Radiogenomics in Renal Cancer Management—Current Evidence and Future Prospects
Abstract
1. Introduction
2. Results
2.1. Radiomics
2.2. Radiomics in Renal Cancer Management
2.2.1. Differentiation of Benign from Cancerous Kidney Tumors, of Angiomyolipoma from RCC, and of Oncocytoma from RCC
2.2.2. Differentiation of Different Subtypes of RCC
2.2.3. Nuclear Grade Prediction
2.2.4. Evaluation of Treatment Response
2.3. Genomic and Molecular Tumor Characterization
2.3.1. Genetics of Clear Cell-Renal Cell Carcinoma (ccRCC)
2.3.2. Von Hippel Lindau Disease
2.3.3. Genetics of Non-Clear Cell-Renal Cell Carcinoma
Papillary Renal Cell Carcinoma
Hereditary Papillary Renal Carcinoma (HpapRCC)
Chromophobe Renal Cell Carcinoma (chRCC)
Birt-Hogg-Dubé (BHD) Syndrome
Renal Medullary Carcinoma
2.3.4. Additional Types of Renal Cell Carcinoma
FH-Deficient and SDH-Deficient Renal Cell Carcinoma
Translocation Renal Cell Carcinoma (T-RCC)
2.3.5. Renal Cell Carcinoma as a Metabolic Disease
2.4. Molecular Imaging in Renal Cancer
2.4.1. Single Photon Emission Computed Tomography (SPECT)/CT with 99mTc-Sestamibi
2.4.2. Positron Emission Tomography (PET)/CT with Radiolabeled Girentuximab
2.4.3. PET/CT with 18F-FDG
2.4.4. Prostate-Specific Membrane Antigen (PSMA)-Targeted PET/CT
2.4.5. PET/CT with 11C-Acetate
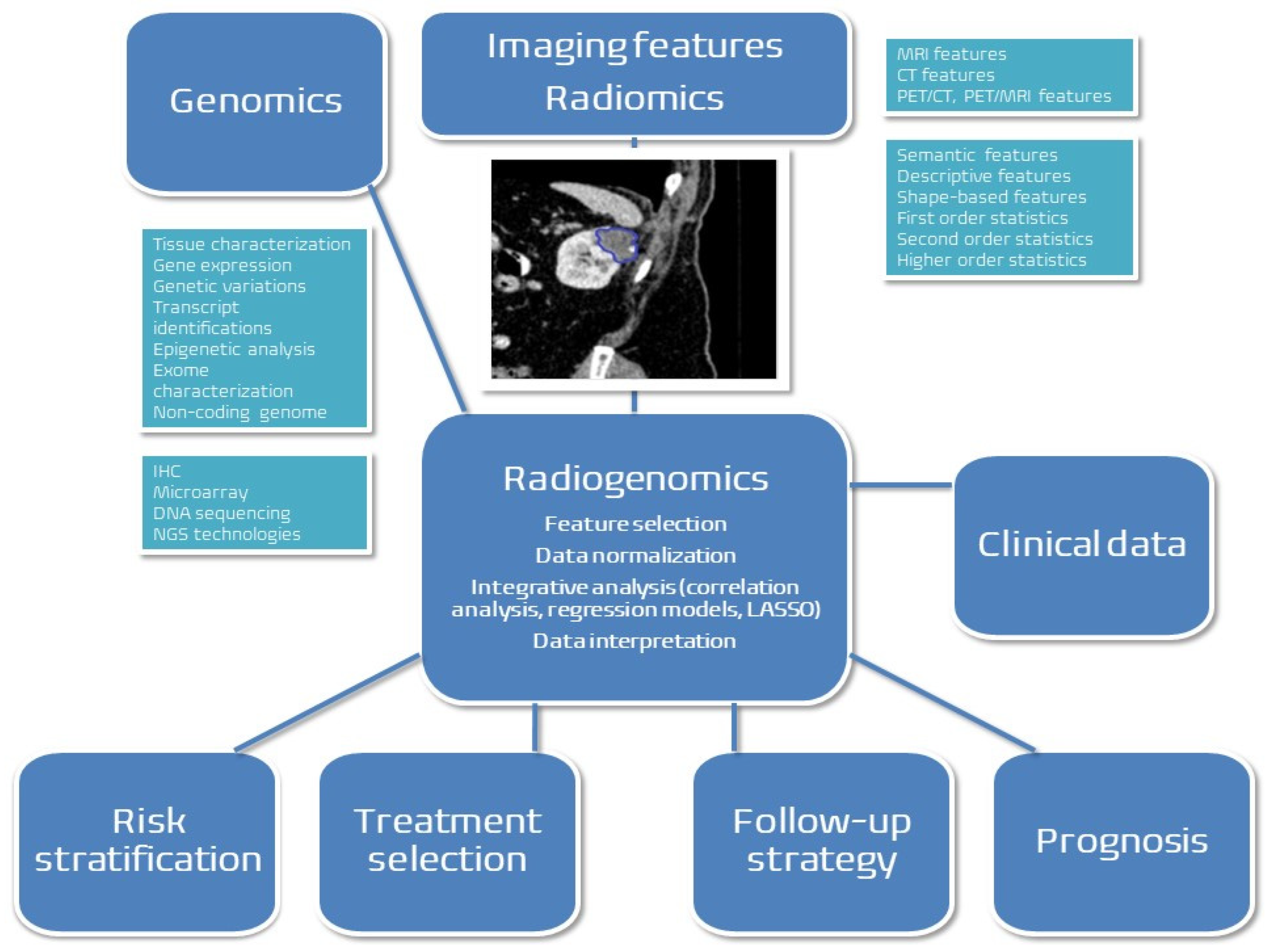
2.5. Radiogenomics in Renal Cancer Management
2.6. Current Challenges, Limitations and Future Perspectives
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA. Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sohlberg, E.M.; Metzner, T.J.; Leppert, J.T. The Harms of Overdiagnosis and Overtreatment in Patients with Small Renal Masses: A Mini-Review. Eur. Urol. Focus 2019, 5, 943–945. [Google Scholar] [CrossRef]
- Leveridge, M.J.; Finelli, A.; Kachura, J.R.; Evans, A.; Chung, H.; Shiff, D.A.; Fernandes, K.; Jewett, M.A.S. Outcomes of Small Renal Mass Needle Core Biopsy, Nondiagnostic Percutaneous Biopsy, and the Role of Repeat Biopsy. Eur. Urol. 2011, 60, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Marchioni, M.; Martel, T.; Bandini, M.; Pompe, R.S.; Tian, Z.; Kapoor, A.; Cindolo, L.; Autorino, R.; Briganti, A.; Shariat, S.F.; et al. Marital Status and Gender Affect Stage, Tumor Grade, Treatment Type and Cancer Specific Mortality in T(1-2) N(0) M(0) Renal Cell Carcinoma. World J. Urol. 2017, 35, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.; Lunardini, S.; Magli, I.A.; Campi, R.; Primiceri, G.; Berardinelli, F.; Amparore, D.; Terracciano, D.; Lucarelli, G.; Schips, L.; et al. Micro-RNAs Predict Response to Systemic Treatments in Metastatic Renal Cell Carcinoma Patients: Results from a Systematic Review of the Literature. Biomedicines 2022, 10, 1287. [Google Scholar] [CrossRef]
- Marchioni, M.; Kriegmair, M.; Heck, M.; Amiel, T.; Porpiglia, F.; Ceccucci, E.; Campi, R.; Minervini, A.; Mari, A.; Van Bruwaene, S.; et al. Development of a Novel Risk Score to Select the Optimal Candidate for Cytoreductive Nephrectomy Among Patients with Metastatic Renal Cell Carcinoma. Results from a Multi-Institutional Registry (REMARCC). Eur. Urol. Oncol. 2021, 4, 256–263. [Google Scholar] [CrossRef]
- Marchioni, M.; Rivas, J.G.; Autran, A.; Socarras, M.; Albisinni, S.; Ferro, M.; Schips, L.; Scarpa, R.M.; Papalia, R.; Esperto, F. Biomarkers for Renal Cell Carcinoma Recurrence: State of the Art. Curr. Urol. Rep. 2021, 22, 31. [Google Scholar] [CrossRef]
- Shui, L.; Ren, H.; Yang, X.; Li, J.; Chen, Z.; Yi, C.; Zhu, H.; Shui, P. The Era of Radiogenomics in Precision Medicine: An Emerging Approach to Support Diagnosis, Treatment Decisions, and Prognostication in Oncology. Front. Oncol. 2021, 10, 570465. [Google Scholar] [CrossRef]
- Chaddad, A.; Kucharczyk, M.J.; Niazi, T. Multimodal Radiomic Features for the Predicting Gleason Score of Prostate Cancer. Cancers 2018, 10, 249. [Google Scholar] [CrossRef]
- Chaddad, A.; Niazi, T.; Probst, S.; Bladou, F.; Anidjar, M.; Bahoric, B. Predicting Gleason Score of Prostate Cancer Patients Using Radiomic Analysis. Front. Oncol. 2018, 8, 630. [Google Scholar] [CrossRef]
- Bardis, M.D.; Houshyar, R.; Chang, P.D.; Ushinsky, A.; Glavis-Bloom, J.; Chahine, C.; Bui, T.-L.; Rupasinghe, M.; Filippi, C.G.; Chow, D.S. Applications of Artificial Intelligence to Prostate Multiparametric MRI (MpMRI): Current and Emerging Trends. Cancers 2020, 12, 1204. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, M.A.; Borrelli, P.; Poulsen, M.H.; Gerke, O.; Enqvist, O.; Ulén, J.; Trägårdh, E.; Constantinescu, C.; Edenbrandt, L.; Lund, L.; et al. Artificial Intelligence-Based versus Manual Assessment of Prostate Cancer in the Prostate Gland: A Method Comparison Study. Clin. Physiol. Funct. Imaging 2019, 39, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Ström, P.; Kartasalo, K.; Olsson, H.; Solorzano, L.; Delahunt, B.; Berney, D.M.; Bostwick, D.G.; Evans, A.J.; Grignon, D.J.; Humphrey, P.A.; et al. Artificial Intelligence for Diagnosis and Grading of Prostate Cancer in Biopsies: A Population-Based, Diagnostic Study. Lancet Oncol. 2020, 21, 222–232. [Google Scholar] [CrossRef]
- Goldenberg, S.L.; Nir, G.; Salcudean, S.E. A New Era: Artificial Intelligence and Machine Learning in Prostate Cancer. Nat. Rev. Urol. 2019, 16, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Raciti, P.; Sue, J.; Ceballos, R.; Godrich, R.; Kunz, J.D.; Kapur, S.; Reuter, V.; Grady, L.; Kanan, C.; Klimstra, D.S.; et al. Novel Artificial Intelligence System Increases the Detection of Prostate Cancer in Whole Slide Images of Core Needle Biopsies. Mod. Pathol. Off. J. U. S. Can. Acad. Pathol. Inc 2020, 33, 2058–2066. [Google Scholar] [CrossRef]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The Bridge between Medical Imaging and Personalized Medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef]
- Kumar, V.; Gu, Y.; Basu, S.; Berglund, A.; Eschrich, S.A.; Schabath, M.B.; Forster, K.; Aerts, H.J.W.L.; Dekker, A.; Fenstermacher, D.; et al. Radiomics: The Process and the Challenges. Magn. Reson. Imaging 2012, 30, 1234–1248. [Google Scholar] [CrossRef]
- Ferro, M.; de Cobelli, O.; Vartolomei, M.D.; Lucarelli, G.; Crocetto, F.; Barone, B.; Sciarra, A.; Del Giudice, F.; Muto, M.; Maggi, M.; et al. Prostate Cancer Radiogenomics—From Imaging to Molecular Characterization. Int. J. Mol. Sci. 2021, 22, 9971. [Google Scholar] [CrossRef]
- Ferro, M.; de Cobelli, O.; Musi, G.; del Giudice, F.; Carrieri, G.; Busetto, G.M.; Falagario, U.G.; Sciarra, A.; Maggi, M.; Crocetto, F.; et al. Radiomics in Prostate Cancer: An up-to-Date Review. Ther. Adv. Urol. 2022, 14, 175628722211090. [Google Scholar] [CrossRef]
- Busetto, G.M.; Del Giudice, F.; Maggi, M.; De Marco, F.; Porreca, A.; Sperduti, I.; Magliocca, F.M.; Salciccia, S.; Chung, B.I.; De Berardinis, E.; et al. Prospective Assessment of Two-Gene Urinary Test with Multiparametric Magnetic Resonance Imaging of the Prostate for Men Undergoing Primary Prostate Biopsy. World J. Urol. 2021, 39, 1869–1877. [Google Scholar] [CrossRef]
- Coy, H.; Hsieh, K.; Wu, W.; Nagarajan, M.B.; Young, J.R.; Douek, M.L.; Brown, M.S.; Scalzo, F.; Raman, S.S. Deep Learning and Radiomics: The Utility of Google TensorFlowTM Inception in Classifying Clear Cell Renal Cell Carcinoma and Oncocytoma on Multiphasic CT. Abdom. Radiol. N. Y. 2019, 44, 2009–2020. [Google Scholar] [CrossRef]
- Yu, H.; Scalera, J.; Khalid, M.; Touret, A.-S.; Bloch, N.; Li, B.; Qureshi, M.M.; Soto, J.A.; Anderson, S.W. Texture Analysis as a Radiomic Marker for Differentiating Renal Tumors. Abdom. Radiol. N. Y. 2017, 42, 2470–2478. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, Z.; Chen, Y.-C.; Zhao, Z.-Y.; Yin, X.-D.; Jiang, H.-B. A Deep Learning-Based Radiomics Model for Differentiating Benign and Malignant Renal Tumors. Transl. Oncol. 2019, 12, 292–300. [Google Scholar] [CrossRef]
- Erdim, C.; Yardimci, A.H.; Bektas, C.T.; Kocak, B.; Koca, S.B.; Demir, H.; Kilickesmez, O. Prediction of Benign and Malignant Solid Renal Masses: Machine Learning-Based CT Texture Analysis. Acad. Radiol. 2020, 27, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, J.; Biggemann, L.; Nietert, M.M.; Beißbarth, T.; Lotz, J.; Kim, H.S.; Trojan, L.; Uhlig, A. Discriminating Malignant and Benign Clinical T1 Renal Masses on Computed Tomography: A Pragmatic Radiomics and Machine Learning Approach. Medicine 2020, 99, e19725. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Feng, Q.-X.; Xu, X.; Zhang, J.; Zhu, F.-P.; Yang, Y.-H.; Zhang, Y.-D. Radiologic-Radiomic Machine Learning Models for Differentiation of Benign and Malignant Solid Renal Masses: Comparison With Expert-Level Radiologists. Am. J. Roentgenol. 2020, 214, W44–W54. [Google Scholar] [CrossRef]
- Chen, F.; Gulati, M.; Hwang, D.; Cen, S.; Yap, F.; Ugwueze, C.; Varghese, B.; Desai, M.; Aron, M.; Gill, I.; et al. Voxel-Based Whole-Lesion Enhancement Parameters: A Study of Its Clinical Value in Differentiating Clear Cell Renal Cell Carcinoma from Renal Oncocytoma. Abdom. Radiol. N. Y. 2017, 42, 552–560. [Google Scholar] [CrossRef]
- Nassiri, N.; Maas, M.; Cacciamani, G.; Varghese, B.; Hwang, D.; Lei, X.; Aron, M.; Desai, M.; Oberai, A.A.; Cen, S.Y.; et al. A Radiomic-Based Machine Learning Algorithm to Reliably Differentiate Benign Renal Masses from Renal Cell Carcinoma. Eur. Urol. Focus 2022, 8, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Said, D.; Hectors, S.J.; Wilck, E.; Rosen, A.; Stocker, D.; Bane, O.; Beksaç, A.T.; Lewis, S.; Badani, K.; Taouli, B. Characterization of Solid Renal Neoplasms Using MRI-Based Quantitative Radiomics Features. Abdom. Radiol. 2020, 45, 2840–2850. [Google Scholar] [CrossRef]
- Xi, I.L.; Zhao, Y.; Wang, R.; Chang, M.; Purkayastha, S.; Chang, K.; Huang, R.Y.; Silva, A.C.; Vallières, M.; Habibollahi, P.; et al. Deep Learning to Distinguish Benign from Malignant Renal Lesions Based on Routine MR Imaging. Clin. Cancer Res. 2020, 26, 1944–1952. [Google Scholar] [CrossRef]
- Massa’a, R.N.; Stoeckl, E.M.; Lubner, M.G.; Smith, D.; Mao, L.; Shapiro, D.D.; Abel, E.J.; Wentland, A.L. Differentiation of Benign from Malignant Solid Renal Lesions with MRI-Based Radiomics and Machine Learning. Abdom. Radiol. 2022, 47, 2896–2904. [Google Scholar] [CrossRef]
- Xu, Q.; Zhu, Q.; Liu, H.; Chang, L.; Duan, S.; Dou, W.; Li, S.; Ye, J. Differentiating Benign from Malignant Renal Tumors Using T2-and Diffusion-Weighted Images: A Comparison of Deep Learning and Radiomics Models Versus Assessment from Radiologists. J. Magn. Reson. Imaging 2022, 55, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Rong, P.; Cao, P.; Zhou, Q.; Zhu, W.; Yan, Z.; Liu, Q.; Wang, W. Machine Learning-Based Quantitative Texture Analysis of CT Images of Small Renal Masses: Differentiation of Angiomyolipoma without Visible Fat from Renal Cell Carcinoma. Eur. Radiol. 2018, 28, 1625–1633. [Google Scholar] [CrossRef]
- Cui, E.-M.; Lin, F.; Li, Q.; Li, R.-G.; Chen, X.-M.; Liu, Z.-S.; Long, W.-S. Differentiation of Renal Angiomyolipoma without Visible Fat from Renal Cell Carcinoma by Machine Learning Based on Whole-Tumor Computed Tomography Texture Features. Acta Radiol. 2019, 60, 1543–1552. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, J.; Zhou, Z.; Dai, Y.; Qian, X. An Effective Radiomics Model for Noninvasive Discrimination of Fat-Poor Angiomyolipoma from Clear Cell Renal Cell Carcinoma. In Proceedings of the 2019 IEEE Symposium Series on Computational Intelligence (SSCI), Xiamen, China, 6–9 December 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 1551–1558. [Google Scholar]
- Ma, Y.; Cao, F.; Xu, X.; Ma, W. Can Whole-Tumor Radiomics-Based CT Analysis Better Differentiate Fat-Poor Angiomyolipoma from Clear Cell Renal Cell Caricinoma: Compared with Conventional CT Analysis? Abdom. Radiol. 2020, 45, 2500–2507. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.; Yang, G.; Wang, Z.; Yan, L.; Miao, W.; Hao, D.; Wu, J.; Zhao, Y.; Gong, A.; Cui, J.; et al. A CT-Based Radiomics Nomogram for Differentiation of Renal Angiomyolipoma without Visible Fat from Homogeneous Clear Cell Renal Cell Carcinoma. Eur. Radiol. 2020, 30, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wu, J.; Sun, L.; Lai, S.; Xu, Y.; Liu, X.; Ma, Y.; Zhen, X. Radiomics of Small Renal Masses on Multiphasic CT: Accuracy of Machine Learning–Based Classification Models for the Differentiation of Renal Cell Carcinoma and Angiomyolipoma without Visible Fat. Eur. Radiol. 2020, 30, 1254–1263. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, X.; Pang, P.; Wen, Y. A CT-Based Tumoral and Mini-Peritumoral Radiomics Approach: Differentiate Fat-Poor Angiomyolipoma from Clear Cell Renal Cell Carcinoma. Cancer Manag. Res. 2021, 13, 1417–1425. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, W.; Xu, X.; Guan, Z.; Pang, P. A Convention-Radiomics CT Nomogram for Differentiating Fat-Poor Angiomyolipoma from Clear Cell Renal Cell Carcinoma. Sci. Rep. 2021, 11, 4644. [Google Scholar] [CrossRef]
- Han, Z.; Zhu, Y.; Xu, J.; Wen, D.; Xia, Y.; Zheng, M.; Yan, T.; Wei, M. Predictive Value of CT-Based Radiomics in Distinguishing Renal Angiomyolipomas with Minimal Fat from Other Renal Tumors. Dis. Markers 2022, 2022, 9108129. [Google Scholar] [CrossRef]
- Kim, T.M.; Ahn, H.; Lee, H.J.; Kim, M.G.; Cho, J.Y.; Hwang, S.I.; Kim, S.Y. Differentiating Renal Epithelioid Angiomyolipoma from Clear Cell Carcinoma: Using a Radiomics Model Combined with CT Imaging Characteristics. Abdom. Radiol. 2022, 47, 2867–2880. [Google Scholar] [CrossRef] [PubMed]
- Razik, A.; Goyal, A.; Sharma, R.; Kandasamy, D.; Seth, A.; Das, P.; Ganeshan, B. MR Texture Analysis in Differentiating Renal Cell Carcinoma from Lipid-Poor Angiomyolipoma and Oncocytoma. Br. J. Radiol. 2020, 93, 20200569. [Google Scholar] [CrossRef]
- Jian, L.; Liu, Y.; Xie, Y.; Jiang, S.; Ye, M.; Lin, H. MRI-Based Radiomics and Urine Creatinine for the Differentiation of Renal Angiomyolipoma With Minimal Fat From Renal Cell Carcinoma: A Preliminary Study. Front. Oncol. 2022, 12, 876664. [Google Scholar] [CrossRef]
- Matsumoto, S.; Arita, Y.; Yoshida, S.; Fukushima, H.; Kimura, K.; Yamada, I.; Tanaka, H.; Yagi, F.; Yokoyama, M.; Matsuoka, Y.; et al. Utility of Radiomics Features of Diffusion-Weighted Magnetic Resonance Imaging for Differentiation of Fat-Poor Angiomyolipoma from Clear Cell Renal Cell Carcinoma: Model Development and External Validation. Abdom. Radiol. 2022, 47, 2178–2186. [Google Scholar] [CrossRef]
- Baghdadi, A.; Aldhaam, N.A.; Elsayed, A.S.; Hussein, A.A.; Cavuoto, L.A.; Kauffman, E.; Guru, K.A. Automated Differentiation of Benign Renal Oncocytoma and Chromophobe Renal Cell Carcinoma on Computed Tomography Using Deep Learning. BJU Int. 2020, 125, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Soule, E.; Cui, E.; Samuel, A.; Shah, S.; Lall, C.; Sundaram, C.; Sandrasegaran, K. Usefulness of CT Texture Analysis in Differentiating Benign and Malignant Renal Tumours. Clin. Radiol. 2020, 75, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, X.; Xia, Y.; Long, L. Value of Radiomics in Differential Diagnosis of Chromophobe Renal Cell Carcinoma and Renal Oncocytoma. Abdom. Radiol. 2020, 45, 3193–3201. [Google Scholar] [CrossRef]
- Raman, S.P.; Chen, Y.; Schroeder, J.L.; Huang, P.; Fishman, E.K. CT Texture Analysis of Renal Masses. Acad. Radiol. 2014, 21, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Sasaguri, K.; Takahashi, N.; Gomez-Cardona, D.; Leng, S.; Schmit, G.D.; Carter, R.E.; Leibovich, B.C.; Kawashima, A. Small (<4 Cm) Renal Mass: Differentiation of Oncocytoma From Renal Cell Carcinoma on Biphasic Contrast-Enhanced CT. Am. J. Roentgenol. 2015, 205, 999–1007. [Google Scholar] [CrossRef]
- Varghese, B.A.; Chen, F.; Hwang, D.H.; Cen, S.Y.; Desai, B.; Gill, I.S.; Duddalwar, V.A. Differentiation of Predominantly Solid Enhancing Lipid-Poor Renal Cell Masses by Use of Contrast-Enhanced CT: Evaluating the Role of Texture in Tumor Subtyping. Am. J. Roentgenol. 2018, 211, W288–W296. [Google Scholar] [CrossRef]
- Varghese, B.A.; Chen, F.; Hwang, D.H.; Cen, S.Y.; Gill, I.S.; Duddalwar, V.A. Differentiating Solid, Non-Macroscopic Fat Containing, Enhancing Renal Masses Using Fast Fourier Transform Analysis of Multiphase CT. Br. J. Radiol. 2018, 91, 20170789. [Google Scholar] [CrossRef] [PubMed]
- Paschall, A.K.; Mirmomen, S.M.; Symons, R.; Pourmorteza, A.; Gautam, R.; Sahai, A.; Dwyer, A.J.; Merino, M.J.; Metwalli, A.R.; Linehan, W.M.; et al. Differentiating Papillary Type I RCC from Clear Cell RCC and Oncocytoma: Application of Whole-Lesion Volumetric ADC Measurement. Abdom. Radiol. N. Y. 2018, 43, 2424–2430. [Google Scholar] [CrossRef] [PubMed]
- Kocak, B.; Yardimci, A.H.; Bektas, C.T.; Turkcanoglu, M.H.; Erdim, C.; Yucetas, U.; Koca, S.B.; Kilickesmez, O. Textural Differences between Renal Cell Carcinoma Subtypes: Machine Learning-Based Quantitative Computed Tomography Texture Analysis with Independent External Validation. Eur. J. Radiol. 2018, 107, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Hwang, S.I.; Lee, H.J. The Classification of Renal Cancer in 3-Phase CT Images Using a Deep Learning Method. J. Digit. Imaging 2019, 32, 638–643. [Google Scholar] [CrossRef]
- Li, Z.-C.; Zhai, G.; Zhang, J.; Wang, Z.; Liu, G.; Wu, G.; Liang, D.; Zheng, H. Differentiation of Clear Cell and Non-Clear Cell Renal Cell Carcinomas by All-Relevant Radiomics Features from Multiphase CT: A VHL Mutation Perspective. Eur. Radiol. 2019, 29, 3996–4007. [Google Scholar] [CrossRef]
- Leng, S.; Takahashi, N.; Gomez Cardona, D.; Kitajima, K.; McCollough, B.; Li, Z.; Kawashima, A.; Leibovich, B.C.; McCollough, C.H. Subjective and Objective Heterogeneity Scores for Differentiating Small Renal Masses Using Contrast-Enhanced CT. Abdom. Radiol. 2017, 42, 1485–1492. [Google Scholar] [CrossRef]
- Yan, L.; Liu, Z.; Wang, G.; Huang, Y.; Liu, Y.; Yu, Y.; Liang, C. Angiomyolipoma with Minimal Fat. Acad. Radiol. 2015, 22, 1115–1121. [Google Scholar] [CrossRef]
- Hoang, U.N.; Mojdeh Mirmomen, S.; Meirelles, O.; Yao, J.; Merino, M.; Metwalli, A.; Marston Linehan, W.; Malayeri, A.A. Assessment of Multiphasic Contrast-Enhanced MR Textures in Differentiating Small Renal Mass Subtypes. Abdom. Radiol. 2018, 43, 3400–3409. [Google Scholar] [CrossRef]
- Li, A.; Xing, W.; Li, H.; Hu, Y.; Hu, D.; Li, Z.; Kamel, I.R. Subtype Differentiation of Small (≤4 Cm) Solid Renal Mass Using Volumetric Histogram Analysis of DWI at 3-T MRI. Am. J. Roentgenol. 2018, 211, 614–623. [Google Scholar] [CrossRef]
- Bektas, C.T.; Kocak, B.; Yardimci, A.H.; Turkcanoglu, M.H.; Yucetas, U.; Koca, S.B.; Erdim, C.; Kilickesmez, O. Clear Cell Renal Cell Carcinoma: Machine Learning-Based Quantitative Computed Tomography Texture Analysis for Prediction of Fuhrman Nuclear Grade. Eur. Radiol. 2019, 29, 1153–1163. [Google Scholar] [CrossRef]
- Ding, J.; Xing, Z.; Jiang, Z.; Chen, J.; Pan, L.; Qiu, J.; Xing, W. CT-Based Radiomic Model Predicts High Grade of Clear Cell Renal Cell Carcinoma. Eur. J. Radiol. 2018, 103, 51–56. [Google Scholar] [CrossRef]
- Shu, J.; Tang, Y.; Cui, J.; Yang, R.; Meng, X.; Cai, Z.; Zhang, J.; Xu, W.; Wen, D.; Yin, H. Clear Cell Renal Cell Carcinoma: CT-Based Radiomics Features for the Prediction of Fuhrman Grade. Eur. J. Radiol. 2018, 109, 8–12. [Google Scholar] [CrossRef]
- Gill, T.S.; Varghese, B.A.; Hwang, D.H.; Cen, S.Y.; Aron, M.; Aron, M.; Duddalwar, V.A. Juxtatumoral Perinephric Fat Analysis in Clear Cell Renal Cell Carcinoma. Abdom. Radiol. 2019, 44, 1470–1480. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Razik, A.; Kandasamy, D.; Seth, A.; Das, P.; Ganeshan, B.; Sharma, R. Role of MR Texture Analysis in Histological Subtyping and Grading of Renal Cell Carcinoma: A Preliminary Study. Abdom. Radiol. 2019, 44, 3336–3349. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wei, Y.; Zhang, H.; Zhang, T.; Yuan, F.; Huang, Z.; Han, F.; Song, B. Grading of Clear Cell Renal Cell Carcinomas by Using Machine Learning Based on Artificial Neural Networks and Radiomic Signatures Extracted From Multidetector Computed Tomography Images. Acad. Radiol. 2020, 27, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Kocak, B.; Durmaz, E.S.; Ates, E.; Kaya, O.K.; Kilickesmez, O. Unenhanced CT Texture Analysis of Clear Cell Renal Cell Carcinomas: A Machine Learning–Based Study for Predicting Histopathologic Nuclear Grade. Am. J. Roentgenol. 2019, 212, W132–W139. [Google Scholar] [CrossRef]
- Lin, F.; Cui, E.-M.; Lei, Y.; Luo, L. CT-Based Machine Learning Model to Predict the Fuhrman Nuclear Grade of Clear Cell Renal Cell Carcinoma. Abdom. Radiol. 2019, 44, 2528–2534. [Google Scholar] [CrossRef]
- Sun, X.; Liu, L.; Xu, K.; Li, W.; Huo, Z.; Liu, H.; Shen, T.; Pan, F.; Jiang, Y.; Zhang, M. Prediction of ISUP Grading of Clear Cell Renal Cell Carcinoma Using Support Vector Machine Model Based on CT Images. Medicine 2019, 98, e15022. [Google Scholar] [CrossRef]
- Cui, E.; Li, Z.; Ma, C.; Li, Q.; Lei, Y.; Lan, Y.; Yu, J.; Zhou, Z.; Li, R.; Long, W.; et al. Predicting the ISUP Grade of Clear Cell Renal Cell Carcinoma with Multiparametric MR and Multiphase CT Radiomics. Eur. Radiol. 2020, 30, 2912–2921. [Google Scholar] [CrossRef]
- Antunes, J.; Viswanath, S.; Rusu, M.; Valls, L.; Hoimes, C.; Avril, N.; Madabhushi, A. Radiomics Analysis on FLT-PET/MRI for Characterization of Early Treatment Response in Renal Cell Carcinoma: A Proof-of-Concept Study. Transl. Oncol. 2016, 9, 155–162. [Google Scholar] [CrossRef]
- Bharwani, N.; Miquel, M.E.; Powles, T.; Dilks, P.; Shawyer, A.; Sahdev, A.; Wilson, P.D.; Chowdhury, S.; Berney, D.M.; Rockall, A.G. Diffusion-Weighted and Multiphase Contrast-Enhanced MRI as Surrogate Markers of Response to Neoadjuvant Sunitinib in Metastatic Renal Cell Carcinoma. Br. J. Cancer 2014, 110, 616–624. [Google Scholar] [CrossRef]
- Boos, J.; Revah, G.; Brook, O.R.; Rangaswamy, B.; Bhatt, R.S.; Brook, A.; Raptopoulos, V. CT Intensity Distribution Curve (Histogram) Analysis of Patients Undergoing Antiangiogenic Therapy for Metastatic Renal Cell Carcinoma. Am. J. Roentgenol. 2017, 209, W85–W92. [Google Scholar] [CrossRef]
- Goh, V.; Ganeshan, B.; Nathan, P.; Juttla, J.K.; Vinayan, A.; Miles, K.A. Assessment of Response to Tyrosine Kinase Inhibitors in Metastatic Renal Cell Cancer: CT Texture as a Predictive Biomarker. Radiology 2011, 261, 165–171. [Google Scholar] [CrossRef]
- Haider, M.A.; Vosough, A.; Khalvati, F.; Kiss, A.; Ganeshan, B.; Bjarnason, G.A. CT Texture Analysis: A Potential Tool for Prediction of Survival in Patients with Metastatic Clear Cell Carcinoma Treated with Sunitinib. Cancer Imaging 2017, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Mains, J.R.; Donskov, F.; Pedersen, E.M.; Madsen, H.H.T.; Thygesen, J.; Thorup, K.; Rasmussen, F. Use of Patient Outcome Endpoints to Identify the Best Functional CT Imaging Parameters in Metastatic Renal Cell Carcinoma Patients. Br. J. Radiol. 2018, 91, 20160795. [Google Scholar] [CrossRef] [PubMed]
- Khene, Z.; Mathieu, R.; Peyronnet, B.; Kokorian, R.; Gasmi, A.; Khene, F.; Rioux-Leclercq, N.; Kammerer-Jacquet, S.-F.; Shariat, S.; Laguerre, B.; et al. Radiomics Can Predict Tumour Response in Patients Treated with Nivolumab for a Metastatic Renal Cell Carcinoma: An Artificial Intelligence Concept. World J. Urol. 2021, 39, 3707–3709. [Google Scholar] [CrossRef] [PubMed]
- DeVita, V.T.; Lawrence, T.S.; Rosenberg, S.A. (Eds.) Cancer: Principles & Practice of Oncology. Primer of the Molecular Biology of Cancer, 3rd ed.; Wolters Kluwer: Philadelphia, PN, USA, 2021; ISBN 978-1-975149-11-6. [Google Scholar]
- Anderson, C. Renal Cancer: Current Status and Innovations; Springer Nature: Berlin/Heidelberg, Germany, 2022; ISBN 3-030-84756-X. [Google Scholar]
- Khaleel, S.; Ricketts, C.; Linehan, W.M.; Ball, M.; Manley, B.; Turajilic, S.; Brugarolas, J.; Hakimi, A. Genetics and Tumor Microenvironment of Renal Cell Carcinoma. Société Int. Urol. J. 2022, 3, 386–396. [Google Scholar]
- Shuch, B.; Vourganti, S.; Ricketts, C.J.; Middleton, L.; Peterson, J.; Merino, M.J.; Metwalli, A.R.; Srinivasan, R.; Linehan, W.M. Defining Early-Onset Kidney Cancer: Implications for Germline and Somatic Mutation Testing and Clinical Management. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 431–437. [Google Scholar] [CrossRef]
- Durinck, S.; Stawiski, E.W.; Pavía-Jiménez, A.; Modrusan, Z.; Kapur, P.; Jaiswal, B.S.; Zhang, N.; Toffessi-Tcheuyap, V.; Nguyen, T.T.; Pahuja, K.B.; et al. Spectrum of Diverse Genomic Alterations Define Non-Clear Cell Renal Carcinoma Subtypes. Nat. Genet. 2015, 47, 13–21. [Google Scholar] [CrossRef]
- Stephens, P.J.; Greenman, C.D.; Fu, B.; Yang, F.; Bignell, G.R.; Mudie, L.J.; Pleasance, E.D.; Lau, K.W.; Beare, D.; Stebbings, L.A.; et al. Massive Genomic Rearrangement Acquired in a Single Catastrophic Event during Cancer Development. Cell 2011, 144, 27–40. [Google Scholar] [CrossRef]
- Sato, Y.; Yoshizato, T.; Shiraishi, Y.; Maekawa, S.; Okuno, Y.; Kamura, T.; Shimamura, T.; Sato-Otsubo, A.; Nagae, G.; Suzuki, H.; et al. Integrated Molecular Analysis of Clear-Cell Renal Cell Carcinoma. Nat. Genet. 2013, 45, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network Comprehensive Molecular Characterization of Clear Cell Renal Cell Carcinoma. Nature 2013, 499, 43–49. [CrossRef] [PubMed]
- Brugarolas, J. Molecular Genetics of Clear-Cell Renal Cell Carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 1968–1976. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, C.J.; Crooks, D.R.; Sourbier, C.; Schmidt, L.S.; Srinivasan, R.; Linehan, W.M. SnapShot: Renal Cell Carcinoma. Cancer Cell 2016, 29, 610–610.e1. [Google Scholar] [CrossRef]
- Nordstrom-O’Brien, M.; van der Luijt, R.B.; van Rooijen, E.; van den Ouweland, A.M.; Majoor-Krakauer, D.F.; Lolkema, M.P.; van Brussel, A.; Voest, E.E.; Giles, R.H. Genetic Analysis of von Hippel-Lindau Disease. Hum. Mutat. 2010, 31, 521–537. [Google Scholar] [CrossRef]
- Nickerson, M.L.; Jaeger, E.; Shi, Y.; Durocher, J.A.; Mahurkar, S.; Zaridze, D.; Matveev, V.; Janout, V.; Kollarova, H.; Bencko, V.; et al. Improved Identification of von Hippel-Lindau Gene Alterations in Clear Cell Renal Tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 4726–4734. [Google Scholar] [CrossRef]
- Stebbins, C.E.; Kaelin, W.G.; Pavletich, N.P. Structure of the VHL-ElonginC-ElonginB Complex: Implications for VHL Tumor Suppressor Function. Science 1999, 284, 455–461. [Google Scholar] [CrossRef]
- Kaelin, W.G. Molecular Basis of the VHL Hereditary Cancer Syndrome. Nat. Rev. Cancer 2002, 2, 673–682. [Google Scholar] [CrossRef]
- Hoffman, M.A.; Ohh, M.; Yang, H.; Klco, J.M.; Ivan, M.; Kaelin, W.G. Von Hippel-Lindau Protein Mutants Linked to Type 2C VHL Disease Preserve the Ability to Downregulate HIF. Hum. Mol. Genet. 2001, 10, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.; Crossey, P.A.; Cairns, P.; Hetherington, J.W.; Richards, F.M.; Jones, M.H.; Bentley, E.; Affara, N.A.; Ferguson-Smith, M.A.; Maher, E.R. Molecular Genetic Investigation of Sporadic Renal Cell Carcinoma: Analysis of Allele Loss on Chromosomes 3p, 5q, 11p, 17 and 22. Br. J. Cancer 1994, 69, 230–234. [Google Scholar] [CrossRef]
- Jiang, F.; Richter, J.; Schraml, P.; Bubendorf, L.; Gasser, T.; Sauter, G.; Mihatsch, M.J.; Moch, H. Chromosomal Imbalances in Papillary Renal Cell Carcinoma: Genetic Differences between Histological Subtypes. Am. J. Pathol. 1998, 153, 1467–1473. [Google Scholar] [CrossRef]
- Jeffers, M.; Schmidt, L.; Nakaigawa, N.; Webb, C.P.; Weirich, G.; Kishida, T.; Zbar, B.; Vande Woude, G.F. Activating Mutations for the Met Tyrosine Kinase Receptor in Human Cancer. Proc. Natl. Acad. Sci. USA 1997, 94, 11445–11450. [Google Scholar] [CrossRef]
- Chen, Y.-B.; Xu, J.; Skanderup, A.J.; Dong, Y.; Brannon, A.R.; Wang, L.; Won, H.H.; Wang, P.I.; Nanjangud, G.J.; Jungbluth, A.A.; et al. Molecular Analysis of Aggressive Renal Cell Carcinoma with Unclassified Histology Reveals Distinct Subsets. Nat. Commun. 2016, 7, 13131. [Google Scholar] [CrossRef]
- Moch, H.; Amin, M.B.; Berney, D.M.; Compérat, E.M.; Gill, A.J.; Hartmann, A.; Menon, S.; Raspollini, M.R.; Rubin, M.A.; Srigley, J.R.; et al. The 2022 World Health Organization Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2022, 82, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Nova-Camacho, L.M.; Martin-Arruti, M.; Ruiz Díaz, I.; Panizo-Santos, Á. Papillary Renal Neoplasm With Reverse Polarity. Arch. Pathol. Lab. Med. 2022, 28, 728–734. [Google Scholar] [CrossRef]
- Argani, P.; Reuter, V.E.; Eble, J.N.; Vlatkovic, L.; Yaskiv, O.; Swanson, D.; Dickson, B.C.; Antonescu, C.R.; Matoso, A.; Gagan, J.; et al. Biphasic Hyalinizing Psammomatous Renal Cell Carcinoma (BHP RCC): A Distinctive Neoplasm Associated With Somatic NF2 Mutations. Am. J. Surg. Pathol. 2020, 44, 901–916. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.; Duh, F.M.; Chen, F.; Kishida, T.; Glenn, G.; Choyke, P.; Scherer, S.W.; Zhuang, Z.; Lubensky, I.; Dean, M.; et al. Germline and Somatic Mutations in the Tyrosine Kinase Domain of the MET Proto-Oncogene in Papillary Renal Carcinomas. Nat. Genet. 1997, 16, 68–73. [Google Scholar] [CrossRef]
- Schmidt, L.; Junker, K.; Nakaigawa, N.; Kinjerski, T.; Weirich, G.; Miller, M.; Lubensky, I.; Neumann, H.P.; Brauch, H.; Decker, J.; et al. Novel Mutations of the MET Proto-Oncogene in Papillary Renal Carcinomas. Oncogene 1999, 18, 2343–2350. [Google Scholar] [CrossRef] [PubMed]
- Dharmawardana, P.G.; Giubellino, A.; Bottaro, D.P. Hereditary Papillary Renal Carcinoma Type I. Curr. Mol. Med. 2004, 4, 855–868. [Google Scholar] [CrossRef]
- Organ, S.L.; Tsao, M.-S. An Overview of the C-MET Signaling Pathway. Ther. Adv. Med. Oncol. 2011, 3, S7–S19. [Google Scholar] [CrossRef]
- Brunelli, M.; Eble, J.N.; Zhang, S.; Martignoni, G.; Delahunt, B.; Cheng, L. Eosinophilic and Classic Chromophobe Renal Cell Carcinomas Have Similar Frequent Losses of Multiple Chromosomes from among Chromosomes 1, 2, 6, 10, and 17, and This Pattern of Genetic Abnormality Is Not Present in Renal Oncocytoma. Mod. Pathol. Off. J. U. S. Can. Acad. Pathol. Inc 2005, 18, 161–169. [Google Scholar] [CrossRef]
- Ball, M.W.; Gorin, M.A.; Drake, C.G.; Hammers, H.J.; Allaf, M.E. The Landscape of Whole-Genome Alterations and Pathologic Features in Genitourinary Malignancies: An Analysis of the Cancer Genome Atlas. Eur. Urol. Focus 2017, 3, 584–589. [Google Scholar] [CrossRef]
- Casuscelli, J.; Weinhold, N.; Gundem, G.; Wang, L.; Zabor, E.C.; Drill, E.; Wang, P.I.; Nanjangud, G.J.; Redzematovic, A.; Nargund, A.M.; et al. Genomic Landscape and Evolution of Metastatic Chromophobe Renal Cell Carcinoma. JCI Insight 2017, 2, e92688. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.F.; Ricketts, C.J.; Wang, M.; Yang, L.; Cherniack, A.D.; Shen, H.; Buhay, C.; Kang, H.; Kim, S.C.; Fahey, C.C.; et al. The Somatic Genomic Landscape of Chromophobe Renal Cell Carcinoma. Cancer Cell 2014, 26, 319–330. [Google Scholar] [CrossRef]
- Pavlovich, C.P.; Walther, M.M.; Eyler, R.A.; Hewitt, S.M.; Zbar, B.; Linehan, W.M.; Merino, M.J. Renal Tumors in the Birt-Hogg-Dubé Syndrome. Am. J. Surg. Pathol. 2002, 26, 1542–1552. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.S.; Linehan, W.M. Molecular Genetics and Clinical Features of Birt-Hogg-Dubé Syndrome. Nat. Rev. Urol. 2015, 12, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Toro, J.R.; Wei, M.-H.; Glenn, G.M.; Weinreich, M.; Toure, O.; Vocke, C.; Turner, M.; Choyke, P.; Merino, M.J.; Pinto, P.A.; et al. BHD Mutations, Clinical and Molecular Genetic Investigations of Birt-Hogg-Dubé Syndrome: A New Series of 50 Families and a Review of Published Reports. J. Med. Genet. 2008, 45, 321–331. [Google Scholar] [CrossRef]
- Schmidt, L.S.; Linehan, W.M. FLCN: The Causative Gene for Birt-Hogg-Dubé Syndrome. Gene 2018, 640, 28–42. [Google Scholar] [CrossRef]
- Baba, M.; Hong, S.-B.; Sharma, N.; Warren, M.B.; Nickerson, M.L.; Iwamatsu, A.; Esposito, D.; Gillette, W.K.; Hopkins, R.F.; Hartley, J.L.; et al. Folliculin Encoded by the BHD Gene Interacts with a Binding Protein, FNIP1, and AMPK, and Is Involved in AMPK and MTOR Signaling. Proc. Natl. Acad. Sci. USA 2006, 103, 15552–15557. [Google Scholar] [CrossRef]
- Hasumi, H.; Baba, M.; Hong, S.-B.; Hasumi, Y.; Huang, Y.; Yao, M.; Valera, V.A.; Linehan, W.M.; Schmidt, L.S. Identification and Characterization of a Novel Folliculin-Interacting Protein FNIP2. Gene 2008, 415, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Msaouel, P.; Malouf, G.G.; Su, X.; Yao, H.; Tripathi, D.N.; Soeung, M.; Gao, J.; Rao, P.; Coarfa, C.; Creighton, C.J.; et al. Comprehensive Molecular Characterization Identifies Distinct Genomic and Immune Hallmarks of Renal Medullary Carcinoma. Cancer Cell 2020, 37, 720–734.e13. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, I.P.M.; Alam, N.A.; Rowan, A.J.; Barclay, E.; Jaeger, E.E.M.; Kelsell, D.; Leigh, I.; Gorman, P.; Lamlum, H.; Rahman, S.; et al. Germline Mutations in FH Predispose to Dominantly Inherited Uterine Fibroids, Skin Leiomyomata and Papillary Renal Cell Cancer. Nat. Genet. 2002, 30, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.-H.; Toure, O.; Glenn, G.M.; Pithukpakorn, M.; Neckers, L.; Stolle, C.; Choyke, P.; Grubb, R.; Middelton, L.; Turner, M.L.; et al. Novel Mutations in FH and Expansion of the Spectrum of Phenotypes Expressed in Families with Hereditary Leiomyomatosis and Renal Cell Cancer. J. Med. Genet. 2006, 43, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Bayley, J.-P.; Launonen, V.; Tomlinson, I.P.M. The FH Mutation Database: An Online Database of Fumarate Hydratase Mutations Involved in the MCUL (HLRCC) Tumor Syndrome and Congenital Fumarase Deficiency. BMC Med. Genet. 2008, 9, 20. [Google Scholar] [CrossRef]
- Ooi, A. Advances in Hereditary Leiomyomatosis and Renal Cell Carcinoma (HLRCC) Research. Semin. Cancer Biol. 2020, 61, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Sporn, M.B.; Liby, K.T. NRF2 and Cancer: The Good, the Bad and the Importance of Context. Nat. Rev. Cancer 2012, 12, 564–571. [Google Scholar] [CrossRef]
- Ohh, M.; Park, C.W.; Ivan, M.; Hoffman, M.A.; Kim, T.Y.; Huang, L.E.; Pavletich, N.; Chau, V.; Kaelin, W.G. Ubiquitination of Hypoxia-Inducible Factor Requires Direct Binding to the Beta-Domain of the von Hippel-Lindau Protein. Nat. Cell Biol. 2000, 2, 423–427. [Google Scholar] [CrossRef]
- Schmidt, L.S.; Linehan, W.M. Genetic Predisposition to Kidney Cancer. Semin. Oncol. 2016, 43, 566–574. [Google Scholar] [CrossRef]
- Vanharanta, S.; Buchta, M.; McWhinney, S.R.; Virta, S.K.; Peçzkowska, M.; Morrison, C.D.; Lehtonen, R.; Januszewicz, A.; Järvinen, H.; Juhola, M.; et al. Early-Onset Renal Cell Carcinoma as a Novel Extraparaganglial Component of SDHB-Associated Heritable Paraganglioma. Am. J. Hum. Genet. 2004, 74, 153–159. [Google Scholar] [CrossRef]
- Ricketts, C.J.; Shuch, B.; Vocke, C.D.; Metwalli, A.R.; Bratslavsky, G.; Middelton, L.; Yang, Y.; Wei, M.-H.; Pautler, S.E.; Peterson, J.; et al. Succinate Dehydrogenase Kidney Cancer: An Aggressive Example of the Warburg Effect in Cancer. J. Urol. 2012, 188, 2063–2071. [Google Scholar] [CrossRef]
- Pollard, P.J.; Brière, J.J.; Alam, N.A.; Barwell, J.; Barclay, E.; Wortham, N.C.; Hunt, T.; Mitchell, M.; Olpin, S.; Moat, S.J.; et al. Accumulation of Krebs Cycle Intermediates and Over-Expression of HIF1alpha in Tumours Which Result from Germline FH and SDH Mutations. Hum. Mol. Genet. 2005, 14, 2231–2239. [Google Scholar] [CrossRef]
- Ivan, M.; Haberberger, T.; Gervasi, D.C.; Michelson, K.S.; Günzler, V.; Kondo, K.; Yang, H.; Sorokina, I.; Conaway, R.C.; Conaway, J.W.; et al. Biochemical Purification and Pharmacological Inhibition of a Mammalian Prolyl Hydroxylase Acting on Hypoxia-Inducible Factor. Proc. Natl. Acad. Sci. USA 2002, 99, 13459–13464. [Google Scholar] [CrossRef]
- Bindra, R.S.; Vasselli, J.R.; Stearman, R.; Linehan, W.M.; Klausner, R.D. VHL-Mediated Hypoxia Regulation of Cyclin D1 in Renal Carcinoma Cells. Cancer Res. 2002, 62, 3014–3019. [Google Scholar] [PubMed]
- Kondo, K.; Kim, W.Y.; Lechpammer, M.; Kaelin, W.G. Inhibition of HIF2alpha Is Sufficient to Suppress PVHL-Defective Tumor Growth. PLoS Biol. 2003, 1, E83. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Klco, J.; Nakamura, E.; Lechpammer, M.; Kaelin, W.G. Inhibition of HIF Is Necessary for Tumor Suppression by the von Hippel-Lindau Protein. Cancer Cell 2002, 1, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Gui, Y.; Gao, S.; Tang, A.; Hu, X.; Huang, Y.; Jia, W.; Li, Z.; He, M.; Sun, L.; et al. Frequent Mutations of Genes Encoding Ubiquitin-Mediated Proteolysis Pathway Components in Clear Cell Renal Cell Carcinoma. Nat. Genet. 2011, 44, 17–19. [Google Scholar] [CrossRef]
- Bardella, C.; El-Bahrawy, M.; Frizzell, N.; Adam, J.; Ternette, N.; Hatipoglu, E.; Howarth, K.; O’Flaherty, L.; Roberts, I.; Turner, G.; et al. Aberrant Succination of Proteins in Fumarate Hydratase-Deficient Mice and HLRCC Patients Is a Robust Biomarker of Mutation Status. J. Pathol. 2011, 225, 4–11. [Google Scholar] [CrossRef]
- Adam, J.; Hatipoglu, E.; O’Flaherty, L.; Ternette, N.; Sahgal, N.; Lockstone, H.; Baban, D.; Nye, E.; Stamp, G.W.; Wolhuter, K.; et al. Renal Cyst Formation in Fh1-Deficient Mice Is Independent of the Hif/Phd Pathway: Roles for Fumarate in KEAP1 Succination and Nrf2 Signaling. Cancer Cell 2011, 20, 524–537. [Google Scholar] [CrossRef]
- Saxena, N.; Maio, N.; Crooks, D.R.; Ricketts, C.J.; Yang, Y.; Wei, M.-H.; Fan, T.W.-M.; Lane, A.N.; Sourbier, C.; Singh, A.; et al. SDHB-Deficient Cancers: The Role of Mutations That Impair Iron Sulfur Cluster Delivery. J. Natl. Cancer Inst. 2016, 108, djv287. [Google Scholar] [CrossRef]
- Xiao, M.; Yang, H.; Xu, W.; Ma, S.; Lin, H.; Zhu, H.; Liu, L.; Liu, Y.; Yang, C.; Xu, Y.; et al. Inhibition of α-KG-Dependent Histone and DNA Demethylases by Fumarate and Succinate That Are Accumulated in Mutations of FH and SDH Tumor Suppressors. Genes Dev. 2012, 26, 1326–1338. [Google Scholar] [CrossRef]
- Argani, P. MiT Family Translocation Renal Cell Carcinoma. Semin. Diagn. Pathol. 2015, 32, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, E.C.; Ricketts, C.J.; Rais-Bahrami, S.; Yang, Y.; Merino, M.J.; Bottaro, D.P.; Srinivasan, R.; Linehan, W.M. Molecular Genetics and Cellular Features of TFE3 and TFEB Fusion Kidney Cancers. Nat. Rev. Urol. 2014, 11, 465–475. [Google Scholar] [CrossRef]
- La Spina, M.; Contreras, P.S.; Rissone, A.; Meena, N.K.; Jeong, E.; Martina, J.A. MiT/TFE Family of Transcription Factors: An Evolutionary Perspective. Front. Cell Dev. Biol. 2020, 8, 609683. [Google Scholar] [CrossRef]
- Argani, P.; Reuter, V.E.; Zhang, L.; Sung, Y.-S.; Ning, Y.; Epstein, J.I.; Netto, G.J.; Antonescu, C.R. TFEB-Amplified Renal Cell Carcinomas: An Aggressive Molecular Subset Demonstrating Variable Melanocytic Marker Expression and Morphologic Heterogeneity. Am. J. Surg. Pathol. 2016, 40, 1484–1495. [Google Scholar] [CrossRef]
- Xia, Q.-Y.; Wang, X.-T.; Ye, S.-B.; Wang, X.; Li, R.; Shi, S.-S.; Fang, R.; Zhang, R.-S.; Ma, H.-H.; Lu, Z.-F.; et al. Novel Gene Fusion of PRCC-MITF Defines a New Member of MiT Family Translocation Renal Cell Carcinoma: Clinicopathological Analysis and Detection of the Gene Fusion by RNA Sequencing and FISH. Histopathology 2018, 72, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, G.; Fanelli, M.; Larocca, A.M.V.; Germinario, C.A.; Rutigliano, M.; Vavallo, A.; Selvaggi, F.P.; Bettocchi, C.; Battaglia, M.; Ditonno, P. Serum Sarcosine Increases the Accuracy of Prostate Cancer Detection in Patients with Total Serum PSA Less than 4.0 Ng/Ml. Prostate 2012, 72, 1611–1621. [Google Scholar] [CrossRef]
- Lucarelli, G.; Ditonno, P.; Bettocchi, C.; Spilotros, M.; Rutigliano, M.; Vavallo, A.; Galleggiante, V.; Fanelli, M.; Larocca, A.M.V.; Germinario, C.A.; et al. Serum Sarcosine Is a Risk Factor for Progression and Survival in Patients with Metastatic Castration-Resistant Prostate Cancer. Future Oncol. Lond. Engl. 2013, 9, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, G.; Rutigliano, M.; Galleggiante, V.; Giglio, A.; Palazzo, S.; Ferro, M.; Simone, C.; Bettocchi, C.; Battaglia, M.; Ditonno, P. Metabolomic Profiling for the Identification of Novel Diagnostic Markers in Prostate Cancer. Expert Rev. Mol. Diagn. 2015, 15, 1211–1224. [Google Scholar] [CrossRef]
- Lucarelli, G.; Loizzo, D.; Ferro, M.; Rutigliano, M.; Vartolomei, M.D.; Cantiello, F.; Buonerba, C.; Di Lorenzo, G.; Terracciano, D.; De Cobelli, O.; et al. Metabolomic Profiling for the Identification of Novel Diagnostic Markers and Therapeutic Targets in Prostate Cancer: An Update. Expert Rev. Mol. Diagn. 2019, 19, 377–387. [Google Scholar] [CrossRef] [PubMed]
- di Meo, N.A.; Loizzo, D.; Pandolfo, S.D.; Autorino, R.; Ferro, M.; Porta, C.; Stella, A.; Bizzoca, C.; Vincenti, L.; Crocetto, F.; et al. Metabolomic Approaches for Detection and Identification of Biomarkers and Altered Pathways in Bladder Cancer. Int. J. Mol. Sci. 2022, 23, 4173. [Google Scholar] [CrossRef]
- Lucarelli, G.; Loizzo, D.; Franzin, R.; Battaglia, S.; Ferro, M.; Cantiello, F.; Castellano, G.; Bettocchi, C.; Ditonno, P.; Battaglia, M. Metabolomic Insights into Pathophysiological Mechanisms and Biomarker Discovery in Clear Cell Renal Cell Carcinoma. Expert Rev. Mol. Diagn. 2019, 19, 397–407. [Google Scholar] [CrossRef] [PubMed]
- di Meo, N.A.; Lasorsa, F.; Rutigliano, M.; Loizzo, D.; Ferro, M.; Stella, A.; Bizzoca, C.; Vincenti, L.; Pandolfo, S.D.; Autorino, R.; et al. Renal Cell Carcinoma as a Metabolic Disease: An Update on Main Pathways, Potential Biomarkers, and Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 14360. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, G.; Rutigliano, M.; Sanguedolce, F.; Galleggiante, V.; Giglio, A.; Cagiano, S.; Bufo, P.; Maiorano, E.; Ribatti, D.; Ranieri, E.; et al. Increased Expression of the Autocrine Motility Factor Is Associated With Poor Prognosis in Patients With Clear Cell-Renal Cell Carcinoma. Medicine (Baltimore) 2015, 94, e2117. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, G.; Galleggiante, V.; Rutigliano, M.; Sanguedolce, F.; Cagiano, S.; Bufo, P.; Lastilla, G.; Maiorano, E.; Ribatti, D.; Giglio, A.; et al. Metabolomic Profile of Glycolysis and the Pentose Phosphate Pathway Identifies the Central Role of Glucose-6-Phosphate Dehydrogenase in Clear Cell-Renal Cell Carcinoma. Oncotarget 2015, 6, 13371–13386. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, G.; Rutigliano, M.; Sallustio, F.; Ribatti, D.; Giglio, A.; Lepore Signorile, M.; Grossi, V.; Sanese, P.; Napoli, A.; Maiorano, E.; et al. Integrated Multi-Omics Characterization Reveals a Distinctive Metabolic Signature and the Role of NDUFA4L2 in Promoting Angiogenesis, Chemoresistance, and Mitochondrial Dysfunction in Clear Cell Renal Cell Carcinoma. Aging 2018, 10, 3957–3985. [Google Scholar] [CrossRef]
- Lucarelli, G.; Ferro, M.; Loizzo, D.; Bianchi, C.; Terracciano, D.; Cantiello, F.; Bell, L.N.; Battaglia, S.; Porta, C.; Gernone, A.; et al. Integration of Lipidomics and Transcriptomics Reveals Reprogramming of the Lipid Metabolism and Composition in Clear Cell Renal Cell Carcinoma. Metabolites 2020, 10, 509. [Google Scholar] [CrossRef]
- Lucarelli, G.; Rutigliano, M.; Loizzo, D.; di Meo, N.A.; Lasorsa, F.; Mastropasqua, M.; Maiorano, E.; Bizzoca, C.; Vincenti, L.; Battaglia, M.; et al. MUC1 Tissue Expression and Its Soluble Form CA15-3 Identify a Clear Cell Renal Cell Carcinoma with Distinct Metabolic Profile and Poor Clinical Outcome. Int. J. Mol. Sci. 2022, 23, 13968. [Google Scholar] [CrossRef]
- Duclos, V.; Iep, A.; Gomez, L.; Goldfarb, L.; Besson, F.L. PET Molecular Imaging: A Holistic Review of Current Practice and Emerging Perspectives for Diagnosis, Therapeutic Evaluation and Prognosis in Clinical Oncology. Int. J. Mol. Sci. 2021, 22, 4159. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.P.; Gorin, M.A.; Solnes, L.B.; Ball, M.W.; Choudhary, A.; Pierorazio, P.M.; Epstein, J.I.; Javadi, M.S.; Allaf, M.E.; Baras, A.S. Correlation of 99mTc-Sestamibi Uptake in Renal Masses with Mitochondrial Content and Multi-Drug Resistance Pump Expression. EJNMMI Res. 2017, 7, 80. [Google Scholar] [CrossRef]
- Gormley, T.S.; Van Every, M.J.; Moreno, A.J. Renal Oncocytoma: Preoperative Diagnosis Using Technetium 99m Sestamibi Imaging. Urology 1996, 48, 33–39. [Google Scholar] [CrossRef]
- Rowe, S.P.; Gorin, M.A.; Gordetsky, J.; Ball, M.W.; Pierorazio, P.M.; Higuchi, T.; Epstein, J.I.; Allaf, M.E.; Javadi, M.S. Initial Experience Using 99mTc-MIBI SPECT/CT for the Differentiation of Oncocytoma from Renal Cell Carcinoma. Clin. Nucl. Med. 2015, 40, 309–313. [Google Scholar] [CrossRef]
- Gorin, M.A.; Rowe, S.P.; Baras, A.S.; Solnes, L.B.; Ball, M.W.; Pierorazio, P.M.; Pavlovich, C.P.; Epstein, J.I.; Javadi, M.S.; Allaf, M.E. Prospective Evaluation of (99m)Tc-Sestamibi SPECT/CT for the Diagnosis of Renal Oncocytomas and Hybrid Oncocytic/Chromophobe Tumors. Eur. Urol. 2016, 69, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Abu-Ghanem, Y.; Powles, T.; Capitanio, U.; Beisland, C.; Järvinen, P.; Stewart, G.D.; Gudmundsson, E.O.; Lam, T.B.; Marconi, L.; Fernandéz-Pello, S.; et al. The Impact of Histological Subtype on the Incidence, Timing, and Patterns of Recurrence in Patients with Renal Cell Carcinoma After Surgery-Results from RECUR Consortium. Eur. Urol. Oncol. 2021, 4, 473–482. [Google Scholar] [CrossRef]
- Tzortzakakis, A.; Gustafsson, O.; Karlsson, M.; Ekström-Ehn, L.; Ghaffarpour, R.; Axelsson, R. Visual Evaluation and Differentiation of Renal Oncocytomas from Renal Cell Carcinomas by Means of 99mTc-Sestamibi SPECT/CT. EJNMMI Res. 2017, 7, 29. [Google Scholar] [CrossRef]
- Asi, T.; Tuncali, M.Ç.; Tuncel, M.; Alkanat, N.E.İ.; Hazir, B.; Kösemehmetoğlu, K.; Baydar, D.E.; Akdoğan, B. The Role of Tc-99m MIBI Scintigraphy in Clinical T1 Renal Mass Assessment: Does It Have a Real Benefit? Urol. Oncol. 2020, 38, 937.e11–937.e17. [Google Scholar] [CrossRef]
- Su, Z.T.; Patel, H.D.; Huang, M.M.; Meyer, A.R.; Pavlovich, C.P.; Pierorazio, P.M.; Javadi, M.S.; Allaf, M.E.; Rowe, S.P.; Gorin, M.A. Cost-Effectiveness Analysis of 99mTc-Sestamibi SPECT/CT to Guide Management of Small Renal Masses. Eur. Urol. Focus 2021, 7, 827–834. [Google Scholar] [CrossRef]
- Baniak, N.; Flood, T.A.; Buchanan, M.; Dal Cin, P.; Hirsch, M.S. Carbonic Anhydrase IX (CA9) Expression in Multiple Renal Epithelial Tumour Subtypes. Histopathology 2020, 77, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Divgi, C.R.; Pandit-Taskar, N.; Jungbluth, A.A.; Reuter, V.E.; Gönen, M.; Ruan, S.; Pierre, C.; Nagel, A.; Pryma, D.A.; Humm, J.; et al. Preoperative Characterisation of Clear-Cell Renal Carcinoma Using Iodine-124-Labelled Antibody Chimeric G250 (124I-CG250) and PET in Patients with Renal Masses: A Phase I Trial. Lancet Oncol. 2007, 8, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Divgi, C.R.; Uzzo, R.G.; Gatsonis, C.; Bartz, R.; Treutner, S.; Yu, J.Q.; Chen, D.; Carrasquillo, J.A.; Larson, S.; Bevan, P.; et al. Positron Emission Tomography/Computed Tomography Identification of Clear Cell Renal Cell Carcinoma: Results from the REDECT Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 187–194. [Google Scholar] [CrossRef]
- Stillebroer, A.B.; Franssen, G.M.; Mulders, P.F.A.; Oyen, W.J.G.; van Dongen, G.A.M.S.; Laverman, P.; Oosterwijk, E.; Boerman, O.C. ImmunoPET Imaging of Renal Cell Carcinoma with (124)I- and (89)Zr-Labeled Anti-CAIX Monoclonal Antibody CG250 in Mice. Cancer Biother. Radiopharm. 2013, 28, 510–515. [Google Scholar] [CrossRef]
- Verhoeff, S.R.; van Es, S.C.; Boon, E.; van Helden, E.; Angus, L.; Elias, S.G.; Oosting, S.F.; Aarntzen, E.H.; Brouwers, A.H.; Kwee, T.C.; et al. Lesion Detection by [89Zr]Zr-DFO-Girentuximab and [18F]FDG-PET/CT in Patients with Newly Diagnosed Metastatic Renal Cell Carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1931–1939. [Google Scholar] [CrossRef]
- Muselaers, C.H.J.; Stillebroer, A.B.; Desar, I.M.E.; Boers-Sonderen, M.J.; van Herpen, C.M.L.; de Weijert, M.C.A.; Langenhuijsen, J.F.; Oosterwijk, E.; Leenders, W.P.J.; Boerman, O.C.; et al. Tyrosine Kinase Inhibitor Sorafenib Decreases 111In-Girentuximab Uptake in Patients with Clear Cell Renal Cell Carcinoma. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2014, 55, 242–247. [Google Scholar] [CrossRef]
- Muselaers, C.H.J.; Boerman, O.C.; Oosterwijk, E.; Langenhuijsen, J.F.; Oyen, W.J.G.; Mulders, P.F.A. Indium-111-Labeled Girentuximab ImmunoSPECT as a Diagnostic Tool in Clear Cell Renal Cell Carcinoma. Eur. Urol. 2013, 63, 1101–1106. [Google Scholar] [CrossRef]
- Rahmim, A.; Zaidi, H. PET versus SPECT: Strengths, Limitations and Challenges. Nucl. Med. Commun. 2008, 29, 193–207. [Google Scholar] [CrossRef]
- Karivedu, V.; Jain, A.L.; Eluvathingal, T.J.; Sidana, A. Role of Positron Emission Tomography Imaging in Metabolically Active Renal Cell Carcinoma. Curr. Urol. Rep. 2019, 20, 56. [Google Scholar] [CrossRef] [PubMed]
- Ozülker, T.; Ozülker, F.; Ozbek, E.; Ozpaçaci, T. A Prospective Diagnostic Accuracy Study of F-18 Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography in the Evaluation of Indeterminate Renal Masses. Nucl. Med. Commun. 2011, 32, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Nakhoda, Z.; Torigian, D.A.; Saboury, B.; Hofheinz, F.; Alavi, A. Assessment of the Diagnostic Performance of (18)F-FDG-PET/CT for Detection and Characterization of Solid Renal Malignancies. Hell. J. Nucl. Med. 2013, 16, 19–24. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Ding, H.-J.; Chen, J.-H.; Chao, C.-H.; Lu, Y.-Y.; Lin, W.-Y.; Kao, C.-H. Meta-Analysis of the Diagnostic Performance of [18F]FDG-PET and PET/CT in Renal Cell Carcinoma. Cancer Imaging Off. Publ. Int. Cancer Imaging Soc. 2012, 12, 464–474. [Google Scholar] [CrossRef]
- Gündoğan, C.; Çermik, T.F.; Erkan, E.; Yardimci, A.H.; Behzatoğlu, K.; Tatar, G.; Okçu, O.; Toktaş, M.G. Role of Contrast-Enhanced 18F-FDG PET/CT Imaging in the Diagnosis and Staging of Renal Tumors. Nucl. Med. Commun. 2018, 39, 1174–1182. [Google Scholar] [CrossRef]
- Alongi, P.; Picchio, M.; Zattoni, F.; Spallino, M.; Gianolli, L.; Saladini, G.; Evangelista, L. Recurrent Renal Cell Carcinoma: Clinical and Prognostic Value of FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, H.-Y.; Lee, S. Role of F-18 FDG PET/CT in the Follow-up of Asymptomatic Renal Cell Carcinoma Patients for Postoperative Surveillance: Based on Conditional Survival Analysis. J. Cancer Res. Clin. Oncol. 2022, 148, 215–224. [Google Scholar] [CrossRef]
- Elahmadawy, M.A.; Elazab, M.S.S.; Ahmed, S.; Salama, M. Diagnostic Value of F-18 FDG PET/CT for Local and Distant Disease Relapse Surveillance in Surgically Treated RCC Patients: Can It Aid in Establishing Consensus Follow up Strategy? Nucl. Med. Rev. Cent. East. Eur. 2018, 21, 85–91. [Google Scholar] [CrossRef]
- Singh, H.; Arora, G.; Nayak, B.; Sharma, A.; Singh, G.; Kumari, K.; Jana, S.; Patel, C.; Pandey, A.K.; Seth, A.; et al. Semi-Quantitative F-18-FDG PET/Computed Tomography Parameters for Prediction of Grade in Patients with Renal Cell Carcinoma and the Incremental Value of Diuretics. Nucl. Med. Commun. 2020, 41, 485–493. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, C.; Li, W.; Chen, X.; Li, Z.; Liao, X.; Cui, Y.; Zhao, G.; Liu, M.; Fu, Z. 2-[18F]FDG PET/CT Parameters Associated with WHO/ISUP Grade in Clear Cell Renal Cell Carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhao, S.; Zuo, C.; Ren, F. FDG PET/CT and CT Findings of Renal Cell Carcinoma With Sarcomatoid Differentiation. AJR Am. J. Roentgenol. 2020, 215, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Kayani, I.; Avril, N.; Bomanji, J.; Chowdhury, S.; Rockall, A.; Sahdev, A.; Nathan, P.; Wilson, P.; Shamash, J.; Sharpe, K.; et al. Sequential FDG-PET/CT as a Biomarker of Response to Sunitinib in Metastatic Clear Cell Renal Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 6021–6028. [Google Scholar] [CrossRef] [PubMed]
- Ueno, D.; Yao, M.; Tateishi, U.; Minamimoto, R.; Makiyama, K.; Hayashi, N.; Sano, F.; Murakami, T.; Kishida, T.; Miura, T.; et al. Early Assessment by FDG-PET/CT of Patients with Advanced Renal Cell Carcinoma Treated with Tyrosine Kinase Inhibitors Is Predictive of Disease Course. BMC Cancer 2012, 12, 162. [Google Scholar] [CrossRef]
- Tabei, T.; Nakaigawa, N.; Kaneta, T.; Ikeda, I.; Kondo, K.; Makiyama, K.; Hasumi, H.; Hayashi, N.; Kawahara, T.; Izumi, K.; et al. Early Assessment with 18F-2-Fluoro-2-Deoxyglucose Positron Emission Tomography/Computed Tomography to Predict Short-Term Outcome in Clear Cell Renal Carcinoma Treated with Nivolumab. BMC Cancer 2019, 19, 298. [Google Scholar] [CrossRef]
- Rowe, S.P.; Gorin, M.A.; Pomper, M.G. Imaging of Prostate-Specific Membrane Antigen with Small-Molecule PET Radiotracers: From the Bench to Advanced Clinical Applications. Annu. Rev. Med. 2019, 70, 461–477. [Google Scholar] [CrossRef]
- Rhee, H.; Blazak, J.; Tham, C.M.; Ng, K.L.; Shepherd, B.; Lawson, M.; Preston, J.; Vela, I.; Thomas, P.; Wood, S. Pilot Study: Use of Gallium-68 PSMA PET for Detection of Metastatic Lesions in Patients with Renal Tumour. EJNMMI Res. 2016, 6, 76. [Google Scholar] [CrossRef]
- Gao, J.; Xu, Q.; Fu, Y.; He, K.; Zhang, C.; Zhang, Q.; Shi, J.; Zhao, X.; Wang, F.; Guo, H. Comprehensive Evaluation of 68Ga-PSMA-11 PET/CT Parameters for Discriminating Pathological Characteristics in Primary Clear-Cell Renal Cell Carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Oyama, N.; Okazawa, H.; Kusukawa, N.; Kaneda, T.; Miwa, Y.; Akino, H.; Fujibayashi, Y.; Yonekura, Y.; Welch, M.J.; Yokoyama, O. 11C-Acetate PET Imaging for Renal Cell Carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 422–427. [Google Scholar] [CrossRef]
- Kotzerke, J.; Linné, C.; Meinhardt, M.; Steinbach, J.; Wirth, M.; Baretton, G.; Abolmaali, N.; Beuthien-Baumann, B. [1-(11)C]Acetate Uptake Is Not Increased in Renal Cell Carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 884–888. [Google Scholar] [CrossRef]
- Ho, C.-L.; Chen, S.; Ho, K.M.T.; Chan, W.K.; Leung, Y.L.; Cheng, K.C.; Wong, K.-N.; Cheung, M.; Wong, K.K. Dual-Tracer PET/CT in Renal Angiomyolipoma and Subtypes of Renal Cell Carcinoma. Clin. Nucl. Med. 2012, 37, 1075–1082. [Google Scholar] [CrossRef]
- Chung, B.I.; Leow, J.J.; Gelpi-Hammerschmidt, F.; Wang, Y.; Del Giudice, F.; De, S.; Chou, E.P.; Song, K.H.; Almario, L.; Chang, S.L. Racial Disparities in Postoperative Complications After Radical Nephrectomy: A Population-Based Analysis. Urology 2015, 85, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Cheung, H.; Wang, Y.; Chang, S.L.; Khandwala, Y.; Del Giudice, F.; Chung, B.I. Adoption of Robot-Assisted Partial Nephrectomies: A Population-Based Analysis of U.S. Surgeons from 2004 to 2013. J. Endourol. 2017, 31, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Nicolazzo, C.; Busetto, G.M.; Del Giudice, F.; Sperduti, I.; Giannarelli, D.; Gradilone, A.; Gazzaniga, P.; de Berardinis, E.; Raimondi, C. The Long-Term Prognostic Value of Survivin Expressing Circulating Tumor Cells in Patients with High-Risk Non-Muscle Invasive Bladder Cancer (NMIBC). J. Cancer Res. Clin. Oncol. 2017, 143, 1971–1976. [Google Scholar] [CrossRef]
- Salciccia, S.; Capriotti, A.L.; Laganà, A.; Fais, S.; Logozzi, M.; De Berardinis, E.; Busetto, G.M.; Di Pierro, G.B.; Ricciuti, G.P.; Del Giudice, F.; et al. Biomarkers in Prostate Cancer Diagnosis: From Current Knowledge to the Role of Metabolomics and Exosomes. Int. J. Mol. Sci. 2021, 22, 4367. [Google Scholar] [CrossRef] [PubMed]
- Giovannone, R.; Busetto, G.M.; Antonini, G.; De Cobelli, O.; Ferro, M.; Tricarico, S.; Del Giudice, F.; Ragonesi, G.; Conti, S.L.; Lucarelli, G.; et al. Hyperhomocysteinemia as an Early Predictor of Erectile Dysfunction: International Index of Erectile Function (IIEF) and Penile Doppler Ultrasound Correlation With Plasma Levels of Homocysteine. Medicine 2015, 94, e1556. [Google Scholar] [CrossRef]
- Salciccia, S.; Del Giudice, F.; Gentile, V.; Mastroianni, C.M.; Pasculli, P.; Di Lascio, G.; Ciardi, M.R.; Sperduti, I.; Maggi, M.; De Berardinis, E.; et al. Interplay between Male Testosterone Levels and the Risk for Subsequent Invasive Respiratory Assistance among COVID-19 Patients at Hospital Admission. Endocrine 2020, 70, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Busetto, G.M.; Giovannone, R.; Antonini, G.; Rossi, A.; Del Giudice, F.; Tricarico, S.; Ragonesi, G.; Gentile, V.; De Berardinis, E. Short-Term Pretreatment with a Dual 5α-Reductase Inhibitor before Bipolar Transurethral Resection of the Prostate (B-TURP): Evaluation of Prostate Vascularity and Decreased Surgical Blood Loss in Large Prostates. BJU Int. 2015, 116, 117–123. [Google Scholar] [CrossRef]
- Alessandrino, F.; Shinagare, A.B.; Bossé, D.; Choueiri, T.K.; Krajewski, K.M. Radiogenomics in Renal Cell Carcinoma. Abdom. Radiol. N. Y. 2019, 44, 1990–1998. [Google Scholar] [CrossRef] [PubMed]
- Sasaguri, K.; Takahashi, N. CT and MR Imaging for Solid Renal Mass Characterization. Eur. J. Radiol. 2018, 99, 40–54. [Google Scholar] [CrossRef]
- Khaleel, S.; Katims, A.; Cumarasamy, S.; Rosenzweig, S.; Attalla, K.; Hakimi, A.A.; Mehrazin, R. Radiogenomics in Clear Cell Renal Cell Carcinoma: A Review of the Current Status and Future Directions. Cancers 2022, 14, 2085. [Google Scholar] [CrossRef] [PubMed]
- Rutman, A.M.; Kuo, M.D. Radiogenomics: Creating a Link between Molecular Diagnostics and Diagnostic Imaging. Eur. J. Radiol. 2009, 70, 232–241. [Google Scholar] [CrossRef]
- Lo Gullo, R.; Daimiel, I.; Morris, E.A.; Pinker, K. Combining Molecular and Imaging Metrics in Cancer: Radiogenomics. Insights Imaging 2020, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Katabathina, V.S.; Marji, H.; Khanna, L.; Ramani, N.; Yedururi, S.; Dasyam, A.; Menias, C.O.; Prasad, S.R. Decoding Genes: Current Update on Radiogenomics of Select Abdominal Malignancies. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2020, 40, 1600–1626. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Zhong, W.; Li, Y.; Yuan, Y.; Zhong, H.; Huang, C.; Huang, J.; Lin, Y.; Huang, J. Characterization of Molecular Heterogeneity Associated With Tumor Microenvironment in Clear Cell Renal Cell Carcinoma to Aid Immunotherapy. Front. Cell Dev. Biol. 2021, 9, 736540. [Google Scholar] [CrossRef] [PubMed]
- Gopal, N.; Yazdian Anari, P.; Turkbey, E.; Jones, E.C.; Malayeri, A.A. The Next Paradigm Shift in the Management of Clear Cell Renal Cancer: Radiogenomics-Definition, Current Advances, and Future Directions. Cancers 2022, 14, 793. [Google Scholar] [CrossRef]
- Karlo, C.A.; Di Paolo, P.L.; Chaim, J.; Hakimi, A.A.; Ostrovnaya, I.; Russo, P.; Hricak, H.; Motzer, R.; Hsieh, J.J.; Akin, O. Radiogenomics of Clear Cell Renal Cell Carcinoma: Associations between CT Imaging Features and Mutations. Radiology 2014, 270, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Tamboli, P.; Vikram, R.; Rao, A. Imaging-Genomic Pipeline for Identifying Gene Mutations Using Three-Dimensional Intra-Tumor Heterogeneity Features. J. Med. Imaging Bellingham Wash 2015, 2, 041009. [Google Scholar] [CrossRef]
- Kocak, B.; Durmaz, E.S.; Ates, E.; Ulusan, M.B. Radiogenomics in Clear Cell Renal Cell Carcinoma: Machine Learning-Based High-Dimensional Quantitative CT Texture Analysis in Predicting PBRM1 Mutation Status. AJR Am. J. Roentgenol. 2019, 212, W55–W63. [Google Scholar] [CrossRef] [PubMed]
- Kocak, B.; Durmaz, E.S.; Kaya, O.K.; Kilickesmez, O. Machine Learning-Based Unenhanced CT Texture Analysis for Predicting BAP1 Mutation Status of Clear Cell Renal Cell Carcinomas. Acta Radiol. Stockh. Swed. 1987 2020, 61, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, Z.; Hannan, R.; Thomas, K.; Pedrosa, I.; Kapur, P.; Brugarolas, J.; Mou, X.; Wang, J. Reliable Gene Mutation Prediction in Clear Cell Renal Cell Carcinoma through Multi-Classifier Multi-Objective Radiogenomics Model. Phys. Med. Biol. 2018, 63, 215008. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Zhang, L.; Qi, Z.; Shen, Q.; Hu, Z.; Chen, F. Identifying BAP1 Mutations in Clear-Cell Renal Cell Carcinoma by CT Radiomics: Preliminary Findings. Front. Oncol. 2020, 10, 279. [Google Scholar] [CrossRef]
- Yin, Q.; Hung, S.-C.; Rathmell, W.K.; Shen, L.; Wang, L.; Lin, W.; Fielding, J.R.; Khandani, A.H.; Woods, M.E.; Milowsky, M.I.; et al. Integrative Radiomics Expression Predicts Molecular Subtypes of Primary Clear Cell Renal Cell Carcinoma. Clin. Radiol. 2018, 73, 782–791. [Google Scholar] [CrossRef]
- Lee, H.W.; Cho, H.-H.; Joung, J.-G.; Jeon, H.G.; Jeong, B.C.; Jeon, S.S.; Lee, H.M.; Nam, D.-H.; Park, W.-Y.; Kim, C.K.; et al. Integrative Radiogenomics Approach for Risk Assessment of Post-Operative Metastasis in Pathological T1 Renal Cell Carcinoma: A Pilot Retrospective Cohort Study. Cancers 2020, 12, 866. [Google Scholar] [CrossRef]
- Lin, P.; Lin, Y.-Q.; Gao, R.-Z.; Wen, R.; Qin, H.; He, Y.; Yang, H. Radiomic Profiling of Clear Cell Renal Cell Carcinoma Reveals Subtypes with Distinct Prognoses and Molecular Pathways. Transl. Oncol. 2021, 14, 101078. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, G.; Sun, Q.; Zhai, G.; Wu, G.; Li, Z.-C. Validation of CT Radiomics for Prediction of Distant Metastasis after Surgical Resection in Patients with Clear Cell Renal Cell Carcinoma: Exploring the Underlying Signaling Pathways. Eur. Radiol. 2021, 31, 5032–5040. [Google Scholar] [CrossRef]
- Shinagare, A.B.; Vikram, R.; Jaffe, C.; Akin, O.; Kirby, J.; Huang, E.; Freymann, J.; Sainani, N.I.; Sadow, C.A.; Bathala, T.K.; et al. Radiogenomics of Clear Cell Renal Cell Carcinoma: Preliminary Findings of The Cancer Genome Atlas-Renal Cell Carcinoma (TCGA-RCC) Imaging Research Group. Abdom. Imaging 2015, 40, 1684–1692. [Google Scholar] [CrossRef]
- Bowen, L.; Xiaojing, L. Radiogenomics of Clear Cell Renal Cell Carcinoma: Associations Between MRNA-Based Subtyping and CT Imaging Features. Acad. Radiol. 2019, 26, e32–e37. [Google Scholar] [CrossRef]
- Marigliano, C.; Badia, S.; Bellini, D.; Rengo, M.; Caruso, D.; Tito, C.; Miglietta, S.; Palleschi, G.; Pastore, A.L.; Carbone, A.; et al. Radiogenomics in Clear Cell Renal Cell Carcinoma: Correlations Between Advanced CT Imaging (Texture Analysis) and MicroRNAs Expression. Technol. Cancer Res. Treat. 2019, 18, 1533033819878458. [Google Scholar] [CrossRef]
- Cianflone, F.; Lazarevic, D.; Palmisano, A.; Fallara, G.; Larcher, A.; Freschi, M.; Dell’Antonio, G.; Scotti, G.M.; Morelli, M.J.; Ferrara, A.M.; et al. Radiomic and GEnomic Approaches for the Enhanced DIagnosis of Clear Cell REnal Cancer (REDIRECt): A Translational Pilot Methodological Study. Transl. Androl. Urol. 2022, 11, 149–158. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, H.; Chen, L.; Luo, Y.; Ma, X.; Zhao, Y. Exploration of an Integrative Prognostic Model of Radiogenomics Features With Underlying Gene Expression Patterns in Clear Cell Renal Cell Carcinoma. Front. Oncol. 2021, 11, 640881. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Lin, C.; Li, X.; Quan, X. Renal Cell Carcinoma: Predicting DNA Methylation Subtyping and Its Consequences on Overall Survival With Computed Tomography Imaging Characteristics. J. Comput. Assist. Tomogr. 2020, 44, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, N.; Jonasch, E.; Zapala, M.; Korn, R.L.; Brooks, J.D.; Ljungberg, B.; Kuo, M.D. The Radiogenomic Risk Score Stratifies Outcomes in a Renal Cell Cancer Phase 2 Clinical Trial. Eur. Radiol. 2016, 26, 2798–2807. [Google Scholar] [CrossRef]
- Zeng, H.; Chen, L.; Wang, M.; Luo, Y.; Huang, Y.; Ma, X. Integrative Radiogenomics Analysis for Predicting Molecular Features and Survival in Clear Cell Renal Cell Carcinoma. Aging 2021, 13, 9960–9975. [Google Scholar] [CrossRef]
- Gao, J.; Ye, F.; Han, F.; Wang, X.; Jiang, H.; Zhang, J. A Novel Radiogenomics Biomarker Based on Hypoxic-Gene Subset: Accurate Survival and Prognostic Prediction of Renal Clear Cell Carcinoma. Front. Oncol. 2021, 11, 739815. [Google Scholar] [CrossRef]
- Gao, J.; Ye, F.; Han, F.; Jiang, H.; Zhang, J. A Radiogenomics Biomarker Based on Immunological Heterogeneity for Non-Invasive Prognosis of Renal Clear Cell Carcinoma. Front. Immunol. 2022, 13, 956679. [Google Scholar] [CrossRef] [PubMed]
- Cen, D.; Xu, L.; Zhang, S.; Chen, Z.; Huang, Y.; Li, Z.; Liang, B. Renal Cell Carcinoma: Predicting RUNX3 Methylation Level and Its Consequences on Survival with CT Features. Eur. Radiol. 2019, 29, 5415–5422. [Google Scholar] [CrossRef]
- Jamshidi, N.; Jonasch, E.; Zapala, M.; Korn, R.L.; Aganovic, L.; Zhao, H.; Tumkur Sitaram, R.; Tibshirani, R.J.; Banerjee, S.; Brooks, J.D.; et al. The Radiogenomic Risk Score: Construction of a Prognostic Quantitative, Noninvasive Image-Based Molecular Assay for Renal Cell Carcinoma. Radiology 2015, 277, 114–123. [Google Scholar] [CrossRef]
- Acosta, P.H.; Panwar, V.; Jarmale, V.; Christie, A.; Jasti, J.; Margulis, V.; Rakheja, D.; Cheville, J.; Leibovich, B.C.; Parker, A.; et al. Intratumoral Resolution of Driver Gene Mutation Heterogeneity in Renal Cancer Using Deep Learning. Cancer Res. 2022, 82, 2792–2806. [Google Scholar] [CrossRef] [PubMed]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Math, M.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; et al. Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef]
- Al-Kadi, O.S.; Watson, D. Texture Analysis of Aggressive and Nonaggressive Lung Tumor CE CT Images. IEEE Trans. Biomed. Eng. 2008, 55, 1822–1830. [Google Scholar] [CrossRef] [PubMed]
- Udayakumar, D.; Zhang, Z.; Xi, Y.; Dwivedi, D.K.; Fulkerson, M.; Haldeman, S.; McKenzie, T.; Yousuf, Q.; Joyce, A.; Hajibeigi, A.; et al. Deciphering Intratumoral Molecular Heterogeneity in Clear Cell Renal Cell Carcinoma with a Radiogenomics Platform. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 4794–4806. [Google Scholar] [CrossRef]
- Mazurowski, M.A. Radiogenomics: What It Is and Why It Is Important. J. Am. Coll. Radiol. JACR 2015, 12, 862–866. [Google Scholar] [CrossRef]
- Bodalal, Z.; Trebeschi, S.; Nguyen-Kim, T.D.L.; Schats, W.; Beets-Tan, R. Radiogenomics: Bridging Imaging and Genomics. Abdom. Radiol. N. Y. 2019, 44, 1960–1984. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yi, X.; Lu, C.; Pang, Y.; Zu, X.; Chen, M.; Guan, X. Background, Applications and Challenges of Radiogenomics in Genitourinary Tumor. Am. J. Cancer Res. 2021, 11, 1936–1945. [Google Scholar]
- Pinker, K.; Shitano, F.; Sala, E.; Do, R.K.; Young, R.J.; Wibmer, A.G.; Hricak, H.; Sutton, E.J.; Morris, E.A. Background, Current Role, and Potential Applications of Radiogenomics: Role and Applications of Radiogenomics. J. Magn. Reson. Imaging 2018, 47, 604–620. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Pei, X.; Yin, X.-P.; Ren, J.-L.; Wang, Y.; Ma, L.-Y.; Du, X.-G.; Gao, B.-L. Radiomics Models Based on Enhanced Computed Tomography to Distinguish Clear Cell from Non-Clear Cell Renal Cell Carcinomas. Sci. Rep. 2021, 11, 13729. [Google Scholar] [CrossRef] [PubMed]
- Horvat, N.; Bates, D.D.B.; Petkovska, I. Novel Imaging Techniques of Rectal Cancer: What Do Radiomics and Radiogenomics Have to Offer? A Literature Review. Abdom. Radiol. 2019, 44, 3764–3774. [Google Scholar] [CrossRef] [PubMed]
- Zanfardino, M.; Pane, K.; Mirabelli, P.; Salvatore, M.; Franzese, M. TCGA-TCIA Impact on Radiogenomics Cancer Research: A Systematic Review. Int. J. Mol. Sci. 2019, 20, 6033. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.X.; Lee, A.M.; Yang, L.; Zhang, P.; Davatzikos, C.; Maris, J.M.; Diskin, S.J. Imaging Genomics in Cancer Research: Limitations and Promises. Br. J. Radiol. 2016, 89, 20151030. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Cho, J.Y. Imaging Findings of Common Benign Renal Tumors in the Era of Small Renal Masses: Differential Diagnosis from Small Renal Cell Carcinoma: Current Status and Future Perspectives. Korean J. Radiol. 2015, 16, 99. [Google Scholar] [CrossRef]
- D’Avella, C.; Abbosh, P.; Pal, S.K.; Geynisman, D.M. Mutations in Renal Cell Carcinoma. Urol. Oncol. 2020, 38, 763–773. [Google Scholar] [CrossRef]
- Benson, K.A.; Murray, S.L.; Doyle, R.; Doyle, B.; Dorman, A.M.; Sadlier, D.; Brennan, E.; Large, M.; Cavalleri, G.L.; Godson, C.; et al. Diagnostic Utility of Genetic Testing in Patients Undergoing Renal Biopsy. Mol. Case Stud. 2020, 6, a005462. [Google Scholar] [CrossRef]
- Dragoescu, E.A.; Liu, L. Indications for Renal Fine Needle Aspiration Biopsy in the Era of Modern Imaging Modalities. CytoJournal 2013, 10, 15. [Google Scholar] [CrossRef]
- Berenguer, R.; Pastor-Juan, M.d.R.; Canales-Vázquez, J.; Castro-García, M.; Villas, M.V.; Mansilla Legorburo, F.; Sabater, S. Radiomics of CT Features May Be Nonreproducible and Redundant: Influence of CT Acquisition Parameters. Radiology 2018, 288, 407–415. [Google Scholar] [CrossRef]
- Coy, H.; Young, J.R.; Douek, M.L.; Brown, M.S.; Sayre, J.; Raman, S.S. Quantitative Computer-Aided Diagnostic Algorithm for Automated Detection of Peak Lesion Attenuation in Differentiating Clear Cell from Papillary and Chromophobe Renal Cell Carcinoma, Oncocytoma, and Fat-Poor Angiomyolipoma on Multiphasic Multidetector Computed Tomography. Abdom. Radiol. 2017, 42, 1919–1928. [Google Scholar] [CrossRef]
- Yap, F.Y.; Varghese, B.A.; Cen, S.Y.; Hwang, D.H.; Lei, X.; Desai, B.; Lau, C.; Yang, L.L.; Fullenkamp, A.J.; Hajian, S.; et al. Shape and Texture-Based Radiomics Signature on CT Effectively Discriminates Benign from Malignant Renal Masses. Eur. Radiol. 2021, 31, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
| Radiogenomics | Advantages | Limitations |
|---|---|---|
| Improvement in screening and management of at-risk individuals [78,79,80,81,82] | Many retrospective studies [203,204] | |
| Assessment of whole tumor molecular pattern [203] | No standardization of protocols [199,203,204,234] | |
| Increasing use of artificial intelligence in statistical models [206,226] | Lack of proper clinical trials [215] | |
| Prediction of overall survival and metastasis [211,212,213,218,219,220,221,222,223,224,225] | Lack of multivariate models of mutation status [214] | |
| Possibility to tailor treatment according to genomic expressions [230,231] | Inter-observer variability [8] | |
| Genetic and epigenetic profiling of scanned lesions [203] | Automatic extraction of features still underpowered [235] | |
| Preoperative identification of benign lesions [238] | High cost of genomic testing to validate data [236] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferro, M.; Musi, G.; Marchioni, M.; Maggi, M.; Veccia, A.; Del Giudice, F.; Barone, B.; Crocetto, F.; Lasorsa, F.; Antonelli, A.; et al. Radiogenomics in Renal Cancer Management—Current Evidence and Future Prospects. Int. J. Mol. Sci. 2023, 24, 4615. https://doi.org/10.3390/ijms24054615
Ferro M, Musi G, Marchioni M, Maggi M, Veccia A, Del Giudice F, Barone B, Crocetto F, Lasorsa F, Antonelli A, et al. Radiogenomics in Renal Cancer Management—Current Evidence and Future Prospects. International Journal of Molecular Sciences. 2023; 24(5):4615. https://doi.org/10.3390/ijms24054615
Chicago/Turabian StyleFerro, Matteo, Gennaro Musi, Michele Marchioni, Martina Maggi, Alessandro Veccia, Francesco Del Giudice, Biagio Barone, Felice Crocetto, Francesco Lasorsa, Alessandro Antonelli, and et al. 2023. "Radiogenomics in Renal Cancer Management—Current Evidence and Future Prospects" International Journal of Molecular Sciences 24, no. 5: 4615. https://doi.org/10.3390/ijms24054615
APA StyleFerro, M., Musi, G., Marchioni, M., Maggi, M., Veccia, A., Del Giudice, F., Barone, B., Crocetto, F., Lasorsa, F., Antonelli, A., Schips, L., Autorino, R., Busetto, G. M., Terracciano, D., Lucarelli, G., & Tataru, O. S. (2023). Radiogenomics in Renal Cancer Management—Current Evidence and Future Prospects. International Journal of Molecular Sciences, 24(5), 4615. https://doi.org/10.3390/ijms24054615















