Proteomics as a New-Generation Tool for Studying Moulds Related to Food Safety and Quality
Abstract
1. Introduction
2. Problems Associated with Moulds in Foodstuffs
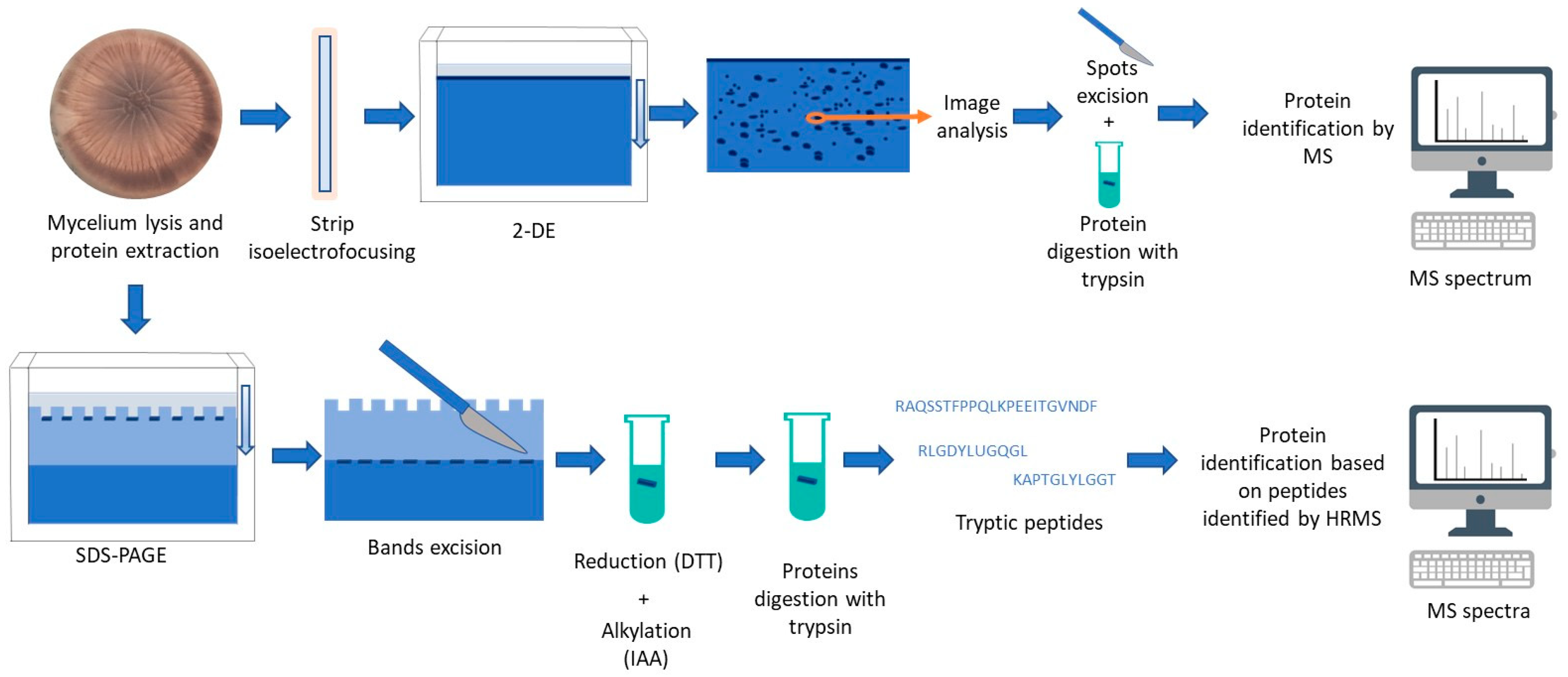
3. Main Characteristics of Proteomics Applied to Moulds in Food
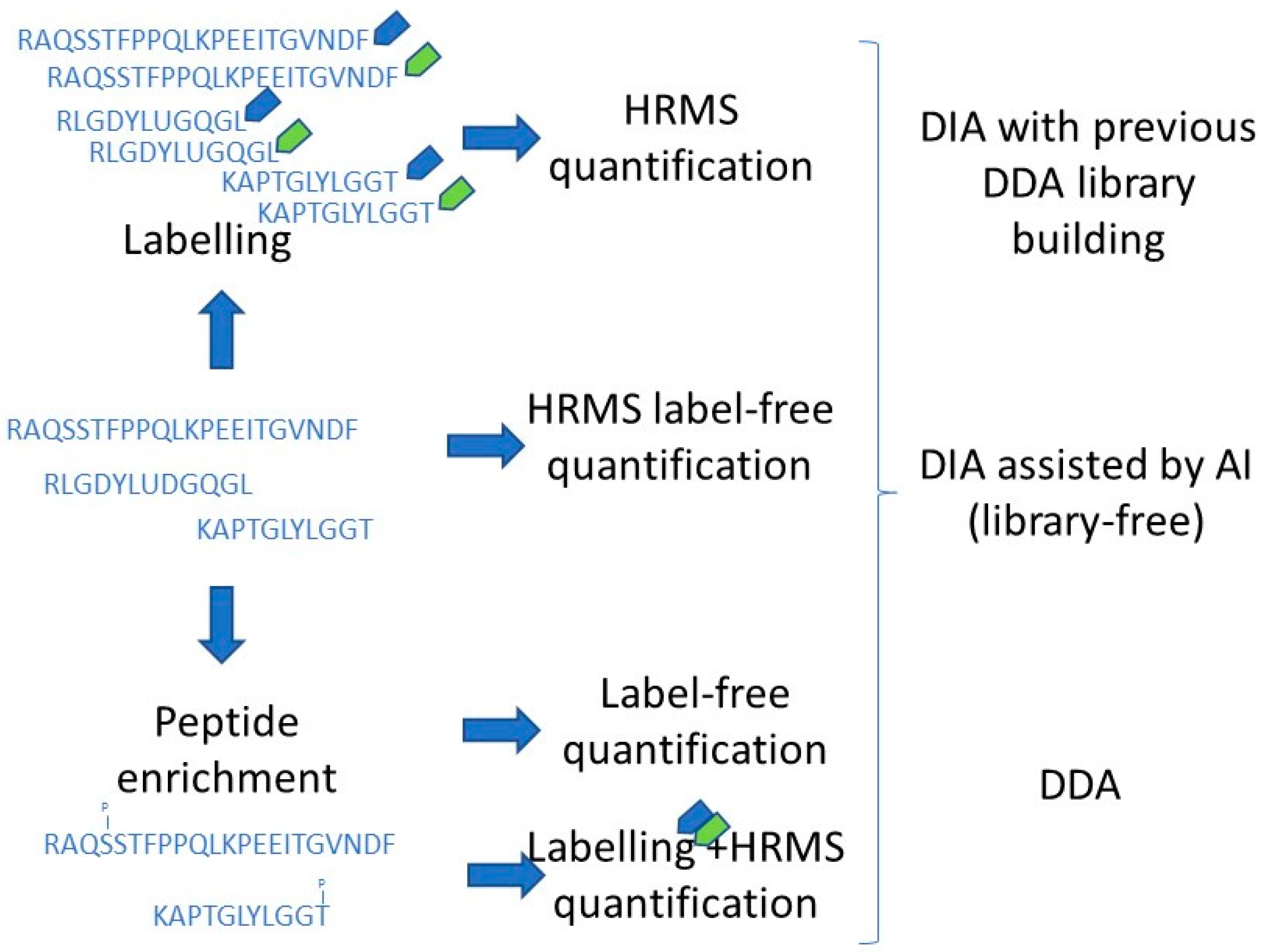
4. Proteomic Strategies for Improving Food Safety and Quality
4.1. Detection and Identification of Moulds
4.2. Study of Mould Growth and Physiology
| Mould | Purpose | Proteomic Methodology Used | Main Proteomic Findings | References |
|---|---|---|---|---|
| Aspergillus flavus | Proteome profile of mycelium | 2-DE and mass spectrometry | Proteins from cellular metabolic process, and AFs a biosynthesis are prevalent. | [96] |
| A. flavus | Proteome during germination of conidia | LC-mass spectrometry | AFs biosynthesis, carbohydrate metabolism, cell wall biogenesis, MAPK pathways, heat shock, autophagy, and dicer-like proteins are expressed at germinating conidia. | [97] |
| A. flavus | Proteome on corn flour | LC-mass spectrometry | ↑ AFs biosynthesis proteins. | [100] |
| A. flavus | Influence of water activity (aw; 0.99 vs. 0.93) | LC-mass spectrometry | ↑ Proteins related to osmotic stress and Afs biosynthesis at 0.99 aw. | [98] |
| A. flavus | Influence of temperature (28 °C vs. 37 °C) | LC-mass spectrometry | Changes on proteins belonging to translation pathways, metabolic pathways, and secondary metabolites biosynthesis. | [55] |
| A. flavus | Influence of temperature (28 °C vs. 37 °C) | LC-mass spectrometry | Changes in abundance of 49 proteins, including AFs pathway, leading to repression of AFs biosynthesis at 37 °C. | [99] |
| A. flavus | Influence of substrate (maize/rice vs. peanut) on AFs production | LC-mass spectrometry | Few differences in protein abundances between crops. Low correlation between transcriptome and proteome data. | [58] |
| Aspergillus carbonarius | Proteomic profile according to OTA b-producing potential | 2-DE and mass spectrometry | CipC protein likely involved in OTA biosynthesis. | [14] |
| Aspergillus ochraceus | Influence of NaCl content (20 g/L vs. 70 g/L) on OTA production | LC-mass spectrometry | Changes in proteins involved in nutrient uptake, membrane integrity, cell cycle, energy metabolism, redox homeostasis, protein synthesis, autophagy, and secondary metabolism. ↑ OTA with 20 g/L NaCl. | [62] |
| Aspergillus niger | Influence of the substrate and culture conditions | 2-DE and mass spectrometry | Extracellular proteome mainly influenced by the carbon source and intracellular proteome by the environmental conditions. | [101] |
| A. niger | Influence of addition of lactate on mycotoxin production | 2-DE and mass spectrometry | Changes in proteins related to acetyl-CoA or NADPH, pentose phosphate pathway, pyruvate metabolism, tricarboxylic acid cycle, ammonium assimilation, fatty acid biosynthesis, and oxidative stress protection. ↑ Fumonisin B2; ↓ OTA. | [102] |
| Penicillium digitatum | Effect of limonene on the growth | LC-mass spectrometry | Changes in proteins related to energy metabolism and antioxidant defence. | [103] |
| Penicillium expansum | Interaction with apple | 2-DE and mass spectrometry | ↑ Proteins related to pathogenesis, secondary metabolism, and patulin biosynthesis. | [7] |
| Interaction with apple | LC-mass spectrometry | ↑ Proteins related to growth, stress tolerance, and virulence. | [104] | |
| Penicillium verrucosum | Effect of short wavelength light | 2-DE and mass spectrometry | Changes in proteins involved in stress response and metabolic processes. ↑ Citrinin; ↓ OTA. | [9] |
| Fusarium proliferatum | Influence of pH (5 vs. 10) on fumonisin production | 2-DE and mass spectrometry | ↑ Proteins involved in fumonisin backbone modification and ↑ fumonisin at pH 10. | [105] |
| Neosartorya pseudofischeri | Resistance to heat (93 °C) | LC-mass spectrometry | ↓ Proteins involved in carbon metabolism, heat stress responses, ROS c elimination, and translation pathways. | [106] |
4.3. Mode of Action of Antifungal Agents against Foodborne Moulds
| Antifungal Agent | Target Mould | Proteomic Method Used | Main Proteomic Findings | References |
|---|---|---|---|---|
| Penicillium chrysogenum | Penicillium nordicum | 2-DE and LC-mass spectrometry | ↓ CWI a and secondary metabolites biosynthesis proteins. | [64] |
| Debaryomyces hansenii | P. nordicum | 2-DE and LC-mass spectrometry | ↓ CWI and secondary metabolites biosynthesis proteins. | [64] |
| D. hansenii | Aspergillus westerdijkiae | LC-mass spectrometry | ↓ CWI and OTA b biosynthesis proteins. | [43] |
| Candida intermedia | Aspergillus carbonarius | LC-mass spectrometry | ↓ Central metabolism, energy production, and stress-response proteins. | [46] |
| Rosmarinus officinalis | P. nordicum | LC-mass spectrometry | ↓ OTA biosynthesis. | [44] |
| R. officinalis | A. westerdijkiae | LC-mass spectrometry | ↓ CWI and OTA biosynthesis proteins. | [70] |
| Protein PgAFP | Aspergillus flavus | 2-DE and LC-mass spectrometry | ↑ Stress-response proteins. ↓ CWI proteins. | [63] |
| Protein PgAFP | A. flavus cultured on calcium-enriched substrate | 2-DE and LC-mass spectrometry | ↑ Oxidative stress-response proteins and secondary metabolites biosynthesis. | [67] |
| Protein PgAFP | Penicillium polonicum | 2-DE and LC-mass spectrometry | ↑ CWI proteins. | [66] |
| Quercetin | A. flavus | LC-mass spectrometry | ↑ Oxidative stress-response proteins. ↓ MAPK pathway and AFs c biosynthesis proteins. | [100] |
| Perilla frutescens | A. flavus | LC-mass spectrometry | ↓ Antioxidative and glycolysis pathway proteins. ↑ Fatty acid, amino acid, pyruvate, and glyoxylic acid metabolism proteins. | [2] |
| α-sarcin and beetin 27 proteins | Penicillium digitatum | LC-mass spectrometry | Changes on cell wall-degrading, stress response, antioxidant, and detoxification mechanisms and metabolic processes proteins. | [112] |
| 2-methoxy-1,4-naphthoquinone | Penicillium italicum | LC-mass spectrometry | Changes on energy generation, NADPH supply, oxidative stress, and pentose phosphate pathway proteins. | [113] |
| Pinocembrin | P. italicum | LC-mass spectrometry | Changes on mitochondrial respiratory chain complexes I and V. ↑ PCD d-related proteins. | [114] |
| Tea tree oil | Botrytis cinerea | LC-mass spectrometry | ↓ TCA e, pyruvate, and amino acid metabolism, and membrane-related pathways. ↑ Sphingolipid metabolism. | [48] |
| Chitosan | Fusarium oxysporum | 2-DE and mass spectrometry | ↓ Virulence proteins and ROS f-degrading enzymes. | [115] |
| Chitosan | Antifungal activity of Rhodotorula mucilaginosa | LC-mass spectrometry | ↑ Energy metabolism, antioxidant and abiotic stress response, and degradation of pathogen cell. | [71] |
| Vitamin C | Antifungal activity of Pichia caribbica | 2-DE and mass spectrometry | ↑ Glucose metabolism enzymes. | [45] |
| Wickerhamomyces anomalus | Resistance of pear to fungi | 2-DE and mass spectrometry | ↑ Resistance-related proteins | [116] |
5. Emerging Proteomics to Be Exploited in Foodstuffs
6. Conclusions and Future Trends
Author Contributions
Funding
Conflicts of Interest
References
- Todrá, F.; Nollet, L.M.L. Proteomics in Food, Priniples and Application, 1st ed.; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-5625-4. [Google Scholar]
- Hu, Z.; Lu, C.; Zhang, Y.; Tong, W.; Du, L.; Liu, F. Proteomic analysis of Aspergillus flavus reveals the antifungal action of Perilla frutescens essential oil by interfering with energy metabolism and defense function. LWT—Food Sci. Technol. 2022, 154, 112660. [Google Scholar] [CrossRef]
- Shi, C.; Maktabdar, M. Lactic acid bacteria as biopreservation against spoilage molds in dairy products—A review. Front. Microbiol. 2022, 12, 4283. [Google Scholar] [CrossRef]
- European Community’s Rapid Alert System for Food and Feed (RASFF). Available online: https://webgate.ec.europa.eu/rasff-window/screen/search (accessed on 17 January 2023).
- Merrill, S.A.; Mazza, A.-M. Reaping the Benefits of Genomic and Proteomic Research: Intellectual Property Rights, Innovation and Public Health; The National Academies Press: Washington, DC, USA, 2006; ISBN 0-309-10067-4. [Google Scholar]
- Yang, L.B.; Dai, X.M.; Zheng, Z.Y.; Zhu, L.; Zhan, X.B.; Lin, C.C. Proteomic analysis of erythritol-producing Yarrowia lipolytica from glycerol in response to osmotic pressure. J. Microbiol. Biotechnol. 2015, 25, 1056–1069. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Li, H.; Liu, F.; Zhang, Y.; Zhang, Q.; Wang, Y.; Li, P. Proteome changes in Penicillium expansum grown in a medium derived from host plant. J. Microbiol. Biotechnol. 2017, 27, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yi, X.; Peng, M.; Zeng, H.; Wang, D.; Li, B.; Tong, Z.; Chang, L.; Jin, X.; Wang, X. Proteomics of Fusarium oxysporum race 1 and race 4 reveals enzymes involved in carbohydrate metabolism and ion transport that might play important roles in banana Fusarium wilt. PLoS ONE 2014, 9, e0113818. [Google Scholar] [CrossRef] [PubMed]
- Stoll, D.A.; Link, S.; Kulling, S.; Geisen, R.; Schmidt-Heydt, M. Comparative proteome analysis of Penicillium verrucosum grown under light of short wavelength shows an induction of stress-related proteins associated with modified mycotoxin biosynthesis. Int. J. Food Microbiol. 2014, 175, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Retanal, C.; Ball, B.; Geddes-Mcalister, J. Post-translational modifications drive success and failure of fungal–host interactions. J. Fungi 2021, 7, 124. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, S.K.; Jung, K.H.; Kim, Y.K.; Hwang, H.C.; Ryu, S.G.; Chai, Y.G. Proteomic analysis of the oxidative stress response induced by low-dose hydrogen peroxide in Bacillus anthracis. J. Microbiol. Biotechnol. 2013, 23, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, D.; Forshey, K.L.; Grimsrud, P.A.; Ané, J.M. Leveraging proteomics to understand plant-microbe interactions. Front. Plant Sci. 2012, 3, 44. [Google Scholar] [CrossRef]
- Giacometti, J.; Tomljanović, A.B.; Josić, D. Application of proteomics and metabolomics for investigation of food toxins. Food Res. Int. 2013, 54, 1042–1051. [Google Scholar] [CrossRef]
- Crespo-Sempere, A.; Gil, J.V.; Martínez-Culebras, P.V. Proteome analysis of the fungus Aspergillus carbonarius under ochratoxin A producing conditions. Int. J. Food Microbiol. 2011, 147, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Akhila, P.P.; Sunooj, K.V.; Navaf, M.; Aaliya, B.; Sudheesh, C.; Sasidharan, A.; Sabu, S.; Mir, S.A.; George, J.; Mousavi Khaneghah, A. Application of innovative packaging technologies to manage fungi and mycotoxin contamination in agricultural products: Current status, challenges, and perspectives. Toxicon 2022, 214, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Sperber, W.H.; Doyle, M.P. Compendium of the Microbiological Spoilage of Foods and Beverages; Springer: New York, NY, USA, 2010; ISBN 978-1-4419-0825-4. [Google Scholar]
- Kilani, J.; Davanture, M.; Simon, A.; Zivy, M.; Fillinger, S. Comparative quantitative proteomics of osmotic signal transduction mutants in Botrytis cinerea explain mutant phenotypes and highlight interaction with cAMP and Ca2+ signalling pathways. J. Proteom. 2020, 212, 103580. [Google Scholar] [CrossRef] [PubMed]
- García, M.V.; Bernardi, A.O.; Copetti, M.V. The fungal problem in bread production: Insights of causes, consequences, and control methods. Curr. Opin. Food Sci. 2019, 29, 1–6. [Google Scholar] [CrossRef]
- Barman, S.; Ghosh, R.; Sengupta, S.; Mandal, N.C. Longterm storage of post-packaged bread by controlling spoilage pathogens using Lactobacillus fermentum C14 isolated from homemade curd. PLoS ONE 2017, 12, e0184020. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Islam, R.; Hasan, S.; Zzaman, W.; Rana, M.R.; Ahmed, S.; Roy, M.; Sayem, A.; Matin, A.; Raposo, A.; et al. A comprehensive review on bio-preservation of bread: An approach to adopt wholesome strategies. Foods 2022, 11, 319. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Ge, B.; Wang, J.; Liu, B.; Ma, J.; Wei, Q.; Zhang, K. iTRAQ-based proteomic analysis reveals the mechanisms of Botrytis cinerea controlled with Wuyiencin. BMC Microbiol. 2019, 19, 280. [Google Scholar] [CrossRef]
- Meng, F.; Lv, R.; Cheng, M.; Mo, F.; Zhang, N.; Qi, H.; Liu, J.; Chen, X.; Liu, Y.; Ghanizadeh, H.; et al. Insights into the molecular basis of biocontrol of Botrytis cinerea by Clonostachys rosea in tomato. Sci. Hortic. 2022, 291, 110547. [Google Scholar] [CrossRef]
- De Simone, N.; Pace, B.; Grieco, F.; Chimienti, M.; Tyibilika, V.; Santoro, V.; Capozzi, V.; Colelli, G.; Spano, G.; Russo, P. Botrytis cinerea and table grapes: A review of the main physical, chemical, and bio-based control treatments in post-harvest. Foods 2020, 9, 1138. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Janisiewicz, W.J.; Nichols, B.; Bauchan, G.; Chao, T.C.; Jurick, W.M. Wound responses of wild apples suggest multiple resistance mechanism against blue mold decay. Postharvest Biol. Technol. 2016, 117, 132–140. [Google Scholar] [CrossRef]
- Alía, A.; Andrade, M.J.; Rodríguez, A.; Reyes-Prieto, M.; Bernáldez, V.; Córdoba, J.J. Identification and control of moulds responsible for black spot spoilage in dry-cured ham. Meat Sci. 2016, 122, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Ojalvo, D.; Rodríguez, A.; Cordero, M.; Bernáldez, V.; Reyes-Prieto, M.; Córdoba, J.J. Characterisation and detection of spoilage mould responsible for black spot in dry-cured fermented sausages. Meat Sci. 2015, 100, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.O.; Lorwy, P.D.; Di Menna, M.E. A note on the identities of organisms causing black spot spoilage of meat. J. Appl. Bacteriol. 1981, 51, 183–187. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Caruso, G.; Cavaliere, C.; Foglia, P.; Samperi, R.; Lagana, A. Multiclass mycotoxin analysis in food, environmental and biological matrices with chromatography/mass spectrometry. Mass Spectrom. Rev. 2011, 31, 466–503. [Google Scholar] [CrossRef] [PubMed]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Escrivá, L.; Oueslati, S.; Font, G.; Manyes, L. Alternaria mycotoxins in food and feed: An overview. J. Food Qual. 2017, 2017, 1569748. [Google Scholar] [CrossRef]
- Ksenija, N. Mycotoxins—Climate impact and steps to prevention based on prediction. Acta Vet. Brno. 2018, 68, 1–15. [Google Scholar] [CrossRef]
- El-Sayed, R.A.; Jebur, A.B.; Kang, W.; El-Demerdash, F.M. An overview on the major mycotoxins in food products: Characteristics, toxicity, and analysis. J. Futur. Foods 2022, 2, 91–102. [Google Scholar] [CrossRef]
- Oguz, H. Mikotoksinler ve Önemi. Turkiye Klin. Vet. Sci. Pharm. Toxicol. 2017, 3, 113–119. [Google Scholar]
- Zadravec, M.; Markov, K.; Lešić, T.; Frece, J.; Petrović, D.; Pleadin, J. Biocontrol methods in avoidance and downsizing of mycotoxin contamination of food crops. Processes 2022, 10, 655. [Google Scholar] [CrossRef]
- Chiminelli, I.; Spicer, L.J.; Maylem, E.R.S.; Caloni, F. Emerging mycotoxins and reproductive effects in animals: A short review. J. Appl. Toxicol. 2022, 42, 1901–1909. [Google Scholar] [CrossRef]
- Van Egmond, H.P.; Schothorst, R.C.; Jonker, M.A. Regulations relating to mycotoxins in food: Perspectives in a global and European context. Anal. Bioanal. Chem. 2007, 389, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Zingales, V.; Taroncher, M.; Martino, P.A.; Ruiz, M.J.; Caloni, F. Climate change and effects on molds and mycotoxins. Toxins 2022, 14, 445. [Google Scholar] [CrossRef] [PubMed]
- Ngum, N.Q.; Babalola, O.O.; Ekwomadu, T.I.; Nleya, N.; Mulunda, M. Six main contributing factors to high levels of mycotoxin contamination in African foods. Toxins 2022, 14, 318. [Google Scholar] [CrossRef]
- Logrieco, A.F.; Miller, J.D.; Eskola, M.; Krska, R.; Ayalew, A.; Bandyopadhyay, R.; Battilani, P.; Bhatnagar, D.; Chulze, S.; De Saeger, S.; et al. The mycotox charter: Increasing awareness of, and concerted action for, minimizing mycotoxin exposure worldwide. Toxins 2018, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- European Commission Commission Regulation (EC) No 118/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 5–24. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32006R1881 (accessed on 17 January 2023).
- Martín-Sánchez, A.M.; Chaves-López, C.; Sendra, E.; Sayas, E.; Fenández-López, J.; Pérez-Álvarez, J.Á. Lipolysis, proteolysis and sensory characteristics of a Spanish fermented dry-cured meat product (salchichón) with oregano essential oil used as surface mold inhibitor. Meat Sci. 2011, 89, 35–44. [Google Scholar] [CrossRef]
- Álvarez, M.; Núñez, F.; Delgado, J.; Andrade, M.J.; Rodrigues, P. Proteomic evaluation of the effect of antifungal agents on Aspergillus westerdijkiae ochratoxin A production in a dry-cured fermented sausage-based medium. Int. J. Food Microbiol. 2022, 379, 109858. [Google Scholar] [CrossRef]
- Álvarez, M.; Delgado, J.; Núñez, F.; Cebrián, E.; Andrade, M.J. Proteomic analyses reveal mechanisms of action of biocontrol agents on ochratoxin A repression in Penicillium nordicum. Food Control 2021, 129, 108232. [Google Scholar] [CrossRef]
- Yang, Q.; Diao, J.; Solairaj, D.; Ngea Guillaume Legrand, N.; Zhang, H. Investigating possible mechanisms of Pichia caribbica induced with ascorbic acid against postharvest blue mold of apples. Biol. Control 2020, 141, 104129. [Google Scholar] [CrossRef]
- Tilocca, B.; Balmas, V.; Hassan, Z.U.; Jaoua, S.; Migheli, Q. A proteomic investigation of Aspergillus carbonarius exposed to yeast volatilome or to its major component 2-phenylethanol reveals major shifts in fungal metabolism. Int. J. Food Microbiol. 2019, 306, 108265. [Google Scholar] [CrossRef] [PubMed]
- Prabawati, E.K.; Turner, M.S.; Bansal, N. Lactiplantibacillus plantarum as an adjunct culture exhibits antifungal activity in shredded Cheddar cheese. Food Control 2023, 144, 109330. [Google Scholar] [CrossRef]
- Wang, N.; Shao, X.; Wei, Y.; Jiang, S.; Xu, F.; Wang, H. Quantitative proteomics reveals that tea tree oil effects Botrytis cinerea mitochondria function. Pestic. Biochem. Physiol. 2020, 164, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.; Gilbert, M.K.; Mack, B.M.; OBrian, G.R.; Rodríguez, A.; Bhatnagar, D.; Payne, G.; Magan, N. Interactions between water activity and temperature on the Aspergillus flavus transcriptome and aflatoxin B1 production. Int. J. Food Microbiol. 2017, 256, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Samaras, A.; Ntasiou, P.; Myresiotis, C.; Karaoglanidis, G. Multidrug resistance of Penicillium expansum to fungicides: Whole transcriptome analysis of MDR strains reveals overexpression of efflux transporter genes. Int. J. Food Microbiol. 2020, 335, 108896. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lu, L.; Li, S.; Zhang, J.; Li, Z.; Wu, S.; Guo, Q.; Liu, H.; Wang, C. Transcriptomic insights into benzenamine effects on the development, aflatoxin biosynthesis, and virulence of Aspergillus flavus. Toxins 2019, 11, 70. [Google Scholar] [CrossRef]
- Álvarez, M.; Rodríguez, A.; Núñez, F.; Silva, A.; Andrade, M.J. In vitro antifungal effects of spices on ochratoxin A production and related gene expression in Penicillium nordicum on a dry-cured fermented sausage medium. Food Control 2020, 114, 107222. [Google Scholar] [CrossRef]
- da Cruz Cabral, L.; Delgado, J.; Patriarca, A.; Rodríguez, A. Differential response to synthetic and natural antifungals by Alternaria tenuissima in wheat simulating media: Growth, mycotoxin production and expression of a gene related to cell wall integrity. Int. J. Food Microbiol. 2019, 292, 48–55. [Google Scholar] [CrossRef]
- Wilmes, A.; Limonciel, A.; Aschauer, L.; Moenks, K.; Bielow, C.; Leonard, M.O.; Hamon, J.; Carpi, D.; Ruzek, S.; Handler, A.; et al. Application of integrated transcriptomic, proteomic and metabolomic profiling for the delineation of mechanisms of drug induced cell stress. J. Proteom. 2013, 79, 180–194. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, S.; Zhong, H.; Yang, Q.; Zhang, F.; Zhuang, Z.; Yuan, J.; Nie, X.; Wang, S. Integrative analyses reveal transcriptome-proteome correlation in biological pathways and secondary metabolism clusters in A. flavus in response to temperature. Sci. Rep. 2015, 5, 14582. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, G.; Hammel, S.; Owens, R.A.; Keane, T.M.; Fitzpatrick, D.A.; Jones, G.W.; Doyle, S. RNA-seq reveals the pan-transcriptomic impact of attenuating the gliotoxin self-protection mechanism in Aspergillus fumigatus. BMC Genom. 2014, 15, 894. [Google Scholar] [CrossRef] [PubMed]
- Barker, B.M.; Kroll, K.; Vödisch, M.; Mazurie, A.; Kniemeyer, O.; Cramer, R.A. Transcriptomic and proteomic analyses of the Aspergillus fumigatus hypoxia response using an oxygen-controlled fermenter. BMC Genom. 2012, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, Y.; Ma, L.; Ma, X.; Liu, Y.; Shan, J.; Ma, K.; Xing, F. Comprehensive transcriptome and proteome analyses reveal the modulation of aflatoxin production by Aspergillus flavus on different crop substrates. Front. Microbiol. 2020, 11, 1497. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, M.; Zhang, C.; Cheng, H.; Gao, Y.; Deng, W.; Li, T. Transcriptome and proteome analyses reveal the regulatory networks and metabolite biosynthesis pathways during the development of Tolypocladium guangdongense. Comput. Struct. Biotechnol. J. 2020, 18, 2081–2094. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Vieira, K.C.; da Silva, H.R.A.; Rocha, I.P.M.; Barboza, E.; Eller, L.K.W. Foodborne pathogens in the omics era. Crit. Rev. Food Sci. Nutr. 2021, 62, 6726–6741. [Google Scholar] [CrossRef] [PubMed]
- Crowther, T.W.; Boddy, L.; Maynard, D.S. The use of artificial media in fungal ecology. Fungal Ecol. 2018, 32, 87–91. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, W.; Yan, H.; Neng, J.; Zheng, Y.; Yang, K.; Xing, F.; Sun, P. iTRAQ proteome analysis of the antifungal mechanism of citral on mycelial growth and OTA production in Aspergillus ochraceus. J. Sci. Food Agric. 2021, 101, 4969–4979. [Google Scholar] [CrossRef]
- Delgado, J.; Owens, R.; Doyle, S.; Asensio, M.A.; Núñez, F. Impact of the antifungal protein PgAFP from Penicillium chrysogenum on the protein profile in Aspergillus flavus. Appl. Microbiol. Biotechnol. 2015, 99, 8701–8715. [Google Scholar] [CrossRef]
- Delgado, J.; Núñez, F.; Asensio, M.A.; Owens, R.A. Quantitative proteomic profiling of ochratoxin A repression in Penicillium nordicum by protective cultures. Int. J. Food Microbiol. 2019, 305, 108243. [Google Scholar] [CrossRef]
- Graves, P.R.; Haystead, T.A.J. Molecular biologist’s guide to proteomics. Microbiol. Mol. Biol. Rev. 2002, 66, 39–63. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.; Owens, R.A.; Doyle, S.; Asensio, M.A.; Núñez, F. Increased chitin biosynthesis contributes to the resistance of Penicillium polonicum against the antifungal protein PgAFP. Appl. Microbiol. Biotechnol. 2016, 100, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.; Owens, R.A.; Doyle, S.; Núñez, F.; Asensio, M.A. Quantitative proteomics reveals new insights into calcium-mediated resistance mechanisms in Aspergillus flavus against the antifungal protein PgAFP in cheese. Food Microbiol. 2017, 66, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.R.; Zhou, W.B.; Yang, X.L.; Tong, A.J.; Hong, J.L.; Guo, W.L.; Li, T.T.; Jia, R.B.; Pan, Y.Y.; Lin, J.; et al. The regulation mechanisms of soluble starch and glycerol for production of azaphilone pigments in Monascus purpureus FAFU618 as revealed by comparative proteomic and transcriptional analyses. Food Res. Int. 2018, 106, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Mironenka, J.; Różalska, S.; Bernat, P. Potential of Trichoderma harzianum and its metabolites to protect wheat seedlings against Fusarium culmorum and 2,4-D. Int. J. Mol. Sci. 2021, 22, 13058. [Google Scholar] [CrossRef]
- Álvarez, M.; Andrade, M.J.; Delgado, J.; Núñez, F.; Román, Á.-C.; Rodrigues, P. Rosmarinus officinalis reduces the ochratoxin A production by Aspergillus westerdijkiae in a dry-cured fermented sausage-based medium. Food Control 2023, 145, 109436. [Google Scholar] [CrossRef]
- Gu, N.; Zhang, X.; Gu, X.; Zhao, L.; Dhanasekaran, S.; Qian, X.; Zhang, H. Proteomic analysis reveals the mechanisms involved in the enhanced biocontrol efficacy of Rhodotorula mucilaginosa induced by chitosan. Biol. Control 2020, 149, 104325. [Google Scholar] [CrossRef]
- Bowman, A.P.; Sawicki, J.; Talaty, N.N.; Buck, W.R.; Yang, J.; Wagner, D.S. Evaluation of quantitative platforms for single target mass spectrometry imaging. Pharmaceuticals 2022, 15, 1180. [Google Scholar] [CrossRef]
- Lesur, A.; Schmit, P.O.; Bernardin, F.; Letellier, E.; Brehmer, S.; Decker, J.; Dittmar, G. Highly multiplexed targeted proteomics acquisition on a TIMS-QTOF. Anal. Chem. 2021, 93, 1383–1392. [Google Scholar] [CrossRef]
- Chalupová, J.; Raus, M.; Sedlářová, M.; Šebela, M. Identification of fungal microorganisms by MALDI-TOF mass spectrometry. Biotechnol. Adv. 2014, 32, 230–241. [Google Scholar] [CrossRef]
- Bahule, C.E.; Martins, L.H.d.S.; Chaúque, B.J.M.; Lopes, A.S. Metaproteomics as a tool to optimize the maize fermentation process. Trends Food Sci. Technol. 2022, 129, 258–265. [Google Scholar] [CrossRef]
- Tomé, L.M.R.; Badotti, F.; Assis, G.B.N.; Fonseca, P.L.C.; da Silva, G.A.; da Silveira, R.M.B.; Costa-Rezende, D.H.; dos Santos, E.R.D.; de Carvalho Azevedo, V.A.; Figueiredo, H.C.P.; et al. Proteomic fingerprinting for the fast and accurate identification of species in the Polyporoid and Hymenochaetoid fungi clades. J. Proteom. 2019, 203, 103390. [Google Scholar] [CrossRef] [PubMed]
- Akimowicz, M.; Bucka-Kolendo, J. MALDI-TOF MS-Application in food microbiology. Acta Biochim. Pol. 2020, 67, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Quéro, L.; Courault, P.; Cellière, B.; Lorber, S.; Jany, J.L.; Puel, O.; Girard, V.; Vasseur, V.; Nodet, P.; Mounier, J. Application of MALDI-TOF MS to species complex differentiation and strain typing of food related fungi: Case studies with Aspergillus section Flavi species and Penicillium roqueforti isolates. Food Microbiol. 2020, 86, 103311. [Google Scholar] [CrossRef] [PubMed]
- Quéro, L.; Girard, V.; Pawtowski, A.; Tréguer, S.; Weill, A.; Arend, S.; Cellière, B.; Polsinelli, S.; Monnin, V.; van Belkum, A.; et al. Development and application of MALDI-TOF MS for identification of food spoilage fungi. Food Microbiol. 2019, 81, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Wigmann, É.F.; Behr, J.; Vogel, R.F.; Niessen, L. MALDI-TOF MS fingerprinting for identification and differentiation of species within the Fusarium fujikuroi species complex. Appl. Microbiol. Biotechnol. 2019, 103, 5323–5337. [Google Scholar] [CrossRef] [PubMed]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Walczak, J.; Buszewski, B. Microbiology neutralization of zearalenone using Lactococcus lactis and Bifidobacterium sp. Anal. Bioanal. Chem. 2018, 410, 943–952. [Google Scholar] [CrossRef]
- Quintilla, R.; Kolecka, A.; Casaregola, S.; Daniel, H.M.; Houbraken, J.; Kostrzewa, M.; Boekhout, T.; Groenewald, M. MALDI-TOF MS as a tool to identify foodborne yeasts and yeast-like fungi. Int. J. Food Microbiol. 2018, 266, 109–118. [Google Scholar] [CrossRef]
- De Souza, T.P.; Evangelista, S.R.; Passamani, F.R.F.; Bertechini, R.; de Abreu, L.R.; Batista, L.R. Mycobiota of Minas artisanal cheese: Safety and quality. Int. Dairy J. 2021, 120, 105085. [Google Scholar] [CrossRef]
- Ertas Onmaz, N.; Gungor, C.; Al, S.; Dishan, A.; Hizlisoy, H.; Yildirim, Y.; Kasap Tekinsen, F.; Disli, H.B.; Barel, M.; Karadal, F. Mycotoxigenic and phylogenetic perspective to the yeasts and filamentous moulds in mould-matured Turkish cheese. Int. J. Food Microbiol. 2021, 357, 109385. [Google Scholar] [CrossRef]
- Penland, M.; Falentin, H.; Parayre, S.; Pawtowski, A.; Maillard, M.B.; Thierry, A.; Mounier, J.; Coton, M.; Deutsch, S.M. Linking Pélardon artisanal goat cheese microbial communities to aroma compounds during cheese-making and ripening. Int. J. Food Microbiol. 2021, 345, 109130. [Google Scholar] [CrossRef] [PubMed]
- Farahani-Kofoet, R.D.; Witzel, K.; Graefe, J.; Grosch, R.; Zrenner, R. Species-specific impact of Fusarium infection on the root and shoot characteristics of asparagus. Pathogens 2020, 9, 509. [Google Scholar] [CrossRef]
- Kleiner, M.; Thorson, E.; Sharp, C.E.; Dong, X.; Liu, D.; Li, C.; Strous, M. Assessing species biomass contributions in microbial communities via metaproteomics. Nat. Commun. 2017, 8, 1558. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, M. Metaproteomics: Much more than measuring gene expression in microbial communities. mSystems 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Fan, W.; Xu, Y. Metaproteomics insights into traditional fermented foods and beverages. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2506–2529. [Google Scholar] [CrossRef] [PubMed]
- Heyer, R.; Schallert, K.; Zoun, R.; Becher, B.; Saake, G.; Benndorf, D. Challenges and perspectives of metaproteomic data analysis. J. Biotechnol. 2017, 261, 24–36. [Google Scholar] [CrossRef]
- Rizo, J.; Guillén, D.; Díaz-Ruiz, G.; Wacher, C.; Encarnación, S.; Sánchez, S.; Rodríguez-Sanoja, R. Metaproteomic insights into the microbial community in Pozol. Front. Nutr. 2021, 8, 714814. [Google Scholar] [CrossRef]
- Xie, M.; An, F.; Wu, J.; Liu, Y.; Shi, H.; Wu, R. Meta-omics reveal microbial assortments and key enzymes in bean sauce mash, a traditional fermented soybean product. J. Sci. Food Agric. 2019, 99, 6522–6534. [Google Scholar] [CrossRef]
- Xie, M.; An, F.; Yue, X.; Liu, Y.; Shi, H.; Yang, M.; Cao, X.; Wu, J.; Wu, R. Characterization and comparison of metaproteomes in traditional and commercial dajiang, a fermented soybean paste in northeast China. Food Chem. 2019, 301, 125270. [Google Scholar] [CrossRef]
- Xie, M.; Wu, J.; An, F.; Yue, X.; Tao, D.; Wu, R.; Lee, Y. An integrated metagenomic/metaproteomic investigation of microbiota in dajiang-meju, a traditional fermented soybean product in Northeast China. Food Res. Int. 2019, 115, 414–424. [Google Scholar] [CrossRef]
- Wang, B.; Wu, Q.; Xu, Y.; Sun, B. Synergistic effect of multiple saccharifying enzymes on alcoholic fermentation for chinese Baijiu production. Appl. Environ. Microbiol. 2020, 86, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pechanova, O.; Pechan, T.; Rodriguez, J.M.; Paul Williams, W.; Brown, A.E. A two-dimensional proteome map of the aflatoxigenic fungus Aspergillus flavus. Proteomics 2013, 13, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Thakur, R.; Goel, G.; Shankar, J. Nano-LC-Q-TOF analysis of proteome revealed germination of Aspergillus flavus conidia is accompanied by MAPK signalling and cell wall modulation. Mycopathologia 2016, 181, 769–786. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhong, H.; Han, X.; Guo, Z.; Yang, W.; Liu, Y.; Yang, K.; Zhuang, Z.; Wang, S. Proteomic profile of Aspergillus flavus in response to water activity. Fungal Biol. 2015, 119, 114–124. [Google Scholar] [CrossRef]
- Georgianna, D.R.; Hawkridge, A.M.; Muddiman, D.C.; Payne, G.A. Temperature-dependent regulation of proteins in Aspergillus flavus: Whole organism stable isotope labeling by amino acids. J. Proteome Res. 2008, 7, 2973–2979. [Google Scholar] [CrossRef]
- Tiwari, S.; Shankar, J. Integrated proteome and HPLC analysis revealed quercetin-mediated inhibition of aflatoxin B1 biosynthesis in Aspergillus flavus. 3 Biotech 2018, 8, 47. [Google Scholar] [CrossRef]
- Lu, X.; Sun, J.; Nimtz, M.; Wissing, J.; Zeng, A.P.; Rinas, U. The intra- and extracellular proteome of Aspergillus niger growing on defined medium with xylose or maltose as carbon substrate. Microb. Cell Fact. 2010, 9, 23. [Google Scholar] [CrossRef]
- Sørensen, L.M.; Lametsch, R.; Andersen, M.R.; Nielsen, P.V.; Frisvad, J.C. Proteome analysis of Aspergillus niger: Lactate added in starch-containing medium can increase production of the mycotoxin fumonisin B2 by modifying acetyl-CoA metabolism. BMC Microbiol. 2009, 9, 255. [Google Scholar] [CrossRef]
- Tao, N.; Chen, Y.; Wu, Y.; Wang, X.; Li, L.; Zhu, A. The terpene limonene induced the green mold of citrus fruit through regulation of reactive oxygen species (ROS) homeostasis in Penicillium digitatum spores. Food Chem. 2019, 277, 414–422. [Google Scholar] [CrossRef]
- Zhang, J.; Meng, D.; Xia, X.; Sun, Y.; Zhao, L.; Zhou, X.; Wang, Y. Profiling the secretomes of Penicillium expansum reveals that a serine carboxypeptidase (PeSCP) is required for the fungal virulence on apple fruit. Physiol. Mol. Plant Pathol. 2022, 122, 101897. [Google Scholar] [CrossRef]
- Li, T.; Gong, L.; Jian, Q.; Duan, X.; Jiang, Y.; Jian, Q.; Chen, F.; Gupta, V.K. Proteomics analysis of Fusarium proliferatum under various initial pH during fumonisin production. J. Proteom. 2017, 164, 59–72. [Google Scholar] [CrossRef]
- Chen, S.; Fan, L.; Song, J.; Zhang, H.; Doucette, C.; Hughes, T.; Campbell, L. Quantitative proteomic analysis of Neosartorya pseudofischeri ascospores subjected to heat treatment. J. Proteom. 2022, 252, 104446. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, Y.; Lin, W.; Yan, H.; Neng, J.; Sun, P. Quantitative proteomic profiling of fungal growth, development, and ochratoxin A production in Aspergillus ochraceus on high- and low-NaCl cultures. Toxins 2021, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Núñez, F.; Lara, M.S.; Peromingo, B.; Delgado, J.; Sánchez-Montero, L.; Andrade, M.J. Selection and evaluation of Debaryomyces hansenii isolates as potential bioprotective agents against toxigenic penicillia in dry-fermented sausages. Food Microbiol. 2015, 46, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Fiori, S.; Urgeghe, P.P.; Hammami, W.; Razzu, S.; Jaoua, S.; Migheli, Q. Biocontrol activity of four non- and low-fermenting yeast strains against Aspergillus carbonarius and their ability to remove ochratoxin A from grape juice. Int. J. Food Microbiol. 2014, 189, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martín, A.; Acosta, R.; Liddell, S.; Núñez, F.; Benito, M.J.; Asensio, M.A. Characterization of the novel antifungal protein PgAFP and the encoding gene of Penicillium chrysogenum. Peptides 2010, 31, 541–547. [Google Scholar] [CrossRef]
- Delgado, J.; Acosta, R.; Rodríguez-Martín, A.; Bermúdez, E.; Núñez, F.; Asensio, M.A. Growth inhibition and stability of PgAFP from Penicillium chrysogenum against fungi common on dry-ripened meat products. Int. J. Food Microbiol. 2015, 205, 23–29. [Google Scholar] [CrossRef]
- Citores, L.; Valletta, M.; Singh, V.P.; Pedone, P.V.; Iglesias, R.; Ferreras, J.M.; Chambery, A.; Russo, R. Deciphering molecular determinants underlying Penicillium digitatum’s response to biological and chemical antifungal agents by tandem mass tag (TMT)-based high-resolution LC-MS/MS. Int. J. Mol. Sci. 2022, 23, 680. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, X.; Li, M.; Li, T.; Duan, X.; Zhang, D. Label-Free proteomic analysis of molecular effects of 2-methoxy-1,4-naphthoquinone on Penicillium italicum. Int. J. Mol. Sci. 2019, 20, 3459. [Google Scholar] [CrossRef]
- Yang, S.; Fan, M.; Li, D.; Zhou, J.; Fan, G.; Peng, L.; Zhang, S. Physiological and iTRAQ-based proteomic analyses reveal the mechanism of pinocembrin against Penicillium italicum through targeting mitochondria. Pestic. Biochem. Physiol. 2020, 167, 104534. [Google Scholar] [CrossRef]
- Elagamey, E.; Abdellatef, M.A.E.; Arafat, M.Y. Proteomic insights of chitosan mediated inhibition of Fusarium oxysporum f. sp. cucumerinum. J. Proteom. 2022, 260, 104560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, L.; Li, B.; Gu, X.; Zhang, X.; Boateng, N.S.; Zhang, H. Molecular dissection of defense response of pears induced by the biocontrol yeast, Wickerhamomyces anomalus using transcriptomics and proteomics approaches. Biol. Control 2020, 148, 104305. [Google Scholar] [CrossRef]
- Geromanos, S.J.; Vissers, J.P.C.; Silva, J.C.; Dorschel, C.A.; Li, G.Z.; Gorenstein, M.V.; Bateman, R.H.; Langridge, J.I. The detection, correlation, and comparison of peptide precursor and product ions from data independent LC-MS with data dependant LC-MS/MS. Proteomics 2009, 9, 1683–1695. [Google Scholar] [CrossRef]
- López Pedrouso, M.; Lorenzo, J.M.; Franco Ruiz, D. IntroductionFood proteomics: Technological advances, current applications and future perpectives. In Food Proteomics; López Pedrouso, M., Lorenzo, J.M., Franco Ruiz, D., Eds.; Elsevier: London, UK, 2022; pp. 1–12. ISBN 978-0-323-90889-4. [Google Scholar]
- Gotti, C.; Roux-Dalvai, F.; Joly-Beauparlant, C.; Mangnier, L.; Leclercq, M.; Droit, A. Extensive and accurate benchmarking of DIA acquisition methods and software tools using a complex proteomic standard. J. Proteome Res. 2021, 20, 4801–4814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ge, W.; Ruan, G.; Cai, X.; Guo, T. Data-Independent Acquisition Mass Spectrometry-Based Proteomics and Software Tools: A Glimpse in 2020. Proteomics 2020, 20, 1900276. [Google Scholar] [CrossRef] [PubMed]
- Sinitcyn, P.; Hamzeiy, H.; Salinas Soto, F.; Itzhak, D.; McCarthy, F.; Wichmann, C.; Steger, M.; Ohmayer, U.; Distler, U.; Kaspar-Schoenefeld, S.; et al. MaxDIA enables library-based and library-free data-independent acquisition proteomics. Nat. Biotechnol. 2021, 39, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Demichev, V.; Messner, C.B.; Vernardis, S.I.; Lilley, K.S.; Ralser, M. DIA-NN: Neural networks and interference correction enable deep proteome coverage in high throughput. Nat. Methods 2020, 17, 41–44. [Google Scholar] [CrossRef]
- Demichev, V.; Szyrwiel, L.; Yu, F.; Teo, G.C.; Rosenberger, G.; Niewienda, A.; Ludwig, D.; Decker, J.; Kaspar-Schoenefeld, S.; Lilley, K.S.; et al. Dia-PASEF data analysis using FragPipe and DIA-NN for deep proteomics of low sample amounts. Nat. Commun. 2022, 13, 3944. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Li, B.; He, Q.-Y.; Wang, Y.; Zhang, J.; Li, B.; He, Q.-Y. Advances of Proteomics in novel PTM discovery: Applications in cancer therapy. Small Methods 2019, 3, 1900041. [Google Scholar] [CrossRef]
- Holstein, E.; Dittmann, A.; Kääriäinen, A.; Pesola, V.; Koivunen, J.; Pihlajaniemi, T.; Naba, A.; Izzi, V. The burden of post-translational modification (PTM)-Disrupting mutations in the tumor matrisome. Cancers 2021, 13, 1081. [Google Scholar] [CrossRef]
- Tikhonov, D.; Kulikova, L.; Kopylov, A.T.; Rudnev, V.; Stepanov, A.; Malsagova, K.; Izotov, A.; Kulikov, D.; Zulkarnaev, A.; Enikeev, D.; et al. Proteomic and molecular dynamic investigations of PTM-induced structural fluctuations in breast and ovarian cancer. Sci. Rep. 2021, 11, 19318. [Google Scholar] [CrossRef] [PubMed]
- Etier, A.; Dumetz, F.; Chéreau, S.; Ponts, N. Post-translational modifications of histones are versatile regulators of fungal development and secondary metabolism. Toxins 2022, 14, 317. [Google Scholar] [CrossRef] [PubMed]
- Tumukunde, E.; Xie, R.; Wang, S. Updates on the functions and molecular mechanisms of the genes involved in Aspergillus flavus development and biosynthesis of aflatoxins. J. Fungi 2021, 7, 666. [Google Scholar] [CrossRef]
- Johnson, L.N.; Lewis, R.J. Structural basis for control by phosphorylation. Chem. Rev. 2001, 101, 2209–2242. [Google Scholar] [CrossRef]
- Mattos, E.C.; Silva, P.; Valero, C.; De Castro, A.; Taft, C.A.; Al-furaiji, N.; Bromley, M.; Mortensen, U.H.; Benz, J.P.; Brown, A. The Aspergillus fumigatus phosphoproteome reveals roles of High-Osmolarity Glycerol Mitogen-Activated Protein Kinases in promoting cell wall damage and caspofungin tolerance. mBio 2020, 11, e02962-19. [Google Scholar] [CrossRef]
- Mattos, E.C.; Palmisano, G.; Goldman, G.H. Phosphoproteomics of Aspergillus fumigatus exposed to the antifungal drug caspofungin. mSphere 2020, 5, 1–16. [Google Scholar] [CrossRef]
- Chelius, C.; Huso, W.; Reese, S.; Doan, A.; Lincoln, S.; Lawson, K.; Tran, B.; Purohit, R.; Glaros, T.; Srivastava, R.; et al. Dynamic transcriptomic and phosphoproteomic analysis during cell wall stress in Aspergillus nidulans. Mol. Cell. Proteom. 2020, 19, 1310–1329. [Google Scholar] [CrossRef] [PubMed]
| Mycotoxins | Major Foods | Mould Sources | Toxic Effects |
|---|---|---|---|
| Aflatoxins: B1, B2, G1, G2, | Cereals, nuts, spices | Aspergillus section Flavi | Hepatotoxic, immunosuppressive, carcinogenic, teratogenic, genotoxic |
| Ochratoxin A | Cacao, dried fruits, wine, cereals, spices, dry-cured meats | Aspergillus section Circumdati Aspergillus section Nigri Penicillium verrucosum, P. viridicatum, P. nordicum | Carcinogenic, teratogenic, genotoxic, immunotoxic |
| Fumonisins: B1, B2 | Maize | Fusarium section Liseola | Carcinogenic, pulmonary oedema, neurotoxic, cardiovascular toxicity |
| Patulin | Apple | P. expansum, Aspergillus clavatus, Bysochlamis nivea | Acute toxicity, neurotoxic, genotoxic, carcinogenic, teratogenic, immunotoxic |
| Trichothecenes: T-2, DON, DAS, HT-2, NIV, etc. a | Cereals | Fusarium acuminatum, F. poae, F. sporotrichioides, F. graminearum, F. colmorum, F. cerealis | Vomiting, diarrhoea, leukopenia, necrotic lesions, haemorrhage, kidney problems, immunosuppressive |
| Zearalenone | Cereals | F. graminearum, F. culmorum, F. equiseti, F. cerealis, F. verticillioides, F. incarnatum | Oestrogenic effects, reproductive toxicity |
| Alternaria mycotoxins: AOH, AME, ALT, TeA, etc. b | Tomato, sunflower seed, cereals | Alternaria sp. | Acute toxicity, cytotoxic, fetotoxic, teratogenic, haematological disorders, oesophageal cancer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez, M.; Andrade, M.J.; Núñez, F.; Rodríguez, M.; Delgado, J. Proteomics as a New-Generation Tool for Studying Moulds Related to Food Safety and Quality. Int. J. Mol. Sci. 2023, 24, 4709. https://doi.org/10.3390/ijms24054709
Álvarez M, Andrade MJ, Núñez F, Rodríguez M, Delgado J. Proteomics as a New-Generation Tool for Studying Moulds Related to Food Safety and Quality. International Journal of Molecular Sciences. 2023; 24(5):4709. https://doi.org/10.3390/ijms24054709
Chicago/Turabian StyleÁlvarez, Micaela, María J. Andrade, Félix Núñez, Mar Rodríguez, and Josué Delgado. 2023. "Proteomics as a New-Generation Tool for Studying Moulds Related to Food Safety and Quality" International Journal of Molecular Sciences 24, no. 5: 4709. https://doi.org/10.3390/ijms24054709
APA StyleÁlvarez, M., Andrade, M. J., Núñez, F., Rodríguez, M., & Delgado, J. (2023). Proteomics as a New-Generation Tool for Studying Moulds Related to Food Safety and Quality. International Journal of Molecular Sciences, 24(5), 4709. https://doi.org/10.3390/ijms24054709








