Protective Effects of Glycine soja Leaf and Stem Extract against Chondrocyte Inflammation and Osteoarthritis
Abstract
1. Introduction
2. Results
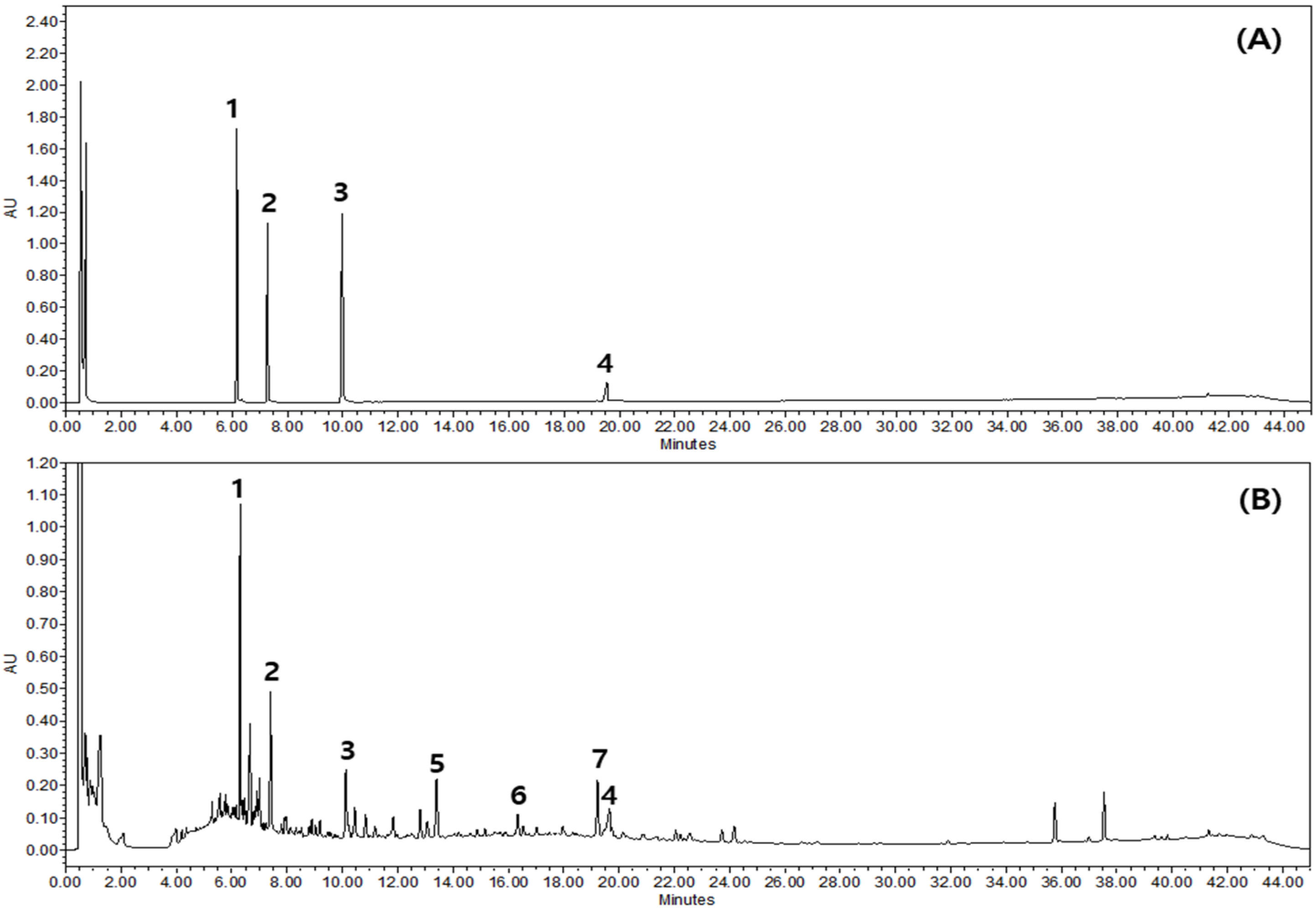
2.1. Chemical Profiling of GSLS
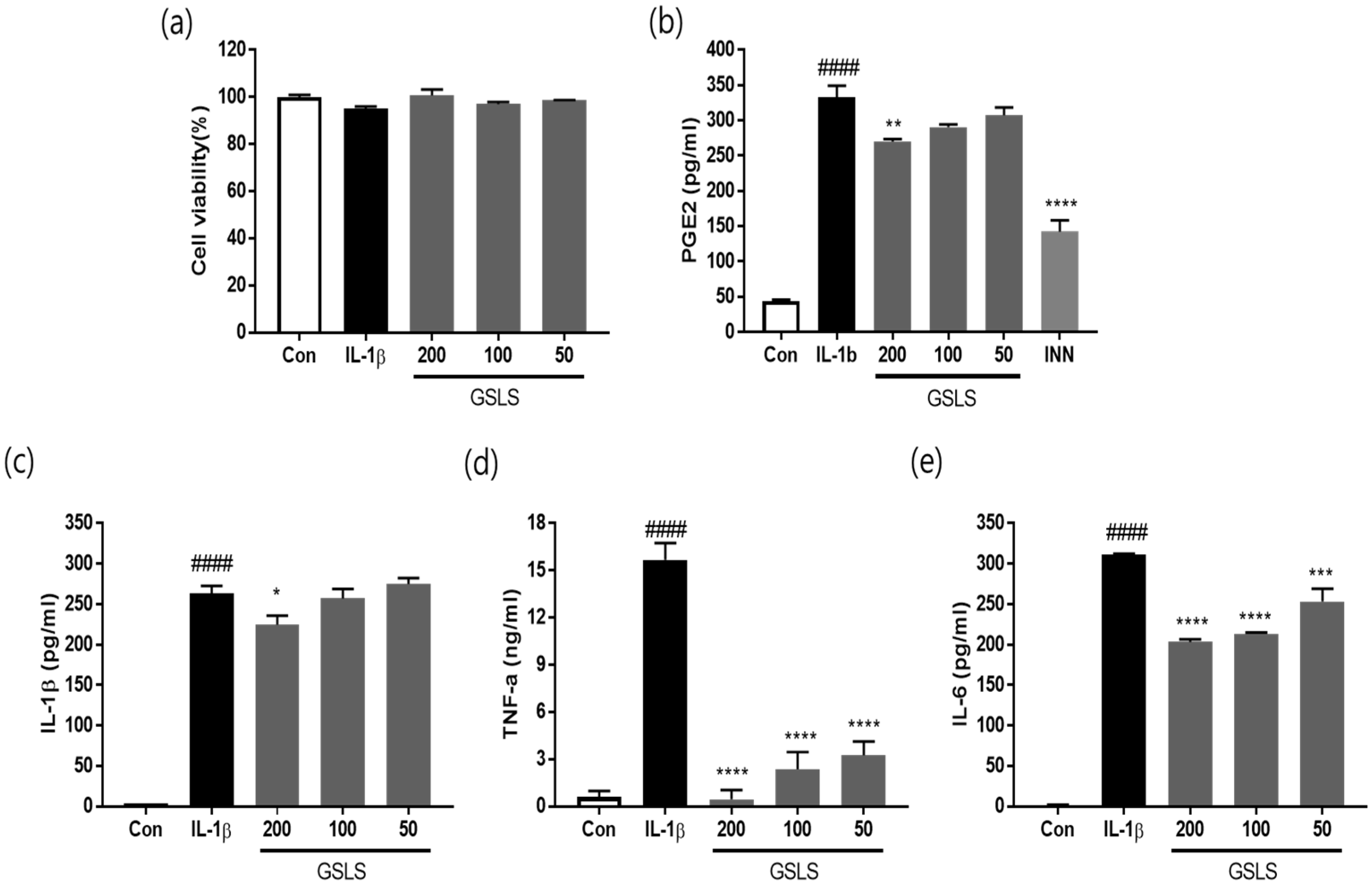
2.2. Effects of GSLS on the Viability in Chondrocytes
2.3. Effects of GSLS on IL-1β, TNF-α, and IL-6 Production
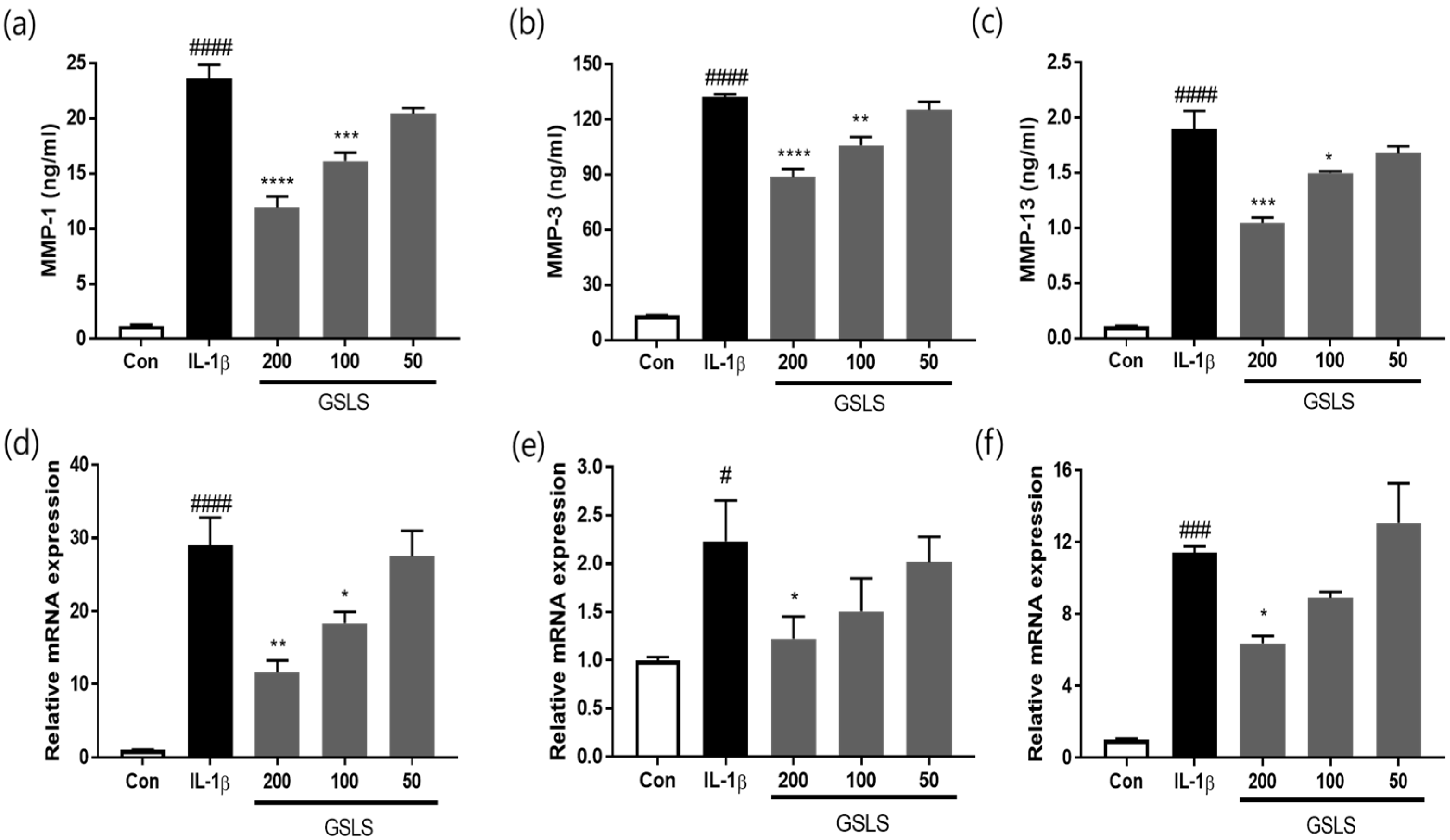
2.4. Effects of GSLS on IL-1β-Induced Extracellular Matrix Degradation
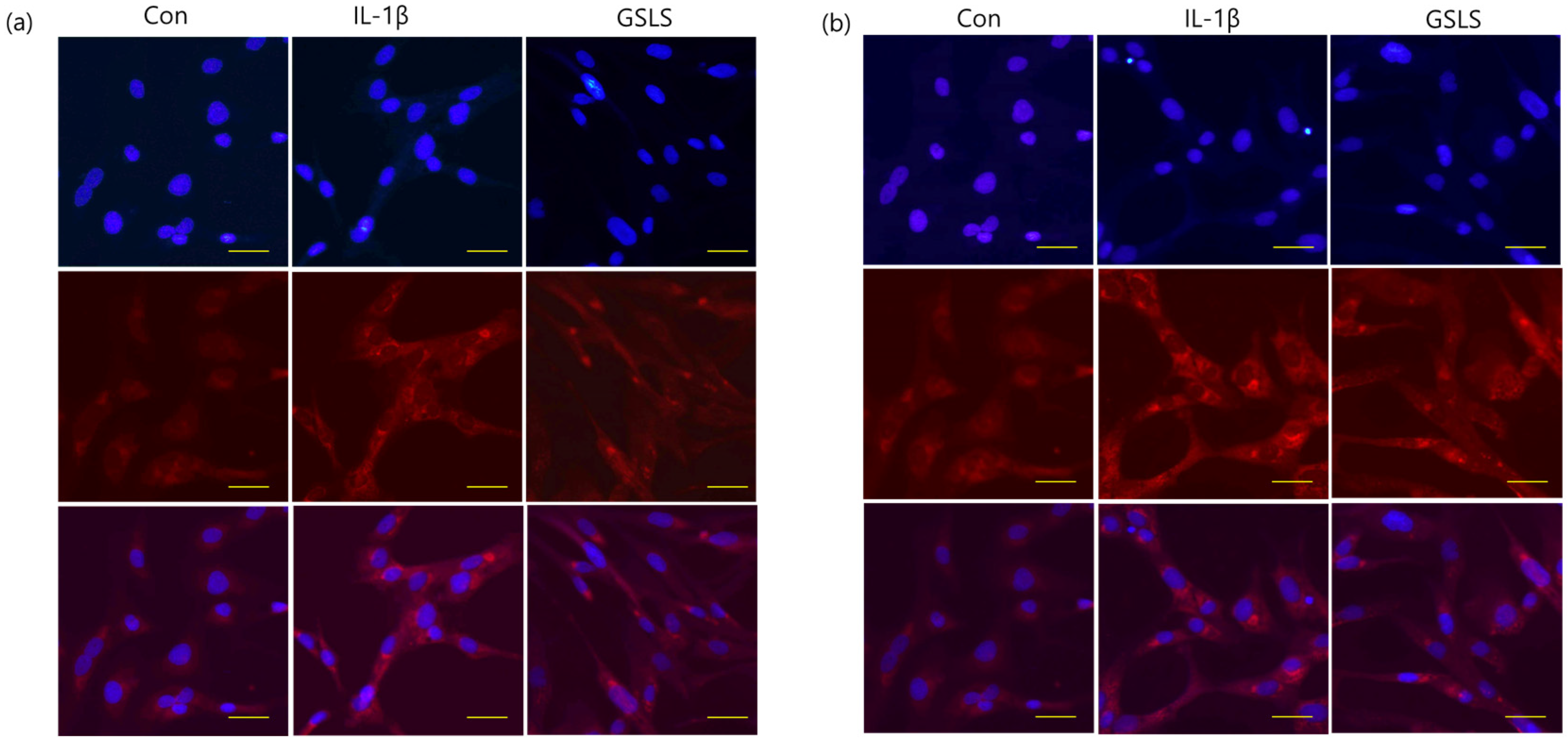
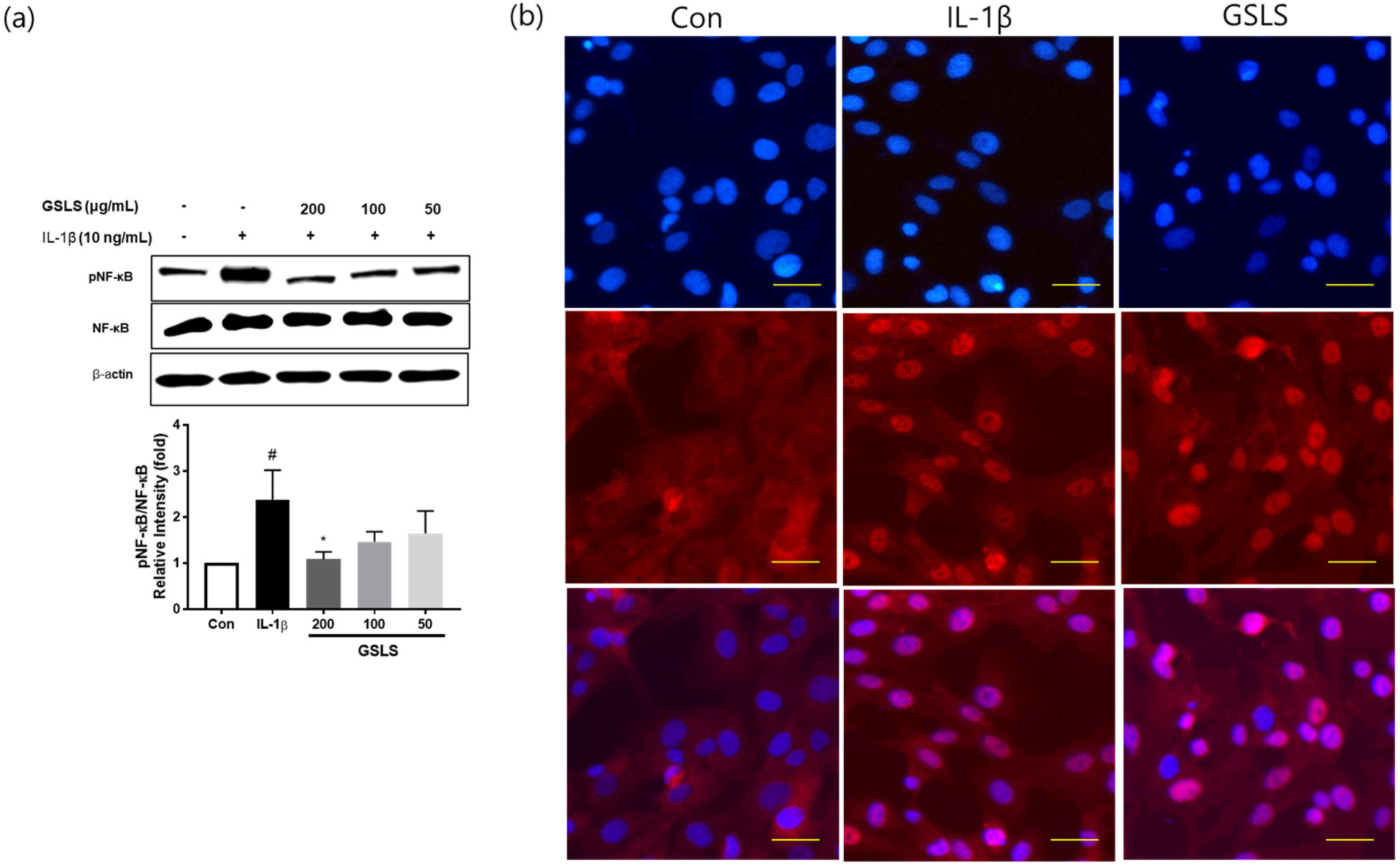
2.5. Effect of GSLS on IL-1β-Induced NF-κB Signal Activation
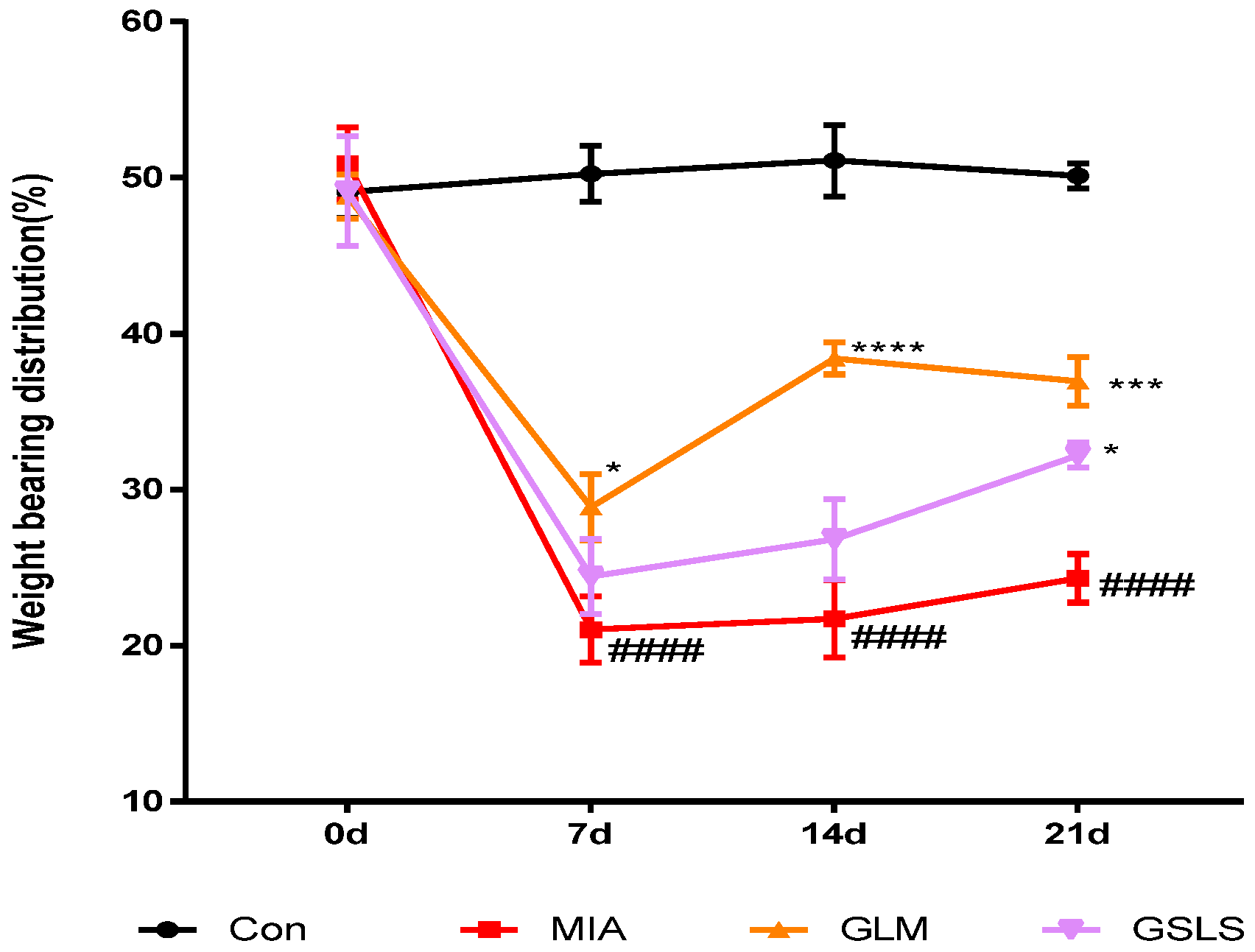
2.6. Effect of GSLS on Joint Pain in Rats with Monosodium Iodoacetate-Induced Osteoarthritis
2.7. Effect of GSLS on Inflammatory Mediators and Cytokines in Rats with Monosodium Iodoacetate-Induced Osteoarthritis
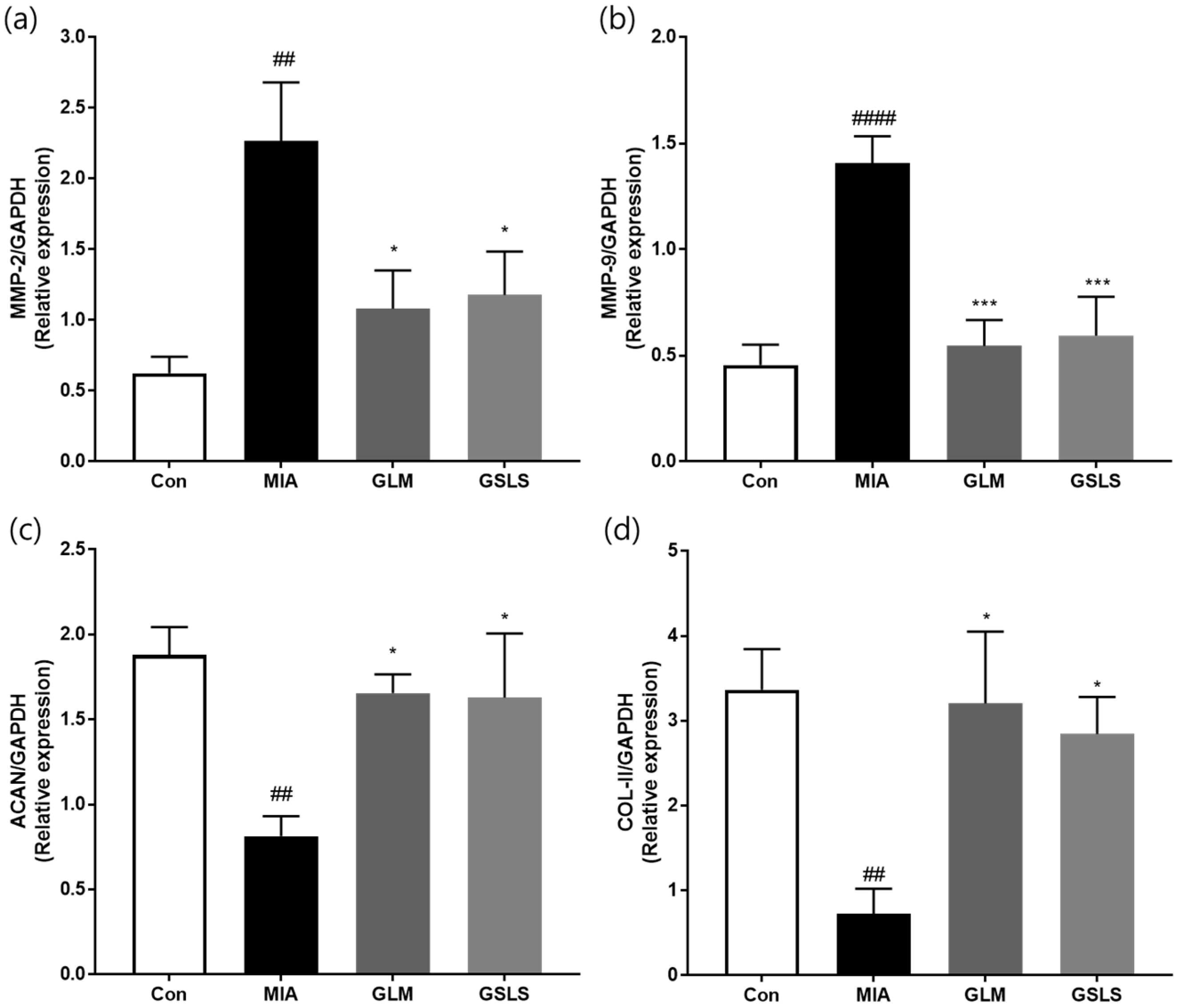
2.8. Effects of GSLS on Cartilage Degradation in Rats with Monosodium Iodoacetate-Induced Osteoarthritis
3. Discussion
4. Materials and Methods
4.1. GSLS Preparation
4.2. Chemical Profiling of GSLS
4.3. Cell Culture
4.4. Cytotoxicity Measurement
4.5. Rat Model of Monosodium Iodoacetate-Induced OA
4.6. Enzyme-Linked Immunosorbent Assay for Proinflammatory Cytokines and Matrix Metalloproteinases
4.7. Western Blot Analysis
4.8. Immunofluorescence Staining
4.9. Total RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.J. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef] [PubMed]
- Guilak, F.; Nims, R.J.; Dicks, A.; Wu, C.L.; Meulenbelt, I. Osteoarthritis as a disease of the cartilage pericellular matrix. Matrix. Biol. 2018, 71–72, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Burmester, G.R.; Feist, E.; Dorner, T. Emerging cell and cytokine targets in rheumatoid arthritis. Nat. Rev. Rheumatol. 2014, 10, 77–88. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef]
- Lark, M.W.; Bayne, E.K.; Flanagan, J.; Harper, C.F.; Hoerrner, L.A.; Hutchinson, N.I.; Singer, I.I.; Donatelli, S.A.; Weidner, J.R.; Williams, H.R.; et al. Aggrecan degradation in human cartilage. Evidence for both matrix metalloproteinase and aggrecanase activity in normal, osteoarthritic, and rheumatoid joints. J. Clin. Investig. 1997, 100, 93–106. [Google Scholar] [CrossRef]
- Henrotin, Y.; Sanchez, C.; Bay-Jensen, A.C.; Mobasheri, A. Osteoarthritis biomarkers derived from cartilage extracellular matrix: Current status and future perspectives. Ann. Phys. Rehabil. Med. 2016, 59, 145–148. [Google Scholar] [CrossRef]
- Kang, Y.H.; Lee, H.J.; Lee, C.J.; Park, J.S. Natural Products as Sources of Novel Drug Candidates for the Pharmacological Management of Osteoarthritis: A Narrative Review. Biomol. Ther. 2019, 27, 503–513. [Google Scholar] [CrossRef]
- Wen, Z.; Ding, Y.; Zhao, T.; Gai, J. Genetic diversity and peculiarity of annual wild soybean (G. soja Sieb. et Zucc.) from various eco-regions in China. Theor. Appl. Genet. 2009, 119, 371–381. [Google Scholar] [CrossRef]
- Jing, C.; Wen, Z.; Zou, P.; Yuan, Y.; Jing, W.; Li, Y.; Zhang, C. Consumption of Black Legumes Glycine soja and Glycine max Lowers Serum Lipids and Alters the Gut Microbiome Profile in Mice Fed a High-Fat Diet. J. Agric. Food Chem. 2018, 66, 7367–7375. [Google Scholar] [CrossRef]
- de Araujo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and their applications: An approach in food chemistry and innovation potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, X.; Yuan, X.; Shi, J.; Zhang, C.; Yan, N.; Jing, C. Comparison of Phenolic and Flavonoid Compound Profiles and Antioxidant and alpha-Glucosidase Inhibition Properties of Cultivated Soybean (Glycine max) and Wild Soybean (Glycine soja). Plants 2021, 10, 813. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Yuan, Y.; Tang, Q.; Zou, P.; Li, Y.; Zhang, C. Extraction optimization, preliminary characterization and antioxidant activities of polysaccharides from Glycine soja. Int. J. Biol. Macromol. 2017, 103, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Y.; Luo, S.H.; Yi, T.S.; Li, C.H.; Luo, Q.; Hua, J.; Liu, Y.; Li, S.H. Secondary metabolites from Glycine soja and their growth inhibitory effect against Spodoptera litura. J. Agric. Food Chem. 2011, 59, 6004–6010. [Google Scholar] [CrossRef]
- Poole, A.R.; Kobayashi, M.; Yasuda, T.; Laverty, S.; Mwale, F.; Kojima, T.; Sakai, T.; Wahl, C.; El-Maadawy, S.; Webb, G.; et al. Type II collagen degradation and its regulation in articular cartilage in osteoarthritis. Ann. Rheum. Dis. 2002, 61 (Suppl. S2), ii78–ii81. [Google Scholar] [CrossRef]
- Hardingham, T. Extracellular matrix and pathogenic mechanisms in osteoarthritis. Curr. Rheumatol. Rep. 2008, 10, 30–36. [Google Scholar] [CrossRef]
- Bonnet, C.S.; Walsh, D.A. Osteoarthritis, angiogenesis and inflammation. Rheumatology 2005, 44, 7–16. [Google Scholar] [CrossRef]
- Chun, J.M.; Lee, A.Y.; Kim, J.S.; Choi, G.; Kim, S.H. Protective Effects of Peucedanum japonicum Extract against Osteoarthritis in an Animal Model Using a Combined Systems Approach for Compound-Target Prediction. Nutrients 2018, 10, 754. [Google Scholar] [CrossRef]
- Lee, Y.M.; Son, E.; Kim, S.H.; Kim, D.S. Anti-Inflammatory and Analgesic Effects of Schisandra chinensis Leaf Extracts and Monosodium Iodoacetate-Induced Osteoarthritis in Rats and Acetic Acid-Induced Writhing in Mice. Nutrients 2022, 14, 1356. [Google Scholar] [CrossRef]
- Pelletier, J.P.; Martel-Pelletier, J.; Abramson, S.B. Osteoarthritis, an inflammatory disease: Potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001, 44, 1237–1247. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Martel-Pelletier, J.; Pelletier, J.P. The role of cytokines in osteoarthritis pathophysiology. Biorheology 2002, 39, 237–246. [Google Scholar] [PubMed]
- van de Loo, F.A.; Joosten, L.A.; van Lent, P.L.; Arntz, O.J.; van den Berg, W.B. Role of interleukin-1, tumour necrosis factor alpha, and interleukin-6 in cartilage proteoglycan metabolism and destruction. Effect of in situ blocking in murine antigen- and zymosan-induced arthritis. Arthritis Rheum. 1995, 38, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Guerne, P.A.; Carson, D.A.; Lotz, M. IL-6 production by human articular chondrocytes. Modulation of its synthesis by cytokines, growth factors, and hormones in vitro. J. Immunol. 1990, 144, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Lotz, M.; Terkeltaub, R.; Villiger, P.M. Cartilage and joint inflammation. Regulation of IL-8 expression by human articular chondrocytes. J. Immunol. 1992, 148, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zhang, X.; Cheang, W.S. Isoflavones daidzin and daidzein inhibit lipopolysaccharide-induced inflammation in RAW264.7 macrophages. Chin. Med. 2022, 17, 95. [Google Scholar] [CrossRef]
- Zha, L.; Chen, J.; Sun, S.; Mao, L.; Chu, X.; Deng, H.; Cai, J.; Li, X.; Liu, Z.; Cao, W. Soyasaponins can blunt inflammation by inhibiting the reactive oxygen species-mediated activation of PI3K/Akt/NF-kB pathway. PLoS ONE 2014, 9, e107655. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, F.; Chen, J.; Huang, S.; Chen, J.; Huang, J.; Li, N.; Sun, S.; Chu, X.; Zha, L. Soyasaponin Bb inhibits the recruitment of toll-like receptor 4 (TLR4) into lipid rafts and its signaling pathway by suppressing the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-dependent generation of reactive oxygen species. Mol. Nutr. Food Res. 2016, 60, 1532–1543. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M.; Plumb, D.A.; Dragomir, C.; Favero, M.; El Hachem, K.; Hashimoto, K.; Roach, H.I.; Olivotto, E.; Borzi, R.M.; et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: Signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur. Cell Mater. 2011, 21, 202–220. [Google Scholar] [CrossRef]
- Mastbergen, S.C.; Jansen, N.W.; Bijlsma, J.W.; Lafeber, F.P. Differential direct effects of cyclo-oxygenase-1/2 inhibition on proteoglycan turnover of human osteoarthritic cartilage: An in vitro study. Arthritis Res. Ther. 2006, 8, R2. [Google Scholar] [CrossRef]
- Tung, J.T.; Arnold, C.E.; Alexander, L.H.; Yuzbasiyan-Gurkan, V.; Venta, P.J.; Richardson, D.W.; Caron, J.P. Evaluation of the influence of prostaglandin E2 on recombinant equine interleukin-1beta-stimulated matrix metalloproteinases 1, 3, and 13 and tissue inhibitor of matrix metalloproteinase 1 expression in equine chondrocyte cultures. Am. J. Vet. Res. 2002, 63, 987–993. [Google Scholar] [CrossRef]
- Jablonska-Trypuc, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzyme. Inhib. Med. Chem. 2016, 31 (Suppl. S1), 177–183. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Woessner, J.F., Jr. Matrix metalloproteinases. J. Biol. Chem. 1999, 274, 21491–21494. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef]
- Vincenti, M.P.; Brinckerhoff, C.E. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: Integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002, 4, 157–164. [Google Scholar] [CrossRef]
- Hulejova, H.; Baresova, V.; Klezl, Z.; Polanska, M.; Adam, M.; Senolt, L. Increased level of cytokines and matrix metalloproteinases in osteoarthritic subchondral bone. Cytokine 2007, 38, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Bay-Jensen, A.C.; Karsdal, M.A.; Siebuhr, A.S.; Zheng, Q.; Maksymowych, W.P.; Christiansen, T.G.; Henriksen, K. The active form of MMP-3 is a marker of synovial inflammation and cartilage turnover in inflammatory joint diseases. BMC Musculoskelet Disord. 2014, 15, 93. [Google Scholar] [CrossRef] [PubMed]
- Mengshol, J.A.; Vincenti, M.P.; Coon, C.I.; Barchowsky, A.; Brinckerhoff, C.E. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: Differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 2000, 43, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, M.P.; Coon, C.I.; Mengshol, J.A.; Yocum, S.; Mitchell, P.; Brinckerhoff, C.E. Cloning of the gene for interstitial collagenase-3 (matrix metalloproteinase-13) from rabbit synovial fibroblasts: Differential expression with collagenase-1 (matrix metalloproteinase-1). Biochem. J. 1998, 331 Pt 1, 341–346. [Google Scholar] [CrossRef]
- Roman-Blas, J.A.; Jimenez, S.A. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthr. Cartil. 2006, 14, 839–848. [Google Scholar] [CrossRef]
- Marcu, K.B.; Otero, M.; Olivotto, E.; Borzi, R.M.; Goldring, M.B. NF-kappaB signaling: Multiple angles to target OA. Curr. Drug. Targets 2010, 11, 599–613. [Google Scholar] [CrossRef]
- Jimi, E.; Fei, H.; Nakatomi, C. NF-kappaB Signaling Regulates Physiological and Pathological Chondrogenesis. Int. J. Mol. Sci. 2019, 20, 6275. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Jung, Y.B.; Seong, S.C.; Park, H.B.; Byun, K.Y.; Lee, D.C.; Song, E.K.; Son, J.H. Clinical efficacy and safety of Lyprinol, a patented extract from New Zealand green-lipped mussel (Perna Canaliculus) in patients with osteoarthritis of the hip and knee: A multicenter 2-month clinical trial. Eur. Ann. Allergy Clin. Immunol. 2003, 35, 212–216. [Google Scholar] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.M.; Son, E.; Kim, S.-H.; Kim, D.-S. Protective Effects of Glycine soja Leaf and Stem Extract against Chondrocyte Inflammation and Osteoarthritis. Int. J. Mol. Sci. 2023, 24, 4829. https://doi.org/10.3390/ijms24054829
Lee YM, Son E, Kim S-H, Kim D-S. Protective Effects of Glycine soja Leaf and Stem Extract against Chondrocyte Inflammation and Osteoarthritis. International Journal of Molecular Sciences. 2023; 24(5):4829. https://doi.org/10.3390/ijms24054829
Chicago/Turabian StyleLee, Yun Mi, Eunjung Son, Seung-Hyung Kim, and Dong-Seon Kim. 2023. "Protective Effects of Glycine soja Leaf and Stem Extract against Chondrocyte Inflammation and Osteoarthritis" International Journal of Molecular Sciences 24, no. 5: 4829. https://doi.org/10.3390/ijms24054829
APA StyleLee, Y. M., Son, E., Kim, S.-H., & Kim, D.-S. (2023). Protective Effects of Glycine soja Leaf and Stem Extract against Chondrocyte Inflammation and Osteoarthritis. International Journal of Molecular Sciences, 24(5), 4829. https://doi.org/10.3390/ijms24054829




