2. Results
We have previously published complete descriptions of the logical design and methodological execution for the AsedaSciences
® SYSTEMETRIC
® Cell Health Screen [
24,
25]. Briefly, it is a multiparametric live-cell phenotypic screen using automated flow cytometry (FC), in which a twelve-parameter acute cellular stress phenotype is classified by a supervised machine learning classifier. The classifier uses a multidimensional logistic regression model in which each dimension is an FC parameter. The training was performed with a 300-compound training set [
24,
25], which consisted of on-market and withdrawn drugs, research compounds, and several agricultural/industrial compounds [
24,
25]. The training set was first divided into binary outcome classes (high toxicity risk and low toxicity risk) using literature, clinical trial results, and market histories (where applicable). Next, all 300 compounds were processed through the FC screen and the empirical data populated distributions within each of the two known outcome classes. These distributions optimized the logistic regression model, defining the dependence of the outcome on each of the 12 FC parameters. The trained classifier subsequently classifies the acute cellular stress phenotype produced when an unknown test compound is applied to the cells. The final classification value, or Cell Health Index (CHI), is a probability value (0–1) representing the maximum likelihood that a test compound’s phenotype belongs in the high toxicity risk outcome class (
Tables S1 and S2). In addition, the classifier can be used to produce the same type of probability score by using only one or a subset of the twelve FC parameters, and this is how it generates the biological fingerprint, comprised of eight phenotypic endpoints. Hence, for example, the cell morphology score is produced by allowing the classifier to only see four FC parameters related to forward scatter and side scatter from one laser. The assay is run with HL-60 cells, not because of any specific appropriateness as a disease model but for two pragmatic reasons. Suspension cell culture enables automated flow cytometry, and during the screen prototyping phase, HL-60 cells empirically produced an optimal dynamic range for the required fluorescent reporter dyes. This resulted in a screen design that was most generalizable across compounds from diverse therapeutic and chemical classes while having relatively low labor intensity and cost.
To have a better understanding of the kinase inhibitor toxophore landscape, we first screened thirty-one literature-reported, late-stage, and clinically approved kinase inhibitors in the Cell Health Screen (
Table 1). The CHI results showed that twenty one inhibitors had higher risk factors, with four in the mid-range and six showing risk at or lower than 0.41. These results are, in part, reflective of on-target effects from these anti-cancer agents that generally target pathways promoting cell growth [
26].
The six kinase inhibitors with lower toxicity risk are potentially the most interesting, as although many kinase inhibitors were developed towards specific targets, in practice, most of these drugs are multi-kinase inhibitors. That leveraging of the ATP pocket across multiple kinases increases their clinical efficacy, but it can also lead to increased toxicity. To target diseases outside of oncology, kinase inhibitors with lower toxicity and likely narrower target specificity are required [
27]. This will enable the treatment of chronic diseases via kinase targets, including inflammation and neurodegeneration, potentially opening the route of personalized medicine [
27,
28,
29].
These six kinase inhibitors with low toxicities include Tofacitinib and Ruxolitinib, which are both Janus kinase (JAK) inhibitors, based on the 7
H-pyrrolo[2,3-
d]pyrimidin-4-amine core scaffold [
30,
31]. Tofacitinib has been FDA-approved for a number of non-oncology indications, including the treatment of psoriatic arthritis, juvenile idiopathic arthritis, and ulcerative colitis [
31,
32,
33]. Ruxolitinib has also been used to treat myelofibrosis, polycythemia vera, and steroid-refractory acute graft-versus-host disease [
31,
34]. Ruxolitinib has more recently been approved for several topical indications, including mild to moderate atopic dermatitis [
35] and the treatment of vitiligo [
36]. Tofacitinib and ruxolitinib are both primarily targeting non-oncology indications, which may, in part, explain these favorable CHI values, as their intended uses would not tolerate high human safety risk. Trametinib and dabrafenib were also in this group of six low-scoring kinase inhibitors. Trametinib is a highly selective allosteric mitogen-activated protein kinase kinase (MEK) inhibitor [
37], that was originally approved for the treatment of malignant melanoma driven by the BRAF V600E mutation in combination with BRAF inhibitors, such as dabrafenib [
38]. More recently, the combination of dabrafenib with trametinib has been approved for BRAF V600-positive advanced or metastatic non-small-cell lung cancer (NSCLC) [
39]. These targeted therapies can be considered a first step towards personalized medicine, where the presence of the BRAF V600 mutation dictates the success of the treatment [
40,
41]. The narrower kinome spectrum of these compounds again may help to explain the favorable CHI values for both trametinib and dabrafenib.
The final two compounds of the six low-scoring kinase inhibitors were erlotinib, a first-generation epidermal growth factor receptor (EGFR) inhibitor [
42], and sapitinib, a second-generation reversible EGFR inhibitor [
43,
44]. The main clinical indication for erlotinib is NSCLC, but there have also been subsequent approvals for the treatment of locally advanced, unresectable, or metastatic pancreatic cancer in combination with gemcitabine [
45,
46]. Whereas sapitinib has an enhanced pharmacologic profile due in part to equipotent inhibition of EGFR, erbB2, and erbB3, showing potent antitumor activity in preclinical cancer models [
43,
47]. Erlotinib and sapitinib are both oncology drugs based on the 4-anilinoquinazoline kinase inhibitor scaffold and are multi-kinase inhibitors, albeit not as promiscuous across the kinome as some inhibitors [
6,
7,
12,
13,
14,
15,
16]. The fact that both of these compounds have a favorable CHI prompted us to further investigate the 4-anilinoquin(az)oline scaffold.
To explore the 4-anilinoquin(az)oline scaffold, we profiled seven focused arrays of compounds, probing the toxicity profile structure-activity relationships of the quinoline/quinazoline scaffold. We synthesized and screened a series of compounds (
1–
112) to follow up on the results of erlotinib and sapatinib, exploring the 4-anilinoquin(az)oline through a series of nucleophilic aromatic displacements of 4-chloroquin(az)olines with a series of anilines in good yields (
Scheme 1) consistent with previous reports [
48,
49,
50,
51,
52,
53].
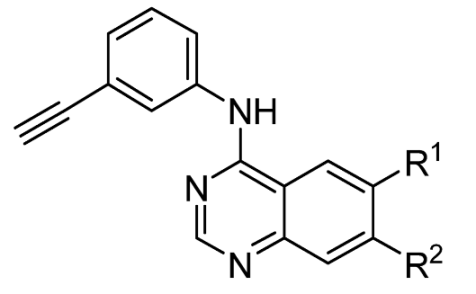
To understand the structural drivers of toxicity on the 4-anilinoquin(az)oline scaffold, we first screened a series of simplified erlotinib-related 4-anilinoquinazolines containing the 3-ethynylaniline (
1–
20) (
Table 2). We first screened
N-(3-ethynylphenyl)-6,7-dimethoxyquinazolin-4-amine (
1) and found that, despite the curtailment of the pendent arms with the removal of the ethylene glycol linker to afford the methoxy groups, the toxicity profile was broadly similar with a CHI of 0.41 (vs. 0.40 for erlotinib). Interestingly, the removal of the 7-position methoxy
2 or the 6-position methoxy
3 resulted in a decrease in the CHI, with
3 showing an almost 40% reduction in CHI. In the fingerprint of each compound, there appears to be a switch in driving CMI toxicity and limited reactive oxygen species (ROS) involvement in
2, while in
3, this trend is reversed. The catechol with the fused methyl spacer
4 cleaned the profile further, with a more than two-fold reduction of CHI compared with erlotinib. The extension of the fused spacer to ethyl
5 resulted in an almost 40% increase in the CHI compared with erlotinib, while the unsubstituted quinazoline
6 showed an increase of 80% in the CHI. The introduction of 6-position fluorine
7 reduced the CHI substantially compared to both the unsubstituted analog
6 and erlotinib. The 6,7-position difluoro
8 had a slightly lower CHI, at 0.26, compared to 0.31 for 6-fluoro
7. The trend of 6-position halogens chloro
9, bromo
10, iodo
11, and trifluoromethyl
12 analogs all showed a almost 50% reduction in CHI compared with erlotinib. Interestingly, switching the halogen from the 6- to 7-position led to an increase in CHI, with the 7-position fluoro
13 having the same CHI as erlotinib at 0.40. The 7-position chloro
14 and bromo
15 had similar fingerprints and an identical CHI of 0.33, an almost 20% reduction in CHI compared with erlotinib. However, unlike the 6-position analogs, the trend did not continue, with the 7-position iodo
16 and trifluoromethyl
17 having a nearly two-fold increase in CHI compared to erlotinib. Switching to the 7-position cyano
18 reduced CHI but still resulted in a 10% increase over erlotinib. The 6-position cyano analog
19 showed a more favorable CHI with more than 50% reduction relative to the 7-position cyano
18. The 6-position methylsulfone
20 also performed favorably with a low CHI of 0.22.
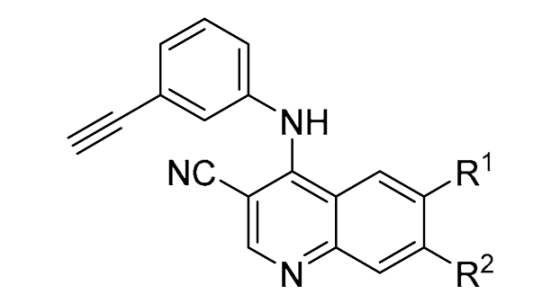
Second, we screened a series of 3-cyanoquinolines containing the 3-ethynylaniline (
21–
32) (
Table 3). The 3-cyanoquinoline still maintains the ability to form a dual hydrogen bond at the hinge region but forces the aniline out of plane to an almost perpendicular angle [
51]. The unsubstituted analog
21 showed a 75% increase in CHI compared to erlotinib, similar to the quinazoline analog
6. However, unlike the 6-position halogen quinazoline analogs
7 and
9–
11, the 3-cyanoquinoline derivatives
22–
25 all showed similar or higher toxicity with relatively high CHI risk indicators. A switch to the 6-position methylsulfone
26 reduced the CHI by more than two-fold compared with erlotinib. A similar level of reduction was also seen in the CHI of the 6-position methoxy
27, potentially related to the electron-donating ability of these two compounds. Screening 4-((3-ethynylphenyl)amino)-6,7-dimethoxyquinoline-3-carbonitrile (
28), we found the same CHI as erlotinib despite several phenotypic endpoint score differences and differing structural features. The 7-position methoxy analog
29 showed a 10% increase in CHI, while the chloro substitution
30 showed a 10% decrease in CHI. The other two 7-position halogens had much higher CHI, with the bromo
31 50% higher and the iodo
32 100% higher compared with erlotinib. The 7-position iodo
32 had a similar profile to the quinazoline counterpart
16.
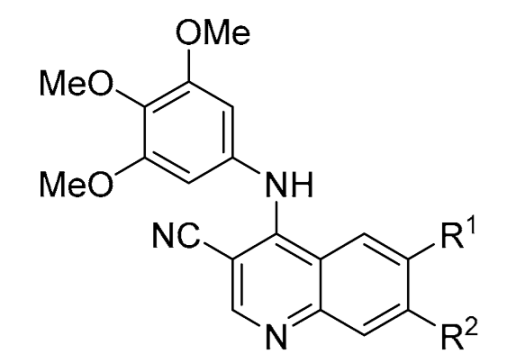
Third, we switched the 3-ethynylaniline with the 3,4,5-trimethoxyaniline and screened a series of 3-cyanoquinolines (
33–
43) (
Table 4), starting with 6,7-dimethoxy-4-((3,4,5-trimethoxyphenyl)amino)quinoline-3-carbonitrile (
33) which had a similar CHI to erlotinib. The removal of either methoxy group
34–
35 resulted in a 25% decrease in CHI with respect to
33 and erlotinib. The unsubstituted analog
36, unlike the 3-ethynylaniline counterparts quinazoline
6 and 3-cyanoquinoline
21, had a lower toxicity risk profile with a nearly two-fold reduction compared with erlotinib’s CHI. The same was the case with the 6-position halogens chloro
37, bromo
38, and iodo
39, showing a 50–75% reduction in CHI compared with erlotinib. The 6-position methyl sulfone
40 showed a 50% spike in the CHI to 0.59, which was an opposite trend to the unsubstituted analogs, with counterparts quinazoline
20 and 3-cyanoquinoline
26 both having shown a lower toxicity risk estimate. Last, the 7-position halogen chloro
41, bromo
42, and iodo
43 all demonstrated a lower CHI than erlotinib. The chloro analog
41 showed a more than 50% reduction in CHI, with the bromo
42 and iodo
43 showing a more modest 10% reduction in CHI. This was, however, a much shallower trend than the matched pair quinazolines and 3-cyanoquinoline 3-ethynylaniline analogs, particularly with the iodo derivatives
16 and
32.
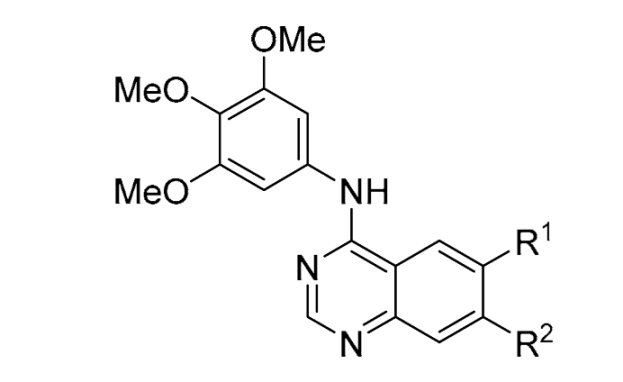
Fourth, we switched from the 3-cyanoquinoline to a series of quinazolines while maintaining the 3,4,5-trimethoxyaniline (
44–
62) (
Table 5). This time, the direct derivative
44 of erlotinib was screened with the 3,4,5-trimethoxyaniline replacement and found to have a 40% lower CHI compared to erlotinib. This effect appears to be mainly driven by a reduction in glutathione depletion (GSH) and a reduction in cell membrane disruption effects (CM). The 7-position mono-methoxy group derivative
45 showed a further reduction with a >50% lower CHI risk estimate than erlotinib, while the 6-position mono-methoxy
46 had only a 20% reduction in CHI. The unsubstituted analog
47 had the lowest CHI of the entire study at 0.13, which contrasted with some of the previous unsubstituted analogs, including
6 and
21, but was more consistent with
36 that had the 3,4,5-trimethoxyaniline present in the compound. Interestingly, the addition of a methyl group in the 6-position
48 caused the CHI to double compared to the unsubstituted derivative
47. Switching the methyl for a fluoro
49 maintained the CHI, as does having a 6,7-position difluoro substitution
50 and 6-position chloro
51. However, increasing the size of the 6-position halogen appeared to be unfavored, with the bromo analog
52 having an almost 50% increase over the CHI of erlotinib and >100% increase in CHI from chloro
51 to bromo
52. The penalty appears to plateau with the 6-position iodo
53 and trifluoromethyl
54 showing only a 10% uptick on the CHI of erlotinib. The 7-position halogens are more favored with low CHI across the fluoro
55, chloro
56, and bromo
57. The 7-position iodo
58 reversed that trend with a 2.5-fold increase compared to bromo
57 and an almost 50% increase compared with erlotinib. The 7-position trifluoromethyl
59 was near parity with erlotinib, with only a 10% increase in CHI. Interestingly, switching to 7-position cyano
60 led to a more favorable CHI with a 60% reduction compared with erlotinib, while the 6-position cyano
61 showed parity with the CHI of erlotinib. Switching to the 6-position methyl sulfone
62 recovered the earlier gain from the 7-position cyano
60 with an identical CHI.
Fifth, building on the encouraging results of the 3,4,5-trimethoxyaniline analogs, we switched to another less common kinase hinge binder, quinoline (
63–
80) (
Table 6). The quinoline has a reduced capacity to form an additional hydrogen bond in the 3-position of the ring system, but the C-H can push the aniline portion of the scaffold out of plane of the quinoline by up to 60 degrees [
51]. Initially, the dimethoxy analog 6,7-dimethoxy-
N-(3,4,5-trimethoxyphenyl)quinolin-4-amine (
63) was screened and, despite a narrow spectrum on the kinome [
48], afforded a CHI of close to with a 130% increase compared to erlotinib. The removal of the 6-position methoxy group to afford
64 reduced the CHI by almost 3-fold from the dimethoxy
63 to a much more favorable 0.32, a 20% reduction compared with erlotinib. Removal of the 7-position methoxy group to produce
65 reduced the CHI with respect to the dimethoxy
63, but the CHI was still 40% greater than erlotinib.
The unsubstituted analog 66 was consistent with 36 and 47 and demonstrated a lower CHI of 0.32. The 6-position fluoro 67 and 6,7-position difluoro 68 analogs both have a similar CHI to the unsubstituted derivative 66 with roughly a 30% reduction compared to erlotinib. Increased size of the halogen resulted in an increased toxicity risk; the chloro derivative 69 had parity with erlotinib, while the bromo 70 showed a 15% increase in CHI and the iodo 71 showed nearly a 70% increase. The 6-position trifluoromethyl 72 returned the CHI to parity with erlotinib, while the introduction of a cyano 73 at the 6-position reduced the CHI by a further 40% to 0.22. While the direct methylsulfone analog 74 had a slightly shallower 30% reduction compared to trifluoromethyl 72, this was still a 40% reduction over erlotinib. Switching to the 7-position with a fluoro substitution 75 was favorable, with a 50% reduction in CHI over erlotinib; conversely, the chloro analog 76 showed an almost 60% increase in CHI. The 7-position bromo 77 showed a >100% improvement over chloro 76 and 30% over erlotinib. The respective iodo 78 was closer to parity with erlotinib with only a 10% reduction; this reduction was extended with the direct trifluoromethyl replacement to afford 79 with a 25% reduction. The 7-position cyano analog 80 showed an additional improvement with a 45% reduction in CHI compared with erlotinib, with the majority of the CHI appearing to be derived from nuclear membrane integrity 1 (NMI1).
Sixth, after we observed different profiles between the 3-ethynylaniline and 3,4,5-trimethoxyaniline, we selected the 6-(trifluoromethyl)quinoline, whose CHI was not only similar to erlotinib but has been shown to maintain cellular penetrance using a nanoBRET in-cell target engagement assay [
48,
49,
50,
51,
52,
53]. We fixed the quinoline and assessed how a series of point changes on the pendent aniline altered the toxicity profile of the scaffold (
81–
100) (
Table 7). A direct replacement of the methoxy groups in
72 with fluorine to afford a 3,4,5-trifluoroaniline
81, resulting in a compound that had an almost 40% lower CHI than erlotinib. The 4-position mono-fluoro
82 was nearly 30% lower, while the 3-position fluoro
83 jumped to 50% lower with a CHI of 0.21 compared to the CHI of 0.40 for erlotinib. The 2-position mono-fluoro
84 changed the trend and showed a 4-fold increase in CHI compared with the 3-position analog
83 and an almost 100% increase compared with the CHI of erlotinib. The 4-chloro-3-fluoroaniline derivative
85 had a similar CHI to the 2-position mono-fluoro
84 and with gefitinib (CHI = 0.80). Moving the chlorine around the ring to afford the 3-chloro-5-fluoroaniline derivative
86 reduced the CHI to 0.51, still about 25% higher than erlotinib.
The
N-(3,4-dichlorophenyl)-6-(trifluoromethyl)quinolin-4-amine (
87) analog showed a significant increase in CHI with over an 80% increase compared with the erlotinib. The 4-position mono-chloro
88 also had a substantial increase of more than 110% relative to the CHI of erlotinib, while the 3-position chloro
89 showed an almost 3-fold drop in CHI compared to the 4-position analog
88. The 3-position analog had a decrease of >30% in CHI compared with erlotinib. Moving the chlorine around the ring to the 2-position
90 afforded a compound with a similar profile to the 4-position derivative
88, where the CHI was 100% increased relative to the CHI for erlotinib. The 4-, 3- and 2-position bromo substitutions,
91–
93, respectively, showed consistent results with the choro analogs
88–
90. However, the larger 3-position iodo
94 broke the trend with over a 50% increase in CHI compared with erlotinib. Interestingly, the introduction of a cyano group at the 4-position
95 was well tolerated with a near 30% reduction in CHI compared with erlotinib, while the 3-position derivative
96 demonstrated a >40% decrease, with the 2-position analog
97 showing just over 20%. The cyano groups followed the same trend as the halogens, albeit with a less pronounced gradient. The 3-position trifluoromethyl
98 was consistent with the iodo
94, likely related to size and/or electronegativity increase [
54,
55] and showed a further increased toxicity risk with a CHI of 0.81, >100% of the corresponding CHI of erlotinib. The 3-ethynylaniline derivative
99 was closer to the 3-cyanoquinoline series than the quinazoline, with a 75% increase in CHI in the presence of a 6-position halogen situated on the quinoline. This difference could be related to both electronics and the overall conformation of the scaffold [
51,
54]. The final analog in this series was the 4-((methylsulfonyl)methyl)aniline derivative
100, which showed a substantially lower CHI with a drop of >40% compared with erlotinib.
Finally, we investigated the direct contribution of the methoxy groups on the aniline ring system of
72, with a series of matched pair analogs (
101–
112) (
Table 8). The removal of the central 4-position methoxy to afford
101 resulted in a compound that had a 60% increase in CHI compared with parent
72. Removing one of the flanking methoxy groups (3-position) to afford
102 had a less pronounced effect on the CHI with a marginal 10% increase compared with parent
72. Having the two methoxy groups in the 2,4-position,
103 was even more favorable, with a net reduction in toxicity risk of 30% compared with parent
72. Intriguingly moving one of the methoxy groups to establish a 2,5-position
104 orientation actually caused a 3-fold increase in toxicity compared with the 2,4-position analog
104 and >100% compared with the CHI of parent
72. The 4-position mono-methoxy
105 showed a favorable toxicity profile with a 20% reduction of the CHI compared with parent
72. The 3-position analog had parity with parent
72, while the 2-position showed a large increase of 100% in CHI compared with parent
72, consistent with the other derivative containing a 2-position methoxy substitution
104. Fusing the 3,4-dimethoxy analog with a methyl spacer to afford
106 was disfavored with an almost >70% premium compared with both parent
72 and
102. The use of an ethyl bridging group provided for a more favorable CHI, where the increase was reduced to only 20%.
The direct switch to a quinazoline 110 from quinoline 72 led to a small 10% increase in CHI, but the removal of the central 4-position methoxy was more favorable on the quinazoline 111 than quinoline 101 and showed an almost 30% reduction compared with parent 72 and almost 90% compared with 101. The final analog in this series 112, the 3-position methoxy, showed a 50% drop in CHI compared to quinoline 106 and parent 72.