Spectroscopic Study of Five-Coordinated Thermal Treated Alumina Formation: FTIR and NMR Applying
Abstract
1. Introduction
2. Results
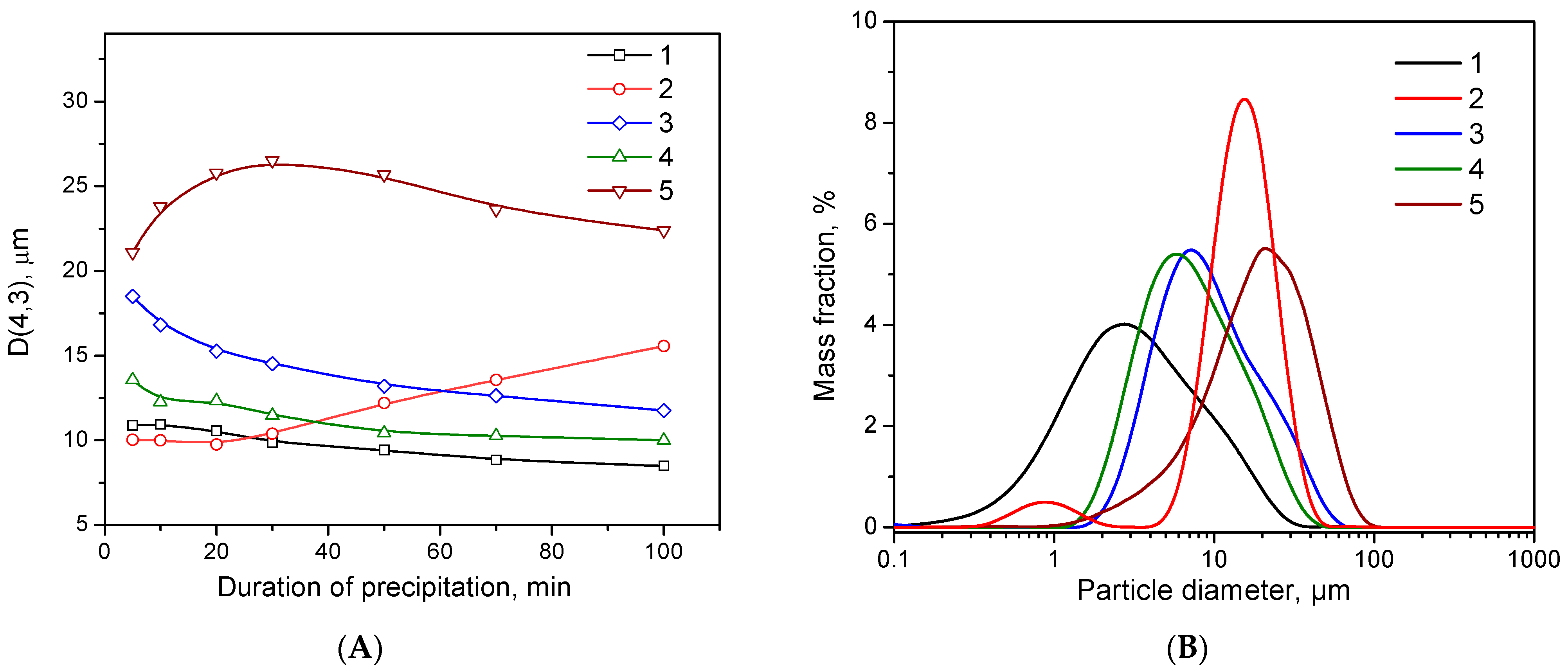
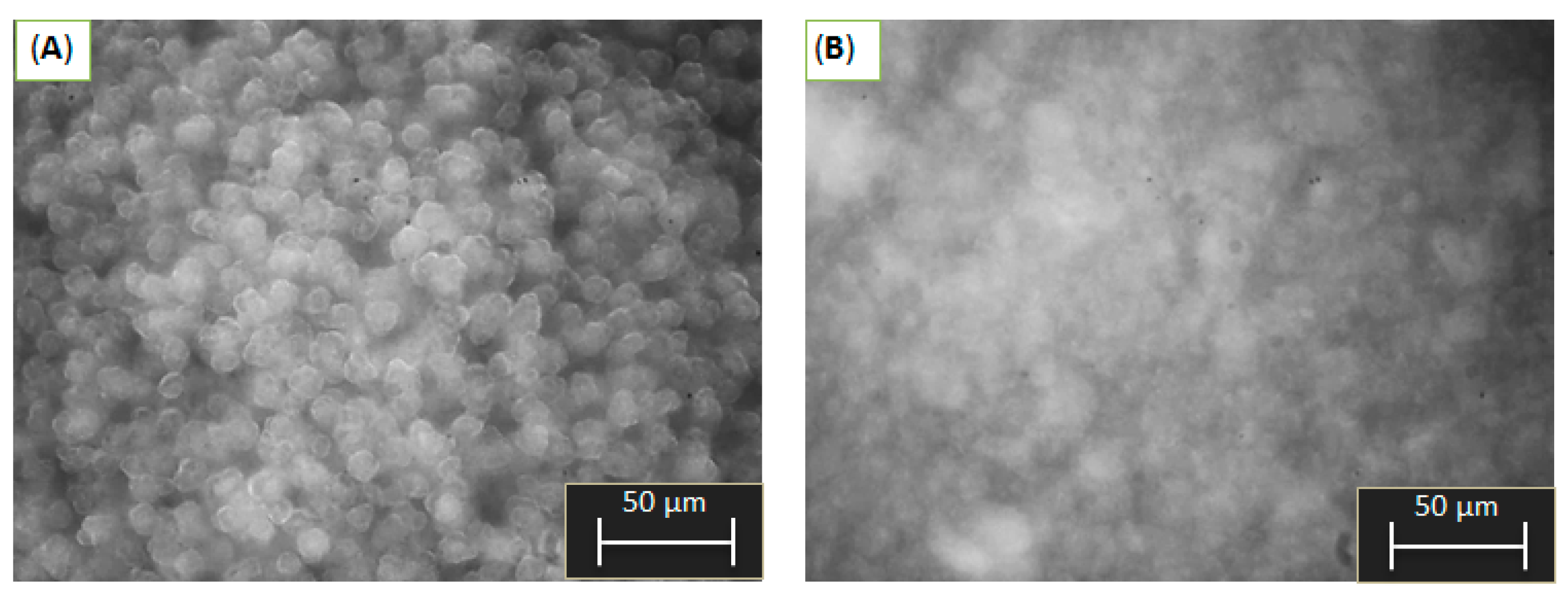
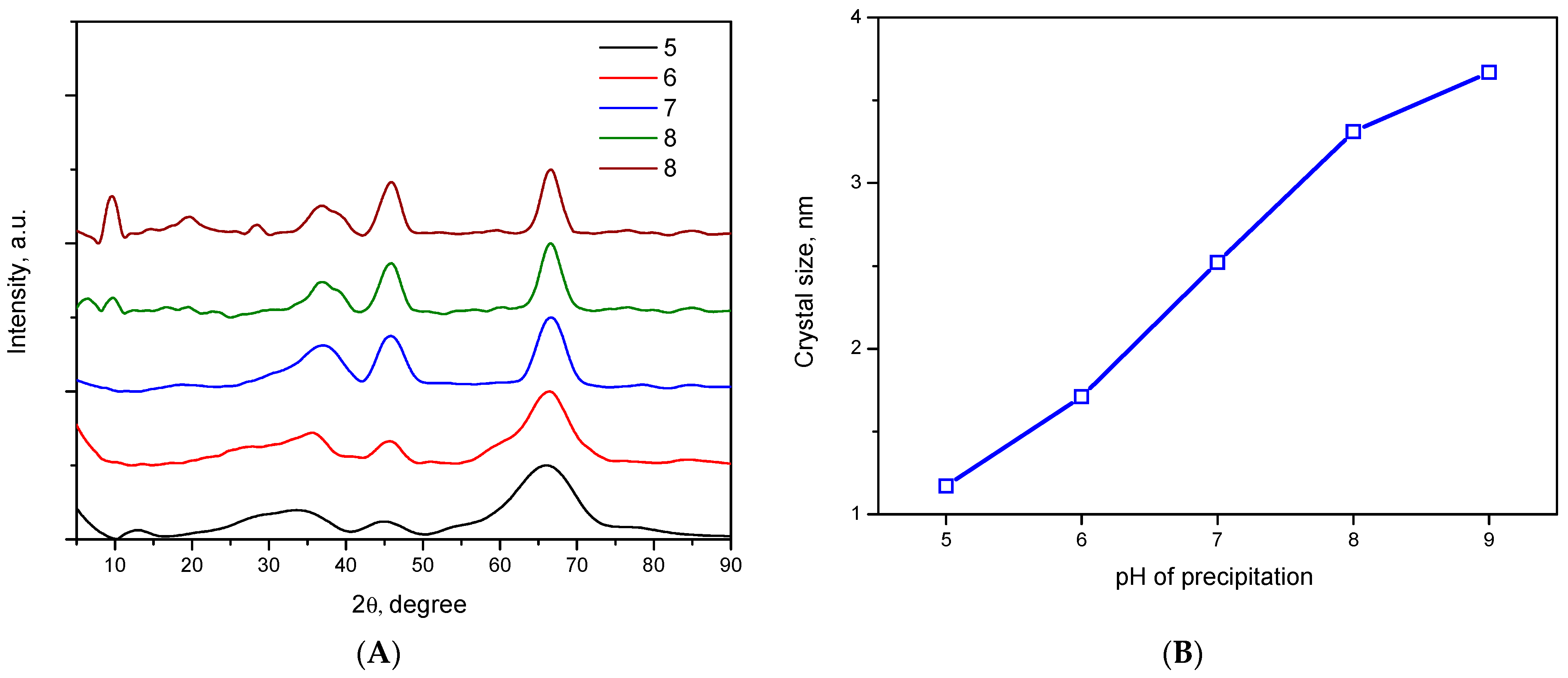
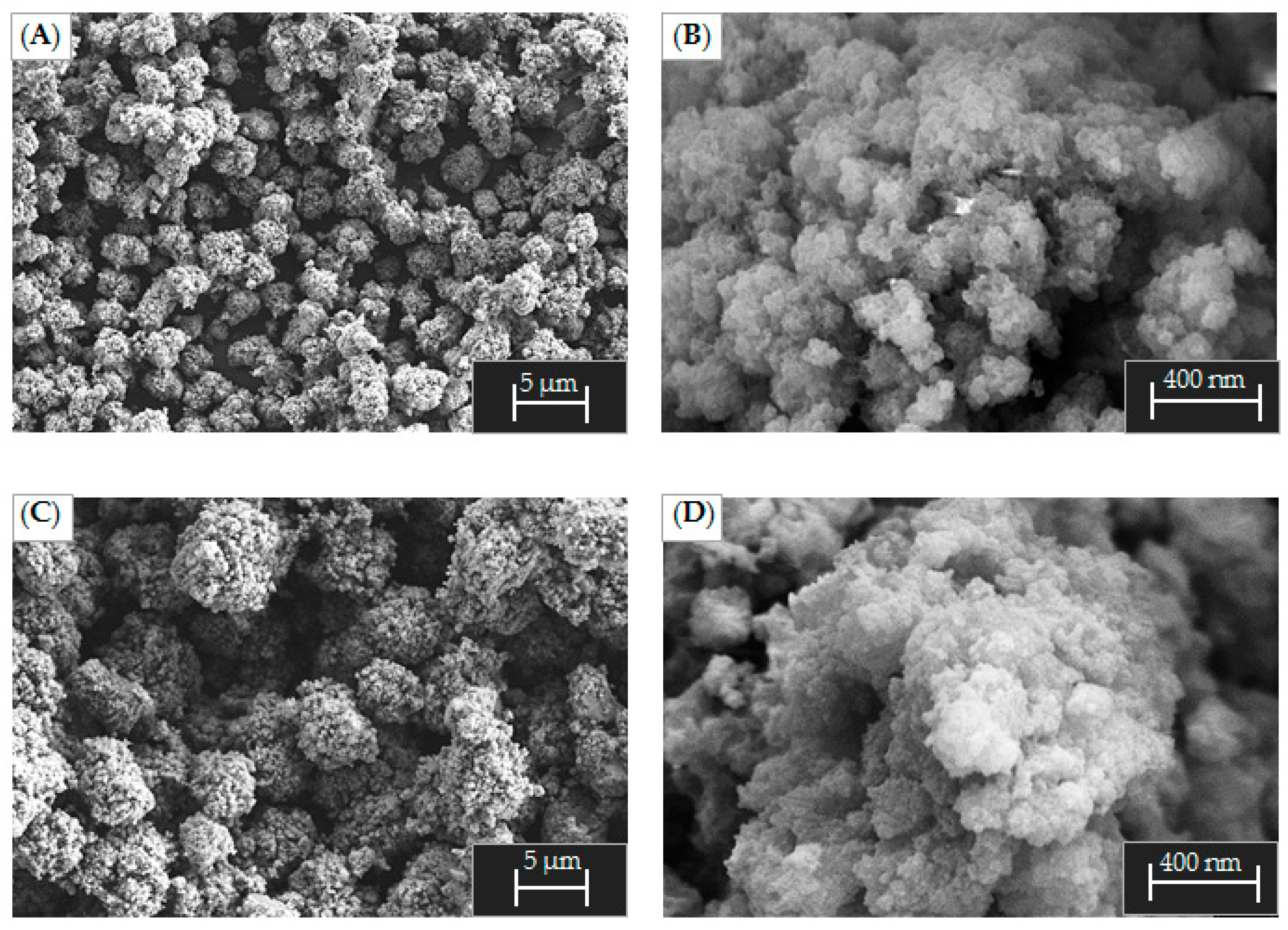
2.1. Particle Size and Morphology Investigation during Precipitation
2.2. Xerogels Composition and Structure
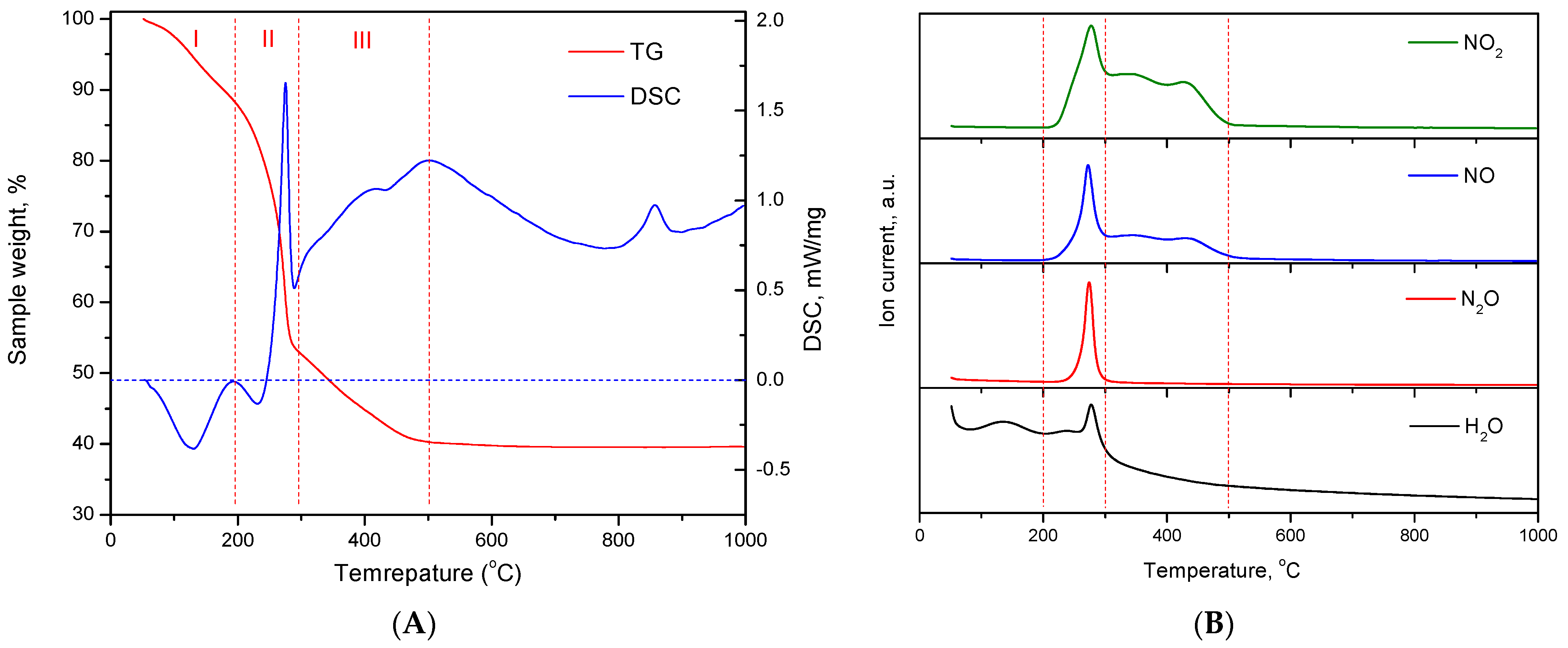
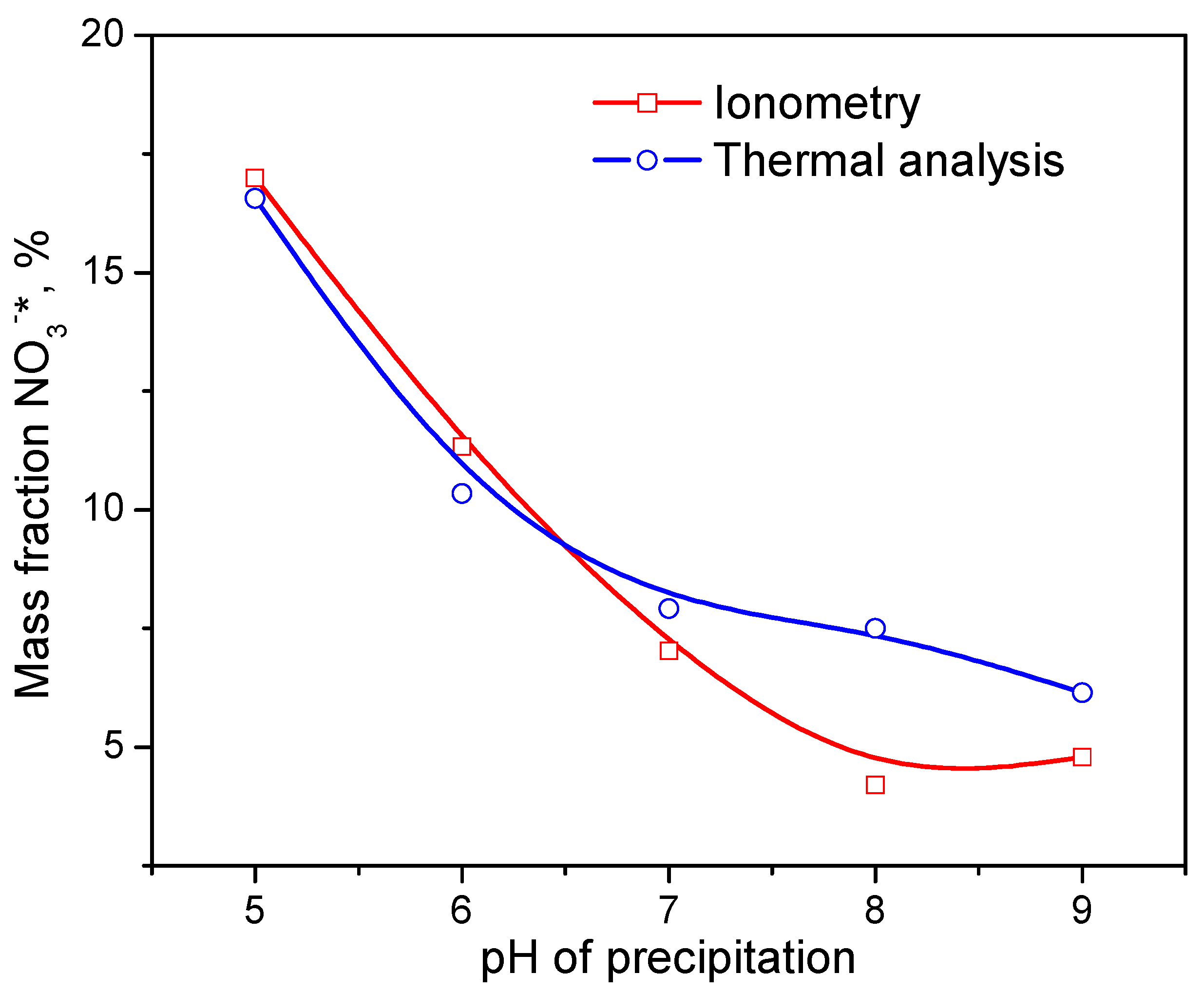
2.3. Chemical Composition of Xerogels
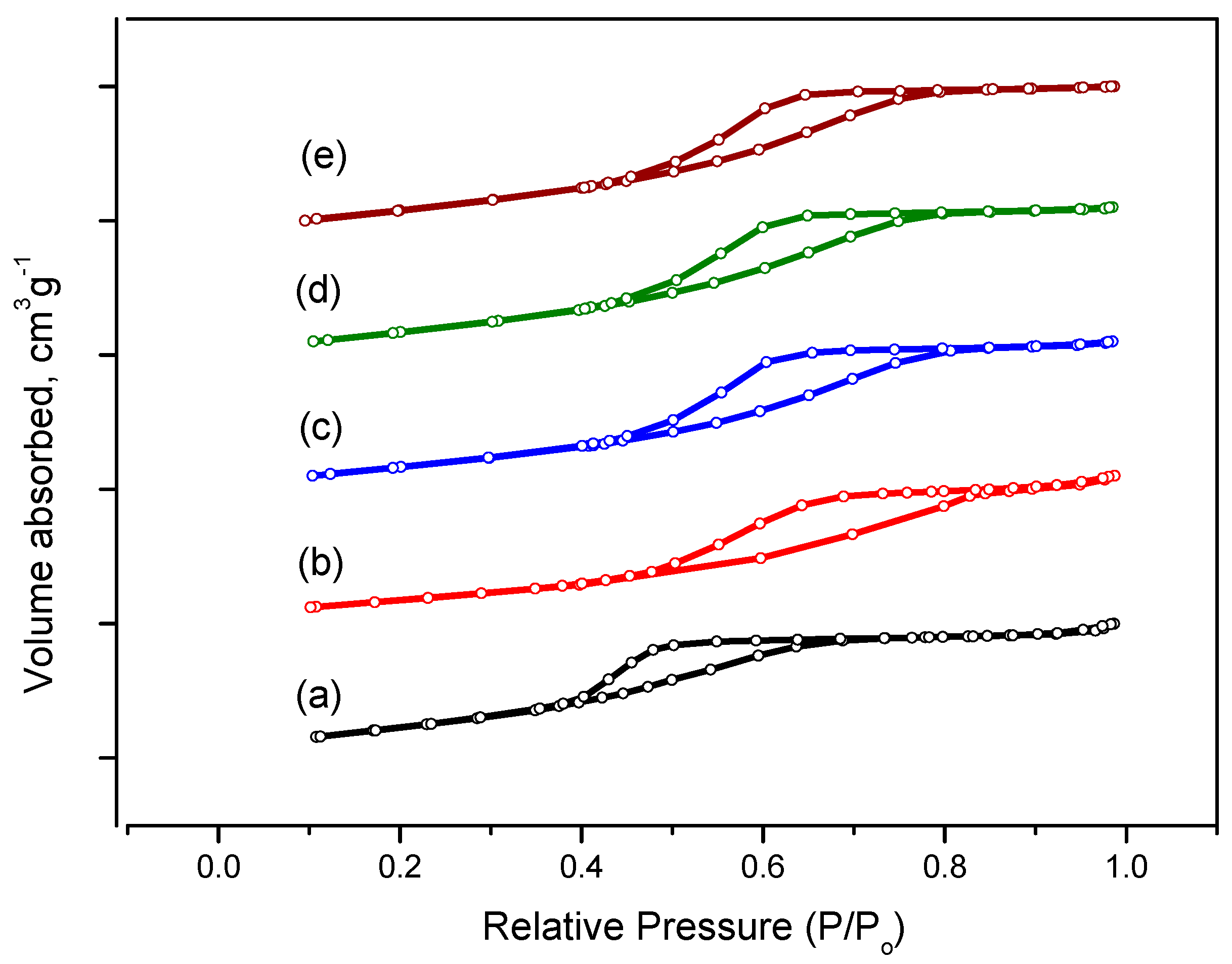
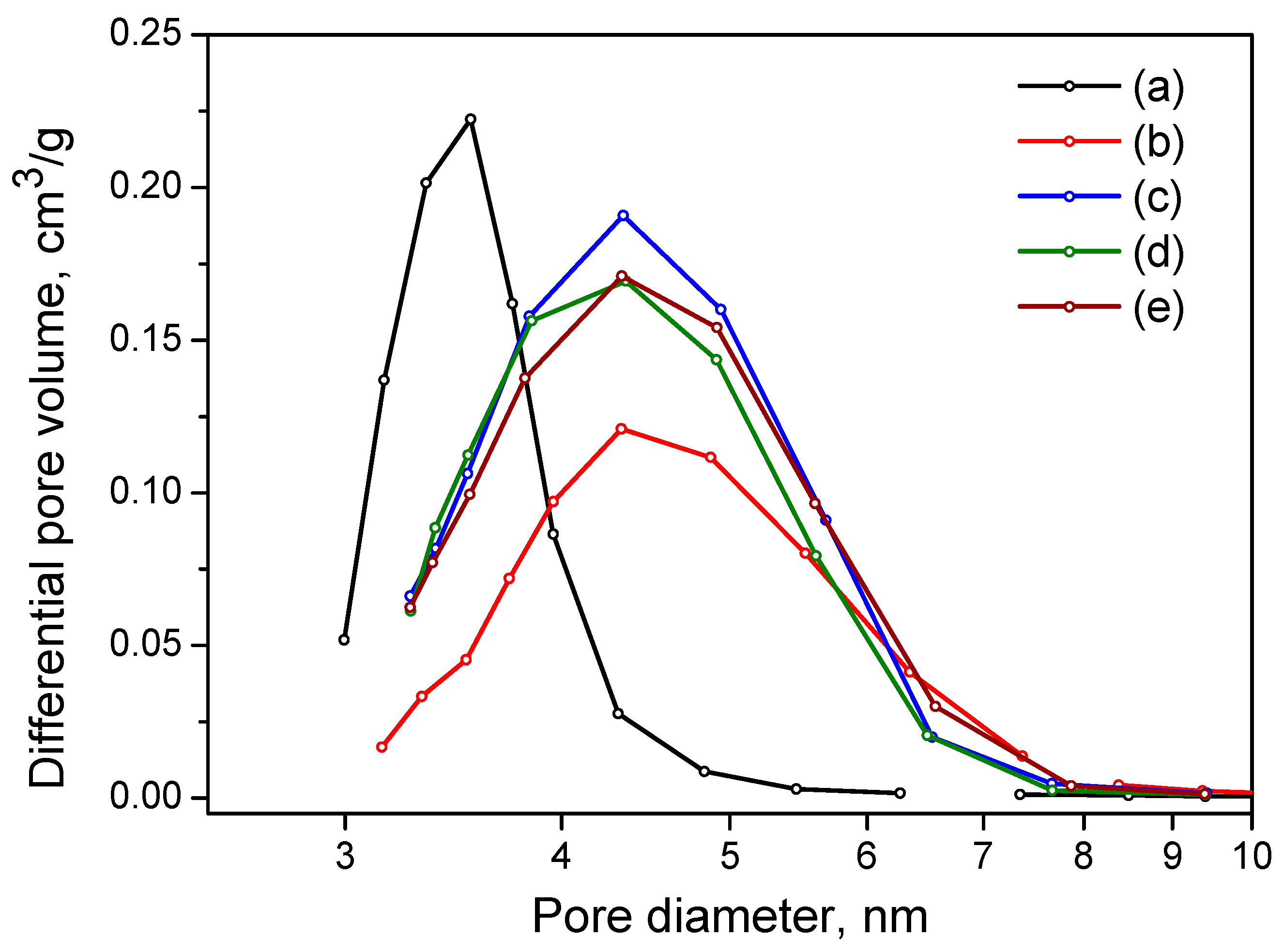
2.4. Aluminum Oxide Samples Research
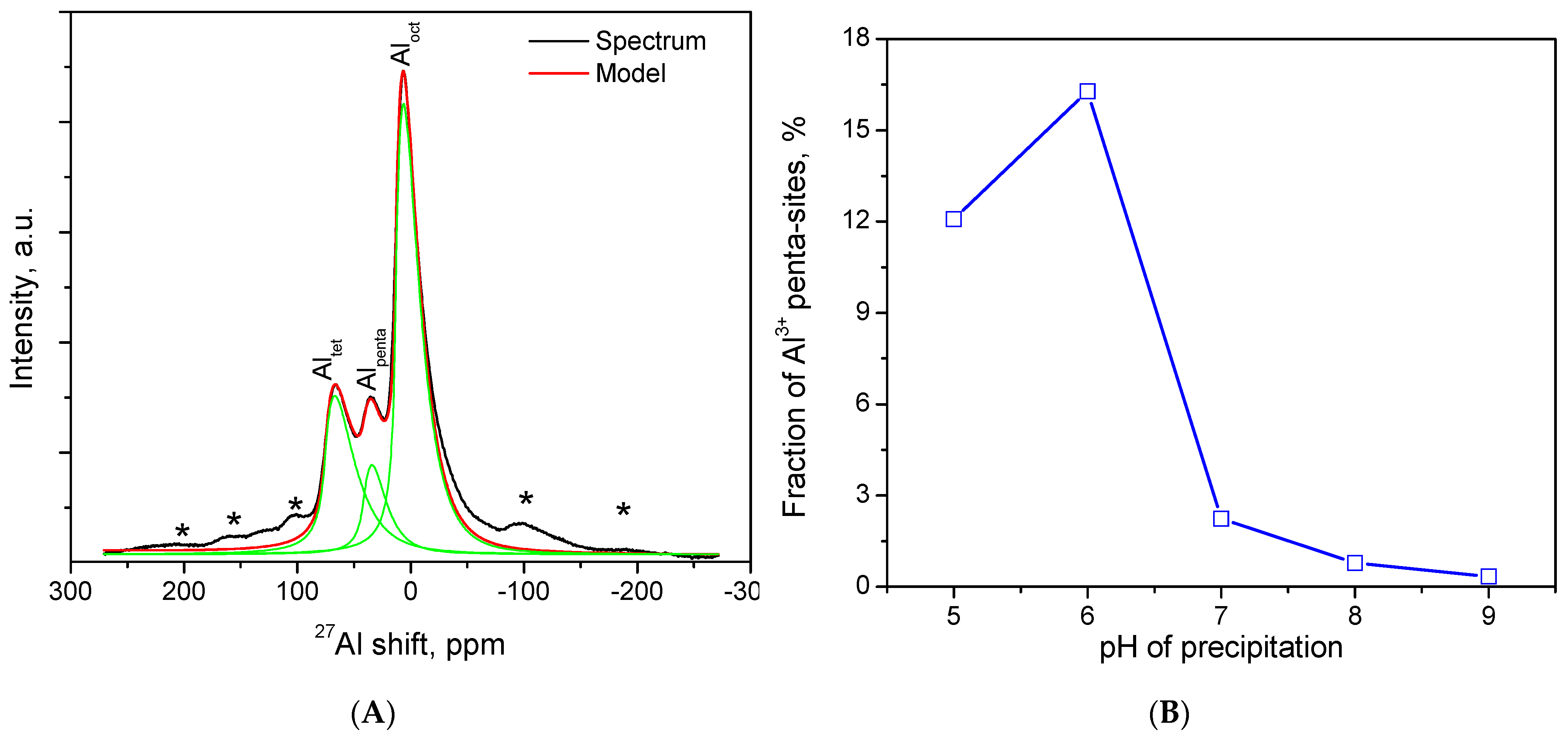
2.5. NMR-Spectum Research
3. Materials and Methods
3.1. Synthesis of Aluminum Oxide
3.2. Research Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Chemical Society. Industrial Alumina Chemicals. Anal. Chem. 1987, 59, 706A. [Google Scholar] [CrossRef]
- Ratnasamy, P.; Knözinger, H. Infrared and optical spectroscopic study of Co-Mo-Al2O3 catalysts. J. Catal. 1978, 54, 155–165. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Jafari, M.; Iranshahi, D. Progress in catalytic naphtha reforming process: A review. Appl. Energy 2013, 109, 79–93. [Google Scholar] [CrossRef]
- Datsko, T.Y.; Zelentsov, V.I. Dependence of the Surface Charge and the Fluorine Adsorption by g-Aluminum Oxide on the Solution Temperature. Surf. Eng. Appl. Electrochem. 2009, 45, 404–410. [Google Scholar] [CrossRef]
- Sattler, J.J.H.B.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef] [PubMed]
- Yashnik, S.A.; Kuznetsov, V.V.; Ismagilov, Z.R. Effect of χ-alumina addition on H2S oxidation properties of pure and modified γ-alumina. Chin. J. Catal. 2018, 39, 258–274. [Google Scholar] [CrossRef]
- Levin, I.; Bendersky, L.A.; Brandon, D.G.; Rühle, M. Cubic to monoclinic phase transformations in alumina. Acta Mater. 1997, 45, 3659–3669. [Google Scholar] [CrossRef]
- Ivanova, A.S.; Litvak, G.S.; Kryukova, G.N.; Tsybulya, S.V.; Paukshtis, E.A. Real structure of metastable forms of aluminum oxide. Kinet. Catal. 2000, 41, 137–141. [Google Scholar] [CrossRef]
- Anna, K.K.; Bogireddy, K.R.; Agarwal, V.; Ramírez Bon, R. Synthesis of α and γ phase of aluminium oxide nanoparticles for the photocatalytic degradation of methylene blue under sunlight: A comparative study. Mater. Lett. 2022, 317, 132085. [Google Scholar] [CrossRef]
- Kureti, S.; Weisweiler, W. A new route for the synthesis of high surface area γ-aluminium oxide xerogel. Appl. Catal. A 2002, 225, 251–259. [Google Scholar] [CrossRef]
- Fernandes, E.P.; Silva, T.S.; Carvalho, C.M.; Selvasembian, R.; Chaukura, N.; Oliveira, L.M.T.M.; Meneghetti, S.M.P.; Meili, L. Efficient adsorption of dyes by γ-alumina synthesized from aluminum wastes: Kinetics, isotherms, thermodynamics and toxicity assessment. J. Environ. Chem. Eng. 2021, 9, 106198. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Allahverdi, A. Production of High Purity α- and γ-Alumina from Aluminum Dross. In Encyclopedia of Renewable and Sustainable Materials; Elsevier: Amsterdam, The Netherlands, 2020; Volume 2, pp. 473–482. [Google Scholar] [CrossRef]
- Kwak, J.H.; Hu, J.; Mei, D.; Yi, C.W.; Kim, D.H.; Peden, C.H.F.; Allard, L.F.; Szanyi, J. Coordinatively unsaturated Al3+ centers as binding sites for active catalyst phases of platinum on γ-Al2O3. Science 2009, 325, 1670–1673. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Yu, X.; Ge, M.; Lin, M.; Yang, X.; Jing, Y. Insights into adsorption mechanism for fluoride on cactus-like amorphous alumina oxide microspheres. Chem. Eng. J. 2018, 345, 252–259. [Google Scholar] [CrossRef]
- Yang, X.; Li, Q.; Lu, E.; Wang, Z.; Gong, X.; Yu, Z.; Guo, Y.; Wang, L.; Guo, Y.; Zhan, W.; et al. Taming the stability of Pd active phases through a compartmentalizing strategy toward nanostructured catalyst supports. Nat. Commun. 2019, 10, 1611. [Google Scholar] [CrossRef]
- Kwak, J.H.; Hu, J.Z.; Kim, D.H.; Szanyi, J.; Peden, C.H.F. Penta-coordinated Al3+ ions as preferential nucleation sites for BaO on γ-Al2O3: An ultra-high-magnetic field 27Al MAS NMR study. J. Catal. 2007, 251, 189–194. [Google Scholar] [CrossRef]
- Lee, J.; Jang, E.J.; Kwak, J.H. Effect of number and properties of specific sites on alumina surfaces for Pt-Al2O3 catalysts. Appl. Catal. A 2019, 569, 16833. [Google Scholar] [CrossRef]
- Jang, E.J.; Lee, J.; Jeong, H.Y.; Kwak, J.H. Controlling the acid-base properties of alumina for stable PtSn-based propane dehydrogenation catalysts. Appl. Catal. A 2019, 572, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.; Pei, C.; Wang, X.; Sun, G.; Zhao, Z. The role of pentacoordinate Al3+ sites of Pt/Al2O3 catalysts in propane dehydrogenation. Fundam. Res. 2022; in press. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, Y.; Jin, F.; Stampfl, C.; Hunger, M.; Baiker, A.; Huang, J. Strongly enhanced acidity and activity of amorphous silica–alumina by formation of pentacoordinated AlV species. J. Catal. 2019, 372, 1–7. [Google Scholar] [CrossRef]
- Du, X.; Wang, Y.; Su, X.; Li, J. Influences of pH value on the microstructure and phase transformation of aluminum hydroxide. Powder Technol. 2009, 192, 40–46. [Google Scholar] [CrossRef]
- Mihaylov, M.Y.; Zdravkova, V.R.; Ivanova, E.Z.; Aleksandrov, H.A.; Petkov, P.S.; Vayssilov, G.N.; Hadjiivanov, K.I. Infrared spectra of surface nitrates: Revision of the current opinions based on the case study of ceria. J. Catal. 2021, 394, 245–258. [Google Scholar] [CrossRef]
- Guoyuan, Z.G.; Wu, C.; Wang, J.; Mo, S.; Zou, Z.; Zhou, B.; Long, F. Space-Confined Effect One-Pot Synthesis of γ-AlO(OH)/MgAl-LDH Heterostructures with Excellent Adsorption Performance. Nanoscale Res. Lett. 2019, 14, 281. [Google Scholar] [CrossRef]
- Chen, H.; Ren, B.; Liu, M.; Qin, T.; Guo, Q.; Li, G.; Gong, D.; Cheng, G.; Chen, J.; Li, B. Facile synthesis of hydroxyl aluminum oxalate based on the hydrothermal reaction of boehmite and oxalic acid. Colloids Surf. A 2022, 639, 128344. [Google Scholar] [CrossRef]
- Litvak, I.; Anker, Y.; Cohen, H. On-line in situ determination of deuterium content in water via FTIR spectroscopy. RSC Adv. 2018, 8, 28472–28479. [Google Scholar] [CrossRef]
- Wu, H.B.; Chan, M.N.; Chan, C.K. FTIR Characterization of Polymorphic Transformation of Ammonium Nitrate. Aerosol Sci. Technol. 2007, 41, 581–588. [Google Scholar] [CrossRef]
- Nakamoto, K. Part B: Applications in coordination, organometallic, and bioinorganic chemistry. In Infrared and Raman Spectra of Inorganic and Coordination Compounds; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Hadjiivanov, K. Identification of Neutral and Charged NxOy Surface Species by IR Spectroscopy. Catal. Rev. 2000, 42, 71–144. [Google Scholar] [CrossRef]
- Wolverton, C.; Hass, K.C. Phase stability and structure of spinel-based transition aluminas. Phys. Rev. B 2000, 63, 024102. [Google Scholar] [CrossRef]
- Massiot, D.; Fayon, F.; Capron, M.; King, I.; Calve, S.; Alonso, B.; Durand, J.-O.; Bujoli, B.; Gan, Z.; Hoatson, G. Modelling one- and two-dimensional solid-state NMR spectra. Magn. Reson. Chem. 2002, 40, 70–76. [Google Scholar] [CrossRef]
- Neuville, D.R.; Cormier, L.; Massiot, D. Al environment in tectosilicate and peraluminous glasses: A 27Al MQ-MAS NMR, Raman, and XANES investigation, Geo-chim. Cosmochim. Acta 2004, 68, 5071–5079. [Google Scholar] [CrossRef]
- Pershina, S.V.; Raskovalov, A.A.; Antonov, B.D.; Yaroslavtseva, T.V.; Reznitskikh, O.G.; Baklanova, Y.V.; Pletneva, E.D. Extreme behavior of Li-ion conductivity in the Li2O–Al2O3–P2O5 glass system. J. Non-Cryst. Solids 2015, 430, 64–72. [Google Scholar] [CrossRef]
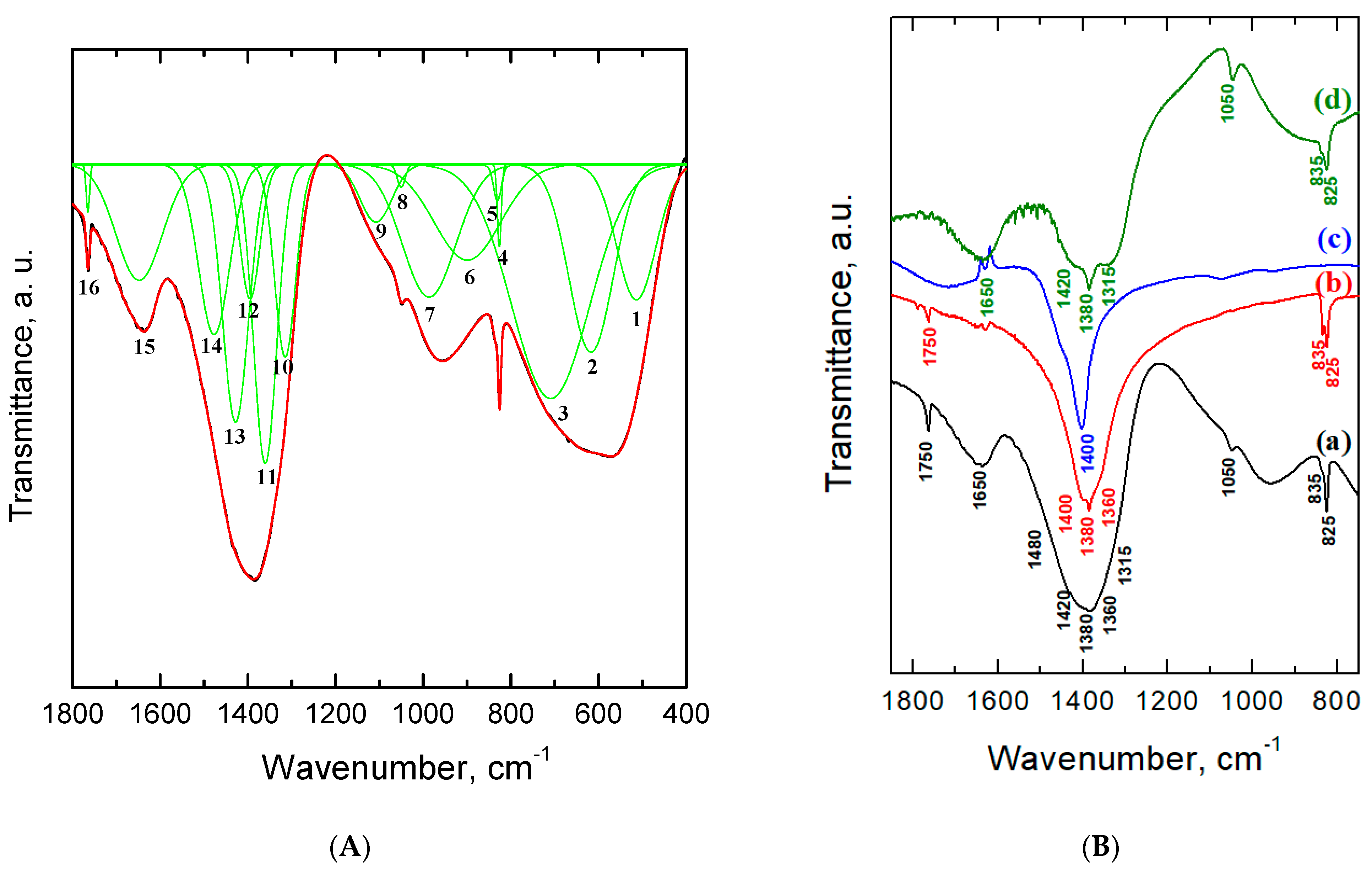
| No of Band | Wavenumber, cm–1 | Description |
|---|---|---|
| 1 | 480 | ν(Al–O) |
| 2 | 535 | ν(Al–O) |
| 3 | 625 | ν(Al–O) |
| 4 | 725 | ν(Al–O) or δ(O–N–O) |
| 5 | 825 | out-of-plane rocking vibration of N–O |
| 6 | 835 | out-of-plane rocking vibration of N–O |
| 7 | 900 | δ(Al–OH) |
| 8 | 985 | δ(Al–OH) |
| 9 | 1050 | δ(Al–O–NO2) or νs(N–O) |
| 10 | 1100 | δ(Al–OH) |
| 11 | 1275 | νs(N–O) |
| 12 | 1315 | νas(N–O) |
| 13 | 1360 | νas(N–O) |
| 14 | 1380 | νas(N–O) |
| 15 | 1420 | νas(N–O) |
| 16 | 1480 | νas(N–O) |
| 17 | 1520 | νas(N–O) |
| 18 | 1650 | vibration of H2O molecules |
| 19 | 1750 | stretches vibration of NH4+ + NO3– |
| Sample | Mass Fraction, % | Mole Ratio | ||||||
|---|---|---|---|---|---|---|---|---|
| Al2O3 | NH4+ | NO3– | H2O | NH4+/Al | NO3–/Al | NO3–*/Al | H2O/Al | |
| Al-5 | 39.6 | 3.1 | 27.8 | 29.4 | 0.22 | 0.58 | 0.35 | 2.10 |
| Al-6 | 33.1 | 7.4 | 36.9 | 22.6 | 0.63 | 0.92 | 0.28 | 1.93 |
| Al-7 | 25.9 | 10.5 | 43.3 | 20.2 | 1.15 | 1.37 | 0.22 | 2.21 |
| Al-8 | 23.7 | 12.7 | 47.9 | 15.7 | 1.52 | 1.66 | 0.15 | 1.88 |
| Al-9 | 21.5 | 9.2 | 36.4 | 32.9 | 1.21 | 1.39 | 0.18 | 4.34 |
| Sample | Peak 1 | Peak 2 | Peak 3 | Total Weight Loss, % | |||
|---|---|---|---|---|---|---|---|
| T, °C | Δm, % | T, °C | Δm, % | T, °C | Δm, % | ||
| Al-5 | 35–200 | 13.63 | 200–279 | 30.55 | 279–600 | 16.56 | 60.74 |
| Al-6 | 35–200 | 12.43 | 200–289 | 44.61 | 289–600 | 10.34 | 67.38 |
| Al-7 | 35–200 | 7.87 | 200–302 | 58.53 | 302–600 | 7.92 | 74.32 |
| Al-8 | 35–200 | 5.8 | 200–303 | 61.53 | 303–600 | 7.5 | 74.83 |
| Al-9 | 35-200 | 5.63 | 200-308 | 66.47 | 308-600 | 6.14 | 78.24 |
| Samples | Surface Area, m2/g | Pore Volume., cm3/g | Pore Size., nm |
|---|---|---|---|
| Al-5 | 206 | 0.23 | 3.5 |
| Al-6 | 200 | 0.33 | 4.3 |
| Al-7 | 210 | 0.43 | 4.3 |
| Al-8 | 240 | 0.38 | 4.3 |
| Al-9 | 236 | 0.40 | 4.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashkovtsev, M.; Tarasova, N.; Baksheev, E.; Rychkov, V.; Zhuravlev, N.; Solodovnikova, P.; Galiaskarova, M. Spectroscopic Study of Five-Coordinated Thermal Treated Alumina Formation: FTIR and NMR Applying. Int. J. Mol. Sci. 2023, 24, 5151. https://doi.org/10.3390/ijms24065151
Mashkovtsev M, Tarasova N, Baksheev E, Rychkov V, Zhuravlev N, Solodovnikova P, Galiaskarova M. Spectroscopic Study of Five-Coordinated Thermal Treated Alumina Formation: FTIR and NMR Applying. International Journal of Molecular Sciences. 2023; 24(6):5151. https://doi.org/10.3390/ijms24065151
Chicago/Turabian StyleMashkovtsev, Maxim, Nataliia Tarasova, Evgeniy Baksheev, Vladimir Rychkov, Nikolai Zhuravlev, Polina Solodovnikova, and Maria Galiaskarova. 2023. "Spectroscopic Study of Five-Coordinated Thermal Treated Alumina Formation: FTIR and NMR Applying" International Journal of Molecular Sciences 24, no. 6: 5151. https://doi.org/10.3390/ijms24065151
APA StyleMashkovtsev, M., Tarasova, N., Baksheev, E., Rychkov, V., Zhuravlev, N., Solodovnikova, P., & Galiaskarova, M. (2023). Spectroscopic Study of Five-Coordinated Thermal Treated Alumina Formation: FTIR and NMR Applying. International Journal of Molecular Sciences, 24(6), 5151. https://doi.org/10.3390/ijms24065151







