Antioxidative and Mitochondrial Protection in Retinal Pigment Epithelium: New Light Source in Action
Abstract
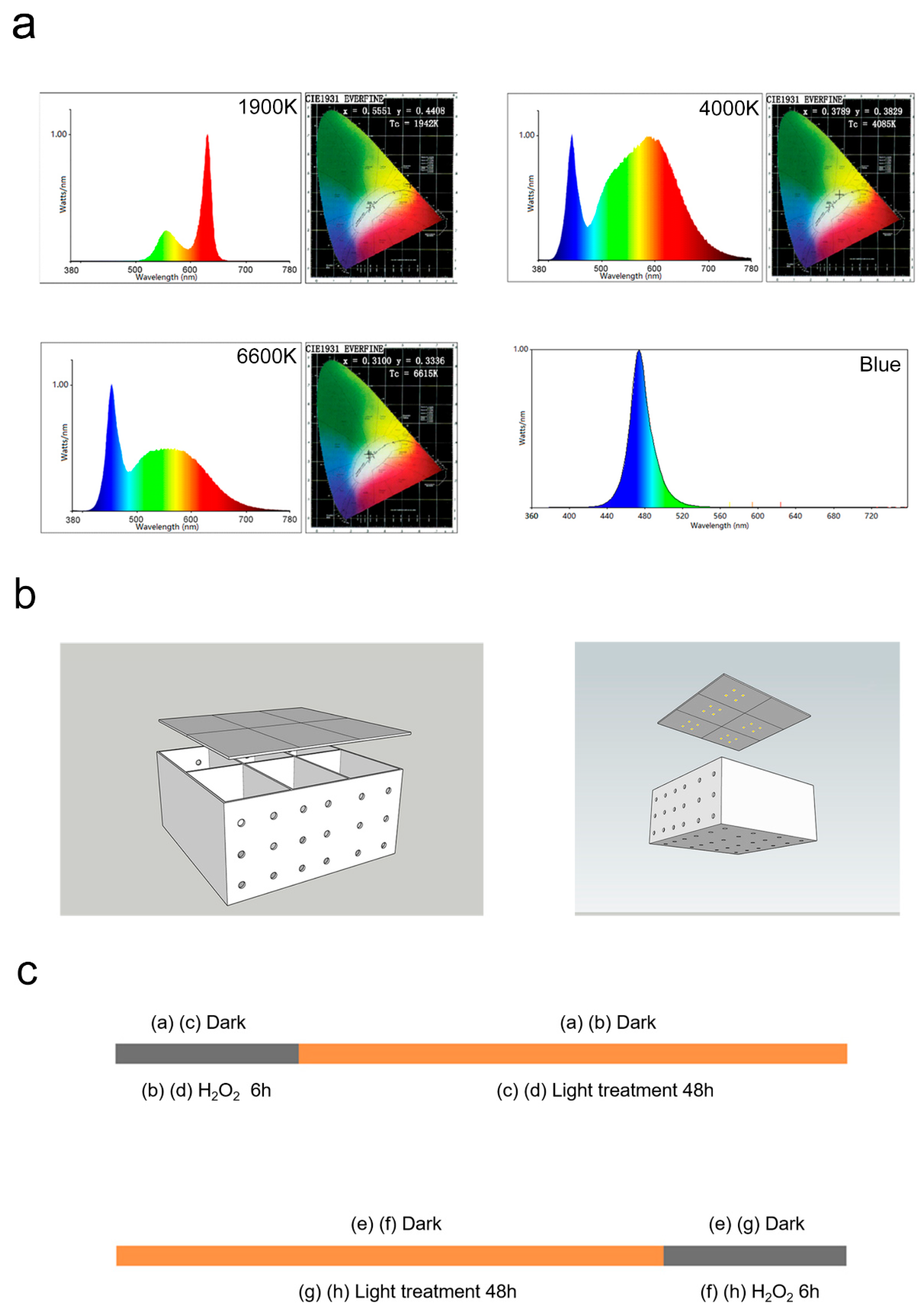
1. Introduction
2. Results and Discussion
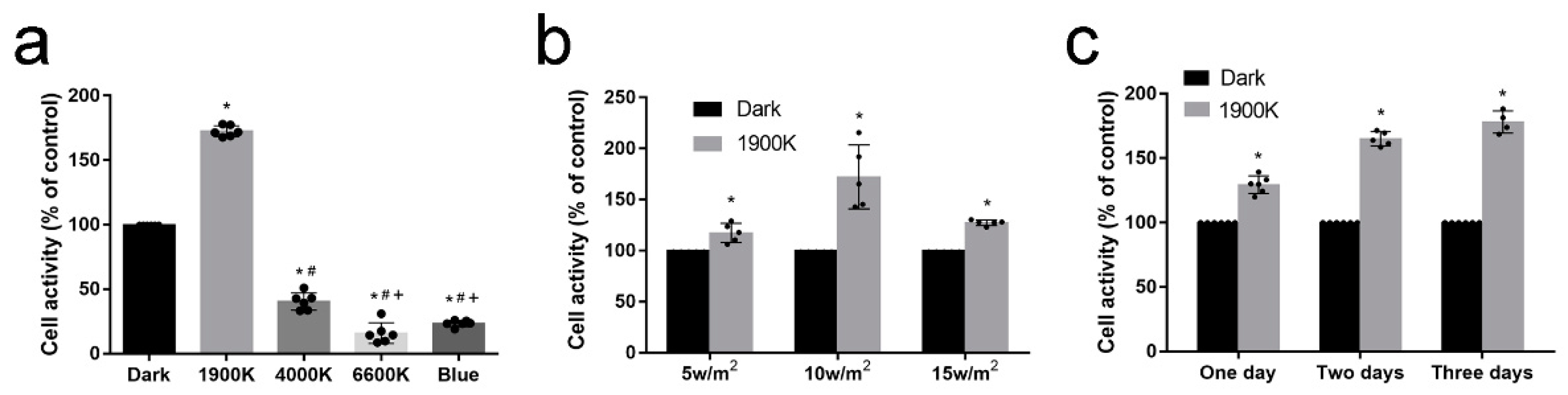
2.1. 1900 K LEDs Regulate Cell Activity According to Irradiance and Light Time
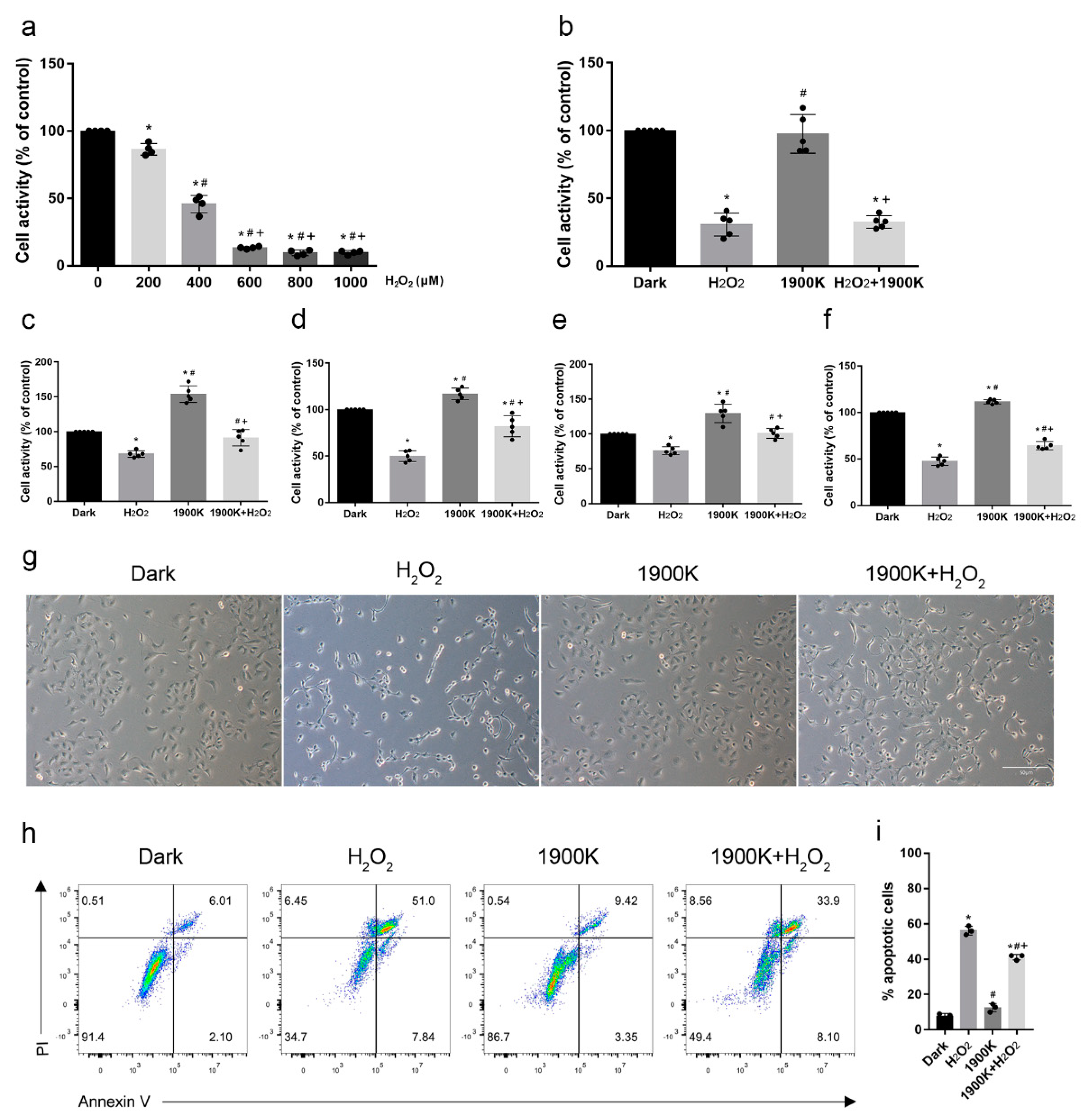
2.2. Pretreatment with 10 W/m2 1900 K LEDs Reduced the Death of ARPE-19 Cells
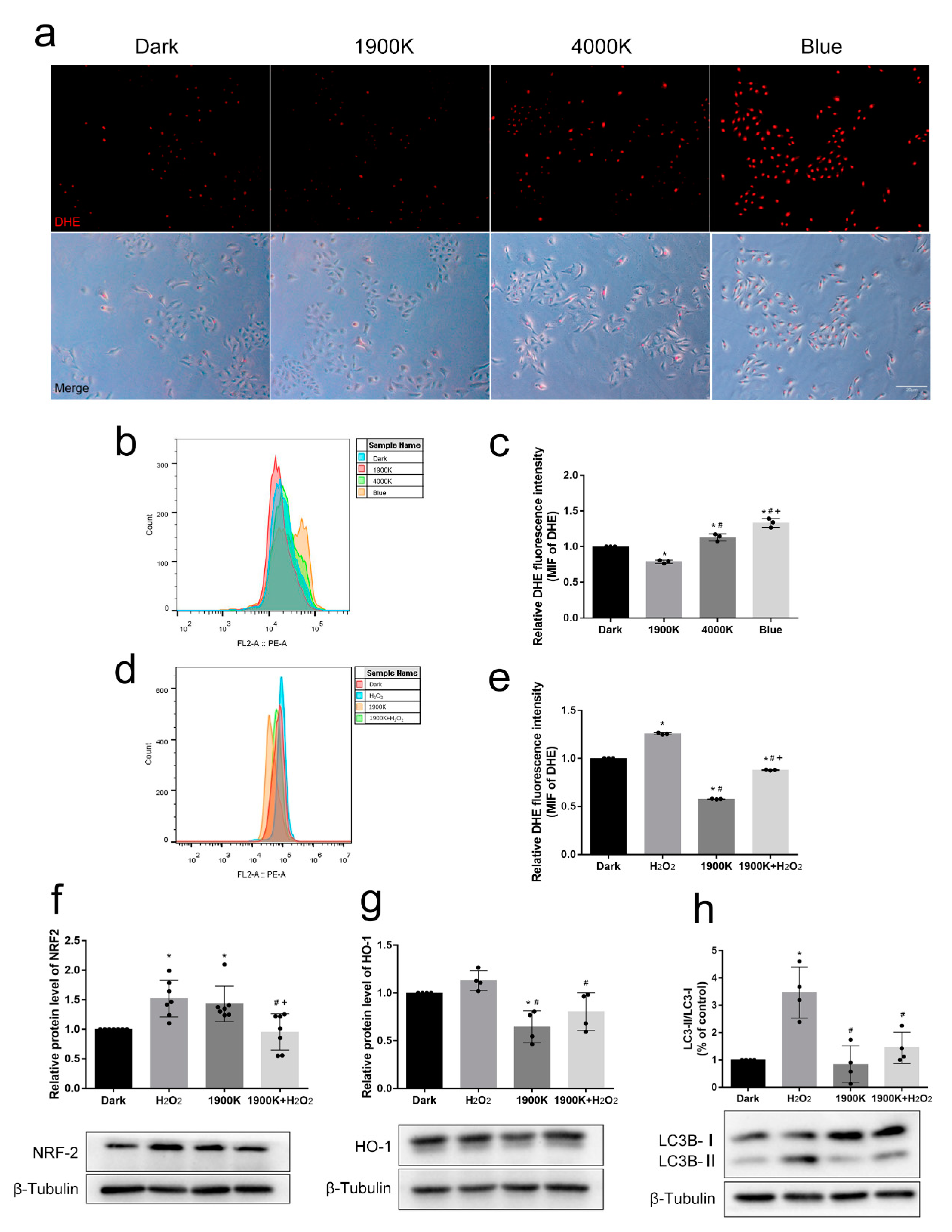
2.3. The 1900 K LEDs Play an Active Role in Antioxidative Stress of ARPE-19 Cells
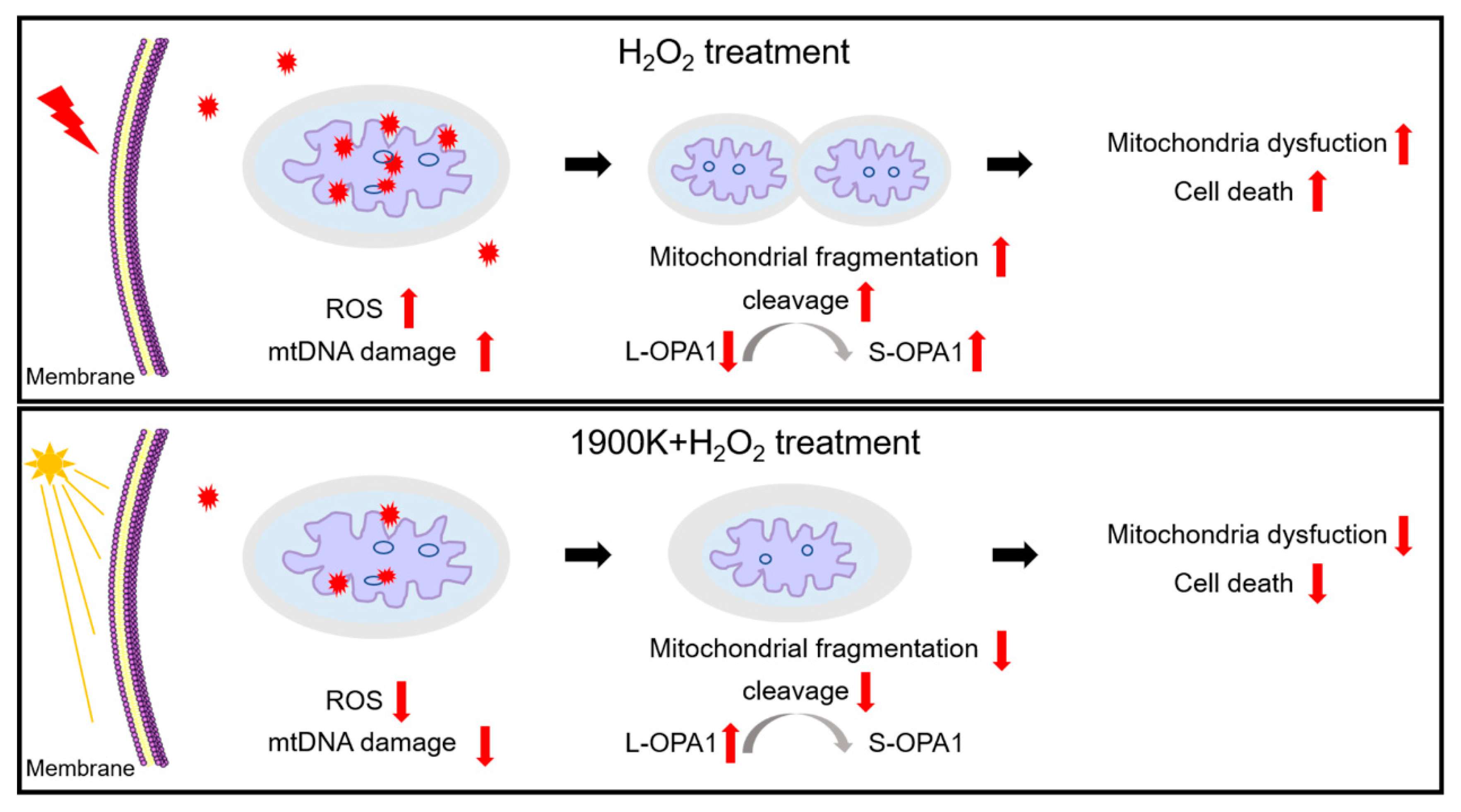
2.4. The 1900 K LEDs Can Reduce Mitochondrial Damage Caused by H2O2 in ARPE-19 Cells
2.5. Irradiation with 1900 K LEDs Did Not Cause Retinal Damage in Zebrafish
3. Materials and Methods
3.1. Cell Culture
3.2. Animals
3.3. Light Treatment and H2O2 Damage
3.4. Light Exposure in Animal Experiments
3.5. Cell Activity Assay
3.6. Cell Death Assay
3.7. ROS Levels Detection
3.8. Mitochondrial Imaging
3.9. Western Blotting
3.10. Detection of mtDNA Damage
3.11. Hematoxylin and Eosin Staining and Histologic Evaluation
3.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shang, Y.-M.; Wang, G.-S.; Sliney, D.; Yang, C.-H.; Lee, L.-L. White light–emitting diodes (LEDs) at domestic lighting levels and retinal injury in a rat model. Environ. Health Perspect. 2014, 122, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Behar-Cohen, F.; Martinsons, C.; Viénot, F.; Zissis, G.; Barlier-Salsi, A.; Cesarini, J.; Enouf, O.; Garcia, M.; Picaud, S.; Attia, D. Light-emitting diodes (LED) for domestic lighting: Any risks for the eye? Prog. Retin. Eye Res. 2011, 30, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Touitou, Y.; Point, S. Effects and mechanisms of action of light-emitting diodes on the human retina and internal clock. Environ. Res. 2020, 190, 109942. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, S.M.; Bee, P.E.; Meyer, N.; Dijk, D.-J.; Drake, R.J. Light therapies to improve sleep in intrinsic circadian rhythm sleep disorders and neuro-psychiatric illness: A systematic review and meta-analysis. Sleep Med. Rev. 2019, 46, 108–123. [Google Scholar] [CrossRef]
- Touitou, Y.; Reinberg, A.; Touitou, D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sci. 2017, 173, 94–106. [Google Scholar] [CrossRef]
- Macchi, M.M.; Bruce, J.N. Human pineal physiology and functional significance of melatonin. Front. Neuroendocr. 2004, 25, 177–195. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Swaab, D.F. The human pineal gland and melatonin in aging and Alzheimer’s disease. J. Pineal Res. 2005, 38, 145–152. [Google Scholar] [CrossRef]
- Guan, Q.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Mechanisms of Melatonin in Obesity: A Review. Int. J. Mol. Sci. 2021, 23, 218. [Google Scholar] [CrossRef]
- Costa, G.; Haus, E.; Stevens, R. Shift work and cancer—Considerations on rationale, mechanisms, and epidemiology. Scand. J. Work. Environ. Health 2010, 36, 163–179. [Google Scholar] [CrossRef]
- Jin, M.; Li, X.; Yan, F.; Chen, W.; Jiang, L.; Zhang, X. The effects of low-color-temperature dual-primary-color light-emitting diodes on three kinds of retinal cells. J. Photochem. Photobiol. B Biol. 2021, 214, 112099. [Google Scholar] [CrossRef]
- Lin, J.; Ding, X.; Hong, C.; Pang, Y.; Chen, L.; Liu, Q.; Zhang, X.; Xin, H.; Wang, X. Several biological benefits of the low color temperature light-emitting diodes based normal indoor lighting source. Sci. Rep. 2019, 9, 7560. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Hanus, J.; Anderson, C.; Wang, S. RPE necroptosis in response to oxidative stress and in AMD. Ageing Res. Rev. 2015, 24 Pt B, 286–298. [Google Scholar] [CrossRef]
- Binder, S.; Stanzel, B.V.; Krebs, I.; Glittenberg, C. Transplantation of the RPE in AMD. Prog. Retin. Eye Res. 2007, 26, 516–554. [Google Scholar] [CrossRef] [PubMed]
- Ebeling, M.C.; Polanco, J.R.; Qu, J.; Tu, C.; Montezuma, S.R.; Ferrington, D.A. Improving retinal mitochondrial function as a treatment for age-related macular degeneration. Redox Biol. 2020, 34, 101552. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Dong, Y.; Wu, C.; Youngblood, H.; Li, Y.; Zong, X.; Li, L.; Xu, T.; Zhang, Q. Effects of prenatal photobiomodulation treatment on neonatal hypoxic ischemia in rat offspring. Theranostics 2021, 11, 1269–1294. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Teo, K.Y.C.; Wood, J.P.M.; Vaze, A.; Chidlow, G.; Ao, J.; Lee, S.-R.; Yam, M.X.; Cornish, E.E.; Fraser-Bell, S.; et al. Preclinical and clinical studies of photobiomodulation therapy for macular oedema. Diabetologia 2020, 63, 1900–1915. [Google Scholar] [CrossRef]
- Yang, L.; Youngblood, H.; Wu, C.; Zhang, Q. Mitochondria as a target for neuroprotection: Role of methylene blue and photobiomodulation. Transl. Neurodegener. 2020, 9, 19. [Google Scholar] [CrossRef]
- Leyane, T.S.; Jere, S.W.; Houreld, N.N. Cellular Signalling and Photobiomodulation in Chronic Wound Repair. Int. J. Mol. Sci. 2021, 22, 11223. [Google Scholar] [CrossRef]
- Xie, C.; Li, X.; Tong, J.; Gu, Y.; Shen, Y. Effects of white light-emitting diode (LED) light exposure with different correlated color temperatures (CCTs) on human lens epithelial cells in culture. Photochem. Photobiol. 2014, 90, 853–859. [Google Scholar] [CrossRef]
- Shen, Y.; Xie, C.; Gu, Y.; Li, X.; Tong, J. Illumination from light-emitting diodes (LEDs) disrupts pathological cytokines expression and activates relevant signal pathways in primary human retinal pigment epithelial cells. Exp. Eye Res. 2016, 145, 456–467. [Google Scholar] [CrossRef]
- Ivandic, B.T.; Ivandic, T. Low-level laser therapy improves vision in patients with age-related macular degeneration. Photomed. Laser Surg. 2008, 26, 241–245. [Google Scholar] [CrossRef]
- Qu, C.; Cao, W.; Fan, Y.; Lin, Y. Near-infrared light protect the photoreceptor from light-induced damage in rats. Adv. Exp. Med. Biol. 2009, 664, 365–374. [Google Scholar] [CrossRef]
- Rutar, M.; Natoli, R.; Albarracin, R.; Valter, K.; Provis, J. 670-nm light treatment reduces complement propagation following retinal degeneration. J. Neuroinflam. 2012, 9, 257. [Google Scholar] [CrossRef] [PubMed]
- Albarracin, R.; Eells, J.; Valter, K. Photobiomodulation protects the retina from light-induced photoreceptor degeneration. Investig. Opthalmol. Vis. Sci. 2011, 52, 3582–3592. [Google Scholar] [CrossRef] [PubMed]
- Merry, G.F.; Munk, M.R.; Dotson, R.S.; Walker, M.G.; Devenyi, R.G. Photobiomodulation reduces drusen volume and improves visual acuity and contrast sensitivity in dry age-related macular degeneration. Acta Ophthalmol. 2016, 95, e270–e277. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, S.N.; Devenyi, R.G.; Munk, M.R.; Croissant, C.L.; Tedford, S.E.; Rückert, R.; Walker, M.G.; Patino, B.E.; Chen, L.; Nido, M.; et al. A double-masked, randomized, sham-controlled, single-center study with photobiomodulation for the treatment of dry age-related macular degeneration. Retina 2020, 40, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Garrido, C.; Gurbuxania, S.; Ravagnanb, L.; Kroemer, G. Heat shock proteins: Endogenous modulators of apoptotic cell death. Biochem. Biophys. Res. Commun. 2001, 286, 433–442. [Google Scholar] [CrossRef]
- Schipper, H.M.; Song, W.; Zukor, H.; Hascalovici, J.R.; Zeligman, D. Heme oxygenase-1 and neurodegeneration: Expanding frontiers of engagement. J. Neurochem. 2009, 110, 469–485. [Google Scholar] [CrossRef]
- Núñez-Álvarez, C.; Barrio, C.S.; Aguado, S.D.O.; Osborne, N.N. Blue light negatively affects the survival of ARPE19 cells through an action on their mitochondria and blunted by red light. Acta Ophthalmol. 2019, 97, e103–e115. [Google Scholar] [CrossRef]
- Tang, Z.; Ju, Y.; Dai, X.; Ni, N.; Liu, Y.; Zhang, D.; Gao, H.; Sun, H.; Zhang, J.; Gu, P. HO-1-mediated ferroptosis as a target for protection against retinal pigment epithelium degeneration. Redox Biol. 2021, 43, 101971. [Google Scholar] [CrossRef] [PubMed]
- Misra, J.R.; Horner, M.A.; Lam, G.; Thummel, C.S. Transcriptional regulation of xenobiotic detoxification in Drosophila. Genes Dev. 2011, 25, 1796–1806. [Google Scholar] [CrossRef] [PubMed]
- Misra, J.R.; Lam, G.; Thummel, C.S. Constitutive activation of the Nrf2/Keap1 pathway in insecticide-resistant strains of Drosophila. Insect Biochem. Mol. Biol. 2013, 43, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Mitter, S.K.; Qi, X.; Beli, E.; Rao, H.V.; Ding, J.; Ip, C.S.; Gu, H.; Akin, D.; Dunn, W.A., Jr.; et al. Oxidative stress-mediated NFκB phosphorylation upregulates p62/SQSTM1 and promotes retinal pigmented epithelial cell survival through increased autophagy. PLoS ONE 2017, 12, e0171940. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Guo, J.; Zhou, L.; Zhu, S.; Wang, C.; Liu, J.; Hu, S.; Yang, M.; Lin, C. Hydrogen Sulfide Protects Retinal Pigment Epithelial Cells from Oxidative Stress-Induced Apoptosis and Affects Autophagy. Oxidative Med. Cell. Longev. 2020, 2020, 8868564. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.; Wang, K.; Liu, X.; Klionsky, D.J. The scaffold protein Atg11 recruits fission machinery to drive selective mitochondria degradation by autophagy. Dev. Cell 2013, 26, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.-H.; Do, Y.-J.; Son, D.; Son, E.; Choi, J.-S.; Kim, E. AIF-independent parthanatos in the pathogenesis of dry age-related macular degeneration. Cell Death Dis. 2017, 8, e2526. [Google Scholar] [CrossRef]
- Legros, F.; Lombès, A.; Frachon, P.; Rojo, M. Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Mol. Biol. Cell 2002, 13, 4343–4354. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.-P.; Shao, Q.; Li, P.-F.; Sun, Y.; Luo, L.-Z.; Yan, X.-Q.; Fan, Z.-Y.; Hu, J.; Zhao, J.; et al. Brain-derived neurotrophic factor mimetic, 7,8-dihydroxyflavone, protects against myocardial ischemia by rebalancing optic atrophy 1 processing. Free Radic. Biol. Med. 2019, 145, 187–197. [Google Scholar] [CrossRef]
- Garcia, I.; Innis-Whitehouse, W.; Lopez, A.; Keniry, M.; Gilkerson, R. Oxidative insults disrupt OPA1-mediated mitochondrial dynamics in cultured mammalian cells. Redox Rep. 2018, 23, 160–167. [Google Scholar] [CrossRef]
- Liang, F.-Q.; Godley, B.F. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: A possible mechanism for RPE aging and age-related macular degeneration. Exp. Eye Res. 2003, 76, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Pawlowska, E.; Szczepanska, J.; Jablkowska, A.; Blasiak, J. Role of Mitochondrial DNA Damage in ROS-Mediated Pathogenesis of Age-Related Macular Degeneration (AMD). Int. J. Mol. Sci. 2019, 20, 2374. [Google Scholar] [CrossRef] [PubMed]
- Yakes, F.M.; Van Houten, B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl. Acad. Sci. USA 1997, 94, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, S.; Lin, H.; Godley, B.F.; Boulton, M.E. Mitochondrial DNA damage and its potential role in retinal degeneration. Prog. Retin. Eye Res. 2008, 27, 596–607. [Google Scholar] [CrossRef]
- Eells, J.T.; Henry, M.M.; Summerfelt, P.; Wong-Riley, M.T.T.; Buchmann, E.V.; Kane, M.; Whelan, N.T. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc. Natl. Acad. Sci. USA 2003, 100, 3439–3444. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Mehrvar, S.; Maleki, S.; Schmitt, H.; Summerfelt, P.; Dubis, A.M.; Abroe, B.; Connor, T.B., Jr.; Carroll, J.; Huddleston, W.; et al. Photobiomodulation preserves mitochondrial redox state and is retinoprotective in a rodent model of retinitis pigmentosa. Sci. Rep. 2020, 10, 20382. [Google Scholar] [CrossRef]
- Sheng, W.; Lu, Y.; Mei, F.; Wang, N.; Liu, Z.-Z.; Han, Y.-Y.; Wang, H.-T.; Zou, S.; Xu, H.; Zhang, X. Effect of Resveratrol on Sirtuins, OPA1, and Fis1 Expression in Adult Zebrafish Retina. Investig. Opthalmol. Vis. Sci. 2018, 59, 4542–4551. [Google Scholar] [CrossRef]
- Liu, X.; Weaver, D.; Shirihai, O.; Hajnóczky, G. Mitochondrial ‘kiss-and-run’: Interplay between mitochondrial motility and fusion–fission dynamics. EMBO J. 2009, 28, 3074–3089. [Google Scholar] [CrossRef]
- Li, J.-Y.; Zhang, K.; Xu, D.; Zhou, W.-C.; Fang, W.-Q.; Wan, Y.-Y.; Yan, D.-D.; Guo, M.-Y.; Tao, J.-X.; Yang, F.; et al. Mitochondrial Fission Is Required for Blue Light-Induced Apoptosis and Mitophagy in Retinal Neuronal R28 Cells. Front. Mol. Neurosci. 2018, 11, 432. [Google Scholar] [CrossRef]
- Lai, Y.; Lin, P.; Chen, M.; Zhang, Y.; Chen, J.; Zheng, M.; Liu, J.; Du, H.; Chen, R.; Pan, X.; et al. Restoration of L-OPA1 alleviates acute ischemic stroke injury in rats via inhibiting neuronal apoptosis and preserving mitochondrial function. Redox Biol. 2020, 34, 101503. [Google Scholar] [CrossRef]
- Gilkerson, R.; De La Torre, P.; Vallier, S.S. Mitochondrial OMA1 and OPA1 as Gatekeepers of Organellar Structure/Function and Cellular Stress Response. Front. Cell Dev. Biol. 2021, 9, 626117. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Wai, T.; Baker, M.J.; Kladt, N.; Schauss, A.C.; Rugarli, E.; Langer, T. The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J. Cell Biol. 2014, 204, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial Fission, Fusion, and Stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef] [PubMed]
- Stella, S.; Geathers, J.; Weber, S.; Grillo, M.; Barber, A.; Sundstrom, J.; Grillo, S. Neurodegeneration, neuroprotection and regeneration in the zebrafish retina. Cells 2021, 10, 633. [Google Scholar] [CrossRef]
- Goldsmith, P.; Harris, W. The zebrafish as a tool for understanding the biology of visual disorders. Semin. Cell Dev. Biol. 2003, 14, 11–18. [Google Scholar] [CrossRef]
- Tsai, S.-R.; Hamblin, M.R. Biological effects and medical applications of infrared radiation. J. Photochem. Photobiol. B Biol. 2017, 170, 197–207. [Google Scholar] [CrossRef]
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.Y.; Carroll, J.D.; Hamblin, M.R. The nuts and bolts of low-level laser (light) therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef]
- Geneva, I.I. Photobiomodulation for the treatment of retinal diseases: A review. Int. J. Ophthalmol. 2016, 9, 145–152. [Google Scholar] [CrossRef]
- Fitzgerald, M.; Hodgetts, S.; Heuvel, C.V.D.; Natoli, R.; Hart, N.S.; Valter, K.; Harvey, A.R.; Vink, R.; Provis, J.; Dunlop, S.A. Red/near-infrared irradiation therapy for treatment of central nervous system injuries and disorders. Rev. Neurosci. 2013, 24, 205–226. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, Q.; Wu, X.; Zhang, D.; Xing, D. Activation of PKA/SIRT1 signaling pathway by photobiomodulation therapy reduces Aβ levels in Alzheimer’s disease models. Aging Cell 2019, 19, e13054. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, H.-T.; Wang, N.; Sheng, W.-W.; Jin, M.; Lu, Y.; Bai, Y.-J.; Zou, S.-Q.; Pang, Y.-L.; Xu, H.; et al. Establishment of an adult zebrafish model of retinal neurodegeneration induced by NMDA. Int. J. Ophthalmol. 2020, 12, 1250–1261. [Google Scholar] [CrossRef] [PubMed]


| Antibody | Source | Catolog.No | Type of Ab | Dilution | MW |
|---|---|---|---|---|---|
| HO-1 | Abcam | Ab13248 | Mouse mAb | 1:1000 | 34.6 |
| NRF2 | Abmart | T55136F | Rabbit mAb | 1:1000 | 110 |
| LC3B | CST | 2775s | Rabbit mAb | 1:1000 | 14/16 |
| DRP1 | CST | #8570 | Rabbit mAb | 1:1000 | 78–82 |
| OPA1 | BD | 612606 | Mouse mAb | 1:1000 | 80–100 |
| β-tubulin | TRANS | HC101-01 | Mouse mAb | 1:2500 | 55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, M.; Zhang, X.-Y.; Ying, Q.; Hu, H.-J.; Feng, X.-T.; Peng, Z.; Pang, Y.-L.; Yan, F.; Zhang, X. Antioxidative and Mitochondrial Protection in Retinal Pigment Epithelium: New Light Source in Action. Int. J. Mol. Sci. 2023, 24, 4794. https://doi.org/10.3390/ijms24054794
Jin M, Zhang X-Y, Ying Q, Hu H-J, Feng X-T, Peng Z, Pang Y-L, Yan F, Zhang X. Antioxidative and Mitochondrial Protection in Retinal Pigment Epithelium: New Light Source in Action. International Journal of Molecular Sciences. 2023; 24(5):4794. https://doi.org/10.3390/ijms24054794
Chicago/Turabian StyleJin, Ming, Xiao-Yu Zhang, Qian Ying, Hai-Jian Hu, Xin-Ting Feng, Zhen Peng, Yu-Lian Pang, Feng Yan, and Xu Zhang. 2023. "Antioxidative and Mitochondrial Protection in Retinal Pigment Epithelium: New Light Source in Action" International Journal of Molecular Sciences 24, no. 5: 4794. https://doi.org/10.3390/ijms24054794
APA StyleJin, M., Zhang, X.-Y., Ying, Q., Hu, H.-J., Feng, X.-T., Peng, Z., Pang, Y.-L., Yan, F., & Zhang, X. (2023). Antioxidative and Mitochondrial Protection in Retinal Pigment Epithelium: New Light Source in Action. International Journal of Molecular Sciences, 24(5), 4794. https://doi.org/10.3390/ijms24054794






