Abstract
The liver is frequently exposed to potentially toxic materials, and it is the primary site of clearance of foreign agents, along with many innate and adaptive immune cells. Subsequently, drug induced liver injury (DILI), which is caused by medications, herbs, and dietary supplements, often occurs and has become an important issue in liver diseases. Reactive metabolites or drug–protein complexes induce DILI via the activation of various innate and adaptive immune cells. There has been a revolutionary development of treatment drugs for hepatocellular carcinoma (HCC) and liver transplantation (LT), including immune checkpoint inhibitors (ICIs), that show high efficacy in patients with advanced HCC. Along with the high efficacy of novel drugs, DILI has become a pivotal issue in the use of new drugs, including ICIs. This review demonstrates the immunological mechanism of DILI, including the innate and adaptive immune systems. Moreover, it aims to provide drug treatment targets, describe the mechanisms of DILI, and detail the management of DILI caused by drugs for HCC and LT.
1. Introduction
Drug-induced liver injury (DILI), an injury to the liver or biliary system caused by medications, herbs, or dietary supplements, accounts for 50% of acute liver failure cases in the United States [1,2]. DILI is classified as intrinsic (or direct) or idiosyncratic according to its pathogenesis [3]. Intrinsic DILI, which is predictable and acute-onset, occurs in a dose-dependent manner and can be reproduced in animal models [2,4]. However, idiosyncratic DILI, the most frequent type, is unpredictable and not dose-related DILI, although a minimum dose of 50 mg/day is usually required for its development [5].
The incidence of DILI varies by study design and cohort. Retrospective cohorts show lower incidence rates of DILI than prospective studies. According to several prospective studies, the annual incidence of DILI is approximately 13.9–19.1 per 100,000 inhabitants [6,7]. DILI can be influenced by multiple factors, such as age, sex, environmental exposure, and genetics, including human leukocyte antigen (HLA) [8,9]. Its diagnosis is based on an appropriate temporal relationship between drug intake and liver injury, along with the exclusion of other possible causes of liver damage, including viral infection and alcohol consumption [2]. The Roussel Uclaf Causality Assessment Method (RUCAM) is the most widely used assessment scale for DILI [10]. Moreover, according to elevated liver enzyme levels, represented as the alanine aminotransferase (ALT)/alkaline phosphatase (ALP) ratio (R), DILI patterns can be determined as follows: hepatocellular pattern (R ≥ 5), cholestatic pattern (R ≤ 2), and mixed pattern (2 > R < 5) [2,11]. Recently, the updated RUCAM of 2016 was introduced to improve the diagnostic accuracy of DILI [12]. According to the updated RUCAM, assessment of DILI is differently suggested according to the pattern of DILI using ALT/ALP ratio (R) at first presentation. The updated RUCAM also presents a check list of differential diagnosis of DILI and criteria for a positive result of DILI following unintentional re-exposure [12]. The diagnosis of DILI can be confounded by several factors, including comedication and concomitant diseases; therefore, causality assessment using the updated RUCAM is important.
Recent studies have suggested that specific human leukocyte antigen (HLA) genotypes, such as HLA-B*5701, are risk factors for the development of DILI in patients receiving some drugs [13,14]. However, HLA genotypes cannot sufficiently explain the risk of DILI. Moreover, microsomal cytochrome P450 (CYP) also play a role in the development of DILI [14]. As CYP is involved in the metabolism of many drugs, various isoforms of CYP, including CYP3A4, may be associated with the development of DILI [15]. Population-based studies have also demonstrated that pre-existing liver disease, concomitant severe skin reactions, and comedications, such as nonsteroidal anti-inflammatory drugs, are associated with the development DILI [6,16]. Furthermore, ferroptosis can also be a potential factor in the pathogenesis of DILI [17]. Ferroptosis, an iron-dependent form of cell death, reduces cystine uptake causing the production of lethal reactive oxygen species, which can lead to the development of DILI [18].
Regarding immunologic perspective, the liver is the primary site of the clearance of foreign chemical agents; thus, it is exposed to many potentially toxic chemicals that can cause hepatocyte damage via mitochondrial dysfunction and oxidative stress [19]. In addition, the liver is an immune organ with abundant innate (e.g., neutrophils, natural killer [NK] cells, and Kupffer cells) and adaptive (T cells and B cells) immune cells [20]. Although the liver is an immunologically tolerant organ, immune responses, including innate and adaptive immune cells, play pivotal roles in the development of DILI. Tyrosine kinase inhibitors (TKIs), such as sorafenib, lenvatinib, and regorafenib, were developed to treat advanced hepatocellular carcinoma (HCC). Moreover, recent studies have demonstrated the high efficacy of immune checkpoint inhibitors (ICIs), including atezolizumab plus bevacizumab, in HCC [21,22]. Along with the high efficacy of these novel drugs, DILI has become a critical issue in ICI use.
In this review, we discuss the immunological perspective of the mechanism of DILI, including the innate and adaptive immune systems (Figure 1). Moreover, we describe the frequency, hepatobiliary manifestations, and mechanism of DILI in patients with HCC treated with TKIs and ICIs. We also demonstrate the development of DILI in liver transplant (LT) patients administered immunosuppressants (ISs).
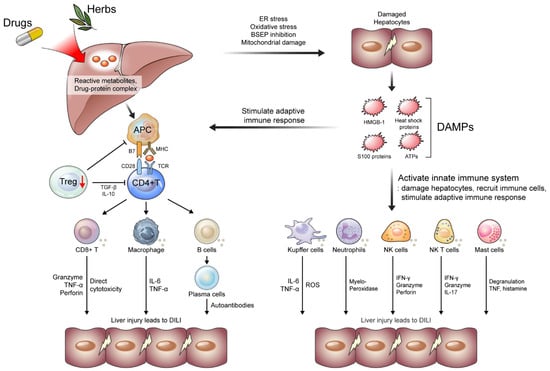
Figure 1.
Mechanisms of the development of drug-induced liver injury (DILI). Reactive metabolites or drug–protein complex causes ER and oxidative stress in hepatocytes. BSEP inhibition and mitochondrial damage also damage hepatocytes, leading to the secretion of DAMPs, including HMGB-1, heat shock proteins, S100 proteins, and ATPs. DAMPs activate innate immune systems and stimulate immune response. Activated innate immune systems (e.g., Kupffer cells, neutrophils, NK cells, NK T cells, and Mast cells) damage hepatocytes, recruit immune cells, and stimulate adaptive immune response. Reactive metabolites or drug–protein complexes are presented by APCs, which lead to activation of adoptive immune response (e.g., T cells and B cells) along with the stimulation of APCs by DAMPs. Meanwhile, Treg cells decrease and fail to maintain immune tolerance. APC, antigen presenting cells; ATPs, adenosine triphosphate; BSEP, bile salt export pump; DAMP, damage-associated molecular patterns; ER, endoplasmic reticulum; HMGB, high-mobility group box; IFN, interferon; IL, interleukin; NK, natural killer; TNF, tumor necrosis factor; Treg, regulatory T cells.
2. Immunological Perspective on DILI Mechanism
2.1. Danger Hypothesis
T cell-mediated liver injury is the cornerstone of DILI development [23]. The hapten hypothesis, which suggests that haptens make the proteins “foreign” and lead to their recognition and destruction by the immune system, was introduced to explain this immune response [24]. However, this hypothesis is insufficient to support the strong immune response in DILI. Subsequently, the danger hypothesis was proposed to redeem the hapten hypothesis. The generation of reactive metabolites or drug–protein complexes damages hepatocytes via several pathways, including oxidative stress, endoplasmic reticulum (ER) stress, bile salt export pump (BSEP) inhibition, and mitochondrial damage [3,25]. Damaged hepatocytes release several damage-associated molecular patterns (DAMPs), such as high-mobility group box (HMGB)-1, heat shock proteins, S100 proteins, and ATPs, which play a pivotal role in the activation of antigen-presenting cells (APCs) by producing a second signal (interaction of CD28 with B7 molecules) [26]. This co-stimulation often refers to a “danger signal” according to the danger hypothesis. Activated APCs lead to the activation of adaptive immune responses, including CD4+, CD8+, and B cells, which cause idiosyncratic DILI [26] (Figure 1).
2.2. Innate Immune Systems in DILI
As discussed above, reactive metabolites or drug–protein complexes can damage hepatocytes via ER and oxidative stress, inhibition of BSEP, and mitochondrial damage [3,25]. Damaged hepatocytes secrete DAMPs, including HMGB-1, heat shock proteins, S100 proteins, and ATPs, which activate the innate immune system and stimulate the immune response [27]. Activated innate immune systems (e.g., Kupffer cells, neutrophils, NK cells, and NK T cells) damage hepatocytes, recruit immune cells, and stimulate adaptive immune response during DILI (Figure 1) [28].
2.2.1. Kupffer Cells
Kupffer cells, resident macrophages in the liver, are important in DILI development. They play key roles in phagocytosis, antigen presentation, and pro-inflammatory cytokines [29]. Traditionally, Kupffer cells can be classified into two types as follows: M1, Kupffer cells that secrete pro-inflammatory cytokines, such as interleukin (IL)-6 and tumor necrosis factor alpha (TNF-α); M2, Kupffer cells secreting potent immunosuppressive cytokines [30,31]. During DILI, Kupffer cells are activated by DAMPs and release pro-inflammatory cytokines and reactive oxygen radicals, along with infiltrated macrophages [32]. Kupffer cells also produce chemokine ligands to recruit monocyte-derived macrophages to the liver during the early phase of inflammation [33]. Activated Kupffer cells can exacerbate liver injury through these pathways.
2.2.2. Neutrophils
Neutrophils, the first-line responders to bacterial and fungal infections, are the most abundant fraction of the innate immune cell group [34]. They defend against infection via phagocytosis, degranulation, and extracellular trapping [35]. Granulocyte colony-stimulating factor is a key regulator of neutrophil generation and maturation. The gut microbiome and metabolites may also play a role in neutrophil function [36]. During infection and inflammation, neutrophils are recruited to the site of inflammation via cytokine and chemokine production [34]. Neutrophils extravasate into the liver parenchyma via chemotactic signal from hepatocytes and other extravasated neutrophils. Extravasated neutrophils directly contact hepatocytes and trigger neutrophil activation. Eventually, abnormally activated neutrophils promote oxidative stress, mitochondrial dysfunction, and necrotic cell death, which can lead to acute liver injury during DILI [36]. Liver injury can be exacerbated by oxidative stress, involving myeloperoxidase and proteolytic enzymes [35,37].
2.2.3. NK Cells
NK cells, the key players in liver immunity, are abundant in the liver, constituting 30–50% of intrahepatic lymphocytes [38]. NK cells have cytotoxic functions and express immunomodulatory cytokines, such as IL-1β, IL-2, IFN-γ, and TNF-α, which can be categorized into subsets according to their characteristics, including cytokines and cytotoxic capabilities [39,40]. These functions can also mediate DILI pathogenesis. The release of cytotoxic granzymes and perforin along with the production of TNF-α and IFN-γ can result in liver injury during DILI [41,42]. The IFN-γ production can mediate the infiltration of immune cells and release of cytokines, which results in hepatocyte apoptosis during DILI [42,43].
2.2.4. NK T Cells
NK T (NKT) cells are unique lymphocytes that have both T and NK cell properties in their phenotype and function [44,45]. NKT cells, characterized by semi-variant T cell receptors (TCRs) and the major histocompatibility complex class I-like molecule CD1d, are pivotal in immunity against pathogens, bridging innate and acquired immunity [46,47,48]. These cells can be activated in both TCR-dependent and -independent manners and stimulate NKT cells to release cytokines, including IFN-γ and IL-17, which can recruit neutrophils, macrophages and activate adaptive immune responses, resulting in acute liver injury (DILI) [49,50]. However, studies have shown that NKT cells also have protective roles in liver injury and cancer immunology [28,51]. Recent studies have also demonstrated the potential role of the gut microbiome as a regulator of NKT cells, with further validation studies needed [51,52].
2.2.5. Mast Cells
Mast cells (MCs) originate from hematopoietic stem cells and play a role in the initiating the response of the innate immune system [53,54]. MCDs are activated by DAMPs, cytokines, and chemokines [55,56,57]. Activated MCs undergo degranulation and release histamines and TNF, which activate the innate immune systems and exacerbate inflammation [58,59,60]. This process stimulates hepatic stellate cells, Kupffer cells, and pro-fibrogenic signaling pathways, which aggravate liver damage and fibrosis [61,62]. Recent studies have also demonstrated that activated MCs affect T cell activation and contribute to adaptive immunity [63,64].
2.3. Adaptive Immune Systems in DILI
The adaptive immune response is stimulated by activated innate immune systems, released DAMPs, and APCs presenting reactive metabolites or drug–protein complexes. The adaptive immune response, a critical process in acute injuries, includes CD4+ and CD8+ T-cell responses and B cell-mediated humoral reactions [65]. During DILI, activated CD4+ and CD8+ T cell and B cells damage hepatocytes. Meanwhile, regulatory T (Treg) cells and their functions are decreased, exacerbating liver injury in DILI [65] (Figure 1).
2.3.1. CD4+ and CD8+ T Cells
Among T cells, CD4+ and CD8+ T cells are the main T lymphocytes in adaptive immune responses and are pivotal during liver injury [66]. The presentation of reactive metabolites or drug-protein complexes by APCs along with signal 2 activates CD4+ Th0 cells, which triggers a subsequent adaptive immune response [25,67]. Among subsets of CD4+ T cells, activated helper T (Th) 1 cells secrete IFN-γ, IL-2, and TNF-α and activate CD8+ T cells during DILI [68,69]. Th2 cells, an important subset of CD4+ T cells, release IL-4 and drive the proliferation and differentiation of B cells, which cause B cell-mediated humoral reactions [70,71]. Infiltrated CD8+ T cells, a major cell killer in adaptive immunity, have direct cytotoxic function and secrete granzymes, perforin, and cytokines, including TNF-α, IL-17 which cause cell death during DILI [65,72]. Indeed, infiltration of cytotoxic T cells (CTLs) may play an important role in fulminant drug-induced hepatic failure [73].
2.3.2. B Cells
B cells originate from hematopoietic stem cells in the bone marrow. After maturation, B cells migrate from the peripheral blood into the spleen and germinal center [74]. As in other liver diseases, B cells participate in immune response and hepatocyte damage during DILI. B cells account for 8% of intrahepatic lymphocytes, which are activated and mature into plasma cells [75]. Plasma cells produce antibodies against proteins and damage hepatocytes during DILI [76]. During DILI, plasma cells can also produce autoantibodies against native proteins, such as cytochrome P450, which exacerbates liver injury [77].
2.3.3. Treg Cells
Treg cells, accounting for 5–10% of CD4+ T cells, are crucial for maintaining immune homeostasis and tolerance in liver disease and transplantation [78,79,80]. Treg cells secrete IL-10 and TGF-β, suppressing the proliferation of CD4+ T and CD8+ T cells and secretion of IFN-γ [81]. Moreover, Treg cells inhibit the proliferation of Th17 cells and release of IL-17 [82]. A recent study demonstrated that Treg cells can be modulated by the gut microbiome in patients with autoimmune diseases, IBD, and transplantation, which might be associated with the pathogenesis of these diseases [83,84,85]. Indeed, a decrease in Treg cells induces an inflammatory response that leads to liver damage [86]. During DILI, intrahepatic Treg numbers and Foxp3 expression decrease, exacerbating liver injury with a decreased IL-10 level [87]. Increasing Treg cell numbers may alleviate liver injury via the secretion of IL-10 and TGF-β, which might be a treatment target for DILI [88,89].
3. DILI Caused by Drugs Treating HCC and LT
3.1. DILI Caused by Drugs Treating HCC
HCC remains a global burden, accounting for 800,000 deaths worldwide [90]. Despite the development of screening protocols and surgical or locoregional treatments for early HCC, diagnosis commonly occurs at the advanced stage [29]. Moreover, approximately half of all patients with HCC experience systemic therapies in their treatment history [91]. In the past decades, sorafenib, a TKI, has been used as the 1st line therapy for advanced HCC. Several TKIs, including lenvatinib, regorafenib, and cabozantinib, have been developed for the 1st and 2nd line treatment of advanced HCC [90]. Recently, immune checkpoint inhibitors, including atezolizumab plus bevacizumab, have shown high efficacy in the treatment of advanced HCC [21,22].
As described above, the liver contains various immune cell types, whose response to ICIs is mostly affected by the tumor microenvironment (TME), which is composed of Treg cells, tumor-associated macrophages (TAMs), cytotoxic T cells, myeloid-derived suppressive cells (MDSCs), and neutrophils [92,93]. The crosslinking between tumor cells and several immune cells causing an immuno-suppressive status has been a treatment target for ICIs to restore the immune response to HCC [94]. During ICI treatment, liver injury can be induced via direct or indirect immune pathways. In this section, we discuss the target, frequency, mechanism, and treatment of DILI caused by drugs for HCC (Table 1).

Table 1.
Hepatobiliary manifestation and frequency of drug-induced liver injury caused by drugs for treating hepatocellular carcinoma and liver transplantation.
3.1.1. Tyrosine Kinase Inhibitors
Several TKIs have been approved for treating advanced HCC (Table 1). Sorafenib targets vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDFGR), c-kit, and Raf, and it can inhibit cancer growth, progression, angiogenesis, and metastasis [95]. Lenvatinib is another multi-kinase inhibitor targeting VEGFR 1-3, PDFGR, fibroblast growth factor (FGF) receptors 1–3, RET, and KIT [96], and it showed non-inferior survival to and better progression-free survival than sorafenib [97]. Regorafenib, approved for HCC patients after sorafenib failure, also targets VEGFR 1-3, PDGFR, FGFR1-2, and RAF [98]. Cabozantinib has also been approved for sorafenib-experienced patients with HCC and targets VEGFR 1-3, MET, and RET [99]. These TKIs reinforce antitumor immunity by increasing dendritic cells (DCs), T-cell infiltration, and PD-1 expression on T cells. Moreover, TKIs also decrease pro-tumor immunity, such as a decrease in MDSCs, Treg cells, and M2 TAMs [100].
During treatment with TKIs, elevated serum aminotransferase levels are common (~50%); however, severe hepatitis with values greater than five times the upper limit of normal is rare [101]. However, several studies have reported that TKI-induced DILI is associated with progressive liver injury and failure [102,103]. Along with DILI, hand–foot syndrome and skin rash can be present in some patients who are administered TKIs, such as sorafenib and regorafenib [101,104,105]. In liver histopathology, hepatocellular necrosis is the most frequent manifestation of TKI-induced DILI, and immune-mediated hepatitis has also developed, including sorafenib-induced DILI [104]. Although the specific mechanism remains unclear, several TKIs, including sorafenib and regorafenib, are metabolized via the CYP 3A4 pathway, which may be associated with the production of a toxic intermediate (Figure 2) [101,105]. The direct effect of inhibition of cellular kinases, such as by lenvatinib and cabozantinib, can be another suggested mechanism for TKI-induced DILI [106,107]. TKI can also induce oxidative stress and apoptotic pathway activations, which can lead to immune response activation and TKI-induced DILI [104,108]. Moreover, several signal transduction pathways, including epidermal growth factor receptor and platelet-derived growth factor receptor, which interact with TKI, play pivotal roles in regulating DILI and are associated with TKI-induced DILI [109].
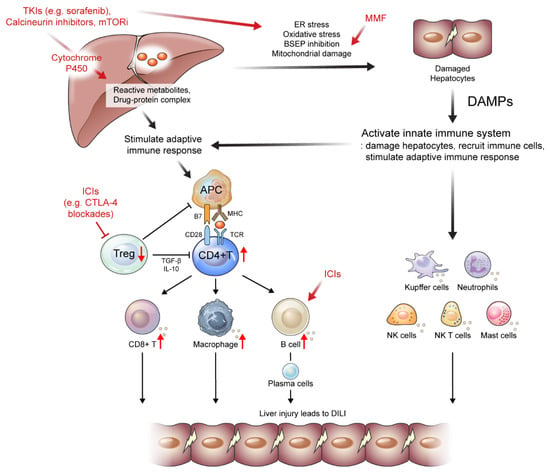
Figure 2.
Suggested mechanisms of drug-induced liver injury (DILI) caused by drugs administered to patients with hepatocellular carcinoma or liver transplantation. Several tyrosine kinase inhibitors (TKIs), calcineurin inhibitors, and mTOR inhibitors are metabolized via the cytochrome P450 pathway, which may be associated with the production of a toxic intermediate. These drugs can also induce oxidative stress and apoptotic pathway activations, which can lead to the activation of immune response. Mycophenolate mofetil can induce mitochondrial damage, which then leads to DILI. Immune checkpoint inhibitors (ICIs) deplete Treg cells inducing the reduction of anti-inflammatory cytokines and proliferation of CD8+ T cells. Moreover, early B cell changes may induce autoreactive B cells, leading to ICI-induced DILI. APC, antigen presenting cells; BSEP, bile salt export pump; DAMP, damage-associated molecular patterns; ER, endoplasmic reticulum; ICIs, immune checkpoint inhibitors; MMF, mycophenolate mofetil; mTORi, mammalian target of rapamycin inhibitors; NK, natural killer; TKI, tyrosine kinase inhibitors; Treg, regulatory T cells;↑, an increase in the indicated cells; ↓, a decrease in the indicated cells; ┤, the reduction and depletion of indicated cells.
Owing to the possibility of DILI, the Food and Drug Administration recommends monitoring liver function with the use of some TKIs, including regorafenib. As TKI-induced DILI usually recovers its discontinuation, appropriate monitoring and dose reduction or temporary cessation can successfully control TKI-induced DILI [101,104].
3.1.2. Immune Check Point Inhibitors
Recently, several ICIs have been approved for HCC treatment. Atezolizumab (an anti-PD-L1 antibody) plus bevacizumab (an anti-VEGF antibody) have changed the treatment landscape and paved the way for the combination therapy, with ICIs showing better overall survival than sorafenib [21]. Moreover, durvalumab (anti-PD-L1 antibody) and tremelimumab (anti-CTLA-4 antibody) also demonstrated superior survival rates compared with sorafenib [110]. In the second-line setting, pembrolizumab (anti-PD-1 antibody) monotherapy and nivolumab (anti-PD-1 antibody) plus ipilimumab (anti-CTLA4 antibody) have been approved for advanced-stage HCC [111,112] (Table 1).
The combination of anti-VEGF drugs with ICIs changes the tumor endothelium, increasing the infiltration of effector immune cells [113]. Moreover, combination therapy has a synergistic effect of increasing antitumor immune cell responses and inhibiting immunosuppressive pathways [114]. Indeed, ICIs that inhibit PD-1 or PD-L1 restore the function of effector CD8+ T cells [115]. CTLA-4 inhibitors activate naïve CD4+ and CD8+ T cells by promoting the interaction between costimulatory signals (B7 with CD28) [116]. Moreover, the addition of anti-VEGF drugs can show synergistic effects via several mechanisms, such as normalization of the vessel, which can lead to improvement in drug delivery and reduction in the immunomodulatory effect of VEGF on TAMs, MDSCs, Treg cells, and effector T cells [117].
ICI-induced DILI is an the immune-related adverse event, which is characterized by elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels [117]. Although the pattern of ICI-induced DILI is heterogeneous, the hepatocellular type is usually frequent [118]. Using the RUCAM model, ICI-induced DILI usually begins 8–12 weeks after ICI initiation, although ICI-induced DILI can occur at any time [119,120]. The incidence of ICI-induced DILI is known to be higher in patients treated with combination therapy (up to 18%) than in those treated with monotherapy (up to 9%) [120,121]. Moreover, as patients with HCC usually have chronic hepatitis or cirrhosis, the incidence of ICI-induced DILI is more frequent than that in patients without liver cancer [122]. According to type and dose of ICIs, the incidence of ICI-induced DILI in any grade ranges from 8% to 20% and is the highest in patients treated with the combination of anti-PD-1 and anti-CTLA4 antibodies [111,123,124,125]. In the diagnosis of ICI-induced DILI, it is essential to exclude other confounding factors, including co-medication, concomitant diseases, and hepatic metastasis, as well as to evaluate the possibility of ICI-induced DILI based on RUCAM [15,126]. Moreover, ICI-induced DILI should be differentiated from autoimmune hepatitis (AIH) [127]. ICI-induced DILI usually has a negative or low titer of antinuclear and anti-smooth muscle antibodies and does not have a female preponderance [120].
Several mechanisms have been proposed to explain ICI-induced DILI development (Table 2 and Figure 2). The first is the reduction and depletion of Treg cells, which are essential immune cells for maintaining tolerance induced by ICI treatment, especially in CTLA-4 blockades [128,129]. The depletion of Treg cells subsequently induces the reduction of anti-inflammatory cytokines and proliferation of CD8+ T cells [130,131]. Moreover, early B cell changes, including elevation of the CD21lo subtype, may induce autoreactive B cells, leading to ICI-induced DILI [132]. Representative histopathologic features of ICI-induced DILI are shown in Figure 2. Liver histopathology showed moderate portal inflammation with CD3+, CD4+, and CD8+ T cell infiltration along with periportal hepatocytic necrosis (Figure 3A–D). Predominant infiltration of histiocytes (CD68+ cells) was identified, along with mild infiltration of CD38+ cells, suggesting the presence of plasma cells (Figure 3E,F). ICI-induced DILI usually presents with lympho-histiocytic infiltration with lobular hepatitis, whereas AIH presents with interface hepatitis with plasma cell infiltration [120]. The gut microbiome may contribute to the development of immune-related adverse events (irAEs), especially immune-related colitis [133]. Gut microbial composition and their changes are associated with various liver disease and may influence the response to cancer immunotherapy [134,135,136]. In this context, the gut microbiota may be a biomarker for predicting irAEs including DILI. Further studies are needed to elucidate the specific pathogenic mechanisms underlying ICI-induced DILI.

Table 2.
Mechanism and treatment of drug-induced liver injury caused by immune checkpoint inhibitor use.
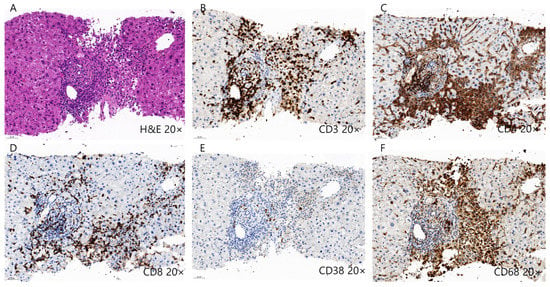
Figure 3.
Histopathology of drug-induced liver injury induced by immune checkpoint inhibitors. (A–D) Liver histopathology shows moderate lymphocytic infiltration, including CD3+, CD4+, and CD8+ T cells along with periportal hepatocytic necrosis. (E,F) Predominant infiltration of CD68+ histiocytes were also identified along with mild infiltration of CD38+ plasma cells.
ICI-induced DILI is asymptomatic in most cases; however, skin reactions (rashes) can occur in some patients [137]. Skin reactions are frequent irAEs after ICI treatment [138]. Moreover, irAEs frequently involve the gastrointestinal tract and endocrine organs, including the thyroid and lung [138]. The severity of ICI-induced DILI is classified according to the Common Terminology Criteria for Adverse Events (CTCAE) of the National Cancer Institute (Table 2) [139]. From grade 2, ICI-induced DILI is treated by stopping ICI along with corticosteroid [140,141]. In grade 2 DILI, 0.5–1 mg/kg/day of prednisolone is recommended, and in grades 3 and 4, the dose rises to 1–2 mg/kg/day of IV methylprednisolone [142]. High dose ursodeoxycholic acid (UDCA) can also be added for patients with cholestasis [118]. In patients with refractory to corticosteroids, mycophenolate mofetil (MMF), azathioprine, or tacrolimus have been used to improve liver function tests [142,143,144]. Although the time to resolution of ICI-induced DILI varies, patients with ICI-induced DILI usually recover within two weeks [145]. Reinduction of ICI after DILI can be applied to patients with grade 2 and 3 DILI, whereas patients with grade 4 DILI must permanently discontinue ICI [146]. Corticosteroids can increase the risk of bacterial infection; therefore, strict evaluation and diagnosis of ICI-induced DILI using the updated RUCAM are needed before the commencement of corticosteroid therapy [12,147]. Moreover, further studies are required to identify and validate predictors for ICI-induced DILI development.
3.2. DILI Casued by Drugs for Treating LT
Immunosuppressants
LT patients generally require life-long ISs due to the risk of graft rejection after LT [148,149]. The most used ISs are calcineurin inhibitors, mycophenolate mofetil (MMF), and the mammalian target of rapamycin inhibitors (mTORi) [150]. Of the calcineurin inhibitors, cyclosporine inhibits the activation of T cells by binding cyclophilin, whereas tacrolimus binds to intracellular proteins and inhibits calcineurin phosphatase activity [150]. Subsequently, the nuclear factors of activated T cells cannot move to the nucleus, which shuts down the production of IL-2, leading to a decrease in T-cell response [151]. MMF, another type of ISs, inhibits the formation of guanosine monophosphate by blocking inosine monophosphate dehydrogenase and suppressing T-cell proliferation [152,153]. The mechanism of action of mTORi, including sirolimus and everolimus, includes the inhibition of serine/threonine kinase activity, a family of phosphatidylinositol-3 kinases (PI3K), which inhibits the PI3K/Akt/mTOR signaling pathway, the transduction signal of IL-2 receptors, and T-cell proliferation [154,155] (Table 1).
Significant elevation of liver function, including AST and ALT, is not frequent with calcineurin inhibitors and mTORi [156,157]. Generally, the abnormalities in liver function tests caused by calcineurin inhibitors and mTORi are asymptomatic [158]. Mechanismically, calcineurin inhibitors and mTORi are mainly metabolized by the cytochrome P450 system (CYP 3A4), which may be associated with DILI. Liver injury can be caused by direct hepatotoxicity or activation of immune cells induced by its metabolites [156,157]. Only a small portion of patients receiving MMF treatment experience elevation in serum liver function [159]. MMF is not usually metabolized by cytochrome P450 enzymes, and MMF-induced DILI may be associated with mitochondrial damage and its immunogenic metabolites [160]. As IS-induced DILI is generally mild and self-limiting, dose reduction or pausing ISs can resolve DILI.
4. Conclusions
The liver contains many innate and adaptive immune cells and, during the development of DILI, reactive metabolites or drug–protein complexes initiate innate and adaptive immune responses, including neutrophils, Kupffer cells, NK cells, CD4+ T cells, CD8+ T cells, and B cells. Multiple activated immune cells damage hepatocytes, leading to DILI. Meanwhile, Treg cells and their functions are suppressed, exacerbating DILI. Understanding the underlying mechanism of DILI may provide clues for future treatment targets for DILI.
The TME, composed of Treg cells, TAMs, cytotoxic T cells, MDSCs, and neutrophils, affects HCC development and responses to TKIs and ICIs. Recently approved ICIs target PD-1/PD-L1 and CTLA-4 to restore the immune response in HCC. An activated immune response can cause irAEs, including DILI, via direct and indirect pathways. DILI caused by TKIs and ICIs is usually asymptomatic and recovers after drug discontinuation. ISs used in LT patients infrequently cause DILI and require regular tests to monitor of liver function. According to the degree of DILI, appropriate treatment with corticosteroids may be needed in severe cases. Along with advances in the treatment of HCC and LT, it is mandatory that future studies elucidate the specific mechanism and appropriate management of DILI.
Author Contributions
Conceptualization, S.K.L. and J.Y.C.; literature search, S.K.L., J.H.K., J.W.J., S.H.B., S.K.Y., E.S.J. and J.Y.C.; writing—original draft preparation, S.K.L.; writing—review and editing, J.Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (no. 2021R1I1A1A01050954; S.K.L.). This study was also supported by a Grant of Translational R&D Project through the Institute for Bio-Medical Convergence, Incheon St. Mary’s Hospital, The Catholic University of Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vuppalanchi, R.; Liangpunsakul, S.; Chalasani, N. Etiology of new-onset jaundice: How often is it caused by idiosyncratic drug-induced liver injury in the United States? Am. J. Gastroenterol. 2007, 102, 558–562. [Google Scholar] [CrossRef]
- Andrade, R.J.; Aithal, G.P.; Björnsson, E.S.; Kaplowitz, N.; Kullak-Ublick, G.A.; Larrey, D.; Karlsen, T.H.; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Drug-induced liver injury. J. Hepatol. 2019, 70, 1222–1261. [Google Scholar] [CrossRef] [PubMed]
- Fontana, R.J. Pathogenesis of idiosyncratic drug-induced liver injury and clinical perspectives. Gastroenterology 2014, 146, 914–928. [Google Scholar] [CrossRef]
- Chalasani, N.P.; Hayashi, P.H.; Bonkovsky, H.L.; Navarro, V.J.; Lee, W.M.; Fontana, R.J. ACG Clinical Guideline: The diagnosis and management of idiosyncratic drug-induced liver injury. Am. J. Gastroenterol. 2014, 109, 950–966; quiz 967. [Google Scholar] [CrossRef] [PubMed]
- Lammert, C.; Einarsson, S.; Saha, C.; Niklasson, A.; Bjornsson, E.; Chalasani, N. Relationship between daily dose of oral medications and idiosyncratic drug-induced liver injury: Search for signals. Hepatology 2008, 47, 2003–2009. [Google Scholar] [CrossRef]
- Sgro, C.; Clinard, F.; Ouazir, K.; Chanay, H.; Allard, C.; Guilleminet, C.; Lenoir, C.; Lemoine, A.; Hillon, P. Incidence of drug-induced hepatic injuries: A French population-based study. Hepatology 2002, 36, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, E.S.; Bergmann, O.M.; Björnsson, H.K.; Kvaran, R.B.; Olafsson, S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013, 144, 1419–1425.e3. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, E.S. Epidemiology and risk factors for idiosyncratic drug-induced liver injury. Semin. Liver Dis. 2014, 34, 115–122. [Google Scholar] [CrossRef]
- Dugan, C.M.; Fullerton, A.M.; Roth, R.A.; Ganey, P.E. Natural killer cells mediate severe liver injury in a murine model of halothane hepatitis. Toxicol. Sci. 2011, 120, 507–518. [Google Scholar] [CrossRef]
- Danan, G.; Benichou, C. Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions of international consensus meetings: Application to drug-induced liver injuries. J. Clin. Epidemiol. 1993, 46, 1323–1330. [Google Scholar] [CrossRef]
- Aithal, G.; Watkins, P.; Andrade, R.; Larrey, D.; Molokhia, M.; Takikawa, H.; Hunt, C.; Wilke, R.; Avigan, M.; Kaplowitz, N.; et al. Case Definition and Phenotype Standardization in Drug-Induced Liver Injury. Clin. Pharmacol. Ther. 2011, 89, 806–815. [Google Scholar] [CrossRef]
- Danan, G.; Teschke, R. RUCAM in Drug and Herb Induced Liver Injury: The Update. Int. J. Mol. Sci. 2016, 17, 14. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.K. Pharmacogenomics of adverse drug reactions. Genome Med. 2013, 5, 5. [Google Scholar] [CrossRef]
- Osanlou, O.; Pirmohamed, M.; Daly, A.K. Chapter Seven-Pharmacogenetics of Adverse Drug Reactions. In Advances in Pharmacology; Brøsen, K., Damkier, P., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 83, pp. 155–190. [Google Scholar]
- Teschke, R. Treatment of Drug-Induced Liver Injury. Biomedicines 2023, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Bonkovsky, H.L.; Fontana, R.; Lee, W.; Stolz, A.; Talwalkar, J.; Reddy, K.R.; Watkins, P.B.; Navarro, V.; Barnhart, H.; et al. Features and Outcomes of 899 Patients With Drug-Induced Liver Injury: The DILIN Prospective Study. Gastroenterology 2015, 148, 1340–1352.e1347. [Google Scholar] [CrossRef]
- Macías-Rodríguez, R.U.; Inzaugarat, M.E.; Ruiz-Margáin, A.; Nelson, L.J.; Trautwein, C.; Cubero, F.J. Reclassifying Hepatic Cell Death during Liver Damage: Ferroptosis—A Novel Form of Non-Apoptotic Cell Death? Int. J. Mol. Sci. 2020, 21, 1651. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Kakisaka, K.; Yoshida, Y.; Suzuki, Y.; Sato, T.; Kuroda, H.; Miyasaka, A.; Takikawa, Y. Serum markers for mitochondrial dysfunction and cell death are possible predictive indicators for drug-induced liver injury by direct acting antivirals. Hepatol. Res. 2018, 48, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.H.; Ju, C.; Ramaiah, S.K.; Uetrecht, J.; Jaeschke, H. Mechanisms of immune-mediated liver injury. Toxicol. Sci. 2010, 115, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef]
- Kim, S.-H.; Naisbitt, D.J. Update on Advances in Research on Idiosyncratic Drug-Induced Liver Injury. Allergy Asthma Immunol. Res. 2016, 8, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Landsteiner, K.; Jacobs, J. Studies on the Sensitization of Animals with Simple Chemical Compounds. J. Exp. Med. 1935, 61, 643–656. [Google Scholar] [CrossRef]
- Kaplowitz, N.; DeLeve, L.D. Drug-Induced Liver Disease; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Cho, T.; Uetrecht, J. How reactive metabolites induce an immune response that sometimes leads to an idiosyncratic drug reaction. Chem. Res. Toxicol. 2017, 30, 295–314. [Google Scholar] [CrossRef]
- Uetrecht, J. Mechanisms of idiosyncratic drug-induced liver injury. Adv. Pharmacol. 2019, 85, 133–163. [Google Scholar] [CrossRef]
- Liu, W.; Zeng, X.; Liu, Y.; Liu, J.; Li, C.; Chen, L.; Chen, H.; Ouyang, D. The Immunological Mechanisms and Immune-Based Biomarkers of Drug-Induced Liver Injury. Front. Pharmacol. 2021, 12, 723940. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.S. Crosstalk between tumor-associated macrophages and neighboring cells in hepatocellular carcinoma. Clin. Mol. Hepatol. 2022, 28, 333–350. [Google Scholar] [CrossRef] [PubMed]
- Rose, K.A.; Holman, N.S.; Green, A.M.; Andersen, M.E.; LeCluyse, E.L. Co-culture of hepatocytes and Kupffer cells as an in vitro model of inflammation and drug-induced hepatotoxicity. J. Pharm. Sci. 2016, 105, 950–964. [Google Scholar] [CrossRef]
- Seo, H.-Y.; Kim, M.-K.; Lee, S.-H.; Hwang, J.S.; Park, K.-G.; Jang, B.K. Kahweol ameliorates the liver inflammation through the inhibition of NF-κB and STAT3 activation in primary Kupffer cells and primary hepatocytes. Nutrients 2018, 10, 863. [Google Scholar] [CrossRef]
- Zhang, J.; Li, N.; Yang, L.; Xie, H.; Yang, Y.; Wang, H.; Wu, C.; Shen, T.; Zhu, Q. Bradykinin contributes to immune liver injury via B2R receptor-mediated pathways in trichloroethylene sensitized mice: A role in Kupffer cell activation. Toxicology 2019, 415, 37–48. [Google Scholar] [CrossRef]
- Mossanen, J.C.; Krenkel, O.; Ergen, C.; Govaere, O.; Liepelt, A.; Puengel, T.; Heymann, F.; Kalthoff, S.; Lefebvre, E.; Eulberg, D.; et al. Chemokine (C-C motif) receptor 2-positive monocytes aggravate the early phase of acetaminophen-induced acute liver injury. Hepatology 2016, 64, 1667–1682. [Google Scholar] [CrossRef]
- Németh, T.; Sperandio, M.; Mócsai, A. Neutrophils as emerging therapeutic targets. Nat. Rev. Drug Discov. 2020, 19, 253–275. [Google Scholar] [CrossRef]
- Jaeschke, H.; Hasegawa, T. Role of neutrophils in acute inflammatory liver injury. Liver Int. 2006, 26, 912–919. [Google Scholar] [CrossRef]
- Li, G.; Lin, J.; Zhang, C.; Gao, H.; Lu, H.; Gao, X.; Zhu, R.; Li, Z.; Li, M.; Liu, Z. Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease. Gut Microbes 2021, 13, 1968257. [Google Scholar] [CrossRef]
- El-Benna, J.; Dang, P.M.; Gougerot-Pocidalo, M.A.; Elbim, C. Phagocyte NADPH oxidase: A multicomponent enzyme essential for host defenses. Arch. Immunol. Ther. Exp. 2005, 53, 199–206. [Google Scholar]
- Klugewitz, K.; Adams, D.H.; Emoto, M.; Eulenburg, K.; Hamann, A. The composition of intrahepatic lymphocytes: Shaped by selective recruitment? Trends Immunol. 2004, 25, 590–594. [Google Scholar] [CrossRef]
- Frey, M.; Packianathan, N.B.; Fehniger, T.A.; Ross, M.E.; Wang, W.C.; Stewart, C.C.; Caligiuri, M.A.; Evans, S.S. Differential expression and function of L-selectin on CD56bright and CD56dim natural killer cell subsets. J. Immunol. 1998, 161, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.L.; Cella, M.; Porter, S.I.; Li, S.; Gurewitz, G.L.; Hong, H.S.; Johnson, R.P.; Oltz, E.M.; Colonna, M. Gene Regulatory Programs Conferring Phenotypic Identities to Human NK Cells. Cell 2019, 176, 348–360.e312. [Google Scholar] [CrossRef]
- Radaeva, S.; Sun, R.; Jaruga, B.; Nguyen, V.T.; Tian, Z.; Gao, B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology 2006, 130, 435–452. [Google Scholar] [CrossRef] [PubMed]
- Fasbender, F.; Obholzer, M.; Metzler, S.; Stöber, R.; Hengstler, J.G.; Watzl, C. Enhanced activation of human NK cells by drug-exposed hepatocytes. Arch. Toxicol. 2020, 94, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Kondo, T.; Ohshima, T.; Fujiwara, H.; Iwakura, Y.; Mukaida, N. A pivotal involvement of IFN-gamma in the pathogenesis of acetaminophen-induced acute liver injury. FASEB J. 2002, 16, 1227–1236. [Google Scholar] [CrossRef]
- Wallace, K.L.; Marshall, M.A.; Ramos, S.I.; Lannigan, J.A.; Field, J.J.; Strieter, R.M.; Linden, J. NKT cells mediate pulmonary inflammation and dysfunction in murine sickle cell disease through production of IFN-gamma and CXCR3 chemokines. Blood 2009, 114, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Bendelac, A.; Lantz, O.; Quimby, M.E.; Yewdell, J.W.; Bennink, J.R.; Brutkiewicz, R.R. CD1 recognition by mouse NK1+ T lymphocytes. Science 1995, 268, 863–865. [Google Scholar] [CrossRef]
- Lantz, O.; Bendelac, A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J. Exp. Med. 1994, 180, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.M.; Bezbradica, J.S.; Van Kaer, L.; Joyce, S. CD1d-Restricted Natural Killer T Cells. In eLS; Wiley: Hoboken, NJ, USA, 2016; pp. 1–27. [Google Scholar] [CrossRef]
- Van Kaer, L.; Parekh, V.V.; Wu, L. Invariant natural killer T cells: Bridging innate and adaptive immunity. Cell Tissue Res. 2011, 343, 43–55. [Google Scholar] [CrossRef]
- Brennan, P.J.; Brigl, M.; Brenner, M.B. Invariant natural killer T cells: An innate activation scheme linked to diverse effector functions. Nat. Rev. Immunol. 2013, 13, 101–117. [Google Scholar] [CrossRef]
- Bhattacharjee, J.; Kirby, M.; Softic, S.; Miles, L.; Salazar-Gonzalez, R.M.; Shivakumar, P.; Kohli, R. Hepatic Natural Killer T-cell and CD8+ T-cell Signatures in Mice with Nonalcoholic Steatohepatitis. Hepatol. Commun. 2017, 1, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Ruf, B.; Heinrich, B.; Greten, T.F. Immunobiology and immunotherapy of HCC: Spotlight on innate and innate-like immune cells. Cell. Mol. Immunol. 2021, 18, 112–127. [Google Scholar] [CrossRef]
- Lin, Q.; Kuypers, M.; Liu, Z.; Copeland, J.K.; Chan, D.; Robertson, S.J.; Kontogiannis, J.; Guttman, D.S.; Banks, E.K.; Philpott, D.J.; et al. Invariant natural killer T cells minimally influence gut microbiota composition in mice. Gut Microbes 2022, 14, 2104087. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Crivellato, E. Mast cell ontogeny: An historical overview. Immunol. Lett. 2014, 159, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Metz, M.; Maurer, M. Mast cells--key effector cells in immune responses. Trends Immunol. 2007, 28, 234–241. [Google Scholar] [CrossRef]
- Marshall, J.S. Mast-cell responses to pathogens. Nat. Rev. Immunol. 2004, 4, 787–799. [Google Scholar] [CrossRef]
- St John, A.L.; Abraham, S.N. Innate immunity and its regulation by mast cells. J. Immunol. 2013, 190, 4458–4463. [Google Scholar] [CrossRef]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2015, 6, 620. [Google Scholar] [CrossRef]
- Dudeck, A.; Dudeck, J.; Scholten, J.; Petzold, A.; Surianarayanan, S.; Köhler, A.; Peschke, K.; Vöhringer, D.; Waskow, C.; Krieg, T.; et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity 2011, 34, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Thorlacius, H. Mast cell-derived tumour necrosis factor-alpha mediates macrophage inflammatory protein-2-induced recruitment of neutrophils in mice. Br. J. Pharmacol. 2005, 145, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Piliponsky, A.M.; Romani, L. The contribution of mast cells to bacterial and fungal infection immunity. Immunol. Rev. 2018, 282, 188–197. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Meurer, S.K.; Liedtke, C.; Huber, M. Mast Cells in Liver Fibrogenesis. Cells 2019, 8, 1429. [Google Scholar] [CrossRef] [PubMed]
- Tolefree, J.A.; Garcia, A.J.; Farrell, J.; Meadows, V.; Kennedy, L.; Hargrove, L.; Demieville, J.; Francis, N.; Mirabel, J.; Francis, H. Alcoholic liver disease and mast cells: What’s your gut got to do with it? Liver Res. 2019, 3, 46–54. [Google Scholar] [CrossRef]
- Katsoulis-Dimitriou, K.; Kotrba, J.; Voss, M.; Dudeck, J.; Dudeck, A. Mast Cell Functions Linking Innate Sensing to Adaptive Immunity. Cells 2020, 9, 2538. [Google Scholar] [CrossRef] [PubMed]
- Suurmond, J.; van Heemst, J.; van Heiningen, J.; Dorjée, A.L.; Schilham, M.W.; van der Beek, F.B.; Huizinga, T.W.J.; Schuerwegh, A.J.M.; Toes, R.E.M. Communication between human mast cells and CD4+ T cells through antigen-dependent interactions. Eur. J. Immunol. 2013, 43, 1758–1768. [Google Scholar] [CrossRef] [PubMed]
- Shuai, Z.; Leung, M.W.Y.; He, X.; Zhang, W.; Yang, G.; Leung, P.S.C.; Eric Gershwin, M. Adaptive immunity in the liver. Cell. Mol. Immunol. 2016, 13, 354–368. [Google Scholar] [CrossRef]
- Kubes, P.; Jenne, C. Immune Responses in the Liver. Annu. Rev. Immunol. 2018, 36, 247–277. [Google Scholar] [CrossRef]
- Björnsson, E.S.; Aithal, G.P. Immune-Mediated Drug-Induced Liver Injury. In Liver Immunology: Principles and Practice; Gershwin, M.E.M., Vierling, J., Tanaka, A.P., Manns, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 491–504. [Google Scholar]
- Dardalhon, V.; Korn, T.; Kuchroo, V.K.; Anderson, A.C. Role of Th1 and Th17 cells in organ-specific autoimmunity. J. Autoimmun. 2008, 31, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Harrington, L.E.; Hatton, R.D.; Mangan, P.R.; Turner, H.; Murphy, T.L.; Murphy, K.M.; Weaver, C.T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005, 6, 1123–1132. [Google Scholar] [CrossRef]
- Walker, J.A.; McKenzie, A.N.J. T(H)2 cell development and function. Nat. Rev. Immunol. 2018, 18, 121–133. [Google Scholar] [CrossRef]
- Björnsson, H.K.; Björnsson, E.S. Drug-induced liver injury: Pathogenesis, epidemiology, clinical features, and practical management. Eur. J. Intern. Med. 2022, 97, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Metkar, S.S.; Wang, B.; Ebbs, M.L.; Kim, J.H.; Lee, Y.J.; Raja, S.M.; Froelich, C.J. Granzyme B activates procaspase-3 which signals a mitochondrial amplification loop for maximal apoptosis. J. Cell Biol. 2003, 160, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Amante, M.F.; Filippini, A.V.; Cejas, N.; Lendoire, J.; Imventarza, O.; Parisi, C. Dress syndrome and fulminant hepatic failure induced by lamotrigine. Ann. Hepatol. 2009, 8, 75–77. [Google Scholar] [CrossRef]
- Pieper, K.; Grimbacher, B.; Eibel, H. B-cell biology and development. J. Allergy Clin. Immunol. 2013, 131, 959–971. [Google Scholar] [CrossRef]
- Robinson, M.W.; Harmon, C.; O’Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell. Mol. Immunol. 2016, 13, 267–276. [Google Scholar] [CrossRef]
- Patel, A.M.; Liu, Y.S.; Davies, S.P.; Brown, R.M.; Kelly, D.A.; Scheel-Toellner, D.; Reynolds, G.M.; Stamataki, Z. The Role of B Cells in Adult and Paediatric Liver Injury. Front. Immunol. 2021, 12, 729143. [Google Scholar] [CrossRef]
- Metushi, I.G.; Sanders, C.; Lee, W.M.; Uetrecht, J. Detection of anti-isoniazid and anti-cytochrome P450 antibodies in patients with isoniazid-induced liver failure. Hepatology 2014, 59, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, C.A.; Shevach, E.M. Cutting edge: Control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J. Immunol. 2001, 167, 1137–1140. [Google Scholar] [CrossRef]
- Lee, S.K.; Park, M.J.; Choi, J.W.; Baek, J.A.; Kim, S.Y.; Choi, H.J.; You, Y.K.; Jang, J.W.; Sung, P.S.; Bae, S.H.; et al. Patient-Derived Avatar Mouse Model to Predict the Liver Immune Homeostasis of Long-Term Stable Liver Transplant Patients. Front. Immunol. 2022, 13, 817006. [Google Scholar] [CrossRef] [PubMed]
- Jhun, J.; Lee, S.H.; Lee, S.K.; Kim, H.Y.; Jung, E.S.; Kim, D.G.; Choi, J.; Bae, S.H.; Yoon, S.K.; Chung, B.H.; et al. Serial Monitoring of Immune Markers Being Represented Regulatory T Cell/T Helper 17 Cell Ratio: Indicating Tolerance for Tapering Immunosuppression after Liver Transplantation. Front. Immunol. 2018, 9, 352. [Google Scholar] [CrossRef]
- Longhi, M.S.; Hussain, M.J.; Mitry, R.R.; Arora, S.K.; Mieli-Vergani, G.; Vergani, D.; Ma, Y. Functional study of CD4+CD25+ regulatory T cells in health and autoimmune hepatitis. J. Immunol. 2006, 176, 4484–4491. [Google Scholar] [CrossRef]
- Grant, C.R.; Liberal, R.; Holder, B.S.; Cardone, J.; Ma, Y.; Robson, S.C.; Mieli-Vergani, G.; Vergani, D.; Longhi, M.S. Dysfunctional CD39(POS) regulatory T cells and aberrant control of T-helper type 17 cells in autoimmune hepatitis. Hepatology 2014, 59, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Tang, X. Gut Microbiota as Regulators of Th17/Treg Balance in Patients With Myasthenia Gravis. Front. Immunol. 2021, 12, 803101. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Jhun, J.; Lee, S.Y.; Choi, S.; Choi, S.S.; Park, M.S.; Lee, S.Y.; Cho, K.H.; Lee, A.R.; Ahn, J.; et al. A decrease in functional microbiomes represented as Faecalibacterium affects immune homeostasis in long-term stable liver transplant patients. Gut Microbes 2022, 14, 2102885. [Google Scholar] [CrossRef]
- Fan, L.; Qi, Y.; Qu, S.; Chen, X.; Li, A.; Hendi, M.; Xu, C.; Wang, L.; Hou, T.; Si, J.; et al. B. adolescentis ameliorates chronic colitis by regulating Treg/Th2 response and gut microbiota remodeling. Gut Microbes 2021, 13, 1826746. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Feng, M.; Gu, J.; Xia, Z.; Zhang, H.; Zheng, S.; Duan, Z.; Hu, R.; Wang, J.; Shi, W.; et al. Restoration of intrahepatic regulatory T cells through MMP-9/13-dependent activation of TGF-β is critical for immune homeostasis following acute liver injury. J. Mol. Cell Biol. 2013, 5, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, L.; Zhang, L.; Jiang, Z. Effect of Adoptive Transfer or Depletion of Regulatory T Cells on Triptolide-induced Liver Injury. Front. Pharmacol. 2016, 7, 99. [Google Scholar] [CrossRef]
- Wang, X.; Sun, R.; Chen, Y.; Lian, Z.X.; Wei, H.; Tian, Z. Regulatory T cells ameliorate acetaminophen-induced immune-mediated liver injury. Int. Immunopharmacol. 2015, 25, 293–301. [Google Scholar] [CrossRef]
- Lee, S.K.; Park, M.J.; Jhun, J.Y.; Beak, J.A.; Choi, J.W.; Rye, J.Y.; Jang, J.W.; Bae, S.H.; Yoon, S.K.; Choi, H.J.; et al. Combination Treatment With Metformin and Tacrolimus Improves Systemic Immune Cellular Homeostasis by Modulating Treg and Th17 Imbalance. Front. Immunol. 2020, 11, 581728. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 6. [Google Scholar] [CrossRef]
- Llovet, J.M.; Montal, R.; Sia, D.; Finn, R.S. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2018, 15, 599–616. [Google Scholar] [CrossRef]
- Zhou, D.; Luan, J.; Huang, C.; Li, J. Tumor-Associated Macrophages in Hepatocellular Carcinoma: Friend or Foe? Gut Liver 2021, 15, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Oura, K.; Morishita, A.; Tani, J.; Masaki, T. Tumor Immune Microenvironment and Immunosuppressive Therapy in Hepatocellular Carcinoma: A Review. Int. J. Mol. Sci. 2021, 22, 5801. [Google Scholar] [CrossRef]
- Okoye, I.S.; Houghton, M.; Tyrrell, L.; Barakat, K.; Elahi, S. Coinhibitory Receptor Expression and Immune Checkpoint Blockade: Maintaining a Balance in CD8(+) T Cell Responses to Chronic Viral Infections and Cancer. Front. Immunol. 2017, 8, 1215. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Hao, Z.; Wang, P. Lenvatinib in Management of Solid Tumors. Oncologist 2020, 25, e302–e310. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Llovet, J.M.; Castet, F.; Heikenwalder, M.; Maini, M.K.; Mazzaferro, V.; Pinato, D.J.; Pikarsky, E.; Zhu, A.X.; Finn, R.S. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2022, 19, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Sorafenib. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Kuroda, D.; Hayashi, H.; Nitta, H.; Imai, K.; Abe, S.; Hashimoto, D.; Chikamoto, A.; Ishiko, T.; Beppu, T.; Baba, H. Successful treatment for sorafenib-induced liver dysfunction: A report of case with liver biopsy. Surg. Case Rep. 2016, 2, 4. [Google Scholar] [CrossRef]
- Van Hootegem, A.; Verslype, C.; Van Steenbergen, W. Sorafenib-induced liver failure: A case report and review of the literature. Case Rep. Hepatol. 2011, 2011, 941395. [Google Scholar] [CrossRef]
- Shah, R.R.; Morganroth, J.; Shah, D.R. Hepatotoxicity of tyrosine kinase inhibitors: Clinical and regulatory perspectives. Drug Saf. 2013, 36, 491–503. [Google Scholar] [CrossRef]
- Regorafenib. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Lenvatinib. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Cabozantinib. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- AlAsmari, A.F.; Ali, N.; AlAsmari, F.; AlAnazi, W.A.; Alqahtani, F.; Alharbi, M.; Alotaibi, F.M.; Aldossari, A.A.; AlSwayyed, M.; Alanazi, M.M.; et al. Elucidation of the Molecular Mechanisms Underlying Sorafenib-Induced Hepatotoxicity. Oxidative Med. Cell. Longev. 2020, 2020, 7453406. [Google Scholar] [CrossRef]
- Han, D.; Shinohara, M.; Ybanez, M.D.; Saberi, B.; Kaplowitz, N. Signal transduction pathways involved in drug-induced liver injury. Handb. Exp. Pharmacol. 2010, 196, 267–310. [Google Scholar] [CrossRef]
- Kelley, R.K.; Sangro, B.; Harris, W.; Ikeda, M.; Okusaka, T.; Kang, Y.K.; Qin, S.; Tai, D.W.; Lim, H.Y.; Yau, T.; et al. Safety, Efficacy, and Pharmacodynamics of Tremelimumab Plus Durvalumab for Patients With Unresectable Hepatocellular Carcinoma: Randomized Expansion of a Phase I/II Study. J. Clin. Oncol. 2021, 39, 2991–3001. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef]
- Yau, T.; Kang, Y.K.; Kim, T.Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat. Rev. Clin. Oncol. 2018, 15, 325–340. [Google Scholar] [CrossRef]
- Ma, L.; Hernandez, M.O.; Zhao, Y.; Mehta, M.; Tran, B.; Kelly, M.; Rae, Z.; Hernandez, J.M.; Davis, J.L.; Martin, S.P.; et al. Tumor Cell Biodiversity Drives Microenvironmental Reprogramming in Liver Cancer. Cancer Cell 2019, 36, 418–430.e416. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.; Araki, K.; Hashimoto, M.; Li, W.; Riley, J.L.; Cheung, J.; Sharpe, A.H.; Freeman, G.J.; Irving, B.A.; Ahmed, R. Role of PD-1 during effector CD8 T cell differentiation. Proc. Natl. Acad. Sci. USA 2018, 115, 4749–4754. [Google Scholar] [CrossRef] [PubMed]
- Maker, A.V.; Attia, P.; Rosenberg, S.A. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J. Immunol. 2005, 175, 7746–7754. [Google Scholar] [CrossRef] [PubMed]
- Friedman, C.F.; Proverbs-Singh, T.A.; Postow, M.A. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol. 2016, 2, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- De Martin, E.; Michot, J.M.; Rosmorduc, O.; Guettier, C.; Samuel, D. Liver toxicity as a limiting factor to the increasing use of immune checkpoint inhibitors. JHEP Rep. 2020, 2, 100170. [Google Scholar] [CrossRef] [PubMed]
- Remash, D.; Prince, D.S.; McKenzie, C.; Strasser, S.I.; Kao, S.; Liu, K. Immune checkpoint inhibitor-related hepatotoxicity: A review. World J. Gastroenterol. 2021, 27, 5376–5391. [Google Scholar] [CrossRef]
- Malnick, S.D.H.; Abdullah, A.; Neuman, M.G. Checkpoint Inhibitors and Hepatotoxicity. Biomedicines 2021, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Brown, Z.J.; Heinrich, B.; Steinberg, S.M.; Yu, S.J.; Greten, T.F. Safety in treatment of hepatocellular carcinoma with immune checkpoint inhibitors as compared to melanoma and non-small cell lung cancer. J. Immunother. Cancer 2017, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.H.R.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Kang, Y.-K.; Kim, T.-Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.-M.; Matilla, A. Nivolumab (NIVO) + Ipilimumab (IPI) Combination Therapy in Patients (Pts) with Advanced Hepatocellular Carcinoma (aHCC): Results from CheckMate 040; American Society of Clinical Oncology: Alexandria, VA, USA, 2019. [Google Scholar]
- Kelley, R.K.; Abou-Alfa, G.K.; Bendell, J.C.; Kim, T.-Y.; Borad, M.J.; Yong, W.-P.; Morse, M.; Kang, Y.-K.; Rebelatto, M.; Makowsky, M. Phase I/II Study of Durvalumab and Tremelimumab in Patients with Unresectable Hepatocellular Carcinoma (HCC): Phase I Safety and Efficacy Analyses; American Society of Clinical Oncology: Alexandria, VA, USA, 2017. [Google Scholar]
- Tsung, I.; Dolan, R.; Lao, C.D.; Fecher, L.; Riggenbach, K.; Yeboah-Korang, A.; Fontana, R.J. Liver injury is most commonly due to hepatic metastases rather than drug hepatotoxicity during pembrolizumab immunotherapy. Aliment. Pharmacol. Ther. 2019, 50, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Zen, Y.; Yeh, M.M. Hepatotoxicity of immune checkpoint inhibitors: A histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug-induced liver injury. Mod. Pathol. 2018, 31, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yao, Z.; Zhou, X.; Zhang, W.; Zhang, X.; Zhang, F. Immune-related adverse events of checkpoint inhibitors: Insights into immunological dysregulation. Clin. Immunol. 2020, 213, 108377. [Google Scholar] [CrossRef]
- Kumar, P.; Saini, S.; Prabhakar, B.S. Cancer immunotherapy with check point inhibitor can cause autoimmune adverse events due to loss of Treg homeostasis. Semin. Cancer Biol. 2020, 64, 29–35. [Google Scholar] [CrossRef]
- Keir, M.E.; Liang, S.C.; Guleria, I.; Latchman, Y.E.; Qipo, A.; Albacker, L.A.; Koulmanda, M.; Freeman, G.J.; Sayegh, M.H.; Sharpe, A.H. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 2006, 203, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef]
- Das, R.; Bar, N.; Ferreira, M.; Newman, A.M.; Zhang, L.; Bailur, J.K.; Bacchiocchi, A.; Kluger, H.; Wei, W.; Halaban, R.; et al. Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J. Clin. Investig. 2018, 128, 715–720. [Google Scholar] [CrossRef]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2017, 28, 1368–1379. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Z.; Mo, Z.S.; Yang, X.H.; Lin, B.L.; Peng, L.; Xu, Y.; Lei, C.Y.; Zhuang, X.D.; Lu, L.; et al. Gut microbiota as prognosis markers for patients with HBV-related acute-on-chronic liver failure. Gut Microbes 2021, 13, 1921925. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Shamsaddini, A.; Fagan, A.; McGeorge, S.; Gavis, E.; Sikaroodi, M.; Brenner, L.A.; Wade, J.B.; Gillevet, P.M. Distinct gut microbial compositional and functional changes associated with impaired inhibitory control in patients with cirrhosis. Gut Microbes 2021, 13, 1953247. [Google Scholar] [CrossRef]
- Temraz, S.; Nassar, F.; Kreidieh, F.; Mukherji, D.; Shamseddine, A.; Nasr, R. Hepatocellular Carcinoma Immunotherapy and the Potential Influence of Gut Microbiome. Int. J. Mol. Sci. 2021, 22, 7800. [Google Scholar] [CrossRef] [PubMed]
- De Martin, E.; Michot, J.-M.; Papouin, B.; Champiat, S.; Mateus, C.; Lambotte, O.; Roche, B.; Antonini, T.M.; Coilly, A.; Laghouati, S.; et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J. Hepatol. 2018, 68, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Riveiro-Barciela, M.; Barreira-Díaz, A.; Vidal-González, J.; Muñoz-Couselo, E.; Martínez-Valle, F.; Viladomiu, L.; Mínguez, B.; Ortiz-Velez, C.; Castells, L.; Esteban, R. Immune-related hepatitis related to checkpoint inhibitors: Clinical and prognostic factors. Liver Int. 2020, 40, 1906–1916. [Google Scholar] [CrossRef] [PubMed]
- Colevas, A.D.; Setser, A. The NCI Common Terminology Criteria for Adverse Events (CTCAE) v 3.0 is the new standard for oncology clinical trials. J. Clin. Oncol. 2004, 22, 6098. [Google Scholar] [CrossRef]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O., 3rd; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef]
- Haanen, J.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv119–iv142. [Google Scholar] [CrossRef] [PubMed]
- Dougan, M.; Wang, Y.; Rubio-Tapia, A.; Lim, J.K. AGA Clinical Practice Update on Diagnosis and Management of Immune Checkpoint Inhibitor Colitis and Hepatitis: Expert Review. Gastroenterology 2021, 160, 1384–1393. [Google Scholar] [CrossRef]
- Huffman, B.M.; Kottschade, L.A.; Kamath, P.S.; Markovic, S.N. Hepatotoxicity After Immune Checkpoint Inhibitor Therapy in Melanoma: Natural Progression and Management. Am. J. Clin. Oncol. 2018, 41, 760–765. [Google Scholar] [CrossRef]
- Iwamoto, K.; Ishitsuka, Y.; Tanaka, R.; Sekine, I.; Fujimoto, M. Azathioprine combination therapy for steroid-refractory hepatic immune system-related adverse events. Eur. J. Dermatol. 2017, 27, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Fu, L.; Qian, Y.; Shang, Z.; Sun, X.; Kong, X.; Gao, Y. Antibiotics enhancing drug-induced liver injury assessed for causality using Roussel Uclaf Causality Assessment Method: Emerging role of gut microbiota dysbiosis. Front. Med. (Lausanne) 2022, 9, 972518. [Google Scholar] [CrossRef]
- Adam, R.; McMaster, P.; O’Grady, J.G.; Castaing, D.; Klempnauer, J.L.; Jamieson, N.; Neuhaus, P.; Lerut, J.; Salizzoni, M.; Pollard, S.; et al. Evolution of liver transplantation in Europe: Report of the European Liver Transplant Registry. Liver Transplant. 2003, 9, 1231–1243. [Google Scholar] [CrossRef]
- Cvetkovski, F.; Hexham, J.M.; Berglund, E. Strategies for Liver Transplantation Tolerance. Int. J. Mol. Sci. 2021, 22, 2253. [Google Scholar] [CrossRef]
- Di Maira, T.; Little, E.C.; Berenguer, M. Immunosuppression in liver transplant. Best Pract. Res. Clin. Gastroenterol. 2020, 46–47, 101681. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.L.; Crabtree, G.R. The mechanism of action of cyclosporin A and FK506. Immunol. Today 1992, 13, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Everson, G.T. Everolimus and mTOR inhibitors in liver transplantation: Opening the “box”. Liver Transplant. 2006, 12, 1571–1573. [Google Scholar] [CrossRef] [PubMed]
- Schmeding, M.; Kiessling, A.; Neuhaus, R.; Heidenhain, C.; Bahra, M.; Neuhaus, P.; Neumann, U.P. Mycophenolate mofetil monotherapy in liver transplantation: 5-year follow-up of a prospective randomized trial. Transplantation 2011, 92, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Parlakpinar, H.; Gunata, M. Transplantation and immunosuppression: A review of novel transplant-related immunosuppressant drugs. Immunopharmacol. Immunotoxicol. 2021, 43, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Lee, S.W.; Jang, J.W.; Bae, S.H.; Choi, J.Y.; Yoon, S.K. Immunological Markers, Prognostic Factors and Challenges Following Curative Treatments for Hepatocellular Carcinoma. Int. J. Mol. Sci. 2021, 22, 10271. [Google Scholar] [CrossRef]
- Tacrolimus. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Sirolimus. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Cyclosporine. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Mycophenolate. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Warren, M.; Mitsinikos, T.; Yanni, G.; Sasaki, M.; Sasaki, A.T.; Thomas, D. Mycophenolate Mofetil Hepatotoxicity Associated With Mitochondrial Abnormality in Liver Transplant Recipients and Mice. J. Pediatr. Gastroenterol. Nutr. 2021, 73, 463–470. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).