Mechanism of Action of Peripheral Nerve Stimulation for Chronic Pain: A Narrative Review
Abstract
1. Introduction
2. Gate Theory
3. History of PNS
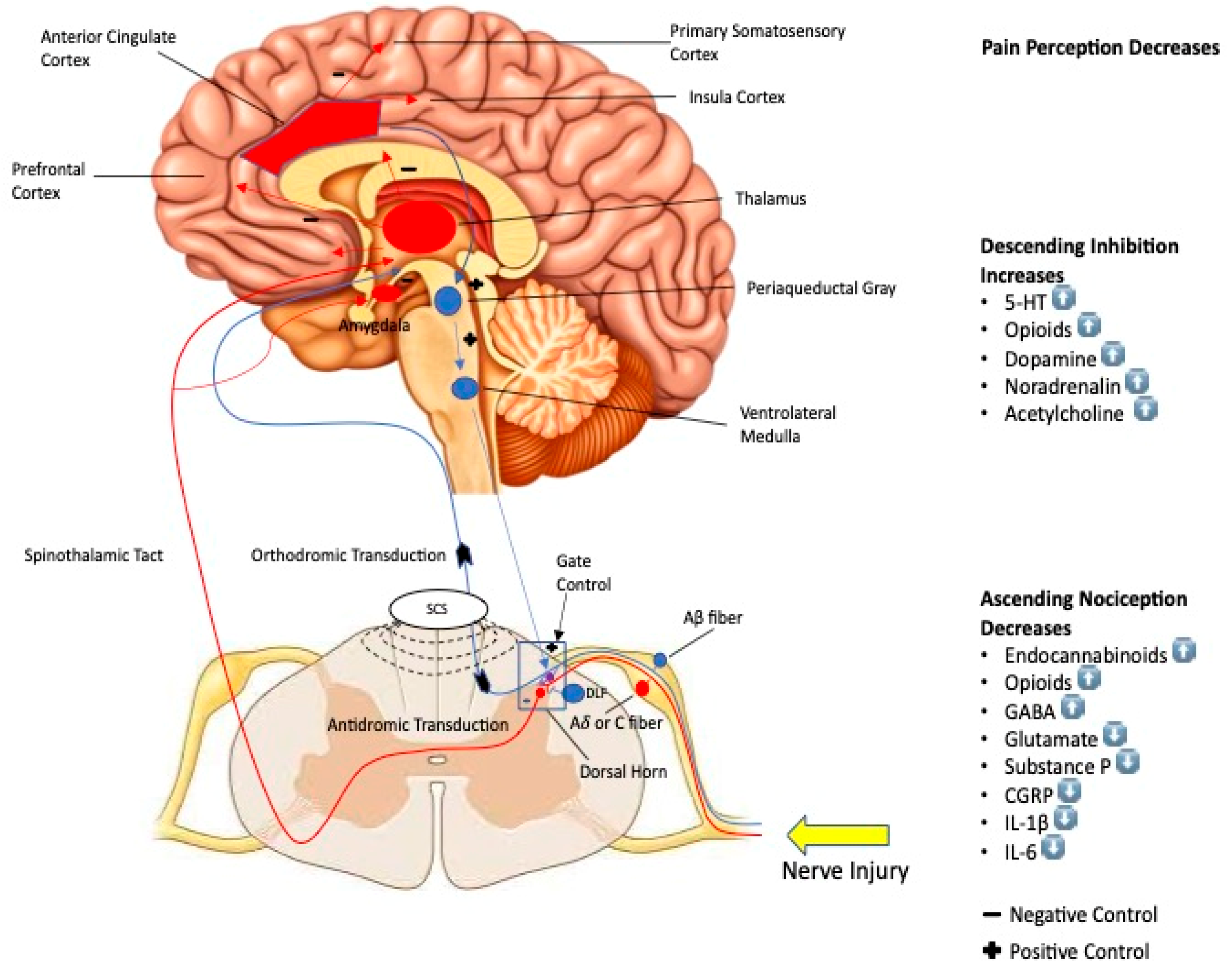
4. Mechanism of Action
4.1. Peripheral Pathway
4.2. Central Pathway
5. Craniofacial PNS
6. PNS in the Acute Perioperative Setting
7. Current Available Devices
8. PNS Safety
9. Future Directions
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Breivik, H.; Collett, B.; Ventafridda, V.; Cohen, R.; Gallacher, D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur. J. Pain 2006, 10, 287–333. [Google Scholar] [CrossRef] [PubMed]
- Tajerian, M.; Alvarado, S.; Millecamps, M.; Vachon, P.; Crosby, C.; Bushnell, M.C.; Stone, L.S. Peripheral nerve injury is associated with chronic, reversible changes in global DNA methylation in the mouse prefrontal cortex. PLoS ONE 2013, 8, e55259. [Google Scholar] [CrossRef]
- Abejón, D.; Pérez-Cajaraville, J. Peripheral nerve stimulation: Definition. Prog. Neurol. Surg. 2011, 24, 203–209. [Google Scholar] [PubMed]
- Ottestad, E.; Orlovich, D.S. History of Peripheral Nerve Stimulation-Update for the 21st Century. Pain Med. 2020, 21, S3. [Google Scholar] [CrossRef]
- Cambiaghi, M.; Sconocchia, S. Scribonius Largus (probably before 1CE-after 48CE). J. Neurol. 2018, 265, 2466–2468. [Google Scholar] [CrossRef] [PubMed]
- Althaus, J. A Treatise on Medical Electricity, Theoretical and Practical; and Its Use in the Treatment of Paralysis, Neuralgia, and Other Diseases. Br. Foreign Med. Chir. Rev. 1859, 24, 157–158. [Google Scholar] [CrossRef]
- Gildenberg, P.L. Evolution of neuromodulation. Stereotact. Funct. Neurosurg. 2005, 83, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Weiner, R.L.; Reed, K.L. Peripheral neurostimulation for control of intractable occipital neuralgia. Neuromodulation 1999, 2, 217–221. [Google Scholar] [CrossRef]
- Slavin, K.V. History of peripheral nerve stimulation. Prog. Neurol. Surg. 2011, 24, 1–15. [Google Scholar]
- Rozand, V.; Grosprêtre, S.; Stapley, P.J.; Lepers, R. Assessment of Neuromuscular Function Using Percutaneous Electrical Nerve Stimulation. J. Vis. Exp. 2015, 103, e52974. [Google Scholar]
- Milby, A.H.; Halpern, C.H.; Baltuch, G.H. Vagus nerve stimulation for epilepsy and depression. Neurotherapeutics 2008, 5, 75–85. [Google Scholar] [CrossRef]
- Melzack, R.; Wall, P.D. Pain mechanisms: A new theory. Science 1965, 150, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Mendell, L.M. Constructing and deconstructing the gate theory of pain. Pain 2014, 155, 210–216. [Google Scholar] [CrossRef]
- Zhou, W.; Benharash, P. Effects and mechanisms of acupuncture based on the principle of meridians. J. Acupunct. Meridian Stud. 2014, 7, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Coutaux, A. Non-pharmacological treatments for pain relief: TENS and acupuncture. Jt. Bone Spine 2017, 84, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Claydon, L.S.; Chesterton, L.S.; Barlas, P.; Sim, J. Dose-specific effects of transcutaneous electrical nerve stimulation (TENS) on experimental pain: A systematic review. Clin. J. Pain 2011, 27, 635–647. [Google Scholar] [CrossRef]
- Caylor, J.; Reddy, R.; Yin, S.; Cui, C.; Huang, M.; Huang, C.; Lerman, I. Spinal cord stimulation in chronic pain: Evidence and theory for mechanisms of action. Bioelectron. Med. 2019, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Banks, G.P.; Winfree, C.J. Chapter 5–Peripheral Nerve Stimulation. In Functional Neurosurgery and Neuromodulation; Raslan, A.M., Burchiel, K.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 35–41. [Google Scholar]
- Sweet, W.H. Control of pain by direct electrical stimulation of peripheral nerves. Clin. Neurosurg. 1976, 23, 103–111. [Google Scholar] [CrossRef]
- Long, D.M. Electrical stimulation for the control of pain. Arch. Surg. 1977, 112, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Law, J.D.; Swett, J.; Kirsch, W.M. Retrospective analysis of 22 patients with chronic pain treated by peripheral nerve stimulation. J. Neurosurg. 1980, 52, 482–485. [Google Scholar] [CrossRef]
- Huntoon, M.A.; Burgher, A.H. Ultrasound-guided permanent implantation of peripheral nerve stimulation (PNS) system for neuropathic pain of the extremities: Original cases and outcomes. Pain Med. 2009, 10, 1369–1377. [Google Scholar] [CrossRef]
- Mobbs, R.J.; Blum, P.; Rossato, R. Mesh electrode for peripheral nerve stimulation. J. Clin. Neurosci. 2003, 10, 476–477. [Google Scholar] [CrossRef] [PubMed]
- Corriveau, M.; Lake, W.; Hanna, A. Nerve Stimulation for Pain. Neurosurg. Clin. N. Am. 2019, 30, 257–264. [Google Scholar] [CrossRef] [PubMed]
- De Leon-Casasola, O.A. Spinal cord and peripheral nerve stimulation techniques for neuropathic pain. J. Pain Symptom Manag. 2009, 38, S28–S38. [Google Scholar] [CrossRef]
- Deer, T.R.; Eldabe, S.; Falowski, S.M.; Huntoon, M.A.; Staats, P.S.; Cassar, I.R.; Boggs, J.W. Peripherally Induced Reconditioning of the Central Nervous System: A Proposed Mechanistic Theory for Sustained Relief of Chronic Pain with Percutaneous Peripheral Nerve Stimulation. J. Pain Res. 2021, 14, 721–736. [Google Scholar] [CrossRef]
- Strand, N.H.; D’Souza, R.; Wie, C.; Covington, S.; Maita, M.; Freeman, J.; Maloney, J. Mechanism of Action of Peripheral Nerve Stimulation. Curr. Pain Headache Rep. 2021, 25, 47. [Google Scholar] [CrossRef]
- Gracely, R.H.; Lynch, S.A.; Bennett, G.J. Painful neuropathy: Altered central processing maintained dynamically by peripheral input. Pain 1992, 51, 175–194. [Google Scholar] [CrossRef]
- Meacham, K.; Shepherd, A.; Mohapatra, D.P.; Haroutounian, S. Neuropathic Pain: Central vs. Peripheral Mechanisms. Curr. Pain Headache Rep. 2017, 21, 28. [Google Scholar] [CrossRef]
- Treede, R.D.; Kenshalo, D.R.; Gracely, R.H.; Jones, A.K. The cortical representation of pain. Pain 1999, 79, 105–111. [Google Scholar] [CrossRef]
- Ballantyne, J.C.; Fishman, S.M.; Rathmell, J.P. Bonica’s Management of Pain; Wolters Kluwer Health: Alphen aan den Rijn, The Netherlands, 2019. [Google Scholar]
- Papuć, E.; Rejdak, K. The role of neurostimulation in the treatment of neuropathic pain. Ann. Agric. Environ. Med. 2013, 1, 14–17. [Google Scholar]
- Lin, T.; Gargya, A.; Singh, H.; Sivanesan, E.; Gulati, A. Mechanism of Peripheral Nerve Stimulation in Chronic Pain. Pain Med. 2020, 21, S6–S12. [Google Scholar] [CrossRef]
- Gasser, H.S. Changes in nerve-potentials produced by rapidly repeated stimuli and their relation to the responsiveness of nerve to stimulation. Am. J. Physiol. 1935, 111, 35–50. [Google Scholar] [CrossRef]
- Franz, D.N.; Iggo, A. Conduction failure in myelinated and non-myelinated axons at low temperatures. J. Physiol. 1968, 199, 319–345. [Google Scholar] [CrossRef]
- Torebjörk, H.E.; Hallin, R.G. Responses in human A and C fibres to repeated electrical intradermal stimulation. J. Neurol. Neurosurg. Psychiatry 1974, 37, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.F.; Hsu, S.T.; Chen, C.C.; Yao, C.H.; Lin, J.H.; Chen, Y.H.; Chen, Y.S. Effects of Electrical Stimulation on Peripheral Nerve Regeneration in a Silicone Rubber Conduit in Taxol-Treated Rats. Materials 2020, 13, 1063. [Google Scholar] [CrossRef]
- Swett, J.E.; Law, J.D. Analgesia with peripheral nerve stimulation, absence of a peripheral mechanism. Pain 1983, 15, 55–70. [Google Scholar] [CrossRef]
- Ahmed, S.; Plazier, M.; Ost, J.; Stassijns, G.; Deleye, S.; Ceyssens, S.; Vanneste, S. The effect of occipital nerve field stimulation on the descending pain pathway in patients with fibromyalgia: A water PET and EEG imaging study. BMC Neurol. 2018, 18, 191. [Google Scholar] [CrossRef]
- Meyer-Frießem, C.H.; Wiegand, T.; Eitner, L.; Maier, C.; Mainka, T.; Vollert, J.; Enax-Krumova, E.K. Effects of Spinal Cord and Peripheral Nerve Stimulation Reflected in Sensory Profiles and Endogenous Pain Modulation. Clin. J. Pain 2019, 35, 111–120. [Google Scholar] [CrossRef]
- Bandeira, J.S.; Antunes, L.D.C.; Soldatelli, M.D.; Sato, J.R.; Fregni, F.; Caumo, W. Functional spectroscopy mapping of pain processing cortical areas during non-painful peripheral electrical stimulation of the accessory spinal nerve. Front. Hum. Neurosci. 2019, 13, 200. [Google Scholar] [CrossRef]
- García-Magro, N.; Negredo, P.; Martin, Y.B.; Nuñez, Á.; Avendaño, C. Modulation of mechanosensory vibrissal responses in the trigeminocervical complex by stimulation of the greater occipital nerve in a rat model of trigeminal neuropathic pain. J. Headache Pain 2020, 21, 96. [Google Scholar] [CrossRef]
- Men, D.S.; Matsui, Y. Peripheral nerve stimulation increases serotonin and dopamine metabolites in rat spinal cord. Brain Res. Bull. 1994, 33, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Schaible, H.G.; Hope, P.J.; Lang, C.W.; Duggan, A.W. Calcitonin Gene-related Peptide Causes Intraspinal Spreading of Substance P Released by Peripheral Stimulation. Eur. J. Neurosci. 1992, 4, 750–757. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, T.; Tiwari, V.; Shu, B.; Zhang, C.; Wang, Y.; Guan, Y. Effects of Combined Electrical Stimulation of the Dorsal Column and Dorsal Roots on Wide-Dynamic-Range Neuronal Activity in Nerve-Injured Rats. Neuromodulation 2015, 18, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xu, Q.; Cheong, Y.K.; Shechter, R.; Sdrulla, A.; He, S.Q.; Guan, Y. Comparison of intensity-dependent inhibition of spinal wide-dynamic range neurons by dorsal column and peripheral nerve stimulation in a rat model of neuropathic pain. Eur. J. Pain 2014, 18, 978–988. [Google Scholar] [CrossRef]
- Chung, J.M.; Fang, Z.R.; Hori, Y.; Lee, K.H.; Willis, W.D. Prolonged inhibition of primate spinothalamic tract cells by peripheral nerve stimulation. Pain 1984, 19, 259–275. [Google Scholar] [CrossRef]
- Bartsch, T.; Goadsby, P.J. Increased responses in trigeminocervical nociceptive neurons to cervical input after stimulation of the dura mater. Brain 2003, 126, 1801–1813. [Google Scholar] [CrossRef]
- Bartsch, T.; Goadsby, P.J. The trigeminocervical complex and migraine: Current concepts and synthesis. Curr. Pain Headache Rep. 2003, 7, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Yecies, T.; Li, S.; Zhang, Y.; Cai, H.; Shen, B.; Wang, J.; Tai, C. Spinal interneuronal mechanisms underlying pudendal and tibial neuromodulation of bladder function in cats. Exp. Neurol. 2018, 308, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.F.; Feng, W.W.; Liu, Y.P.; Dong, Y.B.; Gao, L.; Yang, H.L. Electrical peripheral nerve stimulation relieves bone cancer pain by inducing Arc protein expression in the spinal cord dorsal horn. J. Pain Res. 2018, 11, 599–609. [Google Scholar] [CrossRef]
- Castel-Lacanal, E.; Marque, P.; Tardy, J.; de Boissezon, X.; Guiraud, V.; Chollet, F.; Simonetta-Moreau, M. Induction of Cortical Plastic Changes in Wrist Muscles by Paired Associative Stimulation in the Recovery Phase of Stroke Patients. Neurorehabilit. Neural Repair 2009, 23, 366–372. [Google Scholar] [CrossRef]
- Ceccanti, M.; Onesti, E.; Rubino, A.; Cambieri, C.; Tartaglia, G.; Miscioscia, A.; Inghilleri, M. Modulation of human corticospinal excitability by paired associative stimulation in patients with amyotrophic lateral sclerosis and effects of Riluzole. Brain Stimul. 2018, 11, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Matharu, M.S.; Bartsch, T.; Ward, N.; Frackowiak, R.S.; Weiner, R.; Goadsby, P.J. Central neuromodulation in chronic migraine patients with suboccipital stimulators: A PET study. Brain 2004, 127, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Kupers, R.; Van Laere, K.; Van Calenbergh, F.; Gybels, J.; Dupont, P.; Baeck, A.; Plaghki, L. Multimodal therapeutic assessment of peripheral nerve stimulation in neuropathic pain: Five case reports with a 20-year follow-up. Eur. J. Pain 2011, 15, e1–e9. [Google Scholar]
- De Ridder, D.; Vanneste, S. Burst and Tonic Spinal Cord Stimulation: Different and Common Brain Mechanisms. Neuromodulation 2016, 19, 47–59. [Google Scholar] [CrossRef]
- Stancák, A.; Kozák, J.; Vrba, I.; Tintěra, J.; Vrána, J.; Poláček, H.; Stančák, M. Functional magnetic resonance imaging of cerebral activation during spinal cord stimulation in failed back surgery syndrome patients. Eur. J. Pain 2008, 12, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Strand, N.; D’Souza, R.S.; Hagedorn, J.M.; Pritzlaff, S.; Sayed, D.; Azeem, N.; Deer, T.R. Evidence-Based Clinical Guidelines from the American Society of Pain and Neuroscience for the Use of Implantable Peripheral Nerve Stimulation in the Treatment of Chronic Pain. J. Pain Res. 2022, 15, 2483–2504. [Google Scholar] [CrossRef]
- Gilmore, C.A.; Kapural, L.; McGee, M.J.; Boggs, J.W. Percutaneous Peripheral Nerve Stimulation for Chronic Low Back Pain: Prospective Case Series With 1 Year of Sustained Relief Following Short-Term Implant. Pain Pr. 2020, 20, 310–320. [Google Scholar] [CrossRef]
- Birbaumer, N.; Lutzenberger, W.; Montoya, P.; Larbig, W.; Unertl, K.; Töpfner, S.; Flor, H. Effects of regional anesthesia on phantom limb pain are mirrored in changes in cortical reorganization. J. Neurosci. 1997, 17, 5503–5508. [Google Scholar] [CrossRef]
- Flor, H. The modification of cortical reorganization and chronic pain by sensory feedback. Appl. Psychophysiol. Biofeedback 2002, 27, 215–227. [Google Scholar] [CrossRef]
- Moseley, G.L.; Flor, H. Targeting cortical representations in the treatment of chronic pain: A review. Neurorehabil. Neural. Repair 2012, 26, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, S.; Peeters, R.; De Ridder, D.; Plazier, M.; Menovsky, T.; Sunaert, S. Central effects of occipital nerve electrical stimulation studied by functional magnetic resonance imaging. Neuromodulation 2011, 14, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, S.D.; Dodick, D.W.; Saper, J.; Huh, B.; Slavin, K.V.; Sharan, A.; Mekhail, N. Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: Results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia 2012, 32, 1165–1179. [Google Scholar] [CrossRef] [PubMed]
- Thimineur, M.; De Ridder, D. C2 area neurostimulation: A surgical treatment for fibromyalgia. Pain Med. 2007, 8, 639–646. [Google Scholar] [CrossRef]
- Plazier, M.; Dekelver, I.; Vanneste, S.; Stassijns, G.; Menovsky, T.; Thimineur, M.; De Ridder, D. Peripheral nerve stimulation for fibromyalgia. Prog. Neurol. Surg. 2011, 24, 133–146. [Google Scholar]
- Wood, P.B.; Schweinhardt, P.; Jaeger, E.; Dagher, A.; Hakyemez, H.; Rabiner, E.A.; Chizh, B.A. Fibromyalgia patients show an abnormal dopamine response to pain. Eur. J. Neurosci. 2007, 25, 3576–3582. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, K.V.; Xing, F.; Bruno, K.; Kent, A.R.; Raza, A.; Hurlemann, R.; Kinfe, T.M. A Review of Spinal and Peripheral Neuromodulation and Neuroinflammation: Lessons Learned Thus Far and Future Prospects of Biotype Development. Neuromodulation 2019, 22, 235–243. [Google Scholar] [CrossRef]
- Bari, A.A.; Pouratian, N. Brain imaging correlates of peripheral nerve stimulation. Surg. Neurol. Int. 2012, 3, S260–S268. [Google Scholar] [CrossRef]
- Willoch, F.; Gamringer, U.; Medele, R.; Steude, U.; Tölle, T.R. Analgesia by electrostimulation of the trigeminal ganglion in patients with trigeminopathic pain: A PET activation study. Pain 2003, 103, 119–130. [Google Scholar] [CrossRef]
- Abd-Elsayed, A.; D’Souza, R.S. Peripheral Nerve Stimulation: The Evolution in Pain Medicine. Biomedicines 2021, 10, 18. [Google Scholar] [CrossRef]
- Busch, C.; Smith, O.; Weaver, T.; Vallabh, J.; Abd-Elsayed, A. Peripheral Nerve Stimulation for Lower Extremity Pain. Biomedicines 2022, 10, 1666. [Google Scholar] [CrossRef]
- Mauck, W.D.; Hunt, C.L.; Olatoye, O.O.; Warner, N.S.; Lamer, T.J. Spinal Cord and Peripheral Nerve Stimulation for Painful Disorders. Adv. Anesth. 2019, 37, 163–186. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, R.A.; Swisher, M.W.; Ilfeld, B.M. Percutaneous peripheral nerve stimulation for acute postoperative pain. Pain Manag. 2019, 9, 347–354. [Google Scholar] [CrossRef]
- Xu, J.; Sun, Z.; Wu, J.; Rana, M.; Garza, J.; Zhu, A.C.; Cheng, J. Peripheral Nerve Stimulation in Pain Management: A Systematic Review. Pain Physician 2021, 24, E131–E152. [Google Scholar] [PubMed]
- Ilfeld, B.M.; Grant, S.A.; Gilmore, C.A.; Chae, J.; Wilson, R.D.; Wongsarnpigoon, A.; Boggs, J.W. Neurostimulation for Postsurgical Analgesia: A Novel System Enabling Ultrasound-Guided Percutaneous Peripheral Nerve Stimulation. Pain Pract. 2017, 17, 892–901. [Google Scholar] [CrossRef]
- Ilfeld, B.M.; Gabriel, R.A.; Said, E.T.; Monahan, A.M.; Sztain, J.F.; Abramson, W.B.; Ahmed, S.S. Ultrasound-Guided Percutaneous Peripheral Nerve Stimulation: Neuromodulation of the Sciatic Nerve for Postoperative Analgesia Following Ambulatory Foot Surgery, a Proof-of-Concept Study. Reg. Anesth. Pain Med. 2018, 43, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Ilfeld, B.M.; Said, E.T.; Finneran IV, J.J.; Sztain, J.F.; Abramson, W.B.; Gabriel, R.A.; Robertson, C.M. Ultrasound-Guided Percutaneous Peripheral Nerve Stimulation: Neuromodulation of the Femoral Nerve for Postoperative Analgesia Following Ambulatory Anterior Cruciate Ligament Reconstruction: A Proof of Concept Study. Neuromodulation 2019, 22, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Regnier, S.M.; Chen, J.; Gabriel, R.A.; Chakravarthy, K.V. A review of the StimRouter(®) peripheral neuromodulation system for chronic pain management. Pain Manag. 2021, 11, 227–236. [Google Scholar] [CrossRef]
- Mobbs, R.J.; Nair, S.; Blum, P. Peripheral nerve stimulation for the treatment of chronic pain. J. Clin. Neurosci. 2007, 14, 216–221, discussion 222–223. [Google Scholar] [CrossRef] [PubMed]
- Oswald, J.; Shahi, V.; Chakravarthy, K.V. Prospective case series on the use of peripheral nerve stimulation for focal mononeuropathy treatment. Pain Manag. 2019, 9, 551–558. [Google Scholar] [CrossRef]
- Mattie, R.D.; Kost, J.A.; Washabaugh, E.P.; Lester, D.D.; Zurn, C.A.; Crosby, N.D. Pain relief following 60-day peripheral nerve stimulation (PNS) of the cervical medial branch nerves: A real-world retrospective. In Proceedings of the Pacific Spine Pain Society Annual Conference 2022, Las Vegas, NV, USA, 16–18 September 2022. [Google Scholar]
- Nguyen, V.Q.; Bock, W.C.; Groves, C.C.; Whitney, M.; Bennett, M.E.; Lechman, T.E.; Chae, J. Fully implantable peripheral nerve stimulation for the treatment of hemiplegic shoulder pain: A case report. Am. J. Phys. Med. Rehabil. 2015, 94, 146–153. [Google Scholar] [CrossRef]
- Cohen, S.P.; Gilmore, C.A.; Rauck, R.L.; Lester, D.D.; Trainer, R.J.; Phan, T.; Boggs, J.W. Percutaneous Peripheral Nerve Stimulation for the Treatment of Chronic Pain Following Amputation. Mil. Med. 2019, 184, e267–e274. [Google Scholar] [CrossRef]
- Gilmore, C.; Ilfeld, B.; Rosenow, J.; Li, S.; Desai, M.; Hunter, C.; Boggs, J. Percutaneous peripheral nerve stimulation for the treatment of chronic neuropathic postamputation pain: A multicenter, randomized, placebo-controlled trial. Reg. Anesth. Pain Med. 2019, 44, 637–645. [Google Scholar] [CrossRef]
- Ilfeld, B.M.; Gabriel, R.A.; Saulino, M.F.; Chae, J.; Peckham, P.H.; Grant, S.A.; Boggs, J.W. Infection Rates of Electrical Leads Used for Percutaneous Neurostimulation of the Peripheral Nervous System. Pain Pr. 2017, 17, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Boggs, J.W.; Chae, J.; Bennett, M.E. Peripheral nerve stimulation for pain suppression. In Neuromodulation, 2nd ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 729–740. [Google Scholar]
- Pingree, M.J.; Hurdle, M.F.; Spinner, D.A.; Valimahomed, A.; Crosby, N.D.; Boggs, J.W. Real-world evidence of sustained improvement following 60-day peripheral nerve stimulation treatment for pain: A cross-sectional follow-up survey. Pain Manag. 2022, 12, 611–621. [Google Scholar] [CrossRef]
- Fiala, K.J.; Kim, R.B.; Martens, J.M.; Abd-Elsayed, A. Lumbar Level Peripheral Nerve Stimulation for Low Back Pain. Ochsner. J. 2022, 22, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Kalia, H.; Pritzlaff, S.; Li, A.H.; Ottestad, E.; Gulati, A.; Makous, J.; Chakravarthy, K. Application of the novel Nalu™ Neurostimulation System for peripheral nerve stimulation. Pain Manag. 2022, 12, 795–804. [Google Scholar] [CrossRef]
- Abd-Elsayed, A. Wireless Peripheral Nerve Stimulation for Treatment of Peripheral Neuralgias. Neuromodulation 2020, 23, 827–830. [Google Scholar] [CrossRef] [PubMed]
- Gilligan, C.; Volschenk, W.; Russo, M.; Green, M.; Gilmore, C.; Mehta, V.; Eldabe, S. Three-Year Durability of Restorative Neurostimulation Effectiveness in Patients With Chronic Low Back Pain and Multifidus Muscle Dysfunction. Neuromodulation 2022, 26, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Burton, C.V. Safety and clinical efficacy of implanted neuroaugmentive spinal devices for the relief of pain. Appl. Neurophysiol. 1977, 40, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Günter, C.; Delbeke, J.; Ortiz-Catalan, M. Safety of long-term electrical peripheral nerve stimulation: Review of the state of the art. J. Neuroeng. Rehabil. 2019, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Eldabe, S.; Buchser, E.; Duarte, R.V. Complications of Spinal Cord Stimulation and Peripheral Nerve Stimulation Techniques: A Review of the Literature. Pain Med. 2016, 17, 325–336. [Google Scholar] [CrossRef]
- Helm, S.; Shirsat, N.; Calodney, A.; Abd-Elsayed, A.; Kloth, D.; Soin, A.; Trescot, A. Peripheral Nerve Stimulation for Chronic Pain: A Systematic Review of Effectiveness and Safety. Pain Ther. 2021, 10, 985–1002. [Google Scholar] [CrossRef]
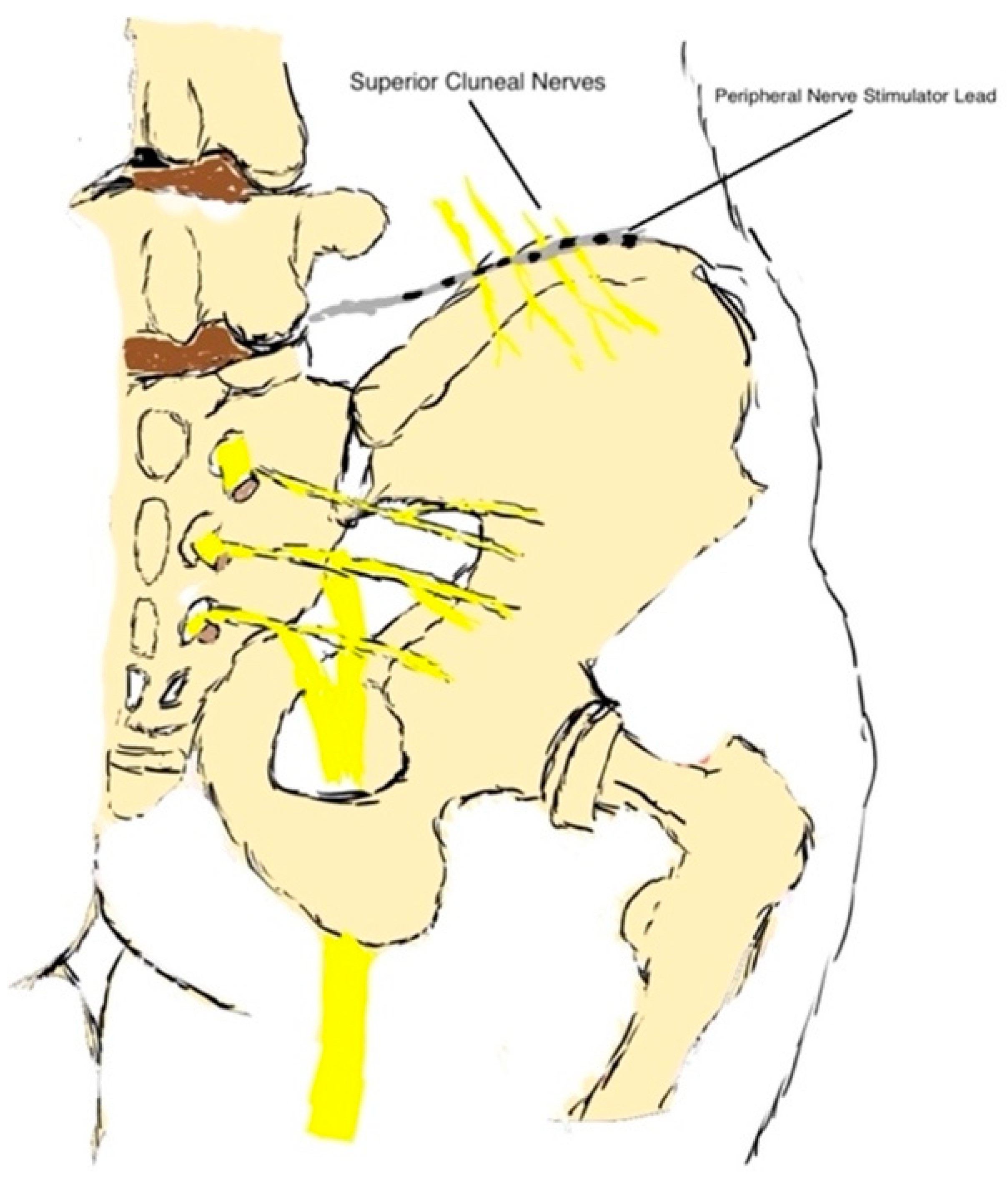
| Head/Neck | Greater Occipital Nerve |
|---|---|
| Upper Extremities | Brachial plexus Suprascapular nerve Axillary nerve Radial nerve Median nerve Ulnar nerve |
| Lower Extremities | Sciatic nerve Obturator nerve Femoral nerve Lateral femoral cutaneous nerve Genicular nerve Saphenous nerve Common peroneal nerve Tibial nerve Sural nerve Superficial peroneal nerve |
| Abdomen/Trunk/Back/Pelvis | Medial branch nerve Ilioinguinal nerve Iliohypogastric nerve Genitofemoral nerve Cluneal nerve Pudendal nerve |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong Sio, L.C.; Hom, B.; Garg, S.; Abd-Elsayed, A. Mechanism of Action of Peripheral Nerve Stimulation for Chronic Pain: A Narrative Review. Int. J. Mol. Sci. 2023, 24, 4540. https://doi.org/10.3390/ijms24054540
Ong Sio LC, Hom B, Garg S, Abd-Elsayed A. Mechanism of Action of Peripheral Nerve Stimulation for Chronic Pain: A Narrative Review. International Journal of Molecular Sciences. 2023; 24(5):4540. https://doi.org/10.3390/ijms24054540
Chicago/Turabian StyleOng Sio, Lady Christine, Brian Hom, Shuchita Garg, and Alaa Abd-Elsayed. 2023. "Mechanism of Action of Peripheral Nerve Stimulation for Chronic Pain: A Narrative Review" International Journal of Molecular Sciences 24, no. 5: 4540. https://doi.org/10.3390/ijms24054540
APA StyleOng Sio, L. C., Hom, B., Garg, S., & Abd-Elsayed, A. (2023). Mechanism of Action of Peripheral Nerve Stimulation for Chronic Pain: A Narrative Review. International Journal of Molecular Sciences, 24(5), 4540. https://doi.org/10.3390/ijms24054540






