Seminal Extracellular Vesicles and Their Involvement in Male (In)Fertility: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
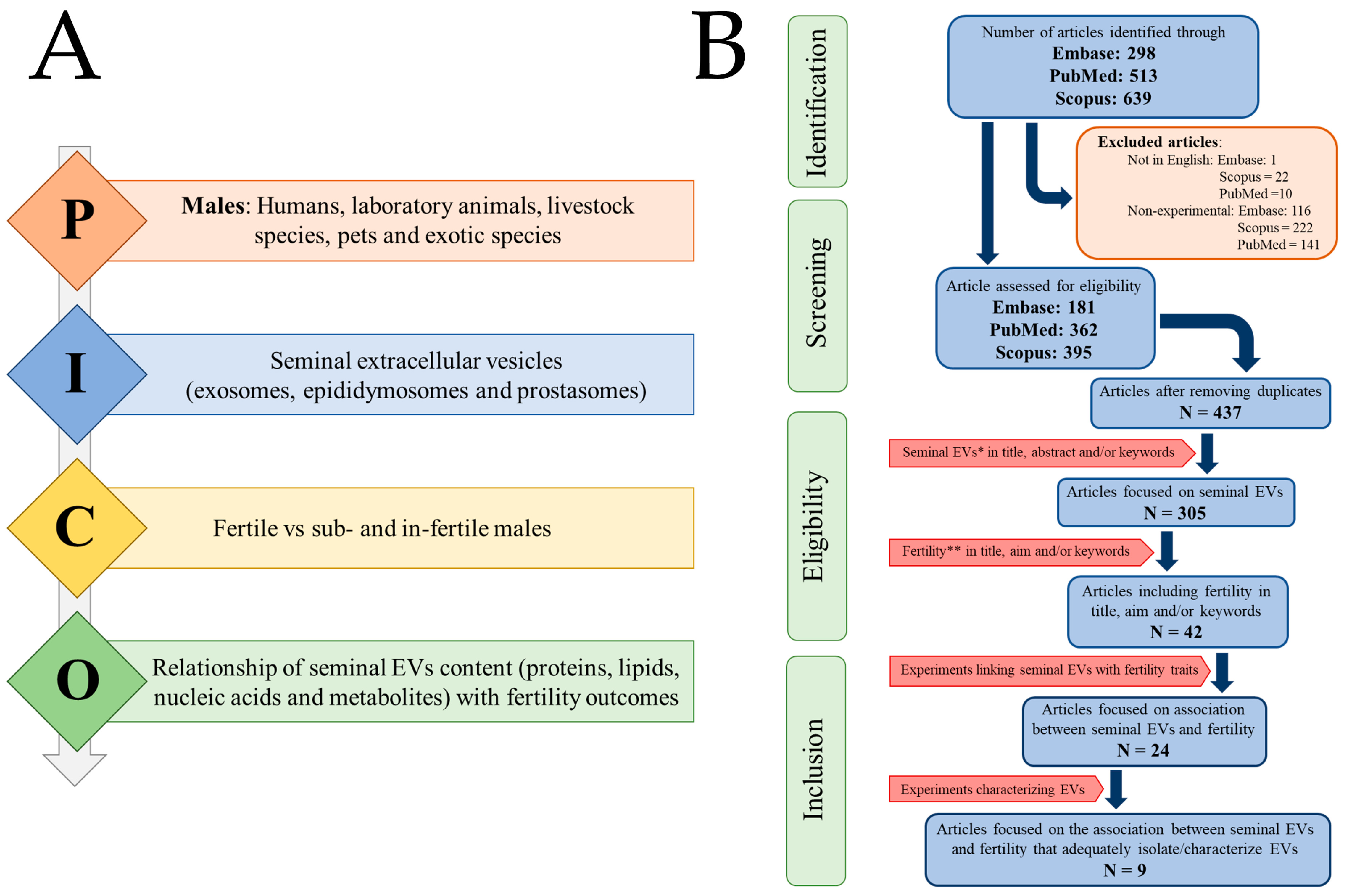
2.1. Selection of Peer-Reviewed and Published Studies
2.2. Screening, Eligibility, and Inclusion/Exclusion Criteria
2.3. Data Extraction
2.4. Bioinformatic Analysis
3. Results
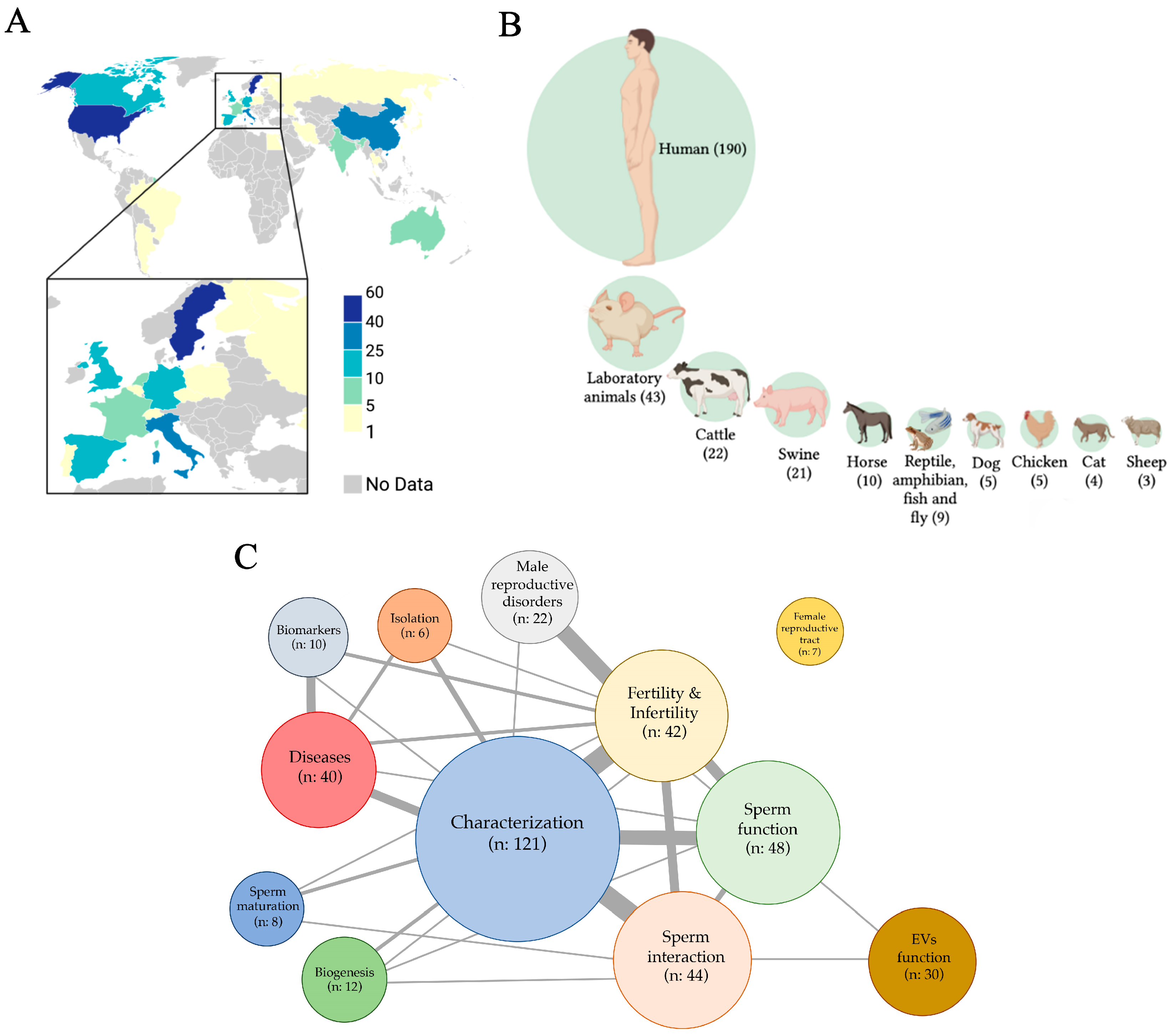
3.1. Search Report
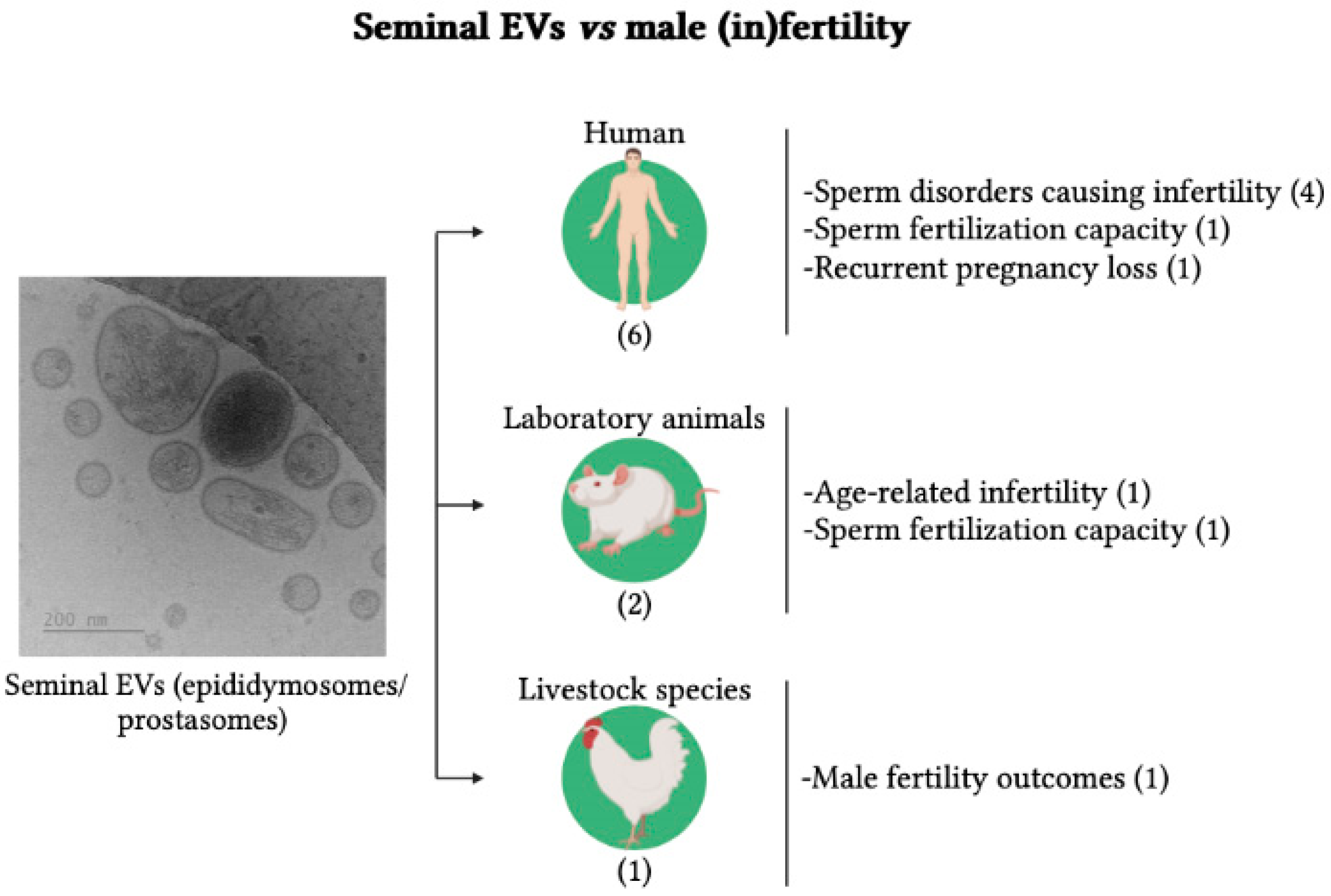
3.2. Seminal EVs vs. Male (In)Fertility
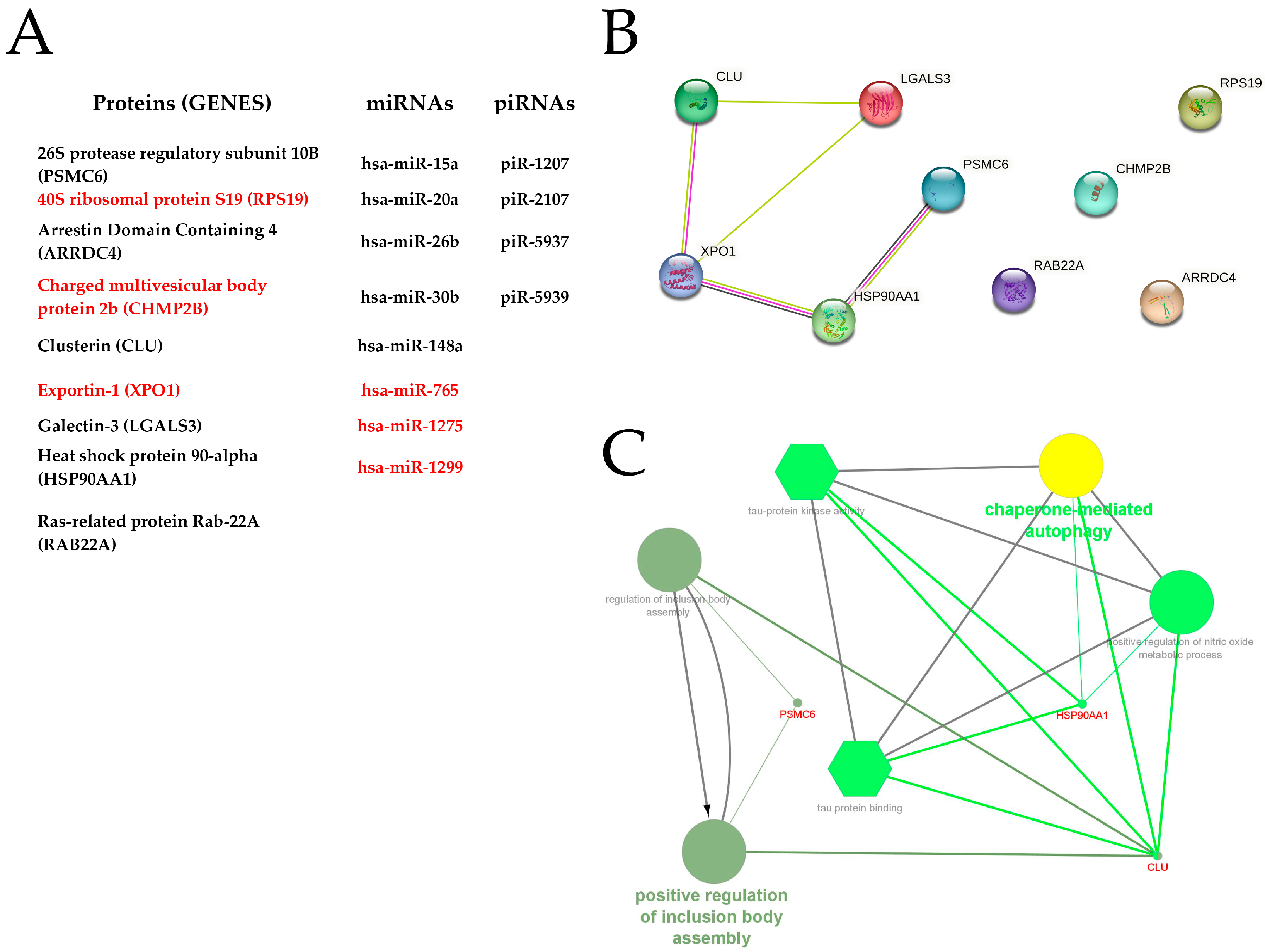
3.3. Bioinformatic Analysis
4. Discussion
4.1. Search Report
4.2. Seminal EVs vs. Male (In)Fertility
4.3. Bioinformatic Analysis
4.4. Strengths and Limitations
4.5. Considerations for Future Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodriguez-Martinez, H.; Martinez, E.A.; Calvete, J.J.; Peña Vega, F.J.; Roca, J. Seminal Plasma: Relevant for Fertility? Int. J. Mol. Sci. 2021, 22, 4368. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Singh, N.K. Emerging Role of Novel Seminal Plasma Bio-Markers in Male Infertility: A Review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 253, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef] [PubMed]
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.A.; Vader, P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Bhatta, B.; Cooks, T. Reshaping the Tumor Microenvironment: Extracellular Vesicles as Messengers of Cancer Cells. Carcinogenesis 2020, 41, 1461–1470. [Google Scholar] [CrossRef]
- Hu, W.; Liu, C.; Bi, Z.Y.; Zhou, Q.; Zhang, H.; Li, L.L.; Zhang, J.; Zhu, W.; Song, Y.Y.Y.; Zhang, F.; et al. Comprehensive Landscape of Extracellular Vesicle-Derived RNAs in Cancer Initiation, Progression, Metastasis and Cancer Immunology. Mol. Cancer 2020, 19, 102. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Barile, L.; Vassalli, G. Exosomes: Therapy Delivery Tools and Biomarkers of Diseases. Pharmacol. Ther. 2017, 174, 63–78. [Google Scholar] [CrossRef]
- Wang, J.; Yue, B.L.; Huang, Y.Z.; Lan, X.Y.; Liu, W.J.; Chen, H. Exosomal RNAs: Novel Potential Biomarkers for Diseases—A Review. Int. J. Mol. Sci. 2022, 23, 2461. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Wrzecińska, M.; Czerniawska-Piątkowska, E.; Kupczyński, R. Exosomes—Spectacular Role in Reproduction. Biomed. Pharmacother. 2022, 148, 112752. [Google Scholar] [CrossRef]
- Tesfaye, D.; Menjivar, N.; Gebremedhn, S. Current Knowledge and the Future Potential of Extracellular Vesicles in Mammalian Reproduction. Reprod. Fertil. Dev. 2021, 34, 174–189. [Google Scholar] [CrossRef]
- Skalnikova, H.K.; Bohuslavova, B.; Turnovcova, K.; Juhasova, J.; Juhas, S.; Rodinova, M.; Vodicka, P. Isolation and Characterization of Small Extracellular Vesicles from Porcine Blood Plasma, Cerebrospinal Fluid, and Seminal Plasma. Proteomes 2019, 7, 17. [Google Scholar] [CrossRef]
- Royo, F.; Théry, C.; Falcón-Pérez, J.M.; Nieuwland, R.; Witwer, K.W. Methods for Separation and Characterization of Extracellular Vesicles: Results of a Worldwide Survey Performed by the ISEV Rigor and Standardization Subcommittee. Cells 2020, 9, 1955. [Google Scholar] [CrossRef]
- Roca, J.; Rodriguez-Martinez, H.; Padilla, L.; Lucas, X.; Barranco, I. Extracellular Vesicles in Seminal Fluid and Effects on Male Reproduction. An Overview in Farm Animals and Pets. Anim. Reprod. Sci. 2021, 246, 106853. [Google Scholar] [CrossRef]
- Höög, J.L.; Lötvall, J. Diversity of Extracellular Vesicles in Human Ejaculates Revealed by Cryo-Electron Microscopy. J. Extracell. Vesicles 2015, 4, 28680. [Google Scholar] [CrossRef]
- Candenas, L.; Chianese, R. Exosome Composition and Seminal Plasma Proteome: A Promising Source of Biomarkers of Male Infertility. Int. J. Mol. Sci. 2020, 21, 7022. [Google Scholar] [CrossRef]
- Ronquist, G. Prostasomes Are Mediators of Intercellular Communication: From Basic Research to Clinical Implications. J. Intern. Med. 2012, 271, 400–413. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Keerthikumar, S.; Chisanga, D.; Ariyaratne, D.; Al Saffar, H.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N.; et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J. Mol. Biol. 2016, 428, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Pathan, M.; Fonseka, P.; Chitti, S.V.; Kang, T.; Sanwlani, R.; Van Deun, J.; Hendrix, A.; Mathivanan, S. Vesiclepedia 2019: A Compendium of RNA, Proteins, Lipids and Metabolites in Extracellular Vesicles. Nucleic Acids Res. 2019, 47, D516–D519. [Google Scholar] [CrossRef] [PubMed]
- Abu-Halima, M.; Ludwig, N.; Hart, M.; Leidinger, P.; Backes, C.; Keller, A.; Hammadeh, M.; Meese, E. Altered Micro-Ribonucleic Acid Expression Profiles of Extracellular Microvesicles in the Seminal Plasma of Patients with Oligoasthenozoospermia. Fertil. Steril. 2016, 106, 1061–1069.e3. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, L.; Lin, H.L.H.; Carvalho, A.V.; Grasseau, I.; Uzbekov, R.; Blesbois, E. First Insights on Seminal Extracellular Vesicles in Chickens of Contrasted Fertility. Reproduction 2021, 161, 489–498. [Google Scholar] [CrossRef]
- Foot, N.J.; Gonzalez, M.B.; Gembus, K.; Fonseka, P.; Sandow, J.J.; Nguyen, T.T.; Tran, D.; Webb, A.I.; Mathivanan, S.; Robker, R.L.; et al. Arrdc4-Dependent Extracellular Vesicle Biogenesis Is Required for Sperm Maturation. J. Extracell. Vesicles 2021, 10, e12113. [Google Scholar] [CrossRef]
- García-Rodríguez, A.; de la Casa, M.; Peinado, H.; Gosálvez, J.; Roy, R. Human Prostasomes from Normozoospermic and Non-Normozoospermic Men Show a Differential Protein Expression Pattern. Andrology 2018, 6, 585–596. [Google Scholar] [CrossRef]
- Hong, Y.; Wu, Y.; Zhang, J.; Yu, C.; Shen, L.; Chen, H.; Chen, L.; Zhou, X.; Gao, F. Decreased PiRNAs in Infertile Semen Are Related to Downregulation of Sperm MitoPLD Expression. Front. Endocrinol. 2021, 12, 696121. [Google Scholar] [CrossRef]
- Jena, S.R.; Nayak, J.; Kumar, S.; Kar, S.; Dixit, A.; Samanta, L. Paternal Contributors in Recurrent Pregnancy Loss: Cues from Comparative Proteome Profiling of Seminal Extracellular Vesicles. Mol. Reprod. Dev. 2021, 88, 96–112. [Google Scholar] [CrossRef]
- Mei, S.; Chen, P.; Lee, C.L.; Zhao, W.; Wang, Y.; Lam, K.K.W.; Ho, P.C.; Yeung, W.S.B.; Fang, C.; Chiu, P.C.N. The Role of Galectin-3 in Spermatozoa-Zona Pellucida Binding and Its Association with Fertilization in Vitro. Mol. Hum. Reprod. 2019, 25, 458–470. [Google Scholar] [CrossRef]
- Vickram, A.S.; Anbarasu, K.; Gulothungan, G.; Thanigaivel, S.; Nanmaran, R.; Palanivelu, J. Characterization of Human Prostasomes Protein Clusterin (Macromolecule)—A Novel Biomarker for Male Infertility Diagnosis and Prognosis. J. Biomol. Struct. Dyn. 2020, 40, 3979–3988. [Google Scholar] [CrossRef]
- Wang, D.; Jueraitetibaike, K.; Tang, T.; Wang, Y.; Jing, J.; Xue, T.; Ma, J.; Cao, S.; Lin, Y.; Li, X.; et al. Seminal Plasma and Seminal Plasma Exosomes of Aged Male Mice Affect Early Embryo Implantation via Immunomodulation. Front. Immunol. 2021, 12, 723409. [Google Scholar] [CrossRef]
- Renneberg, H.; Konrad, L.; Dammshäuser, I.; Seitz, J.; Aumüller, G. Immunohistochemistry of Prostasomes from Human Semen. Prostate 1997, 30, 98–106. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Llorente, A. Current Knowledge on Exosome Biogenesis and Release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef]
- Foot, N.J.; Kumar, S. The Role of Extracellular Vesicles in Sperm Function and Male Fertility. In Subcellular Biochemistry; Springer: Cham, Switzerland, 2021; Volume 97, pp. 483–500. [Google Scholar]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal Experimental Requirements for Definition of Extracellular Vesicles and Their Functions: A Position Statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef]
- Witwer, K.W.; Goberdhan, D.C.I.; O’Driscoll, L.; Théry, C.; Welsh, J.A.; Blenkiron, C.; Buzás, E.I.; Di Vizio, D.; Erdbrügger, U.; Falcón-Pérez, J.M.; et al. Updating MISEV: Evolving the Minimal Requirements for Studies of Extracellular Vesicles. J. Extracell. Vesicles 2021, 10, e12182. [Google Scholar] [CrossRef]
- Deng, Y.; Sun, Z.; Wang, L.; Wang, M.; Yang, J.; Li, G. Biosensor-Based Assay of Exosome Biomarker for Early Diagnosis of Cancer. Front. Med. 2021, 16, 157–175. [Google Scholar] [CrossRef]
- Lu, M.; Yuan, S.; Li, S.; Li, L.; Liu, M.; Wan, S. The Exosome-Derived Biomarker in Atherosclerosis and Its Clinical Application. J. Cardiovasc. Transl. Res. 2019, 12, 68–74. [Google Scholar] [CrossRef]
- Janiszewska, E.; Kratz, E.M. Could the Glycosylation Analysis of Seminal Plasma Clusterin Become a Novel Male Infertility Biomarker? Mol. Reprod. Dev. 2020, 87, 515–524. [Google Scholar] [CrossRef]
- Record, M. Intercellular Communication by Exosomes in Placenta: A Possible Role in Cell Fusion? Placenta 2014, 35, 297–302. [Google Scholar] [CrossRef]
- Leidal, A.M.; Debnath, J. Unraveling the Mechanisms That Specify Molecules for Secretion in Extracellular Vesicles. Methods 2020, 177, 15–26. [Google Scholar] [CrossRef]
- D’Amours, O.; Calvo, É.; Bourassa, S.; Vincent, P.; Blondin, P.; Sullivan, R. Proteomic Markers of Low and High Fertility Bovine Spermatozoa Separated by Percoll Gradient. Mol. Reprod. Dev. 2019, 86, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Barkalina, N.; Jones, C.; Wood, M.J.A.; Coward, K. Extracellular Vesicle-Mediated Delivery of Molecular Compounds into Gametes and Embryos: Learning from Nature. Hum. Reprod. Update 2015, 21, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Machtinger, R.; Laurent, L.C.; Baccarelli, A.A. Extracellular Vesicles: Roles in Gamete Maturation, Fertilization and Embryo Implantation. Hum. Reprod. Update 2016, 22, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Roca, J.; Broekhuijse, M.L.W.J.; Parrilla, I.; Rodriguez-Martinez, H.; Martinez, E.A.; Bolarin, A. Boar Differences in Artificial Insemination Outcomes: Can They Be Minimized? Reprod. Domest. Anim. 2015, 50, 48–55. [Google Scholar] [CrossRef]
- Baixauli, F.; López-Otín, C.; Mittelbrunn, M. Exosomes and Autophagy: Coordinated Mechanisms for the Maintenance of Cellular Fitness. Front. Immunol. 2014, 5, 403. [Google Scholar] [CrossRef]
- Lefèvre, B.; Wolf, J.P.; Ziyyat, A. Sperm-Egg Interaction: Is There a Link between Tetraspanin(s) and GPI-Anchored Protein(S)? Bioessays 2010, 32, 143–152. [Google Scholar] [CrossRef]
- Ambros, V.; Bartel, B.; Bartel, D.P.; Burge, C.B.; Carrington, J.C.; Chen, X.; Dreyfuss, G.; Eddy, S.R.; Griffiths-Jones, S.; Marshall, M.; et al. A Uniform System for MicroRNA Annotation. RNA 2003, 9, 277–279. [Google Scholar] [CrossRef]
| Authors | Species | Isolation Method | Characterization |
|---|---|---|---|
| Abu-Halima et al. (2016) [23] | Human | UC + gradient UC | EV-specific markers (WB: CD63, CD81, CD9 and HSP70) |
| Cordeiro et al. (2021) [24] | Chicken | UC (×2) | Morphology (TEM), EV-specific markers (WB: ANXA5, HSP90A, VCP, and PDCD6IP) |
| Foot et al. (2021) [25] | Mouse | UC | Size distribution and concentration (NTA), EV-specific markers (WB: CD81, Annexin A1, and B-actin) |
| García-Rodríguez et al. (2018) [26] | Human | UC (×2) | Total protein quantification (BCA assay), size distribution and concentration (NTA), morphology (TEM) |
| Hong et al. (2021) [27] | Human | UC | Size distribution and concentration (NTA), Morphology (TEM), EV-specific markers (WB: CD63 and TSG101) |
| Jena et al. (2020) [28] | Human | Filtration + UC | Size distribution (DLS), morphology (SEM), EV-specific markers (WB: CD9 and CD81) |
| Mei et al. (2019) [29] | Human | Filtration | Morphology (TEM), EV-specific markers (WB: CD13) |
| Vickram et al. (2020) [30] | Human | UC + SEC | Size distribution (DLS), morphology (SEM), composition assay (EDX) |
| Wang et al. (2021) [31] | Mouse | UC + filtration | Size distribution and concentration (NTA), morphology (TEM), EV-specific markers (WB: CD63 and TSG101) |
| Authors | Country | Species | Sample Details | Study Question | Main Results |
|---|---|---|---|---|---|
| Abu-Halima et al. (2016) [23] | Germany | Human | Semen from normo- (n:12) and oligoastheno-zoospermic men (n:12) | Whether altered miRNA expression profiles of EVs were related to fertility | Eight miRNAs were differentially expressed between oligoastheno- and normo-zoospermic men |
| Cordeiro et al. (2021) [24] | France | Chicken | Seminal plasma from fertile (n:7) and subfertile (n:6) males | Whether sEVs from fertile and subfertile chickens showed differences in their characteristics and sperm fusion capacity | sEVs from fertile and subfertile roosters showed differences in size, protein composition, and sperm fusion capacity |
| Foot et al. (2021) [25] | Australia | Mouse | Samples from cauda epididymis and vas deferens (n:156) | Whether Arrdc4-/-, a protein involved in EV biogenesis, improves the fertilizing capacity of sperm | Supplementation of Arrdc4-/- spermatozoa with seminal EVs improved their fertilizing capacity |
| García-Rodríguez et al. (2018) [26] | Spain | Human | Semen from normo- (n:12) and non-normo-zoospermic (n:14) men | Whether the protein profile of extracellular vesicles was related to fertility | Two unique proteins identified in the sEVs of normozoospermic males and three in non-normozoospermic males |
| Hong et al. (2021) [27] | China | Human | Semen from normo- (n:41) and astheno-zoospermic (n:42) men | Whether EV piRNAs profile was related to MitoPLD expression and thus to sperm fertility | piRNAs and MitoPLD were reduced in spermatozoa of asthenozoospermic men |
| Jena et al. (2020) [28] | India | Human | Semen from fertile men (n:21) and partners of women with RPL (n:21) | Whether seminal EV proteomic profiling was related to RPL | A total of 106 EV proteins were under- and 71 over-expressed in RPL partners. Fifty-six EV proteins were only expressed in RPL partners. This led to defects in paternal gene expression and embryo development |
| Mei et al. (2019) [29] | China | Human | Semen from men attending infertility center 1 | Whether seminal EV galectin-3 influenced sperm fertilization capacity | Low levels of seminal EV galectin-3 were associated with low fertilization rates |
| Vickram et al. (2020) [30] | India | Human | Semen from normo- (n:35), oligo-, astheno- (n:35), oligoastheno- (n:35) and a-zoospermic (n:10) | Identify new diagnostic and prognostic biomarkers of infertility among proteins encapsulated in prostasomes | Clusterin protein was differentially expressed between normozoospermic and non-normozoospermic samples |
| Wang et al. (2021) [31] | China | Mouse | Seminal plasma from young (n:3) and older males (n:3) | Whether aging affects content of seminal EVs and thus embryo implantation | Aging influenced seminal vesicle size and content. Perfusion of seminal EVs from young males increased implantation rate. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parra, A.; Padilla, L.; Lucas, X.; Rodriguez-Martinez, H.; Barranco, I.; Roca, J. Seminal Extracellular Vesicles and Their Involvement in Male (In)Fertility: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 4818. https://doi.org/10.3390/ijms24054818
Parra A, Padilla L, Lucas X, Rodriguez-Martinez H, Barranco I, Roca J. Seminal Extracellular Vesicles and Their Involvement in Male (In)Fertility: A Systematic Review. International Journal of Molecular Sciences. 2023; 24(5):4818. https://doi.org/10.3390/ijms24054818
Chicago/Turabian StyleParra, Ana, Lorena Padilla, Xiomara Lucas, Heriberto Rodriguez-Martinez, Isabel Barranco, and Jordi Roca. 2023. "Seminal Extracellular Vesicles and Their Involvement in Male (In)Fertility: A Systematic Review" International Journal of Molecular Sciences 24, no. 5: 4818. https://doi.org/10.3390/ijms24054818
APA StyleParra, A., Padilla, L., Lucas, X., Rodriguez-Martinez, H., Barranco, I., & Roca, J. (2023). Seminal Extracellular Vesicles and Their Involvement in Male (In)Fertility: A Systematic Review. International Journal of Molecular Sciences, 24(5), 4818. https://doi.org/10.3390/ijms24054818









