A Novel Hyaluronic Acid Matrix Ingredient with Regenerative, Anti-Aging and Antioxidant Capacity
Abstract
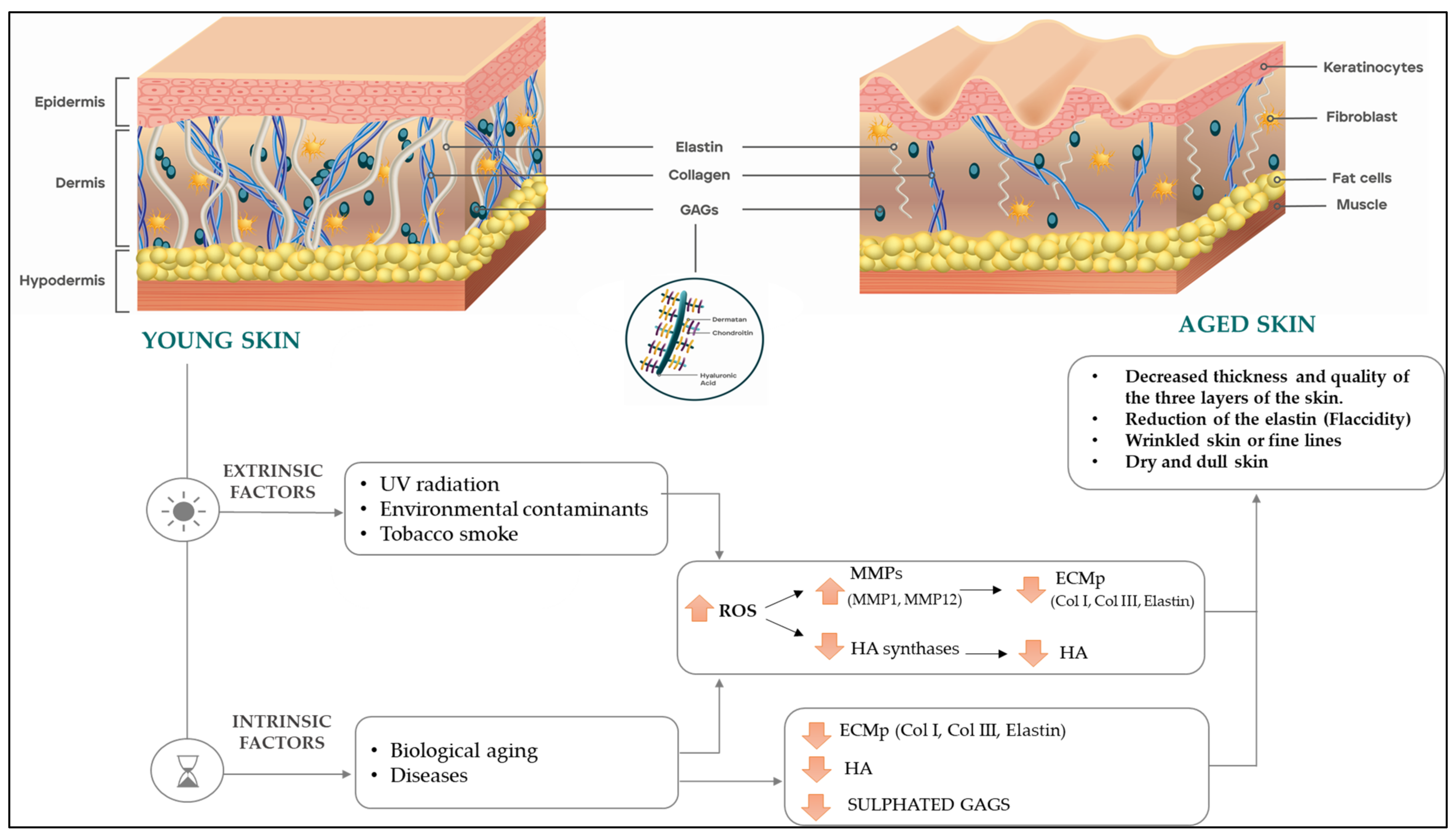
1. Introduction
2. Results
2.1. Extraction, Purification and Physicochemical Characterisation of the HA Matrix
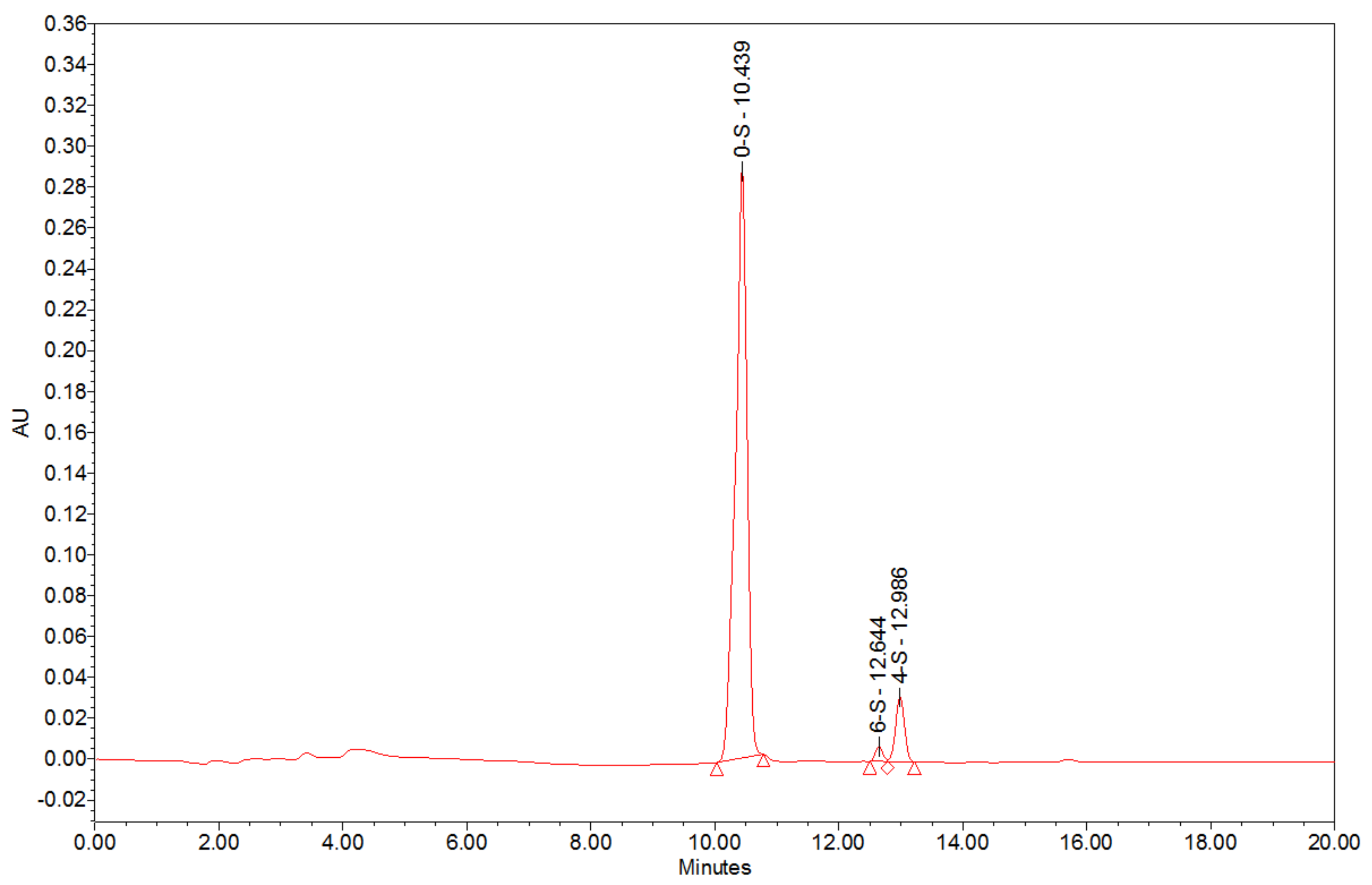
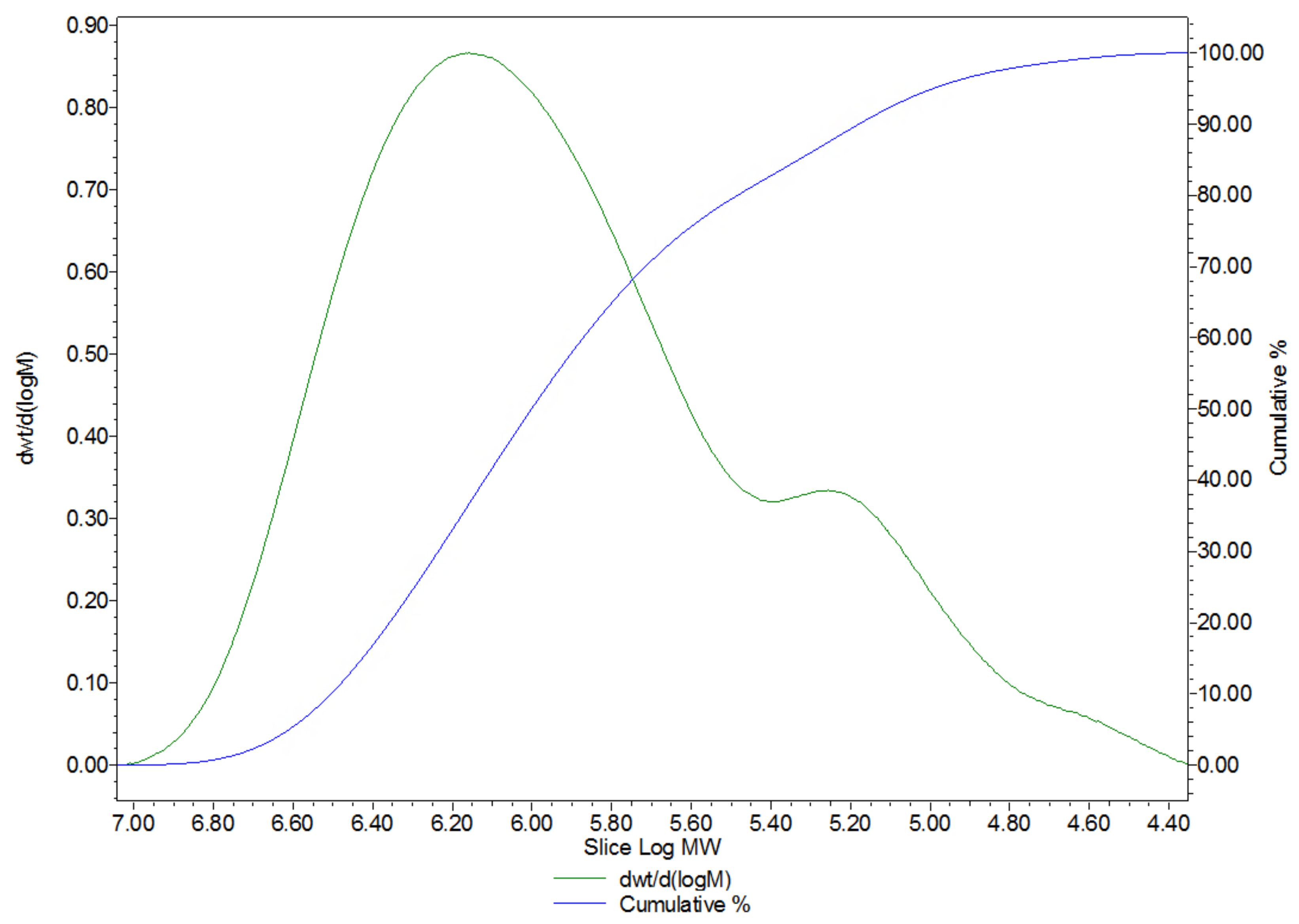
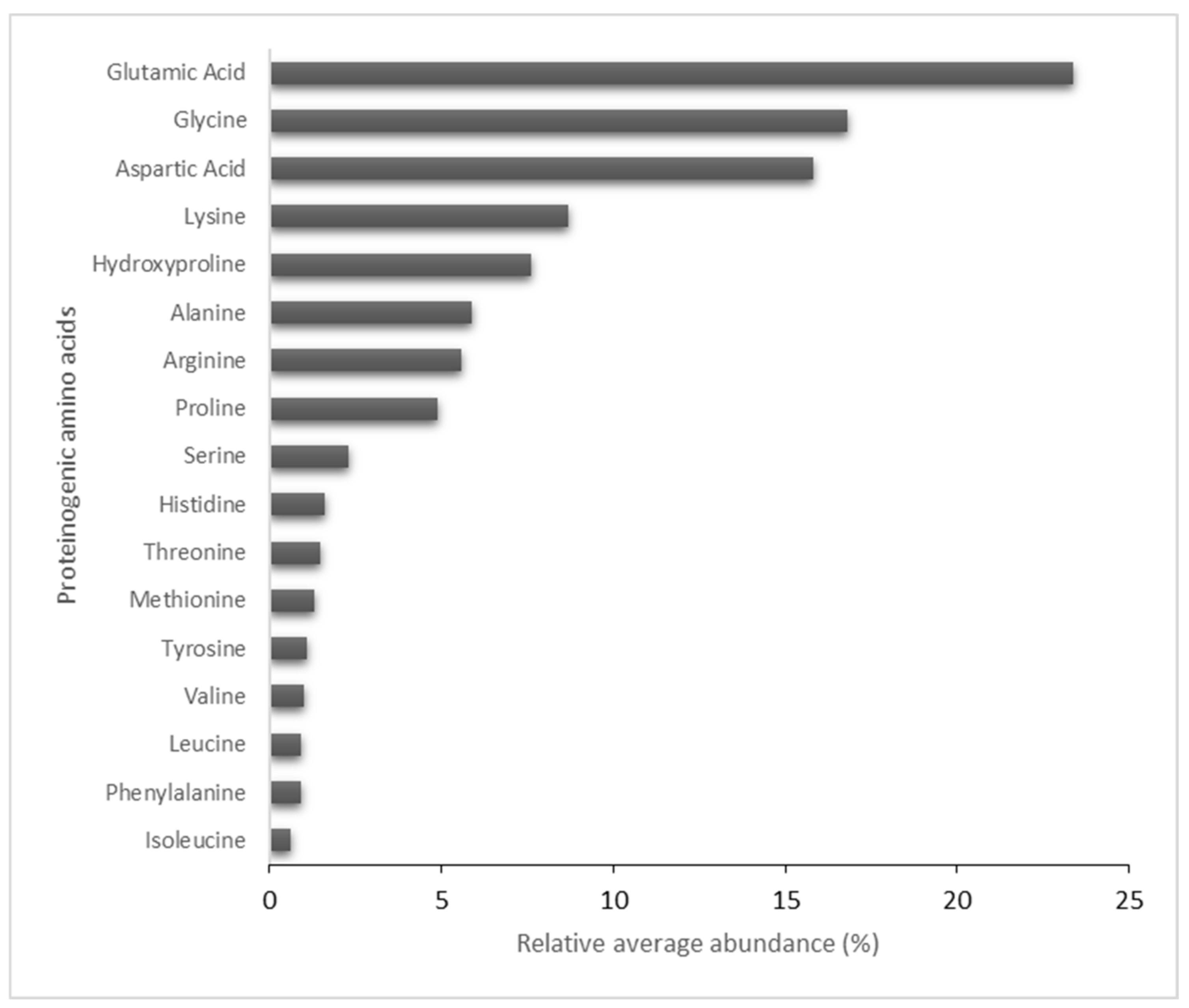
2.2. Compositional Analysis of the HA Matrix
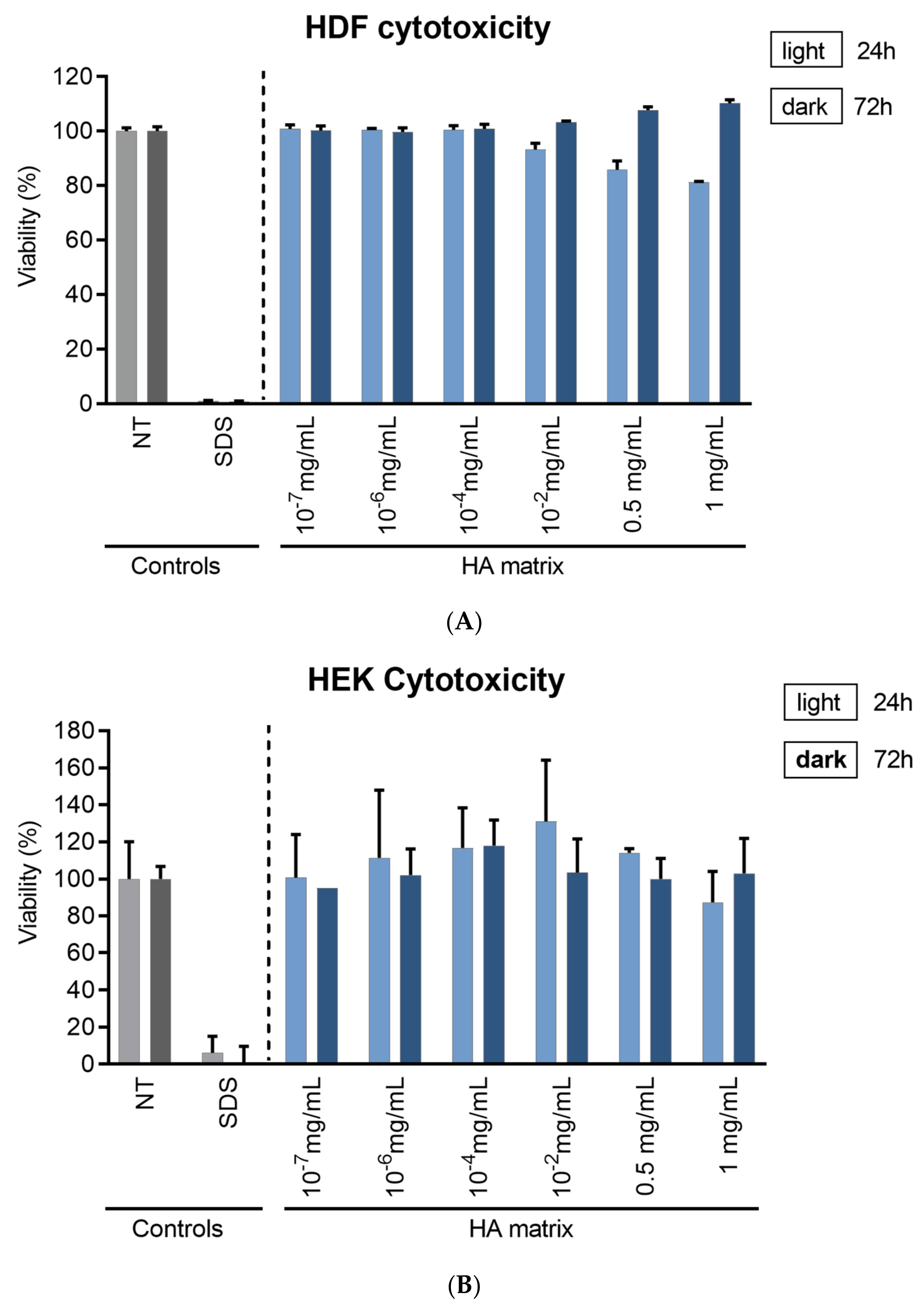
2.3. HA Matrix Effects on Skin Cell Viability
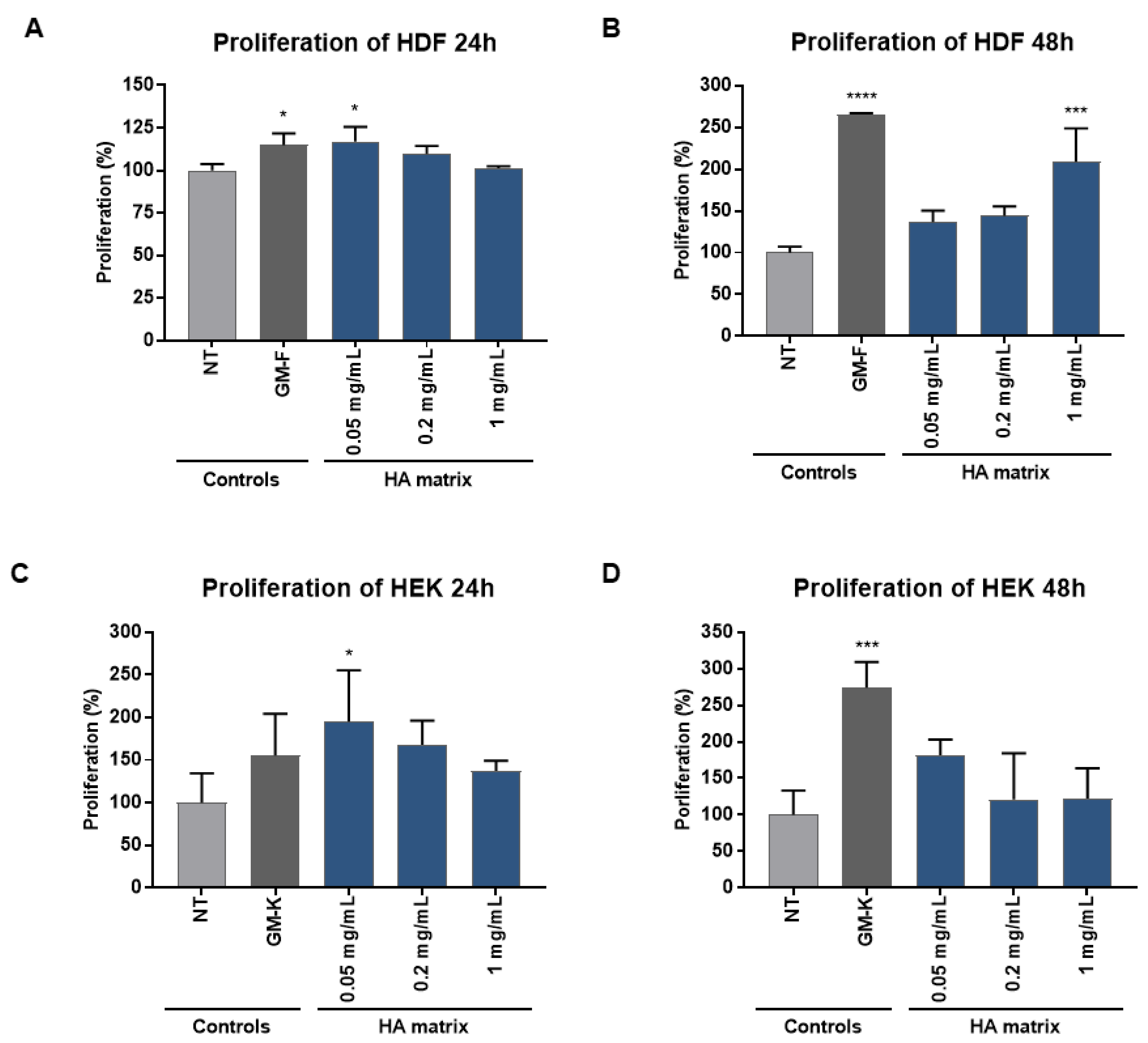
2.4. Stimulation of HDF and HEK Proliferation
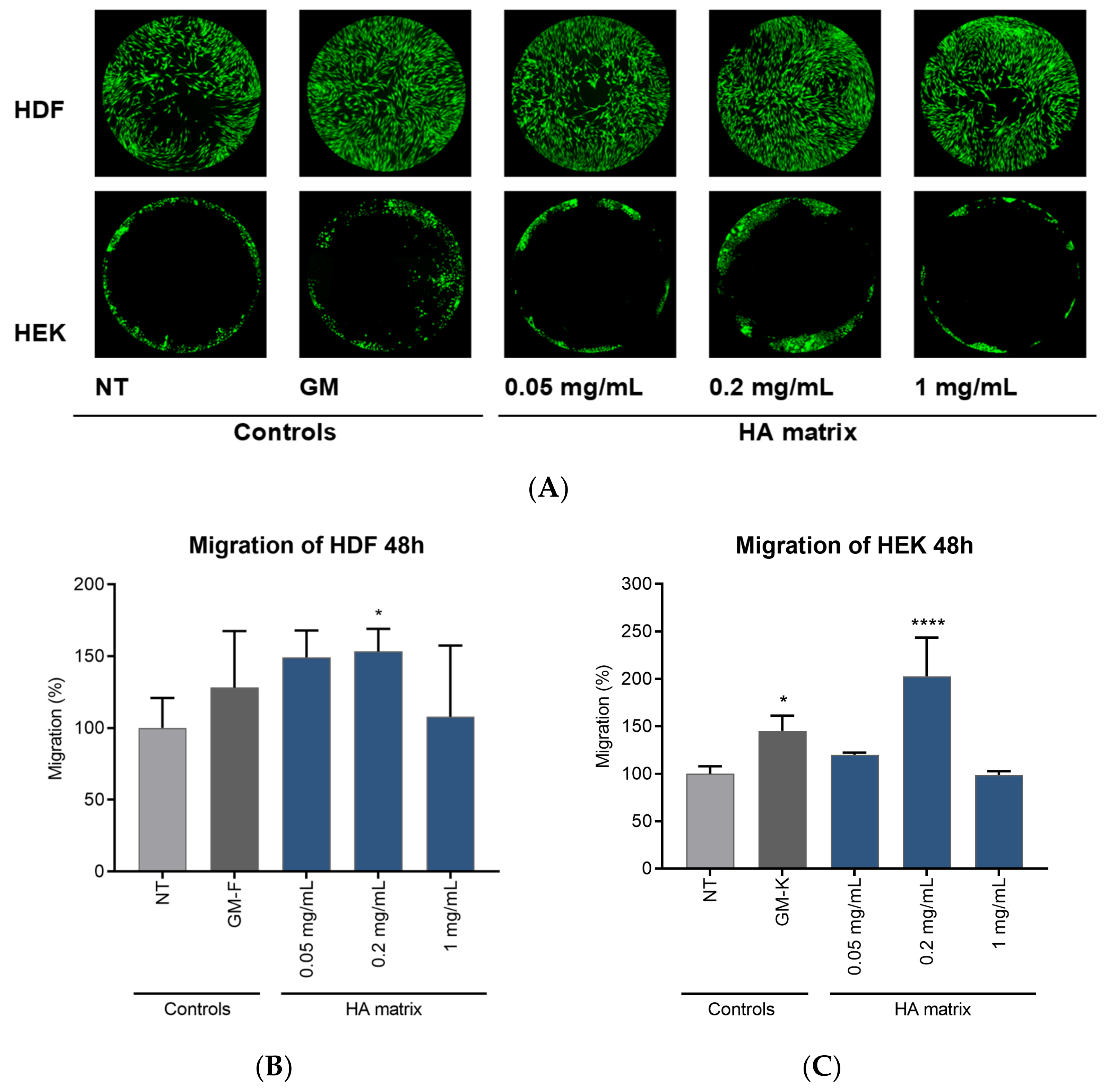
2.5. Effects of HA Matrix on Cell Migration
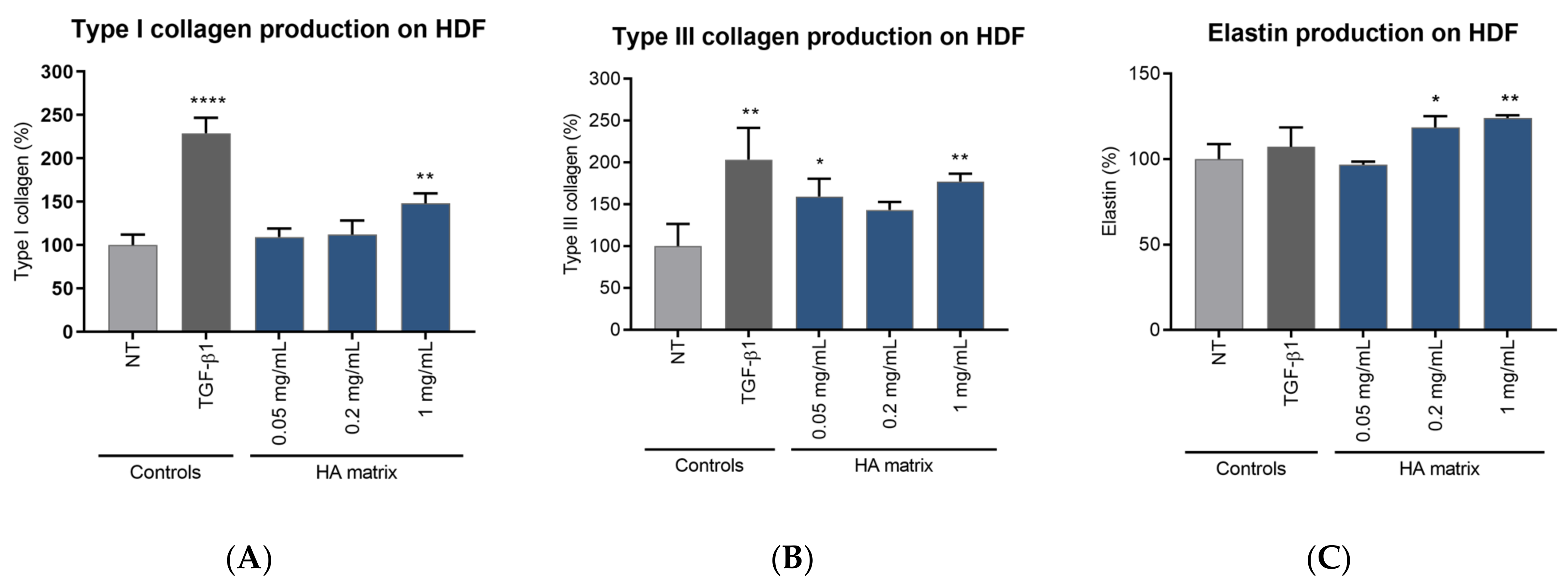
2.6. Induction of ECMp Production
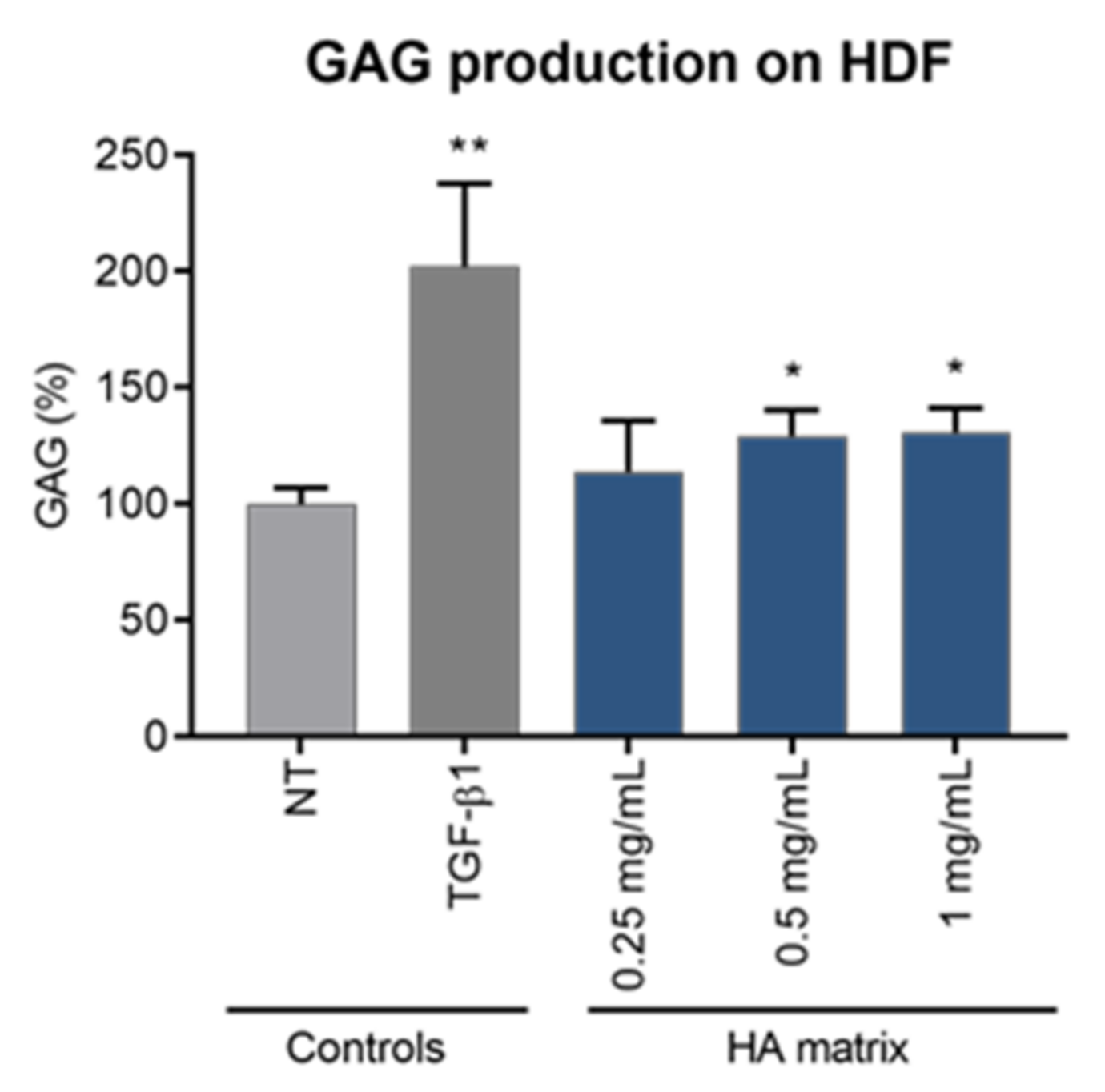
2.7. Induction of Glycosaminoglycan Synthesis
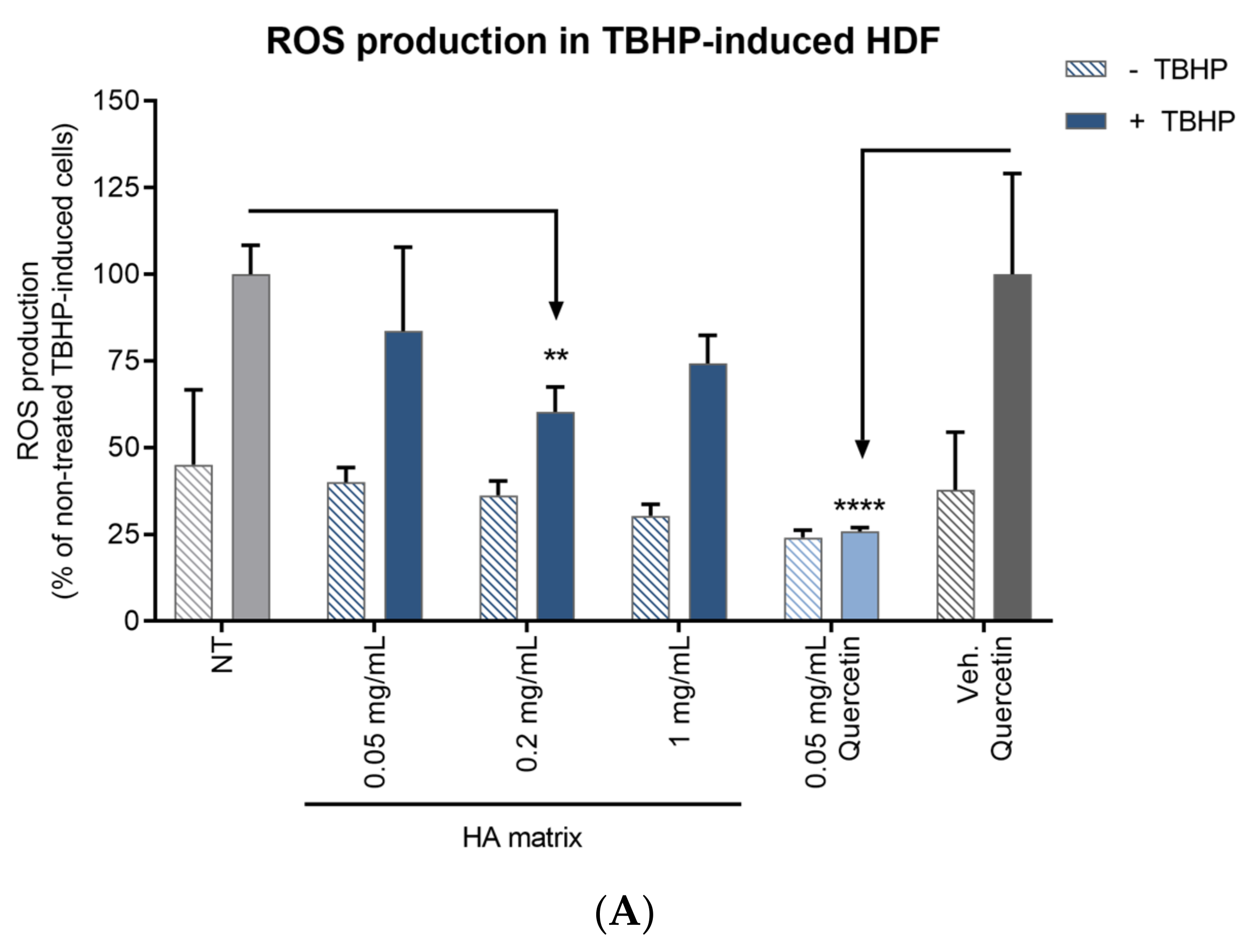
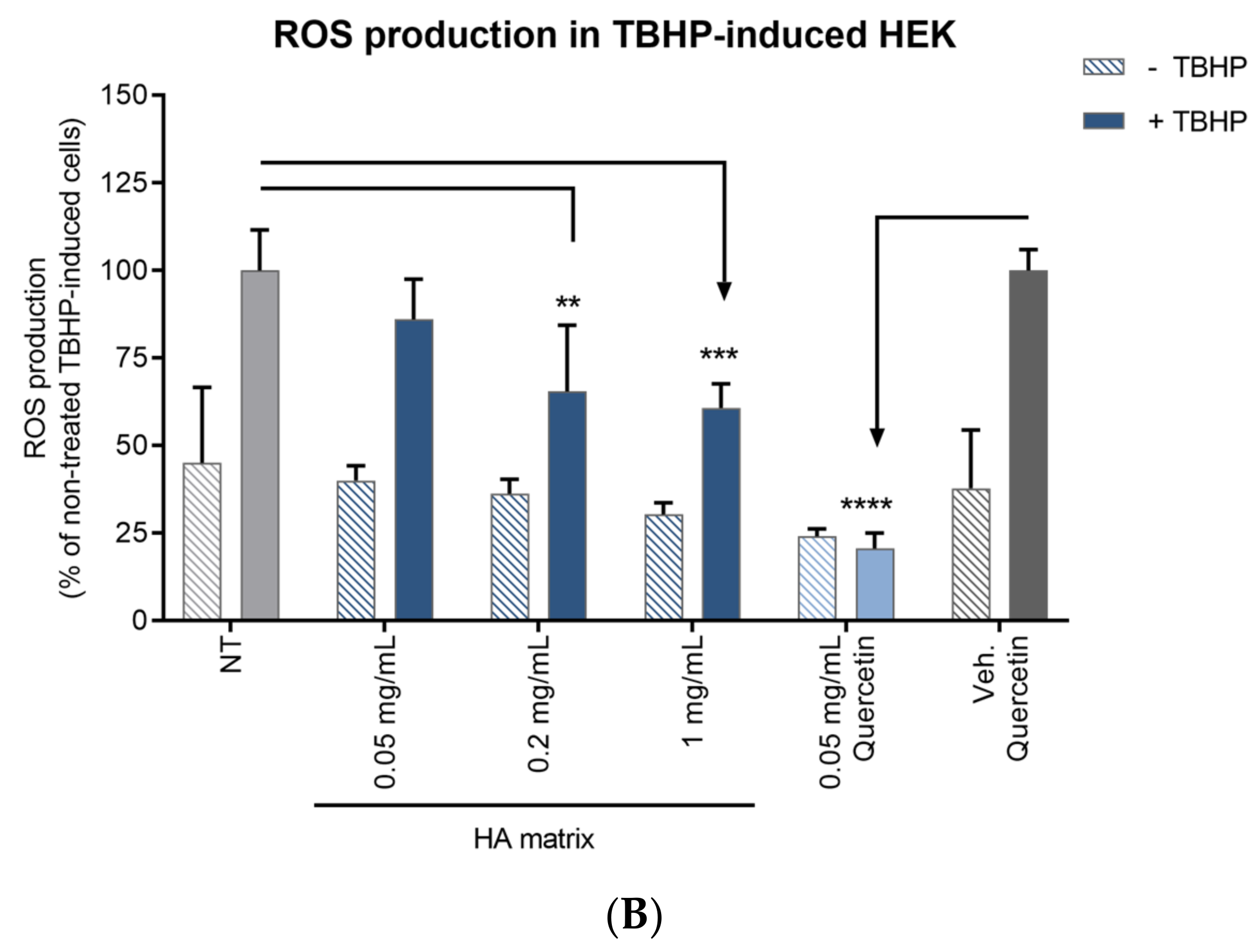
2.8. Antioxidant Activity
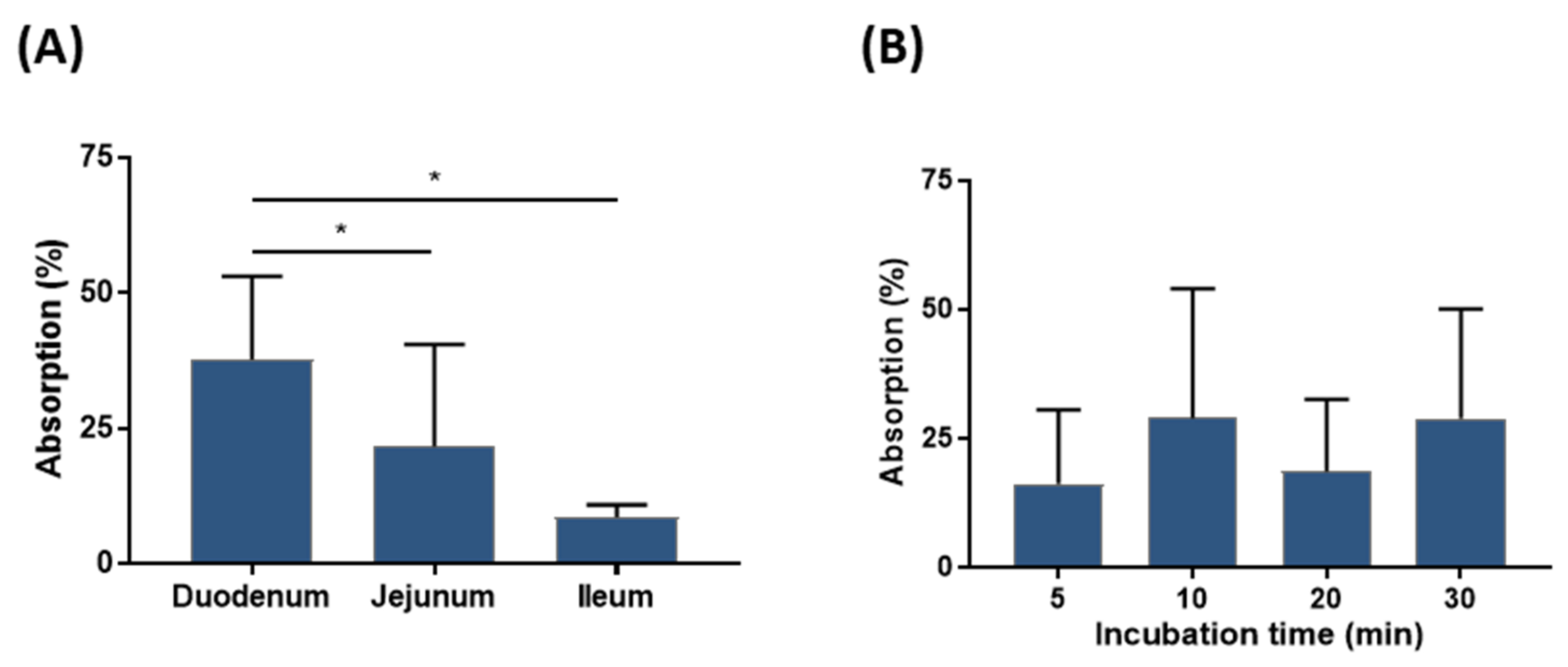
2.9. In Vitro Absorption of the HA Matrix
3. Discussion
4. Materials and Methods
4.1. Extraction and Purification of HA Matrix
4.2. Physicochemical Characterisation
4.2.1. Macroscopic Characteristics
4.2.2. Granulometry
4.2.3. pH Determination
4.2.4. Chloride Levels Determination
4.2.5. Nitrogen Content
4.2.6. Loss on Drying
4.3. Compositional Analysis of Hyaluronic Acid Matrix Ingredient
4.3.1. Disaccharide Profile
4.3.2. HA Content
4.3.3. Sulphated GAGs Content
4.3.4. Molecular Weight Determination
4.3.5. Aminogram
4.4. HA Matrix Biological Activity Assessment
4.4.1. Cell Cultures
4.4.2. In Vitro Cytotoxicity Assay
4.4.3. Cell Proliferation
4.4.4. Cell Migration Assay
4.4.5. Assessment of ECMp Production
4.4.6. Assessment of GAG Production
4.4.7. In Vitro Test of Cell Antioxidant Activity
4.5. In Vitro Absorption Study of the HA Matrix
4.5.1. Everted-Gut Sac Assay
4.5.2. GAG Quantification Assay
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Nutraceuticals for Skin Care: A Comprehensive Review of Human Clinical Studies. Nutrients 2018, 10, E403. [Google Scholar] [CrossRef] [PubMed]
- Low, E.; Alimohammadiha, G.; Smith, L.A.; Costello, L.F.; Przyborski, S.A.; von Zglinicki, T.; Miwa, S. How Good Is the Evidence That Cellular Senescence Causes Skin Ageing? Ageing Res. Rev. 2021, 71, 101456. [Google Scholar] [CrossRef] [PubMed]
- Pullar, J.; Carr, A.; Vissers, M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Bouwstra, J.A.; Helder, R.W.J.; El Ghalbzouri, A. Human Skin Equivalents: Impaired Barrier Function in Relation to the Lipid and Protein Properties of the Stratum Corneum. Adv. Drug Deliv. Rev. 2021, 175, 113802. [Google Scholar] [CrossRef] [PubMed]
- Michelotti, A.; Cestone, E.; De Ponti, I.; Pisati, M.; Sparta, E.; Tursi, F. Oral Intake of a New Full-Spectrum Hyaluronan Improves Skin Profilometry and Ageing: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Eur. J. Dermatol. 2021, 31, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-W.; Kwon, S.-H.; Choi, J.-Y.; Na, J.-I.; Huh, C.-H.; Choi, H.-R.; Park, K.-C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef]
- Wang, S.T.; Neo, B.H.; Betts, R.J. Glycosaminoglycans: Sweet as Sugar Targets for Topical Skin Anti-Aging. CCID 2021, 14, 1227–1246. [Google Scholar] [CrossRef]
- Ajeeshkumar, K.K.; Vishnu, K.V.; Navaneethan, R.; Raj, K.; Remyakumari, K.R.; Swaminathan, T.R.; Suseela, M.; Asha, K.K.; Sreekanth, G.P. Proteoglycans isolated from the bramble shark cartilage show potential anti-osteoarthritic properties. Inflammopharmacology 2019, 27, 175–187. [Google Scholar] [CrossRef]
- Khavkin, J.; Ellis, D.A.F. Aging Skin: Histology, Physiology, and Pathology. Facial Plast. Surg. Clin. N. Am. 2011, 19, 229–234. [Google Scholar] [CrossRef]
- Ruiz Martínez, M.A.; Peralta Galisteo, S.; Castán, H.; Morales Hernández, M.E. Role of Proteoglycans on Skin Ageing: A Review. Int. J. Cosmet. Sci. 2020, 42, 529–535. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation Events and Skin Aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Golden, T.R.; Hinerfeld, D.A.; Melov, S. Oxidative Stress and Aging: Beyond Correlation. Aging Cell 2002, 1, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Little, E.; Quan, H.; Qin, Z.; Voorhees, J.J.; Fisher, G.J. Elevated Matrix Metalloproteinases and Collagen Fragmentation in Photodamaged Human Skin: Impact of Altered Extracellular Matrix Microenvironment on Dermal Fibroblast Function. J. Investig. Dermatol. 2013, 133, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.; Bhatti, H.; Nerusu, K.C.; Bhagavathula, N.; Kang, S.; Fisher, G.J.; Varani, J.; Voorhees, J.J. Matrix Metalloproteinase-1 Is the Major Collagenolytic Enzyme Responsible for Collagen Damage in UV-Irradiated Human Skin. Photochem. Photobiol. 2003, 78, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Van Doren, S.R. Matrix Metalloproteinase Interactions with Collagen and Elastin. Matrix Biol. 2015, 44–46, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Wang, Z.Q.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of Premature Skin Aging Induced by Ultraviolet Light. N. Engl. J. Med. 1997, 337, 1419–1428. [Google Scholar] [CrossRef]
- Quan, T.; Shao, Y.; He, T.; Voorhees, J.J.; Fisher, G.J. Reduced Expression of Connective Tissue Growth Factor (CTGF/CCN2) Mediates Collagen Loss in Chronologically Aged Human Skin. J. Investig. Dermatol. 2010, 130, 415–424. [Google Scholar] [CrossRef]
- Tzellos, T.G.; Klagas, I.; Vtsevanos, K.; Triaridis, S.; Printza, A.; Kyrgidis, A.; Karakiulakis, G.; Zouboulis, C.C.; Papakonstantinou, E. Extrinsic Ageing in the Human Skin Is Associated with Alterations in the Expression of Hyaluronic Acid and Its Metabolizing Enzymes. Exp. Dermatol. 2009, 18, 1028–1035. [Google Scholar] [CrossRef]
- Monzon, M.E.; Fregien, N.; Schmid, N.; Falcon, N.S.; Campos, M.; Casalino-Matsuda, S.M.; Forteza, R.M. Reactive Oxygen Species and Hyaluronidase 2 Regulate Airway Epithelial Hyaluronan Fragmentation. J. Biol. Chem. 2010, 285, 26126–26134. [Google Scholar] [CrossRef]
- Oh, J.-H.; Kim, Y.K.; Jung, J.-Y.; Shin, J.; Chung, J.H. Changes in Glycosaminoglycans and Related Proteoglycans in Intrinsically Aged Human Skin in Vivo: Letter to the Editor. Exp. Dermatol. 2011, 20, 454–456. [Google Scholar] [CrossRef]
- Shuster, S.; Black, M.M.; McVitie, E. The Influence of Age and Sex on Skin Thickness, Skin Collagen and Density. Br. J. Dermatol. 1975, 93, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Pierzak, M.; Kręcisz, B.; Suliga, E. Bioactive Compounds for Skin Health: A Review. Nutrients 2021, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Xiao, Z.; Wu, Y.; Ge, C. Diet and Skin Aging—From the Perspective of Food Nutrition. Nutrients 2020, 12, 870. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Marquez, C.D.; Arteaga-Marin, S.; Rivas-Sánchez, A.; Autrique-Hernández, R.; Castro-Muñoz, R.A. Review on Current Strategies for Extraction and Purification of Hyaluronic Acid. Int. J. Mol. Sci. 2022, 23, 6038. [Google Scholar] [CrossRef] [PubMed]
- Sze, J.H.; Brownlie, J.C.; Love, C.A. Biotechnological production of hyaluronic acid: A mini review. 3 Biotech. 2016, 6, 67. [Google Scholar] [CrossRef]
- Severo da Rosa, C.; Freire Tovar, A.; Mourão, P.; Pereira, R.; Barreto, P.; Henrique Beirão, L. Extraction, quantification and molar mass determination of hyaluronic acid extracted from chicken crest. Ciênc. Rural 2012, 42, 1682–1687. [Google Scholar]
- Kang, D.Y.; Kim, W.S.; Heo, I.S.; Park, Y.H.; Lee, S. Extraction of hyaluronic acid (HA) from rooster comb and characterization using flow field-flow fractionation (FlFFF) coupled with multiangle light scattering (MALS). J. Sep. Sci. 2010, 22, 3530–3536. [Google Scholar] [CrossRef]
- Shi, L. Bioactivities, isolation and purification methods of polysaccharides from natural products: A review. Int. J. Biol. Macromol. 2016, 92, 37–48. [Google Scholar] [CrossRef]
- Boykov, P.Y.; Khabarov, V.N.; Polyak, F.; Selyanin, M.A. Methods of Hyaluronic Acid Production. In Hyaluronic Acid; John Wiley & Sons, Ltd.: Chichester, UK, 2015; pp. 77–95. [Google Scholar]
- Shiedlin, A.; Bigelow, R.; Christopher, W.; Arbabi, S.; Yang, L.; Maier, R.V.; Wainwright, N.; Childs, A.; Miller, R.J. Evaluation of hyaluronan from different sources: Streptococcus zooepidemicus, rooster comb, bovine vitreous, and human umbilical cord. Biomacromolecules 2004, 5, 2122–2127. [Google Scholar] [CrossRef]
- Abdallah, M.M.; Fernández, N.; Matias, A.A.; Bronze, M.D.R. Hyaluronic acid and Chondroitin sulfate from marine and terrestrial sources: Extraction and purification methods. Carbohydr. Polym. 2020, 1, 116441. [Google Scholar] [CrossRef]
- Gruber, F.; Kremslehner, C.; Eckhart, L.; Tschachler, E. Cell Aging and Cellular Senescence in Skin Aging—Recent Advances in Fibroblast and Keratinocyte Biology. Exp. Gerontol. 2020, 130, 110780. [Google Scholar] [CrossRef]
- Mirastschijski, U.; Lupše, B.; Maedler, K.; Sarma, B.; Radtke, A.; Belge, G.; Dorsch, M.; Wedekind, D.; McCawley, L.J.; Boehm, G.; et al. Matrix Metalloproteinase-3 Is Key Effector of TNF-α-Induced Collagen Degradation in Skin. Int. J. Mol. Sci. 2019, 20, 5234. [Google Scholar] [CrossRef]
- Baumann, L.; Bernstein, E.F.; Weiss, A.S.; Bates, D.; Humphrey, S.; Silberberg, M.; Daniels, R. Clinical Relevance of Elastin in the Structure and Function of Skin. Aesthetic Surg. J. Open Forum 2021, 3, ojab019. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and Extrinsic Factors in Skin Ageing: A Review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef]
- Swann, D.A.; Caulfield, J.B. Studies on hyaluronic acid. V. Relationship between the protein content and viscosity of rooster comb dermis hyaluronic acid. Connect. Tissue Res. 1975, 4, 31–39. [Google Scholar] [CrossRef]
- Galvez-Martin, P.; Martinez-Puig, D.; Soto-Fernández, C.; Romero-Rueda, J. Comparative in vitro efficacy of a hyaluronic acid (HA) matrix ingredient containing HA, dermatan sulphate and collagen (Dermial®) versus pure HA from extraction or fermen-tation origin. FASEB J. 2022, 36, 5–7. [Google Scholar] [CrossRef]
- Council of Europe. Sodium Hyaluronate Monograph. In European Pharmacopoeia, 10th ed.; EDQM Commission: Strasbourg, France, 2017; Volume 10. [Google Scholar]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Grant, M.E.; Schor, A.M.; Schor, S.L. Differences between Adult and Foetal Fibroblasts in the Regulation of Hyaluronate Synthesis: Correlation with Migratory Activity. J. Cell Sci. 1989, 94, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Zia, K.M.; Tabasum, S.; Noreen, A.; Ali, M.; Iqbal, R.; Zuber, M. Blends and Composites of Exopolysaccharides; Properties and Applications: A Review. Int. J. Biol. Macromol. 2017, 94, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds 2016, 28, 78–88. [Google Scholar]
- Salwowska, N.M.; Bebenek, K.A.; Żądło, D.A.; Wcisło-Dziadecka, D.L. Physiochemical Properties and Application of Hyaluronic Acid: A Systematic Review. J. Cosmet. Dermatol. 2016, 15, 520–526. [Google Scholar] [CrossRef]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic Acid: The Influence of Molecular Weight on Structural, Physical, Physico-Chemical, and Degradable Properties of Biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef]
- Cyphert, J.M.; Trempus, C.S.; Garantziotis, S. Size Matters: Molecular Weight Specificity of Hyaluronan Effects in Cell Biology. Int. J. Cell Biol. 2015, 2015, 563818. [Google Scholar] [CrossRef]
- Toole, B.P. Hyaluronan and Its Binding Proteins, the Hyaladherins. Curr. Opin. Cell Biol. 1990, 2, 839–844. [Google Scholar] [CrossRef]
- Greco, R.M.; Iocono, J.A.; Ehrlich, H.P. Hyaluronic Acid Stimulates Human Fibroblast Proliferation within a Collagen Matrix. J. Cell. Physiol. 1998, 177, 465–473. [Google Scholar] [CrossRef]
- Tomida, M.; Koyama, H.; Ono, T. Induction of Hyaluronic Acid Synthetase Activity in Rat Fibroblasts by Medium Change of Confluent Cultures. J. Cell. Physiol. 1975, 86, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Ohara, H.; Ichikawa, S.; Matsumoto, H.; Akiyama, M.; Fujimoto, N.; Kobayashi, T.; Tajima, S. Collagen-Derived Dipeptide, Proline-Hydroxyproline, Stimulates Cell Proliferation and Hyaluronic Acid Synthesis in Cultured Human Dermal Fibroblasts. J. Dermatol. 2010, 37, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Zague, V.; do Amaral, J.B.; Rezende Teixeira, P.; de Oliveira Niero, E.L.; Lauand, C.; Machado-Santelli, G.M. Collagen Peptides Modulate the Metabolism of Extracellular Matrix by Human Dermal Fibroblasts Derived from Sun-Protected and Sun-Exposed Body Sites: Collagen Peptides Modulate ECM Metabolism. Cell Biol. Int. 2018, 42, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Ha, G.-H.; Seo, W.-Y.; Kim, C.K.; Kim, K.; Lee, S.B. Human Collagen Alpha-2 Type I Stimulates Collagen Synthesis, Wound Healing, and Elastin Production in Normal Human Dermal Fibroblasts (HDFs). BMB Rep. 2020, 53, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Kim, S.H.; Seo, W.-Y.; Jeong, Y.; Shin, M.C.; Ryu, D.; Lee, S.B.; Choi, Y.J.; Kim, K. Effects of Human Collagen α-1 Type I-Derived Proteins on Collagen Synthesis and Elastin Production in Human Dermal Fibroblasts. BMB Rep. 2021, 54, 329–334. [Google Scholar] [CrossRef]
- Kjellén, L.; Lindahl, U. Proteoglycans: Structures and Interactions. Annu. Rev. Biochem. 1991, 60, 443–475. [Google Scholar] [CrossRef]
- Šoltés, L.; Mendichi, R.; Kogan, G.; Schiller, J.; Stankovská, M.; Arnhold, J. Degradative Action of Reactive Oxygen Species on Hyaluronan. Biomacromolecules 2006, 7, 659–668. [Google Scholar] [CrossRef]
- Antonicelli, F.; Bellon, G.; Debelle, L.; Hornebeck, W. Elastin-Elastases and Inflamm-Aging. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2007; Volume 79, pp. 99–155. ISBN 978-0-12-373913-1. [Google Scholar]
- Halicka, H.D.; Mitlitski, V.; Heeter, J.; Balazs, E.A.; Darzynkiewicz, Z. Darzynkiewicz Attenuation of the Oxidative Burst-Induced DNA Damage in Human Leukocytes by Hyaluronan. Int. J. Mol. Med. 2009, 23, 695–699. [Google Scholar] [CrossRef]
- Lin, Y.-W.; Fang, C.-H.; Meng, F.-Q.; Ke, C.-J.; Lin, F.-H. Hyaluronic Acid Loaded with Cerium Oxide Nanoparticles as Antioxidant in Hydrogen Peroxide Induced Chondrocytes Injury: An In Vitro Osteoarthritis Model. Molecules 2020, 25, 4407. [Google Scholar] [CrossRef]
- Albano, G.D.; Bonanno, A.; Cavalieri, L.; Ingrassia, E.; Di Sano, C.; Siena, L.; Riccobono, L.; Gagliardo, R.; Profita, M. Effect of High, Medium, and Low Molecular Weight Hyaluronan on Inflammation and Oxidative Stress in an In Vitro Model of Human Nasal Epithelial Cells. Mediat. Inflamm. 2016, 2016, 8727289. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, H.; Wu, H.; Wang, C.; Shi, X.; Xie, J.; He, J.; Yang, J. Cytoprotective Effect of Hyaluronic Acid and Hydroxypropyl Methylcellulose against DNA Damage Induced by Thimerosal in Chang Conjunctival Cells. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Avadhani, K.S.; Manikkath, J.; Tiwari, M.; Chandrasekhar, M.; Godavarthi, A.; Vidya, S.M.; Hariharapura, R.C.; Kalthur, G.; Udupa, N.; Mutalik, S. Skin Delivery of Epigallocatechin-3-Gallate (EGCG) and Hyaluronic Acid Loaded Nano-Transfersomes for Antioxidant and Anti-Aging Effects in UV Radiation Induced Skin Damage. Drug Deliv. 2017, 24, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Balogh, L.; Polyak, A.; Mathe, D.; Kiraly, R.; Thuroczy, J.; Terez, M.; Janoki, G.; Ting, Y.; Bucci, L.R.; Schauss, A.G. Absorption, Uptake and Tissue Affinity of High-Molecular-Weight Hyaluronan after Oral Administration in Rats and Dogs. J. Agric. Food Chem. 2008, 56, 10582–10593. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Joumura, T.; Takekuma, Y.; Sugawara, M. Transfer of Orally Administered Hyaluronan to the Lymph. Eur. J. Pharm. Biopharm. 2020, 154, 210–213. [Google Scholar] [CrossRef]
- Kimura, M.; Maeshima, T.; Kubota, T.; Kurihara, H.; Masuda, Y.; Nomura, Y. Absorption of Orally Administered Hyaluronan. J. Med. Food 2016, 19, 1172–1179. [Google Scholar] [CrossRef]
- Göllner, I.; Voss, W.; von Hehn, U.; Kammerer, S. Ingestion of an Oral Hyaluronan Solution Improves Skin Hydration, Wrinkle Reduction, Elasticity, and Skin Roughness: Results of a Clinical Study. J. Evid. Based Complement. Altern. Med. 2017, 22, 816–823. [Google Scholar] [CrossRef]
- Oe, M.; Sakai, S.; Yoshida, H.; Okado, N.; Kaneda, H.; Masuda, Y.; Urushibata, O. Oral Hyaluronan Relieves Wrinkles: A Double-Blinded, Placebo-Controlled Study over a 12-Week Period. Clin. Cosmet. Investig. Dermatol. 2017, 10, 267–273. [Google Scholar] [CrossRef]
- Hsu, T.-F.; Su, Z.-R.; Hsieh, Y.-H.; Wang, M.-F.; Oe, M.; Matsuoka, R.; Masuda, Y. Oral Hyaluronan Relieves Wrinkles and Improves Dry Skin: A 12-Week Double-Blinded, Placebo-Controlled Study. Nutrients 2021, 13, 2220. [Google Scholar] [CrossRef]
- Kawada, C.; Kimura, M.; Masuda, Y.; Nomura, Y. Oral Administration of Hyaluronan Prevents Skin Dryness and Epidermal Thickening in Ultraviolet Irradiated Hairless Mice. J. Photochem. Photobiol. B 2015, 153, 215–221. [Google Scholar] [CrossRef]
- Alam, M.A.; Al-Jenoobi, F.I.; Al-mohizea, A.M. Everted Gut Sac Model as a Tool in Pharmaceutical Research: Limitations and Applications. J. Pharm. Pharmacol. 2012, 64, 326–336. [Google Scholar] [CrossRef]
- Pang, K.S. Modeling of Intestinal Drug Absorption: Roles of Transporters and Metabolic Enzymes (for the Gillette Review Series). Drug Metab. Dispos. 2003, 31, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Pansu, D.; Bellaton, C.; Roche, C.; Bronner, F. Duodenal and Ileal Calcium Absorption in the Rat and Effects of Vitamin D. Am. J. Physiol. Gastrointest. Liver Physiol. 1983, 7, G695–G700. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.; Kuramoto, M.; Takahashi, T.; Nakamura, H.; Nakanishi, Y.; Imasato, Y.; Yoshimura, H. Characteristics of the Gastrointestinal Absorption of Morphine in Rats. Chem. Pharm. Bull. 1989, 37, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Volpi, N.; Mantovani, V.; Galeotti, F.; Bianchi, D.; Straniero, V.; Valoti, E.; Miraglia, N. Oral Bioavailability and Pharmacokinetics of Nonanimal Chondroitin Sulfate and Its Constituents in Healthy Male Volunteers. Clin. Pharmacol. Drug Dev. 2019, 8, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Osawa, Y.; Mizushige, T.; Jinno, S.; Sugihara, F.; Inoue, N.; Tanaka, H.; Kabuyama, Y. Absorption and Metabolism of Orally Administered Collagen Hydrolysates Evaluated by the Vascularly Perfused Rat Intestine and Liver in Situ. Biomed. Res. 2018, 39, 1–11. [Google Scholar] [CrossRef]
- Kawada, C.; Yoshida, T.; Yoshida, H.; Matsuoka, R.; Sakamoto, W.; Odanaka, W.; Sato, T.; Yamasaki, T.; Kanemitsu, T.; Masuda, Y.; et al. Ingested hyaluronan moisturizes dry skin. Nutr. J. 2014, 11, 70. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopeia 2.9.38 Particle-Size Distribution Estimation by Analytical Sieving; Council of Europe: Strasbourg, France, 2010; Volume 10, pp. 392–394. [Google Scholar]
- Council of Europe. European Pharmacopeia 2.5.9 Determination of Nitrogen by Sulfuric Acid Digestion; Council of Europe: Strasbourg, France, 2008; Volume 10, p. 170. [Google Scholar]
- AOAC International. Association of Official Analytical Chemists Official Methods 990.26 and 928.08. In Official Methods of Analysis; AOAC International: Maryland, MD, USA, 2000. [Google Scholar]
- United States Pharmacopoeia. Chondroitin Sulfate Sodium Monograph; United States Pharmacopoeia: North Bethesda, MD, USA, 2020. [Google Scholar]
- Bitter, T.; Muir, H.M. A Modified Uronic Acid Carbazole Reaction. Anal. Biochem. 1962, 4, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe. Heparin sodium monograph. In European Pharmacopoeia, 10th ed.; EDQM Commission: Strasbourg, France, 2020; Volume 10. [Google Scholar]
- Council of Europe. European Pharmacopeia 2.2.56 Amino Acid Analysis; Council of Europe: Strasbourg, France, 2010; Volume 10, pp. 100–106. [Google Scholar]
- López-García, J.; Lehocký, M.; Humpolíček, P.; Sáha, P. HaCaT Keratinocytes Response on Antimicrobial Atelocollagen Substrates: Extent of Cytotoxicity, Cell Viability and Proliferation. JFB 2014, 5, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Rezvanian, M.; Amin, M.C.I.M.; Ng, S.-F. Development and Physicochemical Characterization of Alginate Composite Film Loaded with Simvastatin as a Potential Wound Dressing. Carbohydr. Polym. 2016, 137, 295–304. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5; 2009-Biological Evaluation of Medical Devices-Part 5: Tests for in Vitro Cytotoxicity. International Organization for Standardization: London, UK, 2009.
- Farndale, R.W.; Sayers, C.A.; Barrett, A.J. A Direct Spectrophotometric Microassay for Sulfated Glycosaminoglycans in Cartilage Cultures. Connect. Tissue Res. 1982, 9, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.E.; Akhtar, N.; Botchway, S.; Pennock, C.A. Interaction of 1,9-Dimethylmethylene Blue with Glycosaminoglycans. Ann. Clin. Biochem. 1994, 31, 147–152. [Google Scholar] [CrossRef] [PubMed]
| Test | Result |
|---|---|
| Appearance | Off-white hygroscopic powder |
| Granulometry | 100% through 600 µm |
| pH | 7.1 |
| Chlorides | <1% |
| Nitrogen | 6% |
| Loss on drying | 6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galvez-Martin, P.; Soto-Fernandez, C.; Romero-Rueda, J.; Cabañas, J.; Torrent, A.; Castells, G.; Martinez-Puig, D. A Novel Hyaluronic Acid Matrix Ingredient with Regenerative, Anti-Aging and Antioxidant Capacity. Int. J. Mol. Sci. 2023, 24, 4774. https://doi.org/10.3390/ijms24054774
Galvez-Martin P, Soto-Fernandez C, Romero-Rueda J, Cabañas J, Torrent A, Castells G, Martinez-Puig D. A Novel Hyaluronic Acid Matrix Ingredient with Regenerative, Anti-Aging and Antioxidant Capacity. International Journal of Molecular Sciences. 2023; 24(5):4774. https://doi.org/10.3390/ijms24054774
Chicago/Turabian StyleGalvez-Martin, Patricia, Cristina Soto-Fernandez, Jessica Romero-Rueda, Jesus Cabañas, Anna Torrent, Gloria Castells, and Daniel Martinez-Puig. 2023. "A Novel Hyaluronic Acid Matrix Ingredient with Regenerative, Anti-Aging and Antioxidant Capacity" International Journal of Molecular Sciences 24, no. 5: 4774. https://doi.org/10.3390/ijms24054774
APA StyleGalvez-Martin, P., Soto-Fernandez, C., Romero-Rueda, J., Cabañas, J., Torrent, A., Castells, G., & Martinez-Puig, D. (2023). A Novel Hyaluronic Acid Matrix Ingredient with Regenerative, Anti-Aging and Antioxidant Capacity. International Journal of Molecular Sciences, 24(5), 4774. https://doi.org/10.3390/ijms24054774









