Fibrosis of Peritoneal Membrane, Molecular Indicators of Aging and Frailty Unveil Vulnerable Patients in Long-Term Peritoneal Dialysis
Abstract
1. Introduction
2. Results
2.1. Baseline Characterization of Study Population
2.2. Impact of the Status of the Peritoneal Membrane and Age-Related Indicators in PD-Related Outcomes
2.3. Status of Peritoneal Membrane, Age-Related Indicators and Technical Failure of Peritoneal Dialysis
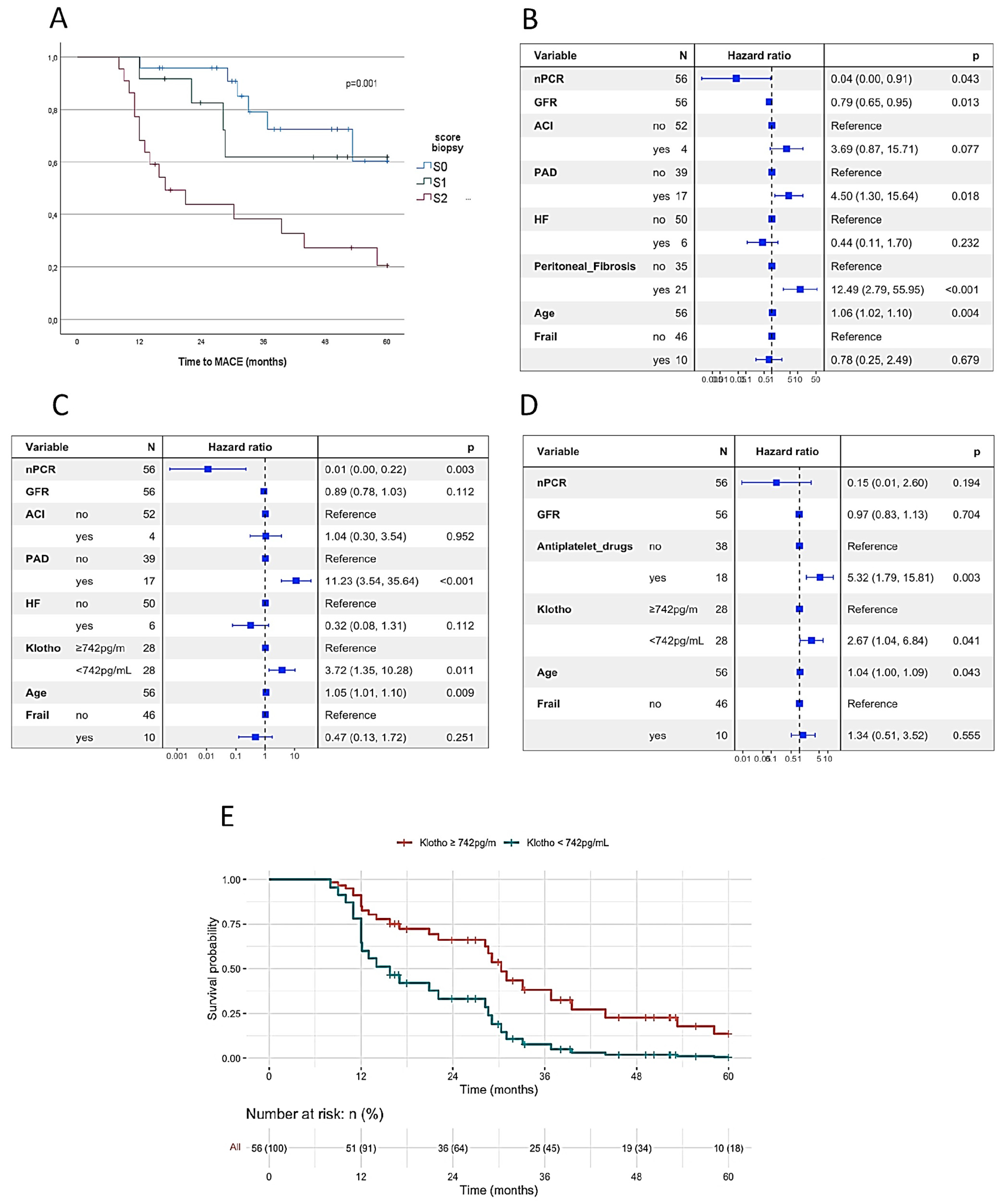
2.4. Peritoneal Membrane, Age-Related Indicators and Major Cardiovascular Event
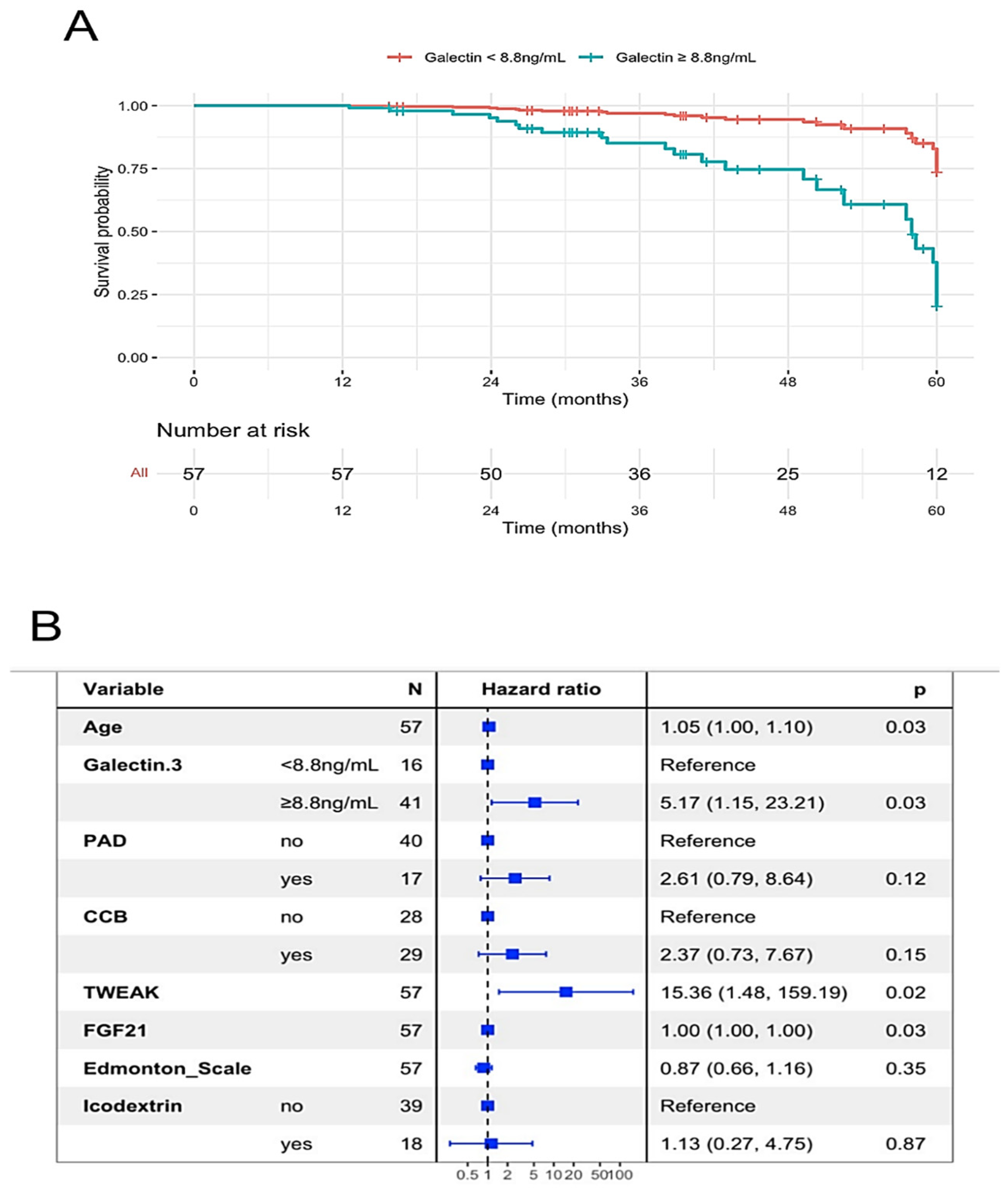
2.5. Peritoneal Membrane, Age-Related Indicators and All-Causes Mortality
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. PD Prescription
4.3. Baseline Variables
- (a)
- Anthropometric, clinical and therapeutic variables
- (b)
- Pre-PD histomorphology score (Biopsy score)
- (c)
- Dialysis Efficacy. The dialysis efficacy was defined by the calculation of the urea clearance index (Kt/Vurea). This index considers the concentration of urea in blood, in the urine (renal clearance) and dialysate (peritoneal clearance), and the body surface. This was obtained from the collection of the PD effluent and of the patient’s urine for 24 h, prior to the scheduled visit at the PD unit. Weekly Kt/V values were calculated according to the recommendations of the kidney disease outcomes quality initiative [11,44] (DOQI). According to K/DOQI guidelines, the cut-off value of Kt/V ≥ 1.7 was set to define Dialysis Efficacy [45].
- (d)
- Profile of Peritoneal Transport. The category of Peritoneal Membrane Transport was determined using the peritoneal equilibration test (PET). The PET and dialysis efficacy were evaluated at the same time. In the night before the PET test, at home, a long overnight dwell of PD was performed using a 1.36% glucose dialysis fluid (isotonic). The next morning at the hospital, the PETs were performed using a 2 L dialysis solution with 3.86% or 4.25% glucose (hypertonic). The dialysate/plasma (D/P) creatinine ratio was measured and used to identify the patients as low (D/P creatinine 0.34–0.50, L), low average (D/P creatinine 0.50 to 0.65, LA), high average (D/P creatinine 0.65 to 0.80, HA) or high (D/P creatinine 0.81–1.03, H) transporters, according to previous definitions [46].
- (e)
- Fluid removal by the peritoneal membrane. The permeability of the membrane to fluid is defined by the ultrafiltration test, which compares the amount of drained dialysate with the 2 L of dialysis fluid instilled at start of the test. Ultrafiltration failure is defined as failure when the target of at least 400 mL of net ultrafiltration during a 4-h period of PD using 3.86% or 4.25% glucose solutions is not achieved (in absence of catheter malposition or mechanical dysfunction).
- (f)
- Residual renal function (RRF). The RRF was obtained through the creatinine clearance, which was calculated by collecting 24 h of urine before blood sampling and using conventional formulas and correcting the result for a body surface area of 1.73 m2/Kg.
- (g)
- Daily protein intake. Nutritional status is an important adequacy parameter in patients on dialysis. The normalized protein catabolic rate (nPCR) was calculated from the urea eliminated in urine and in dialysate and normalized to body weight. The recommended standard value of this parameter is ≥1 g/Kg/day.
- (h)
- Effluent CA125 levels. Effluent levels were measured using electrochemiluminescence (Elecsys, Roche diagnostics).
- (i)
- Levels of serum biomarkers. A panel of proteins related to aging and fibrosis was quantified at study baseline by Enzyme-Linked Immunosorbent Assay (ELISA), i.e., before the start of PD, which consisted of α-Klotho (Bionava assay), galectin-3 and FGF21 (Fibroblast growth factor 21) (Quantikine ELISAS, R&D systems), FGF23 (Fibroblast growth factor 23 c-terminal, Immunotopics), Tweak (Tumor necrosis factor-like weak inducer of apoptosis, Preprotech), TNFα (Tumor necrosis factor alfa, Preprotech) and hr-CRP (ultra-sensitive C-reactive protein assay using a Cobas c702 analyzer, Roche Diagnostics).
- (j)
- Frailty assessment. The Edmonton Frail Scale (FS) was selected as a simple assessment tool comprising eleven items focusing on different frailty dimensions [47].
4.4. Study Outcomes
- -
- PD technique failure refers to ultrafiltration failure, peritonitis, or dialysis inefficacy. Patients were considered with no technical failure when achieving 60 months of follow-up.
- -
- Time for technique failure is the time on PD of each patient in the study until technical failure. Participants dropping PD out for reasons other than technical failure (switching to hemodialysis by option, kidney transplantation, transference to other PD centers or loss to follow-up) were censored.
- (a)
- All-cause mortality
- (b)
- Major Cardiovascular event
- -
- Major Cardiovascular event (MACE) after 3 months on PD. MACEs were defined according to validated clinical criteria and included coronary heart disease (CHD), congestive heart failure (HF), acute myocardial infarction (AMI), acute cerebral infarction (ACI) and cardiac death caused by AMI, arrhythmias or HF. CHD was defined as ≥50% diameter stenosis of coronary arteries by either coronary angiography or CT angiography [48]. HF was diagnosed according to ESC guidelines for the diagnosis and treatment of chronic heart failure [49]. AMI was diagnosed according to ESC guidelines for the management of acute coronary syndromes [50]. ACI was defined as an acute neurological event lasting more than 24 h associated with clinical evidence of ischemic focus of the brain [51]. Cardiac death was defined as death caused by AMI, arrhythmias or CHF.
- -
- Time for MACE was defined for each patient as the time in the study until a MACE. Censored data were defined for those dropping out of the study without MACE or those achieving the end of the study without MACE.
4.5. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, L.; Qureshi, A.R.; Witasp, A.; Lindholm, B.; Stenvinkel, P. Early Vascular Ageing and Cellular Senescence in Chronic Kidney Disease. Comput. Struct. Biotechnol. J. 2019, 17, 721–729. [Google Scholar] [CrossRef]
- Lanzani, C.; Citterio, L.; Vezzoli, G. Klotho: A Link between Cardiovascular and Non-Cardiovascular Mortality. Clin. Kidney J. 2020, 13, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Lozier, M.R.; Sanchez, A.M.; Lee, J.J.; Tamariz, L.J.; Valle, G.A. Comparison of Cardiovascular Outcomes by Dialysis Modality: A Systematic Review and Meta-Analysis. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2019, 39, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Branco, P.; Martins, A.R.; Calça, R.; Mateus, C.; Jervis, M.J.; Rodrigues, A.; Lopes, S.A.; Civantos, E.; Mas-Fontao, S.; Gaspar, A.; et al. Alpha-klotho and peritoneal membrane status: A hypothesis generating study. Eur. J. Clin. Investig. 2022, 53, e13903. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Yang, K.; Zhang, B.; Zhao, J. The Protective Role of Klotho in CKD-Associated Cardiovascular Disease. Kidney Dis. 2020, 6, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Kuro-o, M. The Klotho Proteins in Health and Disease. Nat. Rev. Nephrol. 2019, 15, 27–44. [Google Scholar] [CrossRef]
- Kadoya, H.; Satoh, M.; Nishi, Y.; Kondo, M.; Wada, Y.; Sogawa, Y.; Kidokoro, K.; Nagasu, H.; Sasaki, T.; Kashihara, N. Klotho Is a Novel Therapeutic Target in Peritoneal Fibrosis via Wnt Signaling Inhibition. Nephrol. Dial. Transplant. 2020, 35, 773–781. [Google Scholar] [CrossRef]
- Bao, J.-F.; Hu, P.-P.; She, Q.-Y.; Li, A. A Land of Controversy: Fibroblast Growth Factor-23 and Uremic Cardiac Hypertrophy. J. Am. Soc. Nephrol. 2020, 31, 1423–1434. [Google Scholar] [CrossRef]
- Zoccali, C.; Vanholder, R.; Massy, Z.A.; Ortiz, A.; Sarafidis, P.; Dekker, F.W.; Fliser, D.; Fouque, D.; Heine, G.H.; Jager, K.J.; et al. The Systemic Nature of CKD. Nat. Rev. Nephrol. 2017, 13, 344–358. [Google Scholar] [CrossRef]
- Roh, E.; Hwang, S.Y.; Yoo, H.J.; Baik, S.H.; Cho, B.; Park, Y.S.; Kim, H.J.; Lee, S.-G.; Kim, B.J.; Jang, H.C.; et al. Association of Plasma FGF21 Levels with Muscle Mass and Muscle Strength in a National Multicentre Cohort Study: Korean Frailty and Aging Cohort Study. Age Ageing 2021, 50, 1971–1978. [Google Scholar] [CrossRef]
- Dent, E.; Kowal, P.; Hoogendijk, E.O. Frailty Measurement in Research and Clinical Practice: A Review. Eur. J. Intern. Med. 2016, 31, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Perna, S.; Francis, M.D.; Bologna, C.; Moncaglieri, F.; Riva, A.; Morazzoni, P.; Allegrini, P.; Isu, A.; Vigo, B.; Guerriero, F.; et al. Performance of Edmonton Frail Scale on Frailty Assessment: Its Association with Multi-Dimensional Geriatric Conditions Assessed with Specific Screening Tools. BMC Geriatr. 2017, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Gillerot, G.; Goffin, E.; Michel, C.; Evenepoel, P.; Van Biesen, W.; Tintillier, M.; Stenvinkel, P.; Heimbarger, O.; Lindholm, B.; Nordfors, L.; et al. Genetic and Clinical Factors Influence the Baseline Permeability of the Peritoneal Membrane. Kidney Int. 2005, 67, 2477–2487. [Google Scholar] [CrossRef]
- Morelle, J.; Marechal, C.; Yu, Z.; Debaix, H.; Corre, T.; Lambie, M.; Verduijn, M.; Dekker, F.; Bovy, P.; Evenepoel, P.; et al. AQP1 Promoter Variant, Water Transport, and Outcomes in Peritoneal Dialysis. N. Engl. J. Med. 2021, 385, 1570–1580. [Google Scholar] [CrossRef] [PubMed]
- Rumpsfeld, M.; McDonald, S.P.; Purdie, D.M.; Collins, J.; Johnson, D.W. Predictors of Baseline Peritoneal Transport Status in Australian and New Zealand Peritoneal Dialysis Patients. Am. J. Kidney Dis. 2004, 43, 492–501. [Google Scholar] [CrossRef]
- Teitelbaum, I. Peritoneal Dialysis. N. Engl. J. Med. 2021, 385, 1786–1795. [Google Scholar] [CrossRef]
- Zhou, Q.; Bajo, M.-A.; del Peso, G.; Yu, X.; Selgas, R. Preventing Peritoneal Membrane Fibrosis in Peritoneal Dialysis Patients. Kidney Int. 2016, 90, 515–524. [Google Scholar] [CrossRef]
- Mehrotra, R.; Devuyst, O.; Davies, S.J.; Johnson, D.W. The Current State of Peritoneal Dialysis. J. Am. Soc. Nephrol. 2016, 27, 3238–3252. [Google Scholar] [CrossRef]
- Campbell, S. The Role of TWEAK/Fn14 IN the Pathogenesis of Inflammation and Systemic Autoimmunity. Front. Biosci. 2004, 9, 2273. [Google Scholar] [CrossRef] [PubMed]
- Winkles, J.A. The TWEAK–Fn14 Cytokine–Receptor Axis: Discovery, Biology and Therapeutic Targeting. Nat. Rev. Drug Discov. 2008, 7, 411–425. [Google Scholar] [CrossRef]
- Ebert, T.; Pawelzik, S.-C.; Witasp, A.; Arefin, S.; Hobson, S.; Kublickiene, K.; Shiels, P.G.; Bäck, M.; Stenvinkel, P. Inflammation and Premature Ageing in Chronic Kidney Disease. Toxins 2020, 12, 227. [Google Scholar] [CrossRef]
- Lu, H.; Jia, C.; Wu, D.; Jin, H.; Lin, Z.; Pan, J.; Li, X.; Wang, W. Fibroblast Growth Factor 21 (FGF21) Alleviates Senescence, Apoptosis, and Extracellular Matrix Degradation in Osteoarthritis via the SIRT1-MTOR Signaling Pathway. Cell Death Dis. 2021, 12, 865. [Google Scholar] [CrossRef] [PubMed]
- Moe, O.W. Fibroblast Growth Factor 23: Friend or Foe in Uremia? J. Clin. Investig. 2012, 122, 2354–2356. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Rodríguez, E.; Pizarro-Sánchez, S.; Sanz, A.; Ramos, A.; Sanchez-Niño, M.; Martin-Cleary, C.; Fernandez-Fernandez, B.; Ortiz, A. Inflammatory Cytokines as Uremic Toxins: “Ni Son Todos Los Que Estan, Ni Estan Todos Los Que Son”. Toxins 2017, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, T.; Kobori, H.; Miyazaki, T.; Suzuki, H.; Nishiyama, A.; Ishii, N.; Yamashita, M.; Hayashi, M. Klotho Protein Supplementation Reduces Blood Pressure and Renal Hypertrophy in Db/Db Mice, a Model of Type 2 Diabetes. Acta Physiol. 2019, 225, e13190. [Google Scholar] [CrossRef]
- Pedersen, L.; Pedersen, S.M.; Brasen, C.L.; Rasmussen, L.M. Soluble Serum Klotho Levels in Healthy Subjects. Comparison of Two Different Immunoassays. Clin. Biochem. 2013, 46, 1079–1083. [Google Scholar] [CrossRef]
- Moreno, J.A.; Izquierdo, M.C.; Sanchez-Niño, M.D.; Suárez-Alvarez, B.; Lopez-Larrea, C.; Jakubowski, A.; Blanco, J.; Ramirez, R.; Selgas, R.; Ruiz-Ortega, M.; et al. The Inflammatory Cytokines TWEAK and TNFα Reduce Renal Klotho Expression through NFκB. J. Am. Soc. Nephrol. 2011, 22, 1315–1325. [Google Scholar] [CrossRef]
- Grabner, A.; Faul, C. The Role of Fibroblast Growth Factor 23 and Klotho in Uremic Cardiomyopathy. Curr. Opin. Nephrol. Hypertens. 2016, 25, 314–324. [Google Scholar] [CrossRef]
- de Boer, R.A.; van Veldhuisen, D.J.; Gansevoort, R.T.; Muller Kobold, A.C.; van Gilst, W.H.; Hillege, H.L.; Bakker, S.J.L.; van der Harst, P. The Fibrosis Marker Galectin-3 and Outcome in the General Population. J. Intern. Med. 2012, 272, 55–64. [Google Scholar] [CrossRef]
- Salgado, J.V.; Goes, M.A.; Salgado Filho, N. FGF21 and Chronic Kidney Disease. Metabolism 2021, 118, 154738. [Google Scholar] [CrossRef]
- Sanz, A.B.; Sanchez-Niño, M.D.; Ortiz, A. TWEAK, a Multifunctional Cytokine in Kidney Injury. Kidney Int. 2011, 80, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A.B.; Aroeira, L.S.; Bellon, T.; del Peso, G.; Jimenez-Heffernan, J.; Santamaria, B.; Sanchez-Niño, M.D.; Blanco-Colio, L.M.; Lopez-Cabrera, M.; Ruiz-Ortega, M.; et al. TWEAK Promotes Peritoneal Inflammation. PLoS ONE 2014, 9, e90399. [Google Scholar] [CrossRef]
- Blanco-Colio, L.M.; Martín-Ventura, J.L.; Muñóz-García, B.; Orbe, J.; Páramo, J.A.; Michel, J.-B.; Ortiz, A.; Meilhac, O.; Egido, J. Identification of Soluble Tumor Necrosis Factor-Like Weak Inducer of Apoptosis (STWEAK) as a Possible Biomarker of Subclinical Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Donderski, R.; Stróżecki, P.; Sulikowska, B.; Grajewska, M.; Trafny, R.; Bodnar, M.; Marszałek, A.; Stefańska, A.; Siódmiak, J.; Odrowąż-Sypniewska, G.; et al. Assessment of Peritoneal Membrane Arteriolar Structure in Conjunction with Traditional Cardiovascular System Evaluation in Chronic Kidney Disease (CKD) Stage 5 Patients. Kidney Blood Press. Res. 2018, 43, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Vila Cuenca, M.; Hordijk, P.L.; Vervloet, M.G. Most Exposed: The Endothelium in Chronic Kidney Disease. Nephrol. Dial. Transplant. 2020, 35, 1478–1487. [Google Scholar] [CrossRef]
- Hu, M.C.; Shi, M.; Cho, H.J.; Adams-Huet, B.; Paek, J.; Hill, K.; Shelton, J.; Amaral, A.P.; Faul, C.; Taniguchi, M.; et al. Klotho and Phosphate Are Modulators of Pathologic Uremic Cardiac Remodeling. J. Am. Soc. Nephrol. 2015, 26, 1290–1302. [Google Scholar] [CrossRef]
- Kuro-o, M. Klotho and Calciprotein Particles as Therapeutic Targets against Accelerated Ageing. Clin. Sci. 2021, 135, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Li, Y.M.; Imani, F.; Wojciechowicz, D.; Yang, Z.; Liu, F.T.; Cerami, A. Identification of Galectin-3 as a High-Affinity Binding Protein for Advanced Glycation End Products (AGE): A New Member of the AGE-Receptor Complex. Mol. Med. 1995, 1, 634–646. [Google Scholar] [CrossRef]
- Masola, V.; Bonomini, M.; Borrelli, S.; Di Liberato, L.; Vecchi, L.; Onisto, M.; Gambaro, G.; Palumbo, R.; Arduini, A. Fibrosis of Peritoneal Membrane as Target of New Therapies in Peritoneal Dialysis. Int. J. Mol. Sci. 2022, 23, 4831. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Dounousi, E.; Salmas, M.; Eleftheriadis, T.; Liakopoulos, V. Unfavorable Effects of Peritoneal Dialysis Solutions on the Peritoneal Membrane: The Role of Oxidative Stress. Biomolecules 2020, 10, 768. [Google Scholar] [CrossRef]
- Lau, E.S.; Liu, E.; Paniagua, S.M.; Sarma, A.A.; Zampierollo, G.; Lopez, B.; Diez, J.; Wang, T.J.; Ho, J.E. Galectin-3 Inhibition with Modified Citrus Pectin in Hypertension. JACC Basic Transl. Sci. 2021, 6, 12–21. [Google Scholar] [CrossRef] [PubMed]
- NCT05240131. A Study to Investigate the Safety and Efficacy of GB1211 (a Galectin-3 Inhibitor) in Combination with Atezolizumab in Patients with Non-Small Cell Lung Cancer (NSCLC). Available online: https://clinicaltrials.gov (accessed on 30 August 2022).
- NCT02800629. Blocking Extracellular Galectin-3 in Patients with Osteoarthritis. Available online: https://clinicaltrials.gov (accessed on 30 August 2022).
- National Kidney Foundation Kidney Disease Outcomes Quality Initiative. NKF-K/DOQI Clinical Practice Guidelines for Peritoneal Dialysis Adequacy: Update 2000. Am. J. Kidney Dis. 2001, 37, S65–S136. [Google Scholar] [CrossRef]
- Blake, P.G.; Bargman, J.M.; Brimble, K.S.; Davison, S.N.; Hirsch, D.; McCormick, B.B.; Suri, R.S.; Taylor, P.; Zalunardo, N.; Tonelli, M. Clinical Practice Guidelines and Recommendations on Peritoneal Dialysis Adequacy 2011. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2011, 31, 218–239. [Google Scholar] [CrossRef] [PubMed]
- Twardowski, Z. Peritoneal equilibration test. Perit. Dial. Int. 1987, 7, 138–148. [Google Scholar]
- Rolfson, D.B.; Majumdar, S.R.; Tsuyuki, R.T.; Tahir, A.; Rockwood, K. Validity and Reliability of the Edmonton Frail Scale. Age Ageing 2006, 35, 526–529. [Google Scholar] [CrossRef]
- Fihn, S.D.; Blankenship, J.C.; Alexander, K.P.; Bittl, J.A.; Byrne, J.G.; Fletcher, B.J.; Fonarow, G.C.; Lange, R.A.; Levine, G.N.; Maddox, T.M.; et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS Focused Update of the Guideline for the Diagnosis and Management of Patients with Stable Ischemic Heart Disease. Circulation 2014, 130, 1749–1767. [Google Scholar] [CrossRef]
- McDonagh, T.; Damy, T.; Doehner, W.; Lam, C.S.P.; Sindone, A.; van der Meer, P.; Cohen-Solal, A.; Kindermann, I.; Manito, N.; Pfister, O.; et al. Screening, Diagnosis and Treatment of Iron Deficiency in Chronic Heart Failure: Putting the 2016 European Society of Cardiology Heart Failure Guidelines into Clinical Practice. Eur. J. Heart Fail. 2018, 20, 1664–1672. [Google Scholar] [CrossRef]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Smith, D.; English, J.; Jonhston, C. Cerebrovascular Disease. In Harrisons Manual of Medicine; McGraw-Hill: New York, NY, USA, 2012; Volume 2, pp. 3270–3271. [Google Scholar]
- Therneau, T. A Package for Survival Analysis in R, R Package Version 3.5-3. 2023. Available online: https://CRAN.R-project.org/package=survival (accessed on 30 August 2022).
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000; ISBN 978-1-4419-3161-0. [Google Scholar]
- Hothorn, T.; Lausen, B. Bagging Tree Classifiers for Laser Scanning Images: A Data- and Simulation-Based Strategy. Artif. Intell. Med. 2003, 27, 65–79. [Google Scholar] [CrossRef]
- Hothorn, T.; Lausen, B. Maxstat:Maximally Selected Rank Statistics, R Package Version 07-25; R News. 2002; Volume 2, pp. 3–5. Available online: https://cran.r-project.org/web/packages/maxstat/maxstat.pdf (accessed on 30 August 2022).
| Study Variable | Biopsy Score | ||||
|---|---|---|---|---|---|
| S0 (n = 24) | S1 (n = 12) | S2 (n = 22) | p | ||
| Anthropometric & Clinical data | Women, n (%) | 10 (42) | 4 (33) | 4 (18) | ns |
| Age (years old) | 59 (43–68) | 61 (35–69) | 54 (45–72) | ns | |
| Diabetes mellitus, n (%) | 4 (17) | 3 (25) | 10 (46) | ns | |
| Arterial hypertension, n (%) | 15 (63) | 6 (50) | 14 (74) | ns | |
| Coronaryischemic disease, n (%) | 4 (17) | 4 (33) | 9 (43) | ns | |
| Cerebrovasculardisease, n (%) | 2 (8) | 1 (8) | 1 (5) | ns | |
| Cardiac failure, n (%) | 2 (8) | 1 (8) | 3 (14) | ns | |
| Peripheralarterialdisease, n (%) | 3 (13) | 2 (17) | 12 (55) | 0.004 | |
| PD Prescription & Therapeutics | Icodextrin, n (%) | 6 (25) | 5 (42) | 8 (36) | ns |
| Use of amino acid solution, n (%) | 0 (0) | 2 (17) | 1 (5) | ns | |
| Glucose applied (g/day) | 120 (114–137) | 120 (98–120) | 120 (90–120) | ns | |
| Spironolactone, n (%) | 2 (8) | 1 (8) | 15 (68) | <0.001 | |
| Beta-blockers, n (%) | 2 (8) | 3 (25) | 7 (32) | ns | |
| Other antihypertensives, n (%) | 13 (54) | 7 (58) | 10 (46) | ns | |
| Vitamin D analogues, n (%) | 15 (63) | 10 (83) | 16 (73) | ns | |
| non-calcium Phosphate binders, n (%) | 3 (13) | 3 (25) | 6 (27) | ns | |
| Vitamin D3 supplements, n (%) | 3 (13) | 3 (13) | 5 (23) | ns | |
| Cinacalcet, n (%) | 6 (25) | 3 (25) | 6 (27) | ns | |
| Antiplatelettherapy, n (%) | 5 (21) | 4 (33) | 14 (64) | 0.011 | |
| Statins, n (%) | 5 (21) | 4 (33) | 14 (64) | 0.011 | |
| Erythropoietin/darbepoetin, n (%) | 13 (54) | 5 (42) | 16 (73) | ns | |
| PD-parameters, Nutrition Status & Frailty | Peritoneal transport (H, HA, L, LA), n (%) | 1/14/6/3 (4/58/25/12) | 0/7/4/1 (0/58/33/8) | 1/11/9/1 (5/50/41/5) | ns |
| CA 125 (UI/L) | 25.7 (10.8–33.0) | 16.5 (11.0–37.3) | 16.5 (11.7–23.9) | ns | |
| nPCR (g/Kg/day) | 1.0 (0.76–1.1) | 0.89 (0.79–1.1) | 0.99 (0.79–1.1) | ns | |
| Kt/V | 2.6 (2.1–3.2) | 2.7 (1.8–3.2) | 2.7 (2.0–3.3) | ns | |
| rGFR (mL/min/1.73 m2) | 6.2 (3.6–9.8) | 5.9 (1.1–10.7) | 7.9 (5.9–11.2) | ns | |
| Residual Diuresis (mL) | 1700 (1.25–2350) | 1350 (280–1650) | 1700 (1300–2600) | ns | |
| Non-Frail, n (%) | 18 (75) | 9 (75) | 15 (68) | ns | |
| Study Variable | PD Failure | MACE | |||||
|---|---|---|---|---|---|---|---|
| No (n = 34) | Yes (n = 24) | p | No (n = 31) | Yes (n = 27) | p | ||
| Anthropometric and Clinical data | Women | 10 (29) | 8 (33) | ns | 12 (39) | 6 (22) | ns |
| Age (years old) | 51.0 (37.8–65.5) | 65.0 (53.5–71.8) | 0.006 | 51.0 (37.0–63.0) | 67.0 (52.0–72.0) | 0.003 | |
| Diabetes mellitus | 10 (29) | 7 (29) | ns | 3 (10) | 14 (52) | <0.001 | |
| Arterial hypertension | 21 (66) | 14 (61) | ns | 19 (63) | 16 (64) | ns | |
| Coronary ischemic disease | 10 (30) | 7 (29) | ns | 0 (0) | 17 (65) | <0.001 | |
| Cerebrovascular disease | 3 (9) | 1 (4) | ns | 0 (0) | 4 (15) | 0.038 | |
| Cardiac failure | 3 (9) | 3 (12) | ns | 0 (0) | 6 (23) | 0.006 | |
| Peripheral arterial disease | 7 (21) | 10 (42) | ns | 0 (0) | 17 (63) | <0.001 | |
| rGFR (mL/min/1.73 m2) | 7.9 (5.6–10.2) | 6.1 (2.6–10.3) | ns | 8.9 (5.3–10.4) | 6.0 (2.6–9.3) | ns | |
| Residual Diuresis (mL) | 1700 (1225–2350) | 1500 (600–2500) | ns | 1700 (1300–2400) | 1500 (1000–2350) | ns | |
| PD and Therapeutics Prescription | Icodextrin | 14 (41) | 5 (21) | ns | 10 (32) | 9 (33) | ns |
| Use of amino acid solution | 2 (6) | 1 (4) | ns | 1 (3) | 2 (7) | ns | |
| Glucose applied | 120.0 (90.0–120.0) | 120.0 (120.0–143.8) | ns | 120.0 (90.0–135.0) | 120.0 (90.0–120.0) | ns | |
| Spironolactone | 9 (27) | 9 (38) | ns | 8 (26) | 10 (37) | ns | |
| Beta–blockers | 7 (21) | 5 (21) | ns | 2 (6) | 10 (37) | 0.004 | |
| Calcium channels blockers | 12 (35) | 18 (75) | 0.003 | 16 (52) | 14 (52) | ns | |
| Vitamin D analogues | 26 (77) | 18 (75) | ns | 23 (74) | 21 (78) | ns | |
| Non–calcium Phosphate binders | 9 (27) | 11 (46) | ns | 9 (29) | 11 (41) | ns | |
| Vitamin D3 supplements | 24 (71) | 17 (71) | ns | 20 (64) | 21 (78) | ns | |
| Cinacalcet | 8 (24) | 7 (29) | ns | 9 (29) | 6 (22) | ns | |
| Antiplatelet therapy | 10 (30) | 8 (33) | ns | 0 (0) | 18 (69) | <0.001 | |
| Statins | 10 (30) | 8 (33) | ns | 0 (0) | 18 (69) | <0.001 | |
| Erythropoietin/darbepoetin | 17 (50) | 17 (71) | ns | 15 (48) | 19 (70) | ns | |
| PD-related parameters, Frailty | Peritoneal transport (High) | 20 (59) | 14 (58) | ns | 19 (61) | 15 (56) | ns |
| CA 125 (UI/L) | 14.3 (9.8–25.0) | 25.2 (13.5–36.5) | ns | 15.6 (10.5–32.3) | 16.9 (12.0–36.9) | ns | |
| nPCR (g/Kg/day) | 1.0 (0.81–1.1) | 0.87 (0.77–1.1) | ns | 1.1 (0.88–1.2) | 0.83 (0.78–1.0) | 0.023 | |
| Kt/V | 2.9 (2.1–3.3) | 2.4 (1.9–2.9) | ns | 2.9 (2.4–3.4) | 2.4 (1.8–3.1) | 0.017 | |
| Frail | 4 (12) | 7 (29) | ns | 3 (10) | 8 (30) | ns | |
| Score de Edmonton | 2 (2–3) | 3.5 (2–6) | 0.038 | 2 (2–3) | 4 (2–6) | <0.001 | |
| Membrane Fibrosis and Serum biomarkers | α–Klotho (pg/mL) | 779 (589–1016) | 724 (626–1090) | ns | 803 (640–1119) | 698 (548–958) | ns |
| Galectin–3 (ng/mL) | 9.4 (7.50–10.9) | 10.4 (9.2–11.0) | 0.048 | 9.9 (8.6–10.8) | 10.2 (8.6–11.1) | ns | |
| FGF21(pg/mL) | 1324 (834–2226) | 1435 (980–3135) | ns | 1410 (967–2061) | 1336 (921–2507) | ns | |
| FGF23 (pg/mL) | 748.9 (525.9–862.4) | 744.2 (649.2–848.6) | ns | 685.9 (578.3–858.8) | 761.9 (570.9–850.5) | ns | |
| TWEAK (pg/mL) | 0.14 (0.06–0.49] | 0.12 (0.05–0.28) | ns | 0.11 (0.06–0.48) | 0.16 (0.06–0.27) | ns | |
| TNF–α (pg/mL) | 0.18 (0.13–0.24) | 0.16 (0.14–0.20) | ns | 0.17 (0.12–0.19) | 0.17 (0.15–0.25) | ns | |
| hs–CRP (μg/mL) | 0.35 (0.17–0.48) | 0.39 (0.17–0.90) | ns | 0.39 (0.16–0.54) | 0.35 (0.19–0.63) | ns | |
| Peritoneal membrane fibrosis | 13 (38) | 9 (38) | ns | 5 (16) | 17 (63) | <0.001 | |
| STM | 95.0 (40.0–190.0) | 60.0 (20.0–200.0) | ns | 60.0 (30.0–110.0) | 190.0 (50.0–200.0) | 0.004 | |
| α–Klotho < 742 pg/mL | 16 (47) | 13 (54) | ns | 12 (39) | 17 (63) | ns | |
| Study Variables | Time until Event (Month) HR (95% CI), p Value | ||
|---|---|---|---|
| PD Failure | MACE | ||
| Anthropometric and Clinical data | Women | ns | ns |
| Age (years old) | p = 0.08 | 1.044 (1.012–1.077), p = 0.04 | |
| Diabetes mellitus | ns | 3.717 (1.732–7.978), p = 0.01 | |
| Arterial hypertension | ns | ns | |
| Coronary ischemic disease | ns | 10.063 (4.239–23.894), p = 0.001 | |
| Cerebrovascular disease | ns | 4.206 (1.409–12.582), p = 0.27 | |
| Cardiac failure | ns | p = 0.059 | |
| Peripheral arterial disease | 2.432 (1.066–5.552), p = 0.035 | 8.875 (3.890, 20.248), p = 0.001 | |
| GFRr (mL/min/1.73 m2) | ns | p = 0.110 | |
| Residual Diuresis (mL) | ns | ns | |
| PD and Therapeutics Prescription | Icodextrin | p = 0.099 | ns |
| Use of amino acid solution | ns | ns | |
| Glucose applied | ns | ns | |
| Spironolactone | ns | ns | |
| Beta-blockers | ns | 3.518 (1.579–7.838), p = 0.001 | |
| Calcium channels blockers | 2.88 (1.139–7.236), p = 0.017 | ns | |
| Vitamin D analogues | ns | ns | |
| Non-calcium Phosphate binders | ns | ns | |
| Vitamin D3 supplements | ns | ns | |
| Cinacalcet | ns | ns | |
| Antiplatelet therapy | ns | 12.153 (4.982–29.745), p = 0.001 | |
| Statins | ns | ns | |
| Erythropoietin/darbepoetin | ns | ns | |
| PD-related parameters, Frailty | Peritoneal transport (High) | ns | ns |
| CA 125 (UI/L) | ns | ns | |
| nPCR (g/Kg/day) | ns | 0.093 (0.011–0.802), p = 0.024 | |
| Kt/V | p = 0.078 | 0.475 (0.260–0.869), p = 0.013 | |
| Frail | p = 0.093 | p = 0.073 | |
| Score de Edmonton | p = 0.150 | p = 0.051 | |
| Membrane Fibrosis and Serum biomarkers | α-Klotho (pg/mL) | ns | p = 0.055 |
| Galectin-3 (ng/mL) | 1.271 (0.988–1.635), p = 0.042 | ns | |
| FGF21(pg/mL) | p = 0.146 | ns | |
| FGF23 (pg/mL) | ns | ns | |
| TWEAK (pg/mL) | p = 0.118 | ns | |
| TNF-α (pg/mL) | p = 0.087 | ns | |
| hs-CRP (μg/mL) | ns | ns | |
| Peritoneal membrane fibrosis | ns | 4.181 (1.905–9.175), p = 0.001 | |
| STM | ns | 1.009 (1.003–1.014), p = 0.001 | |
| α-Klotho < 742 pg/mL | ns | p = 0.055 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branco, P.; Calça, R.; Martins, A.R.; Mateus, C.; Jervis, M.J.; Gomes Pinto, D.; Azeredo-Lopes, S.; De Melo Junior, A.F.; Sousa, C.; Civantos, E.; et al. Fibrosis of Peritoneal Membrane, Molecular Indicators of Aging and Frailty Unveil Vulnerable Patients in Long-Term Peritoneal Dialysis. Int. J. Mol. Sci. 2023, 24, 5020. https://doi.org/10.3390/ijms24055020
Branco P, Calça R, Martins AR, Mateus C, Jervis MJ, Gomes Pinto D, Azeredo-Lopes S, De Melo Junior AF, Sousa C, Civantos E, et al. Fibrosis of Peritoneal Membrane, Molecular Indicators of Aging and Frailty Unveil Vulnerable Patients in Long-Term Peritoneal Dialysis. International Journal of Molecular Sciences. 2023; 24(5):5020. https://doi.org/10.3390/ijms24055020
Chicago/Turabian StyleBranco, Patrícia, Rita Calça, Ana Rita Martins, Catarina Mateus, Maria João Jervis, Daniel Gomes Pinto, Sofia Azeredo-Lopes, Antonio Ferreira De Melo Junior, Cátia Sousa, Ester Civantos, and et al. 2023. "Fibrosis of Peritoneal Membrane, Molecular Indicators of Aging and Frailty Unveil Vulnerable Patients in Long-Term Peritoneal Dialysis" International Journal of Molecular Sciences 24, no. 5: 5020. https://doi.org/10.3390/ijms24055020
APA StyleBranco, P., Calça, R., Martins, A. R., Mateus, C., Jervis, M. J., Gomes Pinto, D., Azeredo-Lopes, S., De Melo Junior, A. F., Sousa, C., Civantos, E., Mas-Fontao, S., Gaspar, A., Ramos, S., Morello, J., Nolasco, F., Rodrigues, A., & Pereira, S. A. (2023). Fibrosis of Peritoneal Membrane, Molecular Indicators of Aging and Frailty Unveil Vulnerable Patients in Long-Term Peritoneal Dialysis. International Journal of Molecular Sciences, 24(5), 5020. https://doi.org/10.3390/ijms24055020







