Ceria Nanoparticles Alleviated Osteoarthritis through Attenuating Senescence and Senescence-Associated Secretory Phenotype in Synoviocytes
Abstract
1. Introduction
2. Results
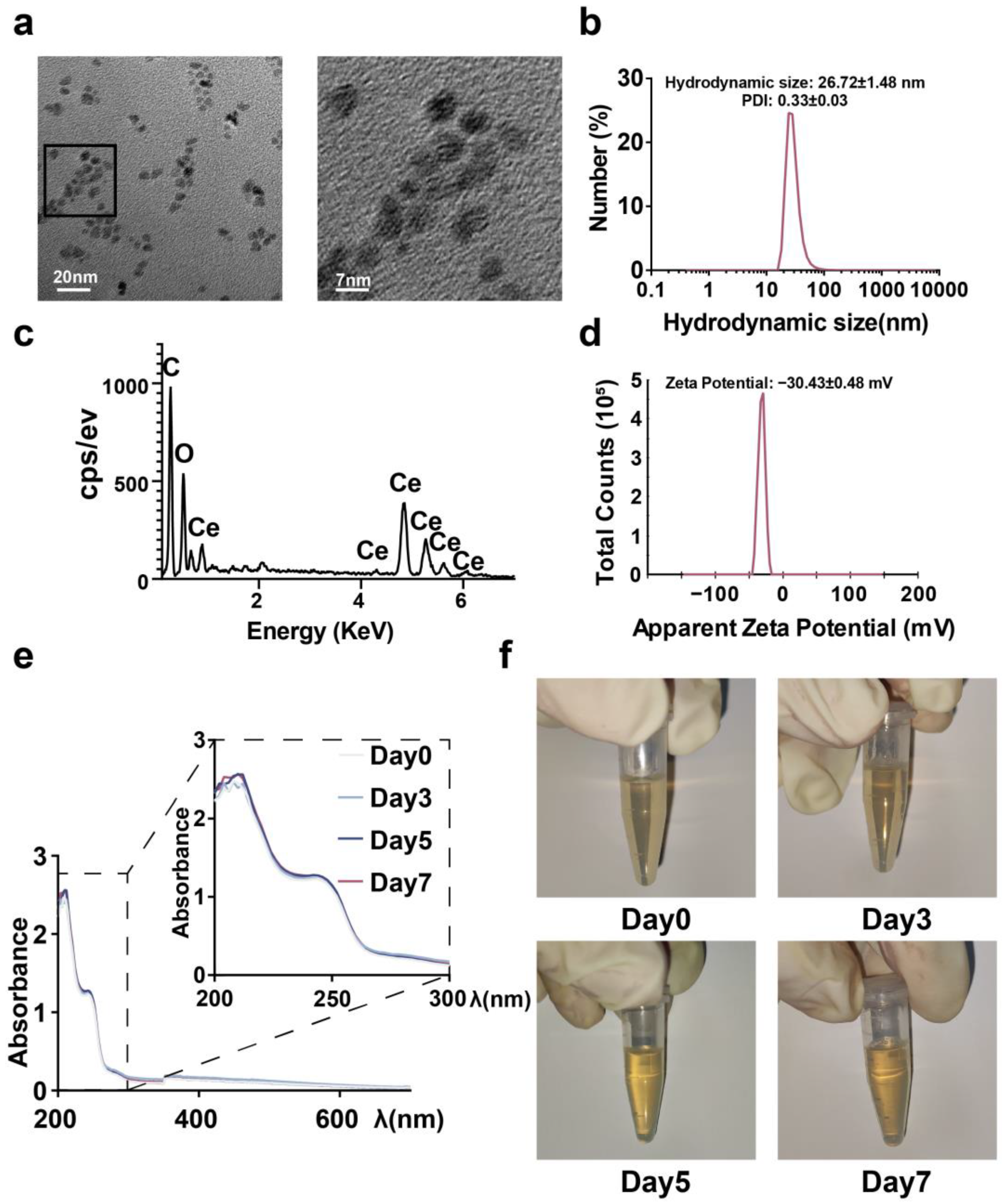
2.1. Synthesis and Characterization of CeNP
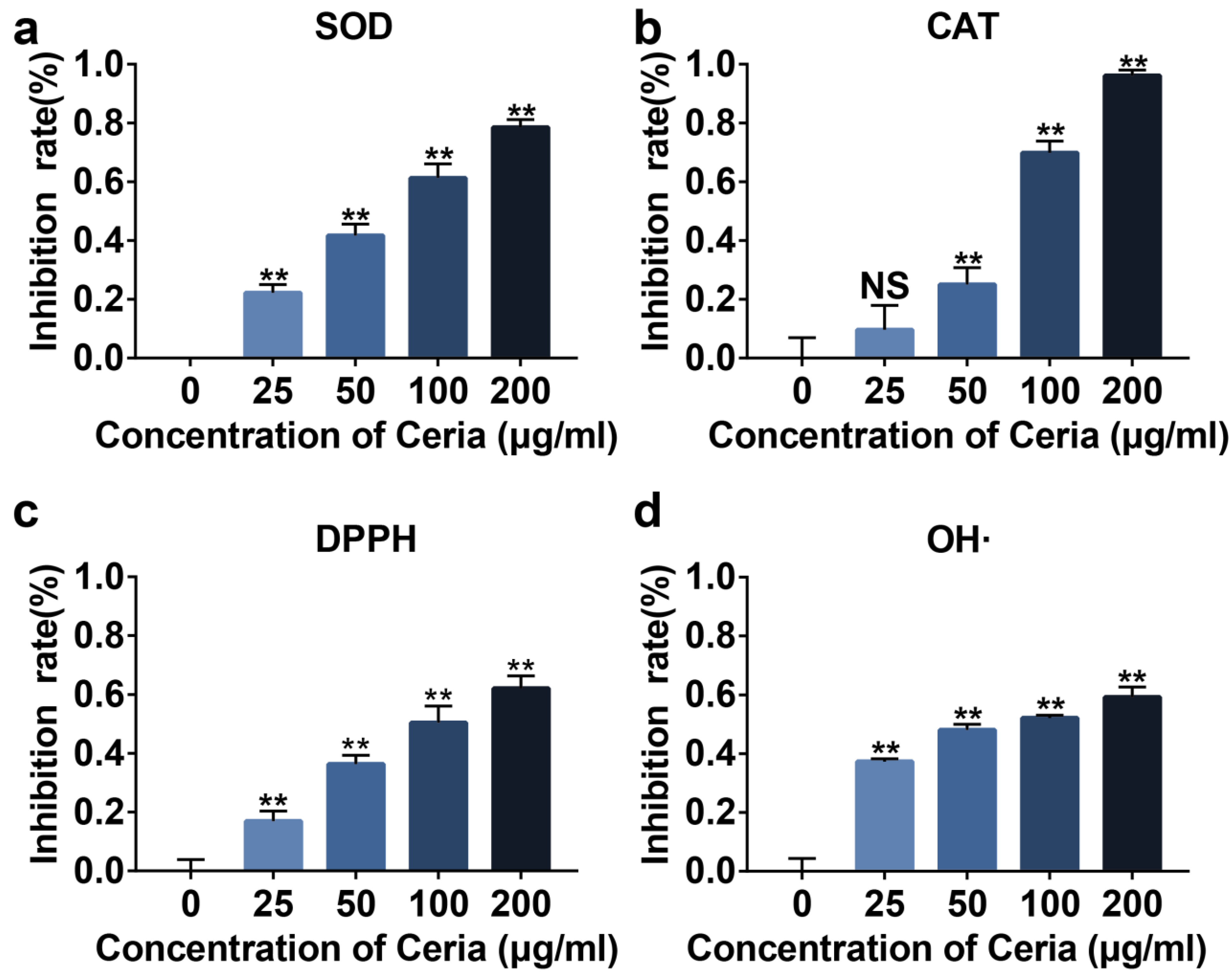
2.2. Extracellular Antioxidant Capacity of CeNP
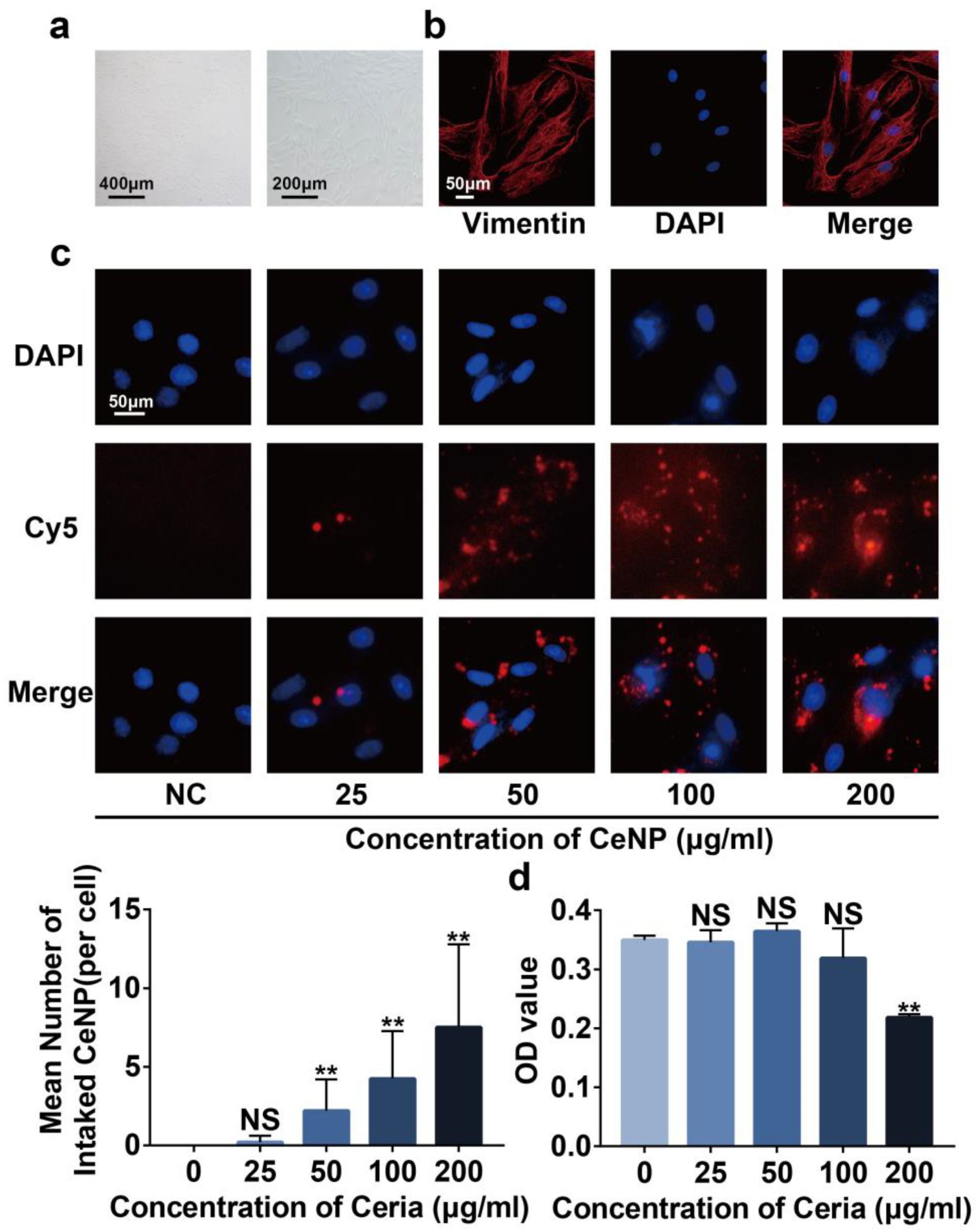
2.3. Cytotoxicity and Cellular Uptake of CeNP
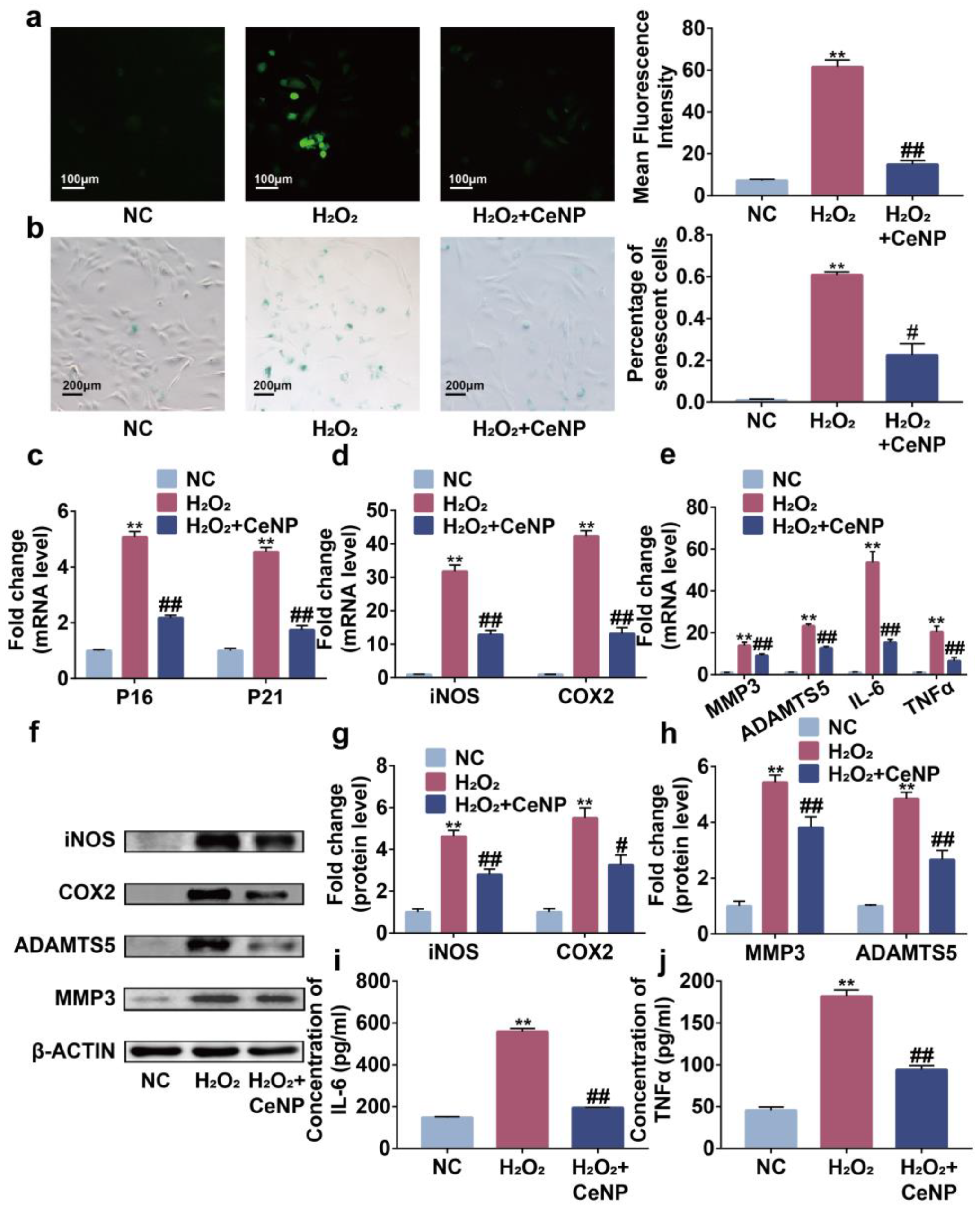
2.4. CeNP Attenuated H2O2-Elicited Senescence and Inhibited SASP in Synoviocytes
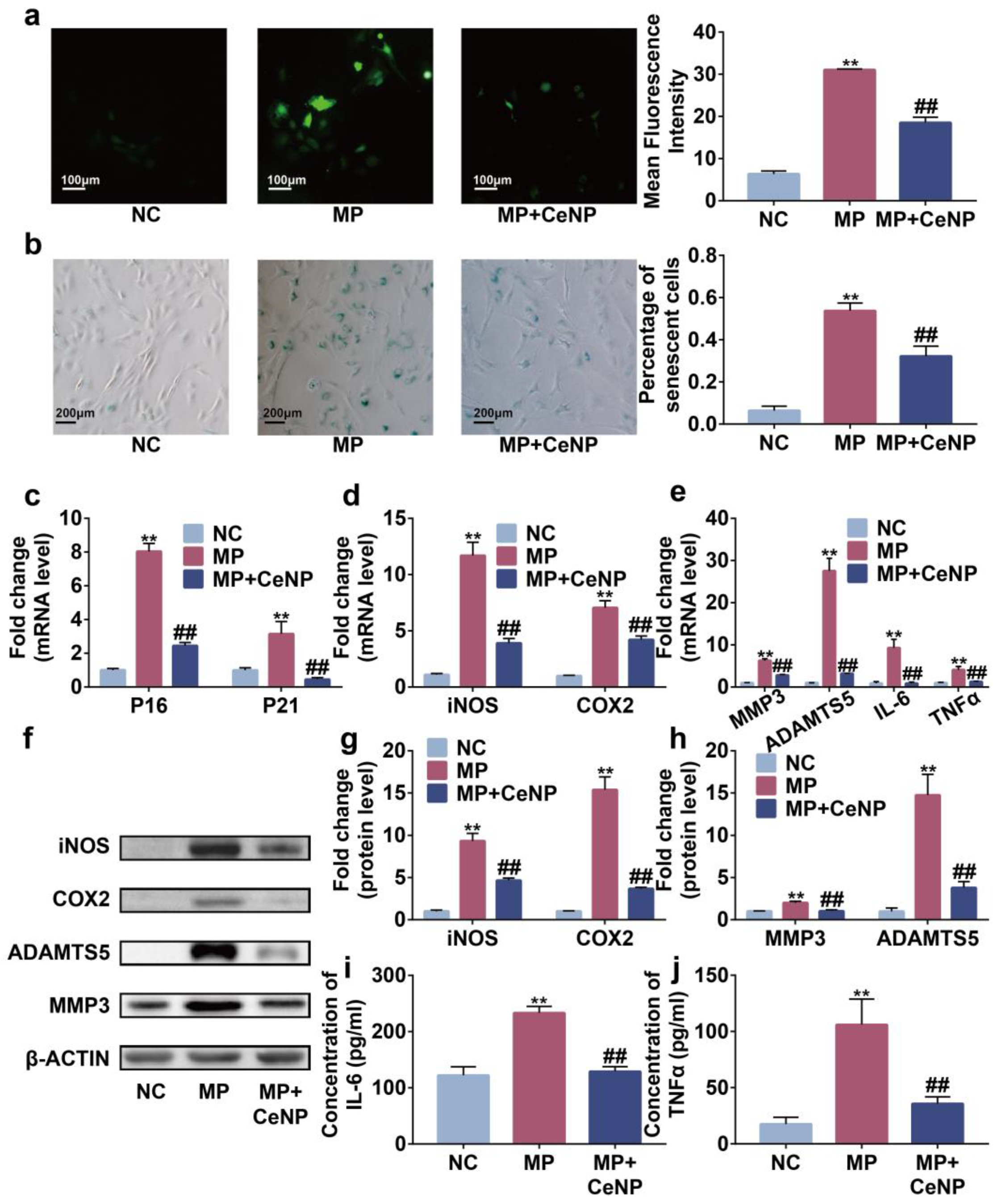
2.5. CeNP Attenuated Senescence and Inhibited SASP in Multiple Passaged Synoviocytes
2.6. CeNP Inhibited the Activation of NFκB Pathway in Senescent Synoviocytes
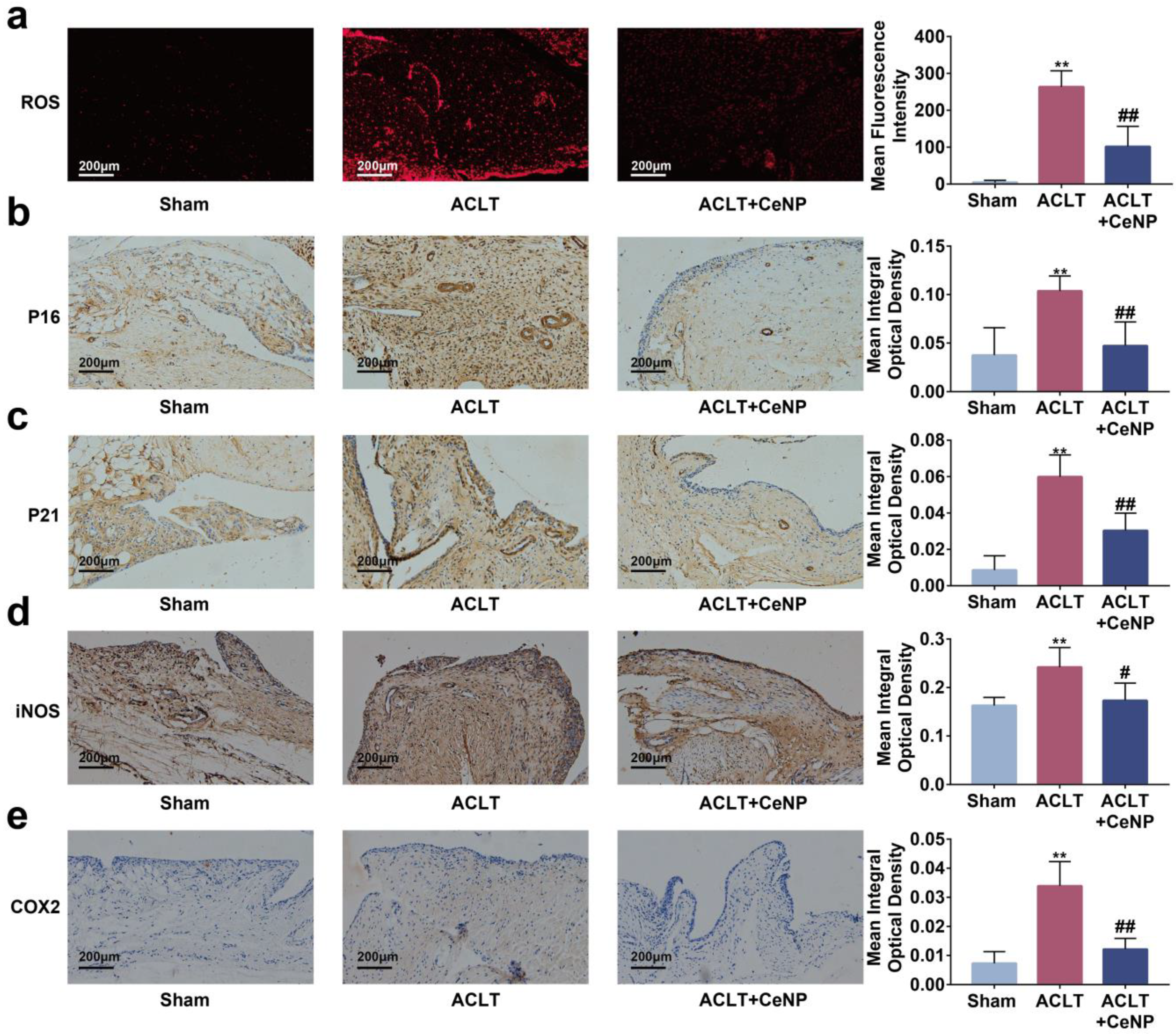
2.7. CeNP Scavenged ROS and Attenuated Senescence of Synoviocytes In Vivo
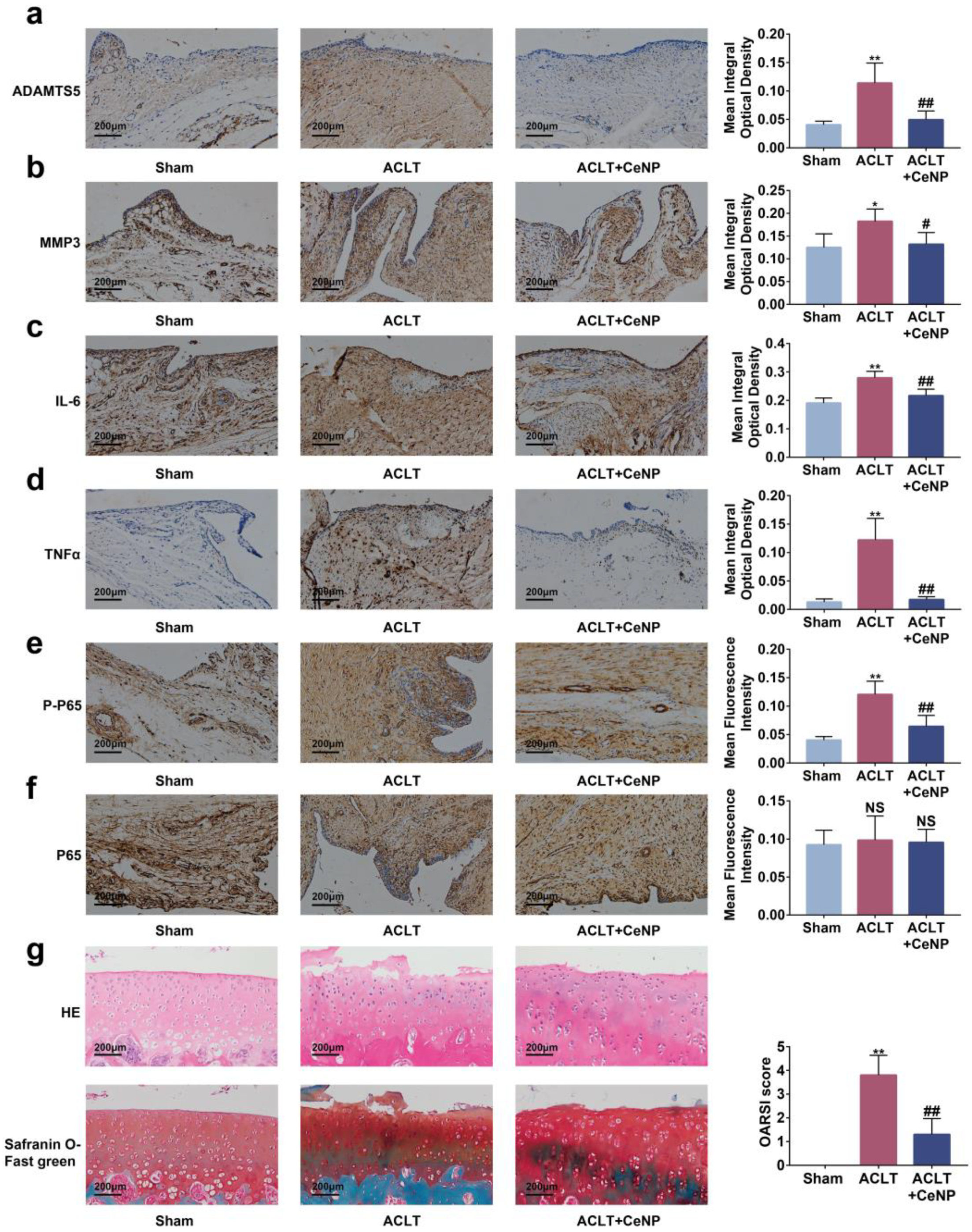
2.8. CeNP Inactivated the NFκB Signaling Pathway and Protected Cartilage In Vivo
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of CeNP
4.3. Characterization of CeNP
4.4. Extracellular Antioxidant Capacity of CeNP
4.5. Cell Isolated and Cultured
4.6. Cellular Uptake of CeNP
4.7. Cytotoxicity Assessment
4.8. ROS Assay
4.9. SA-β-Gal Staining
4.10. Real-Time Quantitative PCR (RT-qPCR)
4.11. Western Blot
4.12. Enzyme-Linked Immunosorbent Assay (ELISA)
4.13. OA Rat Model
4.14. Histology
4.15. Immunohistochemistry
4.16. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T.; Zhang, Y.Q.; Hannan, M.T.; Naimark, A.; Weissman, B.N.; Aliabadi, P.; Levy, D. The Incidence and Natural-History of Knee Osteoarthritis in the Elderly—The Framingham Osteoarthritis Study. Arthritis Rheum. 1995, 38, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Neogi, T.; Guermazi, A.; Roemer, F.; Nevitt, M.C.; Scholz, J.; Arendt-Nielsen, L.; Woolf, C.; Niu, J.; Bradley, L.A.; Quinn, E.; et al. Association of Joint Inflammation with Pain Sensitization in Knee Osteoarthritis: The Multicenter Osteoarthritis Study. Arthritis Rheumatol. 2016, 68, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Siebuhr, A.S.; Bay-Jensen, A.C.; Jordan, J.M.; Kjelgaard-Petersen, C.F.; Christiansen, C.; Abramson, S.B.; Attur, M.; Berenbaum, F.; Kraus, V.; Karsdal, M.A. Inflammation (or synovitis)-driven osteoarthritis: An opportunity for personalizing prognosis and treatment? Scand. J. Rheumatol. 2016, 45, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Freund, A.; Orjalo, A.V.; Desprez, P.Y.; Campisi, J. Inflammatory networks during cellular senescence: Causes and consequences. Trends Mol. Med. 2010, 16, 238–246. [Google Scholar] [CrossRef]
- Greene, M.A.; Loeser, R.F. Aging-related inflammation in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1966–1971. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Zhou, S.Q.; Cai, W.S.; Han, G.T.; Li, J.P.; Chen, M.; Li, H.H. Hypoxia/reoxygenation activates the JNK pathway and accelerates synovial senescence. Mol. Med. Rep. 2020, 22, 265–276. [Google Scholar] [CrossRef]
- Jeon, O.H.; Kim, C.; Laberge, R.M.; Demaria, M.; Rathod, S.; Vasserot, A.P.; Chung, J.W.; Kim, D.H.; Poon, Y.; David, N.; et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 2017, 23, 775–781. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Shao, X.Y.; Gong, W.; Shi, T.S.; Dong, J.; Shi, Y.; Shen, S.Y.; He, Y.; Qin, J.H.; et al. Specific Clearance of Senescent Synoviocytes Suppresses the Development of Osteoarthritis based on Aptamer-Functionalized Targeted Drug Delivery System. Adv. Funct. Mater. 2022, 32, 2109460. [Google Scholar] [CrossRef]
- Chen, X.; Gong, W.; Shao, X.Y.; Shi, T.S.; Zhang, L.; Dong, J.; Shi, Y.; Shen, S.Y.; Qin, J.H.; Jiang, Q.; et al. METTL3-mediated m(6)A modification of ATG7 regulates autophagy-GATA4 axis to promote cellular senescence and osteoarthritis progression. Ann. Rheum. Dis. 2022, 81, 87–99. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (Ros) and Ros-Induced Ros Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Kim, S.; Chung, H.T.; Pae, H.O. Reactive Oxygen Species in the Activation of MAP Kinases. Hydrog. Peroxide Cell Signal. Pt C 2013, 528, 27–48. [Google Scholar]
- Thalhamer, T.; McGrath, M.A.; Harnett, M.M. MAPKs and their relevance to arthritis and inflammation. Rheumatology 2008, 47, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.M.; Haseeb, A.; Ansari, M.Y.; Devarapalli, P.; Haynie, S.; Haqqi, T.M. Wogonin, a plant derived small molecule, exerts potent anti-inflammatory and chondroprotective effects through the activation of ROS/ERK/Nrf2 signaling pathways in human Osteoarthritis chondrocytes. Free Radic. Biol. Med. 2017, 106, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Arra, M.; Swarnkar, G.; Ke, K.; Otero, J.E.; Ying, J.; Duan, X.; Maruyama, T.; Rai, M.F.; O’Keefe, R.J.; Mbalaviele, G.; et al. LDHA-mediated ROS generation in chondrocytes is a potential therapeutic target for osteoarthritis. Nat. Commun. 2020, 11, 3427. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.L.; Chang, C.C.; Yang, Y.W.; Yuan, L.; Xu, L.S.Y.; Ho, C.T.; Li, S.M. Resveratrol Alleviates Rheumatoid Arthritis via Reducing ROS and Inflammation, Inhibiting MAPK Signaling Pathways, and Suppressing Angiogenesis. J. Agric. Food Chem. 2018, 66, 12953–12960. [Google Scholar] [CrossRef]
- Lord, M.S.; Berret, J.F.; Singh, S.; Vinu, A.; Karakoti, A.S. Redox Active Cerium Oxide Nanoparticles: Current Status and Burning Issues. Small 2021, 17, e2102342. [Google Scholar] [CrossRef] [PubMed]
- Barker, E.; Shepherd, J.; Asencio, I.O. The Use of Cerium Compounds as Antimicrobials for Biomedical Applications. Molecules 2022, 27, 2678. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Lai, J.Y. Synthesis, bioactive properties, and biomedical applications of intrinsically therapeutic nanoparticles for disease treatment. Chem. Eng. J. 2022, 435, 134970. [Google Scholar] [CrossRef]
- Yu, H.; Jin, F.Y.; Liu, D.; Shu, G.F.; Wang, X.J.; Qi, J.; Sun, M.C.; Yang, P.; Jiang, S.P.; Ying, X.Y.; et al. ROS-responsive nano-drug delivery system combining mitochondria-targeting ceria nanoparticles with atorvastatin for acute kidney injury. Theranostics 2020, 10, 2342–2357. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Kim, T.; Choi, I.Y.; Soh, M.; Kim, D.; Kim, Y.J.; Jang, H.; Yang, H.S.; Kim, J.Y.; Park, H.K.; et al. Ceria Nanoparticles that can Protect against Ischemic Stroke. Angew. Chem.-Int. Ed. 2012, 51, 11039–11043. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Cha, M.Y.; Kim, D.; Kim, D.K.; Soh, M.; Shin, K.; Hyeon, T.; Mook-Jung, I. Mitochondria-Targeting Ceria Nanoparticles as Antioxidants for Alzheimer’s Disease. ACS Nano 2016, 10, 2860–2870. [Google Scholar] [CrossRef]
- Casals, G.; Perramon, M.; Casals, E.; Portoles, I.; Fernandez-Varo, G.; Morales-Ruiz, M.; Puntes, V.; Jimenez, W. Cerium Oxide Nanoparticles: A New Therapeutic Tool in Liver Diseases. Antioxidants 2021, 10, 660. [Google Scholar] [CrossRef]
- Luo, L.J.; Nguyen, D.D.; Lai, J.Y. Harnessing the tunable cavity of nanoceria for enhancing Y-27632-mediated alleviation of ocular hypertension. Theranostics 2021, 11, 5447–5463. [Google Scholar] [CrossRef]
- Foti, M.C. Use and Abuse of the DPPH center dot Radical. J. Agric. Food Chem. 2015, 63, 8765–8776. [Google Scholar] [CrossRef]
- Qi, W.Z.; Lin, C.X.; Fan, K.; Chen, Z.Y.; Liu, L.L.; Feng, X.F.; Zhang, H.Y.; Shao, Y.; Fang, H.; Zhao, C.; et al. Hesperidin inhibits synovial cell inflammation and macrophage polarization through suppression of the PI3K/AKT pathway in complete Freund’s adjuvant-induced arthritis in mice. Chem. Biol. Interact. 2019, 306, 19–28. [Google Scholar] [CrossRef]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, L.; Fozouni, P.; Evjen, G.; Chandra, V.; Jiang, J.; Lu, C.; Nicastri, M.; Bretz, C.; Winkler, J.D.; et al. SIRT1 is downregulated by autophagy in senescence and ageing. Nat. Cell Biol. 2020, 22, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Pazolli, E.; Luo, X.; Brehm, S.; Carbery, K.; Chung, J.J.; Prior, J.L.; Doherty, J.; Demehri, S.; Salavaggione, L.; Piwnica-Worms, D.; et al. Senescent stromal-derived osteopontin promotes preneoplastic cell growth. Cancer Res. 2009, 69, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Scanzello, C.R.; Goldring, S.R. The role of synovitis in osteoarthritis pathogenesis. Bone 2012, 51, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Celardo, I.; Pedersen, J.Z.; Traversa, E.; Ghibelli, L. Pharmacological potential of cerium oxide nanoparticles. Nanoscale 2011, 3, 1411–1420. [Google Scholar] [CrossRef]
- Lin, W.; Huang, Y.W.; Zhou, X.D.; Ma, Y. Toxicity of cerium oxide nanoparticles in human lung cancer cells. Int. J. Toxicol. 2006, 25, 451–457. [Google Scholar] [CrossRef]
- De Marzi, L.; Monaco, A.; De Lapuente, J.; Ramos, D.; Borras, M.; Di Gioacchino, M.; Santucci, S.; Poma, A. Cytotoxicity and genotoxicity of ceria nanoparticles on different cell lines in vitro. Int. J. Mol. Sci. 2013, 14, 3065–3077. [Google Scholar] [CrossRef]
- Park, E.J.; Choi, J.; Park, Y.K.; Park, K. Oxidative stress induced by cerium oxide nanoparticles in cultured BEAS-2B cells. Toxicology 2008, 245, 90–100. [Google Scholar] [CrossRef]
- Hussain, S.; Al-Nsour, F.; Rice, A.B.; Marshburn, J.; Yingling, B.; Ji, Z.; Zink, J.I.; Walker, N.J.; Garantziotis, S. Cerium dioxide nanoparticles induce apoptosis and autophagy in human peripheral blood monocytes. ACS Nano 2012, 6, 5820–5829. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Guo, W.; Han, L.; Chen, E.; Kong, L.; Wang, L.; Ai, W.; Song, N.; Li, H.; Chen, H. Cerium oxide nanoparticles induce cytotoxicity in human hepatoma SMMC-7721 cells via oxidative stress and the activation of MAPK signaling pathways. Toxicol. In Vitro 2013, 27, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N. Cerium oxide nanostructures: Properties, biomedical applications and surface coatings. 3 Biotech 2022, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.J.; Liu, R.X.; Huan, S.W.; Tang, W.; Zeng, Y.K.; Zhang, J.C.; Yang, J.; Li, Z.Y.; Zhou, Y.; Zha, Z.G.; et al. Senescent skeletal cells cross-talk with synovial cells plays a key role in the pathogenesis of osteoarthritis. Arthritis Res. Ther. 2022, 24, 59. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Ji, S. Cellular senescence: Molecular mechanisms and pathogenicity. J. Cell. Physiol. 2018, 233, 9121–9135. [Google Scholar] [CrossRef] [PubMed]
- Kuyinu, E.L.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Animal models of osteoarthritis: Classification, update, and measurement of outcomes. J. Orthop. Surg. Res. 2016, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Song, D.H.; Kim, S.H.; Jung, Y.; Kim, S.J. Development and characterization of various osteoarthritis models for tissue engineering. PLoS ONE 2018, 13, e0194288. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Adjei, I.M.; Brown, S.B.; Liseth, O.; Sharma, B. Manganese dioxide nanoparticles protect cartilage from inflammation-induced oxidative stress. Biomaterials 2019, 224, 119467. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Xian-Peng, G.; Can, Y.H.; Zhang, C.G.; Zhou, C.Y.; Ma, K.T.; Meng, J.H.; Ma, X.C. Requirement of the NF-κB pathway for induction of Wnt-5A by interleukin-1β in condylar chondrocytes of the temporomandibular joint: Functional crosstalk between the Wnt-5A and NF-κB signaling pathways. Osteoarthr. Cartil. 2011, 19, 111–117. [Google Scholar]
- Goldring, M.B.; Marcu, K.B. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res. Ther. 2009, 11, 224. [Google Scholar] [CrossRef]
- Shakibaei, M.; John, T.; Schulze-Tanzil, G.; Lehmann, I.; Mobasheri, A. Suppression of NF-κB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem. Pharmacol. 2007, 73, 1434–1445. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Knobloch, T.J.; Madhavan, S.; Nam, J.; Agarwal, S.; Agarwal, S. Regulation of chondrocytic gene expression by biomechanical signals. Crit. Rev. Eukaryot. Gene Expr. 2008, 18, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.Y.; Zhou, K.; Li, D.Q.; Xie, X.M.; Jun, F.; Wang, J. Schisantherin A suppresses interleukin-1β-induced inflammation in human chondrocytes via inhibition of NF-κB and MAPKs activation. Eur. J. Pharmacol. 2016, 780, 65–70. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Lee, S.S.; Zhu, H.G.; Contreras, E.Q.; Prakash, A.; Puppala, H.L.; Colvin, V.L. High Temperature Decomposition of Cerium Precursors to Form Ceria Nanocrystal Libraries for Biological Applications. Chem. Mater. 2012, 24, 424–432. [Google Scholar] [CrossRef]
- Kang, D.; Shin, J.; Cho, Y.; Kim, H.S.; Gu, Y.R.; Kim, H.; You, K.T.; Chang, M.J.; Chang, C.B.; Kang, S.B.; et al. Stress-activated miR-204 governs senescent phenotypes of chondrocytes to promote osteoarthritis development. Sci. Transl. Med. 2019, 11, eaar6659. [Google Scholar] [CrossRef]
- Liu, R.F.; Hu, L.; Wu, J.N.; Wang, J.X.; Wang, X.Y.; Liu, Z.Y.; Zhao, Q.D.; Li, W.J.; Song, X.D.; Xiao, J.H. Changes in tumor suppressors and inflammatory responses during hydrogen peroxide-induced senescence in rat fibroblasts. Free Radic. Res. 2022, 56, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.H.; Qiu, B.; Denga, R.H.; Li, H.J.; Xu, X.F.; Shang, X.F. Chondroprotective Effects of Hyaluronic Acid-Chitosan Nanoparticles Containing Plasmid DNA Encoding Cytokine Response Modifier A in a Rat Knee Osteoarthritis Model. Cell. Physiol. Biochem. 2018, 47, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Glasson, S.S.; Chambers, M.G.; Van Den Berg, W.B.; Little, C.B. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr. Cartil. 2010, 18 (Suppl. S3), S17–S23. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Zhuang, H.; Jiang, F.; Zhang, Y.; Zhou, P. Ceria Nanoparticles Alleviated Osteoarthritis through Attenuating Senescence and Senescence-Associated Secretory Phenotype in Synoviocytes. Int. J. Mol. Sci. 2023, 24, 5056. https://doi.org/10.3390/ijms24055056
Ren X, Zhuang H, Jiang F, Zhang Y, Zhou P. Ceria Nanoparticles Alleviated Osteoarthritis through Attenuating Senescence and Senescence-Associated Secretory Phenotype in Synoviocytes. International Journal of Molecular Sciences. 2023; 24(5):5056. https://doi.org/10.3390/ijms24055056
Chicago/Turabian StyleRen, Xunshan, Huangming Zhuang, Fuze Jiang, Yuelong Zhang, and Panghu Zhou. 2023. "Ceria Nanoparticles Alleviated Osteoarthritis through Attenuating Senescence and Senescence-Associated Secretory Phenotype in Synoviocytes" International Journal of Molecular Sciences 24, no. 5: 5056. https://doi.org/10.3390/ijms24055056
APA StyleRen, X., Zhuang, H., Jiang, F., Zhang, Y., & Zhou, P. (2023). Ceria Nanoparticles Alleviated Osteoarthritis through Attenuating Senescence and Senescence-Associated Secretory Phenotype in Synoviocytes. International Journal of Molecular Sciences, 24(5), 5056. https://doi.org/10.3390/ijms24055056





