A NOTCH1 Mutation Found in a Newly Established Ovarian Cancer Cell Line (FDOVL) Promotes Lymph Node Metastasis in Ovarian Cancer
Abstract
1. Introduction
2. Results
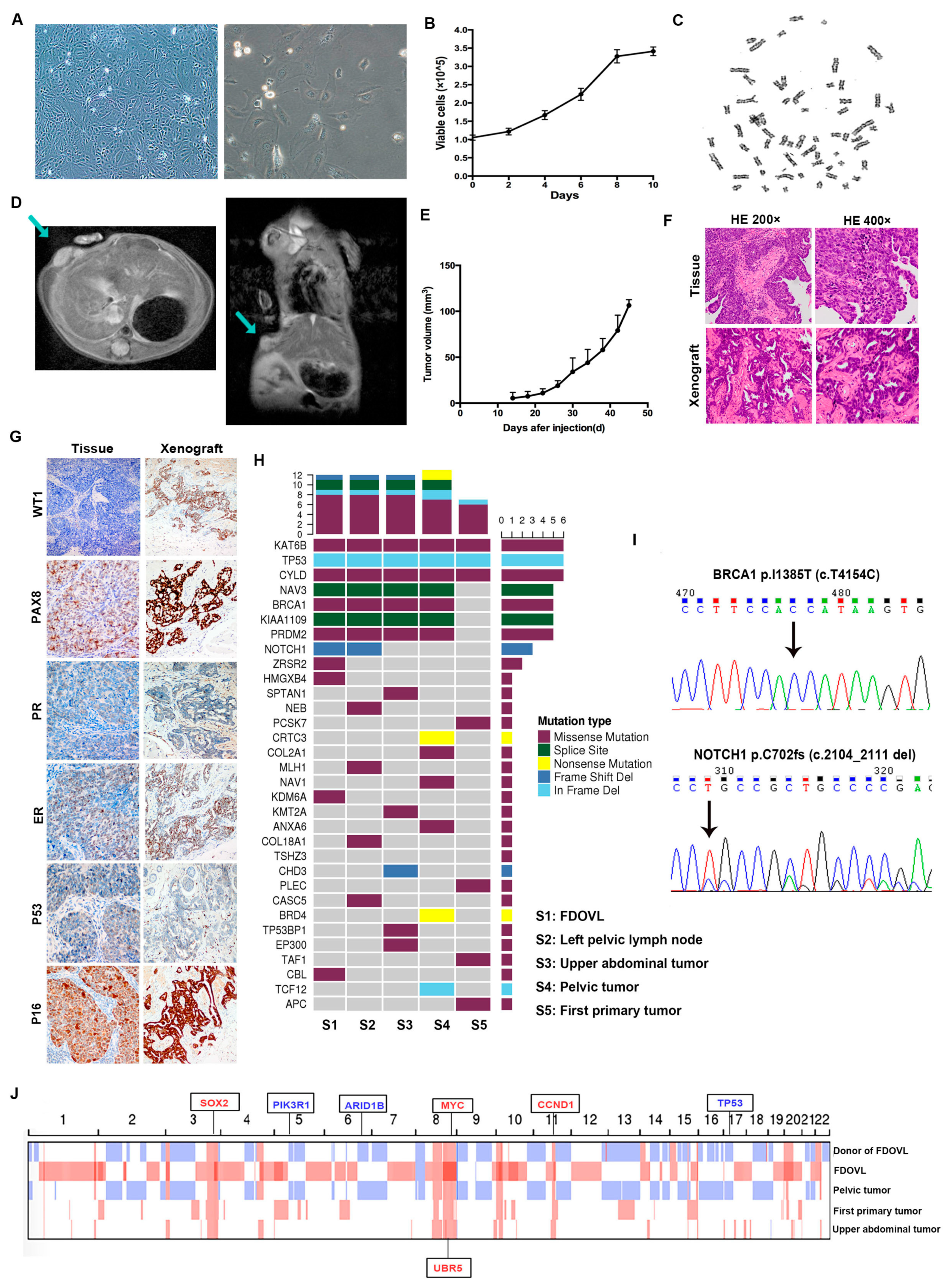
2.1. Morphology, Growth, Transplantation, and Genetic Characteristics of FDOVL Cells
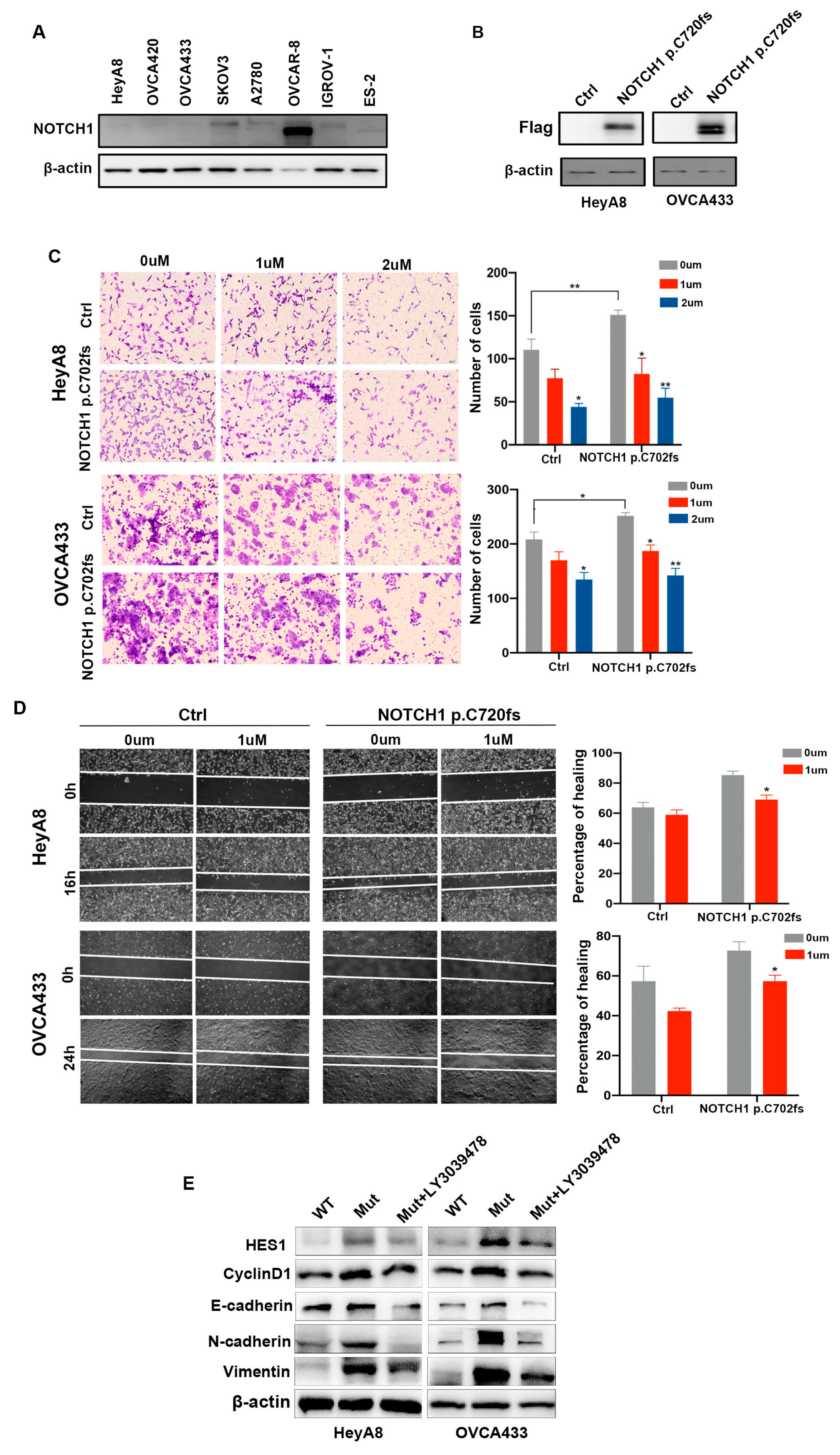
2.2. NOTCH1-p.C720fs Mutation Promotes Epithelial-Mesenchymal Transition (EMT) and Its Effects Could Be Attenuated by a NOTCH1 Inhibitor in Ovarian Cancer Cells
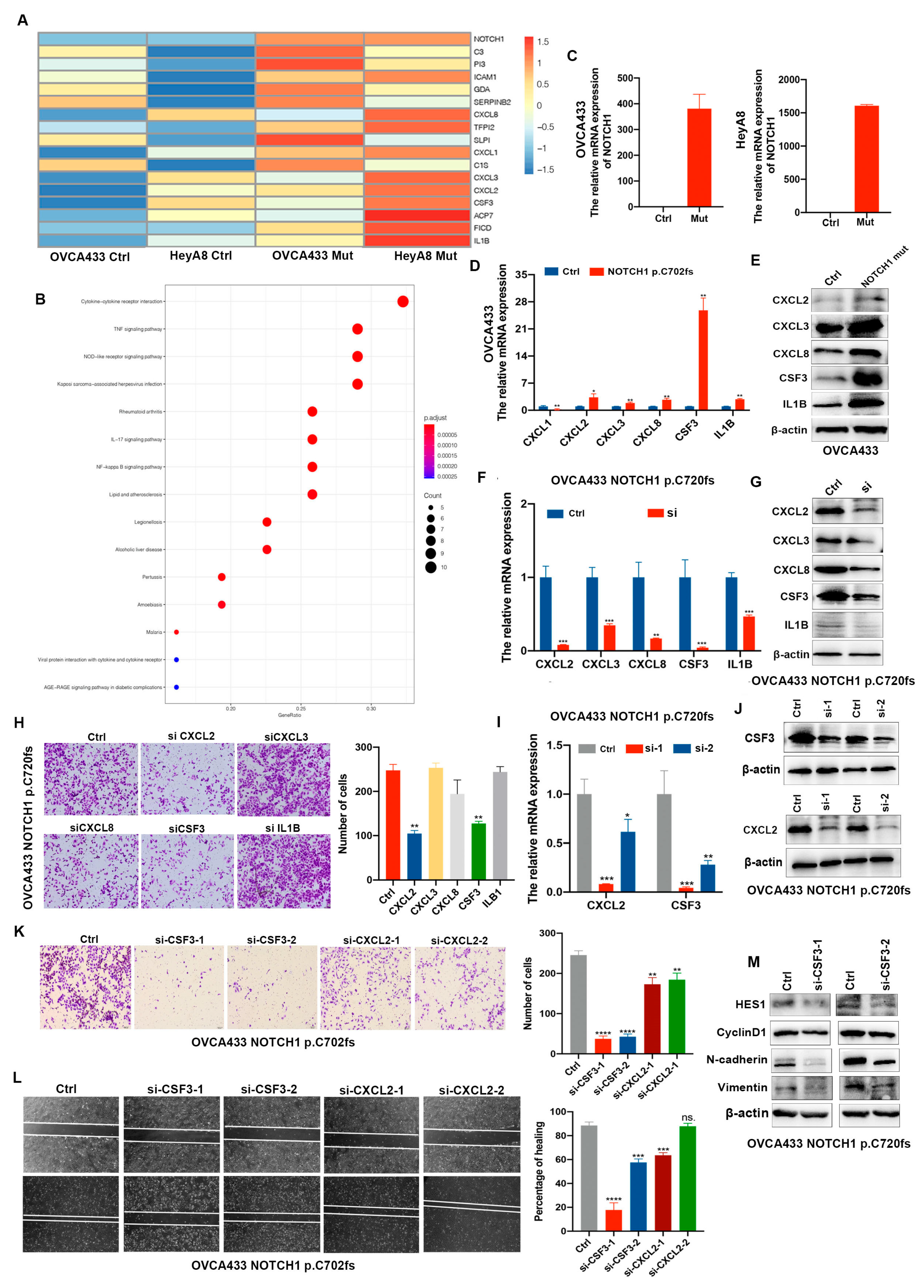
2.3. CSF3 Is Determined as the Downstream Factor by Which NOTCH1-p.C720fs Mutation Affects Epithelial-Mesenchymal Transition
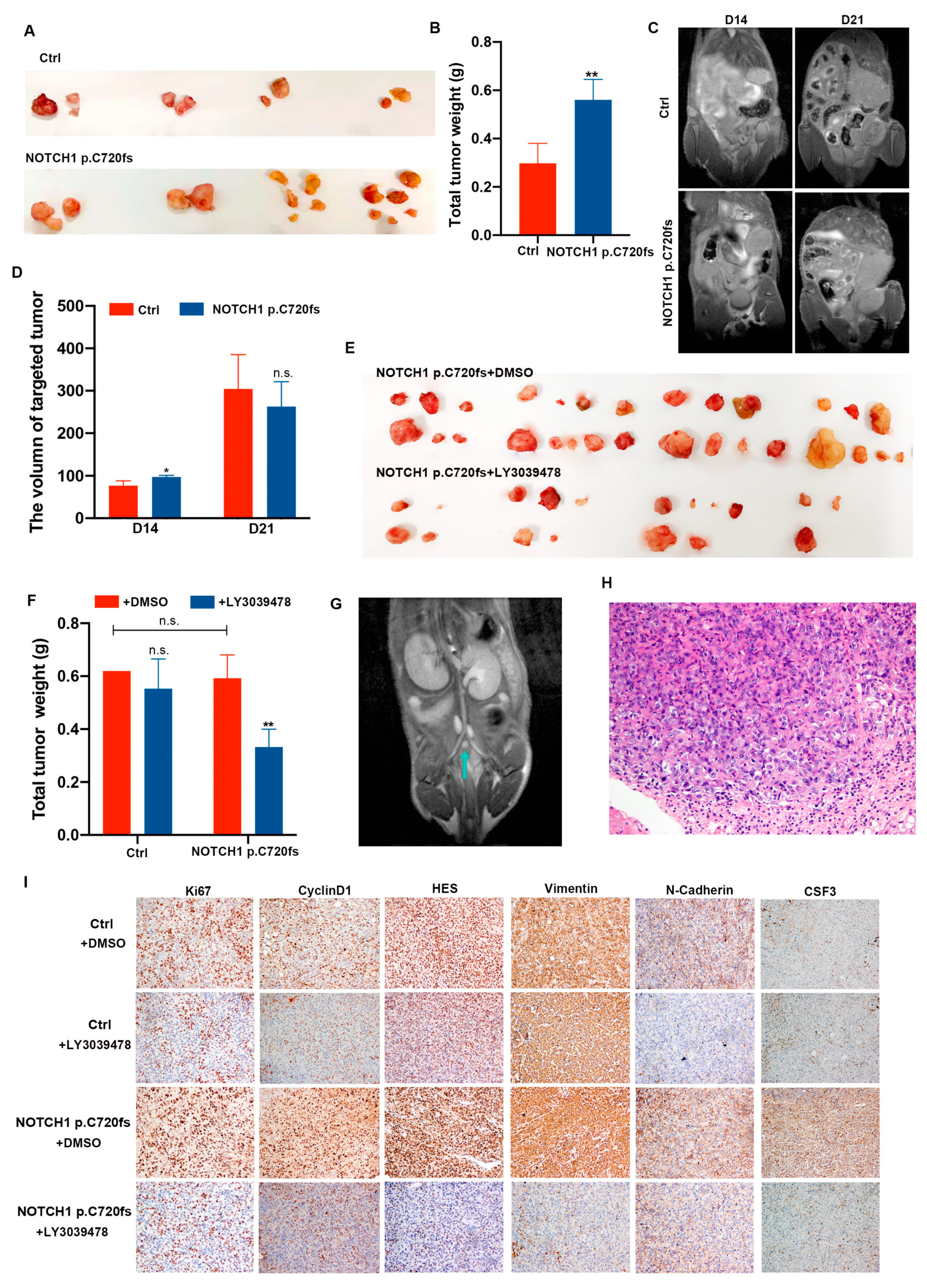
2.4. NOTCH1-p.C720fs Mutation Promotes Implantation and Lymph Node Metastasis of Ovarian Cancer In Vivo, Which Can Be Inhibited by LY3039478
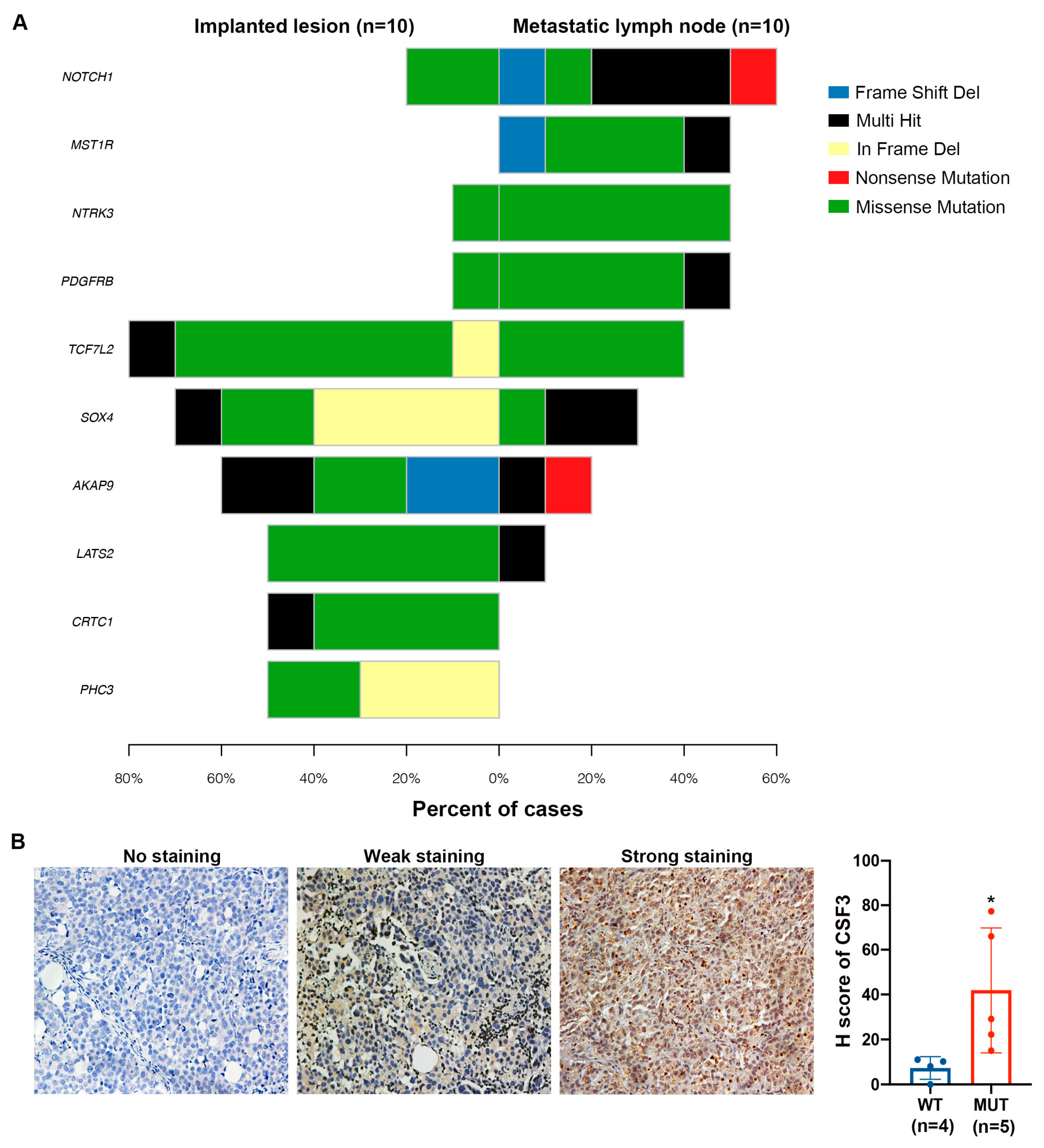
2.5. NOTCH1 Mutations Were more Prominent in Lymph Node Metastases of Ovarian Cancer Than in other Metastatic Sites in the Xenograft Model
3. Discussion
4. Materials and Methods
4.1. Medical History
4.2. Establishment of Cell Lines
4.3. Growth Characteristics
4.4. Chromosome Analysis
4.5. Heterotransplantation of FDOVL Cells
4.6. Histological Analysis and Immunohistochemistry
4.7. Whole-Exome Sequencing (WES) and Sanger Sequencing
4.8. Cell Culture and Viral Transfection
4.9. Western Blot Assay
4.10. Transwell and Wound Healing Assays
4.11. RNA Extraction and Quantitative Real-Time PCR (qRT–PCR)
4.12. RNA Sequencing
4.13. Small Interfering RNA (siRNA) Transfection
4.14. Patients and Specimens
4.15. Establishment and Treatment of Xenografts
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lheureux, S.; Braunstein, M.; Oza, A.M. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Leamon, C.P.; Lovejoy, C.D.; Nguyen, B. Patient selection and targeted treatment in the management of platinum-resistant ovarian cancer. Pharm. Pers. Med. 2013, 6, 113–125. [Google Scholar] [CrossRef]
- Van Driel, W.J.; Koole, S.N.; Sonke, G.S. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 1363–1364. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, N.; Hirai, Y.; Umayahara, K.; Fujiwara, K.; Takizawa, K.; Hasumi, K. Lymph node metastasis in ovarian cancer: Difference between serous and non-serous primary tumors. Gynecol Oncol. 2005, 99, 427–431. [Google Scholar] [CrossRef]
- Di Re, F.; Baiocchi, G.; Fontanelli, R.; Grosso, G.; Cobellis, L.; Raspagliesi, F.; di Re, E. Systematic pelvic and paraaortic lymphadenectomy for advanced ovarian cancer: Prognostic significance of node metastases. Gynecol. Oncol. 1996, 62, 360–365. [Google Scholar] [CrossRef]
- Levy, T.; Migdan, Z.; Aleohin, N.; Shem, B.; Peled, O.; Tal, O.; Elyashiv, O. Retroperitoneal lymph node recurrence of epithelial ovarian cancer: Prognostic factors and treatment outcome. Gynecol. Oncol. 2020, 157, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Naumann, R.W.; Coleman, R.L. Management strategies for recurrent platinum-resistant ovarian cancer. Drugs 2011, 71, 1397–1412. [Google Scholar] [CrossRef]
- Bregenzer, M.E.; Horst, E.N.; Mehta, P.; Novak, C.M.; Repetto, T.; Mehta, G. The Role of Cancer Stem Cells and Mechanical Forces in Ovarian Cancer Metastasis. Cancers 2019, 11, 1008. [Google Scholar] [CrossRef]
- Puente, X.S.; Beà, S.; Valdés-Mas, R.; Villamor, N.; Gutiérrez-Abril, J.; Martín-Subero, J.I.; Munar, M.; Rubio-Pérez, C.; Jares, P.; Aymerich, M.; et al. Non-coding recurrent mutations in chronic lymphocytic leukaemia. Nature 2015, 526, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Nadeu, F.; Delgado, J.; Royo, C.; Baumann, T.; Stankovic, T.; Pinyol, M.; Jares, P.; Navarro, A.; Martín-García, D.; Beà, S.; et al. Clinical impact of clonal and subclonal TP53, SF3B1, BIRC3, NOTCH1, and ATM mutations in chronic lymphocytic leukemia. Blood 2016, 127, 2122–2130. [Google Scholar] [CrossRef]
- Gao, Y.-B.; Chen, Z.-L.; Li, J.-G.; Hu, X.-D.; Shi, X.-J.; Sun, Z.-M.; Zhang, F.; Zhao, Z.-R.; Li, Z.-T.; Liu, Z.-Y.; et al. Genetic landscape of esophageal squamous cell carcinoma. Nat. Genet. 2014, 46, 1097–1102. [Google Scholar] [CrossRef]
- Lin, D.-C.; Hao, J.-J.; Nagata, Y.; Xu, L.; Shang, L.; Meng, X.; Sato, Y.; Okuno, Y.; Varela, A.M. Genomic and molecular characterization of esophageal squamous cell carcinoma. Nat. Genet. 2014, 46, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, Z.; Xiong, X.; Zhong, Y.; Zhang, W.; Dong, Y.; Li, J.; Zhu, Z.; Wu, H.; Gu, W.; et al. Membrane-tethered Notch1 exhibits oncogenic property via activation of EGFR-PI3K-AKT pathway in oral squamous cell carcinoma. J. Cell. Physiol. 2019, 234, 5940–5952. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Huang, C.; Li, Q.; Kazi, S.; Byers, L.; Wang, J.; Johnson, F.; Frederick, M. NOTCH1 Signaling in Head and Neck Squamous Cell Carcinoma. Cells 2020, 9, 2677. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M. Precision medicine for human cancers with Notch signaling dysregulation (Review). Int. J. Mol. Med. 2020, 45, 279–297. [Google Scholar] [CrossRef]
- De Francesco, E.M.; Maggiolini, M.; Musti, A.M. Crosstalk between Notch, HIF-1α and GPER in Breast Cancer EMT. Int. J. Mol. Sci. 2018, 19, 2011. [Google Scholar] [CrossRef]
- Jackstadt, R.; van Hooff, S.R.; Leach, J.D.; Cortes-Lavaud, X.; Lohuis, J.O.; Ridgway, R.A.; Wouters, V.M.; Roper, J.; Kendall, T.J.; Roxburgh, C.S.; et al. Epithelial NOTCH Signaling Rewires the Tumor Microenvironment of Colorectal Cancer to Drive Poor-Prognosis Subtypes and Metastasis. Cancer Cell 2019, 36, 319–336.e317. [Google Scholar] [CrossRef]
- Massard, C.; Azaro, A.; Soria, J.-C.; Lassen, U.; Le Tourneau, C.; Sarker, D.; Smith, C.; Ohnmacht, U.; Oakley, G.; Patel, B.; et al. First-in-human study of LY3039478, an oral Notch signaling inhibitor in advanced or metastatic cancer. Ann. Oncol. 2018, 29, 1911–1917. [Google Scholar] [CrossRef]
- Sambandam, V.; Frederick, M.J.; Shen, L.; Tong, P.; Rao, X.; Peng, S.; Singh, R.; Mazumdar, T.; Huang, C.; Li, Q.; et al. PDK1 Mediates NOTCH1-Mutated Head and Neck Squamous Carcinoma Vulnerability to Therapeutic PI3K/mTOR Inhibition. Clin. Cancer Res. 2019, 25, 3329–3340. [Google Scholar] [CrossRef]
- Murthy, A.; Shao, Y.W.; Narala, S.R.; Molyneux, S.D.; Zúñiga-Pflücker, J.C.; Khokha, R. Notch activation by the metalloproteinase ADAM17 regulates myeloproliferation and atopic barrier immunity by suppressing epithelial cytokine synthesis. Immunity 2012, 36, 105–119. [Google Scholar] [CrossRef]
- Morris, K.T.; Khan, H.; Ahmad, A.; Weston, L.L.; Nofchissey, R.A.; Pinchuk, I.V.; Beswick, E.J. G-CSF and G-CSFR are highly expressed in human gastric and colon cancers and promote carcinoma cell proliferation and migration. Br. J. Cancer 2014, 110, 1211–1220. [Google Scholar] [CrossRef]
- Toh, H.C.; Sun, L.; Soe, Y.; Wu, Y.; Phoon, Y.P.; Chia, W.K.; Wu, J.; Wong, K.Y.; Tan, P. G-CSF induces a potentially tolerant gene and immunophenotype profile in T cells in vivo. Clin. Immunol. 2009, 132, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Morris, K.T.; Castillo, E.F.; Ray, A.L.; Weston, L.L.; Nofchissey, R.A.; Hanson, J.A.; Samedi, V.G.; Pinchuk, I.V.; Hudson, L.G.; Beswick, E.J. Anti-G-CSF treatment induces protective tumor immunity in mouse colon cancer by promoting protective NK cell, macrophage and T cell responses. Oncotarget 2015, 6, 22338–22347. [Google Scholar] [CrossRef]
- Huang, X.; Hu, P.; Zhang, J. Genomic analysis of the prognostic value of colony-stimulating factors (CSFs) and colony-stimulating factor receptors (CSFRs) across 24 solid cancer types. Ann. Transl. Med. 2020, 8, 994. [Google Scholar] [CrossRef] [PubMed]
- Saunders, A.S.; Bender, D.E.; Ray, A.L.; Wu, X.; Morris, K.T. Colony-stimulating factor 3 signaling in colon and rectal cancers: Immune response and CMS classification in TCGA data. PLoS ONE 2021, 16, e0247233. [Google Scholar] [CrossRef]
- Pickup, M.W.; Owens, P.; Gorska, A.E.; Chytil, A.; Ye, F.; Shi, C.; Weaver, V.M.; Kalluri, R.; Moses, H.L.; Novitskiy, S.V. Development of Aggressive Pancreatic Ductal Adenocarcinomas Depends on Granulocyte Colony Stimulating Factor Secretion in Carcinoma Cells. Cancer Immunol. Res. 2017, 5, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Roberti, M.P.; Arriaga, J.M.; Bianchini, M.; Quintá, H.R.; Bravo, A.I.; Levy, E.M.; Mordoh, J.; Barrio, M.M. Protein expression changes during human triple negative breast cancer cell line progression to lymph node metastasis in a xenografted model in nude mice. Cancer. Biol. Ther. 2012, 13, 1123–1140. [Google Scholar] [CrossRef] [PubMed]
- Yates, L.R.; Knappskog, S.; Wedge, D.; Farmery, J.H.; Gonzalez, S.; Martincorena, I.; Alexandrov, L.B.; Van Loo, P.; Haugland, H.K.; Lilleng, P.K.; et al. Genomic Evolution of Breast Cancer Metastasis and Relapse. Cancer Cell 2017, 32, 169–184.e167. [Google Scholar] [CrossRef] [PubMed]
- Connor, A.A.; Denroche, R.E.; Jang, G.H.; Lemire, M.; Zhang, A.; Chan-Seng-Yue, M.; Wilson, G.; Grant, R.C.; Merico, D.; Lungu, I.; et al. Integration of Genomic and Transcriptional Features in Pancreatic Cancer Reveals Increased Cell Cycle Progression in Metastases. Cancer Cell 2019, 35, 267–282.e267. [Google Scholar] [CrossRef]
- Norquist, B.; Wurz, K.A.; Pennil, C.C.; Garcia, R.; Gross, J.; Sakai, W.; Karlan, B.Y.; Taniguchi, T.; Swisher, E.M. Secondary somatic mutations restoring BRCA1/2 predict chemotherapy resistance in hereditary ovarian carcinomas. J. Clin. Oncol. 2011, 29, 3008–3015. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Ye, S.; Xiang, L.; Yang, W.; He, T.; Pei, X.; Guo, L.; Yang, H. Establishment and molecular characterization of a human ovarian clear cell carcinoma cell line (FDOV1). J. Ovarian Res. 2018, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; He, T.; Liu, S.; Zheng, Y.; Xiang, L.; Pei, X.; Wang, Z.; Yang, H. The PIK3CA E542K and E545K mutations promote glycolysis and proliferation via induction of the β-catenin/SIRT3 signaling pathway in cervical cancer. J. Hematol. Oncol. 2018, 11, 139. [Google Scholar] [CrossRef] [PubMed]
| Sample Number | Sample Number | Source of Tumor | NOTCH1 Mutation | Mutation Type | NOTCH1 Mutation | Mutation Type |
|---|---|---|---|---|---|---|
| 1 | JS-3 | Primary | - | NM_017617:exon25:c.C4056A:p.C1352X | SNP (stopgain) | |
| 2 | JS-10 | Primary | - | (1) NM_017617:exon34:c.A6325G:p.I2109V (2) NM_017617:exon12:c.G1993A:p.A665T | SNP (nonsynonymous) | |
| 3 | JS-12 | Primary | NM_017617:exon11:c.C1860G:p.D620E | SNP (nonsynonymous) | (1) NM_017617:exon34:c.A6317C:p.H2106P (2) NM_017617:exon26:c.T4706C:p.L1569P (3) NM_017617:exon11:c.C1860G:p.D620E | SNP (nonsynonymous) |
| 4 | JS-13 | Primary | - | NM_017617:exon34:c.6392delG:p.G2131fs | Indel (frameshift deletion) | |
| 5 | JS-14 | Recurrent | - | (1) NM_017617:exon26:c.T4706C:p.L1569P (2) NM_017617:exon30:c.C5521G:p.R1841G | SNP (nonsynonymous) | |
| 6 | JS-18 | Primary | - | - | ||
| 7 | JS-22 | Primary | NM_017617:exon34:c.A6317C:p.H2106P | SNP (nonsynonymous) | - | |
| 8 | JS-25 | Primary | - | - | ||
| 9 | JS-26 | Primary | - | - | ||
| 10 | JS-33 | Primary | - | NM_017617:exon4:c.C498G:p.C166W | SNP (nonsynonymous) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Ouyang, X.; Jiang, C.; Yin, L.; Yao, Q.; Pei, X.; Ji, Z.; Li, M.; Song, S.; Yang, W.; et al. A NOTCH1 Mutation Found in a Newly Established Ovarian Cancer Cell Line (FDOVL) Promotes Lymph Node Metastasis in Ovarian Cancer. Int. J. Mol. Sci. 2023, 24, 5091. https://doi.org/10.3390/ijms24065091
Jiang W, Ouyang X, Jiang C, Yin L, Yao Q, Pei X, Ji Z, Li M, Song S, Yang W, et al. A NOTCH1 Mutation Found in a Newly Established Ovarian Cancer Cell Line (FDOVL) Promotes Lymph Node Metastasis in Ovarian Cancer. International Journal of Molecular Sciences. 2023; 24(6):5091. https://doi.org/10.3390/ijms24065091
Chicago/Turabian StyleJiang, Wei, Xueyan Ouyang, Chunjuan Jiang, Lina Yin, Qianlan Yao, Xuan Pei, Zhaodong Ji, Ming Li, Shaoli Song, Wentao Yang, and et al. 2023. "A NOTCH1 Mutation Found in a Newly Established Ovarian Cancer Cell Line (FDOVL) Promotes Lymph Node Metastasis in Ovarian Cancer" International Journal of Molecular Sciences 24, no. 6: 5091. https://doi.org/10.3390/ijms24065091
APA StyleJiang, W., Ouyang, X., Jiang, C., Yin, L., Yao, Q., Pei, X., Ji, Z., Li, M., Song, S., Yang, W., Huang, S., Yang, H., & Shan, B. (2023). A NOTCH1 Mutation Found in a Newly Established Ovarian Cancer Cell Line (FDOVL) Promotes Lymph Node Metastasis in Ovarian Cancer. International Journal of Molecular Sciences, 24(6), 5091. https://doi.org/10.3390/ijms24065091





