Proenkephalin as a Novel Prognostic Marker in Heart Failure Patients: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Review and Search Strategy
2.2. Selection Criteria
2.3. Data Abstraction
2.4. Definition of a High Plasma PENK Level
2.5. Definition of Worsening Renal Function
2.6. Statistical Analysis
2.7. Sensitivity Analysis
2.8. Evaluation of Publication Bias
3. Results
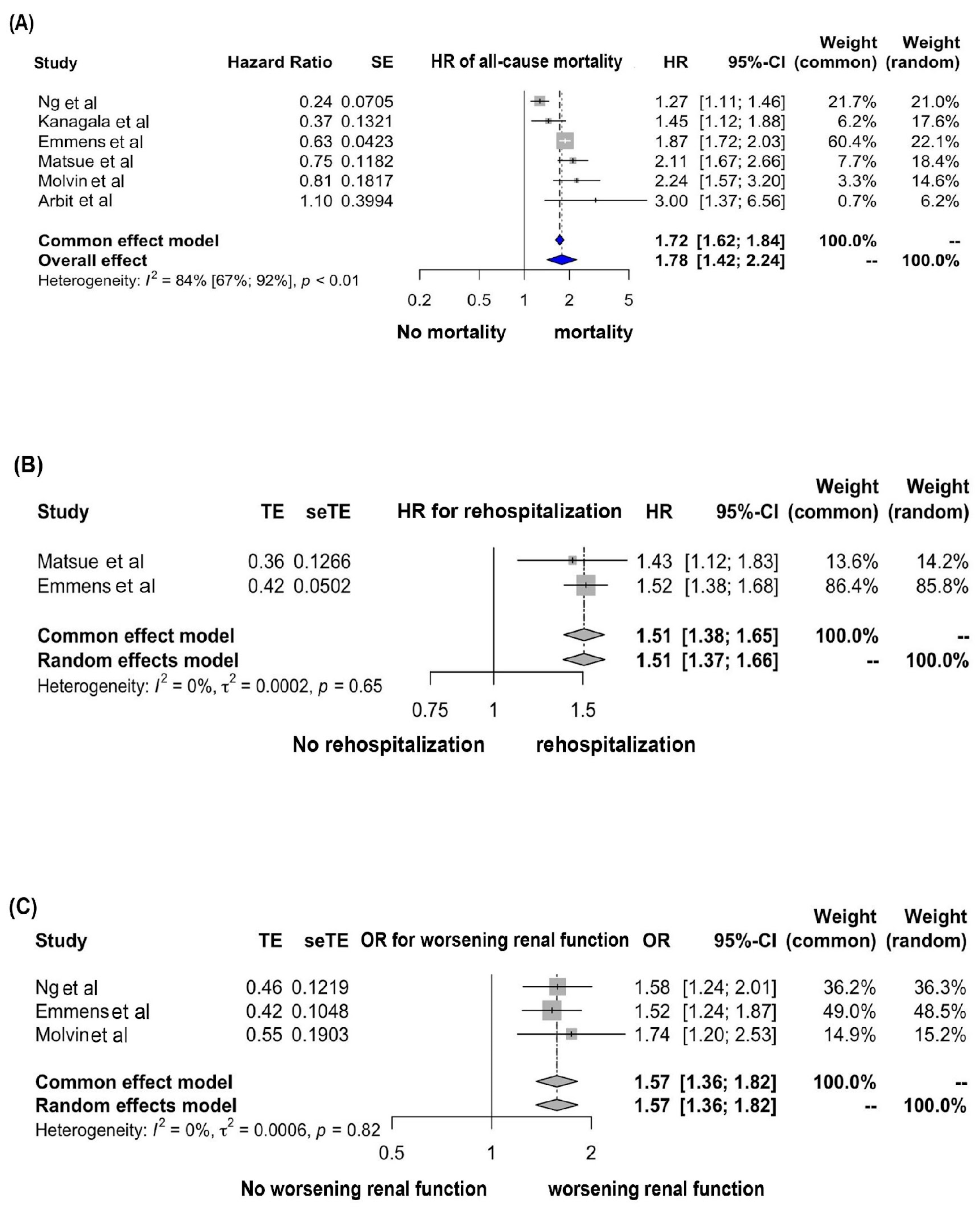
3.1. Association between a High Plasma PENK Level and All-Cause Mortality
3.2. Association between a High Plasma PENK Level and Rehospitalization
3.3. Association between a High Plasma PENK Level and Worsening Renal Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Albert, N.M.; Allen, L.A.; Bluemke, D.A.; Butler, J.; Fonarow, G.C.; Ikonomidis, J.S.; Khavjou, O.; Konstam, M.A.; Maddox, T.M.; et al. Forecasting the impact of heart failure in the United States: A policy statement from the American Heart Association. Circ. Heart Fail. 2013, 6, 606–619. [Google Scholar] [CrossRef]
- Fonarow, G.C. The Acute Decompensated Heart Failure National Registry (ADHERE): Opportunities to improve care of patients hospitalized with acute decompensated heart failure. Rev. Cardiovasc. Med. 2003, 4 (Suppl. S7), S21–S30. [Google Scholar] [PubMed]
- Kanagala, P.; Squire, I.B.; Jones, D.J.L.; Cao, T.H.; Chan, D.C.S.; McCann, G.; Sandhu, J.K.; Quinn, P.A.; McAdam, J.; Marsh, A.-M.; et al. Proenkephalin and prognosis in heart failure with preserved ejection fraction: A GREAT network study. Clin. Res. Cardiol. 2019, 108, 940–949. [Google Scholar] [CrossRef]
- Felker, G.M.; Anstrom, K.J.; Adams, K.F.; Ezekowitz, J.A.; Fiuzat, M.; Houston-Miller, N.; Januzzi, J.L., Jr.; Mark, D.B.; Piña, I.L.; Passmore, G.; et al. Effect of Natriuretic Peptide–Guided Therapy on Hospitalization or Cardiovascular Mortality in High-Risk Patients with Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA 2017, 318, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.L.; Squire, I.B.; Jones, D.J.L.; Cao, T.H.; Chan, D.C.S.; Sandhu, J.K.; Quinn, P.A.; Davies, J.E.; Struck, J.; Hartmann, O.; et al. Proenkephalin, Renal Dysfunction, and Prognosis in Patients with Acute Heart Failure: A GREAT Network Study. J. Am. Coll. Cardiol. 2017, 69, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Arbit, B.; Marston, N.; Shah, K.; Lee, E.L.; Aramin, H.; Clopton, P.; Maisel, A.S. Prognostic Usefulness of Proenkephalin in Stable Ambulatory Patients with Heart Failure. Am. J. Cardiol. 2016, 117, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Matsue, Y.; Ter Maaten, J.M.; Struck, J.; Metra, M.; O’Connor, C.M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; Davison, B.; Cleland, J.G.; et al. Clinical Correlates and Prognostic Value of Proenkephalin in Acute and Chronic Heart Failure. J. Card. Fail. 2017, 23, 231–239. [Google Scholar] [CrossRef]
- Emmens, J.E.; Ter Maaten, J.M.; Damman, K.; van Veldhuisen, D.J.; de Boer, R.A.; Struck, J.; Bergmann, A.; Sama, I.E.; Streng, K.W.; Anker, S.D.; et al. Proenkephalin, an Opioid System Surrogate, as a Novel Comprehensive Renal Marker in Heart Failure. Circ. Heart Fail. 2019, 12, e005544. [Google Scholar] [CrossRef]
- Molvin, J.; Jujic, A.; Navarin, S.; Melander, O.; Zoccoli, G.; Hartmann, O.; Bergmann, A.; Struck, J.; Bachus, E.; Di Somma, S.; et al. Bioactive adrenomedullin, proenkephalin A and clinical outcomes in an acute heart failure setting. Open Heart 2019, 6, e001048. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Herzog, R.; Álvarez-Pasquin, M.J.; Díaz, C.; Del Barrio, J.L.; Estrada, J.M.; Gil, Á. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health 2013, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Easterbrook, P.J.; Berlin, J.A.; Gopalan, R.; Matthews, D.R. Publication bias in clinical research. Lancet 1991, 337, 867–872. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.; Altman, D.G. Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Collaboration and John Wiley & Sons Ltd.: Oxford, UK, 2019; pp. 241–284. [Google Scholar]
- Siong Chan, D.C.; Cao, T.H.; Ng, L.L. Proenkephalin in Heart Failure. Heart Fail. Clin. 2018, 14, 1–11. [Google Scholar] [CrossRef]
- Khorashadi, M.; Beunders, R.; Pickkers, P.; Legrand, M. Proenkephalin: A New Biomarker for Glomerular Filtration Rate and Acute Kidney Injury. Nephron 2020, 144, 655–661. [Google Scholar] [CrossRef]
- Van Den Brink, O.W.V.; Delbridge, L.M.; Rosenfeldt, F.L.; Penny, D.; Esmore, D.S.; Quick, D.; Kaye, D.M.; Pepe, S. Endogenous Cardiac Opioids: Enkephalins in Adaptation and Protection of the Heart. Heart Lung Circ. 2003, 12, 178–187. [Google Scholar] [CrossRef]
- Rubattu, S.; Mennuni, S.; Testa, M.; Mennuni, M.; Pierelli, G.; Pagliaro, B.; Gabriele, E.; Coluccia, R.; Autore, C.; Volpe, M. Pathogenesis of Chronic Cardiorenal Syndrome: Is There a Role for Oxidative Stress? Int. J. Mol. Sci. 2013, 14, 23011–23032. [Google Scholar] [CrossRef]
- Rosenberger, J.; Petrovics, G.; Buzas, B. Oxidative stress induces proorphanin FQ and proenkephalin gene expression in astrocytes through p38- and ERK-MAP kinases and NF-kappaB. J. Neurochem. 2001, 79, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.L.; Hu, H.J.; Zhao, X.J.; Chi, W.W.; Liu, D.M.; Wang, Q.; Cui, W. Urine N-terminal pro-B-type natriuretic peptide and plasma proenkephalin are promising biomarkers for early diagnosis of cardiorenal syndrome type 1 in acute decompensated heart failure: A prospective, double-center, observational study in real-world. Ren. Fail. 2022, 44, 1486–1497. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Publication Committee for the VMAC [Vasodilatation in the Management of Acute CHF] Investigators. Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: A randomized controlled trial. JAMA 2002, 287, 1531–1540. [Google Scholar] [CrossRef]
- Donato, L.J.; Meeusen, J.W.; Lieske, J.C.; Bergmann, D.; Sparwaßer, A.; Jaffe, A.S. Analytical performance of an immunoassay to measure proenkephalin. Clin. Biochem. 2018, 58, 72–77. [Google Scholar] [CrossRef]
- Ng, L.L.; Sandhu, J.K.; Narayan, H.; Quinn, P.A.; Squire, I.B.; Davies, J.E.; Bergmann, A.; Maisel, A.; Jones, D.J. Proenkephalin and prognosis after acute myocardial infarction. J. Am. Coll. Cardiol. 2014, 63, 280–289. [Google Scholar] [CrossRef]
- Braam, B.; Joles, J.A.; Danishwar, A.H.; Gaillard, C.A. Cardiorenal syndrome—Current understanding and future perspectives. Nat. Rev. Nephrol. 2014, 10, 48–55. [Google Scholar] [CrossRef]
- ter Maaten, J.M.; Damman, K.; Verhaar, M.C.; Paulus, W.J.; Duncker, D.J.; Cheng, C.; van Heerebeek, L.; Hillege, H.L.; Lam, C.S.P.; Navis, G.; et al. Connecting heart failure with preserved ejection fraction and renal dysfunction: The role of endothelial dysfunction and inflammation. Eur. J. Heart Fail. 2016, 18, 588–598. [Google Scholar] [CrossRef]
- Arfsten, H.; Goliasch, G.; Bartko, P.E.; Prausmüller, S.; Spinka, G.; Cho, A.; Novak, J.; Mascherbauer, J.; Haslacher, H.; Strunk, G.; et al. Neprilysin inhibition does not alter dynamic of proenkephalin-A 119-159 and pro-substance P in heart failure. ESC Heart Fail. 2021, 8, 2016–2024. [Google Scholar] [CrossRef]

| Kanagala et al. [5] | Ng et al. [7] | Arbit et al. [8] | Matsue et al. [9] | Emmens et al. [10] | Molvin et al. [11] | |
|---|---|---|---|---|---|---|
| Year | 2019 | 2017 | 2016 | 2016 | 2019 | 2019 |
| Country | United Kingdom, Switzerland | United Kingdom, France, Switzerland | USA | Netherlands, Germany, Italy, United Kingdom, Poland, USA | Scotland, United Kingdom | Sweden, Italy |
| Study design | Cohort study | Cohort study | Cohort study | Cohort study | Cohort study | Cohort study |
| Population | Patients with either new onset or worsening HF | Patients with acute HF who presented with acute dyspnea | Patients with HF who were referred for an echocardiogram | Patients with acute HF with renal function impairment | Patients with either new onset or worsening HF | Patients with either new onset or worsening HF |
| Settings (OPD/IPD) | Both | Both | OPD | Both | Both | Both |
| n | 522 | 1908 | 200 | 1589 | 2180 | 530 |
| Mean age ± SD | 76.13 ± 10.73 | 75.66 ± 11.74 | 63.7 ± 12.5 | 71 ± 11 | 69 ± 12 | 76.4 ± 10.7 |
| Time to follow-up (months) | 24 | 12 | 48 | 6 | 21 | 12 |
| Male sex (%) | 48.5 | 62.2 | 97.5 | 66 | 73.2 | 60.2 |
| CAD | 171 | 769 | N/A | N/A | 957 | N/A |
| HT | 425 | 1330 | 143 | 1272 | 228 | N/A |
| DM | 189 | 609 | 49 | 733 | 703 | 194 |
| Loop diuretics (%) | N/A | 65.6 | N/A | N/A | 99.5 | 82.6 |
| Beta-blocker (%) | 62.9 | 53.9 | 55.5 | 75 | 83.1 | 71.7 |
| ACEI/ARB (%) | 74.4 | 61.6 | 34.5 | N/A | 71.7 | 67.9 |
| Plasma PENK measurement | chemiluminometric sandwich immunoassay | chemiluminometric sandwich immunoassay | chemiluminometric sandwich immunoassay | chemiluminometric sandwich immunoassay | chemiluminometric sandwich immunoassay | chemiluminometric sandwich immunoassay |
| PENK cut-off level (pmol/L) | 68.2 | 67 | 67.5 | 83 | 69.4 | N/A |
| All-cause mortality | ||||||
| Hazard ratio for all-cause mortality | 1.45 (1.12–1.88) | 1.27 (1.1–1.45) | 3 (1.4–6.7) | 2.11 (1.68–2.67) | 1.87 (1.72–2.03) | 2.24 (1.57–3.2) |
| Counfounder adjusted | Age, sex, SBP, HR, NYHA class, DM, HT, previous HF, IHD, AF, plasma urea, Cr, sodium, Hb, natriuretic peptide | Age, sex, SBP, HR, renal failure, DM, HF, HT, IHD, eGFR, BUN, NP levels | N/A | Age, sex, SBP, peripheral edema, previous HF rehospitalization, serum Na, log BUN, log Cr, albumin | Age, Hb, beta-blocker use, eGFR, BUN, log UACR, log NT-proBNP, log hs-troponin T, log CRP, log GDF-15, log urinary KIM-1, log urinary NGAL, BIOSTAT risk score | Age, sex, DM, SBP, smoking, AF, prior HF, NT-proBNP |
| Quality assessment | S4C2O2 | S3C2O2 | S3C1O2 | S4C2O2 | S4C2O2 | S3C2O2 |
| Worsening renal function | ||||||
| WRF definition | † | Increase in the plasma creatinine level of more than 0.3 ng/dL or of 50% of the level within 48 h of admission compared with the admission value | † | † | Decrease of more than 25% in the estimated glomerular filtration rate (eGFR) compared with the baseline | Increase in the plasma creatinine level of more than 0.3 ng/dL or of 50% of the level within 48 h of admission compared with the admission value |
| Baseline Cr (mg/dL) | † | 1.43 ± 0.74 | † | † | 1.17 (0.95–1.47) | 1.37 ± 0.76 |
| Adjusted odds ratio for WRF | † | 1.58 (1.24–2.00) | † | † | 1.52 (1.24–1.87) | 1.74 (1.2–2.53) |
| Confounder WRF | † | Age, sex, SBP, HR, renal failure, DM, HF, HT, IHD, eGFR, plasma urea, NP levels | † | † | Plasma NGAL, urinary KIM-1, urinary NGAL, UACR | SBP, ACEI, ARB, beta-blocker, DM, prior HF, Cr, BNP |
| Quality assessment | † | S2C1O2 | † | † | S3C1O2 | S2C2O2 |
| Rehospitalization | ||||||
| Harzard ratio for rehospitalization | ‡ | ‡ | ‡ | 1.43 (1.12–1.84) | 1.52 (1.38–1.68) | ‡ |
| Confounder rehospitalization | ‡ | ‡ | ‡ | Age, sex, SBP, peripheral edema, previous HF rehospitalization, serum Na, log BUN, log Cr, albumin | Age, peripheral edema, SBP, beta-blocker use, previous HF hospitalization, Hb, eGFR, BUN, log UACR, log NT-proBNP, log hs-troponin T, log CRP, log GDF-15, log urinary KIM-1, log urinary NGAL, BIOSTAT risk score | ‡ |
| Quality assessment | ‡ | ‡ | ‡ | S4C2O2 | S4C2O2 | ‡ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siranart, N.; Laohasurayotin, K.; Phanthong, T.; Sowalertrat, W.; Ariyachaipanich, A.; Chokesuwattanaskul, R. Proenkephalin as a Novel Prognostic Marker in Heart Failure Patients: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 4887. https://doi.org/10.3390/ijms24054887
Siranart N, Laohasurayotin K, Phanthong T, Sowalertrat W, Ariyachaipanich A, Chokesuwattanaskul R. Proenkephalin as a Novel Prognostic Marker in Heart Failure Patients: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2023; 24(5):4887. https://doi.org/10.3390/ijms24054887
Chicago/Turabian StyleSiranart, Noppachai, Khamik Laohasurayotin, Tanattida Phanthong, Walit Sowalertrat, Aekarach Ariyachaipanich, and Ronpichai Chokesuwattanaskul. 2023. "Proenkephalin as a Novel Prognostic Marker in Heart Failure Patients: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 24, no. 5: 4887. https://doi.org/10.3390/ijms24054887
APA StyleSiranart, N., Laohasurayotin, K., Phanthong, T., Sowalertrat, W., Ariyachaipanich, A., & Chokesuwattanaskul, R. (2023). Proenkephalin as a Novel Prognostic Marker in Heart Failure Patients: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 24(5), 4887. https://doi.org/10.3390/ijms24054887








