Recent Advances in Tick Antigen Discovery and Anti-Tick Vaccine Development
Abstract
1. Introduction
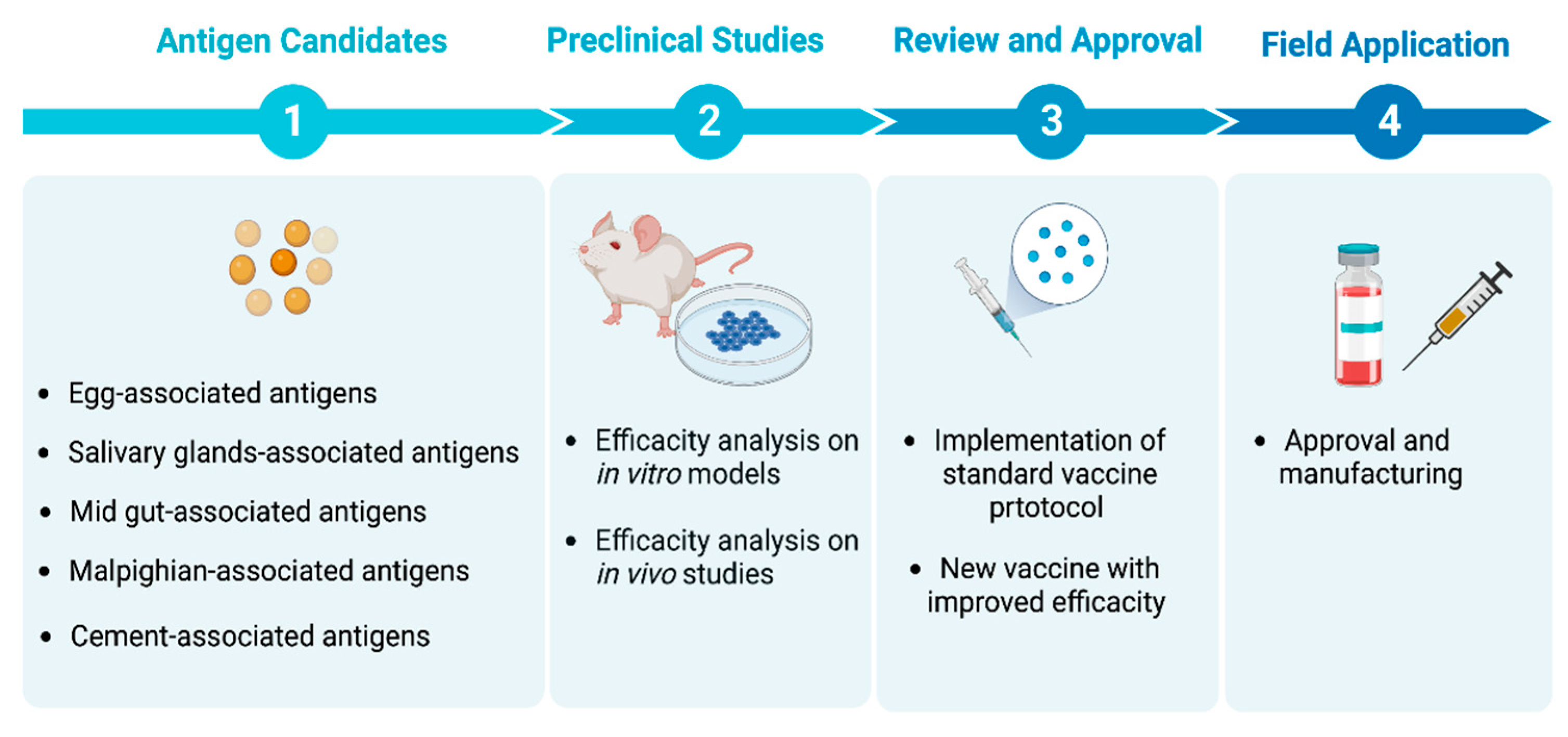
2. Identification of Antigens: A Road Map to Develop an Anti-Tick Vaccine
2.1. Egg-Associated Antigen Candidates
2.2. Salivary Gland-Associated Antigen Candidates
2.3. Midgut-Associated Antigen Candidates
2.4. Malpighian-Associated Antigen Candidates
2.5. Tick-Cement-Associated Antigen Candidates
| Antigen Candidate | Characterization | Feature of Antigen | Species | Experimental Animal | Efficacy or Reduction (%) | References |
|---|---|---|---|---|---|---|
| Egg-associated antigens | ||||||
| BYC | Boophilus yolk pro-cathepsin | Native protein | R. microplus | Cattle | 14–36 | [24] |
| Recombinant protein | R. microplus | Cattle | 25.24 | [26] | ||
| VTDCE | Vitellin-degrading cysteine endopeptidase | Native protein | R. microplus | Cattle | 21 | [31] |
| Vitelin | Vitelin | Native protein | R. microplus | Sheep | 68 | [28] |
| GP80 | Glycoprotein | Native protein | R. microplus | Sheep | 68 | [28] |
| HH-GP80 | Hexahis-GP80 | Native protein | R. microplus | Cattle | 72.80 | [65] |
| FER1 | Ferritin | Recombinant protein | H. longicornis | Rabbit | 34 | [72] |
| FER 2 | - | Recombinant protein | H. longicornis | Rabbit | 49 | [72] |
| FER 2 | - | Recombinant protein | P. schulze | Guinea pigs | [76] | |
| FER 2 | - | Recombinant protein | I. ricinus | Cattle | 63–98 | [75,119] |
| FER 2 | - | Recombinant protein | H. anatolicum | Cattle | 51.2–51.7 | [74] |
| FER 2 | - | Native protein | R. microplus | Cattle | 64 | [119] |
| BmTI N-terminal | Trypsin inhibitor | Synthetic peptide | R. microplus | Cattle | 18.4 | [64] |
| RmLTI | Recombinant protein | R. microplus | Cattle | 32 | [66] | |
| Gut associated antigens | ||||||
| Bm95 | B. microplus 95, peptidase | Recombinant protein | R. microplus | Cattle | 81.27–89 | [34,120] |
| Bm4912 | Glycoprotein | Synthetic peptide | B. microplus | Cattle | 72.4 | [121] |
| Bm7462® | Glycoprotein | Synthetic peptide | Control | 81.05 | [121] | |
| Bm19733 | - | Synthetic peptide | R. microplus | Cattle | 35.87 | [121] |
| Bm7462® | - | Recombinant protein | Cattle | 72.4 | [122] | |
| Ba86 | B. annulatus 86 protein | Recombinant protein | R. microplus | Cattle | 71.5 | [123] |
| Haa86 | H. anatolicum 86 protein | Recombinant protein | H. a. anatolicum | Cattle | 36.5 | [124] |
| TROSPA | Tick receptor for outer surface protein A | Recombinant protein | R. microplus | Cattle | 0 | [14] |
| RmAQP1 | Aquaporin 1 | Recombinant protein | R. microplus | Cattle | 68–75 | [62] |
| RmAQP2 | Aquaporin 2 | Synthetic peptide | R. microplus | Cattle | 25 | [92] |
| OeAQP, OeAQP1 | Aquaporins | Synthetic peptides | O. erraticus | Rabbit | 4.6 | [94] |
| GST | Glutathione S-transferases | Recombinant protein | H. longicornis | Cattle | 57 | [107] |
| ATAQ | Peptidase | Synthetic protein | R. microplus | Cattle | 35 | [125] |
| 5′-nucleotidase | Recombinant protein | R. microplus | Cattle | 0 | [104] | |
| Salivary gland associated antigens | ||||||
| RmSUB | Subolesin, trancription factor | Recombinant protein | R. microplus | Cattle | 51–75 | [99,100] |
| BmSUB | Subolesin | Recombinant protein | R. microplus | Cattle | 37.2–44.1 | [101] |
| Bm91 | B. microplus 91 | Recombinant protein | R. microplus | Cattle | 6 | [35] |
| FSP | Flagelliform silk protein, Glycoprotein | Recombinant protein | R. microplus | Cattle | 62 | [14] |
| UBQ | Ubiquitin | Synthetic peptide | R. microplus | Cattle | 55 | [99] |
| Cement-associated antigens | ||||||
| 64TRPs | Truncated constructs of 64P | R. appendiculatus | Rabbits | 40–70 | [116] | |
| Multi-antigen | ||||||
| Rm39 + Rm76 + Rm180 + Rm239 | R. microplus 39 + R. microplus 76 + R. microplus 180 + R. microplus 239 | Recombinant salivary gland proteins | R. microplus | Cattle | 73.2 | [51] |
| SUB + Bm86 | Subolesin and B. microplus 86 dual vaccine | Recombinant salivary gland (SUB) and gut (Bm86) protein | R. microplus | Cattle | 97 | [126] |
| SUB + IV | Subolesin + heat-inactivated Mycobacterium bovis | Recombinant salivary gland protein and inactivated mycobacterium | R. microplus | Cattle | 65 | [127] |
| Bm95-MSP1a | B. microplus 95-Major surface protein 1a | Recombinant protein | R. microplus | Control | 64 | [102] |
| RmLTI-BmCG-LTB | R. microplus larvae trypsin inhibitors, B. microplus Campo grande heat-labile enterotoxin B | Recombinant protein | R. microplus | Cattle | 55.6 | [128] |
| UBQ-MSP1a | Ubiquitin-major surface protein 1a | Recombinant protein | R. microplus | Cattle | 0 | [102] |
| SUB-MSP1a | Subolesin-Major surface protein 1a | Recombinant protein | R. microplus | Cattle | 81 | [102] |
| Q38 | Recombinant protein | R. microplus | Cattle | 75 | [14,129] | |
| EF1a-MSP1a | Elongation factor 1 alpha-Major surface protein 1a | Recombinant protein | R. microplus | Cattle | 38 | [102] |
| BrRm-MP4 | Reprolysin R. microplus-Metaloprotease 4 | Recombinant protein | R. microplus | Cattle | 60 | [50] |
| pcDNA3.1-HlLIP | Plasmid-Lipocalins | Recombinant plasmid | H. longicornis | Rabbit | 30 | [130] |
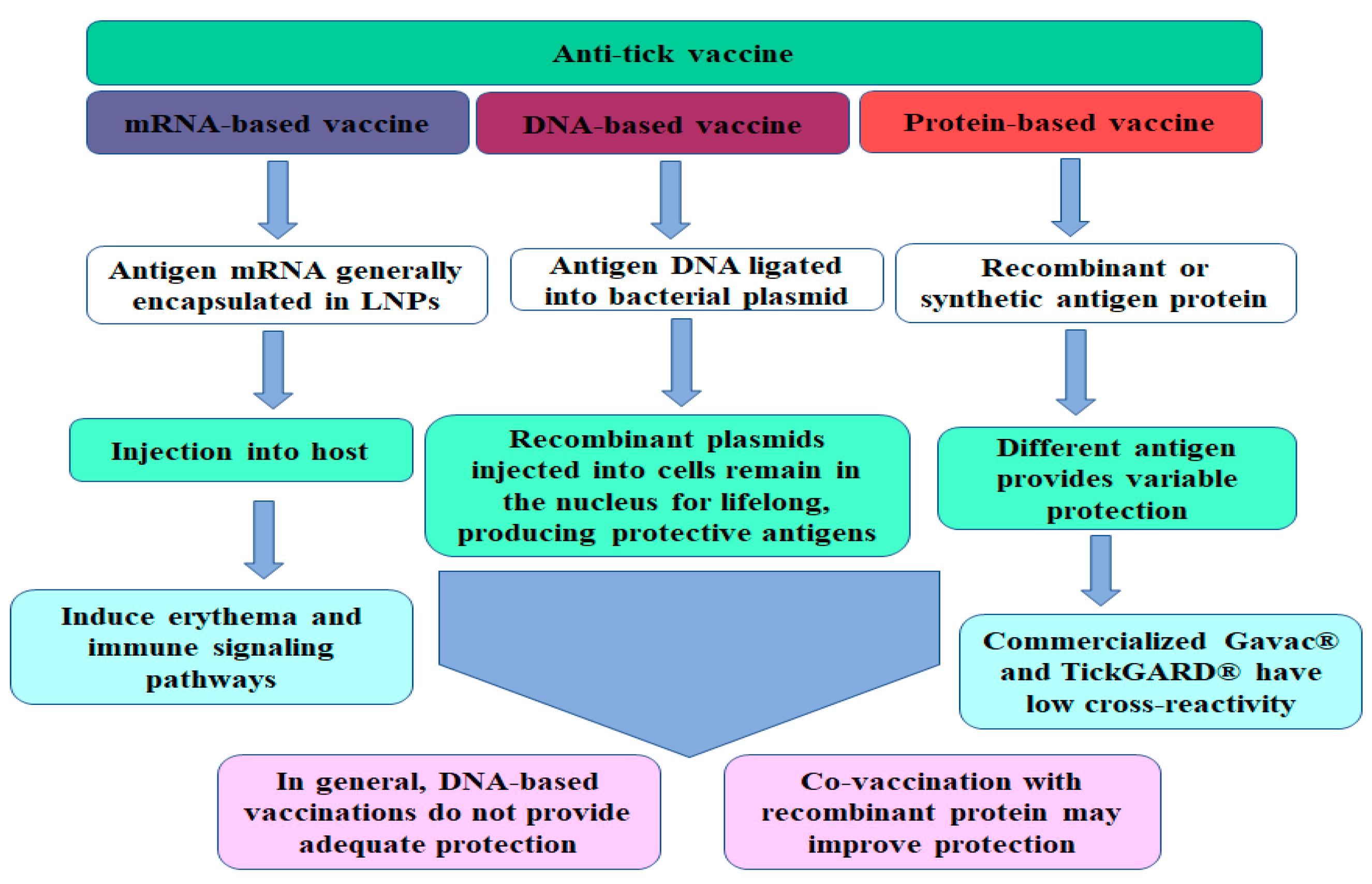
3. The Types of Anti-Tick Vaccines
3.1. DNA-Based Anti-Tick Vaccines
3.2. mRNA Vaccine
3.3. Protein-Based Vaccines
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de la Fuente, J.; Kocan, K.M. Advances in the identification and characterization of protective antigens for recombinant vaccines against tick infestations. Expert Rev. Vaccines 2003, 2, 583–593. [Google Scholar] [CrossRef]
- Weaver, G.V.; Anderson, N.; Garrett, K.; Thompson, A.T.; Yabsley, M.J. Ticks and Tick-Borne Pathogens in Domestic Animals, Wild Pigs, and Off-Host Environmental Sampling in Guam, USA. Front. Vet. Sci. 2022, 8, 803424. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef]
- Beugnet, F.; Marié, J.-L. Emerging arthropod-borne diseases of companion animals in Europe. Vet. Parasitol. 2009, 163, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.G.; Kariuki, H.W.; Aboge, G.O.; Gakuya, D.W.; Maingi, N.; Mulei, C.M. Prevalence of Ticks Infesting Dairy Cattle and the Pathogens They Harbour in Smallholder Farms in Peri-Urban Areas of Nairobi, Kenya. Vet. Med. Int. 2021, 2021, 9501648. [Google Scholar] [CrossRef]
- Graf, J.-F.; Gogolewski, R.; Leach-Bing, N.; Sabatini, G.A.; Molento, M.B.; Bordin, E.L.; Arantes, G.J. Tick control: An industry point of view. Parasitology 2004, 129, S427–S442. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, J. Vaccines for vector control: Exciting possibilities for the future. Vet. J. 2012, 194, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Sparagano, O.; Földvári, G.; Derdáková, M.; Kazimírová, M. New challenges posed by ticks and tick-borne diseases. Biologia 2022, 77, 1497–1501. [Google Scholar] [CrossRef]
- Doolan, D.L.; Apte, S.H.; Proietti, C. Genome-based vaccine design: The promise for malaria and other infectious diseases. Int. J. Parasitol. 2014, 44, 901–913. [Google Scholar] [CrossRef]
- Bragazzi, N.L.; Gianfredi, V.; Villarini, M.; Rosselli, R.; Nasr, A.; Hussein, A.; Martini, M.; Behzadifar, M. Vaccines Meet Big Data: State-of-the-Art and Future Prospects. From the Classical 3Is (“Isolate-Inactivate-Inject”) Vaccinology 1.0 to Vaccinology 3.0, Vaccinomics, and Beyond: A Historical Overview. Front. Public Health 2018, 6, 62. [Google Scholar] [CrossRef]
- Zepp, F. Principles of vaccine design—Lessons from nature. Vaccine 2010, 28 (Suppl. 3), C14–C24. [Google Scholar] [CrossRef]
- Bouazzaoui, A.; Abdellatif, A.; Al-Allaf, F.; Bogari, N.; Al-Dehlawi, S.; Qari, S. Strategies for Vaccination: Conventional Vaccine Approaches Versus New-Generation Strategies in Combination with Adjuvants. Pharmaceutics 2021, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- D’Argenio, D.A.; Wilson, C.B. A Decade of Vaccines: Integrating Immunology and Vaccinology for Rational Vaccine Design. Immunity 2010, 33, 437–440. [Google Scholar] [CrossRef]
- Merino, O.; Antunes, S.; Mosqueda, J.; Moreno-Cid, J.A.; de la Lastra, J.M.P.; Rosario-Cruz, R.; Rodríguez, S.; Domingos, A.; de la Fuente, J. Vaccination with proteins involved in tick–pathogen interactions reduces vector infestations and pathogen infection. Vaccine 2013, 31, 5889–5896. [Google Scholar] [CrossRef] [PubMed]
- White, A.L.; Gaff, H. Review: Application of Tick Control Technologies for Blacklegged, Lone Star, and American Dog Ticks. J. Integr. Pest Manag. 2018, 9, 12. [Google Scholar] [CrossRef]
- Willadsen, P. Anti-tick vaccines. Parasitology 2004, 129, S367–S387. [Google Scholar] [CrossRef]
- de la Fuente, J.; Merino, O. Vaccinomics, the new road to tick vaccines. Vaccine 2013, 31, 5923–5929. [Google Scholar] [CrossRef]
- Hill, C.A.; Wikel, S.K. The Ixodes scapularis Genome Project: An opportunity for advancing tick research. Trends Parasitol. 2005, 21, 151–153. [Google Scholar] [CrossRef]
- Medina, J.M.; Abbas, M.N.; Bensaoud, C.; Hackenberg, M.; Kotsyfakis, M. Bioinformatic Analysis of Ixodes ricinus Long Non-Coding RNAs Predicts Their Binding Ability of Host miRNAs. Int. J. Mol. Sci. 2022, 23, 9761. [Google Scholar] [CrossRef]
- Valle, M.R.; Guerrero, F.D. Anti-tick vaccines in the omics era. Front. Biosci. (Elite Ed.) 2018, 10, 122–136. [Google Scholar] [CrossRef]
- Logullo, C.; Vaz, I.D.S.; Sorgine, M.H.F.; Paiva-Silva, G.O.; Faria, F.S.; Zingali, R.B.; DE Lima, M.F.R.; Abreu, L.; Oliveira, E.F.; Alves, E.W.; et al. Isolation of an aspartic proteinase precursor from the egg of a hard tick, Boophilus microplus. Parasitology 1998, 116, 525–532. [Google Scholar] [CrossRef]
- Kurlovs, A.H.; Li, J.; Cheng, D.; Zhong, J. Ixodes pacificus Ticks Maintain Embryogenesis and Egg Hatching after Antibiotic Treatment of Rickettsia Endosymbiont. PLoS ONE 2014, 9, e104815. [Google Scholar] [CrossRef] [PubMed]
- Sappington, T.W.; Hays, A.R.; Raikhel, A.S. Mosquito vitellogenin receptor: Purification, developmental and biochemical characterization. Insect Biochem. Mol. Biol. 1995, 25, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Vaz, I.D.S.; Logullod, C.; Sorgine, M.; Velloso, F.F.; de Lima, M.F.R.; Gonzales, J.C.; Masuda, H.; Oliveira, P.L.; Masudaa, A. Immunization of bovines with an aspartic proteinase precursor isolated from Boophilus microplus eggs. Vet. Immunol. Immunopathol. 1998, 66, 331–341. [Google Scholar] [CrossRef]
- Leal, A.T.; Seixas, A.; Pohl, P.C.; Ferreira, C.A.; Logullo, C.; Oliveira, P.L.; Farias, S.E.; Termignoni, C.; Vaz, I.D.S.; Masuda, A. Vaccination of bovines with recombinant Boophilus Yolk pro-Cathepsin. Vet. Immunol. Immunopathol. 2006, 114, 341–345. [Google Scholar] [CrossRef]
- Leal, A.T.; Pohl, P.C.; Ferreira, C.A.; Nascimento-Silva, M.C.; Sorgine, M.H.; Logullo, C.; Oliveira, P.L.; Farias, S.E.; Vaz, I.D.S.; Masuda, A. Purification and antigenicity of two recombinant forms of Boophilus microplus yolk pro-cathepsin expressed in inclusion bodies. Protein Expr. Purif. 2006, 45, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, O.; Indrasith, L.S. Metabolic Fates of Yolk Proteins during Embryogenesis in Arthropods. (Arthropods/embryogenesis/yolk proteins/limited proteolysis/protease). Dev. Growth Differ. 1988, 30, 337–346. [Google Scholar] [CrossRef]
- Tellam, R.; Kemp, D.; Riding, G.; Briscoe, S.; Smith, D.; Sharp, P.; Irving, D.; Willadsen, P. Reduced oviposition of Boophilus microplus feeding on sheep vaccinated with vitellin. Vet. Parasitol. 2002, 103, 141–156. [Google Scholar] [CrossRef]
- Boldbaatar, D.; Umemiya-Shirafuji, R.; Liao, M.; Tanaka, T.; Xuan, X.; Fujisaki, K. Multiple vitellogenins from the Haemaphysalis longicornis tick are crucial for ovarian development. J. Insect Physiol. 2010, 56, 1587–1598. [Google Scholar] [CrossRef]
- Seixas, A.; Dos Santos, P.C.; Velloso, F.F.; Vaz, I.D.S.; Masuda, A.; Horn, F.; Termignoni, C. A Boophilus microplus vitellin-degrading cysteine endopeptidase. Parasitology 2003, 126, 155–163. [Google Scholar] [CrossRef]
- Seixas, A.; Leal, A.T.; Nascimento-Silva, M.C.L.; Masuda, A.; Termignoni, C.; Vaz, I.D.S. Vaccine potential of a tick vitellin-degrading enzyme (VTDCE). Vet. Immunol. Immunopathol. 2008, 124, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Jarmey, J.; Riding, G.; Pearson, R.; McKenna, R.; Willadsen, P. Carboxydipeptidase from Boophilus microplus: A “concealed” antigen with similarity to angiotensin-converting enzyme. Insect Biochem. Mol. Biol. 1995, 25, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Willadsen, P.; Smith, D.; Cobon, G.; McKenna, R.V. Comparative vaccination of cattle against Boophilus microplus with recombinant antigen Bm86 alone or in combination with recombinant Bm91. Parasite Immunol. 1996, 18, 241–246. [Google Scholar] [CrossRef]
- García-García, J.C.; Montero, C.; Redondo, M.; Vargas, M.; Canales, M.; Boue, O.; Rodríguez, M.; Joglar, M.; Machado, H.; González, I.L.; et al. Control of ticks resistant to immunization with Bm86 in cattle vaccinated with the recombinant antigen Bm95 isolated from the cattle tick, Boophilus microplus. Vaccine 2000, 18, 2275–2287. [Google Scholar] [CrossRef]
- Lambertz, C.; Chongkasikit, N.; Jittapalapong, S.; Gauly, M. Immune Response of Bos indicus Cattle against the Anti-Tick Antigen Bm91 Derived from Local Rhipicephalus (Boophilus) microplus Ticks and Its Effect on Tick Reproduction under Natural Infestation. J. Parasitol. Res. 2012, 2012, 907607. [Google Scholar] [CrossRef]
- Zivkovic, Z.; Esteves, E.; Almazán, C.; Daffre, S.; Nijhof, A.M.; Kocan, K.M.; Jongejan, F.; De La Fuente, J. Differential expression of genes in salivary glands of male Rhipicephalus (Boophilus)microplus in response to infection with Anaplasma marginale. BMC Genom. 2010, 11, 186. [Google Scholar] [CrossRef]
- Alarcon-Chaidez, F.J.; Sun, J.; Wikel, S.K. Transcriptome analysis of the salivary glands of Dermacentor andersoni Stiles (Acari: Ixodidae). Insect Biochem. Mol. Biol. 2007, 37, 48–71. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthi, N.; Narasimhan, S.; Pal, U.; Bao, F.; Yang, X.F.; Fish, D.; Anguita, J.; Norgard, M.V.; Kantor, F.S.; Anderson, J.F.; et al. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature 2005, 436, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Hovius, J.W.R.; Levi, M.; Fikrig, E. Salivating for Knowledge: Potential Pharmacological Agents in Tick Saliva. PLoS Med. 2008, 5, e43. [Google Scholar] [CrossRef] [PubMed]
- Schuijt, T.J.; Hovius, J.W.R.; van Burgel, N.D.; Ramamoorthi, N.; Fikrig, E.; van Dam, A.P. The Tick Salivary Protein Salp15 Inhibits the Killing of Serum-Sensitive Borrelia burgdorferi Sensu Lato Isolates. Infect. Immun. 2008, 76, 2888–2894. [Google Scholar] [CrossRef]
- Wen, S.; Wang, F.; Ji, Z.; Pan, Y.; Jian, M.; Bi, Y.; Zhou, G.; Luo, L.; Chen, T.; Li, L.; et al. Salp15, a Multifunctional Protein from Tick Saliva With Potential Pharmaceutical Effects. Front. Immunol. 2020, 10, 3067. [Google Scholar] [CrossRef]
- Dai, J.; Wang, P.; Adusumilli, S.; Booth, C.J.; Narasimhan, S.; Anguita, J.; Fikrig, E. Antibodies against a Tick Protein, Salp15, Protect Mice from the Lyme Disease Agent. Cell Host Microbe 2009, 6, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Kolb, P.; Verreiter, J.; Habicht, J.; Bentrop, D.; Wallich, R.; Nassal, M. Soluble cysteine-rich tick saliva proteins Salp15 and Iric-1 from E. coli. FEBS Open Bio 2015, 5, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Kolb, P.; Wallich, R.; Nassal, M. Whole-Chain Tick Saliva Proteins Presented on Hepatitis B Virus Capsid-Like Particles Induce High-Titered Antibodies with Neutralizing Potential. PLoS ONE 2015, 10, e0136180. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Francischetti, I.M.; Mather, T.N.; Ribeiro, J.M. Cloning of a salivary gland metalloprotease and characterization of gelatinase and fibrin(ogen)lytic activities in the saliva of the Lyme disease tick vector Ixodes scapularis. Biochem. Biophys. Res. Commun. 2003, 305, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Harnnoi, T.; Sakaguchi, T.; Nishikawa, Y.; Xuan, X.; Fujisaki, K. Molecular characterization and comparative study of 6 salivary gland metalloproteases from the hard tick, Haemaphysalis longicornis. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 147, 93–101. [Google Scholar] [CrossRef]
- Decrem, Y.; Beaufays, J.; Blasioli, V.; Lahaye, K.; Brossard, M.; Vanhamme, L.; Godfroid, E. A family of putative metalloproteases in the salivary glands of the tick Ixodes ricinus. FEBS J. 2008, 275, 1485–1499. [Google Scholar] [CrossRef]
- Barnard, A.-C.; Nijhof, A.M.; Gaspar, A.R.; Neitz, A.W.; Jongejan, F.; Maritz-Olivier, C. Expression profiling, gene silencing and transcriptional networking of metzincin metalloproteases in the cattle tick, Rhipicephalus (Boophilus) microplus. Vet. Parasitol. 2012, 186, 403–414. [Google Scholar] [CrossRef]
- Ali, A.; Fernando Parizi, L.; Garcia Guizzo, M.; Tirloni, L.; Seixas, A.; da Silva Vaz, I., Jr.; Termignoni, C. Immunoprotective potential of a Rhipicephalus (Boophilus) microplus metalloprotease. Vet. Parasitol. 2015, 207, 107–114. [Google Scholar] [CrossRef]
- Maruyama, S.R.; Garcia, G.R.; Teixeira, F.R.; Brandão, L.G.; Anderson, J.M.; Ribeiro, J.M.C.; Valenzuela, J.G.; Horackova, J.; Veríssimo, C.J.; Katiki, L.M.; et al. Mining a differential sialotranscriptome of Rhipicephalus microplus guides antigen discovery to formulate a vaccine that reduces tick infestations. Parasites Vectors 2017, 10, 206. [Google Scholar] [CrossRef]
- Rodríguez-Mallon, A.; Fernández, E.; Encinosa, P.E.; Bello, Y.; Méndez-Pérez, L.; Ruiz, L.C.; Pérez, D.; González, M.; Garay, H.; Reyes, O.; et al. A novel tick antigen shows high vaccine efficacy against the dog tick, Rhipicephalus sanguineus. Vaccine 2012, 30, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro-Da-Silva, A.; Borges, M.C.; Guilvard, E.; Ouaissi, A. Dual Role of the Leishmania major Ribosomal Protein S3a Homologue in Regulation of T- and B-Cell Activation. Infect. Immun. 2001, 69, 6588–6596. [Google Scholar] [CrossRef] [PubMed]
- Radulović, M.; Kim, T.K.; Porter, L.M.; Sze, S.-H.; Lewis, L.; Mulenga, A. A 24–48 h fed Amblyomma americanum tick saliva immuno-proteome. BMC Genom. 2014, 15, 518. [Google Scholar] [CrossRef] [PubMed]
- Tirloni, L.; Reck, J.; Terra, R.M.S.; Martins, J.R.; Mulenga, A.; Sherman, N.E.; Fox, J.W.; Yates, J.R.; Termignoni, C.; Pinto, A.F.M.; et al. Proteomic Analysis of Cattle Tick Rhipicephalus (Boophilus) microplus Saliva: A Comparison between Partially and Fully Engorged Females. PLoS ONE 2014, 9, e94831. [Google Scholar] [CrossRef]
- El-Sayed, S.A.E.; Rizk, M.A.; Eldoumani, H.; Sorour, S.S.; Terkawi, M.A.; Aboulaila, M.; Igarashi, I.; Sayed-Ahmed, M.Z. Identification and Characterization of P0 Protein as a Vaccine Candidate Against Babesia divergens, Blood Parasite of Veterinary and Zoonotic Importance. Front. Vet. Sci. 2021, 8, 795906. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Mallon, A.; Encinosa, P.E.; Méndez-Pérez, L.; Bello, Y.; Fernández, R.R.; Garay, H.; Cabrales, A.; Méndez, L.; Borroto, C.; Estrada, M.P. High efficacy of a 20 amino acid peptide of the acidic ribosomal protein P0 against the cattle tick, Rhipicephalus microplus. Ticks Tick-Borne Dis. 2015, 6, 530–537. [Google Scholar] [CrossRef]
- Willadsen, P.; Riding, A.G. On the biological role of a proteolytic-enzyme inhibitor from the ectoparasitic tick Boophilus microplus. Biochem. J. 1980, 189, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Kausar, S.; Abbas, M.N.; Qian, C.; Zhu, B.; Sun, Y.; Sun, Y.; Wang, L.; Wei, G.; Maqsood, I.; Liu, C.-L. Serpin-14 negatively regulates prophenoloxidase activation and expression of antimicrobial peptides in Chinese oak silkworm Antheraea pernyi. Dev. Comp. Immunol. 2017, 76, 45–55. [Google Scholar] [CrossRef]
- Kausar, S.; Abbas, M.N.; Qian, C.; Zhu, B.; Gao, J.; Sun, Y.; Wang, L.; Wei, G.; Liu, C. Role of Antheraea pernyi serpin 12 in prophenoloxidase activation and immune responses. Arch. Insect Biochem. Physiol. 2018, 97, e21435. [Google Scholar] [CrossRef]
- Abbas, M.N.; Chlastáková, A.; Jmel, M.A.; Iliaki-Giannakoudaki, E.; Chmelař, J.; Kotsyfakis, M. Serpins in Tick Physiology and Tick-Host Interaction. Front. Cell. Infect. Microbiol. 2022, 12, 892770. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, F.D.; Andreotti, R.; Bendele, K.G.; Cunha, R.C.; Miller, R.J.; Yeater, K.; de León, A.A. P Rhipicephalus (Boophilus) microplus aquaporin as an effective vaccine antigen to protect against cattle tick infestations. Parasit Vectors 2014, 7, 475. [Google Scholar]
- Andreotti, R.; Malavazi-Piza, K.C.; Sasaki, S.D.; Torquato, R.J.S.; Gomes, A.; Tanaka, A.S. Serine Proteinase Inhibitors from Eggs and Larvae of Tick Boophilus microplus: Purification and Biochemical Characterization. Protein J. 2001, 20, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, R. A synthetic bmti n-terminal fragment as antigen in bovine immunoprotection against the tick Boophilus microplus in a pen trial. Exp. Parasitol. 2007, 116, 66–70. [Google Scholar] [CrossRef]
- Andreotti, R.; Gomes, A.; Malavazi-Piza, K.C.; Sasaki, S.D.; Sampaio, C.A.; Tanaka, A.S. BmTI antigens induce a bovine protective immune response against Boophilus microplus tick. Int. Immunopharmacol. 2002, 2, 557–563. [Google Scholar] [CrossRef]
- Andreotti, R.; Cunha, R.C.; Soares, M.A.; Guerrero, F.D.; Leite, F.P.L.; de León, A.A.P. Protective immunity against tick infestation in cattle vaccinated with recombinant trypsin inhibitor of Rhipicephalus microplus. Vaccine 2012, 30, 6678–6685. [Google Scholar] [CrossRef] [PubMed]
- Imamura, S.; da Silva Vaz, I., Jr.; Sugino, M.; Ohashi, K.; Onuma, M. A serine protease inhibitor (serpin) from Haemaphysalis longicornis as an anti-tick vaccine. Vaccine 2005, 23, 1301–1311. [Google Scholar] [CrossRef]
- Jittapalapong, S.; Kaewhom, P.; Pumhom, P.; Canales, M.; De La Fuente, J.; Stich, R.W. Immunization of rabbits with recombinant serine protease inhibitor reduces the performance of adult female Rhipicephalus microplus. Transbound. Emerg. Dis. 2010, 57, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Radulovic, Z.; Mulenga, A. Target validation of highly conserved Amblyomma americanum tick saliva serine protease inhibitor 19. Ticks Tick-borne Dis. 2015, 7, 405–414. [Google Scholar] [CrossRef]
- Ruddell, R.G.; Hoang-Le, D.; Barwood, J.M.; Rutherford, P.S.; Piva, T.J.; Watters, D.J.; Santambrogio, P.; Arosio, P.; Ramm, G.A. Ferritin functions as a proinflammatory cytokine via iron-independent protein kinase C zeta/nuclear factor kappaB-regulated signaling in rat hepatic stellate cells. Hepatology 2009, 49, 887–900. [Google Scholar] [CrossRef]
- Hajdusek, O.; Sojka, D.; Kopacek, P.; Buresova, V.; Franta, Z.; Sauman, I.; Winzerling, J.; Grubhoffer, L. Knockdown of proteins involved in iron metabolism limits tick reproduction and development. Proc. Natl. Acad. Sci. USA 2009, 106, 1033–1038. [Google Scholar] [CrossRef]
- Galay, R.L.; Miyata, T.; Umemiya-Shirafuji, R.; Maeda, H.; Kusakisako, K.; Tsuji, N.; Mochizuki, M.; Fujisaki, K.; Tanaka, T. Evaluation and comparison of the potential of two ferritins as anti-tick vaccines against Haemaphysalis longicornis. Parasites Vectors 2014, 7, 482. [Google Scholar] [CrossRef]
- Kopácek, P.; Hajdusek, O.; Buresova, V.; Daffre, S. Tick innate immunity. Adv. Exp. Med. Biol. 2010, 708, 137–162. [Google Scholar]
- Manjunathachar, H.V.; Kumar, B.; Saravanan, B.C.; Choudhary, S.; Mohanty, A.K.; Nagar, G.; Chigure, G.; Kumar, G.V.P.P.S.R.; Fuente, J.; Ghosh, S. Identification and characterization of vaccine candidates against Hyalomma anatolicum —Vector of Crimean-Congo haemorrhagic fever virus. Transbound. Emerg. Dis. 2019, 66, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Knorr, S.; Anguita, J.; Cortazar, J.T.; Hajdusek, O.; Kopáček, P.; Trentelman, J.J.; Kershaw, O.; Hovius, J.W.; Nijhof, A.M. Preliminary Evaluation of Tick Protein Extracts and Recombinant Ferritin 2 as Anti-tick Vaccines Targeting Ixodes ricinus in Cattle. Front. Physiol. 2018, 9, 1696. [Google Scholar] [CrossRef]
- Githaka, N.W.; Konnai, S.; Isezaki, M.; Goto, S.; Xavier, M.A.; Fujisawa, S.; Yamada, S.; Okagawa, T.; Maekawa, N.; Logullo, C.; et al. Identification and functional analysis of ferritin 2 from the Taiga tick Ixodes persulcatus Schulze. Ticks Tick-Borne Dis. 2020, 11, 101547. [Google Scholar] [CrossRef] [PubMed]
- Xavier, M.; Konnai, S.; Parizi, L.F.; Githaka, N.W.; Isezaki, M.; Goto, S.; Fujisawa, S.; Yamada, S.; Okagawa, T.; Maekawa, N.; et al. Cross-species reactivity of antibodies against Ixodes persulcatus ferritin 2 to Rhipicephalus microplus. Jpn. J. Vet. Res. 2021, 69, 57. [Google Scholar]
- Oleaga, A.; González-Pérez, S.; Peréz-Sánchez, R. First molecular and functional characterisation of ferritin 2 proteins from Ornithodoros argasid ticks. Vet. Parasitol. 2022, 304, 109684. [Google Scholar] [CrossRef]
- Pal, U.; Li, X.; Wang, T.; Montgomery, R.R.; Ramamoorthi, N.; Desilva, A.M.; Bao, F.; Yang, X.; Pypaert, M.; Pradhan, D.; et al. TROSPA, an Ixodes scapularis Receptor for Borrelia burgdorferi. Cell 2004, 119, 457–468. [Google Scholar] [CrossRef]
- Antunes, S.; Galindo, R.C.; Almazán, C.; Rudenko, N.; Golovchenko, M.; Grubhoffer, L.; Shkap, V.; Rosário, V.D.; de la Fuente, J.; Domingos, A. Functional genomics studies of Rhipicephalus (Boophilus) annulatus ticks in response to infection with the cattle protozoan parasite, Babesia bigemina. Int. J. Parasitol. 2012, 42, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Pal, U.; Fikrig, E. Adaptation of Borrelia burgdorferi in the vector and vertebrate host. Microbes Infect. 2003, 5, 659–666. [Google Scholar] [CrossRef]
- Promnares, K.; Kumar, M.; Shroder, D.Y.; Zhang, X.; Anderson, J.F.; Pal, U. Borrelia burgdorferi small lipoprotein Lp6.6 is a member of multiple protein complexes in the outer membrane and facilitates pathogen transmission from ticks to mice. Mol. Microbiol. 2009, 74, 112–125. [Google Scholar] [CrossRef]
- Tilly, K.; Rosa, P.A.; Stewart, P.E. Biology of Infection with Borrelia burgdorferi. Infect. Dis. Clin. N. Am. 2008, 22, 217–234. [Google Scholar] [CrossRef] [PubMed]
- Lahdenne, P.; Porcella, S.F.; Hagman, E.K.; Akins, D.R.; Popova, T.G.; Cox, D.L.; Katona, I.L.; Radolf, J.D.; Norgard, M.V. Molecular characterization of a 6.6-kilodalton Borrelia burgdorferi outer membrane-associated lipoprotein (lp6.6) which appears to be downregulated during mammalian infection. Infect. Immun. 1997, 65, 412–421. [Google Scholar] [CrossRef]
- Hovius, J.W.; van Dam, A.P.; Fikrig, E. Tick-host-pathogen interactions in Lyme borreliosis. Trends Parasitol. 2007, 23, 434–438. [Google Scholar] [CrossRef]
- Di Giorgio, J.P.; Soto, G.; Alleva, K.; Jozefkowicz, C.; Amodeo, G.; Muschietti, J.P.; Ayub, N.D. Prediction of Aquaporin Function by Integrating Evolutionary and Functional Analyses. J. Membr. Biol. 2013, 247, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.N.; Cerdà, J. Evolution and Functional Diversity of Aquaporins. Biol. Bull. 2015, 229, 6–23. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.M.; Ball, A.; Hoppler, S.; Bowman, A.S. Invertebrate aquaporins: A review. J. Comp. Physiol. B 2008, 178, 935–955. [Google Scholar] [CrossRef] [PubMed]
- Stavang, J.A.; Chauvigné, F.; Kongshaug, H.; Cerdà, J.; Nilsen, F.; Finn, R.N. Phylogenomic and functional analyses of salmon lice aquaporins uncover the molecular diversity of the superfamily in Arthropoda. BMC Genom. 2015, 16, 618. [Google Scholar] [CrossRef]
- Verkman, A.S. Novel roles of aquaporins revealed by phenotype analysis of knockout mice. Rev. Physiol. Biochem. Pharmacol. 2005, 155, 31–55. [Google Scholar]
- Holmes, S.P.; Li, D.; Ceraul, S.M.; Azad, A.F. An aquaporin-like protein from the ovaries and gut of American dog tick (Acari: Ixodidae). J. Med. Entomol. 2008, 45, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Scoles, G.A.; Hussein, H.E.; Olds, C.L.; Mason, K.L.; Davis, S.K. Vaccination of cattle with synthetic peptides corresponding to predicted extracellular domains of Rhipicephalus (Boophilus) microplus aquaporin 2 reduced the number of ticks feeding to repletion. Parasites Vectors 2022, 15, 49. [Google Scholar] [CrossRef]
- Contreras, M.; de la Fuente, J. Control of infestations by Ixodes ricinus tick larvae in rabbits vaccinated with aquaporin recombinant antigens. Vaccine 2017, 35, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, R.; Manzano-Román, R.; Obolo-Mvoulouga, P.; Oleaga, A. Function-guided selection of midgut antigens from Ornithodoros erraticus ticks and an evaluation of their protective efficacy in rabbits. Vet. Parasitol. 2019, 272, 1–12. [Google Scholar] [CrossRef]
- Pérez-Sánchez, R.; Cano-Argüelles, A.L.; González-Sánchez, M.; Oleaga, A. First Data on Ornithodoros moubata Aquaporins: Structural, Phylogenetic and Immunogenic Characterisation as Vaccine Targets. Pathogens 2022, 11, 694. [Google Scholar] [CrossRef]
- Ndekezi, C.; Nkamwesiga, J.; Ochwo, S.; Kimuda, M.P.; Mwiine, F.; Tweyongyere, R.; Amanyire, W.; Muhanguzi, D. Identification of Ixodid Tick-Specific Aquaporin-1 Potential Anti-tick Vaccine Epitopes: An in-silico Analysis. Front. Bioeng. Biotechnol. 2019, 7, 236. [Google Scholar] [CrossRef] [PubMed]
- Schuijt, T.J.; Narasimhan, S.; Daffre, S.; DePonte, K.; Hovius, J.W.R.; Veer, C.V.; Van Der Poll, T.; Bakhtiari, K.; Meijers, J.C.M.; Boder, E.T.; et al. Identification and Characterization of Ixodes scapularis Antigens That Elicit Tick Immunity Using Yeast Surface Display. PLoS ONE 2011, 6, e15926. [Google Scholar] [CrossRef]
- Almazán, C.; Kocan, K.M.; Bergman, D.K.; Garcia-Garcia, J.C.; Blouin, E.F.; de la Fuente, J. Identification of protective antigens for the control of Ixodes scapularis infestations using cDNA expression library immunization. Vaccine 2003, 21, 1492–1501. [Google Scholar] [CrossRef]
- Almazán, C.; Lagunes, R.; Villar, M.; Canales, M.; Rosario-Cruz, R.; Jongejan, F.; de la Fuente, J. Identification and characterization of Rhipicephalus (Boophilus) microplus candidate protective antigens for the control of cattle tick infestations. Parasitol. Res. 2010, 106, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Merino, O.; Almazán, C.; Canales, M.; Villar, M.; Moreno-Cid, J.A.; Peña, A.E.; Kocan, K.M.; De La Fuente, J. Control of Rhipicephalus (Boophilus) microplus infestations by the combination of subolesin vaccination and tick autocidal control after subolesin gene knockdown in ticks fed on cattle. Vaccine 2011, 29, 2248–2254. [Google Scholar] [CrossRef]
- Shakya, M.; Kumar, B.; Nagar, G.; de la Fuente, J.; Ghosh, S. Subolesin: A candidate vaccine antigen for the control of cattle tick infestations in Indian situation. Vaccine 2014, 32, 3488–3494. [Google Scholar] [CrossRef]
- Almazán, C.; Moreno-Cantú, O.; Moreno-Cid, J.A.; Galindo, R.C.; Canales, M.; Villar, M.; de la Fuente, J. Control of tick infestations in cattle vaccinated with bacterial membranes containing surface-exposed tick protective antigens. Vaccine 2012, 30, 265–272. [Google Scholar] [CrossRef]
- McKenna, R.V.; Riding, G.A.; Jarmey, J.M.; Pearson, R.D.; Willadsen, P. Vaccination of cattle against the Boophilus microplus using a mucin-like membrane glycoprotein. Parasite Immunol. 1998, 20, 325–336. [Google Scholar] [CrossRef]
- Hope, M.; Jiang, X.; Gough, J.; Cadogan, L.; Josh, P.; Jonsson, N.; Willadsen, P. Experimental vaccination of sheep and cattle against tick infestation using recombinant 5′-nucleotidase. Parasite Immunol. 2010, 32, 135–142. [Google Scholar] [CrossRef]
- de Lima, M.R.; Ferreira, C.; Freitas, D.; Valenzuela, J.; Masuda, A. Cloning and partial characterization of a Boophilus microplus (Acari: Ixodidae) glutathione S-transferase. Insect Biochem. Mol. Biol. 2002, 32, 747–754. [Google Scholar] [CrossRef]
- Jnr, I.D.S.V.; Imamura, S.; Ohashi, K.; Onuma, M. Cloning, expression and partial characterization of a Haemaphysalis longicornis and a Rhipicephalus appendiculatus glutathione S-transferase. Insect Mol. Biol. 2004, 13, 329–335. [Google Scholar] [CrossRef]
- Parizi, L.F.; Utiumi, K.U.; Imamura, S.; Onuma, M.; Ohashi, K.; Masuda, A.; Vaz, I.D.S. Cross immunity with Haemaphysalis longicornis glutathione S-transferase reduces an experimental Rhipicephalus (Boophilus) microplus infestation. Exp. Parasitol. 2011, 127, 113–118. [Google Scholar] [CrossRef]
- Liyou, N.; Hamilton, S.; Elvin, C.; Willadsen, P.; Field, L.M.; James, A.A. Cloning and expression of ecto 5’-nucleotidase from the cattle tick Boophilus microplus. Insect Mol. Biol. 1999, 8, 257–266. [Google Scholar] [CrossRef]
- Liyou, N.; Hamilton, S.; McKenna, R.; Elvin, C.; Willadsen, P. Localisation and functional studies on the 5′-nucleotidase of the cattle tick Boophilus microplus. Exp. Appl. Acarol. 2000, 24, 235–246. [Google Scholar] [CrossRef]
- Pérez-Sánchez, R.; Carnero-Morán, Á.; Soriano, B.; Llorens, C.; Oleaga, A. RNA-seq analysis and gene expression dynamics in the salivary glands of the argasid tick Ornithodoros erraticus along the trophogonic cycle. Parasites Vectors 2021, 14, 170. [Google Scholar] [CrossRef]
- Díaz-Martín, V.; Manzano-Román, R.; Oleaga, A.; Pérez-Sánchez, R. New salivary anti-haemostatics containing protective epitopes from Ornithodoros moubata ticks: Assessment of their individual and combined vaccine efficacy. Vet. Parasitol. 2015, 212, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Moorhouse, D.E.; Tatchell, R.J. The feeding processes of the cattle-tick Boophilus microplus (Canestrini): A study in host-parasite relations. I. Attachment to the host. Parasitology 1966, 56, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Suppan, J.; Engel, B.; Marchetti-Deschmann, M.; Nürnberger, S. Tick attachment cement—Reviewing the mysteries of a biological skin plug system. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1056–1076. [Google Scholar] [CrossRef]
- Alekseev, A.N.; Arumova, E.A.; Vasilieva, I.S. Borrelia burgdorferi sensu lato in the female cement plug of Ixodes persulcatus ticks (Acari, Ixodidae). Exp. Appl. Acarol. 1995, 19, 519–522. [Google Scholar] [CrossRef]
- Alekseev, A.N.; Burenkova, L.A.; Vasilieva, I.S.; Dubinina, H.V.; Chunikhin, S.P. Preliminary studies on virus and spirochete accumulation in the cement plug of ixodid ticks. Exp. Appl. Acarol. 1996, 20, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Trimnell, A.R.; Davies, G.M.; Lissina, O.; Hails, R.S.; Nuttall, P.A. A cross-reactive tick cement antigen is a candidate broad-spectrum tick vaccine. Vaccine 2005, 23, 4329–4341. [Google Scholar] [CrossRef]
- Labuda, M.; Trimnell, A.R.; Ličková, M.; Kazimírová, M.; Davies, G.M.; Lissina, O.; Hails, R.; Nuttall, A.P. An Antivector Vaccine Protects against a Lethal Vector-Borne Pathogen. PLoS Pathog. 2006, 2, e27. [Google Scholar] [CrossRef]
- Willadsen, P. Tick control: Thoughts on a research agenda. Vet. Parasitol. 2006, 138, 161–168. [Google Scholar] [CrossRef]
- Hajdusek, O.; Almazán, C.; Loosova, G.; Villar, M.; Canales, M.; Grubhoffer, L.; Kopacek, P.; de la Fuente, J. Characterization of ferritin 2 for the control of tick infestations. Vaccine 2010, 28, 2993–2998. [Google Scholar] [CrossRef]
- Kumar, A.; Garg, R.; Yadav, C.; Vatsya, S.; Kumar, R.; Sugumar, P.; Chandran, D.; Mangamoorib, L.N.; Bedarkar, S. Immune responses against recombinant tick antigen, Bm95, for the control of Rhipicephalus (Boophilus) microplus ticks in cattle. Vet. Parasitol. 2009, 165, 119–124. [Google Scholar] [CrossRef]
- Patarroyo, J.; Portela, R.; De Castro, R.; Pimentel, J.C.; Guzman, F.; Patarroyo, M.; Vargas, M.; Prates, A.; Mendes, M.D. Immunization of cattle with synthetic peptides derived from the Boophilus microplus gut protein (Bm86). Vet. Immunol. Immunopathol. 2002, 88, 163–172. [Google Scholar] [CrossRef]
- Patarroyo, S.J.; Neves, E.d.S.; Fidelis, C.F.; Tafur-Gomez, G.A.; de Araujo, L.; Vargas, M.I.; Sossai, S.; Prates-Patarroyo, P.A. Bovine immunisation with a recombinant peptide derived from synthetic SBm7462® (Bm86 epitope construct) immunogen for Rhipicephalus microplus control. Ticks Tick Borne Dis. 2020, 11, 101461. [Google Scholar] [CrossRef]
- Canales, M.; Almazán, C.; Naranjo, V.; Jongejan, F.; de la Fuente, J. Vaccination with recombinant Boophilus annulatus Bm86 ortholog protein, Ba86, protects cattle against B. annulatus and B. microplus infestations. BMC Biotechnol. 2009, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Azhahianambi, P.; Ray, D.D.; Chaudhuri, P.; De La Fuente, J.; Kumar, R.; Ghosh, S. Comparative efficacy of rHaa86 and rBm86 against Hyalomma anatolicum anatolicum and Rhipicephalus (Boophilus) microplus. Parasite Immunol. 2012, 34, 297–301. [Google Scholar] [CrossRef]
- Aguirre, A.d.A.R.; Lobo, F.P.; Cunha, R.C.; Garcia, M.V.; Andreotti, R. Design of the ATAQ peptide and its evaluation as an immunogen to develop a Rhipicephalus vaccine. Vet. Parasitol. 2016, 221, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Trentelman, J.J.A.; Teunissen, H.; Kleuskens, J.A.G.M.; van de Crommert, J.; de la Fuente, J.; Hovius, J.W.R.; Schetters, T.P.M. A combination of antibodies against Bm86 and Subolesin inhibits engorgement of Rhipicephalus australis (formerly Rhipicephalus microplus) larvae in vitro. Parasites Vectors 2019, 12, 362. [Google Scholar] [CrossRef]
- Contreras, M.; Kasaija, P.D.; Merino, O.; de la Cruz-Hernandez, N.I.; Gortazar, C.; de la Fuente, J. Oral Vaccination with a Formulation Combining Rhipicephalus microplus Subolesin with Heat Inactivated Mycobacterium bovis Reduces Tick Infestations in Cattle. Front. Cell. Infect. Microbiol. 2019, 9, 45. [Google Scholar] [CrossRef]
- Csordas, B.G.; Cunha, R.C.; Garcia, M.V.; da Silva, S.S.; Leite, F.L.; Andreotti, R. Molecular characterization of the recombinant protein RmLTI-BmCG-LTB: Protective immunity against Rhipicephalus (Boophilus) microplus. PLoS ONE 2018, 13, e0191596. [Google Scholar] [CrossRef]
- Contreras, M.; José, C.S.; Estrada-Peña, A.; Talavera, V.; Rayas, E.; León, C.I.; Núñez, J.L.; de Mera, I.G.F.; de la Fuente, J. Control of tick infestations in wild roe deer (Capreolus capreolus) vaccinated with the Q38 Subolesin/Akirin chimera. Vaccine 2020, 38, 6450–6454. [Google Scholar] [CrossRef]
- Fan, X.; Xu, X.; Wu, Y.; Liu, X.; Yang, F.; Hu, Y. Evaluation of anti-tick efficiency in rabbits induced by DNA vaccines encoding Haemaphysalis longicornis lipocalin homologue. Med. Vet. Èntomol. 2022, 36, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Wikel, S.K. Ticks and Tick-Borne Infections: Complex Ecology, Agents, and Host Interactions. Vet. Sci. 2018, 5, 60. [Google Scholar] [CrossRef]
- de Castro, J.J. Sustainable tick and tickborne disease control in livestock improvement in developing countries. Vet. Parasitol. 1997, 71, 77–97. [Google Scholar] [CrossRef]
- Ghosh, S.; Azhahianambi, P.; Yadav, M.P. Upcoming and future strategies of tick control: A review. J. Vector Borne Dis. 2007, 44, 79–89. [Google Scholar]
- Rodríguez-Vivas, R.; Rivas, A.; Chowell, G.; Fragoso, S.; Rosario, C.; García, Z.; Smith, S.; Williams, J.; Schwager, S. Spatial distribution of acaricide profiles (Boophilus microplus strains susceptible or resistant to acaricides) in southeastern Mexico. Veter- Parasitol. 2007, 146, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cogollo, L.; Rodriguez-Vivas, R.; Ramirez-Cruz, G.; Miller, R. First report of the cattle tick Rhipicephalus microplus resistant to ivermectin in Mexico. Vet. Parasitol. 2010, 168, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Seixas, A.; Oliveira, P.; Termignoni, C.; Logullo, C.; Masuda, A.; Vaz, I.D.S. Rhipicephalus (Boophilus) microplus embryo proteins as target for tick vaccine. Vet. Immunol. Immunopathol. 2012, 148, 149–156. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Salman, M. Current Limitations in the Control and Spread of Ticks that Affect Livestock: A Review. Agriculture 2013, 3, 221–235. [Google Scholar] [CrossRef]
- Wolff, J.A.; Ludtke, J.J.; Acsadi, G.; Williams, P.; Jani, A. Long-term persistence of plasmid DNA and foreign gene expression in mouse muscle. Hum. Mol. Genet. 1992, 1, 363–369. [Google Scholar] [CrossRef]
- Wang, Z.; Troilo, P.J.; Wang, X.; Griffiths, T.G.; Pacchione, S.J.; Barnum, A.B.; Harper, L.B.; Pauley, C.J.; Niu, Z.; Denisova, L.; et al. Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther. 2004, 11, 711–721. [Google Scholar] [CrossRef]
- Manam, S.; Ledwith, B.J.; Barnum, A.B.; Troilo, P.J.; Pauley, C.J.; Harper, L.B.; Ii, T.G.G.; Niu, Z.; Denisova, L.; Follmer, T.T.; et al. Plasmid DNA Vaccines: Tissue Distribution and Effects of DNA Sequence, Adjuvants and Delivery Method on Integration into Host DNA. Intervirology 2000, 43, 273–281. [Google Scholar] [CrossRef]
- Jiao, S.; Williams, P.; Berg, R.K.; Hodgeman, B.A.; Liu, L.; Repetto, G.; Wolff, J.A. Direct Gene Transfer into Nonhuman Primate Myofibers In Vivo. Hum. Gene Ther. 1992, 3, 21–33. [Google Scholar] [CrossRef]
- Mairhofer, J.; Lara, A.R. Advances in Host and Vector Development for the Production of Plasmid DNA Vaccines. Methods Mol. Biol. 2014, 1139, 505–541. [Google Scholar] [CrossRef] [PubMed]
- Myhr, A.I. DNA Vaccines: Regulatory Considerations and Safety Aspects. Curr. Issues Mol. Biol. 2017, 22, 79–88. [Google Scholar] [CrossRef]
- De Rose, R.; McKenna, R.V.; Cobon, G.; Tennent, J.; Zakrzewski, H.; Gale, K.; Wood, P.R.; Scheerlinck, J.-P.Y.; Willadsen, P. Bm86 antigen induces a protective immune response against Boophilus microplus following DNA and protein vaccination in sheep. Vet. Immunol. Immunopathol. 1999, 71, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Kutzler, M.A.; Weiner, D.B. DNA vaccines: Ready for prime time? Nat. Rev. Genet. 2008, 9, 776–788. [Google Scholar] [CrossRef]
- Ghaffarifar, F. Plasmid DNA vaccines: Where are we now? Drugs Today (Barc) 2018, 54, 315–333. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Kinnear, E. Using Plasmids as DNA Vaccines for Infectious Diseases. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef]
- Li, L.; Petrovsky, N. Molecular mechanisms for enhanced DNA vaccine immunogenicity. Expert Rev. Vaccines 2015, 15, 313–329. [Google Scholar] [CrossRef]
- Sayed, M.A.; Kammah, K.M.E.; El-Fiky, Z.A. A preliminary study on the DNA-vaccine for chicken protection against tick Argas persicus (Oken, 1818). Arab. J. Biotech. 2004, 7, 273–282. [Google Scholar]
- Ruiz, L.M.; Orduz, S.; López, E.D.; Guzmán, F.; Patarroyo, M.E.; Armengol, G. Immune response in mice and cattle after immunization with a Boophilus microplus DNA vaccine containing bm86 gene. Vet. Parasitol. 2007, 144, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.-T.; Zhang, J.-C.; Cui, X.-J.; Zheng, J.-J.; Li, R.; Wang, F.; Liu, J.-Z.; Hu, Y.-H. Evaluation of immune protection induced by DNA vaccines from Haemaphysalis longicornis paramyosin in rabbits. Parasites Vectors 2017, 10, 325. [Google Scholar] [CrossRef]
- Tshilwane, S.; Thema, N.; Steyn, H.; van Kleef, M.; Pretorius, A. A multi-epitope DNA vaccine co-administered with monophosphoryl lipid A adjuvant provides protection against tick transmitted Ehrlichia ruminantium in sheep. Vaccine 2019, 37, 4354–4363. [Google Scholar] [CrossRef] [PubMed]
- Matias, J.; Kurokawa, C.; Sajid, A.; Narasimhan, S.; Arora, G.; Diktas, H.; Lynn, G.E.; DePonte, K.; Pardi, N.; Valenzuela, J.G.; et al. Tick immunity using mRNA, DNA and protein-based Salp14 delivery strategies. Vaccine 2021, 39, 7661–7668. [Google Scholar] [CrossRef]
- Sajid, A.; Matias, J.; Arora, G.; Kurokawa, C.; DePonte, K.; Tang, X.; Lynn, G.; Wu, M.-J.; Pal, U.; Strank, N.O.; et al. mRNA vaccination induces tick resistance and prevents transmission of the Lyme disease agent. Sci. Transl. Med. 2021, 13, eabj9827. [Google Scholar] [CrossRef]
- Allen, J.R.; Humphreys, S.J. Immunisation of guinea pigs and cattle against ticks. Nature 1979, 280, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Kemp, D.; Agbede, R.; Johnston, L.; Gough, J. Immunization of cattle against Boophilus microplus using extracts derived from adult female ticks: Feeding and survival of the parasite on vaccinated cattle. Int. J. Parasitol. 1986, 16, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Willadsen, P.; Kemp, D.H. Vaccination with ‘concealed’ antigens for tick control. Parasitol. Today 1988, 4, 196–198. [Google Scholar] [CrossRef]
- Willadsen, P.; Riding, G.A.; McKenna, R.V.; Kemp, D.H.; Tellam, R.L.; Nielsen, J.N.; Lahnstein, J.; Cobon, G.S.; Gough, J.M. Immunologic control of a parasitic arthropod. Identification of a protective antigen from Boophilus microplus. J. Immunol. 1989, 143, 1346–1351. [Google Scholar] [CrossRef]
- Gough, J.M.; Kemp, D.H. Localization of a Low Abundance Membrane Protein (Bm86) on the Gut Cells of the Cattle Tick Boophilus microplus by Immunogold Labeling. J. Parasitol. 1993, 79, 900. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, N.; Matschoss, A.; Pepper, P.; Green, P.; Albrecht, M.; Hungerford, J.; Ansell, J. Evaluation of TickGARDPLUS, a novel vaccine against Boophilus microplus, in lactating Holstein–Friesian cows. Vet. Parasitol. 2000, 88, 275–285. [Google Scholar] [CrossRef]
- de la Fuente, J.; Almazan, C.; Canales, M.; de la Lastra, J.M.P.; Kocan, M.K.; Willadsen, P. A ten-year review of commercial vaccine performance for control of tick infestations on cattle. Anim. Health Res. Rev. 2007, 8, 23–28. [Google Scholar] [CrossRef]
- Rodríguez, M.; Penichet, M.; Mouris, A.; Labarta, V.; Luaces, L.L.; Rubiera, R.; Cordovés, C.; Sánchez, P.; Ramos, E.; Soto, A.; et al. Control of Boophilus microplus populations in grazing cattle vaccinated with a recombinant Bm86 antigen preparation. Vet. Parasitol. 1995, 57, 339–349. [Google Scholar] [CrossRef]
- Parizi, L.F.; Pohl, P.C.; Masuda, A.; Junior, I.D.S.V. New approaches toward anti-Rhipicephalus (Boophilus) microplus tick vaccine. Rev. Bras. Parasitol. Vet. 2009, 18, 1–7. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente, J.; Rodríguez, M.; García-Garí, J.C. Immunological control of ticks through vaccination with Boophilus microplus gut antigens. Ann. N. Y. Acad. Sci. 2000, 916, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Canales, M.; Moreno-Cid, J.A.; Almazan, C.; Villar, M.; de la Fuente, J. Bioprocess design and economics of recombinant BM86/BM95 antigen production for anti-tick vaccines. Biochem. Eng. J. 2010, 52, 79–90. [Google Scholar] [CrossRef]
- de Vos, S.; Zeinstra, L.; Taoufik, A.; Willadsen, P.; Jongejan, F. Evidence for the utility of the Bm86 antigen from Boophilus microplus in vaccination against other tick species. Exp. Appl. Acarol. 2001, 25, 245–261. [Google Scholar] [CrossRef]
- Perez-Perez, D.; Bechara, G.; Machado, R.; Andrade, G.; del Vecchio, R.; Pedroso, M.; Hernández, M.; Farnós, O. Efficacy of the Bm86 antigen against immature instars and adults of the dog tick Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae). Vet. Parasitol. 2010, 167, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Ferreira Leal, B.; Sanchez Ferreira, C.A. Ticks and antibodies: May parasite density and tick evasion influence the outcomes following immunization protocols? Vet. Parasitol. 2021, 300, 109610. [Google Scholar] [CrossRef] [PubMed]
- Odongo, D.; Kamau, L.; Skilton, R.; Mwaura, S.; Nitsch, C.; Musoke, A.; Taracha, E.; Daubenberger, C.; Bishop, R. Vaccination of cattle with TickGARD induces cross-reactive antibodies binding to conserved linear peptides of Bm86 homologues in Boophilus decoloratus. Vaccine 2007, 25, 1287–1296. [Google Scholar] [CrossRef]
- Toaleb, N.I.; Gabr, H.S.M.; El-Shafy, S.A.; Abdel-Rahman, E.H. Evaluation of vaccine candidates purified from the adult ticks of Ornithodoros savignyi (Acari: Argasidae) and Hyalomma dromedarii (Acari: Ixodidae) against tick infestations. J. Parasit. Dis. 2019, 43, 246–255. [Google Scholar] [CrossRef]
- Popara, M.; Villar, M.; Mateos-Hernández, L.; de Mera, I.G.F.; Marina, A.; del Valle, M.; Almazán, C.; Domingos, A.; de la Fuente, J. Lesser protein degradation machinery correlates with higher BM86 tick vaccine efficacy in Rhipicephalus annulatus when compared to Rhipicephalus microplus. Vaccine 2013, 31, 4728–4735. [Google Scholar] [CrossRef]
- Vargas, M.; Montero, C.; Sanchez, D.; Perez, D.; Valdes, M.; Alfonso, A.; Joglar, M.; Machado, H.; Rodriguez, E.; Mendez, L.; et al. Two initial vaccinations with the Bm86-based Gavacplus vaccine against Rhipicephalus (Boophilus) microplus induce similar reproductive suppression to three initial vaccinations under production conditions. BMC Vet. Res. 2010, 6, 43. [Google Scholar] [CrossRef]
- Nijhof, A.M.; Balk, J.A.; Postigo, M.; Rhebergen, A.M.; Taoufik, A.; Jongejan, F. Bm86 homologues and novel ATAQ proteins with multiple epidermal growth factor (EGF)-like domains from hard and soft ticks. Int. J. Parasitol. 2010, 40, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Coumou, J.; Wagemakers, A.; Trentelman, J.J.; Nijhof, A.; Hovius, J.W. Vaccination against Bm86 Homologues in Rabbits Does Not Impair Ixodes ricinus Feeding or Oviposition. PLoS ONE 2014, 10, e0123495. [Google Scholar] [CrossRef] [PubMed]
- Valle, M.R.; Mendez, L.; Valdez, M.; Redondo, M.; Espinosa, C.M.; Vargas, M.; Cruz, R.L.; Barrios, H.P.; Seoane, G.; Ramirez, E.S.; et al. Integrated control of Boophilus microplus ticks in Cuba based on vaccination with the anti-tick vaccine Gavac. Exp. Appl. Acarol. 2004, 34, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Suarez, M.; Rubi, J.; Pérez, D.; Cordova, V.; Salazar, Y.; Vielma, A.; Barrios, F.; Gil, C.A.; Segura, N.; Carrillo, Y.; et al. High impact and effectiveness of Gavac™ vaccine in the national program for control of bovine ticks Rhipicephalus microplus in Venezuela. Livest. Sci. 2016, 187, 48–52. [Google Scholar] [CrossRef]
- Xu, D.; Tang, B.; Wang, Y.; Zhang, L.; Qu, Z.; Shi, W.; Wang, X.; Sun, Q.; Sun, S.; Liu, M. The immune protection induced by a serine protease from the Trichinella spiralis adult administered as DNA and protein vaccine. Acta Trop. 2020, 211, 105622. [Google Scholar] [CrossRef]
- Hassan, I.A.; Wang, Y.; Zhou, Y.; Cao, J.; Zhang, H.; Zhou, J. Cross protection induced by combined Subolesin-based DNA and protein immunizations against adult Haemaphysalis longicornis. Vaccine 2020, 38, 907–915. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, M.N.; Jmel, M.A.; Mekki, I.; Dijkgraaf, I.; Kotsyfakis, M. Recent Advances in Tick Antigen Discovery and Anti-Tick Vaccine Development. Int. J. Mol. Sci. 2023, 24, 4969. https://doi.org/10.3390/ijms24054969
Abbas MN, Jmel MA, Mekki I, Dijkgraaf I, Kotsyfakis M. Recent Advances in Tick Antigen Discovery and Anti-Tick Vaccine Development. International Journal of Molecular Sciences. 2023; 24(5):4969. https://doi.org/10.3390/ijms24054969
Chicago/Turabian StyleAbbas, Muhammad Nadeem, Mohamed Amine Jmel, Imen Mekki, Ingrid Dijkgraaf, and Michail Kotsyfakis. 2023. "Recent Advances in Tick Antigen Discovery and Anti-Tick Vaccine Development" International Journal of Molecular Sciences 24, no. 5: 4969. https://doi.org/10.3390/ijms24054969
APA StyleAbbas, M. N., Jmel, M. A., Mekki, I., Dijkgraaf, I., & Kotsyfakis, M. (2023). Recent Advances in Tick Antigen Discovery and Anti-Tick Vaccine Development. International Journal of Molecular Sciences, 24(5), 4969. https://doi.org/10.3390/ijms24054969






