Molecular Detection and Identification of Plant-Associated Lactiplantibacillus plantarum
Abstract
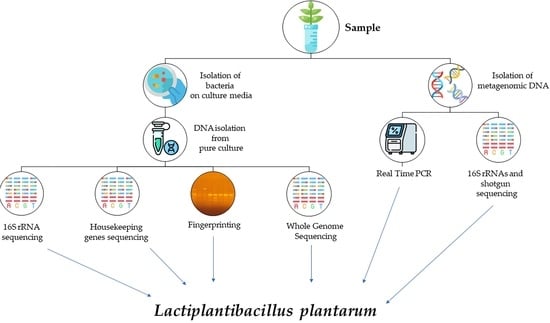
1. Introduction
2. Culture-Dependent Methods
2.1. 16S rRNA Gene Sequencing
2.2. Housekeeping Genes Sequencing
2.3. MultiLocus Sequence Typing (MLST)
2.4. Fingerprinting Methods
2.5. Whole Genome Sequencing (WGS)
3. Culture-Independent Methods
3.1. Real-Time PCR (qPCR)
3.2. 16S rRNAs and Shotgun Next and Third Generation Sequencing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beganović, J.; Kos, B.; Pavunc, A.L.; Uroić, K.; Jokić, M.; Šušković, J. Traditionally produced sauerkraut as source of autochthonous functional starter cultures. Microbiol. Res. 2014, 169, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zang, S.; Zhao, Z.; Li, X. Dynamic changes of bacterial communities and nitrite character during northeastern Chinese sauerkraut fermentation. Food Sci. Biotechnol. 2018, 27, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Cho, E.J.; Yang, S.M.; Kim, M.J.; Kim, H.Y. Novel approaches for the identification of microbial communities in kimchi: MALDI-TOF MS analysis and high-throughput sequencing. Food Microbiol. 2021, 94, 103641. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Díaz, I.M.; Hayes, J.; Medina, E.; Anekella, K.; Daughtry, K.; Dieck, S.; Levi, M.; Price, R.; Butz, N.; Lu, Z.; et al. Reassessment of the succession of lactic acid bacteria in commercial cucumber fermentations and physiological and genomic features associated with their dominance. Food Microbiol. 2017, 63, 217–227. [Google Scholar] [CrossRef]
- Reis, P.J.; Tavares, T.G.; Rocha, J.M.; Malcata, F.X.; Macedo, A.C. Cobrançosa Table Olives: Characterization of Processing Method and Lactic Acid Bacteria Profile throughout Spontaneous Fermentation. Appl. Sci. 2022, 12, 9738. [Google Scholar] [CrossRef]
- Wuyts, S.; Van Beeck, W.; Oerlemans, E.F.; Wittouck, S.; Claes, I.J.; De Boeck, I.; Weckx, S.; Lievens, B.; De Vuyst, L.; Lebeer, S. Carrot juice fermentations as man-made microbial ecosystems dominated by lactic acid bacteria. Appl. Environ. Microbiol. 2018, 84, e00134-18. [Google Scholar] [CrossRef]
- Bah, A.; Ferjani, R.; Fhoula, I.; Gharbi, Y.; Najjari, A.; Boudabous, A.; Ouzari, H.I. Microbial community dynamic in tomato fruit during spontaneous fermentation and biotechnological characterization of indigenous lactic acid bacteria. Ann. Microbiol. 2019, 69, 41–49. [Google Scholar] [CrossRef]
- Wouters, D.; Bernaert, N.; Conjaerts, W.; Van Droogenbroeck, B.; De Loose, M.; De Vuyst, L. Species diversity, community dynamics, and metabolite kinetics of spontaneous leek fermentations. Food Microbiol. 2013, 33, 185–196. [Google Scholar] [CrossRef]
- Pardali, E.; Paramithiotis, S.; Papadelli, M.; Mataragas, M.; Drosinos, E.H. Lactic acid bacteria population dynamics during spontaneous fermentation of radish (Raphanus sativus L.) roots in brine. World J. Microbiol. Biotechnol. 2017, 33, 110. [Google Scholar] [CrossRef]
- So’aib, M.S.; Hamid, K.H.K.; Salihon, J.; Tan, H.L. Phenolic Content, Antioxidant Activity and Biodiversity Changes During Spontaneous Fermentation of Carica Papaya Leaf. J. Teknol. 2020, 82, 65–73. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Liu, D. Biochemical changes and microbial community dynamics during spontaneous fermentation of Zhacai, a traditional pickled mustard tuber from China. Int. J. Food Microbiol. 2021, 347, 109199. [Google Scholar] [CrossRef]
- Ouattara, H.D.; Ouattara, H.G.; Droux, M.; Reverchon, S.; Nasser, W.; Niamke, S.L. Lactic acid bacteria involved in cocoa beans fermentation from Ivory Coast: Species diversity and citrate lyase production. Int. J. Food Microbiol. 2017, 256, 11–19. [Google Scholar] [CrossRef]
- Maidana, S.D.; Ficoseco, C.A.; Bassi, D.; Cocconcelli, P.S.; Puglisi, E.; Savoy, G.; Vignolo, G.; Fontana, C. Biodiversity and technological-functional potential of lactic acid bacteria isolated from spontaneously fermented chia sourdough. Int. J. Food Microbiol. 2020, 316, 108425. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Guo, X.; Zhang, L.; Zhang, W.; Man, C.; Jiang, Y. Characterization and transcriptomic basis of biofilm formation by Lactobacillus plantarum J26 isolated from traditional fermented dairy products. LWT 2020, 125, 109333. [Google Scholar] [CrossRef]
- Mangia, N.P.; Cottu, M.; Mura, M.E.; Murgia, M.A.; Blaiotta, G. Technological Parameters, Anti-Listeria Activity, Biogenic Amines Formation and Degradation Ability of L. plantarum Strains Isolated from Sheep-Fermented Sausage. Microorganisms 2021, 9, 1895. [Google Scholar] [CrossRef]
- Yang, J.; Lu, J.; Zhu, Q.; Tao, Y.; Zhu, Q.; Guo, C.; Fang, Y.; Chen, L.; Koyande, A.K.; Wang, S.; et al. Isolation and characterization of a novel Lactobacillus plantarum MMB-07 from traditional Suanyu for Acanthogobius hasta fermentation. J. Biosci. Bioeng. 2021, 132, 161–166. [Google Scholar] [CrossRef]
- Parichehreh, S.; Tahmasbi, G.; Sarafrazi, A.; Imani, S.; Tajabadi, N. Isolation and identification of Lactobacillus bacteria found in the gastrointestinal tract of the dwarf honey bee, Apis florea Fabricius, 1973 (Hymenoptera: Apidae). Apidologie 2018, 49, 430–438. [Google Scholar] [CrossRef]
- Khusro, A.; Arasu, M.V.; Sahibzada, M.U.K.; Salem, A.Z.; Al-Dhabi, N.A.; Rivas-Caceres, R.R.; Seidel, V.; Choi, K.C. Assessment on in vitro probiotic attributes of Lactobacillus plantarum isolated from horse feces. J. Equine Vet. Sci. 2021, 107, 103769. [Google Scholar] [CrossRef]
- Zhang, N.; Li, C.; Niu, Z.; Kang, H.; Wang, M.; Zhang, B.; Tian, H. Colonization and immunoregulation of Lactobacillus plantarum BF_15, a novel probiotic strain from the feces of breast-fed infants. Food Funct. 2020, 11, 3156–3166. [Google Scholar] [CrossRef]
- Singhal, N.; Singh, N.S.; Mohanty, S.; Kumar, M.; Virdi, J.S. Rhizospheric Lactobacillus plantarum (Lactiplantibacillus plantarum) strains exhibit bile salt hydrolysis, hypocholestrolemic and probiotic capabilities in vitro. Sci. Rep. 2021, 11, 15288. [Google Scholar] [CrossRef]
- Fidanza, M.; Panigrahi, P.; Kollmann, T.R. Lactiplantibacillus plantarum–nomad and ideal probiotic. Front. Microbiol. 2021, 12, 712236. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information’s Genome Database. Available online: https://www.ncbi.nlm.nih.gov/data-hub/genome/?taxon=1590 (accessed on 18 December 2022).
- Siezen, R.J.; van Hylckama Vlieg, J.E. Genomic diversity and versatility of Lactobacillus plantarum, a natural metabolic engineer. Microb. Cell Factories 2011, 10 (Suppl. 1), S3. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, S.; Liu, W.; Kwok, L.Y.; Bilige, M.; Zhang, W. Comparative genomic analysis of 455 Lactiplantibacillus plantarum isolates: Habitat-specific genomes shaped by frequent recombination. Food Microbiol. 2022, 104, 103989. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chao, Y.; Deng, Y.; Piao, M.; Chen, T.; Xu, J.; Zhang, R.; Zhao, J.; Deng, Y. Formation of viable, but putatively non-culturable (VPNC) cells of beer-spoilage lactobacilli growing in biofilms. LWT 2020, 133, 109964. [Google Scholar] [CrossRef]
- Daranas, N.; Roselló, G.; Cabrefiga, J.; Donati, I.; Francés, J.; Badosa, E.; Spinelli, F.; Montesinos, E.; Bonaterra, A. Biological control of bacterial plant diseases with Lactobacillus plantarum strains selected for their broad-spectrum activity. Ann. Appl. Biol. 2019, 174, 92–105. [Google Scholar] [CrossRef]
- Pontonio, E.; Di Cagno, R.; Tarraf, W.; Filannino, P.; De Mastro, G.; Gobbetti, M. Dynamic and assembly of epiphyte and endophyte lactic acid bacteria during the life cycle of Origanum vulgare L. Front. Microbiol. 2018, 9, 1372. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Li, B.; Peters, B.M.; Deng, Y.; Xu, Z.; Shirtliff, M.E. Study on spoilage capability and VBNC state formation and recovery of Lactobacillus plantarum. Microb. Pathog. 2017, 110, 257–261. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, J.; Li, H.; Li, L.; Tu, J.; Fang, H.; Chen, J.; Qian, F. An improved plate culture procedure for the rapid detection of beer-spoilage lactic acid bacteria. J. Inst. Brew. 2014, 120, 127–132. [Google Scholar] [CrossRef]
- Veselá, K.; Kumherová, M.; Klojdová, I.; Solichová, K.; Horáčková, Š.; Plocková, M. Selective culture medium for the enumeration of Lactobacillus plantarum in the presence of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus. LWT 2019, 114, 108365. [Google Scholar] [CrossRef]
- Bujalance, C.; Jiménez-Valera, M.; Moreno, E.; Ruiz-Bravo, A. A selective differential medium for Lactobacillus plantarum. J. Microbiol. Methods 2006, 66, 572–575. [Google Scholar] [CrossRef]
- Imran, M.Y.M.; Reehana, N.; Jayaraj, K.A.; Ahamed, A.A.P.; Dhanasekaran, D.; Thajuddin, N.; Alharbi, N.S.; Muralitharan, G. Statistical optimization of exopolysaccharide production by Lactobacillus plantarum NTMI05 and NTMI20. Int. J. Biol. Macromol. 2016, 93, 731–745. [Google Scholar] [CrossRef]
- Wang, X.; Shao, C.; Liu, L.; Guo, X.; Xu, Y.; Lü, X. Optimization, partial characterization and antioxidant activity of an exopolysaccharide from Lactobacillus plantarum KX041. Int. J. Biol. Macromol. 2017, 103, 1173–1184. [Google Scholar] [CrossRef]
- Yoo, H.; Rheem, I.; Rheem, S.; Oh, S. Optimizing medium components for the maximum growth of Lactobacillus plantarum JNU 2116 using response surface methodology. Korean J. Food Sci. Anim. Resour. 2018, 38, 240. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, D.H.; Kang, H.J.; Shin, M.; Yang, S.Y.; Yang, J.; Jung, Y.H. Enhanced production of γ-aminobutyric acid (GABA) using Lactobacillus plantarum EJ2014 with simple medium composition. LWT 2021, 137, 110443. [Google Scholar] [CrossRef]
- Choi, G.H.; Lee, N.K.; Paik, H.D. Optimization of medium composition for biomass production of Lactobacillus plantarum 200655 using response surface methodology. J. Microbiol. Biotechnol. 2021, 31, 717–725. [Google Scholar] [CrossRef]
- Cerdeira, V.; Bravo-Ferrada, B.M.; Semorile, L.; Tymczyszyn, E. Design of a low-cost culture medium based in whey permeate for biomass production of enological Lactobacillus plantarum strains. Biotechnol. Prog. 2019, 35, e2791. [Google Scholar] [CrossRef]
- Manzoor, A.; Qazi, J.I.; Mukhtar, H.; Rasool, A. Significantly enhanced biomass production of a novel bio-therapeutic strain Lactobacillus plantarum (AS-14) by developing low cost media cultivation strategy. J. Biol. Eng. 2017, 11, 17. [Google Scholar] [CrossRef]
- Coghetto, C.C.; Vasconcelos, C.B.; Brinques, G.B.; Ayub, M.A.Z. Lactobacillus plantarum BL011 cultivation in industrial isolated soybean protein acid residue. Braz. J. Microbiol. 2016, 47, 941–948. [Google Scholar] [CrossRef]
- Unban, K.; Kanpiengjai, A.; Khatthongngam, N.; Saenjum, C.; Khanongnuch, C. Simultaneous bioconversion of gelatinized starchy waste from the rice noodle manufacturing process to lactic acid and maltose-forming α-amylase by Lactobacillus plantarum S21, using a low-cost medium. Fermentation 2019, 5, 32. [Google Scholar] [CrossRef]
- Teusink, B.; van Enckevort, F.H.J.; Francke, C.; Wiersma, A.; Wegkamp, A.; Smid, E.J.; Siezen, R.J. In silico reconstruction of the metabolic pathways of Lactobacillus plantarum: Comparing predictions of nutrient requirements with those from growth experiments. Appl. Environ. Microbiol. 2005, 71, 7253–7262. [Google Scholar] [CrossRef]
- Wegkamp, A.; Teusink, B.; De Vos, W.M.; Smid, E.J. Development of a minimal growth medium for Lactobacillus plantarum. Lett. Appl. Microbiol. 2010, 50, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Woese, C.R.; Fox, G.E. Phylogenetic structure of the prokaryotic domain: The primary kingdoms. Proc. Natl. Acad. Sci. USA 1977, 74, 5088–5090. [Google Scholar] [CrossRef] [PubMed]
- Woese, C.R.; Stackebrandt, E.; Macke, T.J.; Fox, G.E. A phylogenetic definition of the major eubacterial taxa. Syst. Appl. Microbiol. 1985, 6, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.S.; Spakowicz, D.J.; Hong, B.Y.; Petersen, L.M.; Demkowicz, P.; Chen, L.; Leopold, R.S.; Hanson, B.M.; Agresta, H.O.; Gerstein, M.; et al. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat. Commun. 2019, 10, 5029. [Google Scholar] [CrossRef]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley and Sons: New York, NY, USA, 1991; pp. 115–147. [Google Scholar]
- Pan, Q.; Zhang, L.; Li, J.; Chen, T.; Chen, W.; Wang, G.; Yin, J. Characterization of pLP18, a novel cryptic plasmid of Lactobacillus plantarum PC518 isolated from Chinese pickle. Plasmid 2011, 65, 204–209. [Google Scholar] [CrossRef]
- Louca, S.; Doebeli, M.; Parfrey, L.W. Correcting for 16S rRNA gene copy numbers in microbiome surveys remains an unsolved problem. Microbiome 2018, 6, 158. [Google Scholar] [CrossRef]
- Větrovský, T.; Baldrian, P. The variability of the 16S rRNA gene in bacterial genomes and its consequences for bacterial community analyses. PLoS ONE 2013, 8, e57923. [Google Scholar] [CrossRef]
- Lee, C.M.; Sieo, C.C.; Abdullah, N.; Ho, Y.W. Estimation of 16S rRNA gene copy number in several probiotic Lactobacillus strains isolated from the gastrointestinal tract of chicken. FEMS Microbiol. Lett. 2008, 287, 136–141. [Google Scholar] [CrossRef]
- Chokesajjawatee, N.; Santiyanont, P.; Chantarasakha, K.; Kocharin, K.; Thammarongtham, C.; Lertampaiporn, S.; Vorapreeda, T.; Srisuk, T.; Wongsurawat, T.; Jenjaroenpun, P.; et al. Safety assessment of a nham starter culture Lactobacillus plantarum BCC9546 via whole-genome analysis. Sci. Rep. 2020, 10, 10241. [Google Scholar] [CrossRef]
- Kim, E.; Yang, S.M.; Kim, H.B.; Kim, H.Y. Novel specific peaks for differentiating the Lactobacillus plantarum group using matrix-assisted laser desorption ionization time-of-flight mass spectrometry. J. Microbiol. Methods 2020, 178, 106064. [Google Scholar] [CrossRef]
- Hounkpe, B.W.; Chenou, F.; de Lima, F.; De Paula, E.V. HRT Atlas v1. 0 database: Redefining human and mouse housekeeping genes and candidate reference transcripts by mining massive RNA-seq datasets. Nucleic Acids Res. 2021, 49, D947–D955. [Google Scholar] [CrossRef]
- Endo, A.; Tanizawa, Y.; Arita, M. Isolation and Identification of Lactic Acid Bacteria from Environmental Samples. In Lactic Acid Bacteria; Kanauchi, M., Ed.; Humana Press: New York, NY, USA, 2019; pp. 3–13. [Google Scholar]
- Liu, Y.; Štefanič, P.; Miao, Y.; Xue, Y.; Xun, W.; Zhang, N.; Shen, Q.; Zhang, R.; Xu, Z.; Mandic-Mulec, I. Housekeeping gene gyrA, a potential molecular marker for Bacillus ecology study. AMB Express 2022, 12, 133. [Google Scholar] [CrossRef]
- Pérez-Díaz, I.M.; Johanningsmeier, S.D.; Anekella, K.; Pagán-Medina, C.G.; Méndez-Sandoval, L.; Arellano, C.; Price, R.; Daughtry, K.V.; Borges, M.; Bream, C.; et al. Genotypic and phenotypic diversity among Lactobacillus plantarum and Lactobacillus pentosus isolated from industrial scale cucumber fermentations. Food Microbiol. 2021, 94, 103652. [Google Scholar] [CrossRef]
- Huang, C.H.; Chen, C.C.; Lin, Y.C.; Chen, C.H.; Lee, A.Y.; Liou, J.S.; Gu, C.T.; Huang, L. The mutL gene as a genome-wide taxonomic marker for high resolution discrimination of Lactiplantibacillus plantarum and its closely related taxa. Microorganisms 2021, 9, 1570. [Google Scholar] [CrossRef]
- Lee, J.M.; Park, S.H.; Jin, C.-Z.; Kang, M.-K.; Park, D.-J.; Kim, C.-J. The groESL ISR sequence-based species-specific identification of GRAS and non-GRAS Lactiplantibacillus as an alternative to 16S rRNA sequencing. LWT 2021, 147, 111504. [Google Scholar] [CrossRef]
- Das, S.; Dash, H.R.; Mangwani, N.; Chakraborty, J.; Kumari, S. Understanding molecular identification and polyphasic taxonomic approaches for genetic relatedness and phylogenetic relationships of microorganisms. J. Microbiol. Methods 2014, 103, 80–100. [Google Scholar] [CrossRef]
- Yu, A.O.; Goldman, E.A.; Brooks, J.T.; Golomb, B.L.; Yim, I.S.; Gotcheva, V.; Angelov, A.; Bae Kim, E.; Marco, M.L. Strain diversity of plant-associated Lactiplantibacillus plantarum. Microb. Biotechnol. 2021, 14, 1990–2008. [Google Scholar] [CrossRef]
- Yalçınkaya, S.; & Kılıç, G.B.; Kılıç, G.B. Isolation, identification and determination of technological properties of the halophilic lactic acid bacteria isolated from table olives. J. Food Sci. Technol. 2019, 56, 2027–2037. [Google Scholar] [CrossRef]
- Prete, R.; Long, S.L.; Joyce, S.A.; Corsetti, A. Genotypic and phenotypic characterization of food-associated Lactobacillus plantarum isolates for potential probiotic activities. FEMS Microbiol. Lett. 2020, 367, fnaa076. [Google Scholar] [CrossRef]
- Xu, H.; Liu, W.; Zhang, W.; Yu, J.; Song, Y.; Menhe, B.; Zhang, H.; Sun, Z. Use of multilocus sequence typing to infer genetic diversity and population structure of Lactobacillus plantarum isolates from different sources. BMC Microbiol. 2015, 15, 241. [Google Scholar] [CrossRef]
- Chen, Y.; Frazzitta, A.E.; Litvintseva, A.P.; Fang, C.; Mitchell, T.G.; Springer, D.J.; Ding, Y.; Yuan, G.; Perfect, J.R. Next generation multilocus sequence typing (NGMLST) and the analytical software program MLSTEZ enable efficient, cost-effective, high-throughput, multilocus sequencing typing. Fungal Genet. Biol. 2015, 75, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Liou, C.H.; Wu, H.C.; Liao, Y.C.; Lauderdale, T.L.Y.; Huang, I.W.; Chen, F.J. nanoMLST: Accurate multilocus sequence typing using Oxford Nanopore Technologies MinION with a dual-barcode approach to multiplex large numbers of samples. Microb. Genom. 2020, 6, e000336. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.; Vegas, C.; Zavaleta, A.I.; Esteve-Zarzoso, B. Predominance of Strains in Peruvian Amazonian Fruits. Pol. J. Microbiol. 2019, 68, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Lorn, D.; Ho, P.H.; Tan, R.; Licandro, H.; Waché, Y. Screening of lactic acid bacteria for their potential use as aromatic starters in fermented vegetables. Int. J. Food Microbiol. 2021, 350, 109242. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Papadelli, M.; Pardali, E.; Mataragas, M.; Drosinos, E.H. Evaluation of plantaricin genes expression during fermentation of Raphanus sativus roots with a plantaricin-producing Lactobacillus plantarum starter. Curr. Microbiol. 2019, 76, 909–916. [Google Scholar] [CrossRef]
- Limanska, N.; Merlich, A.; Galkin, M.; Vasylieva, N.; Choiset, Y.; Ivanytsia, T.; Choiset, Y.; Vasylieva, N.; Galkin, M.; Merlich, A.; et al. Biofilm formation and genetic diversity of Lactobacillus plantarum strains originated from France and Ukraine. J. Microbiol. Biotechnol. Food Sci. 2019, 8, 1326–1331. [Google Scholar] [CrossRef]
- Santos, A.D.O.D.; Ávila, C.L.D.S.; Soares, C.; Carvalho, B.F.; Schwan, R.F.; Lima, N. Lactic acid bacteria diversity in corn silage produced in Minas Gerais (Brazil). Ann. Microbiol. 2019, 69, 1445–1459. [Google Scholar] [CrossRef]
- Barache, N.; Ladjouzi, R.; Belguesmia, Y.; Bendali, F.; Drider, D. Abundance of Lactobacillus plantarum strains with beneficial attributes in blackberries (Rubus sp.), fresh figs (Ficus carica), and prickly pears (Opuntia ficus-indica) grown and harvested in Algeria. Probiotics Antimicrob. Proteins 2020, 12, 1514–1523. [Google Scholar] [CrossRef]
- Puntillo, M.; Gaggiotti, M.; Oteiza, J.M.; Binetti, A.; Massera, A.; Vinderola, G. Potential of lactic acid bacteria isolated from different forages as silage inoculants for improving fermentation quality and aerobic stability. Front. Microbiol. 2020, 11, 586716. [Google Scholar] [CrossRef]
- Petkova, M.; Gotcheva, V.; Dimova, M.; Bartkiene, E.; Rocha, J.M.; Angelov, A. Screening of Lactiplantibacillus plantarum Strains from Sourdoughs for Biosuppression of Pseudomonas syringae pv. syringae and Botrytis cinerea in Table Grapes. Microorganisms 2022, 10, 2094. [Google Scholar] [CrossRef]
- López, I.; Torres, C.; Ruiz-Larrea, F. Genetic typification by pulsed-field gel electrophoresis (PFGE) and randomly amplified polymorphic DNA (RAPD) of wild Lactobacillus plantarum and Oenococcus oeni wine strains. Eur. Food Res. Technol. 2008, 227, 547–555. [Google Scholar] [CrossRef]
- Reshma, R.S.; Das, D.N. Molecular markers and its application in animal breeding. In Advances in Animal Genomics; Mondal, S., Singh, R.L., Eds.; Academic Press: London, UK, 2021; pp. 123–140. [Google Scholar]
- Figueroa, C.J.; Tang, Y.W.; Taur, Y. Principles and Applications of Genomic Diagnostic Techniques. In Molecular Medical Microbiology Vol 1; Tang, Y.W., Sussman, M., Liu, D., Poxton, I., Schwartzman, J., Eds.; Academic Press: London, UK, 2015; pp. 381–397. [Google Scholar]
- Laref, N.; Belkheir, K. Application of 16S rRNA virtual RFLP for the discrimination of some closely taxonomic-related lactobacilli species. J. Genet. Eng. Biotechnol. 2022, 20, 167. [Google Scholar] [CrossRef]
- Neoh, H.M.; Tan, X.E.; Sapri, H.F.; Tan, T.L. Pulsed-field gel electrophoresis (PFGE): A review of the “gold standard” for bacteria typing and current alternatives. Infect. Genet. Evol. 2019, 74, 103935. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, S.; Park, Y.S. Molecular typing tools for identifying and characterizing lactic acid bacteria: A review. Food Sci. Biotechnol. 2020, 29, 1301–1318. [Google Scholar] [CrossRef]
- Adesulu-Dahunsi, A.T.; Sanni, A.I.; Jeyaram, K.; Banwo, K. Genetic diversity of Lactobacillus plantarum strains from some indigenous fermented foods in Nigeria. LWT 2017, 82, 199–206. [Google Scholar] [CrossRef]
- Hu, J.; Yao, Y.; Yang, Y.; Gao, C.; Zhang, F.; Xia, R.; Ran, C.; Zhang, Z.; Clarke, J.L.; Zhou, Z. Advances of intra-species molecular typing analysis of aquatic probiotics approved by the Chinese Ministry of Agriculture. Rev. Aquac. 2021, 13, 178–188. [Google Scholar] [CrossRef]
- Kleerebezem, M.; Boekhorst, J.; Van Kranenburg, R.; Molenaar, D.; Kuipers, O.P.; Leer, R.; Tarchini, R.; Peters, S.A.; Sandbrink, H.M.; Fiers, M.W.E.J.; et al. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc. Natl. Acad. Sci. USA 2003, 100, 1990–1995. [Google Scholar] [CrossRef]
- Hugenholtz, P.; Chuvochina, M.; Oren, A.; Parks, D.H.; Soo, R.M. Prokaryotic taxonomy and nomenclature in the age of big sequence data. ISME 2021, 15, 1879–1892. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.; Harris, H.M.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Surve, S.; Shinde, D.B.; Kulkarni, R. Isolation, characterization and comparative genomics of potentially probiotic Lactiplantibacillus plantarum strains from Indian foods. Sci. Rep. 2022, 12, 1940. [Google Scholar] [CrossRef]
- Seddik, H.A.; Bendali, F.; Gancel, F.; Fliss, I.; Spano, G.; Drider, D. Lactobacillus plantarum and its probiotic and food potentialities. Probiotics Antimicrob. Proteins 2017, 9, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Gerner-Smidt, P.; Besser, J.; Concepción-Acevedo, J.; Folster, J.P.; Huffman, J.; Joseph, L.A.; Kucerova, Z.; Nichols, M.C.; Schwensohn, C.A.; Tolar, B. Whole genome sequencing: Bridging one-health surveillance of foodborne diseases. Front. Public Health 2019, 7, 172. [Google Scholar] [CrossRef] [PubMed]
- Apruzzese, I.; Song, E.; Bonah, E.; Sanidad, V.S.; Leekitcharoenphon, P.; Medardus, J.J.; Abdalla, N.; Hosseini, H.; Takeuchi, M. Investing in food safety for developing countries: Opportunities and challenges in applying whole-genome sequencing for food safety management. Foodborne Pathog. Dis. 2019, 16, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Deneke, C.; Brendebach, H.; Uelze, L.; Borowiak, M.; Malorny, B.; Tausch, S.H. Species-specific quality control, assembly and contamination detection in microbial isolate sequences with AQUAMIS. Genes 2021, 12, 644. [Google Scholar] [CrossRef]
- Daniel, R. The metagenomics of soil. Nat. Rev. Microbiol. 2005, 3, 470–478. [Google Scholar] [CrossRef]
- Steen, A.D.; Crits-Christoph, A.; Carini, P.; DeAngelis, K.M.; Fierer, N.; Lloyd, K.G.; Thrash, J.C. High proportions of bacteria and archaea across most biomes remain uncultured. ISME J. 2019, 13, 3126–3130. [Google Scholar] [CrossRef]
- Kralik, P.; Ricchi, M. A basic guide to real time PCR in microbial diagnostics: Definitions, parameters, and everything. Front. Microbiol. 2017, 8, 108. [Google Scholar] [CrossRef]
- Kovac, J.; Rolon, M.L.; Naum, M.; Lampel, K.A. DNA-based assays. In Encyclopedia of Dairy Sciences, 3rd ed.; McSweeney, P.L.H., McNamara, J.P., Eds.; Academic Press: New York, NY, USA, 2022; Volume 3, pp. 356–362. [Google Scholar]
- Harshitha, R.; Arunraj, D.R. Real-time quantitative PCR: A tool for absolute and relative quantification. Biochem. Mol. Biol. Educ. 2021, 49, 800–812. [Google Scholar] [CrossRef]
- Kim, E.; Yang, S.M.; Lim, B.; Park, S.H.; Rackerby, B.; Kim, H.Y. Design of PCR assays to specifically detect and identify 37 Lactobacillus species in a single 96 well plate. BMC Microbiol. 2020, 20, 96. [Google Scholar] [CrossRef]
- Jin, Y.J.; Park, Y.K.; Cho, M.S.; Lee, E.S.; Park, D.S. New insight and metrics to understand the ontogeny and succession of Lactobacillus plantarum subsp. plantarum and Lactobacillus plantarum subsp. argentoratensis. Sci. Rep. 2018, 8, 6029. [Google Scholar] [CrossRef]
- Kim, E.; Kim, H.-B.; Yang, S.-M.; Kim, D.; Kim, H.-Y. Real-time PCR assay for detecting Lactobacillus plantarum group using species/subspecies-specific genes identified by comparative genomics. LWT 2020, 138, 110789. [Google Scholar] [CrossRef]
- Choi, C.H.; Kim, E.; Yang, S.M.; Kim, D.S.; Suh, S.M.; Lee, G.Y.; Kim, H.Y. Comparison of Real-Time PCR and Droplet Digital PCR for the Quantitative Detection of Lactiplantibacillus plantarum subsp. plantarum. Foods 2022, 11, 1331. [Google Scholar] [CrossRef]
- Xiong, T.; Chen, J.; Huang, T.; Xie, M.; Xiao, Y.; Liu, C.; Peng, Z. Fast evaluation by quantitative PCR of microbial diversity and safety of Chinese Paocai inoculated with Lactobacillus plantarum NCU116 as the culture starter. LWT 2018, 101, 201–206. [Google Scholar] [CrossRef]
- Pswarayi, F.; Gänzle, M.G. Composition and origin of the fermentation microbiota of mahewu, a Zimbabwean fermented cereal beverage. Appl. Environ. Microbiol. 2019, 85, e03130-18. [Google Scholar] [CrossRef]
- Correa-Galeote, D.; Ghomari, I.; Asehraou, A.; González-López, J. Revealing the bacterial abundance and diversity in brines from started Spanish-style green table olives. LWT 2022, 160, 113212. [Google Scholar] [CrossRef]
- Bell, R.L.; Jarvis, K.G.; Ottesen, A.R.; McFarland, M.A.; Brown, E.W. Recent and emerging innovations in Salmonella detection: A food and environmental perspective. Microb. Biotechnol. 2016, 9, 279–292. [Google Scholar] [CrossRef]
- Schwendimann, L.; Kauf, P.; Fieseler, L.; Gantenbein-Demarchi, C.; Schwenninger, S.M. Development of a quantitative PCR assay for rapid detection of Lactobacillus plantarum and Lactobacillus fermentum in cocoa bean fermentation. J. Microbiol. Methods 2015, 115, 94–99. [Google Scholar] [CrossRef]
- Asano, S.; Shimokawa, M.; Suzuki, K. PCR Analysis Methods for Detection and Identification of Beer-Spoilage Lactic Acid Bacteria. In Lactic Acid Bacteria Methods in Molecular Biology; Kanauchi, M., Ed.; Humana Press: New York, NY, USA, 2019; Volume 1887, pp. 95–107. [Google Scholar]
- Haakensen, M.C.; Butt, L.; Chaban, B.; Deneer, H.; Ziola, B.; Dowgiert, T. horA-Specific Real-Time PCR for Detection of Beer-Spoilage Lactic Acid Bacteria. J. Am. Soc. Brew. Chem. 2007, 65, 157–165. [Google Scholar] [CrossRef]
- Sidstedt, M.; Rådström, P.; Hedman, J. PCR inhibition in qPCR, dPCR and MPS—Mechanisms and solutions. Anal. Bioanal. Chem. 2020, 412, 2009–2023. [Google Scholar] [CrossRef]
- Pervez, M.T.; Abbas, S.H.; Moustafa, M.F.; Aslam, N.; Shah, S.S.M. A Comprehensive Review of Performance of Next-Generation Sequencing Platforms. Biomed. Res. Int. 2022, 2022, 3457806. [Google Scholar] [CrossRef]
- Mitreva, M. The Microbiome in infectious diseases. In Infectious Diseases; Cohen, J., Powderly, W.G., Opal, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1, pp. 68–74. [Google Scholar]
- Sharma, R.; Kumar, A.; Singh, N.; Sharma, K. 16S rRNA gene profiling of rhizospheric microbial community of Eichhornia crassipes. Mol. Biol. Rep. 2021, 48, 4055–4064. [Google Scholar] [CrossRef] [PubMed]
- Bassi, D.; Orrù, L.; Cabanillas Vasquez, J.; Cocconcelli, P.S.; Fontana, C. Peruvian chicha: A focus on the microbial populations of this ancient Maize-based fermented beverage. Microorganisms 2020, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Heo, S.; Na, H.E.; Lee, G.; Kim, J.H.; Kwak, M.S.; Sung, M.H.; Jeong, D.W. Bacterial Community of Galchi-Baechu Kimchi Based on Culture-Dependent and-Independent Investigation and Selection of Starter Candidates. J. Microbiol. Biotechnol. 2022, 32, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Boreczek, J.; Litwinek, D.; Żylińska-Urban, J.; Izak, D.; Buksa, K.; Gawor, J.; Gromadka, R.; Bardowski, J.K.; Kowalczyk, M. Bacterial community dynamics in spontaneous sourdoughs made from wheat, spelt, and rye wholemeal flour. MicrobiologyOpen 2020, 9, e1009. [Google Scholar] [CrossRef]
- Xu, D.; Wang, N.; Rinne, M.; Ke, W.; Weinberg, Z.G.; Da, M.; Bai, J.; Zhang, Y.; Li, F.; Guo, X. The bacterial community and metabolome dynamics and their interactions modulate fermentation process of whole crop corn silage prepared with or without inoculants. Microb. Biotechnol. 2021, 14, 561–576. [Google Scholar] [CrossRef]
- Durazzi, F.; Sala, C.; Castellani, G.; Manfreda, G.; Remondini, D.; De Cesare, A. Comparison between 16S rRNA and shotgun sequencing data for the taxonomic characterization of the gut microbiota. Sci. Rep. 2021, 11, 3030. [Google Scholar] [CrossRef]
- Raghavendra, P.; Pullaiah, T. Pathogen identification using novel sequencing methods. In Advances in Cell and Molecular Diagnostics; Raghavendra, P., Pullaiah, T., Eds.; Academic Press: London, UK, 2018; pp. 161–199. [Google Scholar]
- Meslier, V.; Quinquis, B.; Da Silva, K.; Plaza Oñate, F.; Pons, N.; Roume, H.; Podar, M.; Almeida, M. Benchmarking second and third-generation sequencing platforms for microbial metagenomics. Sci. Data 2022, 9, 694. [Google Scholar] [CrossRef]
- Regalado, J.; Lundberg, D.S.; Deusch, O.; Kersten, S.; Karasov, T.; Poersch, K.; Shirsekar, G.; Weigel, D. Combining whole-genome shotgun sequencing and rRNA gene amplicon analyses to improve detection of microbe–microbe interaction networks in plant leaves. ISME J. 2020, 14, 2116–2130. [Google Scholar] [CrossRef]
- Angeli, D.; Sare, A.R.; Jijakli, M.H.; Pertot, I.; Massart, S. Insights gained from metagenomic shotgun sequencing of apple fruit epiphytic microbiota. Postharvest Biol. Technol. 2019, 153, 96–106. [Google Scholar] [CrossRef]
- Chacón-Vargas, K.; Torres, J.; Giles-Gómez, M.; Escalante, A.; Gibbons, J.G. Genomic profiling of bacterial and fungal communities and their predictive functionality during pulque fermentation by whole-genome shotgun sequencing. Sci. Rep. 2020, 10, 15115. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Bouajila, J.; Pace, M.; Leech, J.; Cotter, P.D.; Souchard, J.P.; Taillandier, P.; Beaufort, S. Metabolome-microbiome signatures in the fermented beverage, Kombucha. Int. J. Food Microbiol. 2020, 333, 108778. [Google Scholar] [CrossRef]
- Tamang, J.P.; Kharnaior, P.; Pariyar, P.; Thapa, N.; Lar, N.; Win, K.S.; Mar, A.; Nyo, N. Shotgun sequence-based metataxonomic and predictive functional profiles of Pe poke, a naturally fermented soybean food of Myanmar. PLoS ONE 2021, 16, e0260777. [Google Scholar] [CrossRef]
- Cai, W.; Wang, Y.; Hou, Q.; Zhang, Z.; Tang, F.; Shan, C.; Yang, X.; Guo, Z. PacBio sequencing combined with metagenomic shotgun sequencing provides insight into the microbial diversity of zha-chili. Food Biosci. 2021, 40, 100884. [Google Scholar] [CrossRef]
| Target Gene | Function | Primer Name | Primer Sequence (5′ → 3′) | References |
|---|---|---|---|---|
| rpoA | α-subunit of RNA polymerase | rpoA-21-F | ATG ATY GAR TTT GAA AAA CC | [56] |
| rpoA-22-R | ACY TTV ATC ATN TCW GVY TC | |||
| pheS | phenylalanyl-tRNA synthase | pheS-21-F | CAY CC NGC HCG YGA YAT GC | [56] |
| pheS-22-R | CCW ARV CCR AAR GCA AAR CC | |||
| dnaK | heat shockresponse | Lpdnak-500F3 1 | CCG TTC TTR TCR ATR TCR AA | [56] |
| Lpdnak-1710R5 1 | GAA AYY CAA GTY GGH GAA GT | |||
| recA | DNA repair and maintenance | planF 2 | CCG TTT ATG CGG AAC ACC TA | [26,56,59,60,61] |
| pREV 2 | TCG GGA TTA CCA AAC ATC AC | |||
| mutL | DNA mismatchrepair | LpmutL-F | TSG AYG TSA AYG TKC AYC C | [57] |
| LpmutL-R | ATG YGG RCA RTT RAA NGG AT | |||
| spLplan-F 2 | GCG RTT GTT CCG TCA GAA T | |||
| spLplan-R 2 | CTT GCA GCC GTG CTG GTT T | |||
| ISR of groESL | intergenic spacer region | Lplan-F 2 | GGA CAA AAG TTG ACC CCA GCG | [58] |
| Lplan-R 2 | ACC GTT GCA GTA GTC GTC CC |
| Primer Name | Primer Sequence (5′ → 3′) | References |
|---|---|---|
| M13 | GAG GGT GGC GGT TCT | [67,69,70,71,72,73] |
| M14 | GAG GGT GGG GCC GTT | [56] |
| LP1 | ACG CGC CCT | [56,74] |
| OPL-05 | ACG CAG GCA C | [56,67,74] |
| COC | AGC AGC GTG G | [56] |
| P1 | GCG GCG TCG CTA ATA CAT GC | [73] |
| P2 | ATG TAA CGC C | [67] |
| P4 | CCG CAG CGT T | [67,73] |
| B10 | CTG CTG GGA C | [73] |
| Target Gene | Function | Primer Name | Primer Sequence(5′ → 3′) | Product Size [bp] | References |
|---|---|---|---|---|---|
| LPXTG | LPxTG-motif cell wall anchor domain protein | Plantarum-F | GCT GGC AAT GCC ATC GTG CT | 147 | [95] |
| Plantarum-R | TCT CAA CGG TTG CTG TAT CG | ||||
| T1PL186F 1 | ACC CCC GTT CCG TCA GA | 186 | [96] | ||
| T1PL186R 1 | ATC ACC GCT TCC CCG CTC ATT | ||||
| bspA | leucine-rich repeat surface protein | LPA187F 2 | GCA TCC CGA CGC TAC TAC ACA | 187 | |
| LPA187R 2 | GAT TTT ATT TGC GTC CCA CTC C | ||||
| ydiC | hypothetical protein | Plantarum_F 1 | CGG CAA CAA GCC ACT AAA CT | 120 | [97] |
| Plantarum_R 1 | TTC TTG ATG GCC CGG GTG TT | ||||
| Argentoratensis_F 2 | TTC TTG ATG GCC CGG GTG TT | 143 | [97,98] | ||
| Argentoratensis_R 2 | GGC TGG ACC ATG GCT AAG AA | ||||
| not stated | hypothetical protein | CMC1F | AGT TTG CCA CAT ATT AGG AAG AGA | 112 | [100] |
| CMC1R | AGG CTC TAA GGG CTA CCT ATA C | ||||
| tal | unknown | Ftal1 | AAC ATT TCG CGG AAC TTG GTT | 160 | [99] |
| Rtal1 | ATC ATC TCT TCG GCC TTG GT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skotniczny, M.; Satora, P. Molecular Detection and Identification of Plant-Associated Lactiplantibacillus plantarum. Int. J. Mol. Sci. 2023, 24, 4853. https://doi.org/10.3390/ijms24054853
Skotniczny M, Satora P. Molecular Detection and Identification of Plant-Associated Lactiplantibacillus plantarum. International Journal of Molecular Sciences. 2023; 24(5):4853. https://doi.org/10.3390/ijms24054853
Chicago/Turabian StyleSkotniczny, Magdalena, and Paweł Satora. 2023. "Molecular Detection and Identification of Plant-Associated Lactiplantibacillus plantarum" International Journal of Molecular Sciences 24, no. 5: 4853. https://doi.org/10.3390/ijms24054853
APA StyleSkotniczny, M., & Satora, P. (2023). Molecular Detection and Identification of Plant-Associated Lactiplantibacillus plantarum. International Journal of Molecular Sciences, 24(5), 4853. https://doi.org/10.3390/ijms24054853