiol. Circ. Physiol. 2016, 310, H153–H173. [Google Scholar] [CrossRef] [PubMed]
- Bournele, D.; Beis, D. Zebrafish models of cardiovascular disease. Hear. Fail. Rev. 2016, 21, 803–813. [Google Scholar] [CrossRef] [PubMed]
- King, G.L.; Kunisaki, M.; Nishio, Y.; Inoguchi, T.; Shiba, T.; Xia, P. Biochemical and Molecular Mechanisms in the Development of Diabetic Vascular Complications. Diabetes 1996, 45, S105–S108. [Google Scholar] [CrossRef] [PubMed]
- Yanyi, S.; Qiuyun, W.; Yuehua, F.; Chunfang, W.; Guoping, L.; Zhenyue, C. Activation of Nkx2.5–Calr–p53 signaling pathway by hyperglycemia induces cardiac remodeling and dysfunction in adult zebrafish. Dis. Model. Mech. 2017, 10, 1217–1227. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, H.-T.; Wang, N.; Sheng, W.-W.; Jin, M.; Lu, Y.; Bai, Y.-J.; Zou, S.-Q.; Pang, Y.-L.; Xu, H.; et al. Establishment of an adult zebrafish model of retinal neurodegeneration induced by NMDA. Int. J. Ophthalmol. 2019, 12, 1250–1261. [Google Scholar] [CrossRef]
- Del Campo, C.H.; Melandez, F. The use of a testosterone intravaginal device to detect oestrus in goats. Acta Veter-Scand. Suppl. 1988, 83, 101–109. [Google Scholar]
- Jung, S.-H.; Kim, Y.S.; Lee, Y.-R.; Kim, J.S. High glucose-induced changes in hyaloid-retinal vessels during early ocular development of zebrafish: A short-term animal model of diabetic retinopathy. Br. J. Pharmacol. 2016, 173, 15–26. [Google Scholar] [CrossRef]
- Hayman, S.R.; Leung, N.; Grande, J.P.; Garovic, V.D. VEGF Inhibition, Hypertension, and Renal Toxicity. Curr. Oncol. Rep. 2012, 14, 285–294. [Google Scholar] [CrossRef]
- González-Reyes, R.E.; Aliev, G.; Avila-Rodrigues, M.; Barreto, G.E. Alterations in Glucose Metabolism on Cognition: A Possible Link Between Diabetes and Dementia. Curr. Pharm. Des. 2016, 22, 812–818. [Google Scholar] [CrossRef]
- Hu, C.; Bai, Y.; Li, J.; Sun, B.; Chen, L. Endocrine disruption and reproductive impairment of methylparaben in adult zebrafish. Food Chem. Toxicol. 2023, 171, 113545. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.-R.; Li, Y.-F.; Zhang, X.-T.; Huang, Y.-H.; Wu, Y.-P.; Ouyang, S.-H.; Tsoi, B.; Yi, R.-N.; Yang, X.; Kurihara, H.; et al. Glucose metabolism disorder is a risk factor in ethanol exposure induced malformation in embryonic brain. Food Chem. Toxicol. 2013, 60, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Dorsemans, A.-C.; Soulé, S.; Weger, M.; Bourdon, E.; D’Hellencourt, C.L.; Meilhac, O.; Diotel, N. Impaired constitutive and regenerative neurogenesis in adult hyperglycemic zebrafish. J. Comp. Neurol. 2017, 525, 442–458. [Google Scholar] [CrossRef]
- Capiotti, K.M.; De Moraes, D.A.; Menezes, F.P.; Kist, L.W.; Bogo, M.R.; Da Silva, R.S. Hyperglycemia induces memory impairment linked to increased acetylcholinesterase activity in zebrafish (Danio rerio). Behav. Brain Res. 2014, 274, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Rocker, A.; Howell, J.; Voithofer, G.; Clark, J.K. Acute effects of hyperglycemia on the peripheral nervous system in zebrafish (Danio rerio) following nitroreductase-mediated β-cell ablation. Am. J. Physiol. Integr. Comp. Physiol. 2019, 316, R395–R405. [Google Scholar] [CrossRef]
- Keating, S.T.; van Diepen, J.A.; Riksen, N.P.; El-Osta, A. Epigenetics in diabetic nephropathy, immunity and metabolism. Diabetologia 2017, 61, 6–20. [Google Scholar] [CrossRef]
- Teramo, K.; Piñeiro-Ramos, J.D. Fetal chronic hypoxia and oxidative stress in diabetic pregnancy. Could fetal erythropoietin improve offspring outcomes? Free. Radic. Biol. Med. 2019, 142, 32–37. [Google Scholar] [CrossRef]
- Timme-Laragy, A.R.; Karchner, S.I.; Hahn, M.E. Gene Knockdown by Morpholino-Modified Oligonucleotides in the Zebrafish (Danio rerio) Model: Applications for Developmental Toxicology. Dev. Toxicol. 2012, 889, 51–71. [Google Scholar] [CrossRef]
- Chen, S.; Huang, J.; Yuan, G.; Chen, Q.; Huang, N.; Xie, F. siRNA specific to Pdx-1 disturbed the formation of the islet in early zebrafish embryos. J. Huazhong Univ. Sci. Technol. 2007, 27, 639–642. [Google Scholar] [CrossRef]
- Baltzis, D.; Eleftheriadou, I.; Veves, A. Pathogenesis and Treatment of Impaired Wound Healing in Diabetes Mellitus: New Insights. Adv. Ther. 2014, 31, 817–836. [Google Scholar] [CrossRef]
- Yang, B.; Yang, L.; Wang, Y.; Maddison, L.A.; Tang, Z.; Haigh, S.; Gong, Y.; Zhang, Y.; Covington, B.A.; Bosma, K.J.; et al. Macrophages and neutrophils are necessary for ER stress-induced β cell loss. Cell Rep. 2022, 40, 111255. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Aravamudhan, S.; Armant, O.; Krüger, M.; Grabher, C. Proteome dynamics in neutrophils of adult zebrafish upon chemically-induced inflammation. Fish Shellfish. Immunol. 2014, 40, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Maddison, L.A.; Zaborska, K.E.; Dai, C.; Yin, L.; Tang, Z.; Zang, L.; Jacobson, D.A.; Powers, A.C.; Chen, W. RIPK3-mediated inflammation is a conserved β cell response to ER stress. Sci. Adv. 2020, 6, eabd7272. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Hopkins, N. vhnf1, the MODY5 and familial GCKD-associated gene, regulates regional specification of the zebrafish gut, pronephros, and hindbrain. Genes Dev. 2001, 15, 3217–3229. [Google Scholar] [CrossRef] [PubMed]
- Lancman, J.J.; Zvenigorodsky, N.; Gates, K.P.; Zhang, D.; Solomon, K.; Humphrey, R.K.; Kuo, T.; Setiawan, L.; Verkade, H.; Chi, Y.-I.; et al. Specification of hepatopancreas progenitors in zebrafish by hnf1ba and wnt2bb. Development 2013, 140, 2669–2679. [Google Scholar] [CrossRef] [PubMed]
- Konadu, B.; Cox, C.; Speed, J.; Gibert, Y. Zebrafish model of Gestational Diabetes. FASEB J. 2021, 35. [Google Scholar] [CrossRef]
- Sarras, M.P., Jr.; Leontovich, A.A.; Olsen, A.S.; Intine, R.V. Impaired tissue regeneration corresponds with altered expression of developmental genes that persists in the metabolic memory state of diabetic zebrafish. Wound Repair Regen. 2013, 21, 320–328. [Google Scholar] [CrossRef]
- Sharchil, C.; Vijay, A.; Ramachandran, V.; Bhagavatheeswaran, S.; Devarajan, R.; Koul, B.; Yadav, D.; Balakrishnan, A. Zebrafish: A Model to Study and Understand the Diabetic Nephropathy and Other Microvascular Complications of Type 2 Diabetes Mellitus. Vet. Sci. 2022, 9, 312. [Google Scholar] [CrossRef]
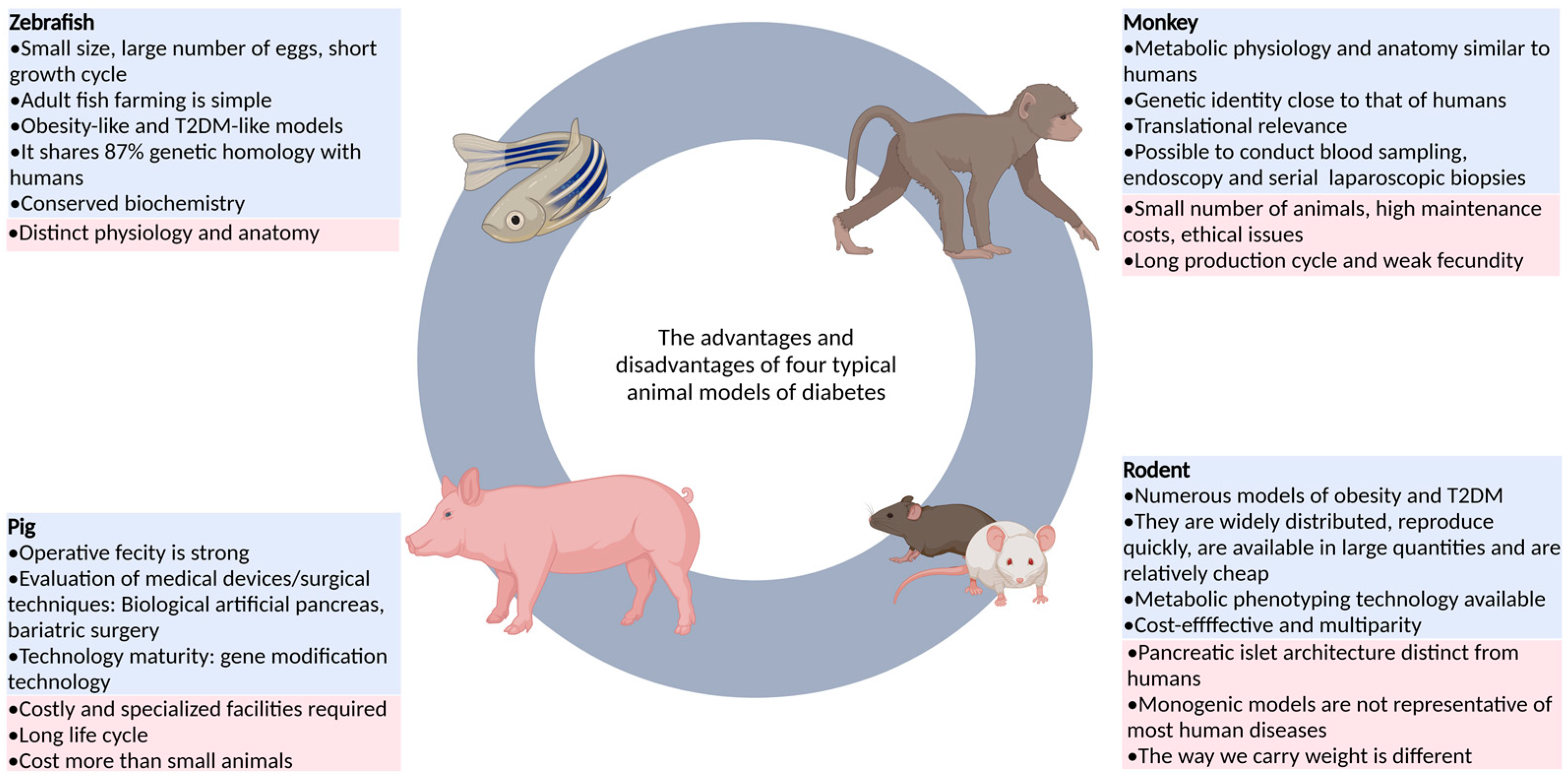
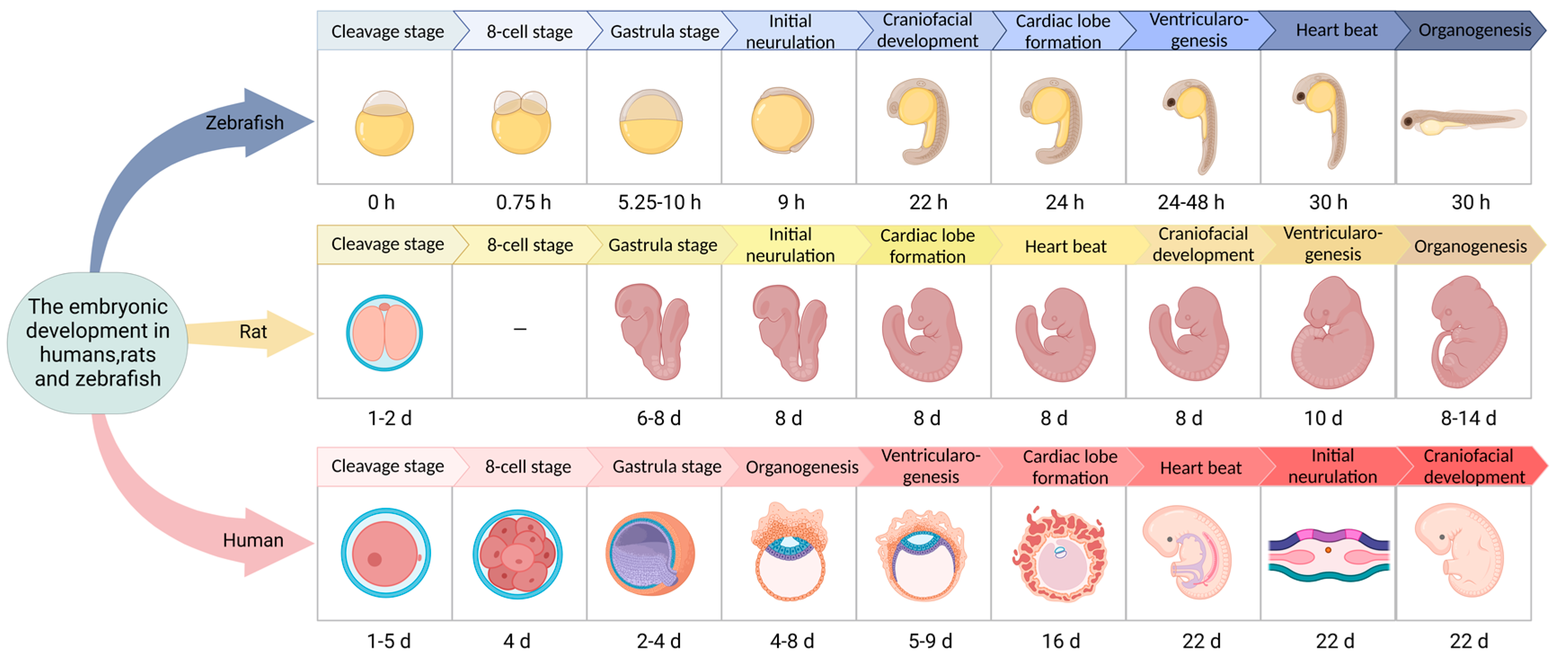
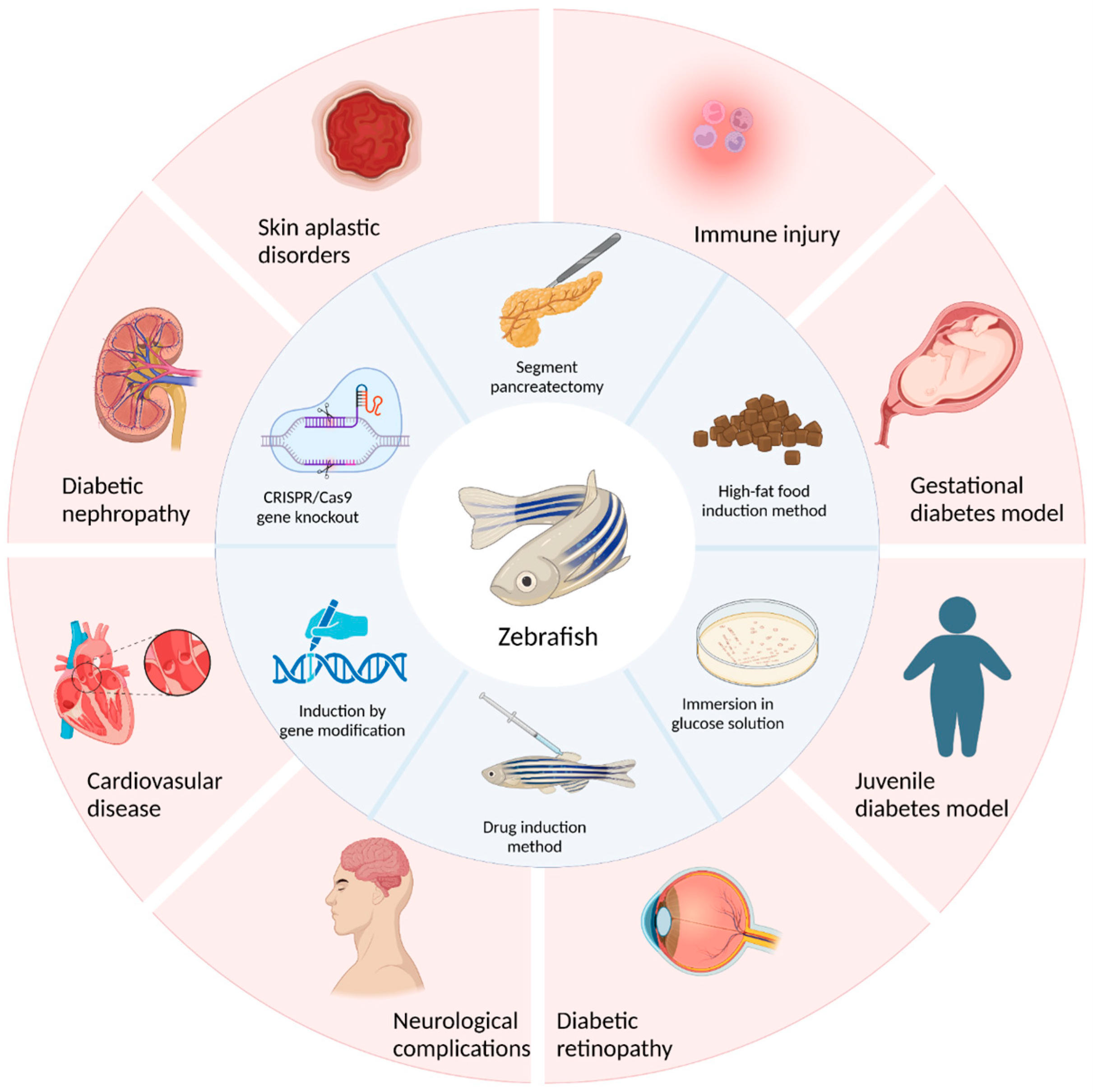
| Category | Animal | Advantage | Disadvantage | References |
|---|---|---|---|---|
| Non-mammalian animals | Zebrafish | As an emerging model, it has the advantages of fast reproduction, copulatory behavior controlled by photoperiod, large number of eggs laid, in vitro fertilization of fertilized eggs, overall transparency of early embryos, easy feeding, and easy use of drugs. | It is an ectothermic animal and lacks brown adipose tissue, so it is still difficult to measure insulin levels and assess insulin resistance. | [15,16,21] |
| Drosophila melanogaster | Drosophila is easy to obtain and operate, and has strong conservation with the lipid-related metabolism genes of mammals, which can be used to study the function of candidate genes related to T2DM. | It is distantly related to humans, and its anatomical structure and physiological function are slightly similar to those of the human body. | [22,23] | |
| Rodent | Rat and mouse | It is highly productive, low-cost, and diverse, covering obesity and diabetes, with advanced tools for genetic modification and metabolic phenotype assessment. | The structure of islets, basal metabolic rate, feeding behavior, immune system, and gut microbiota are less similar to those of humans. | [24,25,26] |
| Large mammals | Pig | The feeding cost is low and wide application is possible. The gene modification technology is relatively mature, and a variety of disease models can be generated through targeted gene editing. | It does not usually lead to diabetes and requires a combination of other means. It also requires special equipment and costs more than small animals. | [13,27,28] |
| Non-human primates | Monkey | The anatomical structure, physiological characteristics, and genetic background are similar to those of humans, and the results of the study are of high clinical relevance. | The number of animals is small, the production cycle is long, the fecundity is weak, the technology is imperfect, and the price is high. | [29] |
| Type | Construction Method | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Type I Diabetes | Surgical resection method | The earliest method for replication of animal models of diabetes. | It requires highly skilled operating techniques and elaborate equipment for researchers, and the trauma to zebrafish is significant, resulting in a low survival rate of zebrafish after surgery. | [109,110] |
| Drug induction method | With the advantages of short time frames, simplicity, ease of mastery, and good repeatability, a large number of models can be induced in a short period of time. | Multiple intraperitoneal injections of streptozotocin are needed, and the operation is complicated. | [111,112,113] | |
| Induction by genetic modification | Transgenic technology can be used to construct zebrafish with specific functions. | Transgenes will damage other genes in the genome, resulting in the loss of other functional genes, and the acquisition of homozygotes takes a long time. | [108,114] | |
| Genetic ablation method | Target cell populations in zebrafish larvae can be effectively removed by the NTR/MTZ system. | This approach has limitations due to the instability and uncertainty of current techniques regarding the genetic aspects. | [108,109,115] | |
| Type II Diabetes | Glucose solution immersion method | The feeding procedure is simple and the diabetic model can be established in about ten days. | In solutions with high concentrations of sugar, the swimming and gill functions of the zebrafish were abnormal, and some zebrafish died in the modeling process. | [116] |
| High-fat food induction method | The feeding procedure is simple. | It takes a long time, and obesity often leads to cardiovascular disease and endocrine abnormalities. | [90] | |
| CRISPR/Cas9 gene knockout method | Knockout zebrafish can be obtained quickly. | In the face of gene editing, the target gene is off-target, and there is genetic instability after editing. | [117] |
| Type of Diabetic Zebrafish Model | Construction Method | Phenotypic Characteristics | References |
|---|---|---|---|
| Juvenile diabetes model | Mutation of hepatic nuclear factor 1β(HNF-1β) gene | Early onset, autosomal dominant inheritance, diabetes mellitus, renal cysts (dysplastic glomerular cystic lesions), and even nondiabetic renal insufficiency. | [149,150] |
| Gestational diabetes model | Glucose immersion method | The total free glucose level of zebrafish embryos in high glucose group fluctuated in a dose-dependent manner, and the average free glucose level increased significantly. | [151] |
| Diabetic cardiovascular disease model | Glucose immersion method | Zebrafish treated with high glucose concentrations gradually showed cardiac hypertrophy, apoptosis, and arrhythmia. Early diastolic dysfunction and late systolic dysfunction occurred in the heart. | [113] |
| Diabetic retinopathy model | Glucose immersion method | The internal layer and core layer of zebrafish in the diabetes model group were significantly lower than those in the normal group, and the IPL was significantly thinner. | [100,109] |
| Neurological complications | Glucose immersion method | Hyperglycemia can regulate acetylcholinesterase function and gene expression in zebrafish, leading to choline dysfunction and eventual memory loss. | [121,123,137] |
| Renal complications | CRISPR/Cas9 gene knockout (CRISPR/Cas9-induced ELMO1 deletion in 48hpf embryos) | The basal glomerular membrane was thickened. | [128,138] |
| Diabetic wound model | Streptozotocin drug induction method | In diabetic zebrafish, fin regeneration and skin wound healing are impaired and persist even after blood sugar levels return to normal. | [152,153] |
| Diabetic immune damage model | The dominant negative mutant of insulin-like growth factor 1 receptor was constructed by using the dominant negative effect. | In this model, ER stress led to an increase in the number of macrophages and neutrophils, and neutrophils attacked beta cells in contact with the macrophages, causing them to be lost. | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Chen, Q.; Liu, Y.; Jin, L.; Peng, R. Research Progress on the Construction and Application of a Diabetic Zebrafish Model. Int. J. Mol. Sci. 2023, 24, 5195. https://doi.org/10.3390/ijms24065195
Cao Y, Chen Q, Liu Y, Jin L, Peng R. Research Progress on the Construction and Application of a Diabetic Zebrafish Model. International Journal of Molecular Sciences. 2023; 24(6):5195. https://doi.org/10.3390/ijms24065195
Chicago/Turabian StyleCao, Yu, Qianqian Chen, Yinai Liu, Libo Jin, and Renyi Peng. 2023. "Research Progress on the Construction and Application of a Diabetic Zebrafish Model" International Journal of Molecular Sciences 24, no. 6: 5195. https://doi.org/10.3390/ijms24065195
APA StyleCao, Y., Chen, Q., Liu, Y., Jin, L., & Peng, R. (2023). Research Progress on the Construction and Application of a Diabetic Zebrafish Model. International Journal of Molecular Sciences, 24(6), 5195. https://doi.org/10.3390/ijms24065195






