Staphylococcus aureus Cell Wall Phenotypic Changes Associated with Biofilm Maturation and Water Availability: A Key Contributing Factor for Chlorine Resistance
Abstract
1. Introduction
2. Results
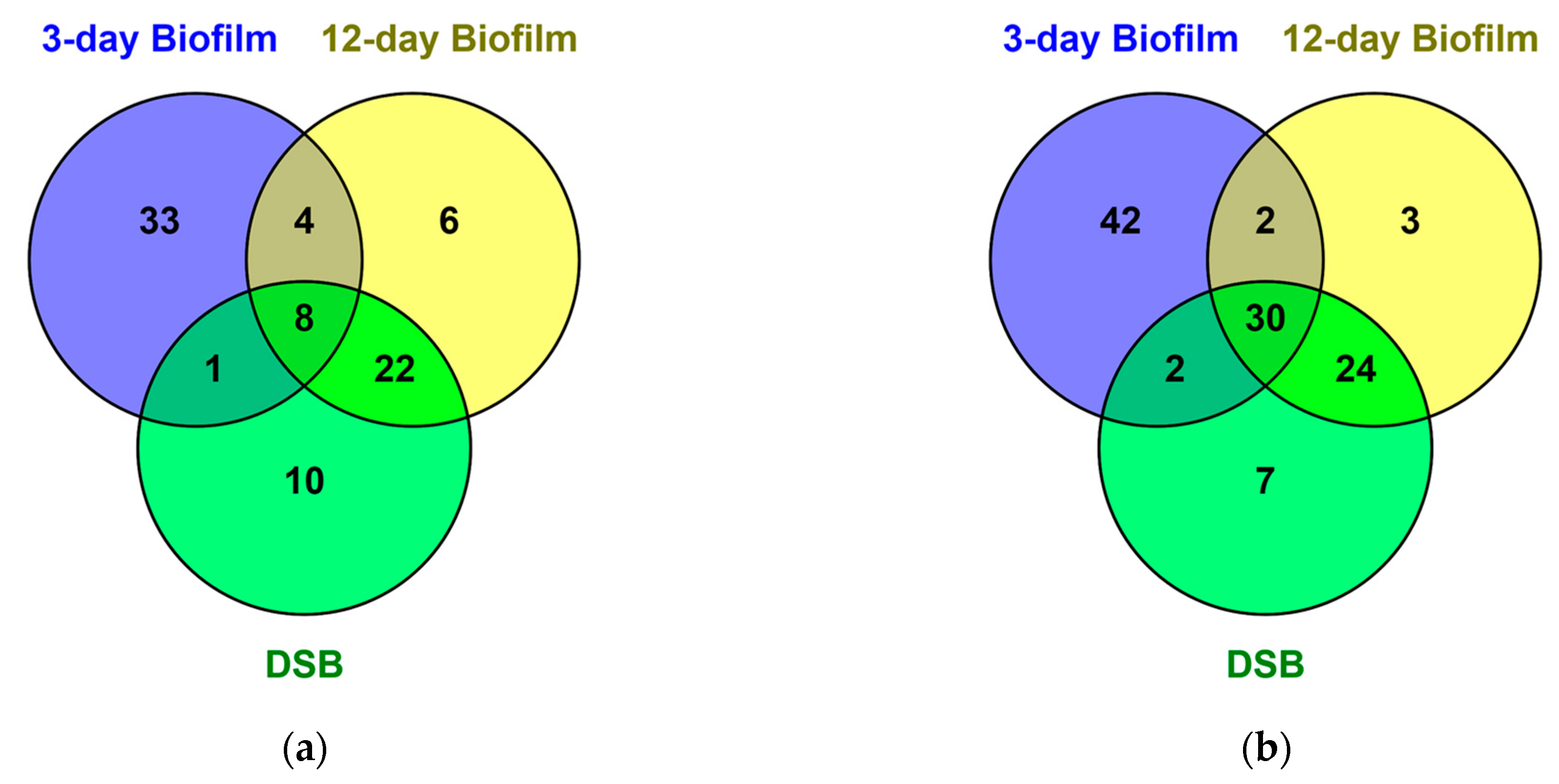
2.1. Proteomics of Biofilms
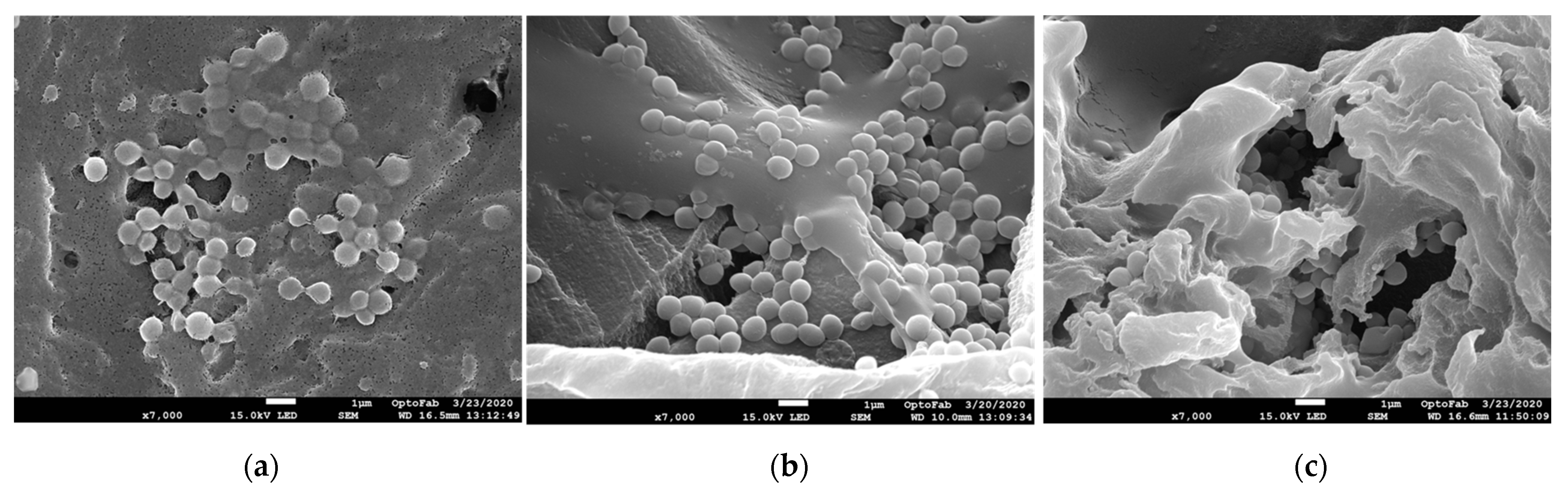
2.2. Scanning Electron Microscopy
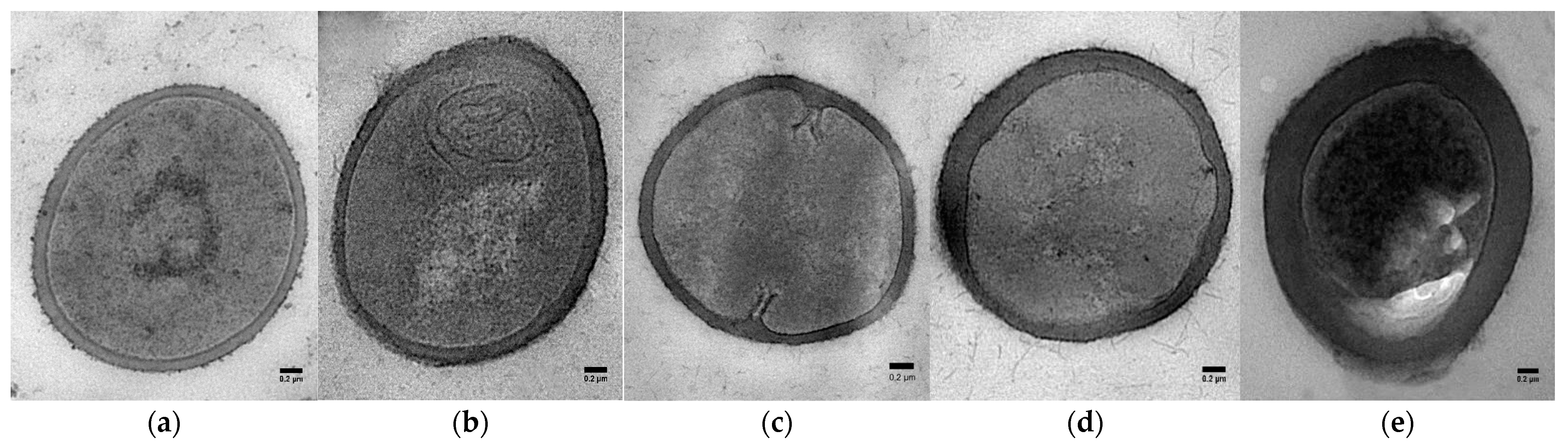
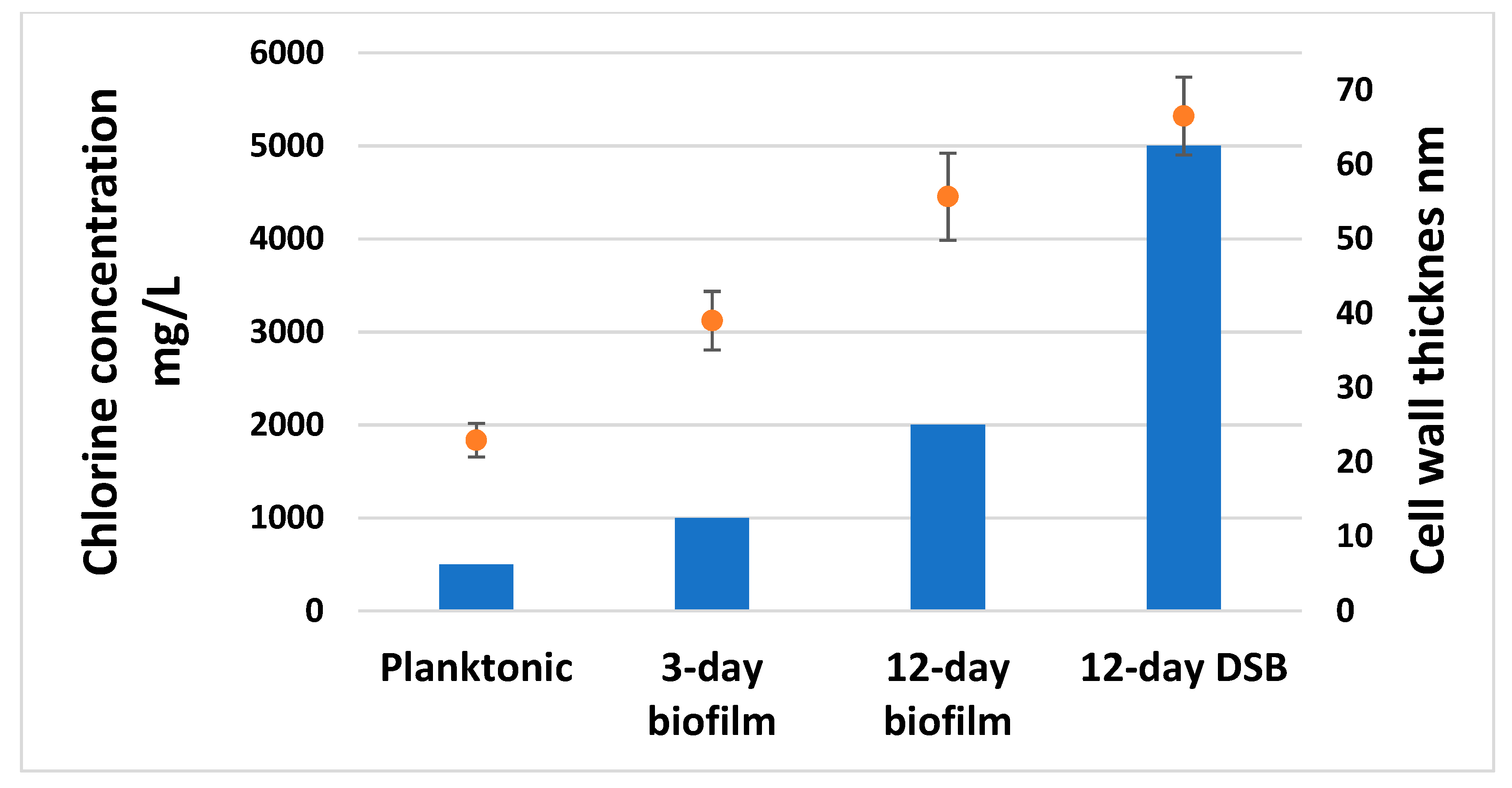
2.3. Transmission Electron Microscopy
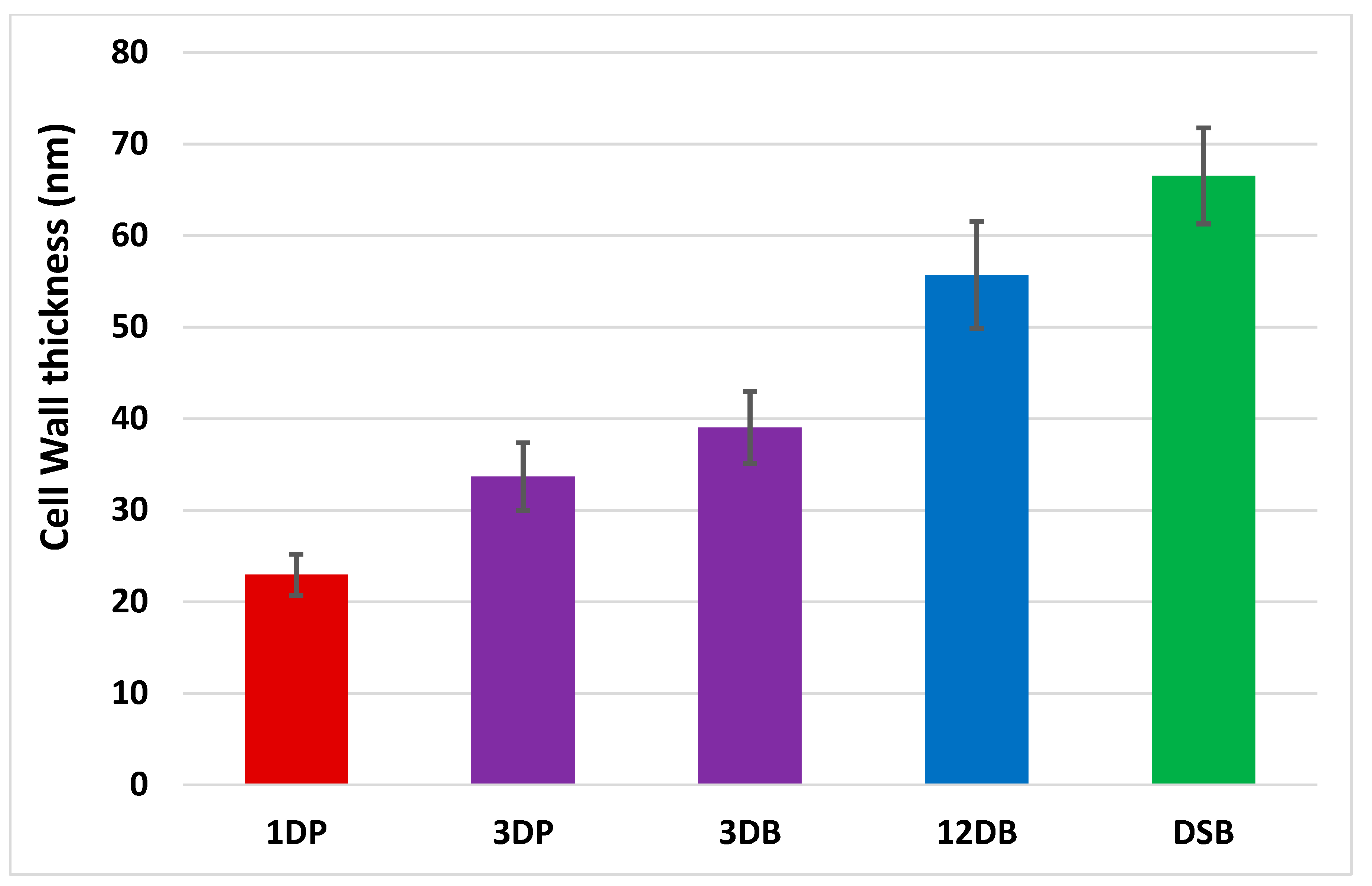
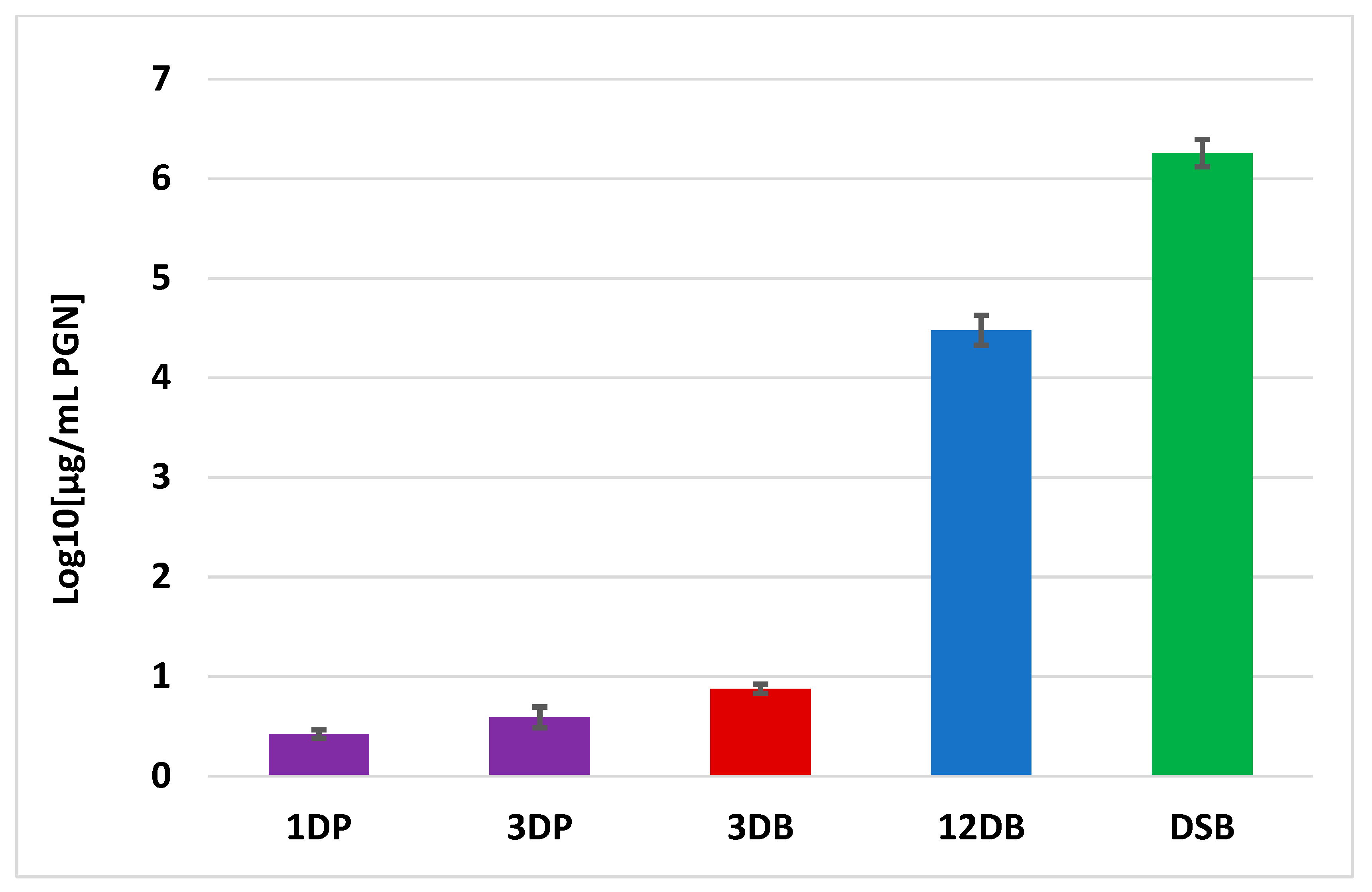
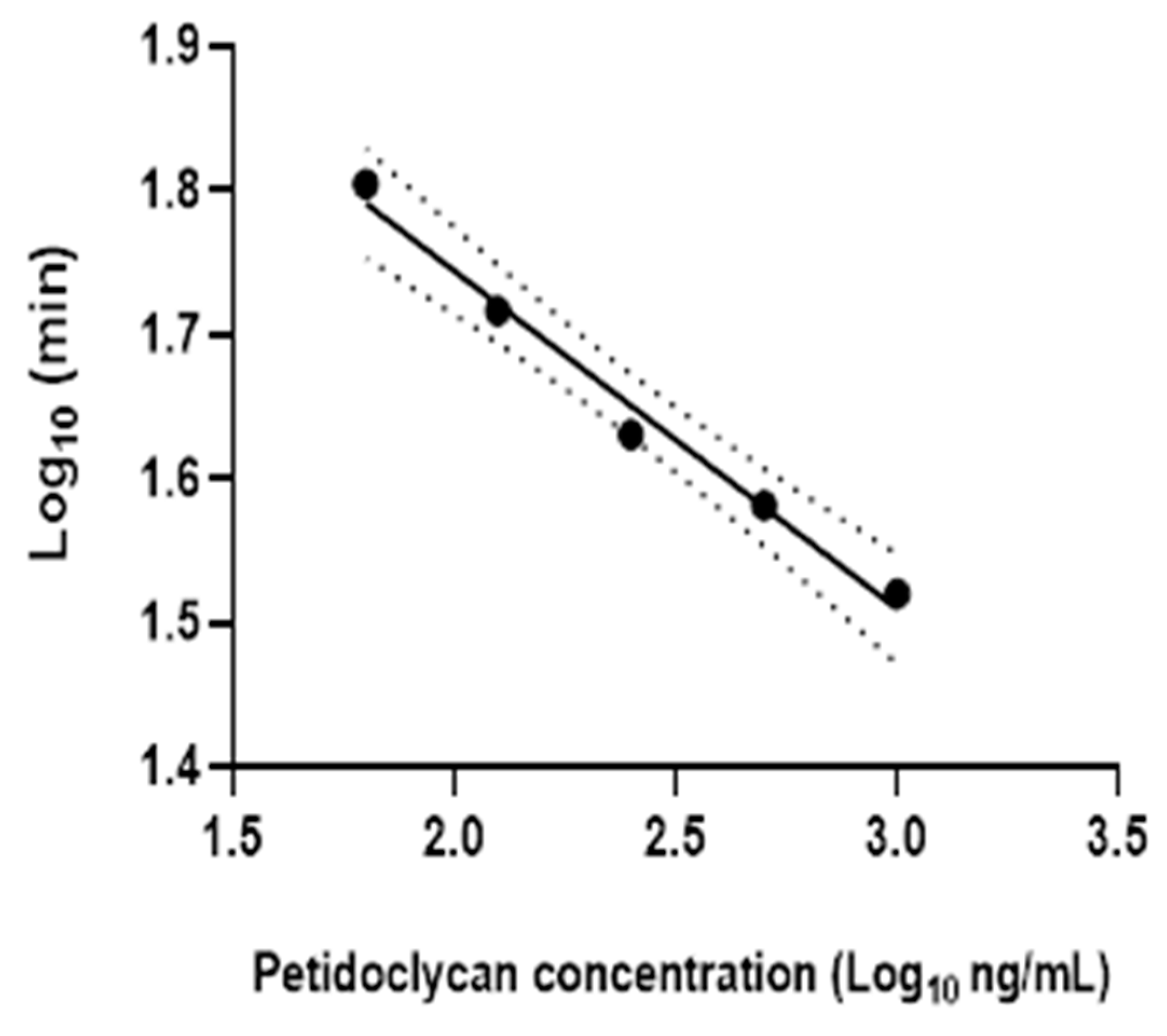
2.4. Peptidoglycan Measurement
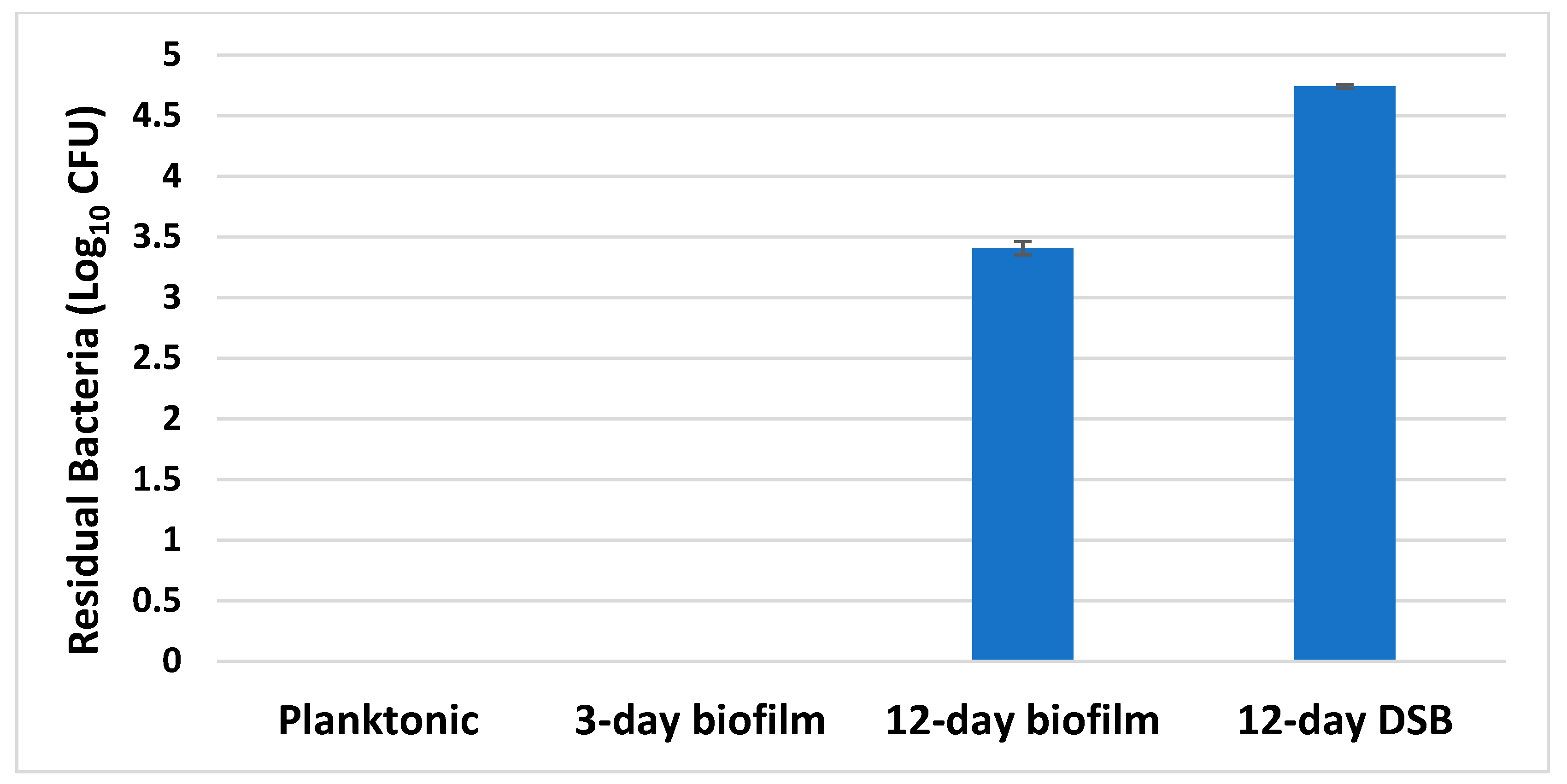
2.5. Disinfectant Efficacy Testing Results
3. Discussion
4. Methods
4.1. Bacterial Culture
4.2. Colony Forming Units Determination
4.3. Proteomics Analysis of Biofilm
4.4. Scanning Electron Microscopy
4.5. Transmission Electron Microscopy
4.6. Peptidoglycan Measurement
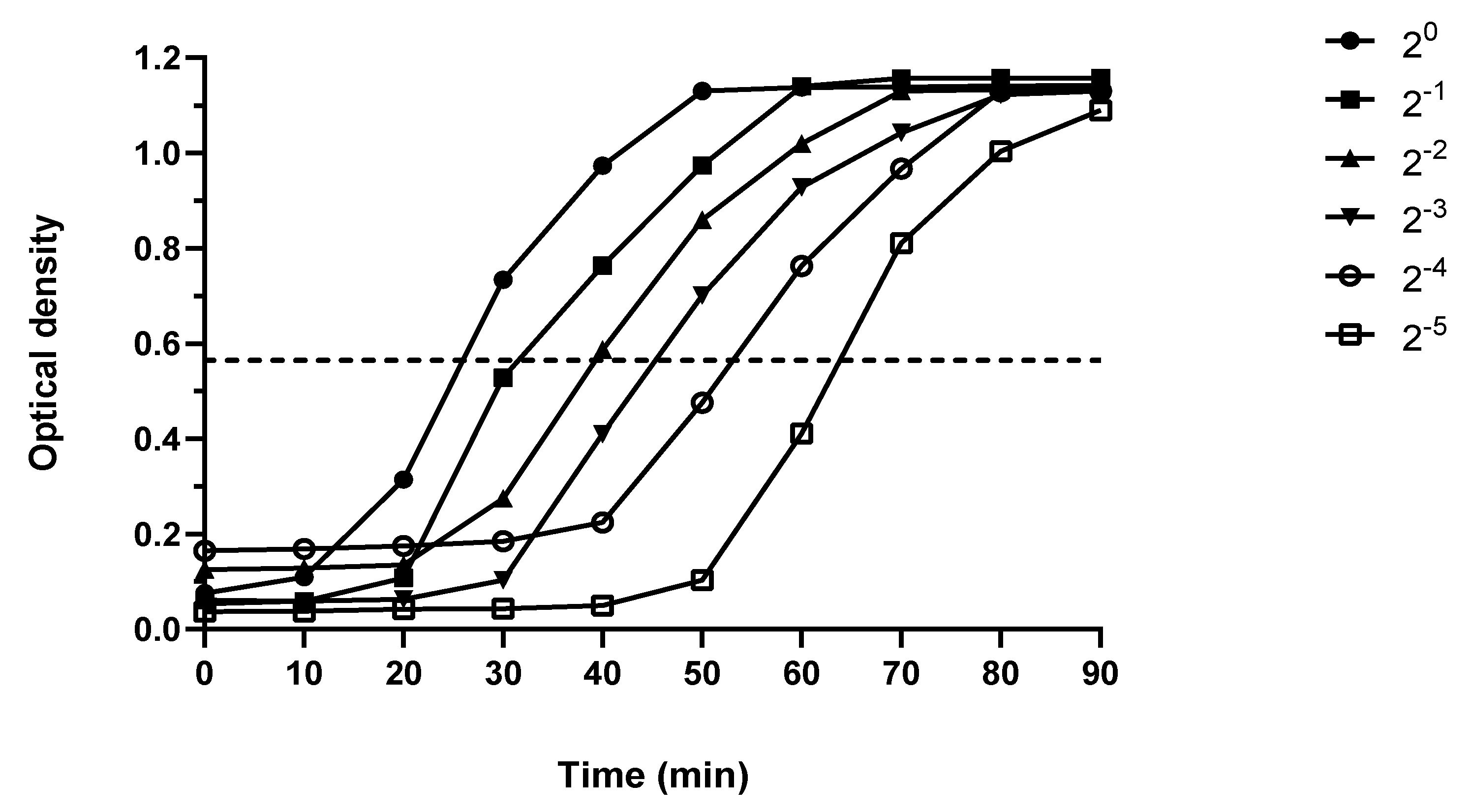
4.6.1. Production of the Standard Curve
4.6.2. Measurement of Peptidoglycan
4.7. Disinfectant Efficacy Testing
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Fux, C.A.; Costerton, J.W.; Stewart, P.S.; Stoodley, P. Survival strategies of infectious biofilms. Trends Microbiol. 2005, 13, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Johani, K.; Gosbell, I.B.; Jacombs, A.; Almatroudi, A.; Whiteley, G.S.; Deva, A.K.; Jensen, S.; Vickery, K. Intensive care unit environmental surfaces are contaminated by multiresistant bacteria in biofilms: Combined results of conventional culture, pyrosequencing, scanning electron microscopy and confocal laser microscopy. J. Hosp. Infect. 2015, 91, 35–44. [Google Scholar] [CrossRef]
- Almatroudi, A.; Tahir, S.; Hu, H.; Chowdhury, D.; Gosbell, I.B.; Jensen, S.O.; Whiteley, G.S.; Deva, A.K.; Glasbey, T.; Vickery, K. Staphylococcus aureus dry-surface biofilms are more resistant to heat treatment than traditional hydrated biofilms. J. Hosp. Infect. 2018, 98, 161–167. [Google Scholar] [CrossRef]
- Alfa, M.J.; Howie, R. Modeling microbial survival in buildup biofilm for complex medical devices. BMC Infect. Dis. 2009, 9, 56. [Google Scholar] [CrossRef]
- Vickery, K.; Deva, A.; Jacombs, A.; Allan, J.; Valente, P.; Gosbell, I.B. Presence of biofilm containing viable multiresistant organisms despite terminal cleaning on clinical surfaces in an intensive care unit. J. Hosp. Infect. 2012, 80, 52–55. [Google Scholar] [CrossRef]
- Ledwoch, K.; Dancer, S.J.; Otter, J.A.; Kerr, K.; Roposte, D.; Maillard, J.-Y. Beware Biofilm! Dry biofilms containing bacterial pathogens on multiple healthcare surfaces; a multicentre study. J. Hosp. Infect. 2018, 100, e47–e56. [Google Scholar] [CrossRef]
- Costa, D.M.; Johani, K.; Melo, D.S.; Lopes, L.K.O.; Lopes Lima, L.K.O.; Tipple, A.F.V.; Hu, H.; Vickery, K. Biofilm contamination of high-touched surfaces in intensive care units: Epidemiology and potential impacts. Lett. Appl. Microbiol. 2019, 68, 269–276. [Google Scholar] [CrossRef]
- Johani, K.; Abualsaud, D.; Costa, D.M.; Hu, H.; Whiteley, G.; Deva, A.; Vickery, K. Characterization of microbial community composition, antimicrobial resistance and biofilm on intensive care surfaces. J. Infect. Public Health 2018, 11, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Otter, J.A.; Vickery, K.; Walker, J.T.; de Lancey Pulcini, E.; Stoodley, P.; Goldenberg, S.D.; Salkeld, J.A.G.; Chewins, J.; Yezli, S.; Edgeworth, J.D. Surface-attached cells, biofilms and biocide susceptibility: Implications for hospital cleaning anddisinfection. J. Hosp. Infect. 2015, 89, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Almatroudi, A.; Gosbell, I.B.; Hu, H.; Jensen, S.O.; Espedido, B.A.; Tahir, S.; Glasbey, T.O.; Legge, P.; Whiteley, G.; Deva, A.; et al. Staphylococcus aureus dry-surface biofilms are not killed by sodium hypochlorite: Implications for infection control. J. Hosp. Infect. 2016, 93, 263–270. [Google Scholar] [CrossRef]
- Chowdhury, D.; Rahman, A.; Hu, H.; Jensen, S.O.; Deva, A.K.; Vickery, K. Effect of disinfectant formulation and organic soil on the efficacy of oxidizing disinfectants against biofilms. J. Hosp. Infect. 2019, 103, e33–e41. [Google Scholar] [CrossRef]
- Parvin, F.; Hu, H.; Whiteley, G.S.; Glasbey, T.; Vickery, K. Difficulty in removing biofilm from dry surfaces. J. Hosp. Infect. 2019, 103, 465–467. [Google Scholar] [CrossRef]
- Ledwoch, K.; Said, J.; Norville, P.; Maillard, J.Y. Artificial dry surface biofilm models for testing the efficacy of cleaning and disinfection. Lett. Appl. Microbiol. 2019, 68, 329–336. [Google Scholar] [CrossRef]
- Russo, P.L.; Stewardson, A.J.; Cheng, A.C.; Bucknall, T.; Mitchell, B.G. The prevalence of healthcare associated infections among adult inpatients at nineteen large Australian acute-care public hospitals: A point prevalence survey. Antimicrob. Resist. Infect. Control 2019, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Tahir, S.; Chowdhury, D.; Legge, M.; Hu, H.; Whiteley, G.; Glasbey, T.; Deva, A.K.; Vickery, K. Transmission of Staphylococcus aureus from dry surface biofilm (DSB) via different types of gloves. Infect. Control Hosp. Epidemiol. 2019, 40, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Creamer, E.; Dorrian, S.; Dolan, A.; Sherlock, O.; Fitzgerald-Hughes, D.; Thomas, T.; Walsh, J.; Shore, A.; Sullivan, D.; Kinnevey, P.; et al. When are the hands of healthcare workers positive for meticillin-resistant Staphylococcus aureus? J. Hosp. Infect. 2010, 75, 107–111. [Google Scholar] [CrossRef]
- Vickery, K.; Hu, H.; Jacombs, A.S.; Bradshaw, D.A.; Deva, A.K. A review of bacterial biofilms and their role in device-associated infection. Healthc. Infect. 2013, 18, 61–66. [Google Scholar] [CrossRef]
- Oliveira, W.F.; Silva, P.M.S.; Silva, R.C.S.; Silva, G.M.M.; Machado, G.; Coelho, L.C.B.B.; Correia, M.T.S. Staphylococcus aureus and Staphylococcus epidermidis infections on implants. J. Hosp. Infect. 2018, 98, 111–117. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, Q.; Zhang, D.; Yan, W. Mechanisms and control strategies of antibiotic resistance in pathological biofilms. J. Microbiol. Biotechnol. 2021, 31, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Espaillat, A.; Cava, F. Bacterial strategies to preserve cell wall integrity against environmental threats. Front. Microbiol. 2018, 9, 2064. [Google Scholar] [CrossRef] [PubMed]
- Barreteau, H.; Kovač, A.; Boniface, A.; Sova, M.; Gobec, S.; Blanot, D. Cytoplasmic steps of peptidoglycan biosynthesis. FEMS Microbiol. Rev. 2008, 32, 168–207. [Google Scholar] [CrossRef] [PubMed]
- Nannini, E.; Murray, B.E.; Arias, C.A. Resistance or decreased susceptibility to glycopeptides, daptomycin, and linezolid in methicillin-resistant Staphylococcus aureus. Curr. Opin. Pharmacol. 2010, 10, 516–521. [Google Scholar] [CrossRef]
- Bayer, A.S.; Schneider, T.; Sahl, H.G. Mechanisms of daptomycin resistance in Staphylococcus aureus: Role of the cell membrane and cell wall. Ann. N. Y. Acad. Sci. 2013, 1277, 139–158. [Google Scholar] [CrossRef] [PubMed]
- García, A.B.; Viñuela-Prieto, J.M.; López-González, L.; Candel, F.J. Correlation between resistance mechanisms in Staphylococcus aureus and cell wall and septum thickening. Infect. Drug Resist. 2017, 10, 353–356. [Google Scholar] [CrossRef]
- Cui, L.; Ma, X.; Sato, K.; Okuma, K.; Tenover, F.C.; Mamizuka, E.M.; Gemmell, C.G.; Kim, M.N.; Ploy, M.C.; El Solh, N.; et al. Cell wall thickening is a common feature of vancomycin resistance in Staphylococcus aureus. J. Clin. Microbiol. 2003, 41, 5–14. [Google Scholar] [CrossRef]
- Ming, T.; Geng, L.; Feng, Y.; Lu, C.; Zhou, J.; Li, Y.; Zhang, D.; He, S.; Li, Y.; Cheong, L.; et al. ITRAQ-Based Quantitative Proteomic Profiling of Staphylococcus aureus under Different Osmotic Stress Conditions. Front. Microbiol. 2019, 10, 1082. [Google Scholar] [CrossRef]
- De Goffau, M.C.; Yang, X.; Van Dijl, J.M.; Harmsen, H.J.M. Bacterial pleomorphism and competition in a relative humidity gradient. Environ. Microbiol. 2009, 11, 809–822. [Google Scholar] [CrossRef]
- Marshall, B.J.; Ohye, D.F.; Christian, J.H. Tolerance of bacteria to high concentrations of NaCl and glycerol in the growth medium. Appl. Microbiol. 1971, 21, 363–364. [Google Scholar] [CrossRef]
- Rahman, M.A.; Amirkhani, A.; Chowdhury, D.; Mempin, M.; Molloy, M.P.; Deva, A.K.; Vickery, K.; Hu, H. Proteome of Staphylococcus aureus Biofilm Changes Significantly with Aging. Int. J. Mol. Sci. 2022, 23, 6415. [Google Scholar] [CrossRef]
- Rahman, M.A.; Amirkhani, A.; Parvin, F.; Chowdhury, D.; Molloy, M.P.; Deva, A.K.; Vickery, K.; Hu, H. One Step Forward with Dry Surface Biofilm (DSB) of Staphylococcus aureus: TMT-Based Quantitative Proteomic Analysis Reveals Proteomic Shifts between DSB and Hydrated Biofilm. Int. J. Mol. Sci. 2022, 23, 2238. [Google Scholar] [CrossRef]
- Peschel, A.; Otto, M.; Jack, R.W.; Kalbacher, H.; Jung, G.; Götz, F. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 1999, 274, 8405–8410. [Google Scholar] [CrossRef]
- Reichmann, N.T.; Cassona, C.P.; Gründling, A. Revised mechanism of D-alanine incorporation into cell wall polymers in Gram-positive bacteria. Microbiology 2013, 159, 1868–1877. [Google Scholar] [CrossRef]
- Gross, M.; Cramton, S.E.; Götz, F.; Peschel, A. Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infect. Immun. 2001, 69, 3423–3426. [Google Scholar] [CrossRef]
- Abdallah, M.; Chataigne, G.; Ferreira-Theret, P.; Benoliel, C.; Drider, D.; Dhulster, P.; Chihib, N.E. Effect of growth temperature, surface type and incubation time on the resistance of Staphylococcus aureus biofilms to disinfectants. Appl. Microbiol. Biotechnol. 2014, 98, 2597–2607. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.B.R.; Ferreira, M.C.S.; Glatthardt, T.; Silvério, M.P.; Chamon, R.C.; Salgueiro, V.C.; Guimarães, L.C.; Alves, E.S.; dos Santos, K.R.N. Osmotic stress induces biofilm production by Staphylococcus epidermidis isolates from neonates. Diagn. Microbiol. Infect. Dis. 2019, 94, 337–341. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Thurnheer, T.; Gmür, R.; Shapiro, S.; Guggenheim, B. Mass transport of macromolecules within an in vitro model of supragingival plaque. Appl. Environ. Microbiol. 2003, 69, 1702–1709. [Google Scholar] [CrossRef]
- Stewart, P.S.; Rayner, J.; Roe, F.; Rees, W.M. Biofilm penetration and disinfection efficacy of alkaline hypochlorite and chlorosulfamates. J. Appl. Microbiol. 2001, 91, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Tabak, M.; Scher, K.; Hartog, E.; Romling, U.; Matthews, K.R.; Chikindas, M.L.; Yaron, S. Effect of triclosan on Salmonella typhimurium at different growth stages and in biofilms. FEMS Microbiol. Lett. 2007, 267, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef]
- Almatroudi, A.; Hu, H.; Deva, A.; Gosbell, I.B.; Jacombs, A.; Jensen, S.O.; Whiteley, G.; Glasbey, T.; Vickery, K. A new dry-surface biofilm model: An essential tool for efficacy testing of hospital surface decontamination procedures. J. Microbiol. Methods 2015, 117, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, Y.; Su, W.; Chai, J.; Xu, L.; Cao, J.; Liu, Y. Encapsulated DNase improving the killing efficiency of antibiotics in staphylococcal biofilms. J. Mater. Chem. B 2020, 8, 4395–4401. [Google Scholar] [CrossRef] [PubMed]
- Wongkaewkhiaw, S.; Kanthawong, S.; Bolscher, J.G.M.; Nazmi, K.; Taweechaisupapong, S.; Krom, B.P. DNase-mediated eDNA removal enhances D-LL-31 activity against biofilms of bacteria isolated from chronic rhinosinusitis patients. Biofouling 2020, 36, 1117–1128. [Google Scholar] [CrossRef]
- Jacombs, A.; Tahir, S.; Hu, H.; Deva, A.K.; Almatroudi, A.; Wessels, W.L.F.; Bradshaw, D.A.; Vickery, K. In vitro and in vivo investigation of the influence of implant surface on the formation of bacterial biofilm in mammary implants. Plast. Reconstr. Surg. 2014, 133, 471e–480e. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parvin, F.; Rahman, M.A.; Deva, A.K.; Vickery, K.; Hu, H. Staphylococcus aureus Cell Wall Phenotypic Changes Associated with Biofilm Maturation and Water Availability: A Key Contributing Factor for Chlorine Resistance. Int. J. Mol. Sci. 2023, 24, 4983. https://doi.org/10.3390/ijms24054983
Parvin F, Rahman MA, Deva AK, Vickery K, Hu H. Staphylococcus aureus Cell Wall Phenotypic Changes Associated with Biofilm Maturation and Water Availability: A Key Contributing Factor for Chlorine Resistance. International Journal of Molecular Sciences. 2023; 24(5):4983. https://doi.org/10.3390/ijms24054983
Chicago/Turabian StyleParvin, Farhana, Md. Arifur Rahman, Anand K. Deva, Karen Vickery, and Honghua Hu. 2023. "Staphylococcus aureus Cell Wall Phenotypic Changes Associated with Biofilm Maturation and Water Availability: A Key Contributing Factor for Chlorine Resistance" International Journal of Molecular Sciences 24, no. 5: 4983. https://doi.org/10.3390/ijms24054983
APA StyleParvin, F., Rahman, M. A., Deva, A. K., Vickery, K., & Hu, H. (2023). Staphylococcus aureus Cell Wall Phenotypic Changes Associated with Biofilm Maturation and Water Availability: A Key Contributing Factor for Chlorine Resistance. International Journal of Molecular Sciences, 24(5), 4983. https://doi.org/10.3390/ijms24054983






