Identification and Functional Analysis of Two Mitoferrins, CsMIT1 and CsMIT2, Participating in Iron Homeostasis in Cucumber
Abstract
1. Introduction
2. Results
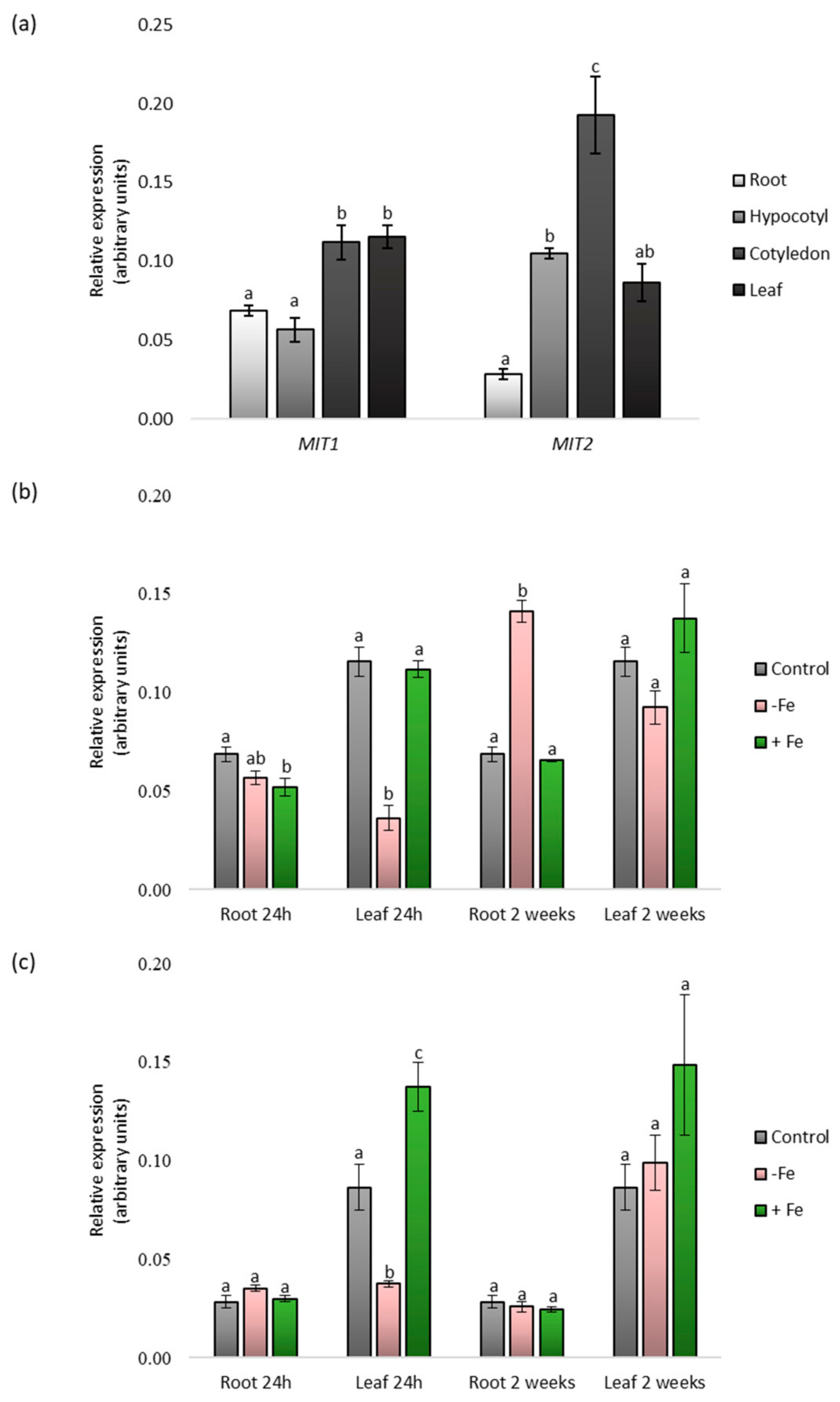
2.1. Identification and Expression Analysis of CsMIT1 and CsMIT2
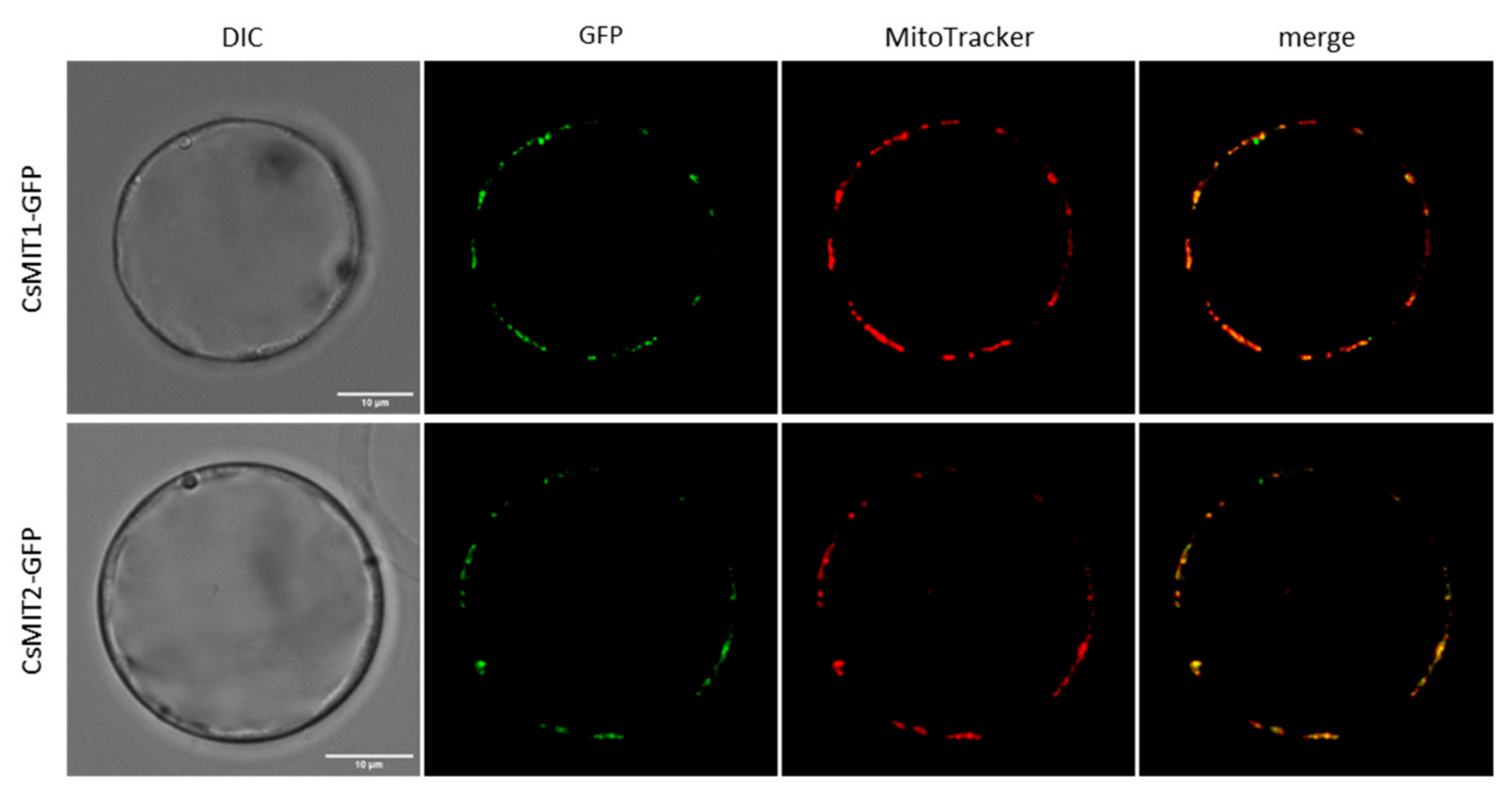
2.2. Mitochondrial Localization of CsMIT1 and CsMIT2 in Arabidopsis thaliana Protoplasts
2.3. Effect of CsMIT1 and CsMIT2 Expression on the Metal-Sensitive Yeast Mutants
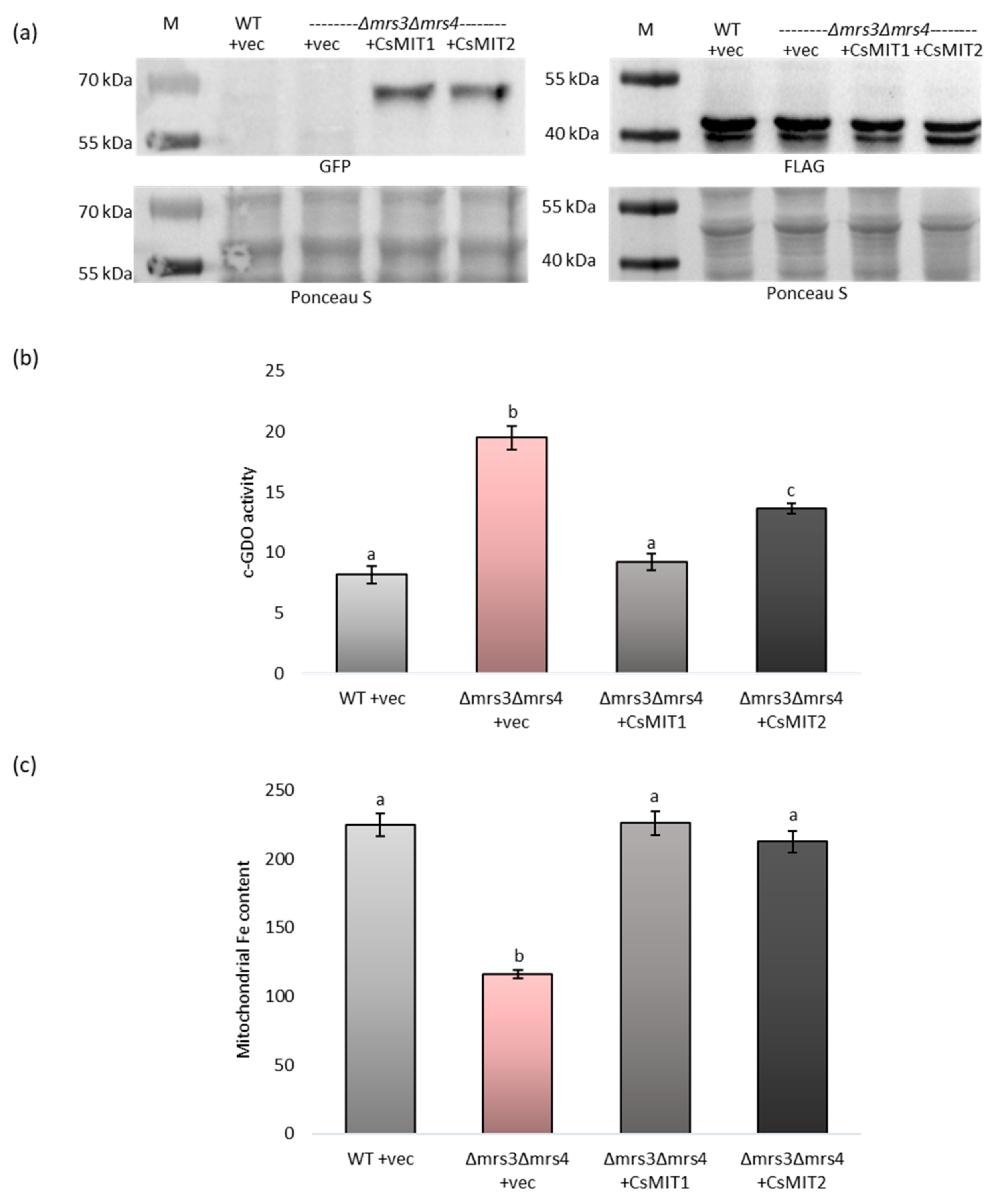
2.4. Effect of CsMIT1 and CsMIT2 Expression on the Cytosolic and Mitochondrial Fe Content in Δmrs3Δmrs4 Cells
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. RNA Extraction and Real-Time PCR
4.3. Cloning of CsMIT1 and CsMIT2
4.4. Yeast Strains, Media and Fluorescence Imaging
4.5. Metal Sensitivity Spot Assays
4.6. Cytosolic Gentisate 1,2-Dioxygenase Assays
4.7. Preparation of Mitochondrial Fraction from Yeast Cells
4.8. Determination of Mitochondrial Heavy Metal Content
4.9. Protein Determination
4.10. Transformation of Arabidopsis thaliana Protoplasts and Confocal Imaging
4.11. Bioinformatics and Accession Numbers
4.12. Statistical Analysis
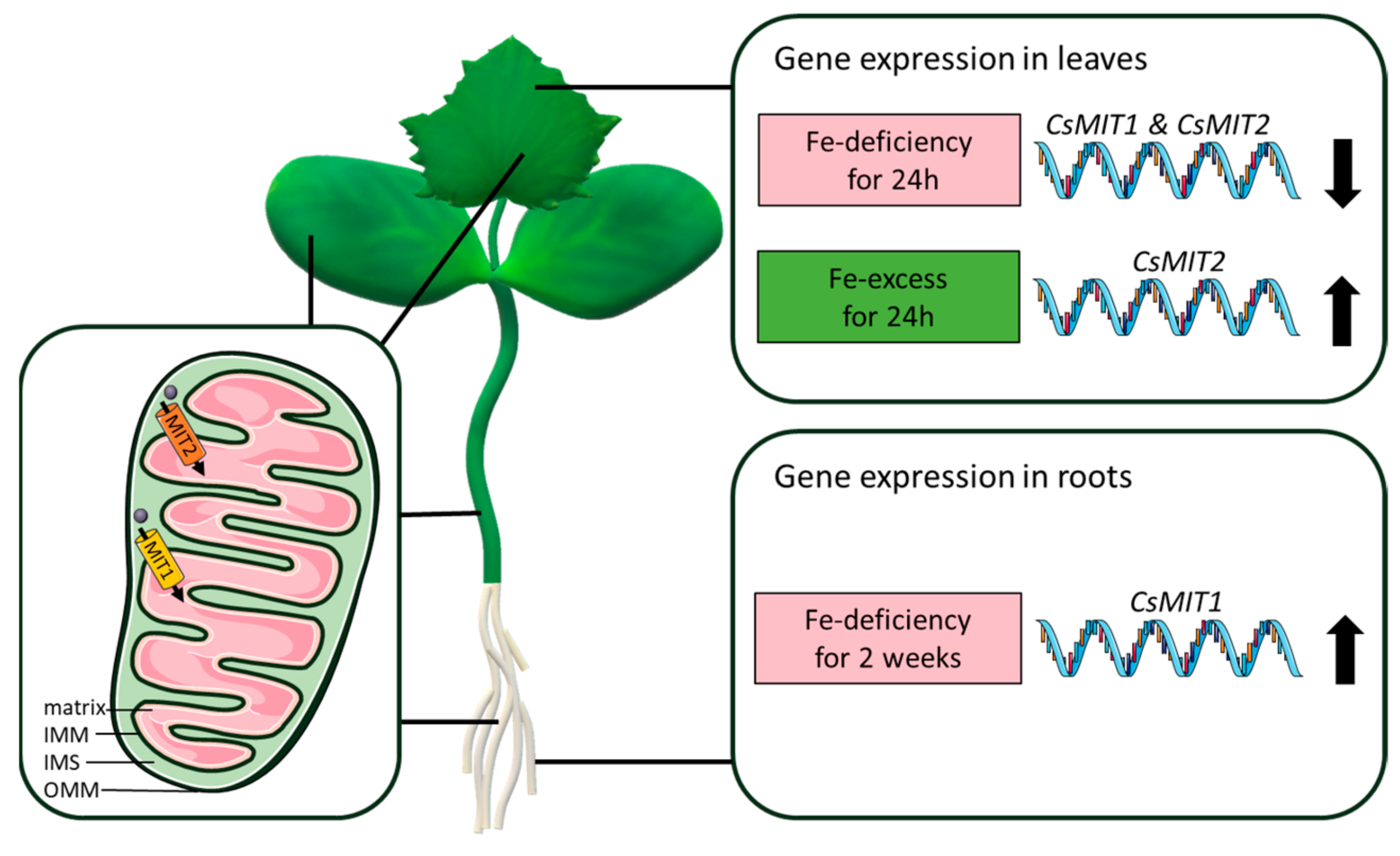
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jain, A.; Connolly, E. Mitochondrial Iron Transport and Homeostasis in Plants. Front. Plant Sci. 2013, 4, 348. [Google Scholar] [CrossRef]
- Connorton, J.M.; Balk, J.; Rodríguez-Celma, J. Iron Homeostasis in Plants—A Brief Overview. Metallomics 2017, 9, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, D.; Sun, W.; Wang, T. The Adaptive Mechanism of Plants to Iron Deficiency via Iron Uptake, Transport, and Homeostasis. Int. J. Mol. Sci. 2019, 20, 2424. [Google Scholar] [CrossRef] [PubMed]
- Santi, S.; Schmidt, W. Dissecting Iron Deficiency-Induced Proton Extrusion in Arabidopsis Roots. New Phytol. 2009, 183, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.L.; Campbell, N.H.; Grotz, N.; Prichard, C.L.; Guerinot, M.L. Overexpression of the FRO2 Ferric Chelate Reductase Confers Tolerance to Growth on Low Iron and Uncovers Posttranscriptional Control. Plant Physiol. 2003, 133, 1102–1110. [Google Scholar] [CrossRef]
- Curie, C.; Panaviene, Z.; Loulergue, C.; Dellaporta, S.L.; Briat, J.-F.; Walker, E.L. Maize Yellow Stripe1 Encodes a Membrane Protein Directly Involved in Fe(III) Uptake. Nature 2001, 409, 346–349. [Google Scholar] [CrossRef]
- Inoue, H.; Kobayashi, T.; Nozoye, T.; Takahashi, M.; Kakei, Y.; Suzuki, K.; Nakazono, M.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. Rice OsYSL15 Is an Iron-Regulated Iron (III) -Deoxymugineic Acid Transporter Expressed in the Roots and Is Essential for Iron Uptake in Early Growth of the Seedlings. J. Biol. Chem. 2009, 284, 3470–3479. [Google Scholar] [CrossRef]
- Eide, D.; Broderius, M.; Fett, J.; Guerinot, M.L. A Novel Iron-Regulated Metal Transporter from Plants Identified by Functional Expression in Yeast. Proc. Natl. Acad. Sci. USA 1996, 93, 5624–5628. [Google Scholar] [CrossRef]
- Vert, G.; Grotz, N.; Deédaldeéchamp, F.; Gaymard, F.; Guerinot, M.L.; Briat, J.-F.; Curie, C. IRT1, an Arabidopsis Transporter Essential for Iron Uptake from the Soil and for Plant Growth. Plant Cell 2002, 14, 1223–1233. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nishizawa, N.K. Iron Uptake, Translocation, and Regulation in Higher Plants. Annu. Rev. Plant Biol. 2012, 63, 131–152. [Google Scholar] [CrossRef]
- Liang, G. Iron Uptake, Signaling, and Sensing in Plants. Plant Commun. 2022, 3, 100349. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Nozoye, T.; Nishizawa, N.K. Iron Transport and Its Regulation in Plants. Free Radic. Biol. Med. 2019, 133, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Punshon, T.; Lanzirotti, A.; Li, L.; Alonso, J.M.; Ecker, J.R.; Kaplan, J.; Guerinot, M.L. Localization of Iron in Arabidopsis Seed Requires the Vacuolar Membrane Transporter VIT1. Science 2006, 314, 1295–1298. [Google Scholar] [CrossRef]
- Gollhofer, J.; Timofeev, R.; Lan, P.; Schmidt, W.; Buckhout, T.J. Vacuolar-Iron-Transporter1-Like Proteins Mediate Iron Homeostasis in Arabidopsis. PLoS ONE 2014, 9, e110468. [Google Scholar] [CrossRef]
- Lanquar, V.; Lelièvre, F.; Bolte, S.; Hamès, C.; Alcon, C.; Neumann, D.; Vansuyt, G.; Curie, C.; Schröder, A.; Krämer, U.; et al. Mobilization of Vacuolar Iron by AtNRAMP3 and AtNRAMP4 Is Essential for Seed Germination on Low Iron. EMBO J. 2005, 24, 4041–4051. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, J.; Baxter, I.R.; Lee, J.; Li, L.; Lahner, B.; Grotz, N.; Kaplan, J.; Salt, D.E.; Guerinot, M.L. The Ferroportin Metal Efflux Proteins Function in Iron and Cobalt Homeostasis in Arabidopsis. Plant Cell 2009, 21, 3326–3338. [Google Scholar] [CrossRef]
- Duy, D.; Wanner, G.; Meda, A.R.; von Wireén, N.; Soll, J.; Philippar, K. PIC1, an Ancient Permease in Arabidopsis Chloroplasts, Mediates Iron Transport. Plant Cell 2007, 19, 986–1006. [Google Scholar] [CrossRef]
- Divol, F.; Couch, D.; Conéjéro, G.; Roschzttardtz, H.; Mari, S.; Curie, C. The Arabidopsis YELLOW STRIPE LIKE4 and 6 Transporters Control Iron Release from the Chloroplast. Plant Cell 2013, 25, 1040–1055. [Google Scholar] [CrossRef]
- Jeong, J.; Cohu, C.; Kerkeb, L.; Pilon, M.; Connolly, E.L.; Guerinot, M.L. Chloroplast Fe(III) Chelate Reductase Activity Is Essential for Seedling Viability under Iron Limiting Conditions. Proc. Natl. Acad. Sci. USA 2008, 105, 10619–10624. [Google Scholar] [CrossRef]
- Mukherjee, I.; Campbell, N.H.; Ash, J.S.; Connolly, E.L. Expression Profiling of the Arabidopsis Ferric Chelate Reductase (FRO) Gene Family Reveals Differential Regulation by Iron and Copper. Planta 2006, 223, 1178–1190. [Google Scholar] [CrossRef]
- Fernie, A.R.; Cavalcanti, J.H.F.; Nunes-Nesi, A. Metabolic Roles of Plant Mitochondrial Carriers. Biomolecules 2020, 10, 1013. [Google Scholar] [CrossRef]
- Haferkamp, I.; Schmitz-Esser, S. The Plant Mitochondrial Carrier Family: Functional and Evolutionary Aspects. Front. Plant Sci. 2012, 3, 2. [Google Scholar] [CrossRef]
- Kunji, E.R.S.; Robinson, A.J. Coupling of Proton and Substrate Translocation in the Transport Cycle of Mitochondrial Carriers. Curr. Opin. Struct. Biol. 2010, 20, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Wiesenberger, G.; Link, T.A.; von Ahsen, U.; Waldherr, M.; Schweyen, R.J. MRS3 and MRS4, Two Suppressors of MtRNA Splicing Defects in Yeast, Are New Members of the Mitochondrial Carrier Family. J. Mol. Biol. 1991, 217, 23–37. [Google Scholar] [CrossRef]
- Bashir, K.; Ishimaru, Y.; Shimo, H.; Nagasaka, S.; Fujimoto, M.; Takanashi, H.; Tsutsumi, N.; An, G.; Nakanishi, H.; Nishizawa, N.K. The Rice Mitochondrial Iron Transporter Is Essential for Plant Growth. Nat. Commun. 2011, 2, 322. [Google Scholar] [CrossRef]
- Bashir, K.; Ishimaru, Y.; Nishizawa, N.K. Identification and Characterization of the Major Mitochondrial Fe Transporter in Rice. Plant Signal. Behav. 2011, 6, 1591–1593. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Dashner, Z.S.; Connolly, E.L. Mitochondrial Iron Transporters (MIT1 and MIT2) Are Essential for Iron Homeostasis and Embryogenesis in Arabidopsis Thaliana. Front. Plant Sci. 2019, 10, 1449. [Google Scholar] [CrossRef]
- Kurt, F.; Kurt, B.; Filiz, E.; Yildiz, K.; Akbudak, M.A. Mitochondrial Iron Transporter (MIT) Gene in Potato (Solanum Tuberosum): Comparative Bioinformatics, Physiological and Expression Analyses in Response to Drought and Salinity. BioMetals 2022, 35, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, Y.; Tsuji, J.; Fu, S.-C.; Tomii, K.; Horton, P.; Imai, K. MitoFates: Improved Prediction of Mitochondrial Targeting Sequences and Their Cleavage Sites. Mol. Cell. Proteom. 2015, 14, 1113–1126. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Salvatore, M.; Emanuelsson, O.; Winther, O.; von Heijne, G.; Elofsson, A.; Nielsen, H. Detecting Sequence Signals in Targeting Peptides Using Deep Learning. Life Sci. Alliance 2019, 2, e201900429. [Google Scholar] [CrossRef]
- Kunji, E.R.S.; Robinson, A.J. The Conserved Substrate Binding Site of Mitochondrial Carriers. Biochim. Biophys. Acta Bioenerg. 2006, 1757, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- King, M.S.; Kerr, M.; Crichton, P.G.; Springett, R.; Kunji, E.R.S. Formation of a Cytoplasmic Salt Bridge Network in the Matrix State Is a Fundamental Step in the Transport Mechanism of the Mitochondrial ADP/ATP Carrier. Biochim. Biophys. Acta Bioenerg. 2016, 1857, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, F. The Mitochondrial Transporter Family SLC25: Identification, Properties and Physiopathology. Mol. Aspects Med. 2013, 34, 465–484. [Google Scholar] [CrossRef] [PubMed]
- Brazzolotto, X.; Pierrel, F.; Pelosi, L. Three Conserved Histidine Residues Contribute to Mitochondrial Iron Transport through Mitoferrins. Biochem. J. 2014, 460, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Christenson, E.T.; Gallegos, A.S.; Banerjee, A. In Vitro Reconstitution, Functional Dissection, and Mutational Analysis of Metal Ion Transport by Mitoferrin-1. J. Biol. Chem. 2018, 293, 3819–3828. [Google Scholar] [CrossRef] [PubMed]
- Froschauer, E.M.; Schweyen, R.J.; Wiesenberger, G. The Yeast Mitochondrial Carrier Proteins Mrs3p/Mrs4p Mediate Iron Transport across the Inner Mitochondrial Membrane. Biochim. Biophys. Acta Biomembr. 2009, 1788, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Savojardo, C.; Bruciaferri, N.; Tartari, G.; Martelli, P.L.; Casadio, R. DeepMito: Accurate Prediction of Protein Sub-Mitochondrial Localization Using Convolutional Neural Networks. Bioinformatics 2020, 36, 56–64. [Google Scholar] [CrossRef]
- Mühlenhoff, U.; Stadler, J.A.; Richhardt, N.; Seubert, A.; Eickhorst, T.; Schweyen, R.J.; Lill, R.; Wiesenberger, G. A Specific Role of the Yeast Mitochondrial Carriers Mrs3/4p in Mitochondrial Iron Acquisition under Iron-Limiting Conditions. J. Biol. Chem. 2003, 278, 40612–40620. [Google Scholar] [CrossRef]
- Li, L.; Miao, R.; Jia, X.; Ward, D.M.; Kaplan, J. Expression of the Yeast Cation Diffusion Facilitators Mmt1 and Mmt2 Affects Mitochondrial and Cellular Iron Homeostasis: Evidence for mitochondrial iron export. J. Biol. Chem. 2014, 289, 17132–17141. [Google Scholar] [CrossRef]
- Wu, H.; Li, L.; Du, J.; Yuan, Y.; Cheng, X.; Ling, H.-Q. Molecular and Biochemical Characterization of the Fe(III) Chelate Reductase Gene Family in Arabidopsis Thaliana. Plant Cell Physiol. 2005, 46, 1505–1514. [Google Scholar] [CrossRef]
- Jain, A.; Wilson, G.; Connolly, E. The Diverse Roles of FRO Family Metalloreductases in Iron and Copper Homeostasis. Front. Plant Sci. 2014, 5, 100. [Google Scholar] [CrossRef] [PubMed]
- Kunji, E.R.S.; King, M.S.; Ruprecht, J.J.; Thangaratnarajah, C. The SLC25 Carrier Family: Important Transport Proteins in Mitochondrial Physiology and Pathology. Physiology 2020, 35, 302–327. [Google Scholar] [CrossRef]
- Ali, M.Y.; Oliva, C.R.; Flor, S.; Griguer, C.E. Mitoferrin, Cellular and Mitochondrial Iron Homeostasis. Cells 2022, 11, 3464. [Google Scholar] [CrossRef]
- Foury, F.; Roganti, T. Deletion of the Mitochondrial Carrier Genes MRS3 and MRS4 Suppresses Mitochondrial Iron Accumulation in a Yeast Frataxin-Deficient Strain. J. Biol. Chem. 2002, 277, 24475–24483. [Google Scholar] [CrossRef]
- Shaw, G.C.; Cope, J.J.; Li, L.; Corson, K.; Hersey, C.; Ackermann, G.E.; Gwynn, B.; Lambert, A.J.; Wingert, R.A.; Traver, D.; et al. Mitoferrin Is Essential for Erythroid Iron Assimilation. Nature 2006, 440, 96–100. [Google Scholar] [CrossRef]
- Troadec, M.-B.; Warner, D.; Wallace, J.; Thomas, K.; Spangrude, G.J.; Phillips, J.; Khalimonchuk, O.; Paw, B.H.; Ward, D.M.; Kaplan, J. Targeted Deletion of the Mouse Mitoferrin1 Gene: From Anemia to Protoporphyria. Blood 2011, 117, 5494–5502. [Google Scholar] [CrossRef]
- Chen, W.; Paradkar, P.N.; Li, L.; Pierce, E.L.; Langer, N.B.; Takahashi-Makise, N.; Hyde, B.B.; Shirihai, O.S.; Ward, D.M.; Kaplan, J.; et al. Abcb10 Physically Interacts with Mitoferrin-1 (Slc25a37) to Enhance Its Stability and Function in the Erythroid Mitochondria. Proc. Natl. Acad. Sci. USA 2009, 106, 16263–16268. [Google Scholar] [CrossRef]
- Dietz, J.V.; Fox, J.L.; Khalimonchuk, O. Down the Iron Path: Mitochondrial Iron Homeostasis and Beyond. Cells 2021, 10, 2198. [Google Scholar] [CrossRef] [PubMed]
- Vigani, G.; Bashir, K.; Ishimaru, Y.; Lehmann, M.; Casiraghi, F.M.; Nakanishi, H.; Seki, M.; Geigenberger, P.; Zocchi, G.; Nishizawa, N.K. Knocking down Mitochondrial Iron Transporter (MIT) Reprograms Primary and Secondary Metabolism in Rice Plants. J. Exp. Bot. 2016, 67, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Migocka, M.; Małas, K.; Maciaszczyk-Dziubinska, E.; Posyniak, E.; Migdal, I.; Szczech, P. Cucumber Golgi Protein CsMTP5 Forms a Zn-Transporting Heterodimer with High Molecular Mass Protein CsMTP12. Plant Sci. 2018, 277, 196–206. [Google Scholar] [CrossRef]
- Migocka, M.; Papierniak, A. Identification of Suitable Reference Genes for Studying Gene Expression in Cucumber Plants Subjected to Abiotic Stress and Growth Regulators. Mol. Breed. 2011, 28, 343–357. [Google Scholar] [CrossRef]
- Niedenthal, R.K.; Riles, L.; Johnston, M.; Hegemann, J.H. Green Fluorescent Protein as a Marker for Gene Expression and Subcellular Localization in Budding Yeast. Yeast 1996, 12, 773–786. [Google Scholar] [CrossRef]
- Voelker, C.; Schmidt, D.; Mueller-Roeber, B.; Czempinski, K. Members of the Arabidopsis AtTPK/KCO Family Form Homomeric Vacuolar Channels in Planta. Plant J. 2006, 48, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Kaplan, J. The Yeast Gene MSC2, a Member of the Cation Diffusion Facilitator Family, Affects the Cellular Distribution of Zinc. J. Biol. Chem. 2001, 276, 5036–5043. [Google Scholar] [CrossRef]
- Li, L.; Kaplan, J. A Mitochondrial-Vacuolar Signaling Pathway in Yeast That Affects Iron and Copper Metabolism. J. Biol. Chem. 2004, 279, 33653–33661. [Google Scholar] [CrossRef]
- Baker Brachmann, C.; Davies, A.; Cost, G.J.; Caputo, E.; Li, J.; Hieter, P.; Boeke, J.D. Designer Deletion Strains Derived from Saccharomyces Cerevisiae S288C: A Useful Set of Strains and Plasmids for PCR-Mediated Gene Disruption and Other Applications. Yeast 1998, 14, 115–132. [Google Scholar] [CrossRef]
- Li, L.; Miao, R.; Bertram, S.; Jia, X.; Ward, D.M.; Kaplan, J. A Role for Iron-Sulfur Clusters in the Regulation of Transcription Factor Yap5-Dependent High Iron Transcriptional Responses in Yeast. J. Biol. Chem. 2012, 287, 35709–35721. [Google Scholar] [CrossRef]
- Gregg, C.; Kyryakov, P.; Titorenko, V.I. Purification of Mitochondria from Yeast Cells. JoVE 2009, 30, e1417. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Thomine, S.; Lelièvre, F.; Debarbieux, E.; Schroeder, J.I.; Barbier-Brygoo, H. AtNRAMP3, a Multispecific Vacuolar Metal Transporter Involved in Plant Responses to Iron Deficiency. Plant J. 2003, 34, 685–695. [Google Scholar] [CrossRef]
- Dobson, L.; Reményi, I.; Tusnády, G.E. CCTOP: A Consensus Constrained TOPology Prediction Web Server. Nucleic Acids Res. 2015, 43, W408–W412. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2—A Multiple Sequence Alignment Editor and Analysis Workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Małas, K.; Kabała, K. Identification and Functional Analysis of Two Mitoferrins, CsMIT1 and CsMIT2, Participating in Iron Homeostasis in Cucumber. Int. J. Mol. Sci. 2023, 24, 5050. https://doi.org/10.3390/ijms24055050
Małas K, Kabała K. Identification and Functional Analysis of Two Mitoferrins, CsMIT1 and CsMIT2, Participating in Iron Homeostasis in Cucumber. International Journal of Molecular Sciences. 2023; 24(5):5050. https://doi.org/10.3390/ijms24055050
Chicago/Turabian StyleMałas, Karolina, and Katarzyna Kabała. 2023. "Identification and Functional Analysis of Two Mitoferrins, CsMIT1 and CsMIT2, Participating in Iron Homeostasis in Cucumber" International Journal of Molecular Sciences 24, no. 5: 5050. https://doi.org/10.3390/ijms24055050
APA StyleMałas, K., & Kabała, K. (2023). Identification and Functional Analysis of Two Mitoferrins, CsMIT1 and CsMIT2, Participating in Iron Homeostasis in Cucumber. International Journal of Molecular Sciences, 24(5), 5050. https://doi.org/10.3390/ijms24055050






