Prediction of Overall Survival by Thymidine Kinase 1 Combined with Prostate-Specific Antigen in Men with Prostate Cancer
Abstract
1. Introduction
2. Results
2.1. Descriptive Evaluation of the Study Cohort and Controls
2.2. Survival in Relation to PSA, TK1 and TK1 + PSA
2.3. Survival in Relation to TK1 and Age
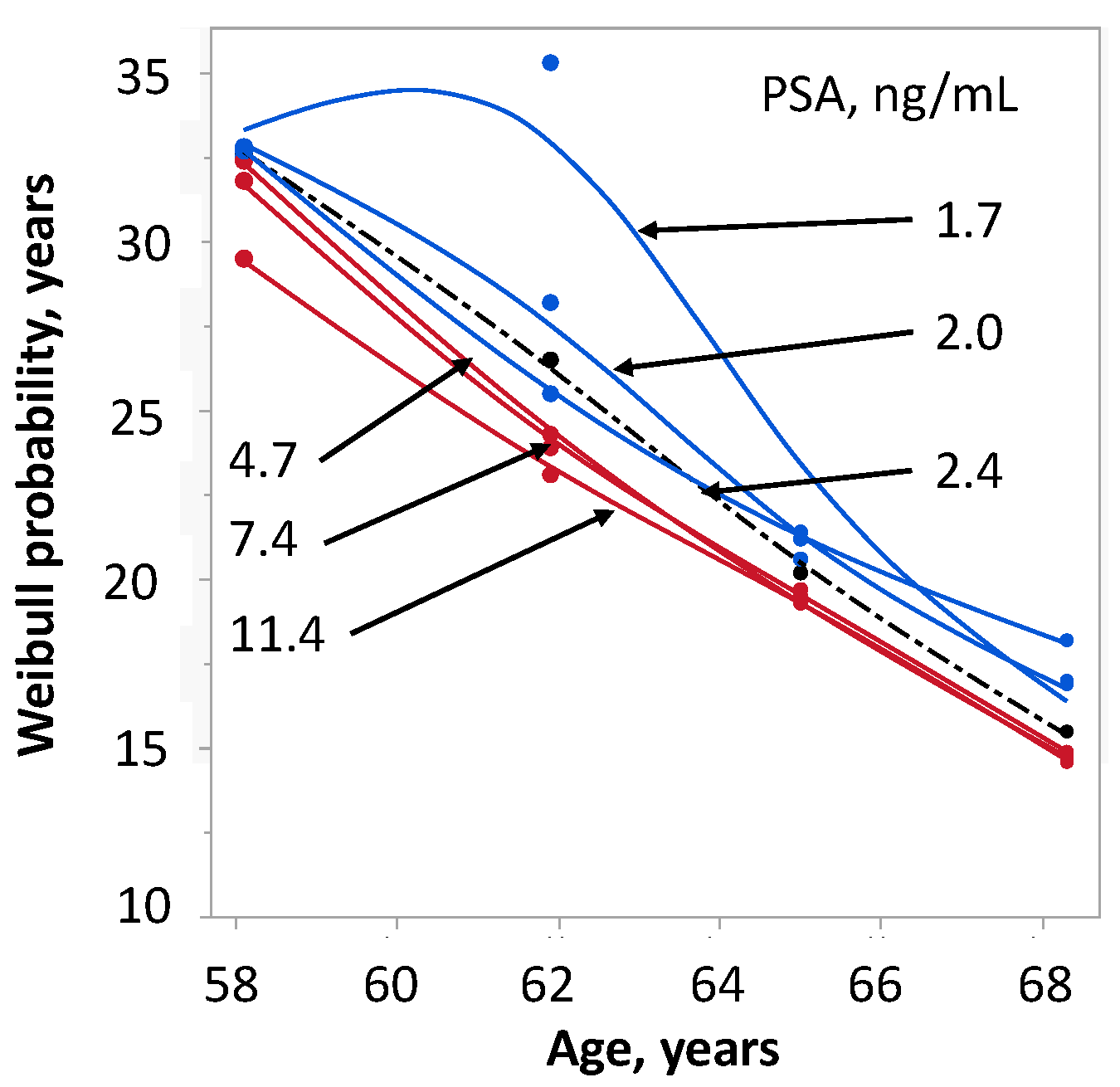
2.4. Survival in Relation to PSA and Age
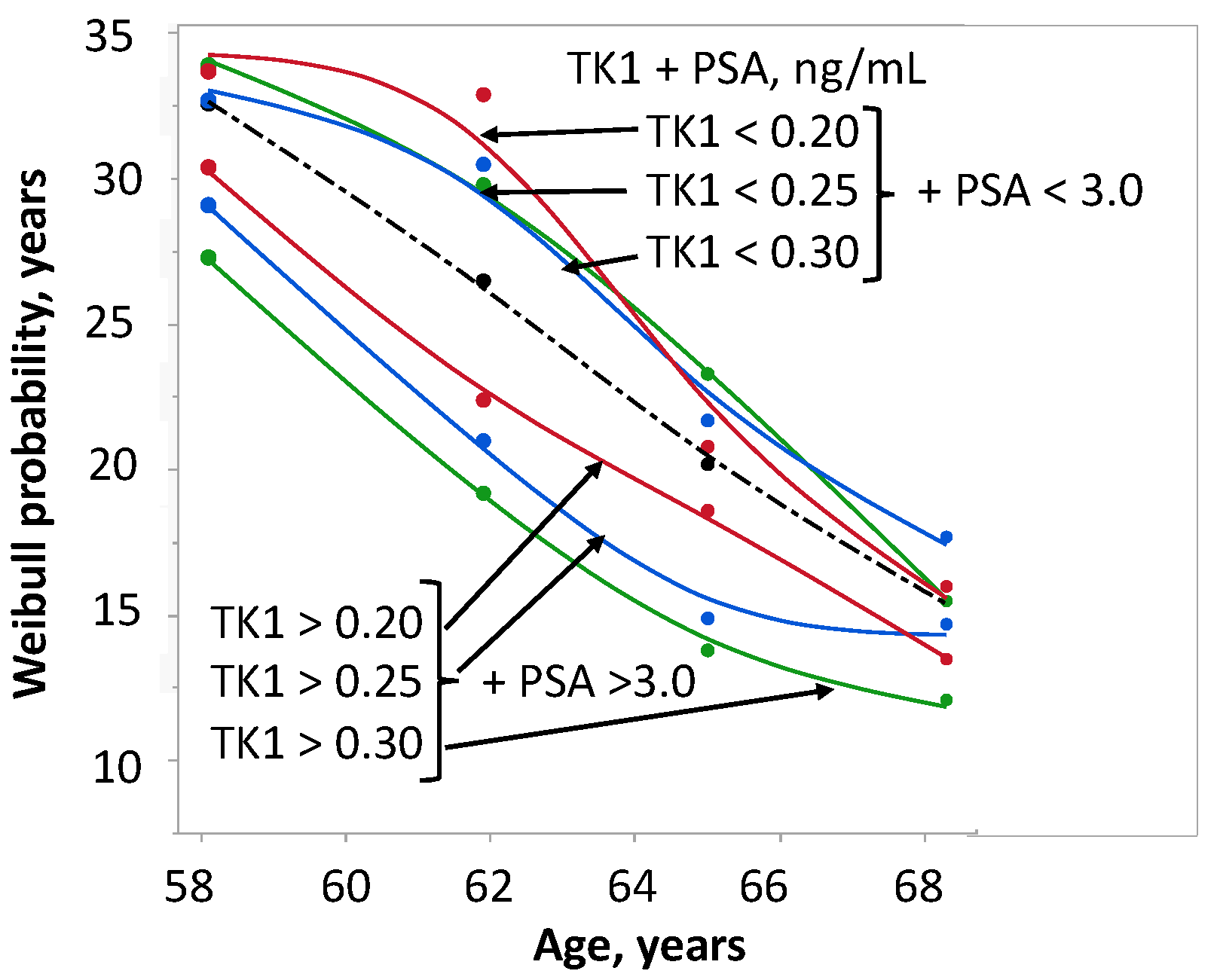
2.5. Survival in Relation to TK1 + PSA and Age
2.6. Survival of Controls in Relation to TK1 and PSA
3. Discussion
4. Material and Methods
4.1. Cohort and Design
4.2. Determination of TK1 Concentration
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rietbergen, J.B.; Schröder, F.H. Screening for prostate cancer—More questions than answers. Acta Oncol. 1998, 37, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J. Biomarkers for prostate cancer: Prostate-specific antigen and beyond. Clin. Chem. Lab. Med. 2020, 58, 326–339. [Google Scholar] [CrossRef]
- Ilic, D.; Djulbegovic, M.; Jung, J.H.; Hwang, E.C.; Zhou, Q.; Cleves, A.; Agoritsas, T.; Dahm, P. Prostate cancer screening with prostate-specific antigen (PSA) test: A systematic review and meta-analysis. BMJ 2018, 362, k3519. [Google Scholar] [CrossRef] [PubMed]
- Hugosson, J.; Roobol, M.J.; Månsson, M.; Tammela, T.L.; Zappa, M.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Carlsson, S.V.; Talala, K.M.; et al. A 16-yr Follow-up of the European Randomized study of Screening for Prostate Cancer. Eur. Urol. 2019, 76, 43–51. [Google Scholar] [CrossRef]
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef]
- Carioli, G.; Bertuccio, P.; Boffetta, P.; Levi, F.; La Vecchia, C.; Negri, E.; Malvezzi, M. European cancer mortality predictions for the year 2020 with a focus on prostate cancer. Ann. Oncol. 2020, 31, 650–658. [Google Scholar] [CrossRef]
- Vickers, A.J.; Sjoberg, D.D.; Ulmert, D.; Vertosick, E.; Roobol, M.J.; Thompson, I.; Heijnsdijk, E.A.; De Koning, H.; Atoria-Swartz, C.; Scardino, P.T.; et al. Empirical estimates of prostate cancer overdiagnosis by age and prostate-specific antigen. BMC Med. 2014, 12, 26. [Google Scholar] [CrossRef]
- Orrason, A.W.; Westerberg, M.; Albertsen, P.; Styrke, J.; Robinson, D.; Garmo, H.; Stattin, P. Diagnostic activity impacts lifetime risk of prostate cancer diagnosis more strongly than life expectancy. PLoS ONE 2022, 17, e0277784. [Google Scholar] [CrossRef] [PubMed]
- Schröder, F.H.; Hugosson, J.; Roobol-Bouts, M.J.; Tammela, T.L.J.; Ciatto, S.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Lilja, H.; Zappa, M.; et al. Screening and prostate-cancer mortality in a randomized European study. N. Engl. J. Med. 2009, 360, 1320–1328. [Google Scholar] [CrossRef]
- Kretschmer, A.; Tilki, D. Biomarkers in prostate cancer—Current clinical utility and future perspectives. Crit. Rev. Oncol. 2017, 120, 180–193. [Google Scholar] [CrossRef]
- Meehan, J.; Gray, M.; Martínez-Pérez, C.; Kay, C.; McLaren, D.; Turnbull, A.K. Tissue- and Liquid-Based Biomarkers in Prostate Cancer Precision Medicine. J. Pers. Med. 2021, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Lotan, T.L.; Tomlins, S.A.; Bismar, T.A.; Van Der Kwast, T.H.; Grignon, D.; Egevad, L.; Kristiansen, G.; Pritchard, C.C.; Rubin, M.; Bubendorf, L. Report From the International Society of Urological Pathology (ISUP) Consultation Conference on Molecular Pathology of Urogenital Cancers. I. Molecular Biomarkers in Prostate Cancer. Am. J. Surg. Pathol. 2020, 44, e15–e29. [Google Scholar] [CrossRef] [PubMed]
- Grönberg, H.; Adolfsson, J.; Aly, M.; Nordström, T.; Wiklund, P.; Brandberg, Y.; Thompson, J.; Wiklund, F.; Lindberg, J.; Clements, M.; et al. Prostate cancer screening in men aged 50–69 years (STHLM3): A prospective population-based diagnostic study. Lancet Oncol. 2015, 16, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Eklund, M.; Nordström, T.; Aly, M.; Adolfsson, J.; Wiklund, P.; Brandberg, Y.; Thompson, J.; Wiklund, F.; Lindberg, J.; Presti, J.C.; et al. The Stockholm-3 (STHLM3) Model can Improve Prostate Cancer Diagnostics in Men Aged 50–69 yr Compared with Current Prostate Cancer Testing. Eur. Urol. Focus. 2018, 4, 707–710. [Google Scholar] [CrossRef]
- Kumar, J.K.; Holmgren, S.; Levedahl, K.H.; Höglund, M.; Venge, P.; Eriksson, S. AroCell TK 210 ELISA for determination of TK1 protein: Age-related reference ranges and comparison with other TK1 assays. Biotechniques 2020, 68, 334–341. [Google Scholar] [CrossRef]
- Munch-Petersen, B. Enzymatic regulation of cytosolic thymidine kinase 1 and mitochondrial thymidine kinase 2: A mini review. Nucleosides Nucleotides Nucleic Acids. 2010, 29, 363–369. [Google Scholar] [CrossRef]
- Chen, Y.L.; Eriksson, S.; Chang, Z.F. Regulation and functional contribution of thymidine kinase 1 in repair of DNA damage. J. Biol. Chem. 2010, 285, 27327–27335. [Google Scholar] [CrossRef]
- Ke, P.Y.; Chang, Z.F. Mitotic degradation of human thymidine kinase 1 is dependent on the anaphase-promoting complex/cyclosome-CDH1-mediated pathway. Mol. Cell. Biol. 2004, 24, 514–526. [Google Scholar] [CrossRef]
- Jagarlamudi, K.K.; Shaw, M. Thymidine kinase 1 as a tumor biomarker: Technical advances offer new potential to an old biomarker. Biomark. Med. 2018, 12, 1035–1048. [Google Scholar] [CrossRef]
- Bitter, E.E.; Townsend, M.H.; Erickson, R.; Allen, C.; O’Neill, K.L. Thymidine kinase 1 through the ages: A comprehensive review. Cell Biosci. 2020, 10, 138. [Google Scholar] [CrossRef]
- Tribukait, B. Early prediction of pathologic response to neoadjuvant treatment of breast cancer: Use of a cell-loss metric based on serum thymidine kinase 1 and tumour volume. BMC Cancer 2020, 20, 440. [Google Scholar] [CrossRef] [PubMed]
- Steel, G.G. Cell loss as a factor in the growth rate of human tumours. Eur. J. Cancer 1967, 3, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Lewenhaupt, A.; Ekman, P.; Eneroth, P.; Nilsson, B. Tumour markers as prognostic aids in prostatic carcinoma. Br. J. Urol. 1990, 66, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Letocha, H.; Eklöv, S.; Gronowitz, S.; Norlén, B.J.; Nilsson, S. Deoxythymidine kinase in the staging of prostatic adenocarcinoma. Prostate. 1996, 29, 15–19. [Google Scholar] [CrossRef]
- Jagarlamudi, K.K.; Zupan, M.; Kumer, K.; Fabjan, T.; Hlebič, G.; Eriksson, S.; Osredkar, J.; Smrkolj, T. The combination of AroCell TK 210 ELISA with Prostate Health Index or prostate-specific antigen density can improve the ability to differentiate prostate cancer from noncancerous conditions. Prostate 2019, 79, 856–863. [Google Scholar] [CrossRef]
- Murtola, T.J.; Raittinen, P.; Tammela, T. Abstract LB-149: Serum thymidine kinase 1 levels predict prostate cancer-specific survival. Cancer Res. 2020, 80, LB-149. [Google Scholar] [CrossRef]
- Li, S.; Zhou, J.; Wang, Y.; Zhang, K.; Yang, J.; Zhang, X.; Wang, C.; Ma, H.; Zhou, J.; He, E.; et al. Serum thymidine kinase 1 is associated with Gleason score of patients with prostate carcinoma. Oncol. Lett. 2018, 16, 6171–6180. [Google Scholar] [CrossRef]
- Lundgren, P.; Tribukait, B.; Kjellman, A.; Norming, U.; Jagarlmudi, K.; Gustafsson, O. Serum thymidine kinase 1 concentration as a predictive biomarker in prostate cancer. Prostate 2022, 82, 911–916. [Google Scholar] [CrossRef]
- Parkes, C.; Wald, N.J.; Murphy, P.; George, L.; Watt, H.C.; Kirby, R.; Knekt, P.; Helzlsouer, K.J.; Tuomilehto, J. Prospective observational study to assess value of prostate specific antigen as screening test for prostate cancer. BMJ 1995, 311, 1340–1343. [Google Scholar] [CrossRef]
- Vickers, A.J.; Cronin, A.M.; Björk, T.; Manjer, J.; Nilsson, P.M.; Dahlin, A.; Bjartell, A.; Scardino, P.T.; Ulmert, D.; Lilja, H.; et al. Prostate specific antigen concentration at age 60 and death or metastasis from prostate cancer: Case-control study. BMJ 2010, 341, c4521. [Google Scholar] [CrossRef]
- Lilja, H.; Cronin, A.M.; Dahlin, A.; Manjer, J.; Nilsson, P.M.; Eastham, J.A.; Bjartell, A.S.; Scardino, P.T.; Ulmert, D.; Vickers, A. Prediction of significant prostate cancer diagnosed 20 to 30 years later with a single measure of prostate-specific antigen at or before age 50. Cancer 2011, 117, 1210–1219. [Google Scholar] [CrossRef]
- Preston, M.A.; Batista, J.L.; Wilson, K.M.; Carlsson, S.V.; Gerke, T.; Sjoberg, D.D.; Dahl, D.M.; Sesso, H.D.; Feldman, A.S.; Gann, P.H.; et al. Baseline Prostate-Specific Antigen Levels in Midlife Predict Lethal Prostate Cancer. J. Clin. Oncol. 2016, 34, 2705–2711. [Google Scholar] [CrossRef] [PubMed]
- Kovac, E.; Carlsson, S.V.; Lilja, H.; Hugosson, J.; Kattan, M.W.; Holmberg, E.; Stephenson, A.J. Association of Baseline Prostate-Specific Antigen Level With Long-term Diagnosis of Clinically Significant Prostate Cancer Among Patients Aged 55 to 60 Years: A Secondary Analysis of a Cohort in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. JAMA Netw. Open 2020, 3, e1919284. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.B.; Miranti, C.K. Disruption of prostate epithelial differentiation pathways and prostate cancer development. Front Oncol. 2013, 3, 273. [Google Scholar] [CrossRef]
- Berges, R.R.; Vukanovic, J.; Epstein, J.; CarMichel, M.; Cisek, L.; Johnson, D.E.; Veltri, R.W.; Walsh, P.C.; Isaacs, J.T. Implication of cell kinetic changes during the progression of human prostatic cancer. Clin. Cancer Res. 1995, 1, 473–480. [Google Scholar]
- Bell, K.J.; Del Mar, C.; Wright, G.; Dickinson, J.; Glasziou, P. Prevalence of incidental prostate cancer: A systematic review of autopsy studies. Int. J. Cancer 2015, 137, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, O.; Khairul-Asri, M.G.; Schubert, T.; Renninger, M.; Stenzl, A.; Gakis, G. Clinicopathological Features and Prognostic Value of Incidental Prostatic Adenocarcinoma in Radical Cystoprostatectomy Specimens: A Systematic Review and Meta-Analysis of 13,140 Patients. J. Urol. 2017, 197, 385–390. [Google Scholar] [CrossRef]
- Zhou, J.; Li, H.; Fang, C.; Gao, P.; Jin, C.; Liu, S.; Zou, R.; Li, J.; Liu, Y.; He, E.; et al. Concentration of human thymidine kinase 1 discover invisible malignant human tumours. Eur. J. Cell Biol. 2022, 101, 151280. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, O.; Norming, U.; Almgård, L.-E.; Fredriksson, A.; Gustavsson, G.; Harvig, B.; Nyman, C.R. Diagnostic methods in the detection of prostate cancer: A study of a randomly selected population of 2400 men. J. Urol. 1992, 148, 1827–1831. [Google Scholar] [CrossRef]
- Lundgren, P.O.; Kjellman, A.; Norming, U.; Gustafsson, O. Association between one-time prostate-specific antigen (PSA) test with free/total PSA ratio and prostate cancer mortality: A 30-year prospective cohort study. BJU Int. 2021, 128, 490–496. [Google Scholar] [CrossRef]
- Catalona, W.J.; Partin, A.W.; Sanda, M.G.; Wei, J.T.; Klee, G.G.; Bangma, C.H.; Slawin, K.M.; Marks, L.S.; Loeb, S.; Broyles, D.L.; et al. A multicenter study of [-2]pro-prostate specific antigen combined with prostate specific antigen and free prostate specific antigen for prostate cancer detection in the 2.0 to 10.0 ng/mL prostate specific antigen range. J. Urol. 2011, 185, 1650–1655, Correction Appears in J. Urol. 2011, 186, 354. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K.; Hille, C.; Scher, H.I. Circulating Tumor Cells in Prostate Cancer: From Discovery to Clinical Utility. Clin. Chem. 2019, 65, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ni, J.; Beretov, J.; Thompson, J.; Graham, P.; Li, Y. Exosomal microRNAs as liquid biopsy biomarkers in prostate cancer. Crit. Rev. Oncol. Hematol. 2020, 145, 102860. [Google Scholar] [CrossRef]
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef] [PubMed]
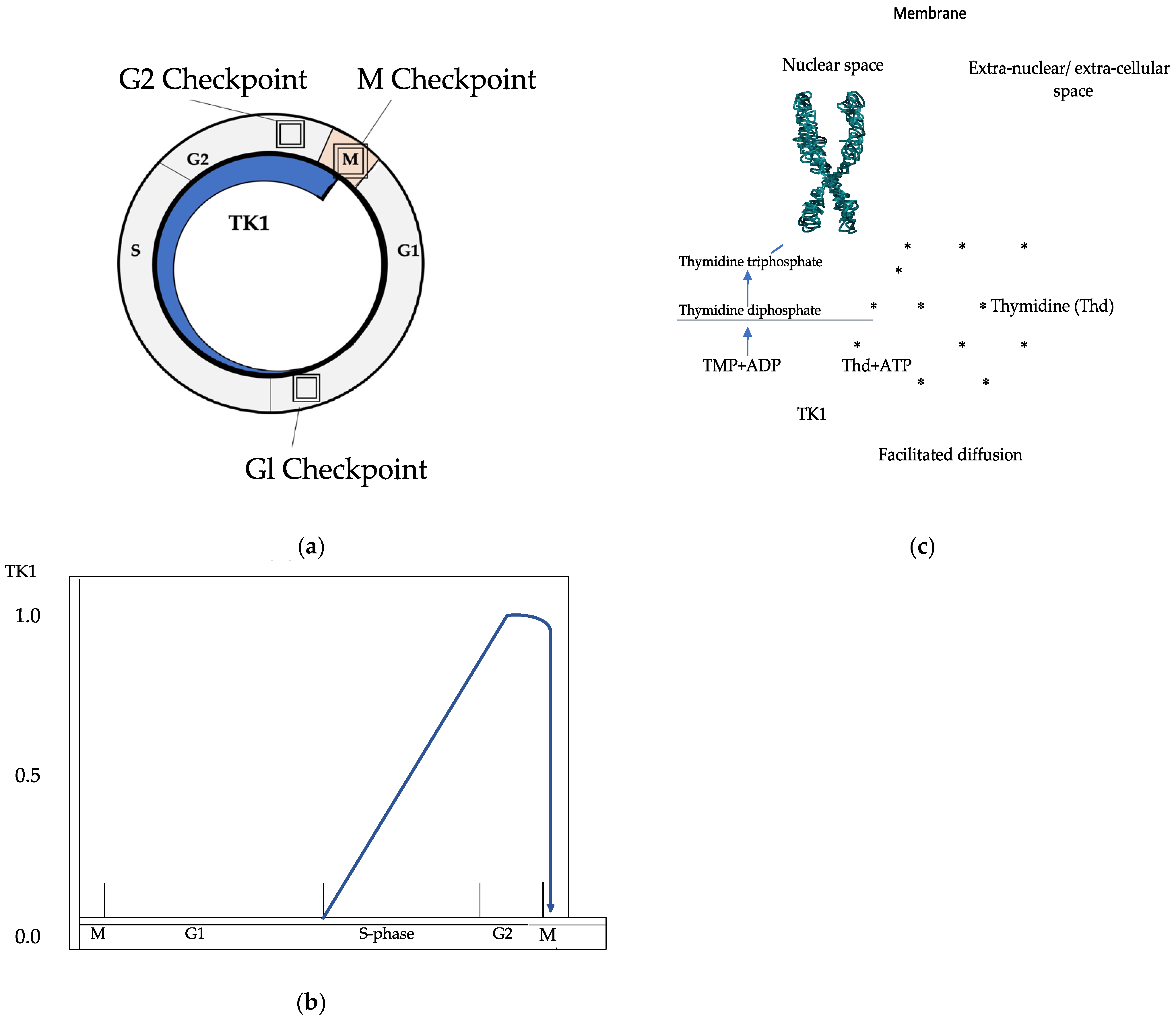
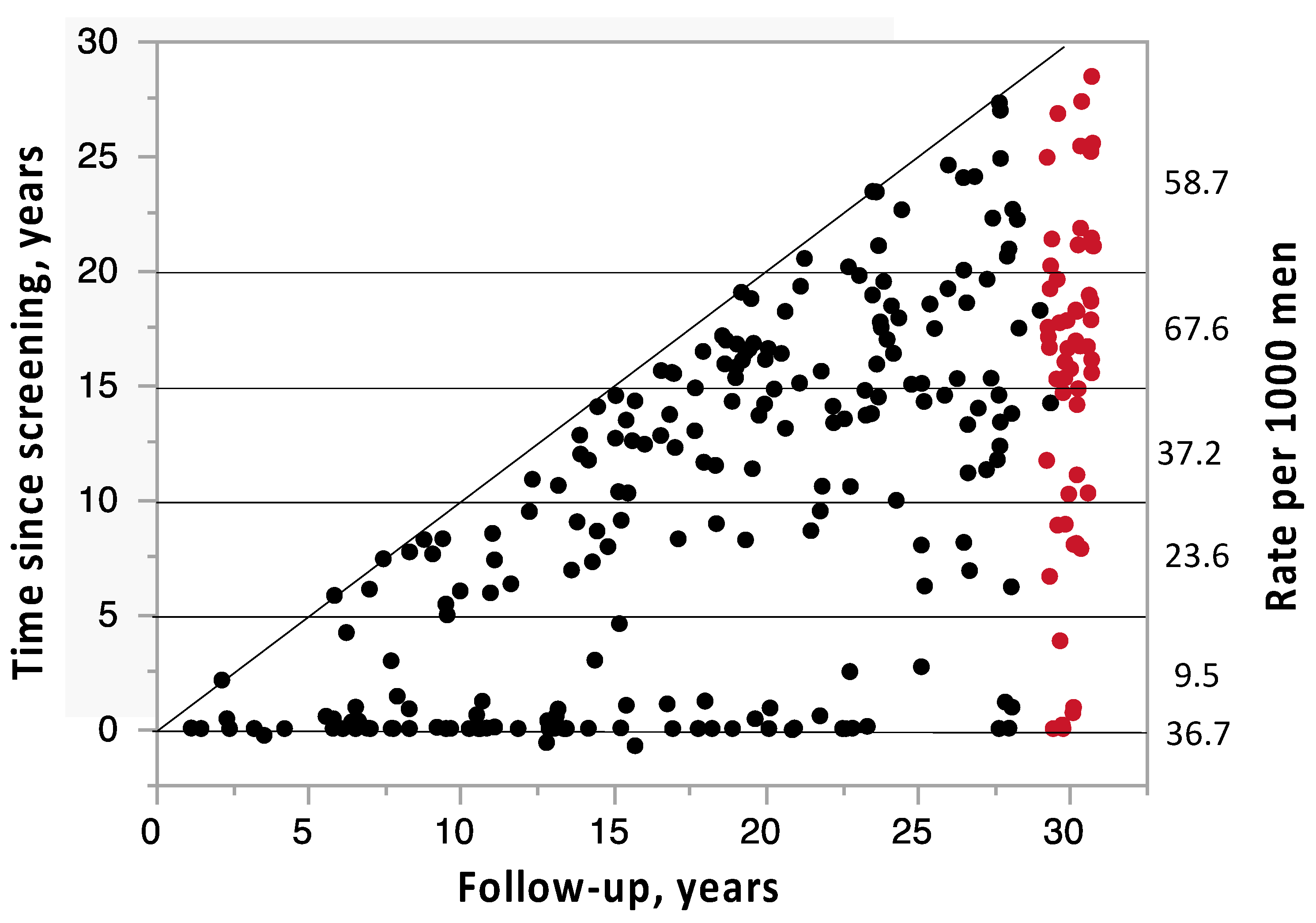

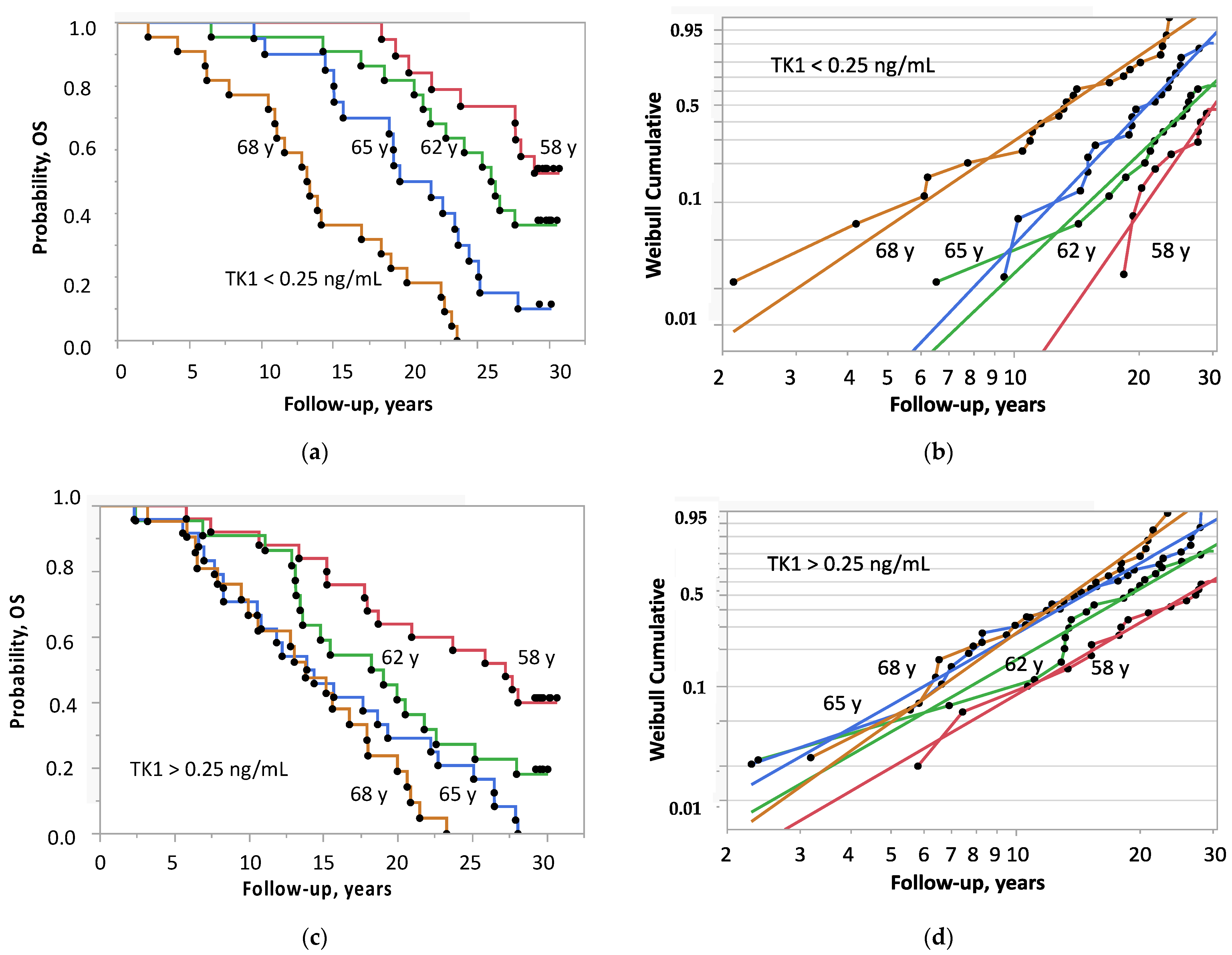

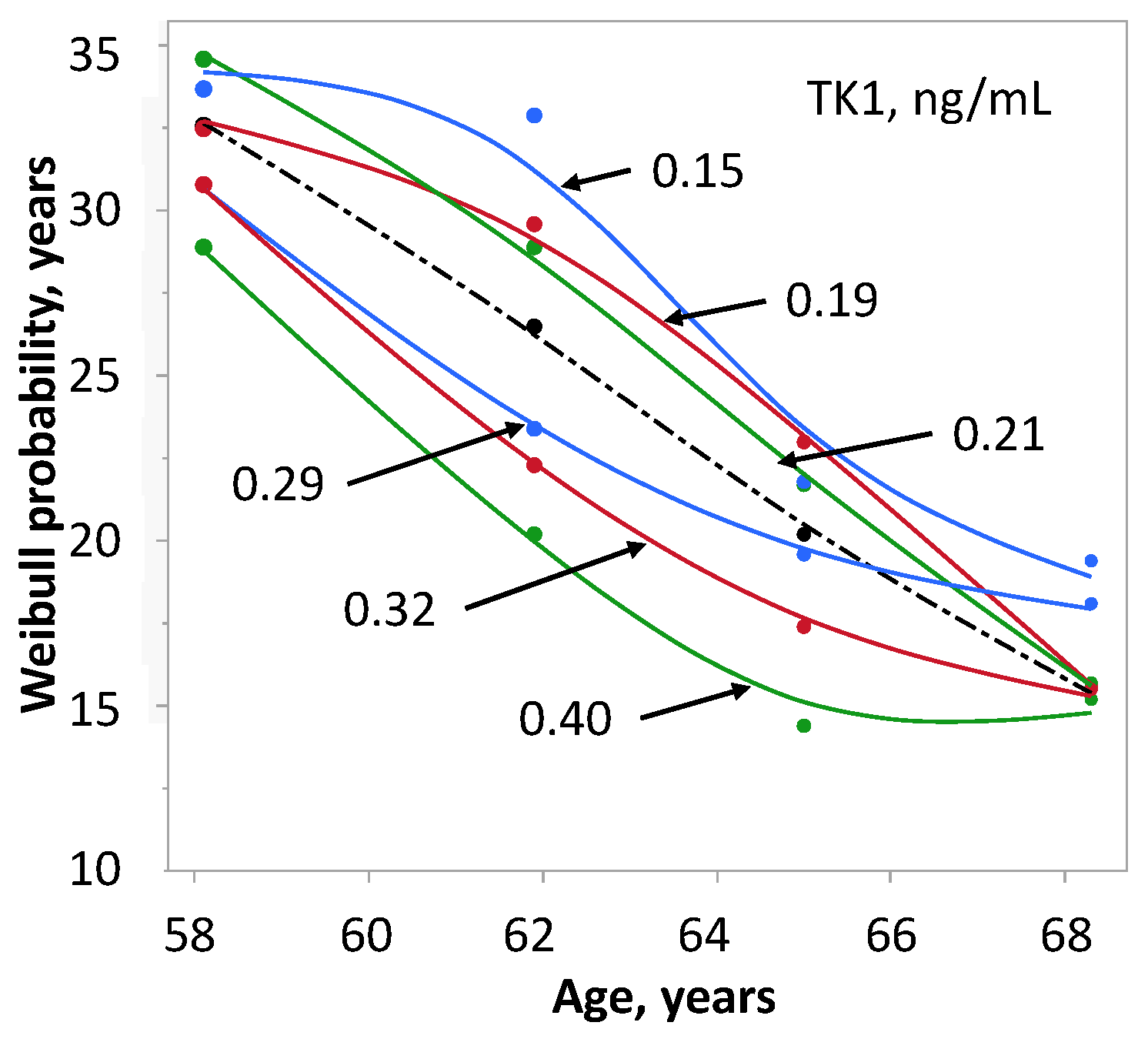
| Variable | Prostate Cancer | Controls | p |
|---|---|---|---|
| Age at screening | 63.2 (6.8) | 63.7 (6.5) | 0.172 |
| Age last follow-up | 84.7 (10.7) | 81.6 (12.5) | 0.0005 |
| Alive last follow-up, n | 58 (20.7%) | 36 (13.2%) | 0.022 |
| PSA, ng/ml | 3.2 (4.5) | 1.8 (0.11) | <0.0001 |
| Free/total PSA | 0.13 (0.09) | 0.15 (0.08) | 0.0015 |
| Prostate volume, cm3 | 23.3 (12.0) | 21.4 (10.4) | 0.004 |
| PSA density, ng/ml/cm3 | 0.09 (0.05) | 0.14 (0.15) | <0.0001 |
| TK1, ng/ml n = 175 | 0.25 (0.14) | 0.23 (0.16) | 0.607 |
| Other malignancies, n 48 (17.7%) | 78 (28.8%) | 0.002 | |
| Age | 77.6 (9.3) | 76.5 (11.4) | 0.843 |
| Time to diagnosis, years | 14.7 (11.4) | 13.6 (10.0) | 0.284 |
| Time between diagnosis and last follow-up, years | 4.89 (1.9) | 1.02 (4.8) | 0.0007 |
| Variable | OR 95% CI | X2 | p |
|---|---|---|---|
| Univariate analysis | |||
| Age at screening, per year | 1.23 (1.17–1.29) | 61.4 | <0.0001 |
| Time screening—Diagnosis, per year | 0.93 (0.91–0.95) | 41.3 | <0.0001 |
| PSA <3.0>, ng/ml | 1.74 (1.24–2.45) | 10.3 | 0.0001 |
| TK1 <0.25>, ng/ml | 1.49 (1.07–2.08) | 5.5 | 0.018 |
| Multivariate analysis | |||
| TK1 <0.25>, ng/ml | 1.46 (1.04–2.03) | 4.9 | 0.026 |
| +Age at screening, per year | 1.23 (1.17–1.29) | 61.3 | <0.0001 |
| TK1 <0.25>, ng/ml | 1.55 (1.11–2.17) | 6.6 | 0.010 |
| +Time screening—Diagnosis, per year | 0.93 (0.91–0.95) | 42.3 | <0.0001 |
| TK1 <0.25>, ng/ml | 1.53 (1.10–2.13) | 6.2 | 0.012 |
| +PSA <3.0>, ng/ml | 1.78 (0.26–2.50) | 11.0 | 0.0009 |
| PSA <3.0>, ng/ml | 1.35 (0.95–1.91) | 2.9 | 0.089 |
| +Age at screening, per year | 1.22 (1.16–1.29) | 59.9 | <0.0001 |
| PSA <3.0>, ng/ml | 1.22 (0.85–1.75) | 1.2 | 0.27 |
| +Time screening—Diagnosis, per year | 0.15 (0.08–0.29) | 32.3 | <0.0001 |
| Age at screening, per year | 1.20 (1.14–1.27) | 49.2 | <0.0001 |
| Time screening—Diagnosis, per year | 0.94 (0.92–0.96) | 28.3 | <0.0001 |
| Variable | Univariate Analysis | Multivariate Analysis A | Multivariate Analysis B | Multivariate Analysis C | ||||
|---|---|---|---|---|---|---|---|---|
| OR 95% CI | p | OR 95% CI | p | OR 95% CI | p | OR 95% CI | p | |
| Age, per year | 1.23 (1.17–1.29) | <0.0001 | 1.21 (1.15–1.28) | <0.0001 | 1.23 (0.17–1.30) | <0.0001 | 1.22 (1.15–1.78) | <0.0001 |
| Time screening- diagnosis of PCa, per year | 0.930 (91–0.95) | <0.0001 | 0.94 (0.91–0.96) | <0.0001 | 0.94 (0.91–0.96) | <0.0001 | ||
| PSA > 3.0 <, ng/ml | 1.74 (1.24–2.45) | 0.001 | 1.07 (0.69–1.65) | 0.77 | ||||
| Free/total PSA < 0.13 > | 0.95 (0.68–1.33) | 0.77 | 1.22 (0.82–1.81) | 0.34 | 1.11 (0.75–1.64) | 0.59 | 1.25 (0.84–1.86) | 0.27 |
| Prostate volume, > 0.23<, cm3 | 1.15 (0.83–1.61) | 0.40 | 1.16 (0.80–1.70) | 0.44 | 1.02 (0.70–1.49) | 0.92 | 1.15 (0.79–1.67) | 0.48 |
| Other malignancies, yes/no | 0.84 (0.54–1.31) | 0.45 | 1.10 (0.70–1.74) | 0.68 | 1.14 (0.72–1.81) | 0.57 | 1.09 (0.69–1.73) | 1.14 |
| TK1 > 0.25 < ng/ml | 1.49 (1.07–2.08) | 0.018 | 1.61 (1.13–2.28) | 0.008 | ||||
| *TK1 <0.25 > + PSA <3.0 >, ng/ml | Sub-group 1: TK1 < 0.25 + PSA < 3.0 ng/ml | Sub-group 2: TK1 < 0.25 + PSA > 3.0 ng/ml | Sub-group 3: TK1 > 0.25 + PSA < 3.0 ng/ml | Sub-group 4: TK1 > 0.25 + PSA > 3.0 ng/ml | ||||
| 4 vs. 1 | 2.61 (1.61–4.23) | <0.0001 | 2.09 (1.21–3.69) | 0.008 | 1.62 (0.92–2.86) | 0.09 | ||
| 4 vs. 2 | 1.69 (1.10–2.60) | 0.016 | 1.85 (0.18–2.89) | 0.007 | 1.94 (1.24–3.02) | 0.004 | ||
| 4 vs. 3 | 1.99 1.26–3.16) | 0.003 | 1.82 (1.07–3.10) | 0.028 | 1.35 (0.78–2.34) | 0.29 | ||
| 2 vs. 1 | 1.54 (0.93–2.55) | 0.09 | 1.13 (0.66–1.93) | 0.65 | 0.84 (0.48–1.45) | 0.53 | ||
| 3 vs. 1 | 1.31 (0.77–2.22) | 0.31 | 1.15 (0.67–1.97) | 0.61 | 1.20 (0.70–2.07) | 0.50 | ||
| 3 vs. 2 | 0.85 (0.52–1.38) | 0.51 | 1.01 (0.59–1.73) | 0.95 | 1.44 (0.83–2.50) | 0.20 | ||
| Variable | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| OR 95% CI | p | OR 95% CI | p | ||
| All controls | Age, per year | 1.07 (1.04–1.10) | <0.0001 | 1.07 (1.03–1.10) | <0.0001 |
| (n = 271) | Malignancies, yes/no | 1.42 (1.07–1.87) | 0.02 | 1.32 (1.0–1.76) | 0.046 |
| TK1, ng/ml per unit | 1.51 (0.92–2.19) | 0.059 | 1.50 (0.97–2.28) | 0.037 | |
| PSA, ng/ml per unit | 1.20 (0.87–1.56) | 0.231 | 1.02 (0.98–1.04) | 0.23 | |
| Controls without malignancies | |||||
| (n = 193) | Age, per year | 1.08 (1.04–1.12) | <0.0001 | 1.08 (1.04–1.12) | <0.0001 |
| TK1, ng/ml per unit | 1.66 (1.03–2.37) | 0.015 | 1.73 (1.08–2.46) | 0.008 | |
| PSA, ng/ml per unit | 1.03 (0.99–1.05) | 0.049 | 1.02 (0.98–1.05) | 0.136 | |
| Prostate volume, | |||||
| cm3 per unit | 1.01 (0.99–1.02) | 0.563 | 0.99 (0.98–1.01) | 0.69 | |
| Controls with malignancies | |||||
| (n = 78) | Age, per year | 1.04 (0.97–1.09) | 0.30 | 1.02 (0.96–1.08) | 0.60 |
| TK1, ng/ml per unit | 0.46 (0.07–2.19) | 0.39 | 0.38 (0.05–1.77) | 0.27 | |
| Diagnose, time since | |||||
| screening, per year. | 0.91 (0.88–0.94) | <0.0001 | 0.91 (0.88–0.94) | <0.0001 | |
| PSA, ng/ml per unit | 0.96 (0.87–1.04) | 0.31 | 0.99 (0.89–1.08) | 0.82 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tribukait, B.; Lundgren, P.-O.; Kjellman, A.; Norming, U.; Nyman, C.R.; Jagarlmundi, K.; Gustafsson, O. Prediction of Overall Survival by Thymidine Kinase 1 Combined with Prostate-Specific Antigen in Men with Prostate Cancer. Int. J. Mol. Sci. 2023, 24, 5160. https://doi.org/10.3390/ijms24065160
Tribukait B, Lundgren P-O, Kjellman A, Norming U, Nyman CR, Jagarlmundi K, Gustafsson O. Prediction of Overall Survival by Thymidine Kinase 1 Combined with Prostate-Specific Antigen in Men with Prostate Cancer. International Journal of Molecular Sciences. 2023; 24(6):5160. https://doi.org/10.3390/ijms24065160
Chicago/Turabian StyleTribukait, Bernhard, Per-Olof Lundgren, Anders Kjellman, Ulf Norming, Claes R. Nyman, Kiran Jagarlmundi, and Ove Gustafsson. 2023. "Prediction of Overall Survival by Thymidine Kinase 1 Combined with Prostate-Specific Antigen in Men with Prostate Cancer" International Journal of Molecular Sciences 24, no. 6: 5160. https://doi.org/10.3390/ijms24065160
APA StyleTribukait, B., Lundgren, P.-O., Kjellman, A., Norming, U., Nyman, C. R., Jagarlmundi, K., & Gustafsson, O. (2023). Prediction of Overall Survival by Thymidine Kinase 1 Combined with Prostate-Specific Antigen in Men with Prostate Cancer. International Journal of Molecular Sciences, 24(6), 5160. https://doi.org/10.3390/ijms24065160







