Bioinspired Synthesis of Magnetic Nanoparticles Based on Iron Oxides Using Orange Waste and Their Application as Photo-Activated Antibacterial Agents
Abstract
1. Introduction
2. Results and Discussion
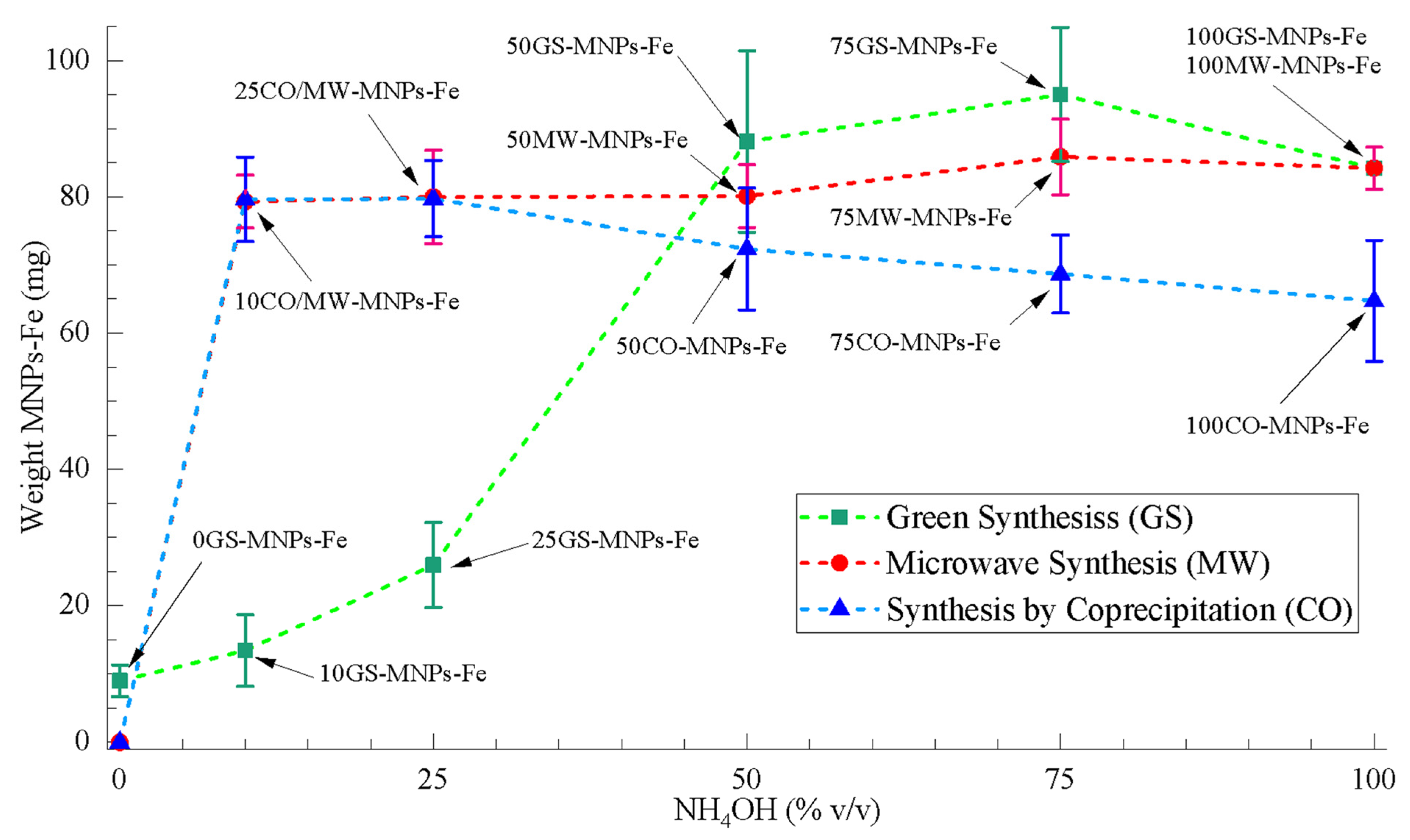
2.1. Weight Obtained of MNPs-Fe
2.2. MNPs-Fe Characterization
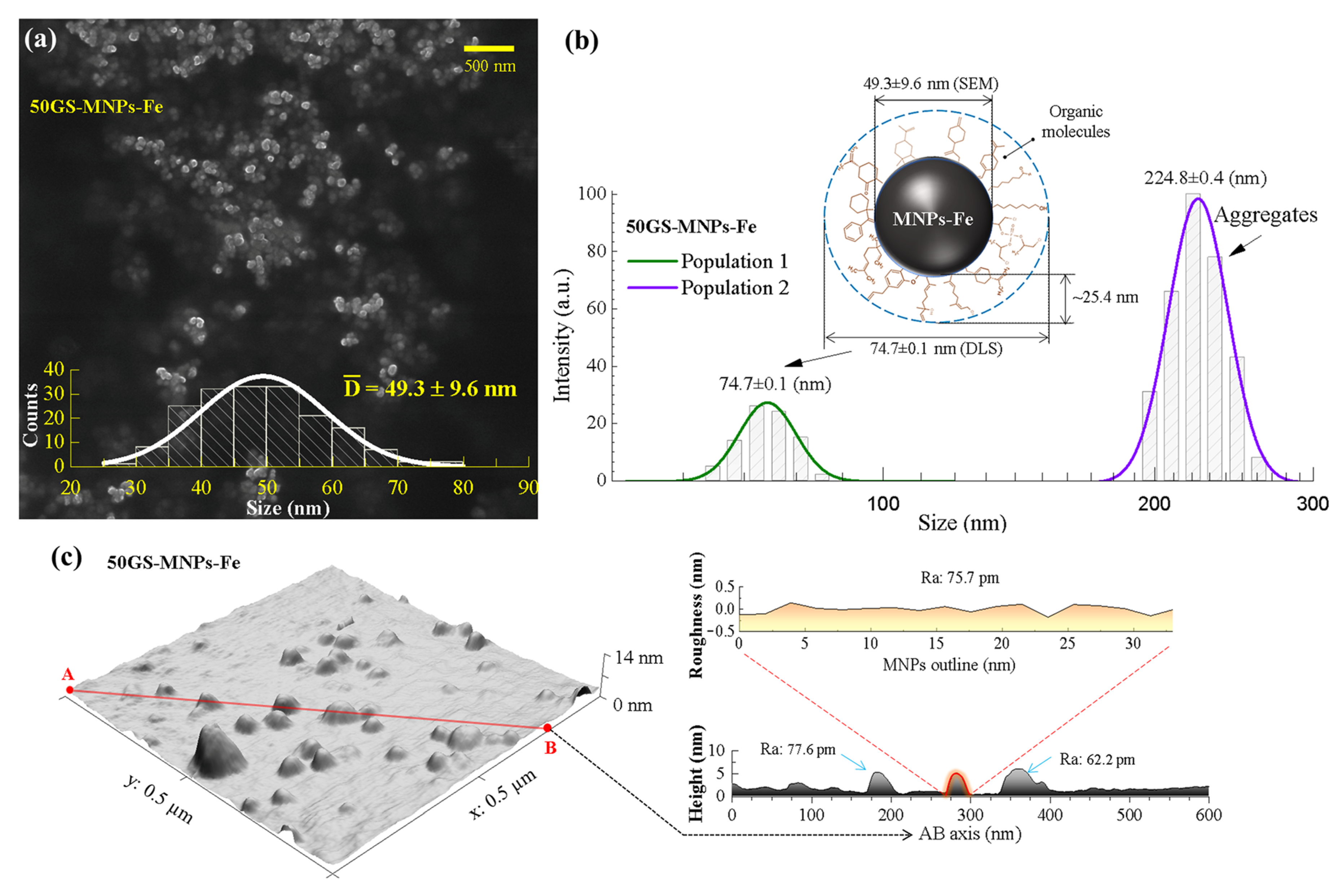
2.2.1. Morphology, Size Distribution, and Topography of MNPs-Fe
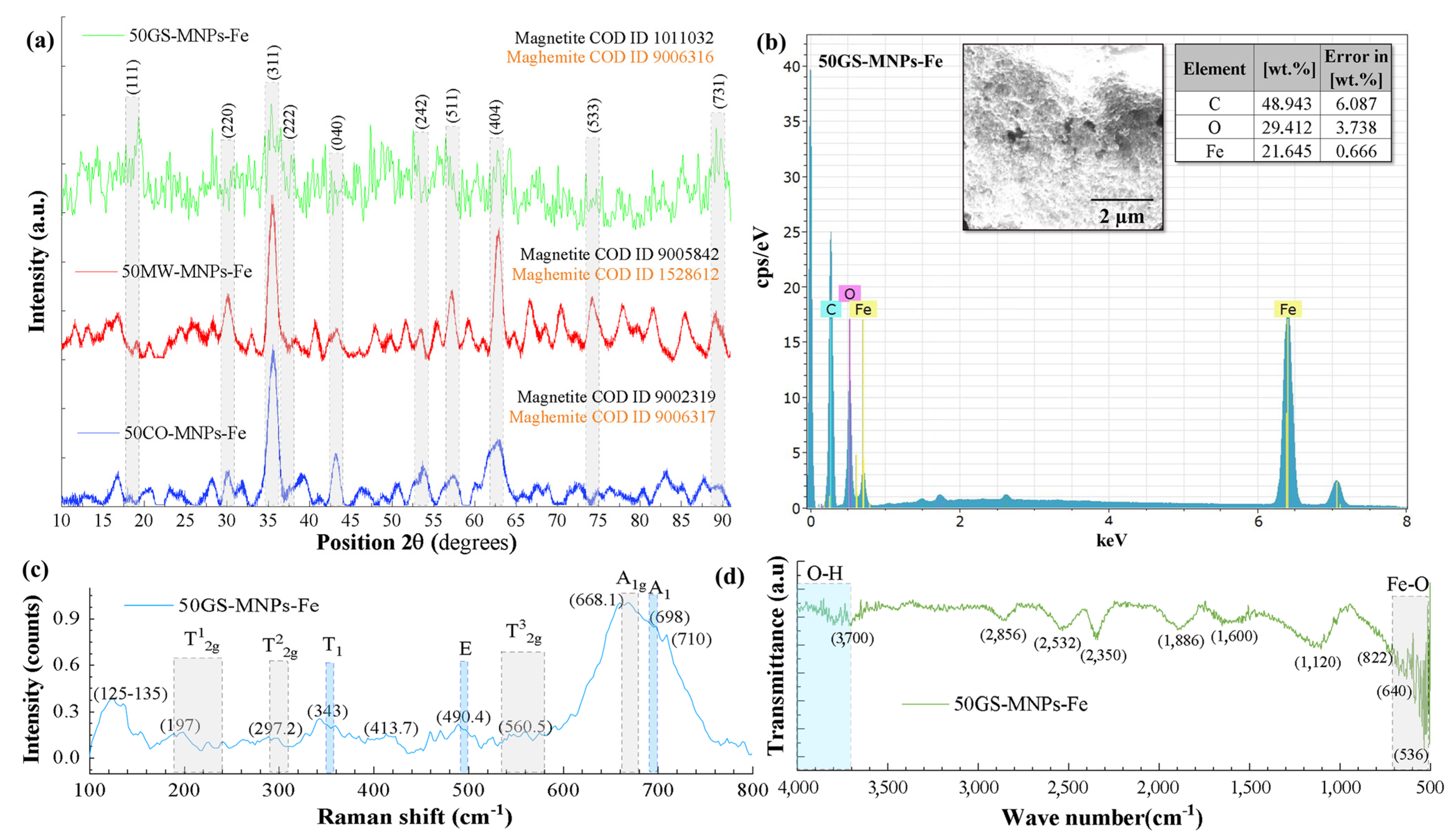
2.2.2. Phase and Chemical Composition of the MNPs-Fe
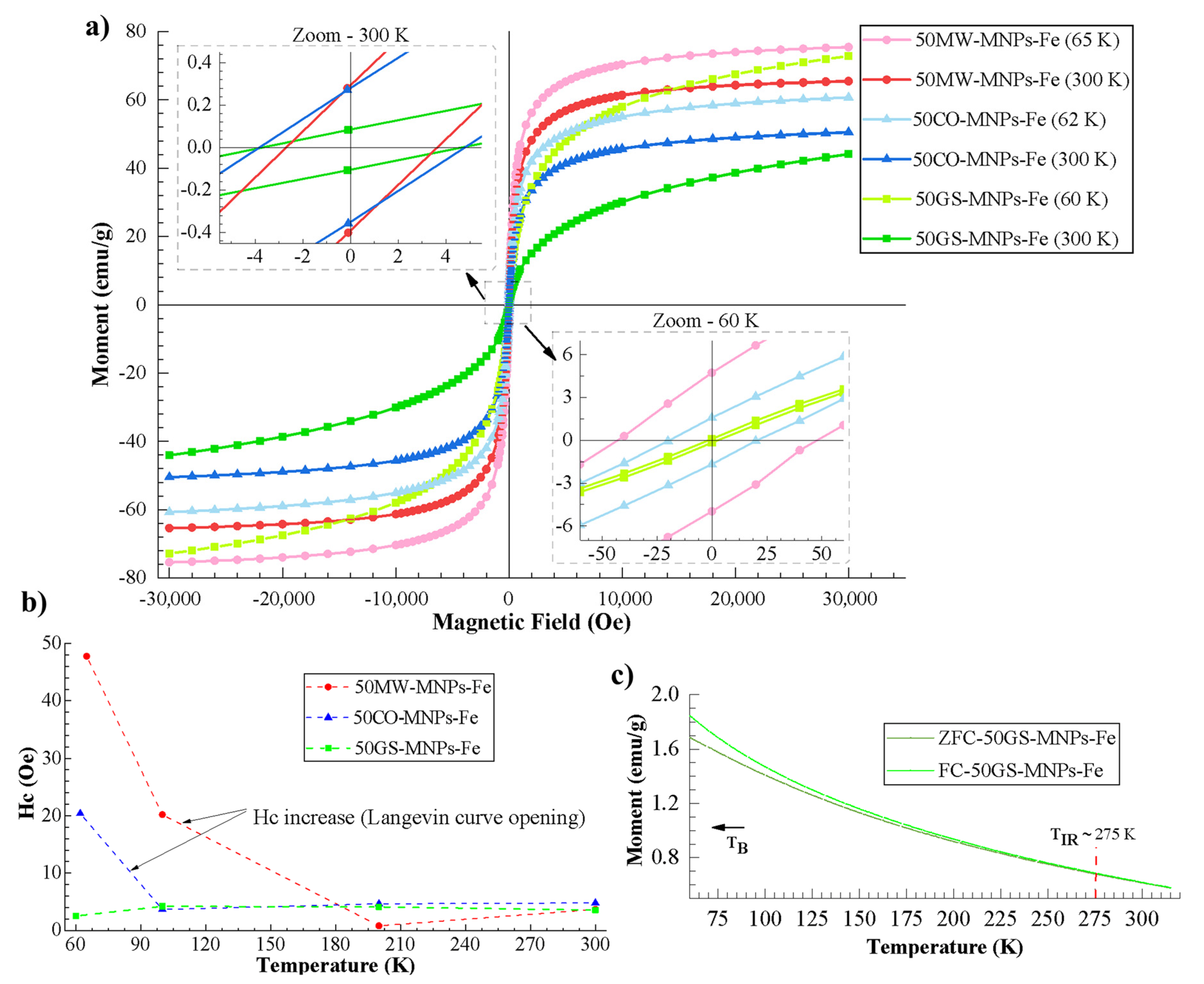
2.2.3. Magnetometry
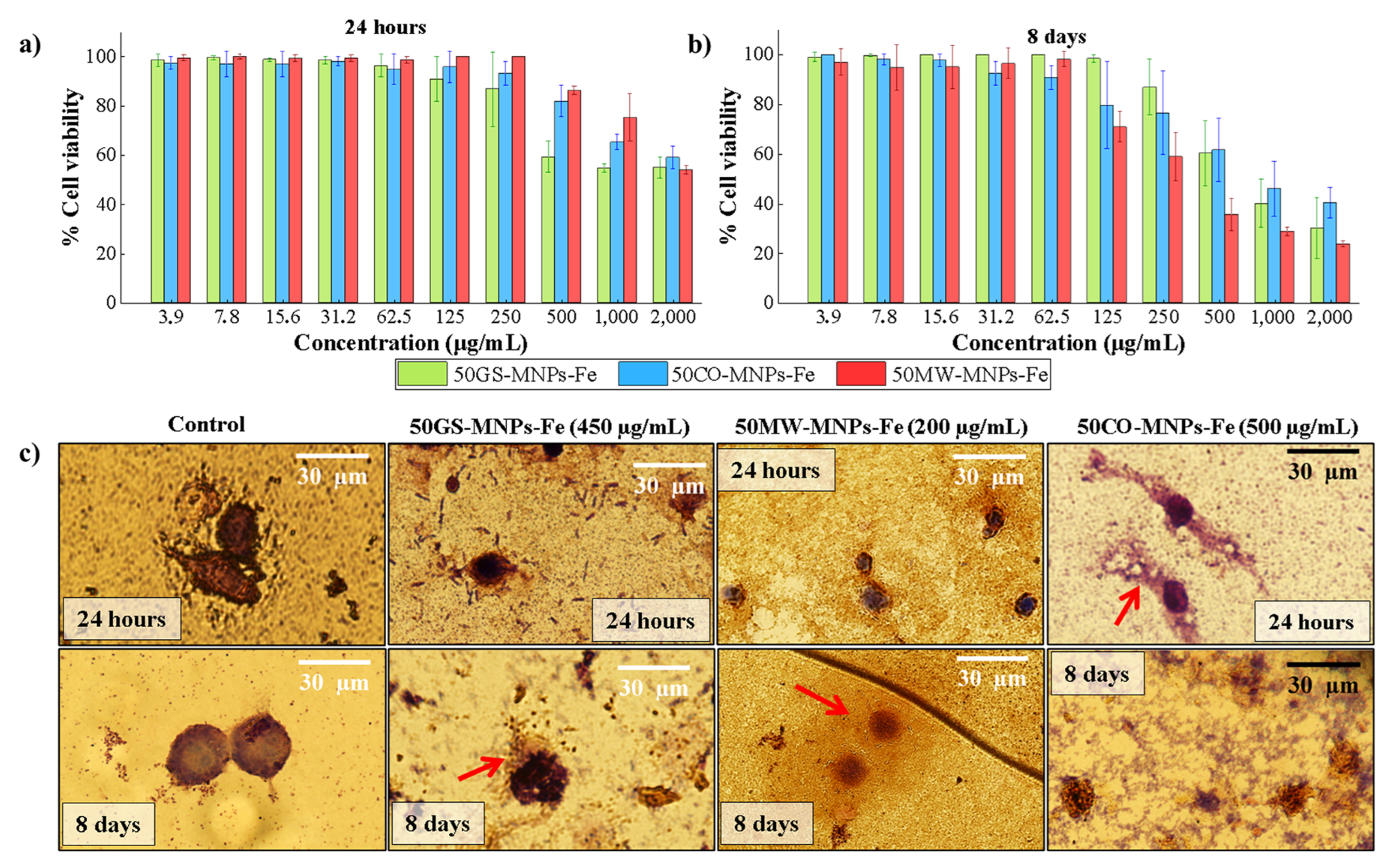
2.3. Cell Viability in ATCC RAW 264.7 Cells
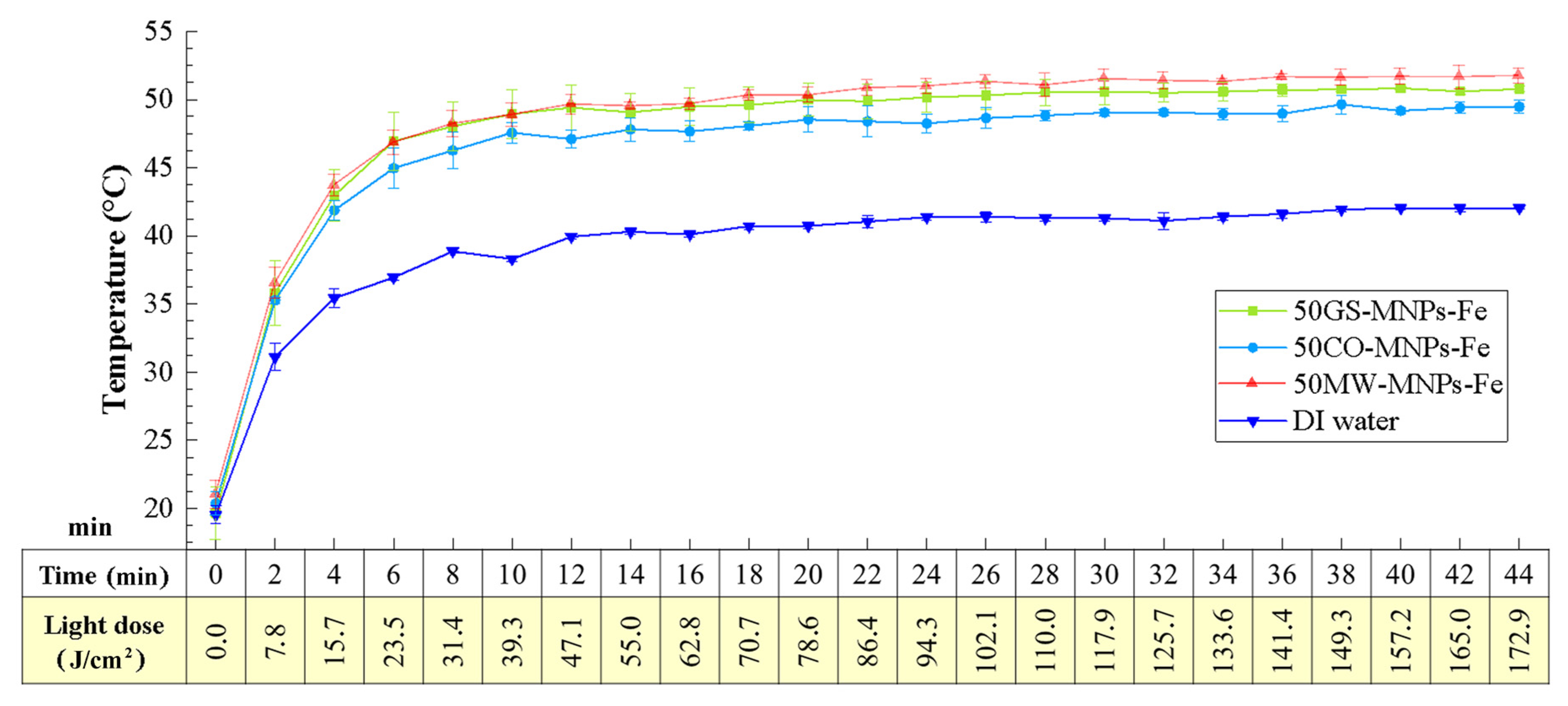
2.4. Photothermal Studies
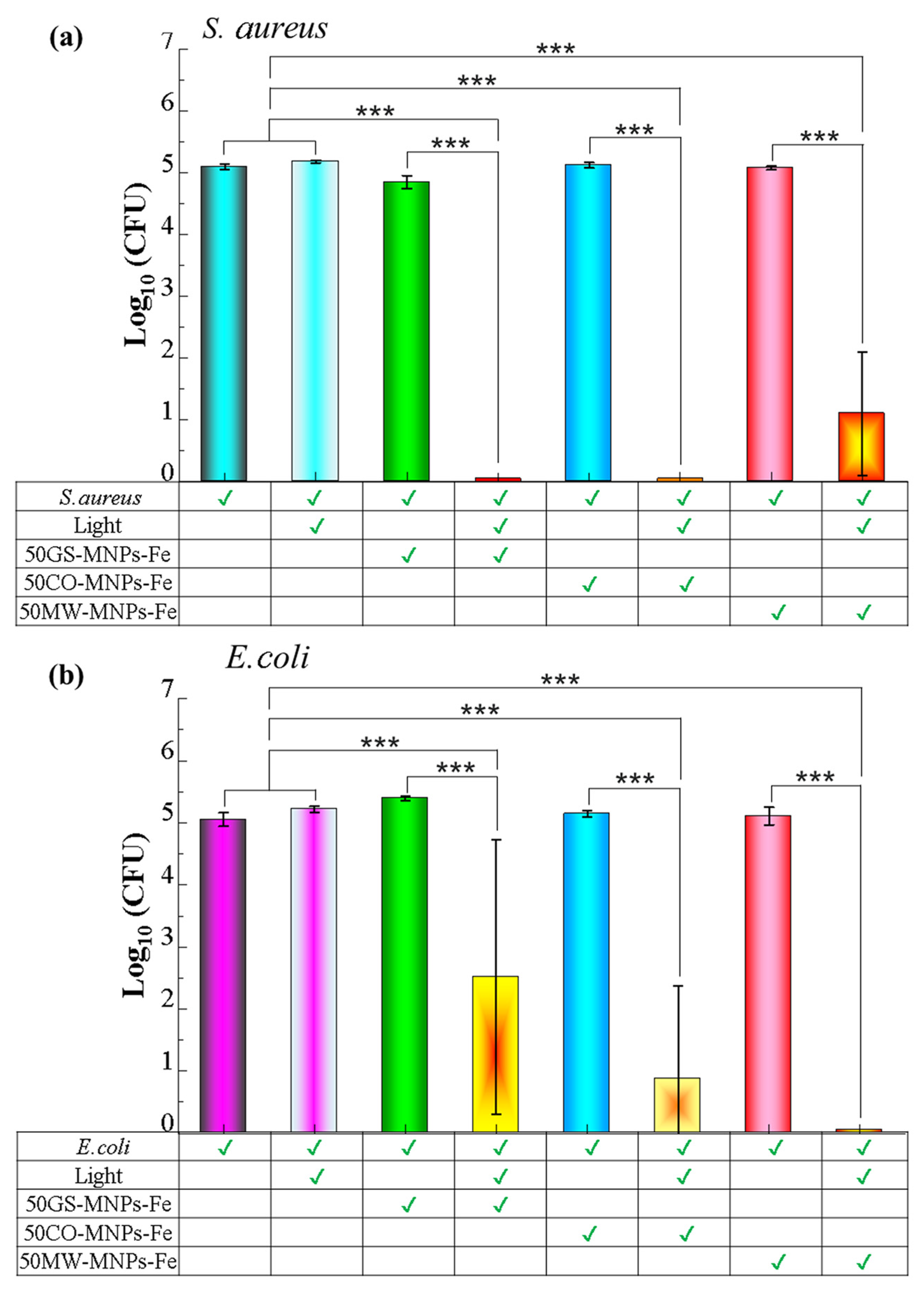
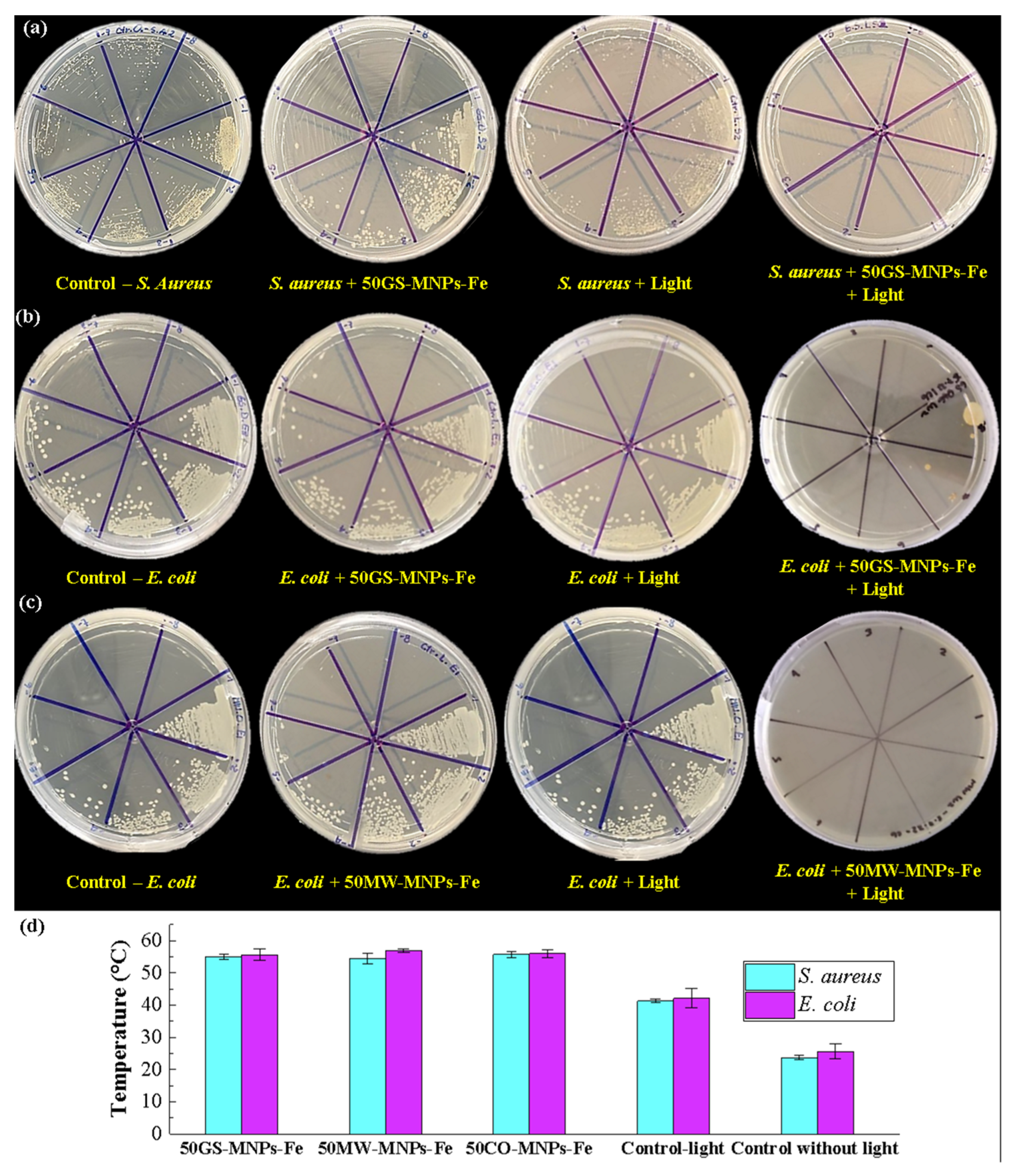
2.5. Antibacterial PTT
3. Materials and Methods
3.1. Materials
3.2. Preparation of Orange Peel Extract
3.3. GS of MNPs-Fe
3.4. MNPs-Fe Characterization
3.5. Cytotoxicity Assay
3.5.1. Cell Culture
3.5.2. Resazurin Cell Viability Assay
3.5.3. Giemsa Staining Assay
3.6. Antibacterial Activity
3.6.1. Thermal Studies
3.6.2. Bacterial Culture
3.6.3. Spread Plate Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Hu, C. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Liu, X.; Zheng, Y.; Chu, P.K.; Zhang, Y.; Wu, S. Graphitic carbon nitride-based materials for photocatalytic antibacterial application. Mater. Sci. Eng. R Rep. 2021, 145, 100610. [Google Scholar] [CrossRef]
- Liang, J.; Li, W.; Chen, J.; Huang, X.; Liu, Y.; Zhang, X.; Shu, W.; Lei, B.; Zhang, H. Antibacterial Activity and Synergetic Mechanism of Carbon Dots against Gram-Positive and Gram-Negative Bacteria. ACS Appl. Bio Mater. 2021, 4, 6937–6945. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.R.; López-abarrategui, C.; De, I.; Gómez, S. Antimicrobial magnetic nanoparticles based-therapies for controlling infectious diseases. Int. J. Pharm. 2018, 555, 356–357. [Google Scholar] [CrossRef]
- Paramanantham, P.; Anju, V.T.; Dyavaiah, M.; Siddhardha, B. Applications of Carbon-Based Nanomaterials for Antimicrobial Photodynamic Therapy. In Nanotechnology in the Life Sciences; Spinger: Berlin/Heidelberg, Germany, 2019; pp. 237–259. [Google Scholar]
- Feng, Y.; Liu, L.; Zhang, J.; Aslan, H.; Dong, M. Photoactive antimicrobial nanomaterials. J. Mater. Chem. B 2017, 5, 8631–8652. [Google Scholar] [CrossRef]
- Smith, M. Antibiotic Resistance Mechanisms. In Journeys in Medicine and Research on Three Continents over 50 Years; ASM Journals: Ames, IA, USA, 2017; pp. 95–99. [Google Scholar] [CrossRef]
- Potbhare, A.K.; Umekar, M.S.; Chouke, P.B.; Bagade, M.B.; Tarik Aziz, S.K.; Abdala, A.A.; Chaudhary, R.G. Bioinspired graphene-based silver nanoparticles: Fabrication, characterization and antibacterial activity. Mater. Today Proc. 2019, 29, 720–725. [Google Scholar] [CrossRef]
- El-Gendy, N.S.; El-Gendy, N.S.; Omran, B.A. Green Synthesis of Nanoparticles for Water Treatment. In Nano and Bio-Based Technologies for Wastewater Treatment; Wiley: Hoboken, NJ, USA, 2019; pp. 205–263. [Google Scholar] [CrossRef]
- Ajinkya, N.; Yu, X.; Kaithal, P.; Luo, H.; Somani, P. Magnetic iron oxide nanoparticle (IONP) synthesis to applications: Present and future. Materials 2020, 13, 4644. [Google Scholar] [CrossRef]
- Xin, Q.; Shah, H.; Nawaz, A.; Xie, W.; Akram, M.Z.; Batool, A.; Tian, L.; Jan, S.U.; Boddula, R.; Guo, B.; et al. Antibacterial Carbon-Based Nanomaterials. Adv. Mater. 2019, 31, 1804838. [Google Scholar] [CrossRef]
- Jalal, M.; Ansari, M.A.; Alzohairy, M.A.; Ali, S.G.; Khan, H.M.; Almatroudi, A.; Raees, K. Biosynthesis of silver nanoparticles from oropharyngeal Candida glabrata isolates and their antimicrobial activity against clinical strains of bacteria and fungi. Nanomaterials 2018, 8, 586. [Google Scholar] [CrossRef]
- Rajendracharu, S.; Betül, K.C. The activation energy and antibacterial investigation of spherical Fe3O4 nanoparticles prepared by Crocus sativus (Saffron) flowers. Biointerface Res. Appl. Chem. Open Access J. 2020, 10, 5951–5959. [Google Scholar] [CrossRef]
- Jaber, M.; Mushtaq, A.; Zhang, K.; Wu, J.; Luo, D.; Yi, Z.; Iqbal, M.Z.; Kong, X. Gram-scale synthesis of splat-shaped Ag-TiO2 nanocomposites for enhanced antimicrobial properties. Beilstein J. Nanotechnol. 2020, 11, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Mushtaq, A.; Tang, Z.; Dempsey, E.; Wu, Y.; Lu, Y.; Tian, C.; Farheen, J.; Kong, X.; Iqbal, M.Z. ROS-responsive Ag-TiO2 hybrid nanorods for enhanced photodynamic therapy of breast cancer and antimicrobial applications. J. Sci. Adv. Mater. Devices 2022, 7, 100417. [Google Scholar] [CrossRef]
- Mushtaq, A.; Zhao, R.; Luo, D.; Dempsey, E.; Wang, X.; Iqbal, M.Z.; Kong, X. Magnetic hydroxyapatite nanocomposites: The advances from synthesis to biomedical applications. Mater. Des. 2021, 197, 109269. [Google Scholar] [CrossRef]
- Jabir, M.S.; Nayef, U.M.; Kadhim, W.K.A. Polyethylene glycol-functionalized magnetic (Fe3O4) nanoparticles: A novel DNA-mediated antibacterial agent. Nano Biomed. Eng. 2019, 11, 18–27. [Google Scholar] [CrossRef]
- Rumyantceva, V.; Rumyantceva, V.; Koshel, E.; Vinogradov, V. Biocide-conjugated magnetite nanoparticles as an advanced platform for biofilm treatment. Ther. Deliv. 2019, 10, 241–250. [Google Scholar] [CrossRef]
- Ling, W.; Wang, M.; Xiong, C.; Xie, D.; Chen, Q.; Chu, X.; Qiu, X.; Li, Y.; Xiao, X. Synthesis, surface modification, and applications of magnetic iron oxide nanoparticles. J. Mater. Res. 2019, 34, 1828–1844. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Chen, Y.; Liu, L.; Mo, A.; Peng, Q. Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment. J. Control. Release 2020, 328, 251–262. [Google Scholar] [CrossRef]
- Jędrzak, A.; Grześkowiak, B.F.; Golba, K.; Coy, E.; Synoradzki, K.; Jurga, S.; Jesionowski, T.; Mrówczyński, R. Magnetite nanoparticles and spheres for chemo-and photothermal therapy of hepatocellular carcinoma in vitro. Int. J. Nanomed. 2020, 15, 7923–7936. [Google Scholar] [CrossRef]
- Songca, S.P.; Adjei, Y. Applications of Antimicrobial Photodynamic Therapy against Bacterial Biofilms. Int. J. Mol. Sci. 2022, 23, 3209. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Atarod, M.; Sajjadi, M.; Sajadi, S.M.; Issaabadi, Z. Plant-Mediated Green Synthesis of Nanostructures: Mechanisms, Characterization, and Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 28. [Google Scholar]
- Dash, A.; Ahmed, M.T.; Selvaraj, R. Mesoporous magnetite nanoparticles synthesis using the Peltophorum pterocarpum pod extract, their antibacterial efficacy against pathogens and ability to remove a pollutant dye. J. Mol. Struct. 2019, 1178, 268–273. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. “Green” synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Potbhare, A.K.; Chaudhary, R.G.; Chouke, P.B.; Yerpude, S.; Mondal, A.; Sonkusare, V.N.; Rai, A.R.; Juneja, H.D. Phytosynthesis of nearly monodisperse CuO nanospheres using Phyllanthus reticulatus/Conyza bonariensis and its antioxidant/antibacterial assays. Mater. Sci. Eng. C 2019, 99, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Maheswari, K.C.; Reddy, P.S. Green Synthesis of Magnetite Nanoparticles through Leaf Extract of Azadirachta indica. JACS Dir. 2016, 2, 189–191. [Google Scholar]
- Venkateswarlu, S.; Rao, Y.S.; Balaji, T.; Prathima, B.; Jyothi, N.V.V. Biogenic synthesis of Fe3O4 magnetic nanoparticles using plantain peel extract. Mater. Lett. 2013, 100, 241–244. [Google Scholar] [CrossRef]
- Šuljagić, M.; Vulić, P.; Jeremić, D.; Pavlović, V.; Filipović, S.; Kilanski, L.; Lewinska, S.; Slawska-Waniewska, A.; Milenković, M.R.; Nikolić, A.S.; et al. The influence of the starch coating on the magnetic properties of nanosized cobalt ferrites obtained by different synthetic methods. Mater. Res. Bull. 2021, 134, 111117. [Google Scholar] [CrossRef]
- Kumar, I.; Mondal, M.; Sakthivel, N. Green Synthesis of Phytogenic Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 37–73. [Google Scholar]
- Bano, S.; Nazir, S.; Nazir, A.; Munir, S.; Mahmood, T.; Afzal, M.; Ansari, F.L.; Mazhar, K. Microwave-assisted green synthesis of superparamagnetic nanoparticles using fruit peel extracts: Surface engineering, T2 relaxometry, and photodynamic treatment potential. Int. J. Nanomed. 2016, 11, 3833–3848. [Google Scholar] [CrossRef]
- Hezam, F.A.; Khalifa, N.O.; Nur, O.; Mustafa, M.A. Synthesis and magnetic properties of Ni0.5MgxZn0.5-xFe2O4 (0.0 ≤ x ≤ 0.5) nanocrystalline spinel ferrites. Mater. Chem. Phys. 2021, 257, 123770. [Google Scholar] [CrossRef]
- Dumani, D.S.; Cook, J.R.; Kubelick, K.P.; Luci, J.J.; Emelianov, S.Y. Photomagnetic Prussian blue nanocubes: Synthesis, characterization, and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102138. [Google Scholar] [CrossRef]
- Amiri, M.; Gholami, T.; Amiri, O.; Pardakhti, A.; Ahmadi, M.; Akbari, A.; Amanatfard, A.; Salavati-Niasari, M. The magnetic inorganic-organic nanocomposite based on ZnFe2O4-Imatinib-liposome for biomedical applications, in vivo and in vitro study. J. Alloys Compd. 2020, 849, 156604. [Google Scholar] [CrossRef]
- Escobar, A.M.; Pizzio, L.R.; Romanelli, G.P. Catalizadores Magnéticos Basados En Óxidos De Hierro: Síntesis, Propiedades Y Aplicaciones. Cienc. Desarro. 2018, 10, 79–101. [Google Scholar] [CrossRef]
- Novoselova, L.Y. Nanoscale magnetite: New synthesis approach, structure and properties. Appl. Surf. Sci. 2021, 539, 148275. [Google Scholar] [CrossRef]
- Shah Saqib, A.N.; Thu Huong, N.T.; Kim, S.W.; Jung, M.H.; Lee, Y.H. Structural and magnetic properties of highly Fe-doped ZnO nanoparticles synthesized by one-step solution plasma process. J. Alloys Compd. 2021, 853, 157153. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Metal oxide nanoparticles as biomedical materials. Biomimetics 2020, 5, 27. [Google Scholar] [CrossRef]
- Dhar, P.K.; Saha, P.; Hasan, M.K.; Amin, M.K.; Haque, M.R. Green synthesis of magnetite nanoparticles using Lathyrus sativus peel extract and evaluation of their catalytic activity. Clean. Eng. Technol. 2021, 3, 100117. [Google Scholar] [CrossRef]
- Silvestri, C.; Silvestri, L.; Forcina, A.; Di Bona, G.; Falcone, D. Green chemistry contribution towards more equitable global sustainability and greater circular economy: A systematic literature review. J. Clean. Prod. 2021, 294, 126137. [Google Scholar] [CrossRef]
- Wang, W.W.; Zhu, Y.J.; Ruan, M.L. Microwave-assisted synthesis and magnetic property of magnetite and hematite nanoparticles. J. Nanoparticle Res. 2007, 9, 419–426. [Google Scholar] [CrossRef]
- Nüchter, M.; Müller, U.; Ondruschka, B.; Tied, A.; Lautenschläger, W. Microwave-assisted chemical reactions. Chem. Eng. Technol. 2003, 26, 1207–1216. [Google Scholar] [CrossRef]
- Henam, S.D.; Ahmad, F.; Shah, M.A.; Parveen, S.; Wani, A.H. Microwave synthesis of nanoparticles and their antifungal activities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 213, 337–341. [Google Scholar] [CrossRef]
- Lazaratos, M.; Karathanou, K.; Mainas, E.; Chatzigoulas, A.; Pippa, N.; Demetzos, C.; Cournia, Z. Coating of magnetic nanoparticles affects their interactions with model cell membranes. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129671. [Google Scholar] [CrossRef]
- Hu, T.; Wang, Z.; Shen, W.; Liang, R.; Yan, D.; Wei, M. Recent advances in innovative strategies for enhanced cancer photodynamic therapy. Theranostics 2021, 11, 3278–3300. [Google Scholar] [CrossRef]
- Estelrich, J.; Antònia Busquets, M. Iron oxide nanoparticles in photothermal therapy. Molecules 2018, 23, 1567. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Chen, J.; Li, Z.Y.; Au, L.; Hartland, G.V.; Li, X.; Marquez, M.; Xia, Y. Gold nanostructures: Engineering their plasmonic properties for biomedical applications. Chem. Soc. Rev. 2006, 35, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.M.; Thorat, N.D.; Shete, P.B.; Bedge, P.A. Comprehensive cytotoxicity studies of superparamagnetic iron oxide nanoparticles. Biochem. Biophys. Rep. 2018, 13, 63–72. [Google Scholar] [CrossRef]
- Peng, Y.; Xia, C.; Cui, M.; Yao, Z.; Yi, X. Effect of reaction condition on microstructure and properties of (NiCuZn)Fe2O4 nanoparticles synthesized via co-precipitation with ultrasonic irradiation. Ultrason. Sonochem. 2021, 71, 105369. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Z.; Li, F.; Xiao, Y.; Zhang, Y.; Bu, T.; Jia, P.; Zhe, T.; Wang, L. Multifunctional Magnetic Copper Ferrite Nanoparticles as Fenton-like Reaction and Near-Infrared Photothermal Agents for Synergetic Antibacterial Therapy. ACS Appl. Mater. Interfaces 2019, 11, 31649–31660. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, A.P.; Feuser, P.E.; Araújo, P.H.H.; Sayer, C. Encapsulation of Magnetic Nanoparticles and Copaíba Oil in Poly(methyl methacrylate) Nanoparticles via Miniemulsion Polymerization for Biomedical Application. Macromol. Symp. 2020, 394, 1–5. [Google Scholar] [CrossRef]
- Hussain, S.M.; Hess, K.L.; Gearhart, J.M.; Geiss, K.T.; Schlager, J.J. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol. Vitr. 2005, 19, 975–983. [Google Scholar] [CrossRef]
- Tousi, M.S.; Sepehri, H.; Khoee, S.; Farimani, M.M.; Delphi, L.; Mansourizadeh, F. Evaluation of apoptotic effects of mPEG-b-PLGA coated iron oxide nanoparticles as a eupatorin carrier on DU-145 and LNCaP human prostate cancer cell lines. J. Pharm. Anal. 2021, 11, 108–121. [Google Scholar] [CrossRef]
- Alarifi, S.; Ali, D.; Alkahtani, S. Iron Oxide Nanoparticles Induce Oxidative Stress, DNA Damage, and Caspase Activation in the Human Breast Cancer Cell Line. Biol. Trace Elem. Res. 2014, 159, 416–424. [Google Scholar] [CrossRef]
- Auffan, M.; Achouak, W.; Rose, J.; Roncato, M.-A.; Chanéac, C.; Waite, D.; Masion, A.; Woicik, J.; Wiesner, M.; Bottero, J.-Y. Relation between the Redox State of Iron-Based Nanoparticles and Their Cytotoxicity toward Escherichia coli. Environ. Sci. Technol. 2008, 42, 6730–6735. [Google Scholar] [CrossRef]
- Wei, H.; Hu, Y.; Wang, J.; Gao, X.; Qian, X.; Tang, M. Superparamagnetic iron oxide nanoparticles: Cytotoxicity, metabolism, and cellular behavior in biomedicine applications. Int. J. Nanomed. 2021, 16, 6097–6113. [Google Scholar] [CrossRef]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Sharma, S.K. Complex Magnetic Nanostructures: Synthesis, Assembly and Applications; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Chen, B.W.; He, Y.C.; Sung, S.Y.; Le, T.T.H.; Hsieh, C.L.; Chen, J.Y.; Wei, Z.H.; Yao, D.J. Synthesis and characterization of magnetic nanoparticles coated with polystyrene sulfonic acid for biomedical applications. Sci. Technol. Adv. Mater. 2020, 21, 471–481. [Google Scholar] [CrossRef]
- López-Téllez, G.; Balderas-Hernández, P.; Barrera-Díaz, C.E.; Vilchis-Nestor, A.R.; Roa-Morales, G.; Bilyeu, B. Green method to form iron oxide nanorods in orange peels for chromium (VI) reduction. J. Nanosci. Nanotechnol. 2013, 13, 2354–2361. [Google Scholar] [CrossRef] [PubMed]
- Kahrilas, G.A.; Wally, L.M.; Fredrick, S.J.; Hiskey, M.; Prieto, A.L.; Owens, J.E. Microwave-Assisted Green Synthesis of Silver Nanoparticles Using Orange Peel Extract. ACS Sustain. Chem. Eng. 2013, 2, 367–376. [Google Scholar] [CrossRef]
- Petcharoen, K.; Sirivat, A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Saxena, N.; Singh, M. Efficient synthesis of superparamagnetic magnetite nanoparticles under air for biomedical applications. J. Magn. Magn. Mater. 2017, 429, 166–176. [Google Scholar] [CrossRef]
- Rahmayanti, M. Synthesis of Magnetite Nanoparticles Using The Reverse Co-precipitation Method With NH4OH as Precipitating Agent and Its Stability Test at Various pH. Nat. Sci. J. Sci. Technol. 2020, 9, 54–58. [Google Scholar] [CrossRef]
- Wroblewski, C.; Volford, T.; Martos, B.; Samoluk, J.; Martos, P. High yield synthesis and application of magnetite nanoparticles (Fe3O4). Magnetochemistry 2020, 6, 22. [Google Scholar] [CrossRef]
- Nnadozie, E.C.; Ajibade, P.A. Green synthesis and characterization of magnetite (Fe3O4) nanoparticles using Chromolaena odorata root extract for smart nanocomposite. Mater. Lett. 2020, 263, 127145. [Google Scholar] [CrossRef]
- Raja, P.M.V.; Barron, A.R. Physical methods in chemistry. Nature 2019, 134, 366–367. [Google Scholar] [CrossRef]
- Marrese, M.; Guarino, V.; Ambrosio, L. Atomic Force Microscopy: A Powerful Tool to Address Scaffold Design in Tissue Engineering. J. Funct. Biomater. 2017, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.; Rasmussen, J.T.; Simonsen, A.C. Roughness analysis of single nanoparticles applied to atomic force microscopy images of hydrated casein micelles. Food Hydrocoll. 2015, 45, 168–174. [Google Scholar] [CrossRef]
- Taimoory, S.M.; Rahdar, A.; Aliahmad, M.; Sadeghfar, F.; Hajinezhad, M.R.; Jahantigh, M.; Shahbazi, P.; Trant, J.F. The synthesis and characterization of a magnetite nanoparticle with potent antibacterial activity and low mammalian toxicity. J. Mol. Liq. 2018, 265, 96–104. [Google Scholar] [CrossRef]
- Silva, V.A.J.; Andrade, P.L.; Silva, M.P.C.; Bustamante, A.D.; De Los Santos Valladares, L.; Albino Aguiar, J. Synthesis and characterization of Fe3O4 nanoparticles coated with fucan polysaccharides. J. Magn. Magn. Mater. 2013, 343, 138–143. [Google Scholar] [CrossRef]
- Tomul, F.; Arslan, Y.; Başoğlu, F.T.; Babuçcuoğlu, Y.; Tran, H.N. Efficient removal of anti-inflammatory from solution by Fe-containing activated carbon: Adsorption kinetics, isotherms, and thermodynamics. J. Environ. Manag. 2019, 238, 296–306. [Google Scholar] [CrossRef]
- Akram, M.A.; Khan, B.A.; Khan, M.K.; Alqahtani, A.; Alshahrani, S.M.; Hosny, K.M. Fabrication and characterization of polymeric pharmaceutical emulgel co-loaded with eugenol and linalool for the treatment of trichophyton rubrum infections. Polymers 2021, 13, 3904. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Syhr, C.; Berensmeier, S. Controlled synthesis of magnetic iron oxide nanoparticles: Magnetite or maghemite? Crystals 2020, 10, 214. [Google Scholar] [CrossRef]
- Rečnik, A.; Nyir-Kósa, I.; Dódony, I.; Pósfai, M. Growth defects and epitaxy in Fe3O4 and γ-Fe2O3 nanocrystals. CrystEngComm 2013, 15, 7539–7547. [Google Scholar] [CrossRef]
- Nemati, Z.; Khurshid, H.; Alonso, J.; Phan, M.H.; Mukherjee, P.; Srikanth, H. From core/shell to hollow Fe/γ-Fe2O3 nanoparticles: Evolution of the magnetic behavior. Nanotechnology 2015, 26, 405705. [Google Scholar] [CrossRef] [PubMed]
- Phan, M.H.; Alonso, J.; Khurshid, H.; Lampen-Kelley, P.; Chandra, S.; Repa, K.S.; Nemati, Z.; Das, R.; Iglesias, Ó.; Srikanth, H. Exchange bias effects in iron oxide-based nanoparticle systems. Nanomaterials 2016, 6, 221. [Google Scholar] [CrossRef] [PubMed]
- Slavov, L.; Abrashev, M.V.; Merodiiska, T.; Gelev, C.; Vandenberghe, R.E.; Markova-Deneva, I.; Nedkov, I. Raman spectroscopy investigation of magnetite nanoparticles in ferrofluids. J. Magn. Magn. Mater. 2010, 322, 1904–1911. [Google Scholar] [CrossRef]
- Chamritski, I.; Burns, G. Infrared- And raman-active phonons of magnetite, maghemite, and hematite: A computer simulation and spectroscopic study. J. Phys. Chem. B 2005, 109, 4965–4968. [Google Scholar] [CrossRef] [PubMed]
- Shebanova, O.N.; Lazor, P. Raman spectroscopic study of magnetite (FeFe2O4): A new assignment for the vibrational spectrum. J. Solid State Chem. 2003, 174, 424–430. [Google Scholar] [CrossRef]
- Salviano, L.B.; Silva Cardoso, T.M.D.S.; Silva, G.C.; Silva Dantas, M.S.; Mello Ferreira, A.D.M. Microstructural assessment of magnetite nanoparticles (Fe3O4) obtained by chemical precipitation under different synthesis conditions. Mater. Res. 2018, 21. [Google Scholar] [CrossRef]
- Jubb, A.M.; Allen, H.C. Vibrational spectroscopic characterization of hematite, maghemite, and magnetite thin films produced by vapor deposition. ACS Appl. Mater. Interfaces 2010, 2, 2804–2812. [Google Scholar] [CrossRef]
- Yvon, H.J. Raman Spectroscopy for Analysis and Monitoring. Horiba Jobin Yvon, Raman Appl Note. 2017, pp. 1–2. Available online: http://www.horiba.com/fileadmin/uploads/Scientific/Documents/Raman/bands.pdf (accessed on 5 February 2022).
- Mitchell, J. Microscopic Identification of Organic Compounds; Wiley: Hoboken, NJ, USA, 1949; Volume 21, pp. 448–461. [Google Scholar]
- Rasouli, E.; Basirun, W.J.; Rezayi, M.; Shameli, K.; Nourmohammadi, E.; Khandanlou, R.; Izadiyan, Z.; Sarkarizi, H.K. Ultrasmall superparamagnetic Fe3O4 nanoparticles: Honey-based green and facile synthesis and in vitro viability assay. Int. J. Nanomed. 2018, 13, 6903–6911. [Google Scholar] [CrossRef]
- Nurbas, M.; Ghorbanpoor, H.; Avci, H. An eco-friendly approach to synthesis and characterization of magnetite (Fe3O4) nanoparticles using Platanus Orientalis L. leaf extract. Dig. J. Nanomater. Biostruct. 2017, 12, 993–1000. [Google Scholar]
- Lobato, N.C.C.; Mansur, M.B.; De Mello Ferreira, A. Characterization and chemical stability of hydrophilic and hydrophobic magnetic nanoparticles. Mater. Res. 2017, 20, 736–746. [Google Scholar] [CrossRef]
- Guardia, P.; Batlle-Brugal, B.; Roca, A.G.; Iglesias, O.; Morales, M.P.; Serna, C.J.; Labarta, A.; Batlle, X. Surfactant effects in magnetite nanoparticles of controlled size. J. Magn. Magn. Mater. 2007, 316, 756–759. [Google Scholar] [CrossRef]
- Kemp, S.J.; Ferguson, R.M.; Khandhar, A.P.; Krishnan, K.M. Monodisperse magnetite nanoparticles with nearly ideal saturation magnetization. RSC Adv. 2016, 6, 77452–77464. [Google Scholar] [CrossRef]
- Wei, Y.; Han, B.; Hu, X.; Lin, Y.; Wang, X.; Deng, X. Synthesis of Fe3O4 nanoparticles and their magnetic properties. Procedia Eng. 2012, 27, 632–637. [Google Scholar] [CrossRef]
- Millan, A.; Urtizberea, A.; Silva, N.J.O.; Palacio, F.; Amaral, V.S.; Snoeck, E.; Serin, V. Surface effects in maghemite nanoparticles. J. Magn. Magn. Mater. 2007, 312, L5–L9. [Google Scholar] [CrossRef]
- Sánchez, O.; Universidad Nacional Mayor de San Marcos. Medicina (B Aires). Available online: http://cybertesis.unmsm.edu.pe/bitstream/handle/cybertesis/4147/Diaz_rc.pdf;jsessionid=CD5A7FF3022F1A5526948369A600356D?sequence=1 (accessed on 12 September 2022).
- Bedanta, S.; Petracic, O.; Kleemann, W. Supermagnetism; Elsevier: Amsterdam, The Netherlands, 2015; Volume 23. [Google Scholar]
- Livesey, K.L.; Ruta, S.; Anderson, N.R.; Baldomir, D.; Chantrell, R.W.; Serantes, D. Beyond the blocking model to fit nanoparticle ZFC/FC magnetisation curves. Sci. Rep. 2018, 8, 11166. [Google Scholar] [CrossRef] [PubMed]
- Guldris, N.; Argibay, B.; Gallo, J.; Iglesias-Rey, R.; Carbó-Argibay, E.; Kolen’Ko, Y.V.; Campos, F.; Sobrino, T.; Salonen, L.M.; Bañobre-López, M.; et al. Magnetite Nanoparticles for Stem Cell Labeling with High Efficiency and Long-Term in vivo Tracking. Bioconjug. Chem. 2017, 28, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, S.K.; Nitti, P.; Stanca, E.; Rochira, A.; Siculella, L.; Raucci, M.G.; Madaghiele, M.; Licciulli, A.; Demitri, C. Mechanical and biological properties of magnesium-and silicon-substituted hydroxyapatite scaffolds. Materials 2021, 14, 6942. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Liu, R. Superparamagnetic α-Fe2O3/Fe3O4 heterogeneous nanoparticles with enhanced biocompatibility. Nanomaterials 2021, 11, 834. [Google Scholar] [CrossRef]
- Hedayatnasab, Z.; Dabbagh, A.; Abnisa, F.; Wan Daud, W.M.A. Synthesis and in-vitro characterization of superparamagnetic iron oxide nanoparticles using a sole precursor for hyperthermia therapy. Mater. Res. Bull. 2020, 132, 110975. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Jain, N.; Parekh, K. Investigating the effect of outer layer of magnetic particles on cervical cancer cells HeLa by magnetic fluid hyperthermia. Cancer Nanotechnol. 2021, 12, 7. [Google Scholar] [CrossRef]
- Huang, J.; Luo, C.; Li, W.; Li, Y.; Zhang, Y.S.; Zhou, J.; Jiang, Q. Eccentric magnetic microcapsules for orientation-specific and dual stimuli-responsive drug release. J. Mater. Chem. B 2015, 3, 4530–4538. [Google Scholar] [CrossRef] [PubMed]
- Jangamreddy, J.R.; Los, M.J. Mitoptosis, a novel mitochondrial death mechanism leading predominantly to activation of autophagy. Hepat. Mon. 2012, 12, 10–12. [Google Scholar] [CrossRef]
- Yusefi, M.; Shameli, K.; Yee, O.S.; Teow, S.Y.; Hedayatnasab, Z.; Jahangirian, H.; Webster, T.J.; Kuča, K. Green synthesis of Fe3O4 nanoparticles stabilized by a Garcinia mangostana fruit peel extract for hyperthermia and anticancer activities. Int. J. Nanomed. 2021, 16, 2515–2532. [Google Scholar] [CrossRef] [PubMed]
- Noval, V.E.; Puentes, C.O.; Carriazo, J.G. Magnetita (Fe3O4): Uma estrutura inorgânica com múltiplas aplicações em catálise heterogénea. Rev. Colomb. Quim. 2017, 46, 42–59. [Google Scholar]
- Frey, P.A.; Reed, G.H. The ubiquity of iron. ACS Chem. Biol. 2012, 7, 1477–1481. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, C.; Pucci, C.; Ciofani, G. Nanostructured carriers as innovative tools for cancer diagnosis and therapy. APL Bioeng. 2019, 3, 011502. [Google Scholar] [CrossRef] [PubMed]
- Wiart, M.; Tavakoli, C.; Hubert, V.; Hristovska, I.; Dumot, C.; Parola, S.; Lerouge, F.; Chauveau, F.; Canet-Soulas, E.; Pascual, O.; et al. Use of metal-based contrast agents for in vivo MR and CT imaging of phagocytic cells in neurological pathologies. J. Neurosci. Methods 2023, 383, 109729. [Google Scholar] [CrossRef]
- Romero, M.P.; Marangoni, V.S.; de Faria, C.G.; Leite, I.S.; Silva, C.D.C.C.E.; Maroneze, C.M.; Pereira-da-Silva, M.A.; Bagnato, V.S.; Inada, N.M. Graphene Oxide Mediated Broad-Spectrum Antibacterial Based on Bimodal Action of Photodynamic and Photothermal Effects. Front. Microbiol. 2020, 10, 2995. [Google Scholar] [CrossRef]
- Ashkbar, A.; Rezaei, F.; Attari, F.; Ashkevarian, S. Treatment of breast cancer in vivo by dual photodynamic and photothermal approaches with the aid of curcumin photosensitizer and magnetic nanoparticles. Sci. Rep. 2020, 10, 21206. [Google Scholar] [CrossRef]
- Sundaram, P.; Abrahamse, H. Phototherapy combined with carbon nanomaterials (1D and 2D) and their applications in cancer therapy. Materials 2020, 13, 4830. [Google Scholar] [CrossRef]
- Qing, G.; Zhao, X.; Gong, N.; Chen, J.; Li, X.; Gan, Y.; Wang, Y.; Zhang, Z.; Zhang, Y.; Guo, W.; et al. Thermo-responsive triple-function nanotransporter for efficient chemo-photothermal therapy of multidrug-resistant bacterial infection. Nat. Commun. 2019, 10, 4336. [Google Scholar] [CrossRef] [PubMed]
- Mascolo, M.C.; Pei, Y.; Ring, T.A. Room Temperature Co-Precipitation Synthesis of Magnetite Nanoparticles in a Large ph Window with Different Bases. Materials 2013, 6, 5549–5567. [Google Scholar] [CrossRef] [PubMed]
- Hussein-Al-Ali, S.H.; El Zowalaty, M.E.; Hussein, M.Z.; Geilich, B.M.; Webster, T.J. Synthesis, characterization, and antimicrobial activity of an ampicillin-conjugated magnetic nanoantibiotic for medical applications. Int. J. Nanomed. 2014, 9, 3801–3814. [Google Scholar] [CrossRef]
- Luz, M.; Pisciotti, M. Estudio del proceso de calentamiento de nanopartículas magnéticas con campos magnéticos AC para su utilización en el tratamiento de tumores por hipertermia. Ph.D. Thesis, Universidad Nacional de Cuyo, Mendoza, Argentina, 2009. [Google Scholar]
- Zheng, B.; Zhang, M.; Xiao, D.; Jin, Y.; Choi, M.M.F. Fast microwave synthesis of Fe3O4 and Fe3O4/Ag magnetic nanoparticles using Fe2+ as precursor. Inorg. Mater. 2010, 46, 1106–1111. [Google Scholar] [CrossRef]
- Alamar, B. Resazurin Cell Viability Assay Kit; Biotium Glowing Products for Science: Fremont, CA, USA, 2015; Volume 14998, pp. 1919–1920. [Google Scholar]
- Bioarray, C. Resazurin Cell Viability Assay. 2022. Available online: https://www.creative-bioarray.com/support/resazurin-cell-viability-assay.htm (accessed on 12 September 2022).
| Sample | Mass Yield | Phases |
|---|---|---|
| 0GS-MNPs-Fe | 1.12% | 35.3% Magnetite, 64.7% Hematite |
| 25GS-MNPs-Fe | 3.71% | 39.5% Magnetite, 53% Maghemite, 7.5% Hematite |
| 50GS-MNPs-Fe | 29.36% | 47.0% Magnetite, 53.0% Maghemite |
| 75GS-MNPs-Fe | 18.40% | 47.0% Magnetite, 53.0% Maghemite |
| 100GS-MNPs-Fe | 91.50% | 6.6% Magnetite, 93.4% Maghemite |
| 25CO-MNPs-Fe | 89.08% | 88.0% Magnetite, 12.0% Maghemite |
| 50CO-MNPs-Fe | 80.15% | 63.5% Magnetite, 36.5% Maghemite |
| 75CO-MNPs-Fe | 74.69% | 8.9% Magnetite, 91.1% Maghemite |
| 100CO-MNPs-Fe | 72.56% | 98.0% Magnetite, 2.0% Maghemite |
| 25MW-MNPs-Fe | 87.73% | 34.1% Magnetite, 65.9% Maghemite |
| 50MW-MNPs-Fe | 89.59% | 91.2% Magnetite, 8.8% Maghemite |
| 75MW-MNPs-Fe | 93.43% | 10.0% Magnetite, 90.0% Maghemite |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, D.G.; Garzón-Romero, C.; Salazar, M.A.; Lagos, K.J.; Campaña, K.O.; Debut, A.; Vizuete, K.; Rivera, M.R.; Niebieskikwiat, D.; Benitez, M.J.; et al. Bioinspired Synthesis of Magnetic Nanoparticles Based on Iron Oxides Using Orange Waste and Their Application as Photo-Activated Antibacterial Agents. Int. J. Mol. Sci. 2023, 24, 4770. https://doi.org/10.3390/ijms24054770
García DG, Garzón-Romero C, Salazar MA, Lagos KJ, Campaña KO, Debut A, Vizuete K, Rivera MR, Niebieskikwiat D, Benitez MJ, et al. Bioinspired Synthesis of Magnetic Nanoparticles Based on Iron Oxides Using Orange Waste and Their Application as Photo-Activated Antibacterial Agents. International Journal of Molecular Sciences. 2023; 24(5):4770. https://doi.org/10.3390/ijms24054770
Chicago/Turabian StyleGarcía, David Giancarlo, Cristina Garzón-Romero, Mateo Alejandro Salazar, Karina J. Lagos, Kleber Orlando Campaña, Alexis Debut, Karla Vizuete, Miryan Rosita Rivera, Dario Niebieskikwiat, Maria J. Benitez, and et al. 2023. "Bioinspired Synthesis of Magnetic Nanoparticles Based on Iron Oxides Using Orange Waste and Their Application as Photo-Activated Antibacterial Agents" International Journal of Molecular Sciences 24, no. 5: 4770. https://doi.org/10.3390/ijms24054770
APA StyleGarcía, D. G., Garzón-Romero, C., Salazar, M. A., Lagos, K. J., Campaña, K. O., Debut, A., Vizuete, K., Rivera, M. R., Niebieskikwiat, D., Benitez, M. J., & Romero, M. P. (2023). Bioinspired Synthesis of Magnetic Nanoparticles Based on Iron Oxides Using Orange Waste and Their Application as Photo-Activated Antibacterial Agents. International Journal of Molecular Sciences, 24(5), 4770. https://doi.org/10.3390/ijms24054770









